Abstract
Introduction
This short case series presents the results from 5 patients with bilateral chronic diabetic macular edema (DME), 12 months after they were initially treated with ILUVIEN® [0.2 μg/day fluocinolone acetonide (FAc)].
Methods
Ten eyes from five patients with pseudophakic lenses were investigated. Patients had bilateral, chronic DME and had received prior laser and anti-VEGF therapy. Visual and anatomic outcomes were investigated 12 months post-FAc implant in both eyes.
Results
At baseline, central retinal thickness (CRT) was 645.3 ± 176.1 microns (mean ± standard deviation), intraocular pressure (IOP) was 13.7 ± 3.6 mmHg and visual acuity (VA) was 44.5 ± 18.6 Early Treatment Diabetic Retinopathy Study (ETDRS) letters. Mean CRT improved at 6 months (341.7 ± 169.7 microns) and 12 months (287.4 ± 103.1 microns) and there were concurrent improvements in VA (ETDRS letters were 56 ± 16 and 55 ± 16 at 6 and 12 months, respectively). Mean IOP was stable throughout and ≤21 mmHg. Left and right eyes were compared in the 5 patients by plotting changes in CFT, IOP and VA at 12 months, from baseline levels.
Conclusion
This bilateral case series demonstrates the effectiveness of a sustained, controlled low dose of FAc in the management of bilateral DME over a 12-month period. The FAc implant has shown to work well in treatment of bilateral DME, although longer follow-up of these patients is still needed.
Funding
Publication charges were funded by Alimera Sciences Ltd.
Electronic supplementary material
The online version of this article (doi:10.1007/s40123-016-0049-3) contains supplementary material, which is available to authorized users.
Keywords: Bilateral diabetic macular edema, Central retinal thickness, Fluocinolone acetonide implant, Intraocular pressure, Visual acuity
Case Series
In everyday clinical practice, patients frequently present with bilateral diabetic macular edema (DME), yet there is a paucity of reported data on the bilateral use of DME therapies [1]. ILUVIEN® [fluocinolone acetonide (FAc) implant] is indicated for the treatment of vision impairment associated with chronic DME, considered insufficiently responsive to available therapies [2]. A single implant in the affected eye is recommended, with the fellow eye being available for therapy but not at the same time or visit as the first eye [2]. This inevitably means that treatment of the fellow eye is delayed; however, early intervention is important in the management of DME as prolonged edema can lead to irreversible damage and permanent vision loss [3].
The structural and functional responses following bilateral intravitreal injections of the FAc implant have been reported previously [4]. The objective of this case series is to report the structural and functional responses 12 months after intravitreal injection of the FAc implant.
Data are presented from 10 eyes. The demographics for the group and prior therapies are presented in Table 1. Prior to intravitreal injection of the FAc implant, all patients had received at least one macular laser therapy for DME. Patients had also received an average of 8.9 (range 3–19) intravitreal injections of an anti-VEGF and 1.2 (range 0–3) intravitreal injections of triamcinolone acetonide. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Table 1.
Patient demographics and baseline values
| Patient number | Gender | Age | Diabetes type | Eye number | Left or right eye | IOP-lowering drops | Prior medical treatment for DME | Date of 0.2 μ/g day FAc implant | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Laser | RBZ | IVTA | ||||||||
| 1 | Male | 70 | II | 1 | LE | N | 1× Grid | 9× | 1× | July 29, 2014 |
| 2 | RE | N | 1× Grid | 9× | 1× | September 9, 2014 | ||||
| 2 | Female | 58 | II | 3 | RE | Lumigan/azarga | 1× Focal | 19× | 1× | June 10, 2014 |
| 4 | LE | Lumigan/azarga | 2× Focal | 19× | 1× | September 23, 2014 | ||||
| 3 | Male | 44 | I | 5 | RE | Latanoprost/timolol | 1× Grid | 6× | 3× | July 25, 2014 |
| 6 | LE | Latanoprost/timolol | 1× Grid | 6× | 2× | October 30, 2014 | ||||
| 4 | Male | 82 | II | 7 | RE | N | 1× Grid | 3× | 2× | April 10, 2014 |
| 8 | LE | N | 1× Grid | 3× | 1× | July 11, 2014 | ||||
| 5 | Male | 63 | I | 9 | LE | N | 1× Grid | 6× | 0 | July 18, 2014 |
| 10 | RE | N | 1× Grid | 9× | 0 | October 21, 2014 | ||||
DME diabetic macular edema, FAc fluocinolone acetonide, IOP intraocular pressure, IVTA intravitreal triamcinolone, LE left eye, RE right eye, RBZ ranibizumab
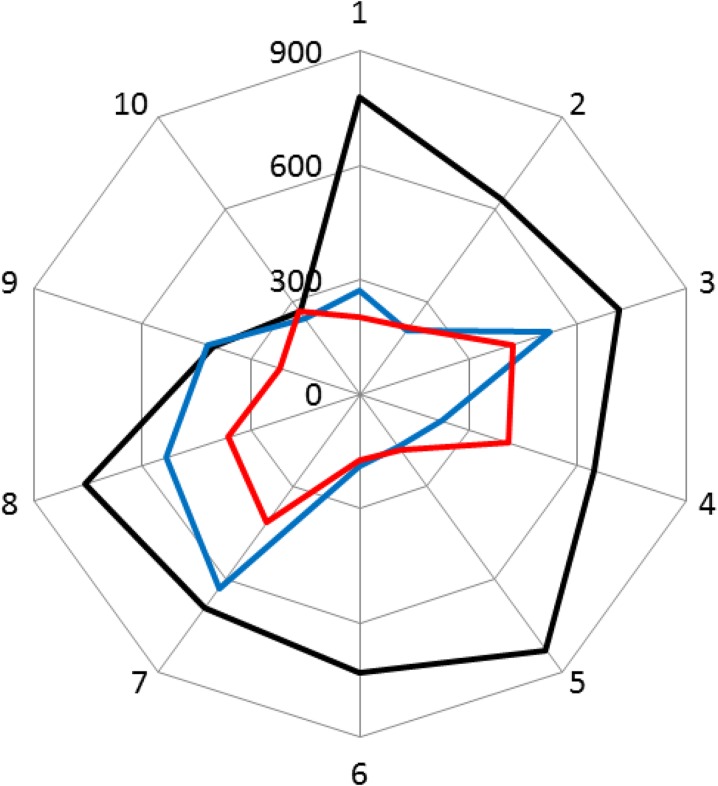
Figure 1 plots central retinal thickness (CRT) for each patient and Table 2 shows the mean changes from baseline. There was a decrease in CRT for 9 of the 10 patients at month 6 or month 12. A single patient (patient 9) initially showed a small (+16 microns) increase in CRT at 6 months but a much greater reduction (−182 microns) at 12 months indicating a delayed response, whereas for patient 10 the changes at months 6 and 12 were comparatively smaller (−22 microns at month 6 and +2 microns at months 12). Overall, mean CRT decreased by −303.6 ± 238.7 microns (−42.1 ± 31.5%) and −357.9 ± 200.3 microns (−50.9 ± 24.2%) at 6 and 12 months, respectively, from a baseline of 645.3 ± 176.1 microns.
Fig. 1.
Individual patient (eyes 1–10) plots of central retinal thickness (microns) at baseline (black line) and 6 months (blue line) and 12 months (red line) after intravitreal injection of the fluocinolone acetonide implant
Table 2.
Mean visual acuity, central retinal thickness and intraocular pressure at baseline and 6 and 12 months after intravitreal injection of the fluocinolone acetonide implant
| Measure | Baseline | 6 months | 12 months |
|---|---|---|---|
| Visual acuity, ETDRS letters | 44.5 ± 18.6 | +11.0 ± 13.1 | +10.5 ± 13.0 |
| Central retinal thickness, µm | 645.3 ± 176.1 | −303.6 ± 238.7 | −357.9 ± 200.3 |
| Intraocular pressure, mmHg | 13.7 ± 3.6 | +1.8 ± 4.5 | +2.3 ± 4.0 |
Data are presented as mean ± standard deviation
ETDRS Early Treatment Diabetic Retinopathy Study
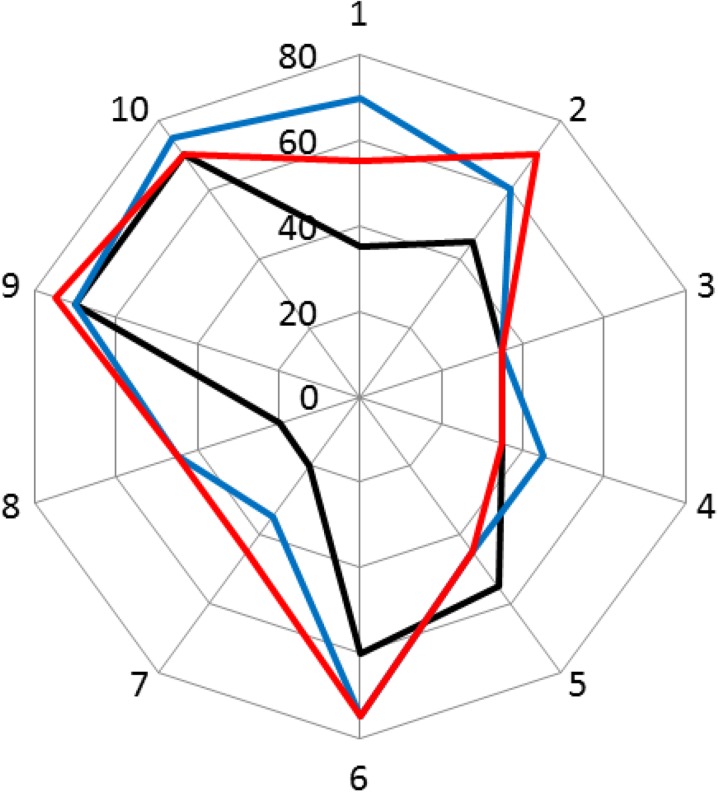
Figure 2 plots visual acuity (VA) in Early Treatment Diabetic Retinopathy Study (ETDRS) letters for each patient and Table 2 shows the mean changes from baseline. At 6 and 12 months, VA was sustained or improved in 9 out of 10 patients with letter gains from baseline ranging between 0 and 35 ETDRS letters. Overall, mean VA increased by 11.0 ± 13.1 and 10.5 ± 13.0 ETDRS letters after 6 and 12 months, respectively, from a baseline of 44.5 ± 18.6 ETDRS letters.
Fig. 2.
Visual acuity in Early Treatment Diabetic Retinopathy Study ETDRS letters for each patient (numbered 1–10) at baseline (black line), 6 months (blue line) and 12 months (red line) after intravitreal injection of the fluocinolone acetonide implant
Intraocular pressure (IOP) was also measured at baseline (mean of 13.7 ± 3.6 mmHg), 6 months (mean 15.5 ± 4.0 mmHg) and 12 months (mean 16.0 ± 3.3 mmHg). Table 2 shows the mean changes from baseline. In all cases, IOP remained ≤21 mmHg.
Conclusion
The patients followed up in our bilateral case series show clinical improvement up to 12 months after intravitreal FAc implantation. Over 12 months, nine out of ten patients had sustained and improved VA with mean improvements of 10.5 letters, and a mean reduction of −357.9 microns in CRT from baseline with no patients experiencing a rise of IOP above 21 mmHg.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Dr Elaraoud and Mr Quhill are the guarantors for this article, and take responsibility for the integrity of the work as a whole. The authors would like to thank Mr C Brand and Mr N Acharya, whose patients are included in this case series. The authors also thank Mr Attawan for his contribution.
The study was performed at the Royal Hallamshire Hospital, Glossop Road, Sheffield, S10 2JF, United Kingdom. No funding was received for the conduct of the study. The publication of this article was supported by Alimera Sciences Ltd. Medical writing assistance for this study was provided and funded by Alimera Sciences Ltd.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Dr Elaraoud and Dr H Quhill have nothing to disclose. Mr F Quhill has attended advisory boards and speaker engagements and has been remunerated for these by Alimera Sciences.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/C4B4F0600D18DB1A.
References
- 1.Hanhart J, Tiosano L, Averbukh E, Banin E, Hemo I, Chowers I. Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye (Lond). 2014;28(6):646–653. doi: 10.1038/eye.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summary of Product Characteristics for ILUVIEN. https://www.medicines.org.uk/emc/medicine/27636. Accessed Feb 29, 2016.
- 3.Virgili G, Parravano M, Menchini F, et al. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;10:CD007419. doi: 10.1002/14651858.CD007419.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Elaraoud I, Attawan A, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (ILUVIEN) in patients with diabetic macular edema. Ophthalmol Ther. 2016;1–10. doi:10.1007/s40123-016-0045-7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.