Abstract
Low-abundance regulatory peptides, including metabolically important gut hormones, have shown promising therapeutic potential. Here, we present a streamlined mass spectrometry-based platform for identifying and characterizing low-abundance regulatory peptides in humans. We demonstrate the clinical applicability of this platform by studying a hitherto neglected glucose- and appetite-regulating gut hormone, namely, oxyntomodulin. Our results show that the secretion of oxyntomodulin in patients with type 2 diabetes is significantly impaired, and that its level is increased by more than 10-fold after gastric bypass surgery. Furthermore, we report that oxyntomodulin is co-distributed and co-secreted with the insulin-stimulating and appetite-regulating gut hormone glucagon-like peptide-1 (GLP-1), is inactivated by the same protease (dipeptidyl peptidase-4) as GLP-1 and acts through its receptor. Thus, oxyntomodulin may participate with GLP-1 in the regulation of glucose metabolism and appetite in humans. In conclusion, this mass spectrometry-based platform is a powerful resource for identifying and characterizing metabolically active low-abundance peptides.
Keywords: Gut hormones, GLP-1, Low-abundant peptides, Mass-spectrometry, Proteomics
Highlights
-
•
In the pursuit of identifying metabolic peptides in humans we developed a streamlined mass-spectrometry based platform
-
•
Our platform was used to investigate a gut derived glucose and appetite regulatory peptide, oxyntomodulin
-
•
Levels of oxyntomodulin are reduced in subjects with type 2 diabetes and increased after gastric bypass surgery
The human plasma comprises a variety of peptides with importance for metabolic health. Identification of such peptides has been exploited for developing glucose-lowering therapies, such as incretin-based therapy. We therefore developed a mass-spectrometry based platform for identification of peptides in humans and by applying this platform we characterized a peptide hormone oxyntomodulin secreted from the intestine in response to glucose. Our data suggest that oxyntomodulin is down-regulated in subjects with type 2 diabetes and up-regulated after bariatric surgery. In summary, the collected data indicate that oxyntomodulin may co-orchestrate appetite and glucose regulatory effects together with incretin hormones.
1. Introduction
Investigation of low-abundance peptides (peptides circulating at the low picomolar range) has provided insights into human physiology and pathophysiology. For example, the identification of the incretin hormone, glucagon-like peptide-1 (GLP-1), paved the way to strategies for the treatment of diabetes and obesity (Holst, 2013b, Sadry and Drucker, 2013, Wewer Albrechtsen et al., 2014). Furthermore, low-abundance peptides derived from the gastrointestinal tract, including GLP-1 (Supplementary Fig. 1), are crucial mediators of the weight-reducing and antidiabetic actions of bariatric surgery (Madsbad et al., 2014, Madsbad and Holst, 2014). Detection and characterization of low-abundance peptides are, therefore, of major clinical interest (Bouillon et al., 2015, Gillette and Carr, 2013a, Keshishian et al., 2007a, Lin et al., 2009, Meng et al., 2011, Sadry and Drucker, 2013).
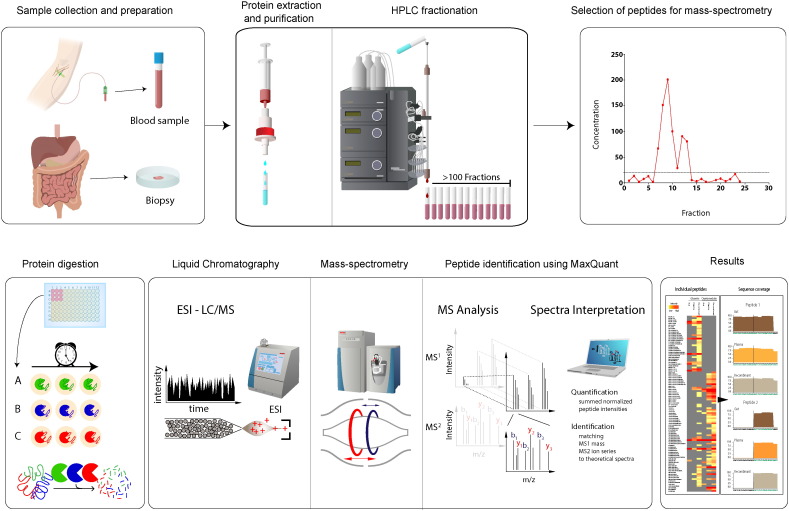
Fig. 1.
A streamlined platform for detection of low-abundant peptides.
This figure summarizes the key components in our mass-spectrometry based platform. Human blood or tissue samples are purified using C18 materials and subsequently proteins are separated using high-performance liquid chromatography. Different proteases are then added to the 96 wells to initiate protein digestion and formation of peptides. The samples are subsequently purified using C18 materials and subjected to liquid chromatography coupled to mass spectrometry (LC–MS/MS) followed by data analysis using MaxQuant.
Therefore, we developed a streamlined, unbiased mass spectrometry-based platform for the characterization of low-abundance peptides (Fig. 1.). Importantly, and in contrast to current targeted mass spectrometry-based detection methods (Fonslow et al., 2011, Gillette and Carr, 2013b, Keshishian et al., 2007b, Parker and Borchers, 2014, Surinova et al., 2011) (e.g., the SISCAPA technology), this method functions without prior immune-based fractionation/precipitation, which makes this method unbiased and suitable for biomarker discovery. The clinical applicability of the platform was validated by detecting and characterizing physiological aspects of a hitherto neglected gut hormone, oxyntomodulin.
2. Materials & methods
2.1. The perfused mouse intestine
Male C57BL/6J mice (10 weeks, purchased from Taconic, Denmark; n = 4) were used for perfusion of the proximal intestine. Animals were kept on a 12:12 h light–dark cycle with free access to standard chow and water, and allowed to acclimatize for one week before use. The animals were anaesthetized by intraperitoneal injection with a mixture of ketamine/xylazine (Ketamine 90 mg/kg (Ketaminol Vet.; MSD Animal Health, Madison, NJ, USA) + Xylazine 10 mg/kg (Rompun Vet.; Bayer Animal Health, Leverkusen, Germany). The proximal small intestine was perfused via a catheter (0.7 mm) in the aorta for inflow of perfusion medium through the superior mesenteric artery at a flow rate of 2.2 ml/min as previously described. The stomach, kidneys, spleen, colon and distal small intestine were tied off and removed to prevent perfusion, thus only the proximal small intestine was perfused. The venous effluent was collected in 1 min periods via a catheter (0.9 mm) inserted into the portal vein, now exclusively draining the perfused segment. The intestinal lumen was perfused with saline at a flow rate of 0.04 ml/min. The perfusion medium (a modified Krebs Ringer bicarbonate buffer containing, in addition, 0.1% bovine serum albumin (Merck KGaA, Darmstadt, Germany), 5% Dextran T-70 (Dextran Products Limited, Scarborough, Canada), 3.5 mmol/l glucose, and 5 mmol/l of each of pyruvate, fumarate, and glutamate) was gassed with a 95% O2/5% CO2 mixture to achieve pH 7.3–7.4 and maintained at 37 °C during the experiment. After the intestinal perfusion was established, the animals were exsanguinated and the intestine was allowed to stabilise for approximately 30 min before the experiment was started. Test substances (Neuromedin C (10 nmol/l) and KCl (70 mmol/l) was infused through a sidearm with a syringe infusion pump (0.11 ml/min) for 5–10 min followed by a resting period of 20 min. Chemicals were obtained from Sigma Aldrich (St. Louis, MO, USA) unless otherwise stated. GLP-1 concentrations in venous effluent samples were measured, using a C-terminally directed (antiserum (#89,390), which reacts fully with intact GLP-1 and its primary metabolite (Kuhre et al., 2014a, Kuhre et al., 2014b). Sample-size estimation was based on a previous study (Svendsen et al., 2016).
2.2. In vitro degradation of oxyntomodulin in human plasma
Blood from 10 healthy volunteers was collected into pre-chilled EDTA tubes and centrifuged for 20 min at 2800 rpm at 4 °C. Plasma was separated immediately, pooled and aliquoted in two portions. Next, a DPP-4 inhibitor (valine pyrrolidide, a gift from Novo Nordisk A/S, Bagsværd, Denmark; final concentration 0.01 mmol/l) was added to one portion of plasma and to one portion of assay buffer. Known amounts of oxyntomodulin (corrected according to QAAA) were added to plasma portions and buffer portions to increase concentrations by 0 (solvent only), 200 pmol/l. Subsequently, both plasma and buffer portions were left at room temperature for 4 h and measured according to protocol.
2.3. Tissue and plasma preparation
Tissue was homogenized in 1% (v/v) trifluoroacetic acid (TFA) (Cat. No. TS-28904, Thermo Fisher Scientific, MA, USA) with a 5 mm steel bead and a bead mill (TissueLyzer, Qiagen instruments AG, Hombrechtikon, Switzerland) at 30 Hz for 2 × 2 min, left to stand for 1 h at room temperature and then centrifuged (3.300 × g, 10 min, RT). Tissue extracts or pooled plasma (N = 9) were partially purified using Sep-Pak pH-resistant tc18 cartridges (Cat. no. WAT036810, Waters, MA, USA) as described previously (Kuhre et al., 2014a, Kuhre et al., 2014b), with peptides being eluted with 70% ethanol containing 0.1% TFA and dried under a gentle stream of compressed air overnight.
Liquid chromatography experiments: 1000 μL of reconstituted samples was injected in a rHPLC (Akta purifier, Amersham Biosciences, Vydac, Columbia, MD 21044, United States cat# 218tp5415, C18, 150 mm × 4.6 mm × 5 μM). The column was eluted with a linear gradient of 10–50% acetonitrile in 0.1% formic acid and ammonium acetate) over 50 min and fractions were automatically sampled in 500 μL portions, evaporated in a SpeedVac (Thermo Fisher Scientific, Odense, Denmark) and stored at − 80 °C until mass-spectrometry analysis or ELISA measurement.
2.4. Mass-spectrometry based proteomic analysis
Protein pellets (HPLC fraction after evaporation) were resuspended in digestion buffer with the proteases: LysC: 6/2 M urea/thiourea, 50 mM ammonium bicarbonate pH 7.5; Trypsin: 1.5/0.5 M urea/thiourea, 50 mM ammonium bicarbonate pH 7.5; and chymotrypsin: 0.3/0.2 M urea/thiourea, 50 mM ammonium bicarbonate pH 7.5). Digestion of ~ 0.2 μg protein was carried out with 1 μg of the respective enzyme. In order to further increase the sequence coverage, partially cleaved peptides were generated by digesting for 5, 10, 20, 40, 60, 120 and 720 min. Samples from all time points of a respective protease were pooled and desalted on stage tips (Rappsilber et al., 2007).
LC–MS/MS: We first separated peptides on a Thermo Scientific EASY-nLC 1000 HPLC system (Thermo Fisher Scientific, Odense, Denmark). Columns (75 μm inner diameter, 20 cm length) were in-house packed with 1.9 μm C18 particles (Dr. Maisch GmbH, Germany). Peptides were loaded in buffer A (0.5% formic acid) and separated with a gradient from 5% to 60% buffer B (80% acetonitrile, 0.5% formic acid) within 60 min at 250 nl/min. The column temperature was set to 50 °C. The LC was directly coupled to a quadrupole Orbitrap mass spectrometer (Scheltema et al., 2014) (Q Exactive HF, Thermo Fisher Scientific) via a nanoelectrospray source. The Q Exactive was operated in a data dependent mode. The survey scan range was set to 300 to 1650 m/z, with a resolution of 60,000 (Q Exactive HF) or 70,000 (Q Exactive). The up to the 10 most abundant isotope patterns with a charge ≥ 2 were subjected to high collision fragmentation (Olsen et al., 2007) at a normalized collision energy of 27 (Q Exactive HF) or 25 (Q Exactive) and a resolution of 15,000 (Q Exactive HF) or 17,500 (Q Exactive) at m/z 200. Dynamic exclusion of sequenced peptides was set to 20s. Thresholds for ion injection time and ion target values were set to 20 ms and 3*E6 for the survey scans and 120 ms (25 ms Q Exactive HF) and 1E5 for the MS/MS scans, respectively. Data was acquired using the Xcalibur software (Thermo Scientific).
Data analysis and statistics: We processed the raw data with MaxQuant software (v 1.5.3.2) (4). We employed the Andromeda search engine (Cox and Mann, 2008), which is integrated into MaxQuant, to search MS/MS spectra against the human UniProtKB FASTA database (59,345 forward entries; version of June 2012). Enzyme specificity was set to semispecific. Peptides had to have a minimum length of 7 amino acids to be considered for identification. Carbamidomethylation was set as fixed modification, acetylation (N-terminus), deamidation and methionine oxidation were set as variable modifications. A false discovery rate (FDR) cut-off of 1% was applied at the peptide level. The cut-off score (delta score) for accepting individual MS/MS was 17. For bioinformatic analysis as well as visualization we used the open PERSEUS environment, which is part of MaxQuant. For several calculations and plots we also used the R framework. Identified peptides were mapped to GCG. In order to display quantitative evidence for overlapping peptides, intensities of identified peptides were summed and plotted per amino acid residue. All data is deposited for public access using the PRIDE Archive.
2.5. Statistics
Specificity evaluation: For each assay, the concentrations measured after addition of glucagon, oxyntomodulin or glicentin were plotted against the calculated concentrations (after subtraction of plasma zero values), and linear regression analyses were performed. The regression coefficient r2 shows the fit of the line, and the slope of the fitted linear lines corresponds to the recovery in the tested assay (100% recovery equates to full cross-reactivity, while a high regression coefficient indicates that findings were consistent and reproducible over the full tested range); for the slopes of each line for each peptide and assay, P-values were calculated for the null hypothesis: horizontal line. For the precision study, a one-way ANOVA for repeated measurements followed by Bonferroni post hoc analysis was performed, comparing the spiked samples and the baseline (non-spiked human plasma). For mouse gut perfusion studies, the Pearson correlation coefficient was used to assess the co-secretion of oxyntomodulin and GLP-1. Clinical samples: Two-way ANOVA with repeated measurement was used to determine significant differences between the oxyntomodulin and glicentin concentration during meal challenges. Incremental area under the curve (AUC) was calculated using the trapezoidal rule using baseline as predictor. For testing of normality and homoscedasticity in datasets we applied the Shapiro–Wilk test (swilk command) and drafted residual plots. To test whether oxyntomodulin responses changed after gastric bypass surgery, we performed a generalized regression model (ANCOVA) with oxyntomodulin as dependent variable as model 1 and glicentin as model 2, time from surgery being the independent variable and GLP-2 concentrations as co-variates. P < 0.05 was considered significant. Calculations were made using GraphPad Prism version 6.04 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com and STAT14, Boston, MA, USA. Adobe CS6 software suite (California, USA) was used for illustrations.
2.6. Study approval
Human samples are from studies by Hartmann (Hartmann et al., 2013) (NCT01700686), Lund (NCT02475421) and Wewer Albrechtsen (Wewer Albrechtsen et al., 2015). The studies were conducted according to the latest revision of the Helsinki Declaration, and approved by the Scientific-Ethical Committee of the Capital Region of Denmark (H-2-2010-064) and by the Danish Data Protection Agency. Written informed consent was received from participants prior to inclusion in the studies. The animal studies were conducted in accordance with international guidelines (National Institutes of Health publication no. 85–23, revised 1985, and Danish legislation governing animal experimentation, 1987), and were carried out after permission had been granted by the Animal Experiments Inspectorate, Ministry of Justice, Denmark. For patient characteristic of the gastric bypass operated and surgical procedures (Bonfils et al., 2015), see Supplementary material & methods, and Supplementary Table 1.
3. Results
3.1. A streamlined mass spectrometry-based platform
We initially performed a number of pilot studies that included optimization of blood and tissue handling for mass spectrometry (see Materials & methods). To separate high-abundance proteins (e.g., human albumin and immunoglobulins) from the low-abundance peptides of interest, we designed an isolation and fractionation approach: Plasma or tissue extracts were placed in a syringe containing C18 materials (SepPak) (Fig. 1), and the eluted peptides (low-abundance peptides, see Supplementary Fig. 1) were subsequently fractionated by high performance liquid chromatography (HPLC) using a gradient optimized for detection of the low-abundance peptides (Fig. 1). The platform thereby allows collection of fractions (> 100) containing the low-abundance peptides (the peptidome) for subsequent mass spectrometry analysis. We employed proteases with different specificities to maximize sequence coverage, and we used varying digestion times to increase the number of unique peptides. Subsequently, the low-abundance peptides were digested with specific proteases followed by desalting on C18-filled tips (stage tips) (Aebersold and Mann, 2003, Kulak et al., 2014, Rappsilber et al., 2007); they were then and analyzed using state of the art mass spectrometry (Meissner et al., 2013, Scheltema et al., 2014) that provided extreme sensitivity (~ 1 pg/ml). Data were analyzed with the MaxQuant software suite, which enabled reliable detection of the low-abundance peptides with false discovery rates (FDR) below 1%. For validation of our platform, we spiked several low-abundance peptides (the peptides shown in Supplementary Fig. 1.) into PBS buffer and human plasma. Importantly, the low-abundance peptides (Supplementary Fig. 2.) were detected independent of matrix (buffer or plasma) in triplicate assays, with estimated recoveries of (42 ± 8%) and coefficients of variation < 90% and inter-assay variation of less than 10% (for further details see Supplementary materials and methods).
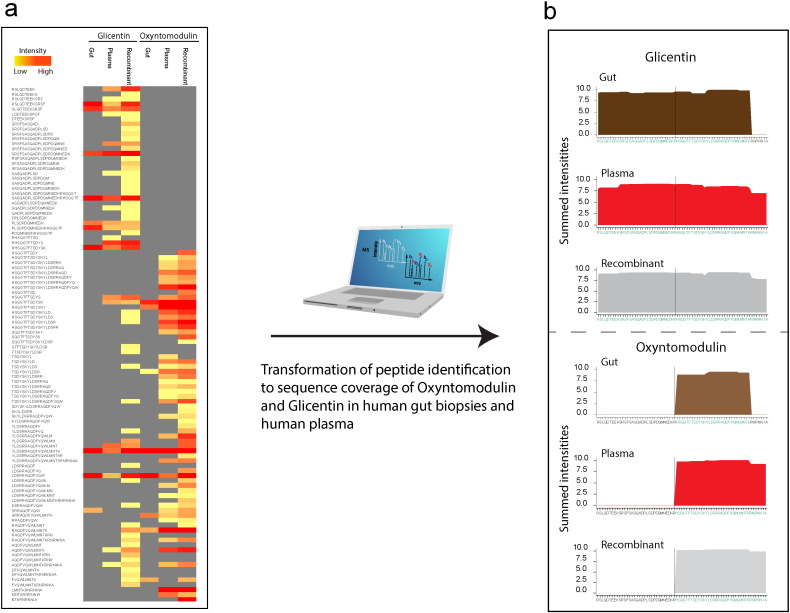
Fig. 2.
Proteomic profiling of plasma from gastric bypass operated patients and human gut biopsies.
A: Illustrates intensities of individual peptides identified of oxyntomodulin and glicentin in human gut, plasma and from recombinant controls. B: Illustrates sequence coverage of oxyntomodulin and glicentin in human gut, plasma and recombinant controls. Evidence for coverage of the respective hormone is depicted as the summed peptide intensities per amino acid for all employed proteases (see Material & Methods). 10 biological replicates and 3 technical replicates are shown.
To demonstrate the clinical applicability of the mass spectrometry-based platform, we analyzed plasma samples from gastric bypass patients (N = 10), who were expected to have high levels of low-abundance glucose- and appetite-regulating proglucagon-derived peptides. We also analyzed gut extracts (n = 4) from the human small intestine, which was also expected to have high levels.
3.2. Oxyntomodulin is secreted from the gut in response to glucose
Interestingly, subjecting human plasma and gut biopsies to our platform provided complete sequence coverage for several proglucagon-derived peptides (Fig. 2 and Supplementary Fig. 2.). False discovery rate (FDR) levels were less than 1%, demonstrating that the platform is robust even across tissue types (plasma and tissue biopsies). In addition, we validated the results from our immune-based oxyntomodulin detection method using an FDA-based evaluation approach: epitope mapping, HPLC fractionation, and ELISA evaluation (Supplementary Tables 2, 3 ,4, and 5, Supplementary Figs. 3. and 4). We hereby demonstrate that the platform can be used to identify glucose- and appetite-regulating hormones, such as oxyntomodulin, glicentin or GLP-1, in human plasma and gut biopsies.
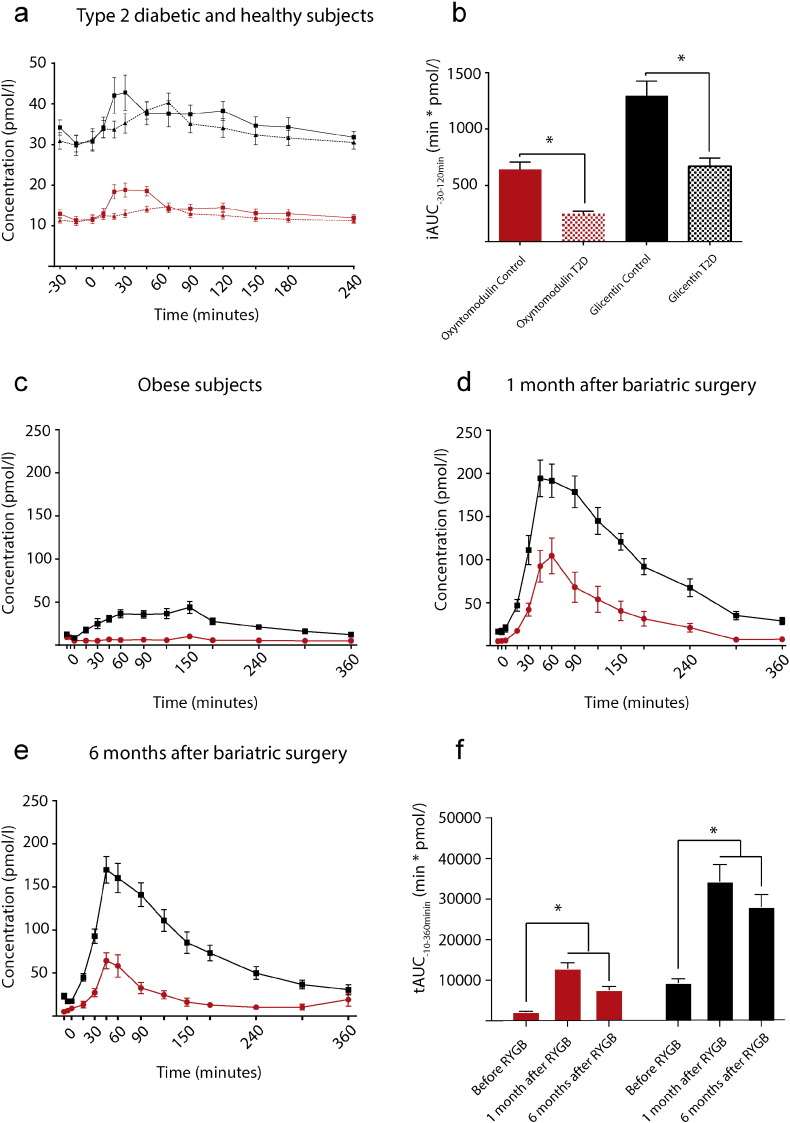
Fig. 3.
Oxyntomodulin responses are blunted in type 2 diabetic subjects and 10-fold elevated after gastric bypass surgery.
This figure illustrates the concentrations of oxyntomodulin (red) and glicentin (black) in A: 10 healthy subjects (squares, full line) and 10 patients with type 2 diabetes (triangles, dotted line) during a standard OGTT challenge; B: Illustrates calculated incremental AUC of data presented in A; 18 obese non diabetic patients during a standardized meal challenge before (C), 1 month after gastric bypass (D) and 6 months after gastric bypass (E). F: tAUC calculated based on data from C,D,E. Data are mean ± SEM. Asterisk (*) represents statistical significant differences by a unpaired t-test (B) or one-way ANOVA correcting for multiple testing by Bonferroni post hoc analysis (F).
Interestingly, our data suggest that oxyntomodulin is expressed in the human small intestine and is secreted to the circulation after stimulation with nutrients, which is in contrast to the previous speculation that it is a product made in circulation by the cleavage of other proglucagon-derived proteins. Intrigued by these results, we decided to investigate the secretory patterns of oxyntomodulin in obese subjects before and after undergoing a gastric bypass operation. Furthermore, to investigate if oxyntomodulin can be linked to the pleiotropic pathophysiology of type 2 diabetes, we analyzed plasma (using the extensively validated oxyntomodulin ELISA) during an oral glucose load in subjects with type 2 diabetes and matched healthy controls.
3.3. Type 2 diabetes and gastric bypass surgery remodulate secretory profiles of oxyntomodulin
Oxyntomodulin and glicentin levels increased (P < 0.01) from 12 to 20 pmol/l and 30 to 42 pmol/l, respectively, during an oral glucose tolerance test (OGTT) in healthy subjects (Fig. 4A). Oxyntomodulin and glicentin responses during the OGTT were (P < 0.01) lower in patients with type 2 diabetes (P < 0.01) (Fig. 3, A and B): The incremental area under the curve (iAUC) for oxyntomodulin was 639 ± 69 min × pmol/l in healthy subjects and 248 ± 21 min × pmol/l in patients with type 2 diabetes (P < 0.01), and the iAUC for glicentin was 1296 ± 131 min × pmol/l in healthy subjects and 670 ± 71 min × pmol/l in patients with type 2 diabetes (P < 0.01). In obese subjects, neither oxyntomodulin nor glicentin levels were significantly different compared to healthy subjects (P = 0.21) (Fig. 3C), but the levels of both hormones were increased by more than 5-fold after gastric bypass operations (Fig. 3, D and F, P = 0.001). The ratios between oxyntomodulin and glicentin were relatively constant in all groups, varying between 30 and 40%.
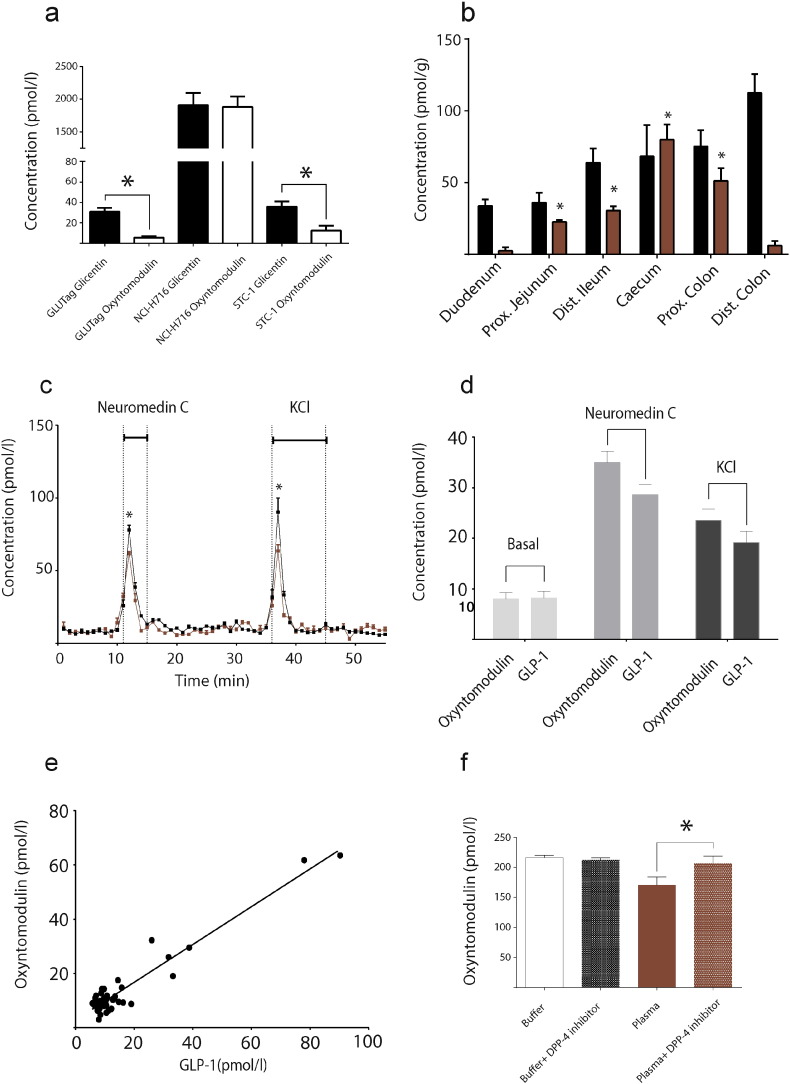
Fig. 4.
Oxyntomodulin is co-distributed, co-secreted with GLP-1 and act through same receptor.
A: Concentrations of extractable oxyntomodulin (white boxes) and glicentin (black boxes) normalized to protein content in GLUtag, NCI-H716 and STC-1 cells, the most frequently used cell models in incretin biology. B: Total GLP-1 tissue concentrations (black boxes) and oxyntomodulin concentrations (red boxes) increase significantly (P ˂ 0.001) along the gastrointestinal tract in mice (n = 10). C: Secretion of GLP-1 (black curve) and oxyntomodulin (red curve) from perfused proximal small intestine (n = 4). Secretion was significantly (P ˂ 0.05) increased by infusion of neuromedin C (10 mM) and KCL (70 mM). D: Averaged levels of GLP-1 and oxyntomodulin during basal period compared to stimulation-period with either neuromedin C (grey) or KCl (black). Oxyntomodulin and GLP-1 secretion increased in parallel during both neuromedin C and KCl stimulation. E: Correlation plot using data from C; the correlation coefficient was 0.91 (R2). Data are illustrated as mean ± SEM. * represent statistical significant differences using a one-way ANOVA correcting for multiple testing by Bonferroni post hoc analysis (B) or a paired t-test (C). Differences between GLP-1 and oxyntomodulin were not significant. F: Levels of oxyntomodulin in buffer ± DPP-4 inhibition and human plasma ± DPP-4 inhibition. Measured levels of oxyntomodulin were higher (P = 0.011) in plasma but not in buffer (P = 0.34) upon DPP-4 inhibition.
3.4. Oxyntomodulin and GLP-1 are co-distributed and co-secreted
We then investigated whether the secretion of oxyntomodulin and GLP-1 are co-distributed and co-secreted. First, we investigated if the enteroendocrine GLP-1-producing cells expressed oxyntomodulin. Indeed, three GLP-1-producing cell lines expressed comparable amounts of oxyntomodulin and GLP-1 (Fig. 4A). Then, analysis of purified extracts from the mouse gastrointestinal tract (Fig. 4B) demonstrated that concentrations of oxyntomodulin increased (P ˂ 0.01) from the proximal jejunum to the distal colon (23 ± 1 pmol/g to 73 ± 21 pmol/g) when compared to the duodenum. A similar pattern from the proximal jejunum to the proximal colon was observed for GLP-1 (35 ± 6 pmol/g to 111 ± 12 pmol/g, Pearson correlation coefficient of 0.921 P = 0.037)).
Finally, to study the (co-)secretion of oxyntomodulin and GLP-1, we isolated and perfused the small intestine of four mice. Oxyntomodulin secretion (Fig. 4C) increased (~ 9-fold, P ˂ 0.001) during infusion of neuromedin C (7 ± 4 pmol/l to a peak of 62 ± 3 pmol/l) and KCl (8 ± 2 pmol/l to peak of 64 ± 9 pmol/l), which were used to stimulate enteroendocrine proglucagon-containing cell secretion (termed L-cells). Importantly, these responses were in parallel with the secretion of GLP-1 (~ 10-fold, P˂0.001, during infusion of neuromedin C (7 ± 2 pmol/l to peak at 78 ± 7 pmol/l) and KCl (10 ± 2 pmol/l to peak at 90 ± 19 pmol/l)). Oxyntomodulin and GLP-1 secretions were similar, as illustrated by Fig. 4D and E, with a Pearson correlation coefficient of 0.95 (P ˂ 0.001), suggesting that these low-abundance peptides detected by the platform are co-distributed and are co-secreted.
3.5. Oxyntomodulin is degraded by the enzyme dipeptidyl-peptidase and act through the GLP-1 receptor
It is well known that GLP-1 secreted from the gastrointestinal tract is rapidly cleaved by the enzyme dipeptidyl peptidase-4 (DPP-4), which means that only ~ 8% of newly secreted GLP-1 reaches the pancreas in its metabolically active form (Hansen et al., 1999, Hjollund et al., 2011). DPP-4 inhibitors are now used to treat hyperglycemia in patients with type 2 diabetes, as these drugs inhibit the degradation of GLP-1. We hypothesized that oxyntomodulin are also degraded by DPP-4. To address this, we incubated oxyntomodulin in human plasma (from 10 healthy subjects) with or without the addition of a DPP-4 inhibitor (valine pyrrolidide). Inhibition of DPP-4 resulted in 21% higher levels of oxyntomodulin in plasma (170 ± 11 versus 206 ± 15 pmol/l, P = 0.011) but not in buffer, indicating a plasma-dependent effect (Fig. 4F). DPP-4 inhibitors could therefore have a pleiotropic effect not solely dependent on GLP-1. Finally, we tested if oxyntomodulin mediates its effect through the GLP-1R. To do this, we treated GLP-1R (a Gi-protein coupled receptor)-transfected cells (HEK293) with oxyntomodulin and simultaneously blocked the GLP-1R (supplementary Fig. 5). GLP-1 and oxyntomodulin both exhibited robust stimulations of cAMP levels (the down-stream signaling molecule of the receptor), however, when we blocked the GLP-1R, the signal was significantly attenuated.
4. Discussion
We here demonstrate the clinical applicability of an unbiased mass spectrometry-based platform in the pursuit of identifying low-abundance regulatory peptides such as oxyntomodulin and GLP-1. The platform was used to identify glucose- and appetite-regulating peptides both in circulation and in the gastrointestinal tract. In summary, we show that oxyntomodulin is expressed in the human and mouse small intestine, is co-distributed and co-secreted to the circulation with GLP-1, and is degraded by the same enzyme as GLP-1, and we show that both low-abundance peptides are elevated by more than 10-fold after gastric bypass surgery and attenuated in subjects with type 2 diabetes. Oxyntomodulin may therefore act together with GLP-1 in regulating blood glucose and appetite in humans, which is consistent with results of other studies that have injected exogenous oxyntomodulin into humans (Bagger et al., 2015, Baldissera et al., 1988, Pocai, 2014, Sandoval and D'Alessio, 2015, Schjoldager et al., 1988).
Our clinical observations are surprising and intriguing. First, it is well established that secretion of GLP-1 is blunted in some subjects with type 2 diabetes, and recent large-scale human studies suggest that the decreased GLP-1 responses may contribute to the development of type 2 diabetes (Calanna et al., 2013, Færch et al., 2015, Meier and Nauck, 2010, Nauck et al., 2011). Our data demonstrate that levels of oxyntomodulin are also attenuated in some subjects with type 2 diabetes, suggesting that oxyntomodulin may contribute to the pleiotropic pathophysiology of diabetes. However, our data do not demonstrate causality of attenuated levels of oxyntomodulin and the development of type 2 diabetes. Future studies may therefore investigate the molecular background, of lower levels of oxyntomodulin, and finally assess oxyntomodulin in a large cohort as done for GLP-1 (Færch et al., 2014).
In addition, exaggerated secretion of GLP-1 after gastric bypass surgery has been suggested to contribute markedly to the antidiabetic effect of this operation (Madsbad et al., 2014, Madsbad and Holst, 2014). We demonstrate that during a standardized meal test, oxyntomodulin levels do increase, but by ~ 10-fold, supporting the notion that the beneficial effects of the gastric bypass intervention may involve several glucose- and appetite-regulating gut hormones. The mechanism(s) underlying the altered secretory profiles of oxyntomodulin in gastric bypass operated subjects may include an abnormal passage of nutrients to a site (1–2 m distal to the duodenum) resulting in extreme exposure of nutrients to oxyntomodulin-expressing cells (Holst, 2013a). and as a consequence expression of oxyntomodulin in the gastrointestinal mucosa may increase (Rhee et al., 2015). It may be speculated that it is these gastrointestinal adaptation(s) that drives the dramatic changes in plasma levels of oxyntomodulin. Interestingly, increased levels of oxyntomodulin may be of importance appetite regulation, and in addtion glucose homeostasis, and altered levels hereof could therefore have clinical relevance supported by a human study demonstrating decreased hunger and reduced food intake upon administration of oxyntomodulin at supraphysiological levels (Cohen et al., 2003).
The concentrations of proteins and peptides in plasma range from picomolar to millimolar, a factor of 109, and this constitutes the main challenge for analyzing low-abundance peptides in plasma (Mann et al., 2013). Immune-based detection methods utilize the extreme binding energy of antibodies, which may have equilibrium constants reaching values of 1012 l/mol, thereby giving these methods the potential to detect very low concentrations. However, immune-based methods often suffer from lack of specificity and interference, the so-called matrix effects, i.e., sensitivity to other components in plasma, including a variety of high-abundance plasma molecules or proteins (e.g., albumin and immunoglobulins); these can lead to unspecific interference in antibody–antigen interaction and distort the results, particularly at the lower end of the detection range (Kuhre et al., 2014a, Kuhre et al., 2014b). Importantly, the current mass spectrometry-based methods for detecting and characterizing low-abundance peptides are immune-based and can therefore only be used in a targeted manner (Lee et al., 2015), which is undesirable for biomarker discovery.
In pursuit of improving the identification and profiling of low-abundance peptides, we developed a streamlined, mass spectrometry-based platform for the characterization of low-abundance peptides in blood and in tissue. As illustrated in Fig. 1, the developed pipeline involves an isolation and fractionation approach for the identification and quantification of peptides of interest using state of the art mass spectrometry. Importantly, our platform does not employ immune-based fractionation or precipitation and is therefore completely unbiased. We here provide a method to identify an un-labeled, low-abundance peptide in human plasma without prior immuno-based precipitation/purification.
In summary, low-abundance peptides are key mediators of glucose and appetite regulation, and the platform presented in the current study may be a resource for identifying low-abundance peptides in humans. We show that oxyntomodulin is co-distributed and co-secreted with the insulin-stimulating and appetite-regulating gut hormone GLP-1, is inactivated by the same protease (DPP-4) as GLP-1 and acts through its receptor therefore oxyntomodulin may be of importance for regulating glucose homeostasis and appetite in humans.
Funding sources
NNF Center for Basic Metabolic Research, University of Copenhagen. Novo Nordisk Foundation (13563). Aase og Ejnar Danielsens Fond. Holger Rabitz fond. Læge Johannes Nicolaj Krogsgaard og hustru Else Krogsgaards minde-legat for medicinsk forskning og medicinske studenter ved Københavns Universitet. (The Danish Council for Independent Research (DFF-1333-00206A), Augustinus Foundation 14-0962, European Molecular Biology Organisation (EMBO) and the European Foundation for the Study of Diabetes (EFSD).
Author contributions
NJWA, DH, RA, BS, ST, SLJ, REK, MH, CJ, AF, AL, TV, FKK, HV, CFD, FM, MM, JJH, BH provided substantial contribution to the concept and design; NJWA, DH, RA, BS, ST, SLJ, REK, MH, CJ, AF, AL, TV, FKK, HV, CFD, FM, MM, JJH, BH substantially contributed to analysis and interpretation of data; NJWA, DH, BS, CFD, MM and JJH drafted the manuscript; RA, BS, ST, SLJ, REK, MH, CJ, AF, AL, TV, FKK, HV, FM and BH revised the manuscript critically for important intellectual content. All authors have provided final approval of the version to be published. JJH is responsible for the integrity of the work as a whole.
Conflict of interest statement
The funding source(s) did not have any impact on the design, data analysis or writing of the paper. The authors had no financial relationships with any other organisations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgement
The authors would like to thank Mercodia A/S (Sylveniusgatan 8A, SE-754 50, Uppsala, Sweden) for providing oxyntomodulin assays. Mercodia provided assay kits without any restrictions regarding to study design nor interpretation of the results. We are very grateful for the help of the laboratory technicians Ramaya Kweder (RK), Lene Albæk (LA) and Sofie Pilgaard (SP) for outstanding technical assistance. LA, SP and RK affiliation: Department for Biomedical Sciences, University of Copenhagen, Denmark. Finally, we are grateful for graphical assistance by Musa Büyükuslu.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.03.034.
Appendix A. Supplementary data
Supplementary material
References
- Aebersold R., Mann M. Mass spectrometry-based proteomics. Nature. 2003;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- Bagger J.I., Holst J.J., Hartmann B., Andersen B., Knop F.K., Vilsboll T. Effect of oxyntomodulin, glucagon, GLP-1 and combined glucagon + GLP-1 infusion on food intake, appetite and resting energy expenditure. J. Clin. Endocrinol. Metab. 2015 doi: 10.1210/jc.2015-2335. (p. jc20152335) [DOI] [PubMed] [Google Scholar]
- Baldissera F.G., Holst J.J., Knuhtsen S., Hilsted L., Nielsen O.V. Oxyntomodulin (glicentin-(33-69)): pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul. Pept. 1988;21(1–2):151–166. doi: 10.1016/0167-0115(88)90099-7. [DOI] [PubMed] [Google Scholar]
- Bonfils P.K., Taskiran M., Damgaard M., Goetze J.P., Floyd A.K., Funch-Jensen P., Kristiansen V.B., Stockel M., Bouchelouche P.N., Gadsboll N. Roux-en-Y gastric bypass alleviates hypertension and is associated with an increase In mid-regional pro-atrial natriuretic peptide in morbid obese patients. J. Hypertens. 2015;33(6):1215–1225. doi: 10.1097/HJH.0000000000000526. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Drucker D.J., Ferrannini E., Grinspoon S., Rosen C.J., Zimmet P. The past 10 years — new hormones, new functions, new endocrine organs. Nat. Rev. Endocrinol. 2015 doi: 10.1038/nrendo.2015.142. (vol. advance online publication) [DOI] [PubMed] [Google Scholar]
- Calanna S., Christensen M., Holst J.J., Laferrère B., Gluud L.L., Vilsbøll T., Knop F.K. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56(5):965–972. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M.A., Ellis S.M., le Roux C.W., Batterham R.L., Park A., Patterson M., Frost G.S., Ghatei M.A., Bloom S.R. Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocrinol. Metab. 2003;88(10):4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26(12):1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Færch K., Torekov S.S., johansen N.B., Witte R., Jonsson A.E., Pedersen O., Hansen T., Lauritzen T. A. S., JJ Holst, Vistisen D., M.E., J Reduced release of GLP-1 in subgroups of prediabetes and type 2 diabetes. Diabetes. 2014;1748-P [Google Scholar]
- Færch K., Torekov S.S., Vistisen D., Johansen N.B., Witte D.R., Jonsson A., Pedersen O., Hansen T., Lauritzen T., Sandbæk A., Holst J.J., Jørgensen M.E. Glucagon-like peptide-1 (GLP-1) response to oral glucose is reduced in pre-diabetes, screen-detected type 2 diabetes and obesity, and influenced by sex: the ADDITION-PRO study. Diabetes. 2015 doi: 10.2337/db14-1751. [DOI] [PubMed] [Google Scholar]
- Fonslow B.R., Carvalho P.C., Academia K., Freeby S., Xu T., Nakorchevsky A., Paulus A., Yates J.R. Improvements In proteomic metrics of low abundance proteins through proteome equalization using ProteoMiner prior to MudPIT. J. Proteome Res. 2011;10(8):3690–3700. doi: 10.1021/pr200304u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette M.A., Carr S.A. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat. Methods. 2013;10(1):28–34. doi: 10.1038/nmeth.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette M.A., Carr S.A. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat. Methods. 2013;10(1):28–34. doi: 10.1038/nmeth.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L., Deacon C.F., Orskov C., Holst J.J. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140(11):5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- Hartmann B., VH, Bonfils P., Hansen M., Wewer, Albrechtsen N.J., EP, Floyd A., Svenningsen A., Holst J.J. The effect of gastric bypass treatment for obesity on hormones related to bone re-modeling and intestinal growth. Eur. Calcif. Tissue Soc. Lissabon. 2013;1:111. [Google Scholar]
- Hjollund, KR, Deacon, CF & Holst, JJ 2011, ‘Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1 (GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs’, paper presented to Diabetologia, 2011, < http://dx.doi.org/10.1007/s00125-011-2168-7>. [DOI] [PubMed]
- Holst J.J. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr. Opin. Pharmacol. 2013 doi: 10.1016/j.coph.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Holst J.J. Incretin hormones and the satiation signal. Int. J. Obes. (Lond) 2013;37(9):1161–1168. doi: 10.1038/ijo.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H., Addona T., Burgess M., Kuhn E., Carr S.A. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol. Cell. Proteomics. 2007;6(12):2212–2229. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H., Addona T., Burgess M., Kuhn E., Carr S.A. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol. Cell. Proteomics. 2007;6(12):2212–2229. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhre R.E., Albrechtsen N.J.W., Hartmann B., Deacon C.F., Holst J.J. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J. Diabetes Complications. 2014 doi: 10.1016/j.jdiacomp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Kuhre R.E., Albrechtsen N.W., Windelov J.A., Svendsen B., Hartmann B., Holst J.J. GLP-1 amidation efficiency along the length of the intestine in mice, rats and pigs and in GLP-1 secreting cell lines. Peptides. 2014;55:52–57. doi: 10.1016/j.peptides.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Kulak N.A., Pichler G., Paron I., Nagaraj N., Mann M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods. 2014;11(3):319–324. doi: 10.1038/nmeth.2834. [DOI] [PubMed] [Google Scholar]
- Lee A.Y.H., Chappell D.L., Bak M.J., Judo M., Liang L., Churakova T., Ayanoglu G., Castro-Perez J., Zhou H., Previs S., Souza S.C., Lassman M.E., Laterza O.F. Multiplexed quantification of proglucagon-derived peptides by immunoaffinity enrichment and tandem mass Spectrometry after a meal tolerance test. Clin. Chem. 2015 doi: 10.1373/clinchem.2015.244251. [DOI] [PubMed] [Google Scholar]
- Lin B., White J.T., Wu J., Lele S., Old L.J., Hood L., Odunsi K. Deep depletion of abundant serum proteins reveals low-abundant proteins as potential biomarkers for human ovarian cancer. Proteomics Clin. Appl. 2009;3(7):853–861. doi: 10.1002/prca.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsbad S., Dirksen C., Holst J.J. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–164. doi: 10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- Madsbad S., Holst J.J. GLP-1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes. 2014;63(10):3172–3174. doi: 10.2337/db14-0935. [DOI] [PubMed] [Google Scholar]
- Mann M., Kulak N., Nagaraj N., Cox Jr. The coming age of complete, accurate, and ubiquitous proteomes. Mol. Cell. 2013;49(4):583–590. doi: 10.1016/j.molcel.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Meier J.J., Nauck M.A. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired β-cell function? Diabetes. 2010;59(5):1117–1125. doi: 10.2337/db09-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner F., Scheltema R.A., Mollenkopf H.J., Mann M. Direct proteomic quantification of the secretome of activated immune cells. Science. 2013;340(6131):475–478. doi: 10.1126/science.1232578. [DOI] [PubMed] [Google Scholar]
- Meng R., Gormley M., Bhat V.B., Rosenberg A., Quong A.A. Low abundance protein enrichment for discovery of candidate plasma protein biomarkers for early detection of breast cancer. J. Proteomics. 2011;75(2):366–374. doi: 10.1016/j.jprot.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Nauck M.A., Vardarli I., Deacon C.F., Holst J.J., Meier J.J. Secretion of glucagon-like peptide-1 (GLP-1) In type 2 diabetes: what is up, what is down? Diabetologia. 2011;54(1):10–18. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- Olsen J.V., Macek B., Lange O., Makarov A., Horning S., Mann M. Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods. 2007;4(9):709–712. doi: 10.1038/nmeth1060. [DOI] [PubMed] [Google Scholar]
- Parker C.E., Borchers C.H. Mass spectrometry based biomarker discovery, verification, and validation — quality assurance and control of protein biomarker assays. Mol. Oncol. 2014;8(4):840–858. doi: 10.1016/j.molonc.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A. Action and therapeutic potential of oxyntomodulin. Mol. Metab. 2014;3(3):241–251. doi: 10.1016/j.molmet.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappsilber J., Mann M., Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2(8):1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- Rhee N.A., Wahlgren C.D., Pedersen J., Mortensen B., Langholz E., Wandall E.P., Friis S.U., Vilmann P., Paulsen S.J., Kristiansen V.B., Jelsing J., Dalbøge L.S., Poulsen S.S., Holst J.J., Vilsbøll T., Knop F.K. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia. 2015;58(10):2254–2258. doi: 10.1007/s00125-015-3696-3. [DOI] [PubMed] [Google Scholar]
- Sadry S.A., Drucker D.J. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat. Rev. Endocrinol. 2013;9(7):425–433. doi: 10.1038/nrendo.2013.47. [DOI] [PubMed] [Google Scholar]
- Sandoval D.A., D'Alessio D.A. Vol. 95. 2015. (Physiology of Proglucagon Peptides: Role of Glucagon and GLP-1 in Health and Disease). [DOI] [PubMed] [Google Scholar]
- Scheltema R.A., Hauschild J.-P., Lange O., Hornburg D., Denisov E., Damoc E., Kuehn A., Makarov A., Mann M. The Q exactive HF, a benchtop mass spectrometer with a pre-filter, high-performance quadrupole and an ultra-high-field orbitrap analyzer. Mol. Cell. Proteomics. 2014;13(12):3698–3708. doi: 10.1074/mcp.M114.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjoldager B.T., Baldissera F.G., Mortensen P.E., Holst J.J., Christiansen J. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and Insulin secretion in man. Eur. J. Clin. Invest. 1988;18(5):499–503. doi: 10.1111/j.1365-2362.1988.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Surinova S., Schiess R., Hüttenhain R., Cerciello F., Wollscheid B., Aebersold R. On the development of plasma protein biomarkers. J. Proteome Res. 2011;10(1):5–16. doi: 10.1021/pr1008515. [DOI] [PubMed] [Google Scholar]
- Svendsen B., Pais R., Engelstoft M.S., Milev N.B., Richards P., Christiansen C.B., Egerod K.L., Jensen S.M., Habib A.M., Gribble F.M., Schwartz T.W., Reimann F., Holst J.J. GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J. Endocrinol. 2016;228(1):39–48. doi: 10.1530/JOE-15-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer Albrechtsen N.J., Bak M.J., Hartmann B., Christensen L.W., Kuhre R.E., Deacon C., Holst J.J. Stability of glucagon-like peptide-1 and glucagon in human plasma. Endocr. Connect. 2015 doi: 10.1530/EC-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer Albrechtsen N.J., Kuhre R.E., Deacon C.F., Holst J.J. Targeting the intestinal L-cell for obesity and type 2 diabetes treatment. Expert. Rev. Endocrinol. Metab. 2014;9(01):61–72. doi: 10.1586/17446651.2014.862152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material