Abstract
Introduction
We describe a proactive method using electronic patient records (EPR) to identify pseudophakic patients with diabetic macular oedema (DMO) that might benefit from treatment with 0.2 µg/day fluocinolone acetonide (FAc; ILUVIEN®) implant.
Methods
Our EPR audit tool (Medisoft®) identified diabetic patients (May 2011–December 2014) with National Screening Committee-confirmed grade M1 maculopathy. Searches segmented this DMO patient population into patient groups who: (1) had received ranibizumab therapy, (2) had received ≥2 macular laser treatments, or (3) were unsuitable for macular laser or ranibizumab therapy. Pre-specified criteria identified patients insufficiently responsive to treatment, and their electronic case notes were flagged for clinicians to consider FAc, based on National Institute for Health and Care Excellence (NICE) TA301.
Results
Using this methodology, 138 patients with DMO were identified, of whom 87 were assigned to group 1, 32 to group 2, and 29 to group 3 (10 patients were included in both groups 2 and 3). From these, 28 different pseudophakic eyes were identified as suitable for treatment with FAc, based on insufficient response to prior treatment.
Conclusion
EPR audit offers a real-world methodology to efficiently identify patients that might benefit from treatment with FAc. Limitations apply, and thorough documentation of lens status and ocular comorbidities is vital; however, this approach was more rapid than prospective recruitment through the clinic. Flagging patient records using EPR audit offers a practical process for application to clinical practice, thereby optimizing patient care in line with NICE TA301 guidelines.
Funding
Alimera Sciences Ltd.
Electronic supplementary material
The online version of this article (doi:10.1007/s40123-016-0053-7) contains supplementary material, which is available to authorized users.
Keywords: Diabetic macular oedema, Fluocinolone acetonide, ILUVIEN, NICE, Patient records
Introduction
Diabetic retinopathy (DR) is a common, vision-limiting vascular complication of diabetes with a prevalence of approximately 35% [1, 2]. A visual complication manifesting in 6.8% of patients with DR is diabetic macular oedema (DMO), a chronic condition and the most common cause of DR-related vision-loss, particularly in patients with type 2 diabetes [2, 3]. 14–25% of patients diagnosed with diabetes appear to develop DMO within 10 years of the initial diagnosis [4].
Given the association between DMO and vision loss, its early and effective management is critical. However, as a chronic and persistent visual complication that does not follow the natural disease course of DR, it is difficult to manage [3]. Historically, the standard of care was laser therapy, and this approach is still widely used. More recently, the preferred standard of care in patients with DMO has become anti-VEGF therapy [5]. This approach is supported by the outcomes from several large, prospective, randomized clinical trials demonstrating improvements in DMO and associated visual function [6–8]. However, a notable proportion of patients remain insufficiently responsive to anti-VEGF treatment in clinical practice, as shown by a cohort of patients (n = 190) in the RIDE and RISE trials (ClinicalTrials.gov identifiers: NCT00473382 and NCT00473330, respectively), who crossed over from sham to ranibizumab treatment after two years. In this patient group, the improvement in VA after 1 year of therapy was notably less when compared with 1-year gain in VA in patients who initiated ranibizumab at the start of the study [mean 2.8 vs. 11.1 Early Treatment Diabetic Retinopathy (ETDRS) letter gain, respectively] [7, 8] suggesting that patients with a longer duration of DMO (chronic DMO) were less likely to achieve a response with ranibizumab.
Diabetic macular oedema is a multifactorial disease, involving the up-regulation of multiple inflammatory cytokines in addition to VEGF [9]. Consequently, corticosteroids, which have a broader-spectrum of activity, may represent an alternative therapeutic option for patients with chronic DMO. Data from the Fluocinolone Acetonide in Diabetic Macular Edema [FAME (ClinicaTrials.gov identifier. NCT00344968)] trials indicate efficacy of 0.2 µg/day FAc implant in DMO [17]. In Europe, 0.2 μg/day FAc implant is approved for the treatment of vision impairment associated with chronic DMO considered insufficiently responsive to available therapies [10]. In the UK, the National Institute for Health and Care Excellence (NICE; TA301) recommends 0.2 μg/day FAc implant as an option for treating chronic DMO that is insufficiently responsive to available therapies in eyes that are pseudophakic [11].
The timely implementation of NICE technology appraisals (TAs) is an ethical responsibility and an opportunity to enhance patient care. National Health Service (NHS) trusts have 3 months to implement guidance and a proactive, methodical approach is needed to optimise TA implementation. Medisoft® (Medisoft Ltd., Leeds, UK) is an electronic patient records (EPR) database, which in our practice contains the details for a large diabetic patient population. Here, we report a methodology that utilizes this EPR audit tool to identify patients who may have sub-optimal responses to current first-line treatment options (anti-VEGF or laser) and consequently, who might benefit from 0.2 μg/day FAc implant. This methodology can be approached from different angles to optimally identify patient groups that meet pre-specified criteria for insufficient response or treatment failure. The data presented within this manuscript are based on searches using EPR records for patients within the Calderdale and Huddersfield NHS Foundation Trust, which serves a population of approximately 22,500 patients with type 1 and type 2 diabetes in Calderdale and South Kirklees, UK. To our knowledge, no prior studies have reported the use of EPR audit to identify patients with DMO suitable for subsequent therapy.
Methods
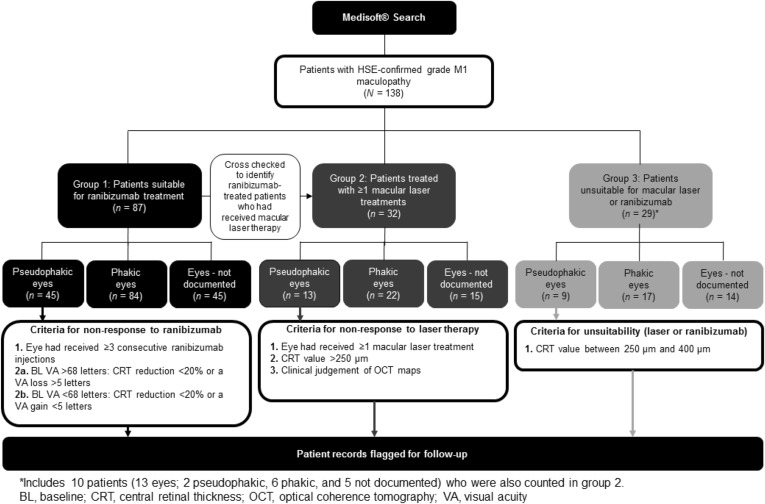
Using the Medisoft EPR tool, searches spanning May 2011–May 2014 were conducted using pre-determined search terms (see the Appendix in the supplementary material), tailored to identify all patients referred to the Calderdale and Huddersfield NHS Foundation Trust with National Screening Committee (NSC)-confirmed grade M1 maculopathy [i.e., exudate within 1 disc diameter (DD) of the centre of the fovea, circinate or group of exudates within the macula, retinal thickening within 1DD of the centre of the fovea (if stereo available) or any microaneurysm or haemorrhage within 1DD of the centre of the fovea only if associated with a best visual acuity (VA) of ≤6/12 (if no stereo)]. Identified patients were further divided into 3 pre-specified patient groups that might include patients who could benefit from 0.2 μg/day FAc implant (Fig. 1). For each of these patient groups, information on patient demographics and disease history were gathered, as was information relating to therapeutic interventions, VA (ETDRS letter score) and anatomical measures of DMO [e.g., central retinal thickness (CRT) according to optical coherence tomography (OCT) map, µm]. Patients who might benefit from 0.2 μg/day FAc were identified in each of the 3 patient groups (Fig. 1), based on the demographic and disease characteristics, and the corresponding electronic case notes were flagged as potentially suitable for therapy with a note for the clinician to consider 0.2 μg/day FAc implant based on TA301. The selection process and methodology for identifying patients is outlined for each group below. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Fig. 1.
Schematic illustrating the three different patient groups identified using the Medisoft® EPR tool. EPR electronic patient records
Group 1: Patients Insufficiently Responsive to Ranibizumab
The EPR audit tool was used to identify patients among those patients with NSC-confirmed grade M1 maculopathy who had received ranibizumab treatment (Fig. 1). Patient records were exported for identified patients, excluding any with non-DMO indications. For this analysis, treatment success was evaluated in eyes that had received ≥3 consecutive ranibizumab injections. The selection of this time point for the assessment of response is supported by the results of the Early Anti-VEGF Response and Long-term Efficacy (EARLY) study, which showed that long-term response to anti-VEGF therapy in patients with DMO could be predicted after three injections (ClinicaTrials.gov identifier. NCT00445003) [12]. Patients with a baseline VA of >68 letters were considered unresponsive to ranibizumab if they achieved less than a 20% reduction in CRT or a loss in VA of >5 letters. In patients who had a baseline VA of <68 letters, non-response to ranibizumab treatment was defined as less than 20% reduction in CRT or a gain in VA of <5 letters. The exported patient records were reviewed to determine which pseudophakic patients (to comply with NICE TA301) [11] with DMO were insufficiently responsive to ranibizumab based on these response criteria, and their patient records flagged accordingly.
Group 2: Patients Unresponsive to Macular Laser Therapy
The EPR audit tool was used to identify which patients with NSC-confirmed grade M1 maculopathy had received more than one macular laser treatment (Fig. 1, group 2). In addition, those patients assigned to group 1 during the EPR search, having received ranibizumab, were cross-checked and any duplicate patient entries deleted; therefore, all patients included in group 2 were ranibizumab naïve. Records for patients with non-DMO indications were excluded.
The selected patient records were exported and reviewed to identify pseudophakic patients with DMO unresponsive to macular laser therapy. Patients who had received more than one laser treatment with persistent DMO (based on clinical judgement of OCT maps and CRT values >250 µm) were deemed to have failed laser therapy.
Group 3: Patients Unsuitable for Ranibizumab or Macular Laser Treatment
Those patients with NSC-confirmed grade M1 maculopathy who were originally identified and who did not conform to the criteria specified for inclusion in group 1 or 2 were included in group 3 (excluding all patients with non-DMO indications; Fig. 1). These patients had CRT greater than 250 µm but less than 400 µm and so were considered unsuitable for ranibizumab treatment in the UK, based on NICE TA274 guidance (recommends ranibizumab only in patients with CRT >400 µm) [13]. Consequently, patient records were screened to identify those patients where macular laser therapy might not be optimal and anti-VEGF therapy is not recommended, but who might benefit from early intervention with 0.2 μg/day FAc implant to reduce CRT and manage DMO progression effectively. Of note, this patient group included some patients who were unsuitable for laser or ranibizumab therapy but who had received prior laser therapy. Consequently, such patients who had received two or more laser treatments were ‘counted twice’ as they were also included in group 2.
Results
Overall, the EPR search identified 138 patients with DMO, whose demographic characteristics were broadly as anticipated (Table S1 in the supplementary material). The average patient age was 64 years [range 27–86 years; standard deviation (SD) ±13.1 years], and most patients were male (n = 85). The majority of patients had type 2 diabetes (n = 112). Of the 138 patients (264 eyes), 67 eyes were pseudophakic, 123 were phakic, and in the remaining 74 eyes the lens status was not documented (Fig. 1).
Group 1: Patients Insufficiently Responsive to Ranibizumab
Overall, 87 patients had received treatment with ranibizumab and were allocated to group 1 (Table S1). Of the 87 patients, 26 eyes from 26 patients were pseudophakic and had received ≥3 consecutive ranibizumab injections.
At baseline, 16/26 patients had a best-corrected (BC) VA of <68 letters, and 10 patients had a BCVA of >68 letters; mean BCVA was 57.5 letters (range 24–75 years; SD ±14.2 years). Baseline CRT ranged from 320 to 880 µm, with a mean CRT of 473.8 µm (SD ±111.8 µm). Three eyes were excluded from further analysis; 1 patient was awaiting follow-up at the time of analysis and 1 had concomitant wet age-related macular degeneration (wAMD). The third patient was not insufficiently responsive to ranibizumab based on the defined study criteria; however, they received 0.2 µg/day FAc implant based on physician assessment.
Based on the pre-specified parameters for insufficient response following ranibizumab therapy, 13 eyes were determined to have failed ranibizumab therapy and consequently were considered suitable for 0.2 μg/day FAc implant; in these eyes there was a mean reduction in CRT of −110.3 µm (SD ±142.8 µm) and a change in BCVA of −5.7 letters (SD ±12.1 letters) from pre-ranibizumab baseline was reported. In the remaining 10/26 eyes, which were responding to ranibizumab, a mean reduction in CRT of −203.7 microns (SD ±99.2) and mean increase in BCVA of 9.4 letters (SD ±10.6 letters) was reported. Table 1 presents the data on prior interventions as well as CRT and BCVA at baseline and following ranibizumab therapy for each eye.
Table 1.
Prior therapeutic interventions and CRT and BCVA at baseline and post-ranibizumab (≥3 consecutive injections) in pseudophakic patients with diabetic macular oedema
| Eye | Laser | RBZ | Baseline CRT (µm) | Post-RBZ CRT (µm) | Change in CRT from baseline (µm, %) | Baseline BCVA (letters) | Post-RBZ BCVA (letters) | Change in BCVA from baseline (letters) | Failed RBZ, consider for 0.2 μg/day FAc implant |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 7 | 549 | 599 | 50, 9.1 | 24 | 35 | 11 | Y |
| 2 | 6 | 880 | 356 | −524, −59.5 | 35 | 37 | 2 | Y | |
| 3 | 1 | 3 | 433 | 282 | −151, −34.9 | 35 | 45 | 10 | N |
| 4 | 3 | 435 | 286 | −149, −34.3 | 40 | 75 | 35 | N | |
| 5 | 1 | 5 | 638 | 214 | −424, −66.5 | 43 | 58 | 15 | N |
| 6 | 1 | 3 | 457 | 321 | −136. −29.8 | 45 | 55 | 10 | aw f/u |
| 7 | 1 | 6 | 425 | 201 | −224, −52.7 | 48 | 28 | −20 | Y |
| 8 | 2 | 5 | 555 | 470 | −85, −15.3 | 50 | 45 | −5 | Y |
| 9 | 6 | 480 | 329 | −151, −31.5 | 51 | 25 | −26 | Y | |
| 10 | 1 | 3 | 320 | 242 | −78, −24.4 | 54 | 65 | 11 | Excludeda |
| 11 | 4 | 427 | 276 | −151, −35.4 | 55 | 44 | −11 | Y | |
| 12 | 1 | 7 | 550 | 468 | −82, −14.9 | 55 | 69 | 14 | Y |
| 13 | 8 | 480 | 439 | −41, −8.5 | 60 | 48 | −12 | Y | |
| 14 | 1 | 5 | 417 | 327 | −90, −21.6 | 60 | 60 | 0 | Y |
| 15 | 1 | 4 | 519 | 264 | −255, −49.1 | 64 | 75 | 11 | N |
| 16 | 5 | 338 | 288 | −50, −14.8 | 67 | 74 | 7 | Excludedb | |
| 17 | 1 | 4 | 408 | 408 | 0, 0.0 | 70 | 61 | −9 | Y |
| 18 | 2 | 4 | 427 | 375 | −52, −12.2 | 70 | 50 | −20 | Y |
| 19 | 2 | 4 | 411 | 289 | −122, −29.7 | 70 | 75 | 5 | N |
| 20 | 2 | 3 | 421 | 257 | −164, −39.0 | 70 | 75 | 5 | N |
| 21 | 5 | 434 | 284 | −150, −34.6 | 70 | 74 | 4 | N | |
| 22 | 3 | 522 | 298 | −224, −42.9 | 70 | 67 | −3 | N | |
| 23 | 3 | 5 | 410 | 311 | −99, −24.1 | 70 | 70 | 0 | N |
| 24 | 1 | 3 | 590 | 291 | −299, −50.7 | 72 | 84 | 12 | N |
| 25 | 1 | 8 | 388 | 344 | −44, −11.3 | 73 | 75 | 2 | Y |
| 26 | 3 | 404 | 364 | −40, −9.9 | 75 | 75 | 0 | Y | |
| Overall mean (±SD) | 473.8 (111.8) | 330.1 (88.6) | −143.7 (126.0) | 57.5 (14.2) | 59.4 (16.7) | +1.8 (13.0) | N = 26 eyes | ||
| Mean (±SD) in RBZ failures | 491.5 (130.9) | 381.2 (99.4) | −110.3 (142.8) | 55.8 (14.9) | 50.2 (16.8) | −5.7 (12.1) | N = 13 eyes | ||
| Mean (±SD) in RBZ successes | 481.3 (81.5) | 277.6 (27.1) | −203.7 (99.2) | 60.4 (14.8) | 69.8 (11.0) | +9.4 (10.6) | N = 10 eyes | ||
aw f/u awaiting follow-up, BCVA best-corrected visual acuity, CRT central retinal thickness, FAc fluocinolone acetonide, N no, RBZ ranibizumab, SD standard deviation, Y yes (and have subsequently been treated with a 0.2 μg/day FAc implant)
aPatient was not insufficiently responsive to RBZ based on the defined study criteria; however, they received 0.2 µg/day FAc implant based on physician assessment
bPatient had concomitant wet age-related macular degeneration
Group 2: Patients Unresponsive to Macular Laser Therapy
Overall, 50 eyes from 32 patients were ranibizumab naïve and had received more than 1 macular laser treatment, of which 13 were pseudophakic and a further 15 had unknown lens status. The majority of patients received more than 2 laser treatments and 5 eyes reported co-pathologies (Table 2). At the time of analysis, the most recent CRT ranged from 219 to 368 µm (mean 286.9 µm; SD ±53.3 µm) and the most recent BCVA from 42 to 82 letters. The majority of patients (n = 16) had a BCVA in line with driving vision (70 letters) or better. Table 2 presents the data on functional and anatomical visual measurements for each eye.
Table 2.
Prior laser therapy, co-pathologies, and CRT and BCVA at referral (baseline) and most recent measure (post-laser) in pseudophakic patients and patients with unknown lens status (e.g., may be pseudophakic) with diabetic macular oedema who had received more than one prior laser therapy
| Eye | Pseudophakic/unknown | Co-pathologies | Laser | CRT at referral (µm) | Most recent CRT (µm) | Change in CRT from baseline (µm) | BCVA at referral (letters) | Most recent BCVA (letters) | Change in BCVA from baseline (µm) | Failed laser, consider for 0.2 μg/day FAc implant |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pseudophakic | 2 | 325 | 348 | 23 | 90 | 70 | −20 | Y | |
| 2 | Pseudophakic | BRVO, CRVO | 2 | 342 | 219 | −123 | 70 | 61 | −9 | N |
| 3 | Pseudophakic | 6 | 431 | 246 | −185 | 70 | 68 | −2 | N | |
| 4 | Pseudophakic | 4 | 318 | 304 | −14 | 75 | 59 | −16 | Y | |
| 5 | Pseudophakic | 2 | 317 | 318 | 1 | 60 | 45 | −15 | Y | |
| 6 | Pseudophakic | 3 | 249 | 226 | −23 | 75 | 82 | 7 | N | |
| 7 | Pseudophakic | 4 | 211 | 262 | 51 | 85 | 74 | −11 | Y | |
| 8 | Pseudophakic | 2 | 266 | 346 | 80 | 70 | 44 | −26 | Y | |
| 9 | Pseudophakic | 2 | 250 | 269 | 19 | 75 | 42 | −33 | Y | |
| 10 | Pseudophakic | FED | 2 | 245 | 259 | 14 | 75 | 70 | −5 | Y |
| 11 | Pseudophakic | FED | 2 | 257 | 300 | 43 | 75 | 75 | 0 | Y |
| 12 | Pseudophakic | 3 | 392 | 268 | −124 | 55 | 55 | 0 | Y | |
| 13 | Pseudophakic | 3 | 275 | 264 | −11 | 75 | 70 | −5 | Y | |
| 14 | UNK | 2 | 228 | 227 | −1 | 60 | 75 | 15 | Na | |
| 15 | UNK | 3 | 290 | 352 | 62 | 85 | 70 | −15 | Na | |
| 16 | UNK | 2 | 264 | 265 | 1 | 85 | 75 | −10 | Na | |
| 17 | UNK | 3 | 265 | 270 | 5 | 85 | 75 | −10 | Na | |
| 18 | UNK | 4 | 288 | 260 | −28 | 75 | 75 | 0 | Na | |
| 19 | UNK | 2 | 262 | 270 | 8 | 70 | 70 | 0 | Na | |
| 20 | UNK | 4 | 250 | 337 | 87 | 60 | 60 | 0 | Na | |
| 21 | UNK | 4 | 268 | 368 | 100 | 85 | 70 | −15 | Na | |
| 22 | UNK | HZ keratitis | 4 | 279 | 264 | −15 | 85 | 70 | −15 | Na |
| 23 | UNK | Amblyopia | 3 | 283 | 362 | 79 | 55 | 60 | 5 | Na |
| 24 | UNK | 2 | 419 | 291 | −128 | 95 | 60 | −35 | Na | |
| 25 | UNK | 2 | 254 | 290 | 36 | 90 | 58 | −32 | Na | |
| 26 | UNK | 2 | 259 | 277 | 18 | 85 | 64 | −21 | Na | |
| 27 | UNK | 2 | 277 | 309 | 32 | 90 | 75 | −15 | Na | |
| 28 | UNK | 3 | 265 | 263 | −2 | 90 | 80 | −10 | Na | |
| Overall mean (±SD) | 286.8 (53.3) | 286.9 (41.8) | +0.2 (68.1) | 76.6 (11.4) | 66.1 (10.5) | −10.5 (12.2) | N = 28 eyes | |||
| Mean (±SD) in pseudophakic laser failures | 285.6 (52.4) | 293.8 (34.6) | +8.2 (54.7) | 73.5 (10.3) | 60.4 (13.1) | −13.1 (11.1) | N = 10 eyes | |||
| Mean (±SD) in pseudophakic laser successes | 340.7 (91.0) | 230.3 (14.0) | −110.3 (81.7) | 71.7 (2.9) | 70.3 (10.7) | −1.3 (8.0) | N = 3 eyes | |||
BCVA best-corrected visual acuity, BRVO branch retinal vein occlusion, CRT central retinal thickness, CRVO central retinal vein occlusion, FAc fluocinolone acetonide, FED Fuch’s endothelial dystrophy, HZ herpes zoster, N no, SD standard deviation, UNK unknown, Y yes
aUNK patients are considered unsuitable for 0.2 μg/day FAc implant, as they are assumed to be phakic (recent cataract surgeries are recorded on Medisoft®)
Based on the pre-specified criteria, 10/13 pseudophakic eyes were deemed to have failed macular laser therapy and thus were considered potential candidates for 0.2 μg/day FAc implant. In these patients, the mean change in CRT and BCVA from baseline (referral) were +8.2 µm (SD ±54.7 µm) and −13.1 letters (SD ±11.1 letters), respectively. Patients with unknown lens status were considered unsuitable for 0.2 μg/day FAc implant at this time, as their lens status was assumed to be phakic.
Group 3: Patients with Centre-Involving DMO Unsuitable for Ranibizumab or Macular Laser Treatment
Overall, 40 eyes from 29 patients were allocated to group 3 (Table S1). In those patients (n = 23) who were pseudophakic or with unknown lens status, 11 eyes had previously received 1–3 laser treatments and 3 eyes reported co-pathologies (Table 3). BCVA at referral ranged from 50 to 90 letters (mean 78.4 letters; SD ±12.0 letters), and appeared relatively stable with a mean recent BCVA at the time of analysis of 74.3 letters (range 54–90 letters; SD ±8.1 letters). CRT was <400 µm in all patients and ranged from 228 to 345 µm (mean 297.2 µm; SD ±31.1 µm). Of the 23 eyes, 5 pseudophakic eyes were selected as suitable candidates for 0.2 μg/day FAc implant [eye 4 was also counted in group 2 (eye 1)]. All 5 patients had good VA (mean 72.0 letters; SD ±12.7 letters) and CRT >250 µm (mean 303.0 µm; SD ±28.5 letters). Patients with unknown lens status were considered unsuitable for 0.2 μg/day FAc implant at this time, as their lens status was assumed to be phakic.
Table 3.
Prior therapeutic interventions, CRT, and BCVA in patients with centre-involving diabetic macular oedema who were pseudophakic or who had unknown lens status (e.g., may be pseudophakic)
| Eye | Pseudophakic/unknown | Co-pathologies | Prior interventions | CRT (µm) | BCVA at referral (letters) | Most recent BCVA (letters) | Consider for 0.2 μg/day FAc implanta |
|---|---|---|---|---|---|---|---|
| 1 | Pseudophakic | Pallor of optic disc | None | 266 | 73 | 75 | N |
| 2 | Pseudophakic | None | 279 | 70 | 70 | Y | |
| 3 | Pseudophakic | None | 293 | 60 | 70 | Y | |
| 4 | Pseudophakic | ×2 laser | 325 | 90 | 70 | Y | |
| 5 | Pseudophakic | None | 265 | 90 | 75 | Y | |
| 6 | Pseudophakic | BRVO, CRVO | ×2 laser | 342 | 70 | 61 | N |
| 7 | Pseudophakic | Corneal scar | ×1 laser | 324 | 50 | 54 | N |
| 8 | Pseudophakic | None | 326 | 70 | 75 | Y | |
| 9 | Pseudophakic | None | 307 | 75 | 75 | N | |
| 10 | UNK | ×2 laser | 228 | 60 | 75 | N# | |
| 11 | UNK | ×3 laser | 290 | 85 | 70 | N# | |
| 12 | UNK | ×2 laser | 264 | 85 | 75 | N# | |
| 13 | UNK | None | 328 | 75 | 75 | N# | |
| 14 | UNK | None | NN | 90 | NN | N# | |
| 15 | UNK | None | 300 | 85 | 85 | N# | |
| 16 | UNK | ×1 laser | 285 | 90 | 70 | N# | |
| 17 | UNK | ×1 laser | 310 | 75 | NN | N# | |
| 18 | UNK | ×2 laser | 277 | 90 | 75 | N# | |
| 19 | UNK | None | 345 | 90 | 85 | N# | |
| 20 | UNK | None | 306 | 85 | 85 | N# | |
| 21 | UNK | None | 273 | 90 | 90 | N# | |
| 22 | UNK | ×3 laser | 265 | 90 | 80 | N# | |
| 23 | UNK | ×1 laser | 341 | 66 | 70 | N# | |
| Mean (±SD) in pseudophakic patients | 303.0 (28.5) | 72.0 (12.7) | 69.4 (7.4) | N = 9 | |||
| Overall mean (±SD) | 297.2 (31.1) | 78.4 (12.0) | 74.3 (8.1) | N = 23 | |||
BCVA best-corrected visual acuity, BRVO branch retinal vein occlusion, CRT central retinal thickness, CRVO central retinal vein occlusion, FAc fluocinolone acetonide, N no, SD standard deviation, UNK unknown. NN, no number
aSome patients were considered unsuitable for 0.2 µg/day FAc implant based on physician assessment and disease history (e.g., patients with a history of glaucoma/raised intra-ocular pressure were deemed unsuitable for 0.2 µg/day FAc implant)
bUNK patients are considered unsuitable for 0.2 μg/day FAc implant, as they are assumed to be phakic (recent cataract surgeries are recorded on Medisoft®)
Discussion
In this study, we used a simple, EPR-based methodology to identify patients insufficiently responsive to their current treatments and consequently potential candidates for 0.2 μg/day FAc implant. Using the defined search criteria, 13 pseudophakic eyes from 87 patients were identified as unresponsive to ranibizumab and potentially suitable for 0.2 μg/day FAc implant (and have subsequently been treated). Similarly, of the pseudophakic eyes identified in groups 2 and 3, 10 and 5 eyes, respectively, were considered potential candidates for 0.2 μg/day FAc implant. Of these, 7 and 2 patients, respectively, have received a 0.2 μg/day FAc implant.
NICE TA301 criteria state that pseudophakic patients with DMO can be treated with 0.2 μg/day FAc implant where other standard therapies have shown an insufficient response [11]. To identify appropriate patients who are insufficiently responsive and who might benefit from this treatment, a practical approach with broad applicability is required. This study demonstrates the utility of an EPR audit tool to identify appropriate patients, based on pre-defined search criteria. Different search criteria were applied, allowing the identification of patients who were either insufficiently responsive, or unsuitable for conventional treatments. The time required to identify patients with this approach was in the order of a few days, compared with the long process (months) of prospective identification through the clinic.
The anti-VEGF injection, ranibizumab, reaches therapeutic stability following treatment initiation with 3 consecutive monthly injections; after this time, the majority of potential visual gain has been achieved [14, 15]. Consequently, for patients treated with ranibizumab in this study, success was evaluated in patients who had received ≥3 consecutive ranibizumab injections. The criteria for treatment success were defined as a function of baseline VA. In patients with good baseline vision (≥68 letters), but anatomical evidence for vision-threatening DMO (e.g., CRT ≥400 µm), the aim of treatment was to improve DMO (≥20% reduction in CRT) or a gain of vision (>5 letters). However, in patients with worse vision (<68 letters), the aim of treatment was considered to be an improvement in DMO or maintenance of visual function (<5 letter gain). It is arguably of particular importance to identify treatment failure early in this latter group, as a retrospective analysis of the FAME studies demonstrated that visual outcomes were better in patients with chronic DMO with less deterioration in baseline BCVA whether they were treated with intermittent therapies or 0.2 μg/day FAc implant; however, patients in the latter group achieved notably better outcomes [16].
A potentially overlooked cohort is those patients with DMO who have a CRT <400 µm. It is assumed that, as they cannot access ranibizumab therapy in the UK due to a lack of evidence for cost-effectiveness in this patient group [13], they will be treated with macular laser therapy. However, a proportion of patients exist for whom laser therapy would be potentially damaging, for example if the laser needs to be applied close to the centre of the retina. In addition, patients may have CRT <400 µm, but have received prior laser therapy that had been unsuccessful. This group of patients has a need for effective intervention to optimise outcomes and limit the damaging effects of progressive DMO, and thus represent a subgroup that may benefit from 0.2 μg/day FAc implant. Here, patients with CRT <400 µm but >250 µm who were not suitable for laser or ranibizumab therapy were reviewed to consider their suitability for 0.2 μg/day FAc implant, identifying a further 5 pseudophakic eyes whose records were flagged accordingly.
In this study, we have shown the potential to use the EPR audit tool effectively, proactively, and rapidly to identify patients who are insufficiently responsive or failing to respond to current standard-of-care treatments. In addition, we propose a practical mechanism for the clinical application of this methodology through the flagging of individual patient records. This approach allows the efficient and timely identification of patients who might benefit from 0.2 μg/day FAc implant, which has both ethical and socioeconomic implications. Earlier, effective treatment has the potential for improved visual outcomes and subsequent quality of life, which in turn affects the patients’ ability to work and the frequency of treatment visits required. While the current study has illustrated that there may be a therapeutic solution for pseudophakic patients with insufficient response to their current therapy, there remains a significant gap in care options for phakic patients that is not currently addressed. Additionally, this retrospective approach could highlight a number of other patient groups that are not being effectively treated, and for whom there is a question of optimal management.
Limitations
There are several important considerations and potential limitations to consider. Complete documentation of current lens status and the presence of ocular comorbidities is necessary for the effective identification of suitable patients, and whilst paper and EPR systems are in concurrent use, it is likely that neither record will be complete. However, in the UK there is a paperless initiative in place, which mandates that all patient records should be fully digital by April 2018, with a clear move towards a paper-light system by 2015 [17]. This will require complete digital documentation, both facilitating and enhancing the value of an EPR audit system such as that described here. However, more extensive digital patient records will not obviate the need for appropriate consideration of optimal search terms. Additionally, this study was performed within the context of the UK healthcare system using a specific EPR tool. However, the objectives of the study are relevant to other healthcare systems and the methodology is likely to be transferable to other technology platforms; the flexibility of EPR tools allows the use of locally derived criteria for the selection of specific patient populations.
Conclusions
To conclude, EPR audit offers a real-world and readily applicable methodology for optimizing treatment options in patients with DMO.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Ackowledgments
Medical writing support was provided by QXV Comms (an Ashfield business, part of UDG Healthcare plc), Macclesfield, UK, and was fully funded by Alimera Sciences Ltd. The research and publication of this article was supported by Alimera Sciences Ltd. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Farhat Butt has received travel grants to attend ophthalmology conferences, and has received part of a medical education grant to fund the time spent collecting data for this work from Alimera Sciences. Rehna Khan has received travel grants to attend ophthalmology conferences, and has received part of the above medical education grant to support eye clinic nurse training and purchase of equipment for the eye department. There are no other financial declarations relevant to this work. Saadia Chaudhry and Kamron Khan declare no potential conflict of interest.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhance content for this article go to www.medengine.com/Redeem/D5C4F06018C15E1D.
References
- 1.Frank RN. Diabetic retinopathy. NEJM. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conceicao L, Pires I, Cuncha-Vaz J. Diabetic Macular Edema. In: Rui B, Cunha-Vaz J, editors. Optical coherence tomography. Berlin: Spinger Verlag; 2012. pp. 1–21. [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/S0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 5.Tripathy K, Sharma YR, et al. Recent advances in management of diabetic macular edema. Curr Diabetes Rev. 2015;11:79–97. doi: 10.2174/1573399811999150324120640. [DOI] [PubMed] [Google Scholar]
- 6.Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4:151–169. doi: 10.1177/2042018813512360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–2022. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency. SPC: ILUVIEN 190 micrograms intravitreal implant in applicator [online]. https://www.medicines.org.uk/emc/medicine/27636. Accessed Feb 10, 2015.
- 11.NICE guidelines [TA301], 2013 [online]. https://www.nice.org.uk/guidance/ta301. Accessed Mar 23, 2016.
- 12.Dugel PU, Campbell J, Holekamp N, Kiss S, Loewenstein A, Augustin A, Ma J, Ho A, Patel V, Whitcup S, Gonzalez V. Long-term response to anti-VEGF therapy for DME can be predicted after 3 injections—an analysis of the Protocol I data. Presented at the American Academy of Ophthalmology in 2015.
- 13.NICE TA274. Ranibizumab for treating diabetic macular oedema (rapid review of technology appraisal guidance 274) [online]. https://www.nice.org.uk/guidance/ta274. Accessed Mar 23, 2016.
- 14.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell P, Bressler N, Tolley K, et al. Patient-reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: randomized clinical trial. JAMA Ophthalmol. 2013;131:1339–1347. doi: 10.1001/jamaophthalmol.2013.4592. [DOI] [PubMed] [Google Scholar]
- 16.Downey L, Chakravarthy U. Exploratory analyses of long-term visual outcomes based on baseline vision in patients with chronic and nonchronic diabetic macular oedema (DMO) treated with fluocinolone acetonide (FAc) [abstract no. 221]. Royal College of Ophthalmologists Annual Congress 19–21 May 2015.
- 17.Department of Health. Jeremy Hunt challenges NHS to go paperless [online]. http://digitalchallenge.dh.gov.uk/2013/01/16/paperless/. Accessed Dec 9, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.