Abstract
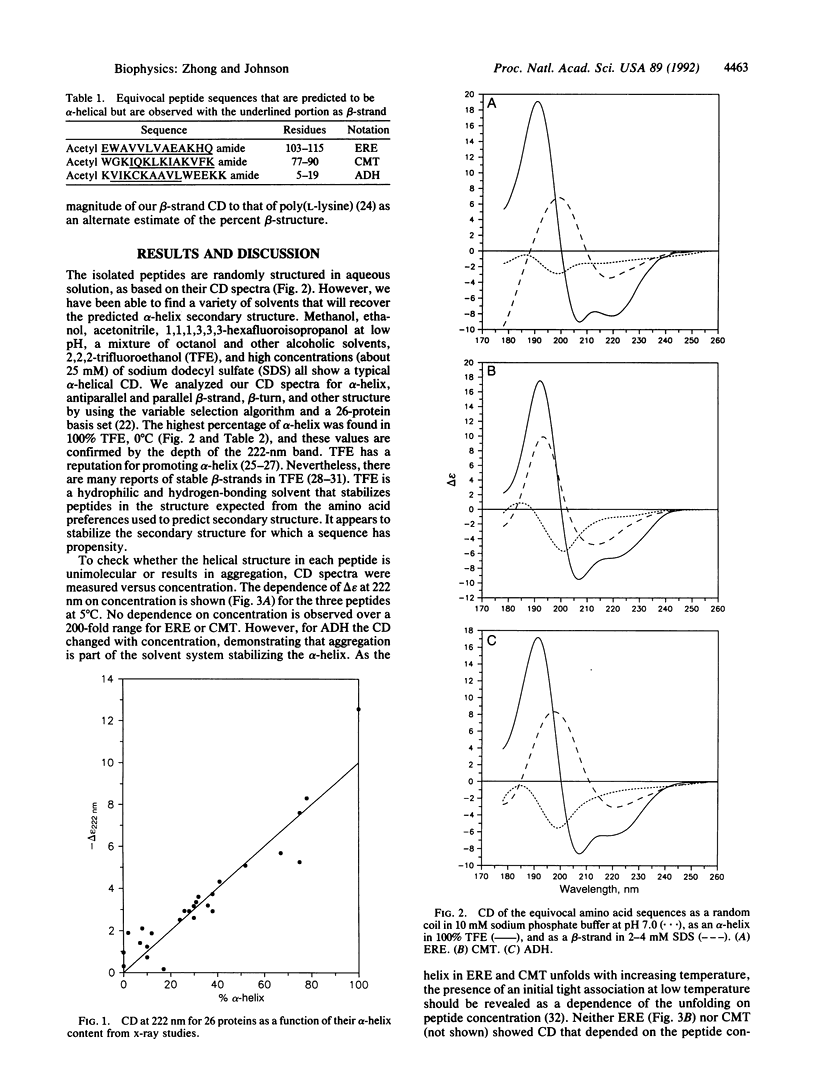
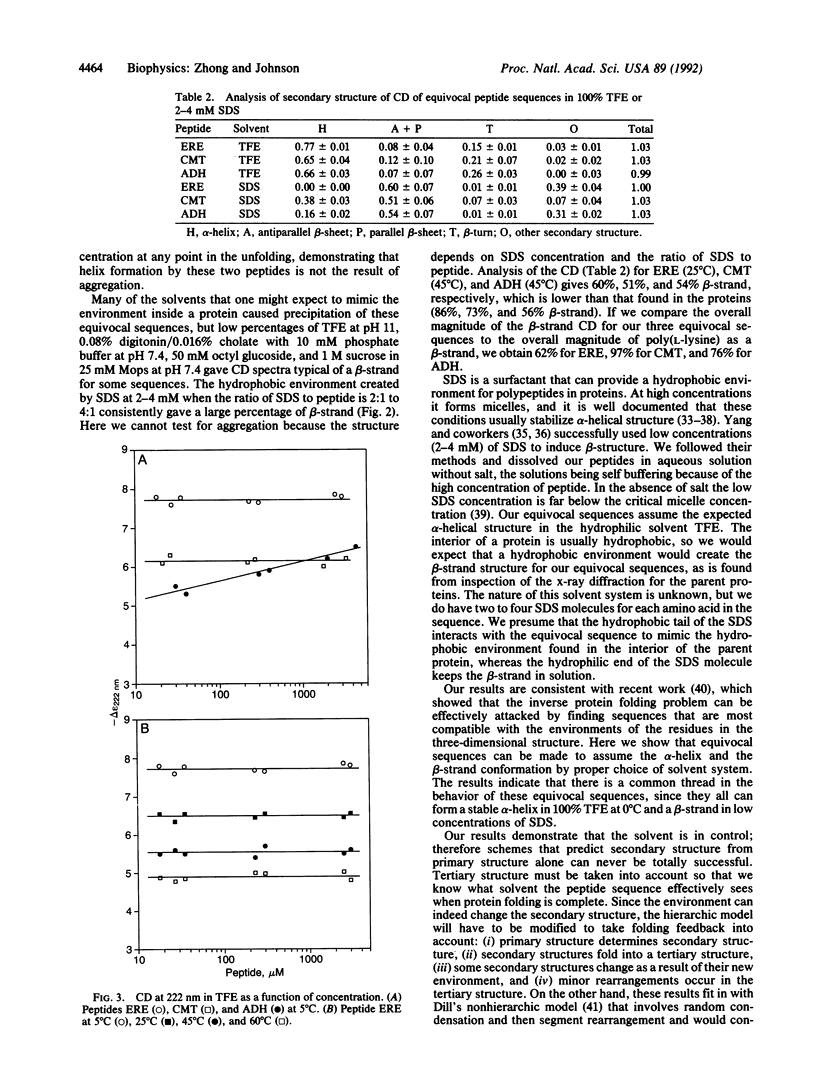
Three equivocal amino acid sequences were synthesized that are predicted to be alpha-helical from amino acid preference but are found to be primarily beta-strand from x-ray diffraction of their respective proteins. In some solvent systems we recover the alpha-helical structure predicted by amino acid preference, whereas in other systems we mimic the interior of the protein and produce a beta-strand. These results are experimental proof that the environment is important in determining the secondary structure formed by an amino acid sequence; therefore schemes that predict secondary structure from amino acid sequence alone can never be totally successful.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balcerski J. S., Pysh E. S., Bonora G. M., Toniolo C. Vacuum ultraviolet circular dichroism of beta-forming alkyl oligopeptides. J Am Chem Soc. 1976 Jun 9;98(12):3470–3473. doi: 10.1021/ja00428a013. [DOI] [PubMed] [Google Scholar]
- Bowie J. U., Lüthy R., Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991 Jul 12;253(5016):164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Abarbanel R. M., Kuntz I. D., Fletterick R. J. Turn prediction in proteins using a pattern-matching approach. Biochemistry. 1986 Jan 14;25(1):266–275. doi: 10.1021/bi00349a037. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Silverton E. W., Davies D. R. Refined crystal structure of gamma-chymotrypsin at 1.9 A resolution. Comparison with other pancreatic serine proteases. J Mol Biol. 1981 Jun 5;148(4):449–479. doi: 10.1016/0022-2836(81)90186-8. [DOI] [PubMed] [Google Scholar]
- Colonna-Cesari F., Perahia D., Karplus M., Eklund H., Brädén C. I., Tapia O. Interdomain motion in liver alcohol dehydrogenase. Structural and energetic analysis of the hinge bending mode. J Biol Chem. 1986 Nov 15;261(32):15273–15280. [PubMed] [Google Scholar]
- Dill K. A. Dominant forces in protein folding. Biochemistry. 1990 Aug 7;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- Edelman J., White S. H. Linear optimization of predictors for secondary structure. Application to transbilayer segments of membrane proteins. J Mol Biol. 1989 Nov 5;210(1):195–209. doi: 10.1016/0022-2836(89)90300-8. [DOI] [PubMed] [Google Scholar]
- Elwell M. L., Schellman J. A. Stability of phage T4 lysozymes. I. Native properties and thermal stability of wild type and two mutant lysozymes. Biochim Biophys Acta. 1977 Oct 26;494(2):367–383. doi: 10.1016/0005-2795(77)90166-0. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hennessey J. P., Jr, Johnson W. C., Jr Information content in the circular dichroism of proteins. Biochemistry. 1981 Mar 3;20(5):1085–1094. doi: 10.1021/bi00508a007. [DOI] [PubMed] [Google Scholar]
- Holley L. H., Karplus M. Protein secondary structure prediction with a neural network. Proc Natl Acad Sci U S A. 1989 Jan;86(1):152–156. doi: 10.1073/pnas.86.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirgensons B. Circular dichroism and conformation of human alpha1-antitrypsin. Biochim Biophys Acta. 1977 Aug 23;493(2):352–358. doi: 10.1016/0005-2795(77)90191-x. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Secondary structure of proteins through circular dichroism spectroscopy. Annu Rev Biophys Biophys Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kelly M. M., Pysh E. S., Bonora G. M., Toniolo C. Vacuum ultraviolet circular dichroism of protected homooligomers derived from L-leucine. J Am Chem Soc. 1977 May 11;99(10):3264–3266. doi: 10.1021/ja00452a010. [DOI] [PubMed] [Google Scholar]
- Kim Y. C., Grable J. C., Love R., Greene P. J., Rosenberg J. M. Refinement of Eco RI endonuclease crystal structure: a revised protein chain tracing. Science. 1990 Sep 14;249(4974):1307–1309. doi: 10.1126/science.2399465. [DOI] [PubMed] [Google Scholar]
- Levin J. M., Robson B., Garnier J. An algorithm for secondary structure determination in proteins based on sequence similarity. FEBS Lett. 1986 Sep 15;205(2):303–308. doi: 10.1016/0014-5793(86)80917-6. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Johnson W. C., Jr, Modrich P. Prediction of secondary structure for Eco RI endonuclease. J Biol Chem. 1984 Oct 10;259(19):11666–11667. [PubMed] [Google Scholar]
- Manavalan P., Johnson W. C., Jr Variable selection method improves the prediction of protein secondary structure from circular dichroism spectra. Anal Biochem. 1987 Nov 15;167(1):76–85. doi: 10.1016/0003-2697(87)90135-7. [DOI] [PubMed] [Google Scholar]
- McClarin J. A., Frederick C. A., Wang B. C., Greene P., Boyer H. W., Grable J., Rosenberg J. M. Structure of the DNA-Eco RI endonuclease recognition complex at 3 A resolution. Science. 1986 Dec 19;234(4783):1526–1541. doi: 10.1126/science.3024321. [DOI] [PubMed] [Google Scholar]
- Merutka G., Stellwagen E. Analysis of peptides for helical prediction. Biochemistry. 1989 Jan 10;28(1):352–357. doi: 10.1021/bi00427a048. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Kallenbach N. R. Persistence of the alpha-helix stop signal in the S-peptide in trifluoroethanol solutions. Biochemistry. 1989 Jun 13;28(12):5256–5261. doi: 10.1021/bi00438a050. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Kallenbach N. R. Stabilization of the ribonuclease S-peptide alpha-helix by trifluoroethanol. Proteins. 1986 Nov;1(3):211–217. doi: 10.1002/prot.340010303. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Ooi T. Amino acid sequence homology applied to the prediction of protein secondary structures, and joint prediction with existing methods. Biochim Biophys Acta. 1986 May 12;871(1):45–54. doi: 10.1016/0167-4838(86)90131-7. [DOI] [PubMed] [Google Scholar]
- Pongor S., Szalay A. A. Prediction of homology and divergence in the secondary structure of polypeptides. Proc Natl Acad Sci U S A. 1985 Jan;82(2):366–370. doi: 10.1073/pnas.82.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N., Sejnowski T. J. Predicting the secondary structure of globular proteins using neural network models. J Mol Biol. 1988 Aug 20;202(4):865–884. doi: 10.1016/0022-2836(88)90564-5. [DOI] [PubMed] [Google Scholar]
- Skolnick J., Kolinski A. Simulations of the folding of a globular protein. Science. 1990 Nov 23;250(4984):1121–1125. doi: 10.1126/science.250.4984.1121. [DOI] [PubMed] [Google Scholar]
- Sweet R. M. Evolutionary similarity among peptide segments is a basis for prediction of protein folding. Biopolymers. 1986 Aug;25(8):1565–1577. doi: 10.1002/bip.360250813. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Ikeda K., Yang J. T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981 Feb 3;20(3):566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Yang J. T. Helical formation of a 13-residue C-peptide analogue of ribonuclease A in sodium dodecyl sulfate solution. Biopolymers. 1988 Mar;27(3):423–430. doi: 10.1002/bip.360270306. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Yang J. T. Sequence-dependent conformations of short polypeptides in a hydrophobic environment. Mol Cell Biochem. 1981 Oct 30;40(2):109–122. doi: 10.1007/BF00224754. [DOI] [PubMed] [Google Scholar]
- Zvelebil M. J., Barton G. J., Taylor W. R., Sternberg M. J. Prediction of protein secondary structure and active sites using the alignment of homologous sequences. J Mol Biol. 1987 Jun 20;195(4):957–961. doi: 10.1016/0022-2836(87)90501-8. [DOI] [PubMed] [Google Scholar]


