Abstract
Study Objectives:
Determine the prevalence of central sleep apnea (CSA) in a large community-based cohort using current definitions and contrast the clinical characteristics of subjects with CSA to those with obstructive sleep apnea (OSA) and no sleep apnea.
Methods:
A cross sectional analysis of baseline data from 5,804 participants of the Sleep Heart Health study was performed. Subjects meeting contemporary diagnostic criteria for CSA and Cheyne Stokes respiration (CSR) were compared to those without sleep apnea and those with OSA. Demographic data, medical comorbidities, medication use, and sleep related symptoms were compared between the groups
Results:
The prevalences of CSA and Cheyne Stokes respiration (CSR) in this sample were 0.9 (95% confidence intervals [CI]: 0.7–1.2)% and 0.4 (95% CI: 0.3–0.6)%, respectively. Individuals with CSA were older, had lower body mass indexes (BMI), lower Epworth Sleepiness Scale scores, and were more likely to be male than individuals with obstructive sleep apnea OSA. Among those with self-reported heart failure (HF), OSA was much more common at 55.1% (95% CI: 45.6–64.6) than CSA 4.1% (95% CI: 0.3–7.9).
Conclusions:
This is the largest community-based study of the prevalence and characteristics of CSA to date and demonstrates a prevalence of CSA that is intermediate to those previously noted. Contrary to prior data from clinic based samples, individuals with heart failure were much more likely to have OSA than CSA.
Citation:
Donovan LM, Kapur VK. Prevalence and characteristics of central compared to obstructive sleep apnea: analyses from the sleep heart health study cohort. SLEEP 2016;39(7):1353–1359.
Keywords: central sleep apnea, obstructive sleep apnea, cheyne stokes respiration, heart failure
Significance.
According to contemporary criteria in the largest sleep cohort available, the current work demonstrates CSA prevalence to be 0.9% in adults aged 40 and older. Among community based subjects with heart failure, OSA is noted to be much more common than CSA.
INTRODUCTION
Central sleep apnea (CSA) is characterized by recurrent cessation or attenuation of respiration during sleep resulting from a decline or absence of ventilatory effort.1 In heart failure patients, a pattern of periodic central apneas with a crescendodecrescendo pattern of intervening breaths known as Cheyne Stokes respiration (CSR) is associated with increased mortality.2,3 The prevalence of CSA (defined as a central apnea hypopnea index [AHI] ≥ 10) in a community cohort of 741 men was noted to be 0.4% overall, but 1.1% in those aged 65 and older.4 In a later cohort of 2,911 men aged 65 and older, the prevalence of CSA (CAI ≥ 5) was appreciably higher (7.5%).5 CSA is associated with conditions including heart failure (HF), stroke (CVA), atrial fibrillation (AF), renal failure (CKD), and medications (e.g., long acting opiates).6–10
Most information regarding risk factors and clinical features of CSA comes from clinical populations enriched in predis-posing conditions such as opiate use, congestive heart failure, and stroke.6,9,10 Such samples are subject to bias based on referral patterns for subspecialty care. One could imagine that medical comorbidities would potentially be overrepresented in the tertiary academic centers from which most of these clinic-based cohorts were derived.
The comparison of the prevalence and clinical characteristics of CSA and CSR to individuals with OSA and no sleep apnea in a community based population that includes women has not been performed. Such an evaluation could provide a less biased determination of associations and potentially discover novel associations. Data from the Sleep Heart Health Study (SHHS) were queried to perform this evaluation.11
METHODS
The SHHS was a community-based prospective cohort study conducted from 1995 through 2006, which recruited 6,441 men and women aged 40 and older. At baseline, the investigators obtained polysomnography (PSG), EKG, blood pressure, anthropomorphic measures, and questionnaires regarding health, medication use, and sleep.12,13 Subjects underwent in home PSG using 5-lead electroencephalogram, submental electromyogram, thoracic and abdominal inductive plethysmography, oronasal thermistor, two-lead electrocardiogram, mercury gauge sensor for body position, and finger pulse oximeter.12 PSG data were scored at a central location according to standardized criteria.14 Data for this study were obtained through the National Sleep Research Resource (NSRR), which possesses data for 5,804 of the 6,441 participants.15 The use of this data by the authors was approved by the NSRR. Each participating institution's institutional review board approved the SHHS protocol at the time of the study, and the investigators obtained informed consent from all participants.
The current International Classification of Sleep Disorders (ICSD-3) contains 6 definitions of CSA which apply to adult patients. Each of these definitions requires, a central AHI (central apneas + central hypopneas/h sleep) ≥ 5 with the central apneas and hypopneas accounting for more than 50% of all apneas and hypopneas.1
SHHS PSG scoring noted the presence of periodic breathing (PB) if 10 consecutive minutes of Cheyne-Stokes breathing pattern was present but did not differentiate central from obstructive hypopneas. In light of this limitation, we defined a general CSA group (CSA-G) with both a central apnea index (CAI) ≥ 5 and a CAI greater than the obstructive apnea index (OAI), and a Cheyne-Stokes respiration group (CSR) that included subjects meeting criteria for general CSA and PB. By definition, all individuals with CSR were included in the CSA-G group. In order to create the purest comparison of OSA and CSA, the group with predominant OSA and a possible component of CSA (presence of PB or CAI ≥ 5/h) were not included in our OSA group in the comparisons performed and will be referred to as predominant OSA with a CSA component.
The apnea hypopnea index (AHI 4%) was defined as the rate of apneas plus hypopneas with ≥ 4% oxygen desaturation per hour. Individuals without PB or CAI < 5 but with an AHI 4% ≥ 5 were classified as obstructive sleep apnea (OSA), and individuals with an AHI 4% ≤ 5 were classified as no sleep-disordered breathing (SDB). A small number (n = 157) of individuals who had AHI 4% ≥ 5, did not meet criteria for CSA, but also had presence of PB or CAI ≥ 5 were classified in the predominant OSA with a CSA component group.
Categorical variables such as medications used or presence of comorbidities were compared between the groups using a likelihood ratio χ2 test or Fisher exact test if necessary. Comparisons of continuous variables were made among the 3 groups with one-way ANOVA with subsequent pairwise Tukey HSD test. Values for categorical variables are expressed as mean ± standard deviation (SD). As this is an exploratory analysis, multiple comparisons were not accounted for. For symptoms, individuals were dichotomized to those reporting “often” and more frequent compared to those reporting less frequent symptoms. Basic statistics were performed with JMP Pro statistical software (volume 11.0, 2013; SAS Institute, Cary, NC).
RESULTS
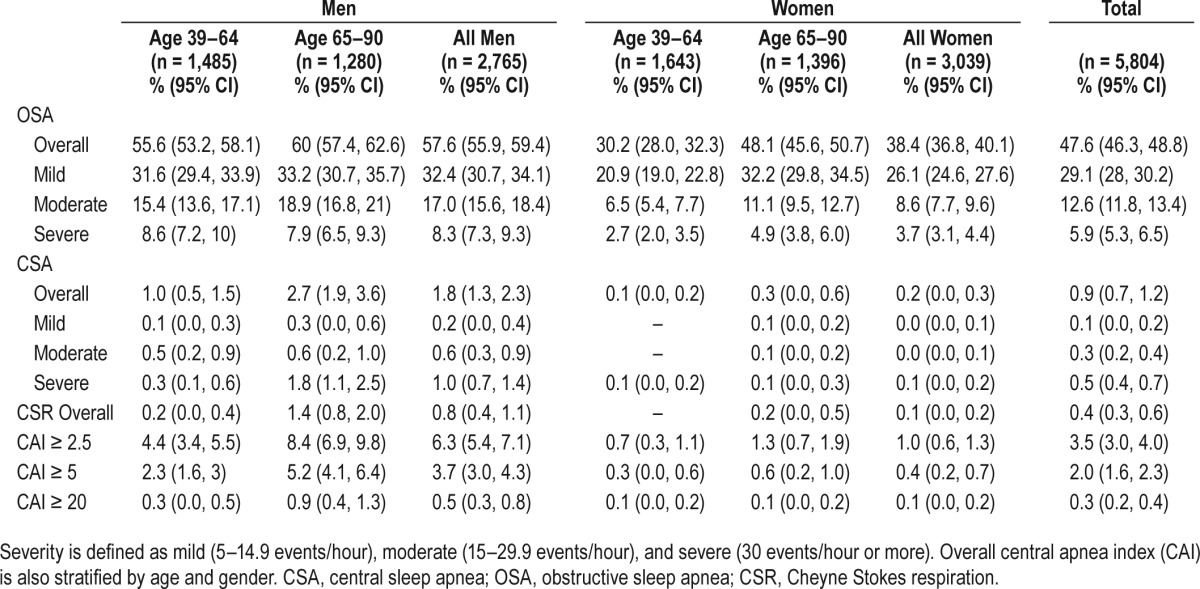
Of the 5,804 participants, 48.8% (n = 2,830) had no SDB, 47.6% (n = 2,762) had OSA, 2.7% had predominant OSA with a CSA component (n = 157), 0.9% (n = 55) had CSA-G, and 0.4% (n = 24) had CSR. Individuals with CSA-G were older (69.1 years) and much more likely to be male (90.9%) than those with OSA (Table 1). Blood pressure was greater in OSA and CSA-G groups than those without SDB. Although severity of SDB was highest in the CSA-G and CSR groups, sleepiness by ESS was highest in the OSA group. The prevalence of OSA and CSA-G, and central apnea index greater than 2.5, 5, and 20, respectively are included in Table 2. CSA-G was much more common among men and in those age 65 years and older.
Table 1.
Demographic and basic sleep characteristics by group.
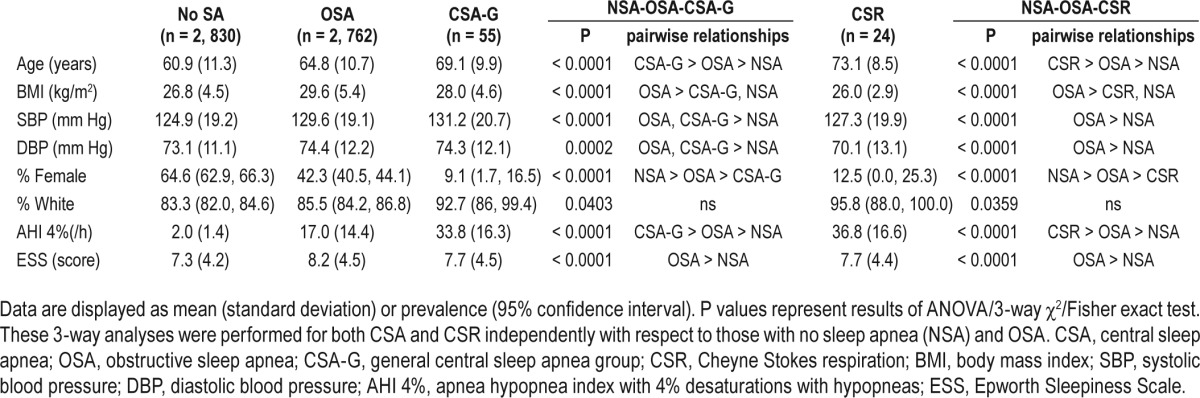
Table 2.
Prevalence of obstructive and central sleep apnea by gender.
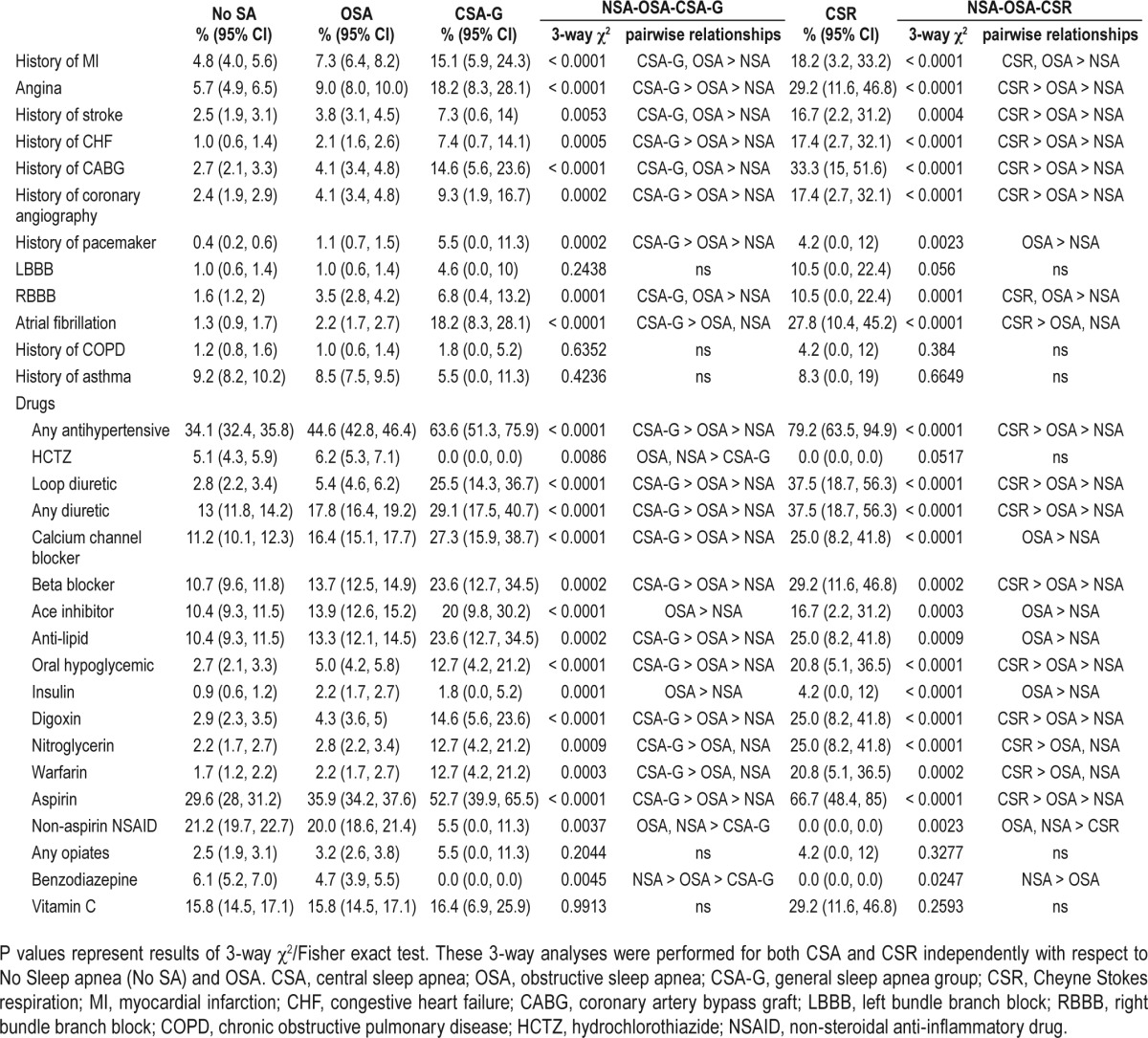
Those with CSA-G were more likely to have a self-reported history of cardiovascular disease (e.g., angina, CHF) or cardiovascular procedure (e.g. coronary angiography) than those with OSA and those without SDB. Subjects with CSR mirrored this trend, although they were also more likely to have had coronary artery bypass grafting (CABG) and stroke. EKG demonstrated atrial fibrillation more frequently in subjects with CSA-G and CSR. Concordant with stated histories, subjects with CSA-G and CSR were more likely to be treated with drugs used for coronary artery disease (e.g., aspirin, antihypertensives, and anti-lipid agents). Benzodiazepine use was less common in CSA-G (0%) than OSA (4.7%; P = 0.02) (Table 3). Notably, non-aspirin NSAID use was much less common in individuals with CSA-G (5.5%) and CSR (0%) than OSA (20%), although total NSAID use (aspirin and non-aspirin NSAIDs) was not significantly different between those with OSA (50.1%) and CSA-G (56.4%, P = 0.36) or CSR (66.7%, P = 0.11).
Table 3.
Prevalence of comorbidities and prescription drug use by sleep-disordered breathing classification.
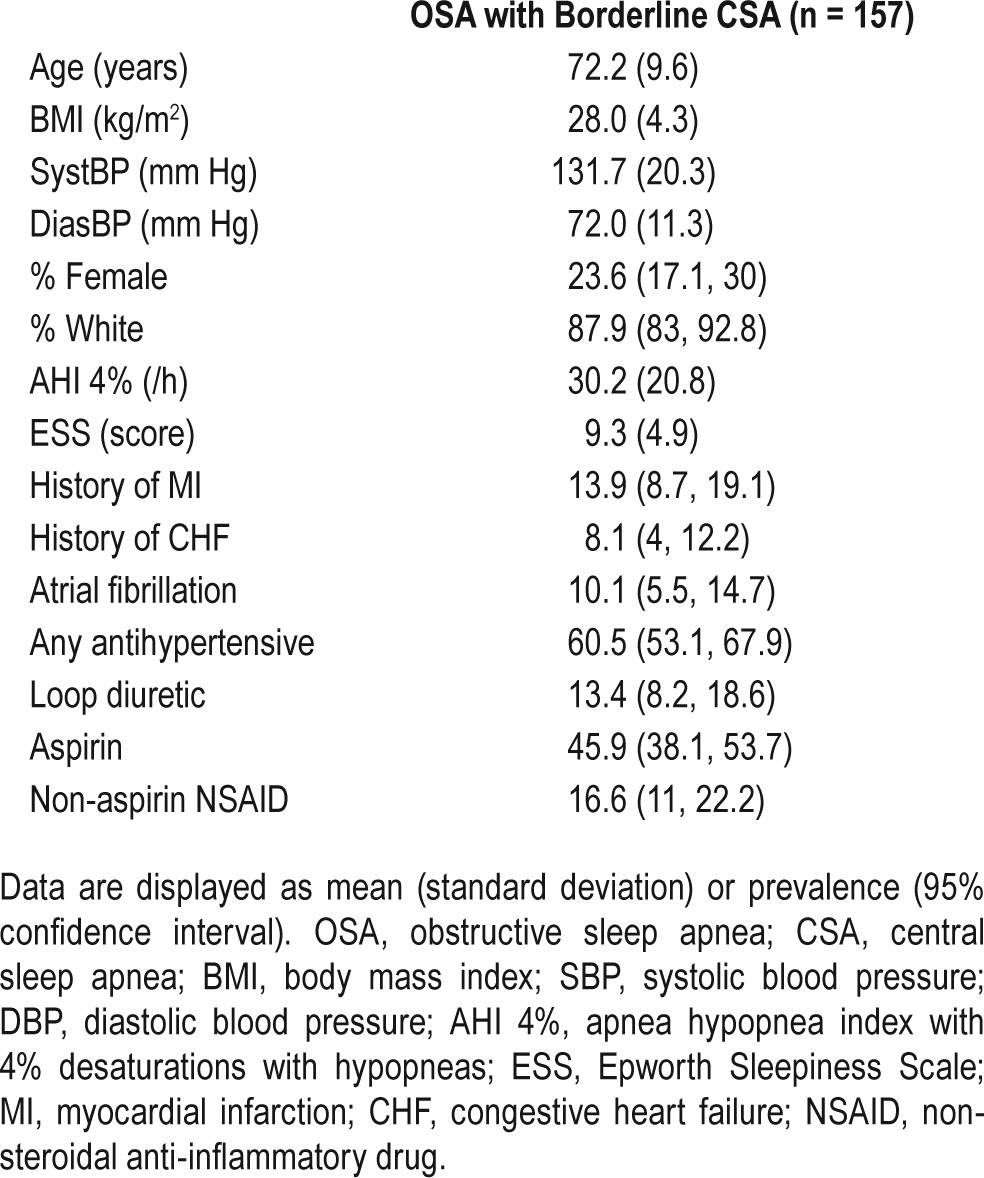
Those 157 subjects with predominant OSA and a CSA component comprised 2.7% (95% CI 2.3,3.1) of the overall sample. These individuals were elderly (72.2 ± 9.6 years old) and predominantly male (87.9%). Compared to CSA-G, rates of self reported MI and CHF were similar. Rates of atrial fibrillation, antihypertensive use, loop diuretic use, and non-aspirin NSAID use were intermediate between OSA and CSA (Table 4).
Table 4.
Demographic and key characteristics of subjects with predominant OSA and a CSA component.
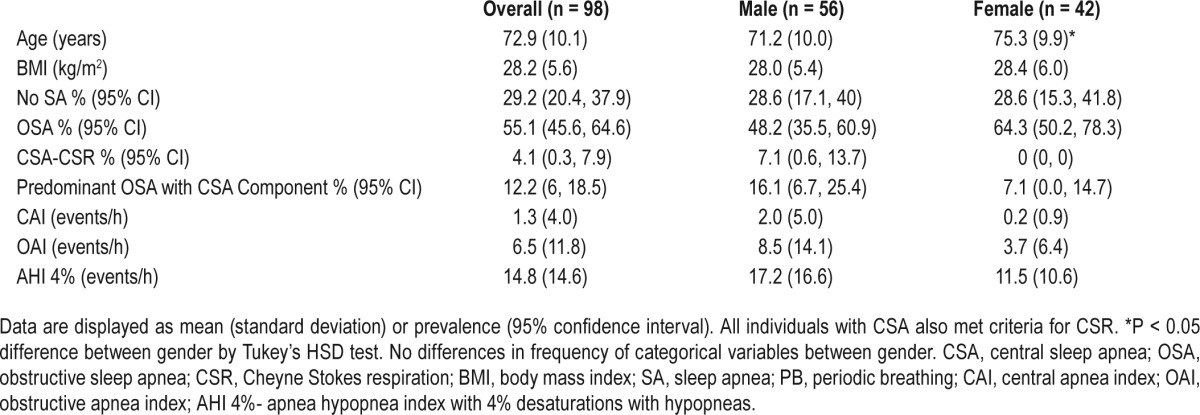
Despite the fact that individuals with CSA-G and CSR were more likely to have self-described HF, those with self-reported heart failure in this sample (n = 98, 57% male) were more likely to have OSA (n = 54; 55%) than CSA-G (n = 4; 4%) or predominant OSA with a CSA component (n = 12, 12.2%). All individuals with CSA-G in this sample were classified as having CSR (Table 5). When the sample was limited to those with heart failure and moderate to severe SDB (AHI 4% ≥ 15, n = 32), pure OSA was noted in 89.9% (n = 32) of subjects, and only 11.1% (n = 4) had CSR.
Table 5.
Characteristics of subjects with stated heart failure history stratified by gender.
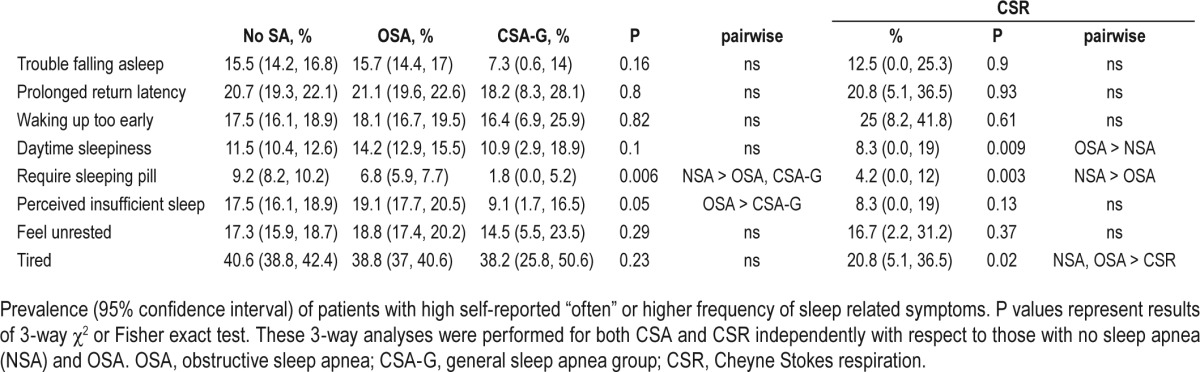
Self-reported sleep questionnaire responses were also analyzed (Table 6). The prevalence of subjects reporting a high frequency of perceived insufficient sleep was lower in the CSA-G group (9.1%) than those with OSA (19.1%). The prevalence of individuals reporting a high frequency of feeling tired was lower in the CSR group (20.8%) than both OSA (38.8%) and no SDB (40.6%) groups, though the prevalence was not lower in the CSA-G group (38.2%). Congruent with the ESS data presented in Table 1, the prevalence of those reporting a high frequency of perceived daytime sleepiness was higher in OSA (14.2%) than no SDB (11.5%), but no significant pairwise relationships existed with CSA-G (10.9%) or CSR (8.3%). Frequent sleeping pill use was less common in CSA-G (1.8%) than those without SDB (9.2%) and was not significantly different from the OSA group (6.8%). Symptoms of insomnia were not statistically different for CSA-G and CSR groups relative to no SDB and OSA groups.
Table 6.
Prevalence of sleep symptoms by group.
DISCUSSION
This is the largest community based study of CSA to date, and the only such study that includes both men and women. It is also the first community based study to evaluate the prevalence of Cheyne-Stokes respiration.
The prevalence of CSA (defined as CSA-G in our sample) was noted to be quite low (0.9%) relative to the prevalence of OSA. When stratified by age and gender, the prevalence of CSA differs from prior reports. Among the male participants in the SHHS sample, the prevalence of CSA was 1.8%, more than double the overall prevalence of male participants cited in Bixler et al. (0.4%). This disparity exists even among similarly defined age groups. Among those age 65 and older, Bixler et al. noted a CSA prevalence of 1.1% in men,4 in contrast to 2.7% in SHHS. Some of this discrepancy may relate to differences in defining central sleep apnea. A modified form of the ICSD-3 classification was used in our analysis, while a central apnea index (CAI) ≥ 10 was employed previously. Using identical CAI cutoffs, prevalence of CAI ≥ 2.5 was higher in the male members of SHHS (6.3%) compared to that of Bixler et al. (2.2%), although prevalence of CAI ≥ 20 was similar (0.5% in both).4 Another large male cohort from the osteoporotic fractures in men (MrOS) study found the prevalence of CAI ≥ 5 to be 7.2%, almost double the prevalence found in SHHS at this cut-point (3.7 %). The older average age in Mehra et al. (76.4 years vs. 63.1 in the SHHS sample) potentially accounts for the higher prevalence of CSA. The prevalence of OAI ≥ 5 among men in the SHHS (26.7%) is also lower than that noted in Mehra et al. (35.1%). Sampling of older individuals in the MrOS and SHHS cohorts is likely responsible for the higher rates of CSA in both of these studies in comparison to Bixler et al. However, the rising prevalence from the Bixler study (published in 1998, with unclear recruitment time) to the SHHS (recruited from 1995–1998 for initial study),16 and MrOS (performed sleep studies from 2003 to 2005) out of proportion to the increases in OSA raises the possibility of rising prevalence of CSA. Increasing rates of heart failure, renal failure, and atrial fibrillation17–19 in particular may be tied to a rise in CSA prevalence. The initial prevalence of objectively measured atrial fibrillation was in fact three times higher in MrOS than in the male participants of SHHS (4.7%20 vs. 1.5%), although prevalence of heart failure by questionnaire was similar (6%21 vs. 6.9%). Further research on contemporary populations with careful attention to central versus obstructive events will be required to evaluate shifting trends in the prevalence of central and obstructive sleep apnea.22
Similar to prior work from clinic based samples with older participants and smaller community based populations, SHHS participants with CSA-G and CSR were older, had lower BMI, and were more likely to be male than those with OSA.4,6,23 The known strong associations with ischemic heart disease, heart failure, and atrial fibrillation were also noted.6,24,25 Additionally, although those with CSA-G and CSR were more likely to have heart failure than those with OSA, among subjects with heart failure the prevalence of OSA vastly exceeded CSA-G and CSR. This deviates from prior clinic-based investigations of heart failure subjects in which the prevalence of CSA either exceeded6 or nearly matched that of OSA.24 This discrepancy may relate to a lower severity of heart failure in the SHHS sample. Another explanation of the lower frequency of CSA-G and CSR in our heart failure population lies in the relatively high prevalence of women in our heart failure population (43%), as women have much lower risk of CSA. The population of Javaheri et al.6 was 100% male, and the population of Sin et al.24 was 85% male. Our findings would suggest that for the typical heart failure patient in the community, OSA is much more likely to be the etiology of SDB than CSA, and that clinical suspicion and pretest probabilities should be adjusted accordingly.
Despite greater severity of SDB in the CSA-G and CSR groups, sleep related symptoms were of similar or lower severity than OSA and no SDB groups. In heart failure populations, severity of CSR and OSA has not been associated with daytime sleepiness.26,27 Congruent with these findings, subjects with CSA-G and CSR in our sample tended to have lower prevalence of perceived sleepiness and lower Epworth Sleepiness Scale scores than those with OSA and were similar to those without SDB, although these findings did not meet statistical significance. A common clinical perception exists that individuals with CSA predominantly exhibit insomnia related complaints, although there are a paucity of data to support this assertion.28 Our data would argue the opposite as those with CSA-G have lower rates of perceived insufficient sleep and tended to report fewer problems with initial and return sleep latency as well as early morning awakenings than OSA and no SDB groups, although these differences did not meet statistical significance. Our data do not indicate that frequency of sleep related symptoms can reliably differentiate OSA from CSA.
Those with predominant OSA and a CSA component were not evaluated extensively in our analyses since our aim was to compare OSA and CSA. This group possessed one or more features of CSA (periodic breathing or CAI ≥ 5), but did not meet full criteria for CSA and thus would qualify as OSA under current ICSD criteria. Borderline CSA comprised a sizeable minority of the subject population (2.7%), and not surprisingly had characteristics similar to the CSA group. This group may represent those at risk for treatment emergent central apneas with the initiation of positive airway pressure therapy.29,30 This borderline or “mixed” group merits further research as it likely represents a distinct entity that could benefit from targeted therapies of respiratory instability.30
A novel association noted in this analysis is the lower prevalence of non-aspirin NSAID use in individuals with CSA-G. A potential mechanism for this association is the prevention of augmented hypoxic sensitivity in the carotid body by NSAIDs. Inflammation plays a role in the acclimatization of the carotid body to hypoxia,31 and in rodents exposed to chronic intermittent hypoxia, ibuprofen reduces the augmented response to subsequent hypoxia.32 Augmented chemosensitivity and ventilatory responsiveness underlies the phenomenon of periodic breathing, Cheyne-Stokes respiration, and recurrent central apneas.33,34 It is equally likely, however, that non-aspirin NSAID use was simply avoided in those already on aspirin in order to avoid concomitant use of two agents in the same class, and similar rates of overall NSAID use among those with OSA, CSA, and CSR would underlie this. While non-aspirin NSAIDs lack an acetyl group and may have a different anti-inflammatory profile—particularly with regard to neutro-phil endothelial attachment,35 there is no evidence to suggest an anti-inflammatory effect on the carotid body would be substantially different between aspirin and non-aspirin NSAIDs. It is also possible that non-aspirin NSAID medications could have been avoided in individuals with cardiovascular disease. However, the association between NSAIDs and cardiovascular disease did not receive widespread attention until 2005, 7 years after SHHS recruitment ended, when cardiovascular disease was linked with the COX-2 inhibitors,36,37 and subsequently with non-selective COX inhibitors.38,39 Further work will be required to elucidate the significance of this finding regarding non-aspirin NSAIDs.
Other medication related findings are also of interest. Vitamin C has been shown to play a role similar to NSAIDs in blunting hypoxic response after chronic hypoxia.32 However, no differences in the prevalence of Vitamin C use were found between CSA-G or CSR with OSA and those without SDB in this analysis. Prior research from small samples indicates that benzodiazepines can improve central sleep apnea40 and symptomatic periodic breathing.41 Potentially congruent with this finding, those with CSA-G had a lower rate of benzodiazepine use. The lack of an association between the prevalence of opiate use with CSA-G or CSR is somewhat unexpected given prior associations of opiate use with central sleep apnea.9,42 Our negative result may simply reflect paucity of opiate users in the sample (n = 166, 2.9%) and the low average daily dose of those on opiates (7.2 morphine equivalents/day). Nevertheless, prior analysis in a selected population of opiate users noted similar prevalence of OSA and CSA.43
The current work has several limitations. One is the absence of scored central hypopneas that precludes determination of a central AHI. The use of CAI greater than or equal to 5 instead of a central AHI would serve to underestimate the prevalence of central sleep apnea in this sample, as ICSD-3 includes central hypopneas in the central event cutoff of 5. Additionally, the vast majority of comorbidities including heart failure were based on self-report, and as a result, we were not able to objectively confirm and assess the type and severity of heart failure. Furthermore, analyses in this work included multiple comparisons increasing the likelihood of type 1 error, and the cross-sectional nature of the analyses limits inferences that can be made regarding associations.
This analysis of SHHS participants confirms a number of previously described associations regarding CSA, and estimates the prevalence of CSA in women for the first time in a large non-clinic based cohort. Contrary to prior work, those with heart failure were much more likely to have OSA than CSA. The overall burden of CSA relative to OSA in this population may be of less severity than previously thought.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Susan Redline, Michael Rueschman, and the National Sleep Research Resource for making the data from SHHS available.
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 3.Findley LJ, Zwillich CW, Ancoli-Israel S, Kripke D, Tisi G, Moser KM. Cheyne-Stokes breathing during sleep in patients with left ventricular heart failure. Southern Med J. 1985;78:11–5. doi: 10.1097/00007611-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 5.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 7.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–7. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 8.Leung RS, Huber MA, Rogge T, Maimon N, Chiu KL, Bradley TD. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28:1543–6. doi: 10.1093/sleep/28.12.1543. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–56. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- 10.Bonnin-Vilaplana M, Arboix A, Parra O, Garcia-Eroles L, Montserrat JM, Massons J. Cheyne-stokes respiration in patients with first-ever lacunar stroke. Sleep Disord. 2012;2012:257890. doi: 10.1155/2012/257890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 12.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 13.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45:683–92. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Tosteson T, Boucher MA, Millman RP. Measurement of sleep-related breathing disturbances in epidemiologic studies. Assessment of the validity and reproducibility of a portable monitoring device. Chest. 1991;100:1281–6. doi: 10.1378/chest.100.5.1281. [DOI] [PubMed] [Google Scholar]
- 15.Dean DA, Goldberger AL, Mueller R, et al. Scaling up scientific discovery in sleep medicine: the National Sleep Research Resource. Sleep. 2016;39:1151–64. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 17.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–98. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 18.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 19.McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–9. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 20.Thadani SR, Ristow B, Blackwell T, et al. Relationship of bisphosphonate therapy and atrial fibrillation/flutter: outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. Chest. 2016;149:1173–80. doi: 10.1016/j.chest.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javaheri S, Blackwell T, Ancoli-Israel S, et al. Sleep disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193:561–8. doi: 10.1164/rccm.201503-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 24.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 25.Mehra R, Stone KL, Varosy PD, et al. Nocturnal Arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–55. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Midgren B, Mared L, Franklin KA, Berg S, Erhardt L, Cline C. Cheyne-Stokes respiration is not related to quality of life or sleepiness in heart failure. Clin Respir J. 2010;4:30–6. doi: 10.1111/j.1752-699X.2009.00139.x. [DOI] [PubMed] [Google Scholar]
- 27.Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–22. doi: 10.1001/archinte.166.16.1716. [DOI] [PubMed] [Google Scholar]
- 28.Al-Jawder SE, Bahammam AS. Comorbid insomnia in sleep-related breathing disorders: an under-recognized association. Sleep Breath. 2012;16:295–304. doi: 10.1007/s11325-011-0513-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee SA, Lee GH, Chung YS, Kim WS. Clinical, polysomnographic, and CPAP titration features of obstructive sleep apnea: mixed versus purely obstructive type. J Neurol Sci. 2015;355:150–4. doi: 10.1016/j.jns.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Rao H, Thomas RJ. Complex sleep apnea. Curr Treatment Options Neurol. 2013;15:677–91. doi: 10.1007/s11940-013-0260-7. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, He L, Stensaas L, Dinger B, Fidone S. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am J Physiol Lung Cel Mol Physiol. 2009;296:L158–66. doi: 10.1152/ajplung.90383.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popa D, Fu Z, Go A, Powell FL. Ibuprofen blocks time-dependent increases in hypoxic ventilation in rats. Respir Physiol Neurobiol. 2011;178:381–6. doi: 10.1016/j.resp.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman KR, Bruce EN, Gothe B, Cherniack NS. Possible mechanisms of periodic breathing during sleep. J Appl Physiol. 1988;64:1000–8. doi: 10.1152/jappl.1988.64.3.1000. [DOI] [PubMed] [Google Scholar]
- 34.Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Gonzalez F, Gonzalez-Alvaro I, Campanero MR, et al. Prevention of in vitro neutrophil-endothelial attachment through shedding of L-selectin by nonsteroidal antiinflammatory drugs. J Clin Invest. 1995;95:1756–65. doi: 10.1172/JCI117853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 37.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–91. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 38.Farkouh ME, Greenberg JD, Jeger RV, et al. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66:764–70. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction: a nationwide cohort study. Circulation. 2011;123:2226–35. doi: 10.1161/CIRCULATIONAHA.110.004671. [DOI] [PubMed] [Google Scholar]
- 40.Bonnet MH, Dexter JR, Arand DL. The effect of triazolam on arousal and respiration in central sleep apnea patients. Sleep. 1990;13:31–41. doi: 10.1093/sleep/13.1.31. [DOI] [PubMed] [Google Scholar]
- 41.Dubowitz G. Effect of temazepam on oxygen saturation and sleep quality at high altitude: randomised placebo controlled crossover trial. BMJ. 1998;316:587–9. doi: 10.1136/bmj.316.7131.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogri M, Desai H, Webster L, Grant BJ, Mador MJ. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13:49–57. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 43.Rose AR, Catcheside PG, McEvoy RD, et al. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. J Clin Sleep Med. 2014;10:847–52. doi: 10.5664/jcsm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]


