Abstract
Cardiovascular disease remains the principal cause of death and disability among patients with diabetes mellitus. Diabetes exacerbates mechanisms underlying atherosclerosis and heart failure. Unfortunately, these mechanisms are not adequately modulated by therapeutic strategies focusing solely on optimal glycemic control with currently available drugs or approaches. In the setting of multi-factorial risk reduction with statins and other lipid lowering agents, anti-hypertensive therapies, and anti-hyperglycemic treatment strategies, cardiovascular complication rates are falling, yet remain higher for patients with diabetes than for those without. This review considers the mechanisms, history, controversies, new pharmacologic agents, and recent evidence for current guidelines for cardiovascular management in the patient with diabetes mellitus to support evidence-based care in the patient with diabetes and heart disease outside of the acute care setting.
Introduction
Reducing atherosclerotic cardiovascular disease (ASCVD) burden in diabetes is a major clinical imperative that should be prioritized to reduce premature death, improve quality of life, and lessen individual and economic burdens of associated morbidities, decreased work productivity, and high cost of medical care. Atherosclerotic cardiovascular disease remains the principal cause of death and disability among patients with diabetes mellitus, especially in those with type 2 diabetes in whom it typically occurs 14.6 years earlier,1 with greater severity, and with more diffuse distribution than in individuals without diabetes.2,3 Furthermore, about two-thirds of deaths in people with diabetes are due to cardiovascular disease: of these, approximately 40% are from ischemic heart disease, 15% from other forms of heart disease, principally congestive heart failure, and about 10% from stroke. Among those with diabetes, excess risks of death from any cause and of ASCVD mortality are particularly prominent in those with younger age, higher burden of glycemia, and greater renal complications, compared to those without.4 Although the incidences of diabetes-related complications including cardiovascular disease have decreased over the past two decades, patients with diabetes continue to have significantly increased risk for vascular complications as compared with individuals without diabetes (Figure 1).5 An estimated 382 million people worldwide have diabetes, and this number is expected to reach 592 million by the year 2035,6 underscoring the global impact of ASCVD in diabetes.
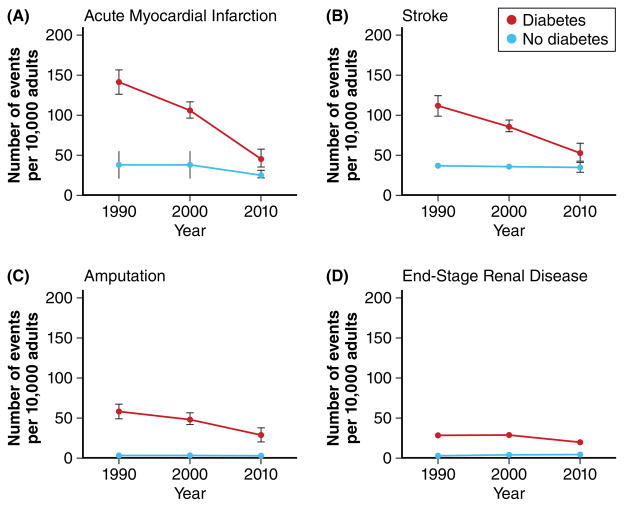
Figure 1. Rates of vascular diseases are decreasing in persons with diabetes but are still higher than in persons without diabetes: Twenty years of surveillance.
Age-standardized rates of selected vascular diseases in individuals with or without diabetes in the years 1990, 2000, and 2010. A: Acute myocardial infarction; B: Stroke; C: Amputation; D: End-stage renal disease. Red: Individuals with diabetes. Blue: Individuals without diabetes. Error bars indicate 95% confidence intervals. Data from Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. New Engl J Med. 2014;370;1514–1523.
Key manifestations of ASCVD in diabetes include advanced atherosclerosis manifest as coronary heart disease, ischemic stroke, peripheral artery disease, and heart failure. Understanding the mechanisms, strategies for and challenges with managing ASCVD and heart failure risk in diabetes, as well as the potential cardiovascular risks and benefits of glucose-lowering drugs, is important for managing cardiovascular disease in diabetes. In this clinical update, we review the current understanding of the mechanisms of ASCVD and heart failure in diabetes, management of these cardiovascular conditions in the diabetes population, and special considerations for treatment of diabetes in patients with ASCVD and/or heart failure. We discuss evidence-based management and areas of uncertainty for ischemic heart disease and heart failure therapies in type 2 diabetes, as well as the impact of diabetes medications on cardiovascular risks. A structured review of the published literature, involving searches of English-language manuscripts for clinical trials and meta-analyses of those trials that may inform treatment decisions for each section was performed by the authors. Case series and non-randomized trials were not considered for inclusion.
Epidemiology of ASCVD in diabetes
The high prevalence of coronary and peripheral artery disease in individuals with diabetes has been recognized for over a century,7–9 yet the ability to improve cardiovascular event rates by glucose lowering per se has remained elusive. In the landmark Framingham Heart Study published in 1979, Kannel and McGee first prospectively demonstrated a higher incidence of cardiovascular disease across all age groups for individuals with diabetes (defined at the time by random blood glucose of ≥150 mg/dL [8.3 mmol/L]) compared to those without, with an even greater impact of diabetes on cardiovascular morbidity and mortality for women than for men.10 The increased risk for ASCVD in diabetes could not be fully accounted for by associated traditional cardiovascular risk factors, and the presence of diabetes in conjunction with other risk factors appeared to cause a synergistic rather than additive additional risk. Importantly, the observed effect of diabetes on ASCVD risk was potentially underestimated in the Framingham Heart study, which also included persons with abnormal glucose tolerance (defined then as a random blood glucose >120 mg/dL).
Subsequent studies confirmed the importance of diabetes as an ASCVD risk factor in diverse populations and suggested diabetes as a risk equivalent for established coronary heart disease, although this remains somewhat controversial.11,12 Persons with diabetes but without a prior myocardial infarction were demonstrated to have high risk of myocardial infarction (20.2% incidence over 7 years), similar to that of individuals with a prior myocardial infarction but no diabetes as shown in the East-West study conducted within the Finnish population.13 Likewise, comparable hazard ratios for cardiovascular death were found in persons age 30 years or older with diabetes requiring glucose-lowering medications but without prior myocardial infarction as compared with persons with a prior myocardial infarction, among 3.3 million individuals from Denmark including 71,801 with diabetes and 79,575 with prior myocardial infarction but without diabetes.14 Furthermore, 21% of all deaths among Alaska Natives with diabetes are from ischemic heart disease.15 Taken together across multiple populations, these studies support the designation of diabetes as a risk equivalent for coronary heart disease16 and highlight the need to better understand ASCVD and to optimize treatment among patients with diabetes.
Mechanisms of increased ASCVD risk and mortality in type 2 diabetes
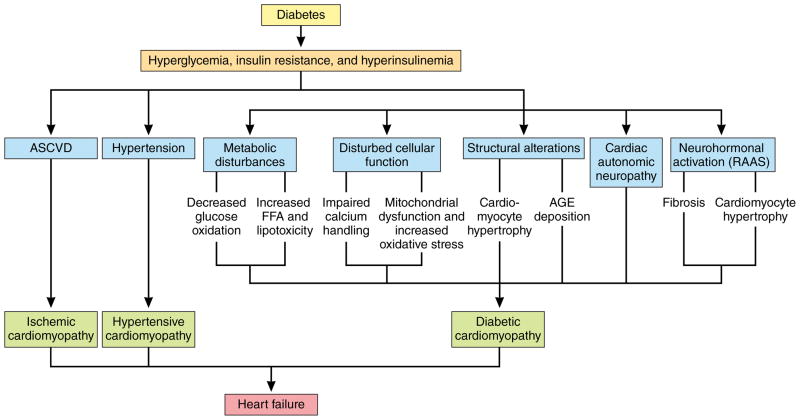
Multiple cellular and molecular pathophysiologic factors participate in ASCVD,17–20 creating the “perfect storm” for atherosclerosis. Patients with type 2 diabetes have greater atherosclerotic plaque burden, higher atheroma volume, and smaller coronary artery lumen diameter than persons without diabetes.21 A general overview of atherogenesis, atherosclerosis progression, and atherothrombosis in diabetes is presented in graphic form in Figure 2. Although numerous processes may contribute to ASCVD in diabetes,22–25 only the following will be described to provide therapeutic context: hyperglycemia, insulin resistance and/or hyperinsulinemia, dyslipidemia, inflammation, reactive oxygen species, endothelial dysfunction, hypercoagulability, and vascular calcification. This discussion is intended to provide a framework for the review of clinical trial evidence and thus is not comprehensive.
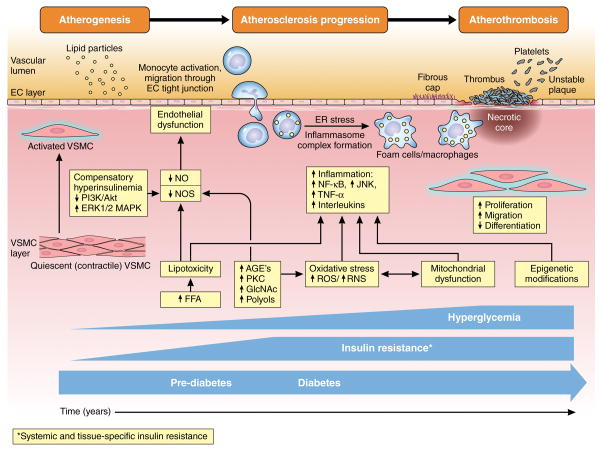
Figure 2. Development and progression of atherosclerosis in diabetes.
Insulin resistance is present before the onset of pre-diabetes or diabetes, and increases progressively over time, while hyperglycemia develops in pre-diabetes and worsens with development of diabetes. Insulin resistance with impairment of insulin signaling, hyperinsulinemia and hyperglycemia contribute to multiple processes including elevated free fatty acids (FFA), advanced glycation end product (AGE) production, protein kinase C (PKC) activation, oxidative stress, mitochondrial dysfunction, and epigenetic modifications, which together contribute to endothelial dysfunction and inflammation resulting in activation of vascular smooth muscle cells (VSMC), endothelial cells (EC), and monocytes. Concentrations of modified (oxidized) LDL are higher in diabetes, and are retained in the subendothelial layer of vulnerable sections of the vasculature. Circulating leukocytes attach and migrate through the endothelial wall into the VSMC layer of the intimal media. These monocytes engulf retained lipoproteins and transform into lipid-laden foam cells/macrophages producing proteinases and inflammatory mediators including tumor necrosis factor alpha (TNF-α) and interleukins. Stress responses including inflammasome complex formation and endoplasmic reticulum (ER) stress result in macrophage proliferation and inflammatory activation with resultant macrophage and VSMC phenotypic switch (proliferation, migration, and dedifferentiation). In response to vascular injury, VSMC secrete collagen to form a fibrous cap, which promotes atherosclerotic plaque stability. However, when stable lesions remodel inward, progressive stenosis of arteries occurs. Plaques can become vulnerable with thinning of the fibrous cap and apoptosis of macrophages in advanced atherosclerotic lesions, where impaired efferocytosis (phagocytic clearance) of lipid laden macrophages results in formation of a necrotic core accelerating vascular inflammation, necrosis, thrombosis. The resulting unstable atherosclerotic lesion complex is prone to sudden expansion from acute thrombus formation forming a nidus for platelet thrombosis, hemorrhage of atherosclerotic plaque microvessels, and rupture of the fibrous cap.
AGE: advanced glycation end-product. Akt: protein kinase B. EC: endothelial cell. NOS: nitric oxide synthase. ER: endoplasmic reticulum. ER: endoplasmi reticulum. ERK: extracellular signal-regulated kinase. FFA: free fatty acids. GlcNAc: N-Acetylglucosamine. IL: interleukin. JNK: c-Jun N-terminal kinase. LDL: low-density lipoprotein. MAPK: mitogen-activated protein kinase. NF-κβ: nuclear factor-kappa beta. NO: nitric oxide. PKC: protein kinase C. PI3K: phosphoinositide 3-kinase. ROS: reactive oxygen species. RNS: reactive nitrogen species. TNF-α: tumor necrosis factor-alpha. VSMC: vascular smooth muscle cell.
Role of hyperglycemia
Diabetes is diagnosed based on fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), 2-hour glucose after 75-gram oral glucose load ≥200 mg/dL (11.1 mmol/L), hemoglobin A1c ≥6.5% (48 mmol/mol), or random plasma glucose ≥200 mg/dL confirmed by repeat testing in the absence of signs and/or symptoms of hyperglycemia or hyperglycemic crisis.26 Diabetes is a heterogeneous disorder with hyperglycemia required for its diagnosis, and despite markedly different genetic and mechanistic etiologies, both type 1 and type 2 diabetes are associated with higher prevalence of ASCVD. Therefore, it is natural to consider hyperglycemia among the causes for accelerated ASCVD observed in patients with diabetes.
Abundant epidemiologic data supports the association between hyperglycemia and increased cardiovascular risk.27–32 There is strong evidence demonstrating greater risk for ASCVD with increasing dysglycemia,33–40 with an estimated 11–16% increase in cardiovascular events for every 1% increase in HbA1c.30,41 The Swedish National Diabetes Register provided compelling evidence for HbA1c as a predictor of fatal and nonfatal coronary heart disease, fatal and nonfatal stroke, fatal and nonfatal cardiovascular disease, fatal cardiovascular disease, and total mortality in a study of 18,334 persons with type 2 diabetes followed over a mean of 5.6 years.32 The relationship between HbA1c and macrovascular disease appears linear and not J-shaped and is observed in subgroups of patients with shorter (≤7 years) and longer duration of diabetes, previous history of cardiovascular disease, and different types of glycemic therapy (oral hypoglycemia agent or insulin). Likewise, a 12% increase in ASCVD risk for every 18 mg/dL (1 mmol/L) increase in fasting glucose above 105 mg/dL33,34 and a similar 13% increased hazard ratio for vascular death for every 18 mg/dL increase in fasting serum glucose above 100 mg/dL (5.6 mmol/L) was demonstrated by the Emerging Risk Factors Collaboration group,34 with comparable findings in numerous studies.35–40
In vitro studies and in vivo models in which hyperglycemia is induced in the absence of elevated lipids are consistent with a direct effect of hyperglycemia on endothelial dysfunction,42,43 atherosclerotic lesion severity and complexity,44 and plaque burden.23 A commonly used pre-clinical diabetes model involves streptozotocin-treatment,45,46 which is toxic to pancreatic β-cells, in an atherosclerosis-prone animal such as an LDL-receptor or apo-E deficient mouse.47 Hyperglycemia results in atherosclerotic lesion formation which can be prevented by intensive insulin therapy,48 while accelerated atherosclerosis develops in the setting of hypercholesterolemia. Similarly, pigs develop atherosclerotic lesions closely mimicking those observed in humans when fed a high-fat high-cholesterol diet after streptozotocin-induced diabetes, developing accelerated atherosclerosis in the aorta and coronary arteries with complex lesions, hemorrhage, and calcification.44,49,50
Acute hyperglycemia can attenuate endothelial function and reduce nitric oxide (NO) bioavailability51 while increasing endothelial cell leukocyte adhesion,43,52 mediated in part by increased oxidative stress and inflammation. Increased flux through the aldose reductase pathway,53 synthesis of diacylglycerol with protein kinase C activation,54 and production of advanced glycation end (AGE) products contribute to activation of endothelial cell receptor for AGEs.55,56 Vascular smooth muscle cells (VSMC) undergo phenotypic switching from a quiescent, contractile state to an activated, proliferative, migratory, dedifferentiated state in the setting of hyperglycemia.57 High glucose concentrations lead to macrophage inflammation and enhancement of response to inflammation,58 and even transient hyperglycemia leads to epigenetic changes with activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway that persists even after return to normoglycemia.59 Under experimental conditions to simulate glycemic variability in individuals with diabetes, 6 hours of hyperglycemia alternating with normoglycemia within a 24 hour period promotes worsening of endothelial function and increased oxidative stress as compared with continuous hyperglycemia even at serum glucose concentrations as high as 282 mg/dL (15.6 mmol/L).60 Together these represent distinct and overlapping mechanisms by which hyperglycemia can promote atherogenesis and accelerate the progression of atherosclerosis (Figure 2).
Role of insulin resistance/hyperinsulinemia
Epidemiologic evidence strongly associates insulin resistance with cardiovascular risk in humans.40,61–68 People with insulin resistance have higher rates of hypertension, dyslipidemia, and impaired glucose tolerance,65,67,69,70 which contribute to development, progression, and complexity of atherosclerosis. Impairment of insulin signaling at multiple points in the insulin signaling pathway in endothelial cells,71–77 VSMC44,78–80 and macrophages22,23,58 promotes development and progression of atherosclerosis, as does the proinflammatory state induced in insulin resistance (Figure 2).22,23,81
Both systemic and tissue-specific vascular insulin resistance contribute to atherosclerosis development and plaque vulnerability.79 In type 2 diabetes, there is selective impairment of insulin signaling through phosphoinositide 3-kinase/protein kinase B, which mediates the metabolic effects of insulin to maintain normal glucose metabolism,82–84 whereas signaling via the extracellular signal-regulated (ERK)-1/2 mitogen-activated protein (MAP) kinase pathway generally remains intact.85–87 Compensatory hyperinsulinemia overstimulates the ERK 1/2-MAP kinase pathway, promoting development or progression of atherosclerosis.79,81 In the apoE-deficient mouse model of atherosclerosis, knocking out the insulin receptor selectively in vascular endothelial cells leads to severe atherosclerosis.77 Furthermore, insulin signaling impairment disrupts activation of endothelial nitric oxide synthase (eNOS) in endothelial cells74 and production of NO with resulting endothelial dysfunction.88–91 VSMC undergo phenotypic modulation22,44,78,92–95 with selective insulin signaling impairment,22,44,78,93 which may participate in atherosclerosis progression. Impaired insulin signaling has complex effects in macrophages, depending on the macrophage sub-type.23,96–98 Multiple additional inflammatory signaling pathways may be activated with insulin resistance and may also stimulate atherosclerotic processes.79,99–101 (Figure 2)
Approaches to target diabetes with insulin sensitization initially held great promise to simultaneously treat hyperglycemia and reduce residual cardiac risk. While metformin continues to be the first choice agent for diabetes management, thiazolidinediones have been neutral to beneficial with regard to ASCVD event rates in for secondary prevention in type 2 diabetes,102 and insulin-sparing therapies have not been shown to be preferential to insulin-provisional therapies in patients with type 2 diabetes and stable ischemic heart disease.103–105 It remains incompletely understood whether the hypothesis of a central pathophysiologic role for insulin resistance promoting both diabetes and ASCVD is incorrect, or whether there are off-target effects of some of these drugs that attenuate potential ASCVD benefits in patients with type 2 diabetes despite effective glucose lowering. Supporting the insulin resistance hypothesis, targeting insulin resistance using the thiazolidinedione pioglitazone reduces fatal or nonfatal stroke or myocardial infarction by 24% and incident diabetes by 52% in patients with insulin resistance and recent ischemic stroke or transient ischemic attack but without type 2 diabetes, albeit with no difference in all-cause mortality between groups.106
Role of diabetes dyslipidemia
Diabetes and dyslipidemia commonly occur together, with lipid abnormalities affecting 60–70% of type 2 diabetes,107 and hyperglycemia accelerates atheroma formation in the setting of diabetic dyslipidemia.48 LDL-cholesterol particles are more atherogenic in diabetes even in the absence of overt increased LDL concentration,108 with small, dense particles that are particularly prone to modification.109 Diabetic dyslipidemia is also characterized by elevated triglycerides, low HDL-cholesterol, and higher concentrations of apolipoprotein B-containing particles.107,110–112 Mechanisms underlying diabetic dyslipidemia remain incompletely understood. Lipid changes are observed in insulin-resistant persons with normal glucose tolerance and in those with metabolic syndrome years before clinical diagnosis of type 2 diabetes,113 suggesting either co-associations of independent disorders or a pathophysiologic role for insulin resistance, rather than hyperglycemia, in the development of diabetic dyslipidemia.113,114
Metabolism of very low-density lipoprotein (VLDL), the main transporter for fasting triglycerides, is insulin-regulated at multiple levels.111,112 Insulin suppresses lipolysis and regulates circulating free fatty acids, which are substrates for VLDL-cholesterol assembly and secretion. In the liver, insulin mediates transfer of triglycerides to apoB and regulates lipoprotein lipase activity to delipidate VLDL-cholesterol. Lipoprotein lipase activity can be disrupted by increased circulating free fatty acids and inhibited by apolipoprotein CIII, while apolipoprotein CIII hinders hepatic uptake of triglyceride-rich lipoproteins, and is itself inhibited by insulin. Thus, in the insulin resistant state, hypertriglyceridemia may be a consequence of elevated free fatty acid level and decreased degradation of apolipoprotein-B leading to overproduction of VLDL-cholesterol, impaired lipoprotein lipase activity, and decreased hepatic uptake of VLDL with reduced VLDL-cholesterol clearance.
Other lipid abnormalities observed in diabetes can be attributed in part to elevated triglycerides. The transfer of triglycerides from triglyceride-rich lipoproteins to HDL- and LDL-cholesterol is facilitated by cholesteryl ester transfer protein (CETP).111,112 Hypertriglyceridemia stimulates CETP activity, resulting in HDL- and LDL-cholesterol with high triglyceride content. Enrichment with triglycerides makes HDL particles subject to increased catabolism, lowering plasma HDL-cholesterol concentration, while triglyceride-enriched LDL particles undergo hydrolysis, decreasing particle size. Elevated free fatty acids impair insulin signaling and cause subclinical inflammation with subsequent pancreatic beta-cell dysfunction.107,115,116 Free fatty acid elevation may also be involved in terminal arrhythmias117 and induction of a prothrombotic state.118 The CETP inhibitor torcetrapib raises HDL-cholesterol concentration but also improves hyperglycemia.119 Furthermore, recombinant HDL-cholesterol infusions improve glucose dysregulation in patients with type 2 diabetes.120 These data suggest a role for HDL-cholesterol in glucose metabolism. Proposed mechanisms include anti-inflammatory properties of HDL-cholesterol and the central role of HDL-cholesterol in mediating reverse cholesterol transport, or cholesterol efflux, which may subsequently improve insulin sensitivity and/or secretion.107,121,122 Recent investigations suggest a role for impaired HDL function, with decreased cholesterol efflux capacity in diabetes.123 However, to date pharmacologic means to raise HDL cholesterol levels have not been associated with both glucose lowering and improved cardiovascular outcomes, as discussed below in the section on non-statin lipid lowering trials.
Role of inflammation
Parallel epidemics of obesity, diabetes and ASCVD suggest common molecular mechanisms for these diseases and novel therapeutic targets. Increased inflammatory markers and mediators are found in obesity,124 with increasing numbers of components of the metabolic syndrome,125 and predict incident hypertension,126 type 2 diabetes,127,128 and cardiovascular event rates.129,130 High-sensitivity C-reactive protein, a marker of inflammation, adds cardiovascular prognostic information beyond traditional risk factors in all major cohorts evaluated.131,132
Hyperlipidemia within the atherosclerotic plaque results in recruitment and migration of monocytes and other immune and inflammatory cells into the vascular subendothelial layer. Recruited monocytes differentiate into macrophages or dendritic cells. Activated macrophages express scavenger receptors to facilitate engulfment of both native and oxidized low density lipoprotein, forming foam cells which, along with other inflammatory cells increase production of chemokines and cytokines. These mechanisms operate in a feed forward cycle, promoting atherosclerotic lesion progression within the inflammatory milieu.22,23,58 Atherosclerotic lesions contain T-cells in addition to macrophages, but T-cells from individuals with diabetes have been found to have a predominance of the pro-inflammatory Th-1 phenotype.23 In addition to deleterious effects of low density lipoprotein on macrophage and foam cells, cholesterol crystals themselves within the atherosclerotic lesion can activate the NACHT, LRR and PYD domains-containing protein 3 inflammasome complex.133 This results in increased transcription of both NF-κB regulated gene products and interleukin-1β, provides an additional feed forward mechanism to amplify the deleterious effects of cholesterol particles that have accumulated in lipid rich plaque,133,134 which may participate in the accelerated atherosclerosis of diabetic dyslipidemia.
In addition to the interleukin-1β signaling pathway, diverse other cellular stress pathways (including tumor necrosis factor-α, oxidized LDL, the receptor for advanced glycation end-products (RAGE),56 reactive oxygen species (ROS), members of the protein kinase C enzyme family, and endoplasmic reticulum stress), many of which are increased in diabetes, can all activate the NF-κB transcription factor pathway.100 NF-κB in turn regulates expression of pro-atherogenic molecules including surface proteins, cytokines, and chemokines. Inhibiting this pathway attenuates the development of atherosclerosis in murine models.135,136 However, in vivo studies yield conflicting results with some pro-atherogenic and some anti-atherogenic effects,137,138 indicating the pathway has complex roles in atherosclerosis.
Whether targeting inflammation per se will reduce cardiovascular event rates is under investigation in multiple large scale clinical trials with diverse agents,139,140 including targeting of interleukin-1β with the monoclonal antibody canakinumab,141 tumor necrosis factor-α using etanercept, interleukin-6 using tocilizumab, interleukin-1 receptor with anakinra, and multiple inflammatory targets including the nucleotide-binding leucine-rich repeat-containing pyrin receptor 3 inflammasome with colchicine142 or multiple targets with low dose methotrexate.143,144 Low dose methotrexate has been reported to reduce cardiovascular event rates by 20% or more in patients with rheumatoid arthritis or psoriatic arthritis.145 Additional ongoing investigations target alternative inflammatory pathways including oxidized LDL-cholesterol, lipoprotein-associated phospholipase A2, secretory PLA2, P-selectin, and leukotrienes, among others.146 Given the complexity of the interactions between the inflammatory, metabolic and vascular pathways, future studies will need to address the clinical benefits of modulating these individual pathways as well as their inter-relationships.
Role of reactive oxygen species
ROS and reactive nitrogen species (RNS) are primarily produced through activity of the electron transport chain in mitochondria, and by other pathways including xanthine oxidase, lipoxygenase, myeloperoxidase and nitric oxide (NO) synthase. Alterations in electron transport chain activity result in increased electrochemical gradients and free radical leakage. Inactivation and degradation of ROS/RNS are regulated by complex networks of proteins and signaling pathways including superoxide dismutase, catalase, glutathione peroxidase, peroxiredoxins and thioredoxins. ROS/RNS participate in compartmentalized signaling pathways that are essential for normal cardiovascular physiology.147 However, excess ROS/RNS from mitochondrial injury, abnormal vascular hemodynamics, and/or hyperglycemia leads to oxidative stress, with increased cell proliferation, migration, endoplasmic reticulum stress, autophagy, senescence, and necrosis.148 This is manifest as hypertension from vascular endothelial dysfunction, reperfusion injury in patients with underlying occlusive atherosclerosis, and accelerated atherosclerosis. Hyperglycemia causes increased production of ROS via formation of Amadori products, which are oxidized to form AGEs that in turn activate RAGE to stimulate NADPH Oxidase-1 with intracellular ROS production.149
Role of endothelial dysfunction
Endothelial function is attenuated in both type 1 and type 2 diabetes.150,151 Even short exposure to high glucose concentrations is sufficient to reduce nitric oxide bioavailability and endothelial dependent vasodilation.42,51,152 Endothelial dysfunction may be an independent risk marker for cardiovascular events.75,76,153,154 Dysfunctional endothelium promotes leukocyte and platelet adhesion, thrombosis, and inflammation.76 Insulin stimulates endothelial nitric oxide synthase (eNOS)-induced production of nitric oxide by endothelial cells via the PI3-kinase/Akt pathway, and defects along the insulin signaling pathway seen in insulin resistance and diabetes result in decreased eNOS activity and decreased nitric oxide production, promoting endothelial dysfunction.74,155 Production of the vasoconstrictors endothelin-1 and angiotensin II are increased in the presence of compensatory hyperinsulinemia and contribute further to endothelial dysfunction and hypertension.75,156,157 Patients with type 2 diabetes also have abnormal VSMC function158 and ROS/RNS, which exacerbate diabetes-associated endothelial dysfunction.159
Role of hypercoagulability
Patients with diabetes are at increased risk for recurrent atherothrombosis.160 Experimentally-induced hyperinsulinemia and hyperglycemia results in elevated circulating tissue factor procoagulant activity and other prothrombotic proteins.161 Patients with diabetes are more thrombogenic162 and have elevated concentrations of plasminogen activator inhibitor-1 (PAI-1) antigen, von Willebrand factor-antigen, and fibrinogen, which are exacerbated by poor glycemic control.163 Higher concentrations of coagulation factors (II, V, VII, VIII, X) and lower anticoagulant (protein C) are also related to blood glucose concentration.164 These prothombotic processes may contribute to atherothrombosis in diabetes, and a recent trial using the thrombin receptor antagonist vorapaxar for secondary prevention of ASCVD in diabetes demonstrated lower major vascular events rates.165
Role of vascular calcification
Individuals with diabetes are more likely to have calcified atherosclerotic lesions166 which occur in more advanced, complex atherosclerotic lesions.167 Coronary calcium score as measured by electron-beam computed tomography is an independent risk factor for cardiovascular events and all-cause mortality in persons with or without diabetes.166,168,169 Individuals with diabetes have higher coronary artery calcification scores than those without diabetes170 and calcified plaque burden similar to that of older individuals without diabetes.170 In addition, persons with diabetes are at particular risk of developing peripheral artery disease with a predilection for the distal tibial artery circulation. Tibial artery calcification is associated with increased risk of limb amputation and all-cause mortality.171 Underlying mechanisms for this may be related to the role of hyperglycemia with development of AGEs which accelerate vascular calcification.172 Hyperglycemia also leads to increased post-translational protein modification, including modification by O-linked N-acetylglucosamine (O-GlcNAc). O-Glc-N-acylation starts a cascade of pro-atherogenic pathways which potentiates vascular calcification.173 In addition, altered regulation of osteoprotegerin and osteocalcin may promote arterial calcification in diabetes.174 Lastly, as noted above, diabetes is associated with arterial inflammation with increased levels of tumor necrosis factor-alpha, which is a mediator of arterial calcification.175
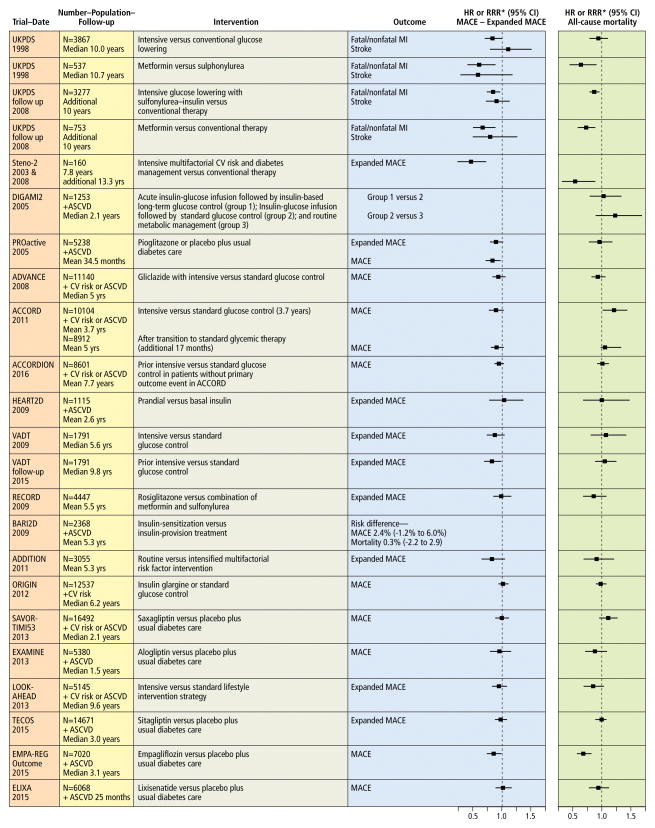
Atherosclerotic Cardiovascular Risk Reduction in Diabetes
Targeting individual cardiovascular risk factors reduces ASCVD risk in diabetes, but addressing multiple risk factors simultaneously may synergistically reduce cardiovascular event risk even further. This hypothesis is supported by the Steno-2 study, in which 160 participant with type 2 diabetes and albuminuria were randomized to intensive versus conventional control of glycemia, blood pressure, and lipids, and followed for a mean of 7.8 years.176 The trial showed a statistically and clinically significant 53% reduction [hazard ratio (HR) 0.47; 95% confidence interval (CI), 0.24 to 0.73] for the primary composite cardiovascular event endpoint. Cardiovascular risk differences between intensive and conventional therapy separated after 1 year. The number needed-to-treat was 5 over 7.8 years to achieve this magnitude of ASCVD risk reduction. The Steno-2 trial was not designed to identify which interventions were most effective, but use of statin and antihypertensive drugs may have accounted for much of the cardiovascular benefit. A secondary analysis of the BARI-2D trial evaluating multiple interventions also supports concurrent risk factor control lowers total mortality and the composite of death, myocardial infarction, and stroke in patients with type 2 diabetes and established coronary heart disease.177 These findings must be interpreted with caution, as the trial was not conducted with randomization to multifactorial control, yet BARI-2D demonstrates not only improved cardiovascular outcomes among those who achieved control of multiple risk factors using a comprehensive approach, but also the feasibility of protocol-guided multi-risk factor targeted intensive medical therapy. While achievement of multiple treatment goals in diabetes care has improved over time, currently only 14.3% of U.S. adults with type 2 diabetes are at recommended goals for HbA1c, blood pressure, and LDL-cholesterol.178
Evidence for ASCVD risk reduction in diabetes by addressing risk factors individually from clinical trials of lipid lowering, blood pressure-lowering, aspirin therapy, lifestyle, and glucose-lowering therapies is presented below, beginning with risk factors having the strongest evidence to date for ASCVD risk reduction in diabetes.
Lipid-lowering therapy
There is strong high level evidence from randomized clinical trials that lipid-lowering therapy with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-Co-A) reductase inhibitors (statins) reduces ASCVD event rates in diabetes, with some benefits potentially attributable to non-lipid-lowering, anti-inflammatory effects of statins.134,179–182 The most recent American College of Cardiology/American Heart Association guidelines recognize patients with diabetes between the ages of 40 and 75 years as one of the four principal groups to benefit from statins and recommend treatment with a moderate intensity statin or a high intensity statin for individuals with a ≥7.5% 10-year risk of cardiovascular disease.132 In those less than 40 or above 75 years of age, guidelines recommend individualizing statin therapy based on the benefits of ASCVD risk reduction versus the potential for adverse effects, interactions with other drugs, and patient preference.132
These recommendations are based on multiple lines of evidence. Statin lowering of LDL-cholesterol levels by 39 mg/dL (1 mmol/L) in high risk individuals reduces coronary mortality risk 19%, as demonstrated in a meta-analysis by the Cholesterol Treatment Trialists’ (CTT) Collaboration. Magnitude of mortality benefits were similar for those with or without diabetes as seen in subgroup analyses.183 A 21% reduction in major vascular events occurred per 1 mmol/L reduction in LDL-cholesterol, irrespective of prior history of vascular disease, gender, age, body mass index (BMI), or baseline systolic or diastolic blood pressure, smoking status, estimated glomerular filtration rate, cholesterol, or predicted annual risk of major vascular events; this finding was confirmed in a separate meta-analysis of 14 randomized trials from the same group.184 One study, the Collaborative Atorvastatin Diabetes Study (CARDS) trial, specifically assessed patients with type 2 diabetes, including 2838 patients in the United Kingdom and Ireland with a mean baseline LDL-cholesterol of 117 mg/dL (3.0 mmol/L) randomized to atorvastatin 10 mg daily or placebo. A 37% reduction in the primary cardiovascular composite outcome (time to first occurrence of acute coronary heart disease event, coronary revascularization, or stroke) was observed for atorvastatin compared to placebo assigned groups. The Treating to New Targets (TNT) study examined whether lowering LDL-cholesterol below the threshold recommended at the time (100 mg/dL, 2.59 mmol/L) would result in greater cardiovascular risk reduction.185 The study included 1501 patients with diabetes and coronary heart disease who were randomized to atorvastatin 10 mg versus 80 mg daily, lowering LDL-cholesterol levels to a mean of 98.6 mg/dL (2.55 mmol/L) versus 77 mg/dL (1.99 mmol/L), respectively. There was a 25% reduction in major cardiovascular events (composite of coronary heart disease death, nonfatal non-procedure-related myocardial infarction, resuscitated cardiac arrest and fatal or nonfatal stroke) after a median of 4.9 years of treatment. This study provides further evidence for more aggressive LDL-lowering to reduce ASCVD in diabetes.
Statins and Diabetes
While there are clear benefits of statins to reduce cardiovascular events and mortality in patients with or at risk for ASCVD, 183,184 statins also modestly accelerate the development of diabetes in individuals with pre-existing risk factors.186–189 In the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), which involved participants without diabetes but with LDL cholesterol levels below 3.4 mmol/L (130 mg/dl) and high-sensitivity C-reactive protein concentrations of 2.0 mg/L or higher, the hazard ratio for newly diagnosed diabetes was increased 25% in the rosuvastatin group compared to the placebo group.186 Despite the increase in the risk of new-onset diabetes, the participants previously considered to be at low cardiovascular risk had clinically important cardiovascular event reductions over a median follow-up period of only 1.9 years, with a hazard rate 44% lower with rosuvastatin compared to placebo for the combined primary end point of myocardial infarction, stroke, arterial revascularization, hospitalization for unstable angina, or death from cardiovascular causes. Almost two-thirds of participants had at least 1 major risk factor for developing diabetes: metabolic syndrome as defined by the 2005 American Heart Association/National Heart, Lung, and Blood Institute consensus criteria, impaired fasting glucose (as defined by fasting serum glucose of equal to or greater than 100 and less than 126 mg/dL [5.55 to 7.00 mmol/L]), BMI 30 kg/m2 or higher, or HbA1c greater than 6%.190 As expected, incident diabetes was 28% higher those with than without any major diabetes risk factor, and statins accelerated the average time to diagnosis of diabetes by 5·4 weeks. However, statin allocation reduced the risk for the primary endpoint both in participants with and without a major risk factor for diabetes, such that in patients with one or more risk factor for diabetes, in total 54 new cases of diabetes were diagnosed, while 93 first major cardiovascular events or deaths, or 134 total cardiovascular events or deaths were avoided.
Several meta-analyses have now been performed examining the risk of developing diabetes in statin-treated individuals, and relative risks are somewhat lower overall than that found in the index JUPITER trial.187–189 Diabetes relative risk was 13% higher with no heterogeneity across 5 trials [including HPS (Heart Protection Study),191 LIPID (Long-Term Intervention with Pravastatin in Ischaemic Disease),192 ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial),193 JUPITER,186 and CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure)194] with a total of 57,593 patients, mean follow-up of 3.9 years, and 2,082 incident cases of diabetes.187 Addition of WOSCOPS (West of Scotland Coronary Prevention Study)195 to the analysis introduced significant heterogeneity and attenuated risk, which no longer retained statistical significance as WOSCOPS reported a protective effect of pravastatin versus placebo on the incidence of diabetes. In a second meta-analysis, a 9% increase in risk for incident diabetes was found (odds ratio (OR) 1.09, 95% CI 1.02–1.17), including 13 trials with 91,140 participants and development of diabetes in 4,278 patients over a mean of 4 years.188 Investigators were contacted to obtain unpublished data regarding incident diabetes resulting in 7 additional trials (and both JUPITER and WOSCOPS) included as compared with the other meta-analysis. In this analysis little heterogeneity was found across trials despite the inclusion of WOSCOPS, potentially attributable to different criteria used to diagnose diabetes. Statin associated diabetes risk may be slightly higher in women.196 A meta-regression analysis showed the highest diabetes risk was associated with older age, but not baseline BMI or LDL-cholesterol level.188 One extra case of diabetes resulted from treating 225 (95% CI 150–852) patients with statins for 4 years, while 5.4 vascular events were prevented. In the larger context, given that statins are used by approximately 24 million Americans, the population-attributable risk of statin-associated diabetes is not small. However, considering the many treatments for diabetes and the importance of cardiovascular event reduction, providers should not avoid using statins when indicated solely due to concern for risk of diabetes.
Potential effects, if any, of statin-induced diabetes on the development of long-term microvascular complications remain unknown, and current epidemiologic data are reassuring. With recent lower lipid target goals and increasing use of statins, as well as improved screening, early detection, and multifactorial interventions, the age-adjusted percentage of adults with diabetes reporting visual impairment197 and the incidence of end-stage renal disease in adults with diabetes198 have decreased over the past few decades. Furthermore, the 10-year risk of myocardial infarction or stroke (approximately 25%) is markedly higher than that of blindness or renal failure (approximately 1 to 2%) for patients with recent-onset diabetes impacting risk-to-benefit considerations.199
The risk of developing diabetes appears to be related to statin potency200 and dose.189 Cellular mechanisms underlying the increased incidence of diabetes remain incompletely understood. Genome-wide studies do not reveal associations between genes that regulate HMG-CoA reductase or LDL-cholesterol metabolism and type 2 diabetes. Statins may interfere with beta-cell insulin secretion either by decreasing Ca2+-dependent insulin secretion or by interfering with isoprenylation of guanosine triphosphate –binding proteins.201 Statin inhibition of isoprenoid biosynthesis may lead to lower expression of insulin signaling proteins in adipocytes and to reduced glucose transporter expression or translocation.202 Fasting insulin levels may increase modestly, suggesting that insulin resistance may be increased, but euglycemic hyperinsulinemic clamp studies do not show consistent changes in insulin sensitivity.203 Other off-target effects may also be involved.
Overall, the risk of incident diabetes with statin therapy is present but vastly outweighed by the actual cardiovascular benefits.204 Patients should be educated regarding the risk of incident diabetes with statins as with other risk-benefit of all therapies.132 Lifestyle modification should be encouraged to lower cardiovascular risk and that for developing diabetes.205 Patients on statins at higher risk for but without pre-existing type 2 diabetes should undergo periodic screening for diabetes with fasting glucose and HbA1c, and if type 2 diabetes develops, standard of care and national guidelines should be used to manage diabetes.206,207
Non-statin lipid lowering
Although statins are effective in reducing ASCVD risk in diabetes, residual cardiovascular risk remains,185,191,208 and further lowering of lipids may be of value. While most studies do not demonstrate other pharmacologic class agents provide additional benefit, the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) supports use of a non-statin LDL-lowering strategy to further lower cardiovascular risk.209 Vytorin is a combination of simvastatin and ezetimbe, which reduces intestinal cholesterol absorption. The primary composite cardiovascular endpoint of cardiovascular death, nonfatal myocardial infarction, unstable angina requiring rehospitalization, coronary revascularization, or nonfatal stroke was 6% lower with ezetimibe compared to placebo administered with simvastatin. Ezetimibe lowered LDL-cholesterol 24% despite the relatively low baseline concentrations, which was an unexpectedly potent effect on LDL-cholesterol lowering. Twenty-seven percent of the study population had diabetes, and there was heterogeneity in response with a greater 14% cardiovascular benefit among those with diabetes. This trial supports the hypothesis that lower LDL-cholesterol targets may be important to reduce residual ASCVD risk in patients with diabetes.
The question remains whether targeting the diabetic lipid abnormalities of high triglycerides, low HDL-cholesterol, and small LDL-cholesterol particle size will result in further benefit. Most trials examining effects of fibrates on cardiovascular risk were completed before statin therapy became widely instituted and included individuals with diabetes only as a subgroup. More recent trials include the lipid arms of Action to Control Cardiovascular Risk in Diabetes (ACCORD)210 which examined fenofibrate versus placebo on a background of simvastatin therapy, and the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial192 involving fenofibrate monotherapy versus placebo. In ACCORD, all patients were randomized to intensive glycemic control (targeting an HbA1c of 6.0%) or standard therapy (targeting HbA1c of 7–7.9%);211 a subset of patients were enrolled in the ACCORD Lipid trial and were randomized in a 2 × 2 factorial design to receive simvastatin plus fenofibrate or placebo. Inclusion in the ACCORD-Lipid substudy did not require high triglycerides and low HDL-cholesterol levels, a group which might benefit most from fibrate therapy.210 Although there was no difference in the annual rate of the primary composite outcome of major adverse cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) for the fenofibrate compared to placebo group, pre-specified subgroup analysis revealed 29% fewer events in those with baseline triglyceride at or above 204 mg/dL (2.31 mmol/L) and HDL-cholesterol at or below 34 mg/dL (0.88 mmol/L). These results are consistent with the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study of 9,975 individuals with type 2 diabetes not on statin therapy, randomized to micronized fenofibrate versus placebo for 5 years.192 No effect of fenofibrate was seen on the primary outcome of coronary events (coronary heart disease death or non-fatal myocardial infarction) in the entire cohort, although a 14% cardiovascular event reduction was observed in the subgroup with baseline low HDL-cholesterol (p=0.02), and a similar trend was observed in those with baseline high triglyceride (p=0.07). Interpretation of the FIELD study is complicated due to higher rates of add-on statin use in the placebo-assigned treatment group, which might be expected to attenuate differences between groups. In total, four studies consistently demonstrate favorable effects of fibrates in the subgroup of patients with the specific lipid phenotype of high triglyceride and low HDL-cholesterol,191,192,210,212 but one must be cautious in interpretation of subgroup analysis, especially when the primary outcome of the trial was not positive, and benefit of adding a fibrate to statin therapy for reducing risk of cardiovascular events in patients with type 2 diabetes remains unproven.213 Further studies are needed to determine if persons with diabetes who have elevated triglyceride and low HDL cholesterol concentrations may realize a cardiovascular benefit with the addition of a fibrate.
In Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH),214 a study that evaluated addition of niacin to intensive statin therapy [with simvastatin plus ezetimibe if needed to maintain LDL of 40 to 80 mg/dL (1.04 to 2.07 mmol/L)] in patients with established cardiovascular disease and low HDL-cholesterol [median baseline HDL-cholesterol of 35 mg/dL (0.91 mmol/L), IQR 31–39 mg/dl], approximately one-third of participants had diabetes. No difference in the primary composite endpoint was observed despite increased mean HDL-cholesterol from 35 to 42 mg/dL (0.91 to 1.09 mmol/L), lowering triglycerides from 164 to 122 mg/dL (1.85 to 1.38 mmol/L), and lowering LDL-cholesterol from 74 to 62 mg/dL (1.92 to 1.61 mmol/L). The trial was stopped 18 months early, after a mean follow-up period of 3 years, for lack of efficacy and an unexpected higher rate of ischemic stroke in the niacin group, although the overall rate was low, so it remains uncertain whether this was a true effect versus a chance occurrence. The trial must be interpreted with caution as the rate of the primary composite cardiovascular endpoint was lower than projected, consistent with recent trends, so the protocol was amended to change the primary endpoint of high-risk acute coronary syndrome to include hospitalization for acute coronary syndrome and symptom-driven coronary or cerebral revascularization. Furthermore, there have been no other studies prior to or since showing a causal link between niacin and stroke. Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) included more than 8,000 patients with diabetes (32.3% of the study population) and compared extended-release niacin versus placebo on a background of statin therapy in high risk patients with prior vascular disease, but was likewise stopped early because of futility.215
Raising HDL cholesterol levels with the use of CETP inhibitors216,217 has not been demonstrated to reduce ASCVD risk, and torcetrapib unexpectedly increased ASCVD events, cardiovascular and non-cardiovascular death.217 Although pharmacologically increasing HDL-cholesterol concentrations has not been demonstrated to reduce ASCVD, it is possible that HDL-cholesterol function, or cholesterol efflux capacity, is a more important determinant of cardiovascular risk.218
Given the data presented above, the opinions of the authors are that ezetimibe may represent a reasonable choice for additional cardiovascular risk reduction, especially in those with diabetes and acute coronary syndrome. Consideration can also be given to adding fibrate therapy for an individual with diabetes and residual hypertriglyceridemia with low HDL-cholesterol levels once the patient is on goal statin therapy. The data do not support the specific use of niacin in diabetes, although it may be an alternative for those with true intolerance to statin therapy. These opinions are consistent with the most recent Standards of Medical Care for Diabetes by the American Diabetes Association (ADA),219 which cite the Level A evidence above to state that the addition of ezetimibe to moderate-intensity statin therapy may be considered for patients with a recent acute coronary syndrome with LDL cholesterol ≥50 mg/dL (1.3 mmol/L) or for those patients who cannot tolerate high intensity statin therapy. It was noted that combination therapy with a statin and fibrate has not been shown to improve ASCVD outcomes in the broad diabetes population and is generally not recommended, but therapy with statin and fenofibrate may be considered for men with both triglyceride level ≥204 mg/dL (2.3 mmol/L) and HDL cholesterol level ≤34 mg/dL (0.9 mmol/L). Lastly, combination therapy with statin and niacin was not generally recommended. Of note, the Scientific Statement on Prevention of Cardiovascular Disease in type 2 diabetes by the American Heart Association and American Diabetes Association (AHA/ADA) does not recommend addition of a fibrate to statin therapy.220
Non-statin LDL-lowering with PCSK9 inhibition
The newest class of LDL-lowering medications consists of monoclonal antibodies to proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is present in hepatocytes, and binds to and targets the LDL receptor for degradation.221,222 PCSK9 inhibitors prevent the degradation of LDL receptors, allowing for increased removal of LDL-cholesterol from the circulation. Individuals with loss-of-function PCSK9 mutations have lower LDL-cholesterol levels and lower coronary heart disease incidence,223,224 while most cases of familial hypercholesterolemia result from gain-of-function mutations, elevated LDL-cholesterol concentrations and resulting early ASCVD.225,226 Statins upregulate PCSK9, which may be the reason that LDL-cholesterol lowering with statins reaches a plateau.227 Alirocumab and evolucamab are two PCSK9 inhibitors recently approved by the Food and Drug Administration (FDA) based on their efficacy at lowering LDL-cholesterol concentrations and initial safety based on relatively small trials, with additional clinical trials ongoing.
Alirocumab was approved by the FDA in July 2015 for use in addition to diet and maximally tolerated statin therapy in adult patients with heterozygous familial hypercholesterolemia or patients with clinical ASCVD who require additional lowering of LDL-cholesterol. Doses of 75–150 mg subcutaneous administered every two weeks lowered LDL-cholesterol concentrations by 36–59% compared with placebo in patients with hypercholesterolemia and/or at high cardiovascular risk as add-on therapy to background statins, on maximally-tolerated statins, or as monotherapy.228–234 Three longer-term studies ranged only from 24–78 weeks229–231 but showed effects to lower LDL-cholesterol are durable over this timespan. A post-hoc analysis of the effect of alirocumab on cardiovascular outcomes was performed in the Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM) trial231 and showed a 48% reduction in major adverse cardiovascular events (MACE HR 0.52, 95% CI 0.31–0.90, nominal P=0.02), but this was based on adverse event reporting and these early trials were not designed as formal cardiovascular outcome trials (CVOT). Thus definitive evidence of cardiovascular benefit awaits completion of a large ongoing trial in persons with a recent acute coronary syndrome event (NCT01663402), with planned enrollment of an estimated 18,600 patients and expected completion in late 2017.235
The initial studies of alirocumab did not show an increased risk of diabetes but did suggest a smaller LDL-cholesterol lowering effect in diabetes.230 Larger populations will need to be studied to substantiate these effects. The most common side effects include itching, swelling, pain, or bruising at the injection site, nasopharyngitis, and flu. Allergic reactions such as hypersensitivity vasculitis have also been reported. A number of additional clinical trials are underway examining the safety and efficacy of alirocumab in statin-intolerant patients,236 patients at high cardiovascular risk on maximally-tolerated statins,237 as an add-on to statin therapy,238 and in patients with familial hypercholesterolemia (FH).239
Evolocumab is the second PCSK9 drug currently approved by the FDA to lower LDL-cholesterol levels. Evolocumab was approved by the FDA in August 2015 for use in patients with heterozygous or homozygous familial hypercholesterolemia, or clinical ASCVD (e.g. history of myocardial infarction or stroke), on diet therapy and maximally-tolerated statin therapy who require additional LDL-cholesterol lowering. Evolocumab appears to have similar magnitude LDL-cholesterol lowering effects as alirocumab, but these agents have not been compared in head-to-head investigations. In the evolocumab development program, cardiovascular outcomes were also examined as an exploratory endpoint in trials primarily designed to evaluate the LDL-cholesterol lowering effect of the drug. In a 48-week open-label study of evolocumab, patients were enrolled from either the phase-2 Open-Label Study of Long-Term Evaluation against LDL Cholesterol -1 (OSLER-1) or phase-3 (OSLER-2) trials.240 A dose of evolocumab 420 mg subcutaneous given monthly (in OSLER-1 and OSLER-2) or 140 mg every 2 weeks (OSLER-2) lowered LDL-cholesterol by 61% at 12 weeks, an effect that was sustained at 58% at 48 weeks, with an absolute LDL-cholesterol reduction of 70.5 mg/dL (1.83 mmol/L) to a mean LDL-cholesterol level of 48 mg/dL (1.24 mmol/L). Likewise, durability of evolocumab lowering of LDL-cholesterol was sustained for 52 weeks in the study of patients with familial hypercholesterolemia or mixed hyperlipidemia either alone or added on to low versus high intensity statin therapy.241 Additional beneficial effects on serum lipoproteins included decreased apolipoprotein B, lipoprotein (a), triglycerides and non-HDL-cholesterol. Evolocumab has been shown to lower LDL-cholesterol levels in patients with statin intolerance for 12 weeks as compared with statin plus ezetimibe,242 as an add-on to moderate or high intensity statin therapy for 12 weeks,243 and as monotherapy for 12 weeks.244 The pre-specified exploratory outcome of adjudicated cardiovascular events (including death, coronary events of myocardial infarction, unstable angina requiring hospitalization, or coronary revascularization, and cerebrovascular events of stroke or transient ischemic attack, and heart failure requiring hospitalization) was reduced by 53% at 1 year (hazard ratio 0.47, 95% CI 0.28–0.78, P=0.003). The safety profile appeared to be acceptable at this early stage,240 although the duration of follow-up is short for chronic disease, and the sample size is small to detect less common potential safety signals. Briefly, injection-site reactions were reported in 4.3% of patients on evolocumab and led to discontinuation of the drug in 0.2%. New evolocumab-binding (neutralizing) antibodies were detected in 0.3% in the evolocumab group but in a surprising number of patients (also 0.3%) in the standard-therapy group. Antibody titers were transient in patients who underwent repeat testing, and no neutralizing antibodies against evolocumab were detected. The Further Cardiovascular Outcomes Research with PCSK9 inhibition in Subjects with Elevated Risk (FOURIER) (NCT01764633) plans enrollment of 27,500 high-risk patients with cardiovascular disease on background statin therapy, with a primary MACE endpoint and is also expected to complete in late 2017. Additionally, two CVOT are underway with bococizumab, a PCSK9 inhibitor that has not yet been approved by the FDA: SPIRE-1 (NCT01975376) and SPIRE-2 (NCT01975389).
Although PCSK9 inhibition has a potent and apparent durable effect to lower LDL-cholesterol, the available clinical trial evidence regarding cardiovascular benefit is currently exploratory and preliminary, and definitive evidence is needed for reduction of cardiovascular outcomes. Furthermore, clinical trial data examining the effect of PCSK9 inhibitors on LDL-lowering and cardiovascular endpoints in patients with diabetes are needed, as the effects in this population remain uncertain.
Blood pressure control and angiotensin converting enzyme inhibitor (ACE-I)/angiotensin receptor blocker (ARB) therapy
The age-adjusted prevalence of hypertension is 57.3% in U.S. adults with diabetes as compared with 28.6% in those without diabetes, with higher rates seen in older persons.245 Elevated blood pressure has unequivocally been shown to increase the risk of both micro- and macrovascular disease in diabetes,246,247 and blood pressure control reduces the risk of death and both micro- and macrovascular complications in type 2 diabetes.248,249 Initial trials investigated intensive versus moderate blood pressure control with secondary aims to also test particular drug classes. The Appropriate Blood Pressure Control in Diabetes (ABCD) trial was a prospective, randomized, controlled trial of intensive (diastolic blood pressure below 75 mmHg) versus moderate (diastolic blood pressure between 80 to 89 mmHg) antihypertensive therapy in 950 individuals with type 2 diabetes followed for 5 years.250 There was a further randomization to nisoldipine or enalapril. The trial was halted early due to marked lowering of cardiovascular complications (nonfatal myocardial infarction, all myocardial infarction, and myocardial infarction plus cardiovascular death) in patients randomized to enalapril. However at the point of early termination of the randomization between the two drugs, the study had insufficient number of events to test any benefit of intensive blood pressure lowering on cardiovascular outcomes.
The benefits of angiotensin converting enzyme (ACE) inhibition observed in the ABCD trial were extended in the Heart Outcomes Prevention Evaluation (HOPE) study, which randomized 3,577 subjects with diabetes and a prior cardiovascular event (coronary artery disease, stroke, or peripheral artery disease) or at least one other cardiovascular risk factor (elevated total cholesterol, low HDL-cholesterol, hypertension, known microalbuminuria, or current smoking) to either ramipril 10 mg daily or placebo, as well as vitamin E 400 IU versus placebo in a 2 × 2 factorial design.251 The trial was stopped 6 months early because of a 25% relative risk reduction in the combined primary outcome of myocardial infarction, stroke, or cardiovascular death in the group randomized to ramipril (95% CI 12–36, P=0.0004), and similar reductions in separate components of the primary outcome. The absolute risk reduction by ramipril was 4.5%, and this benefit remained significant even after adjustment for changes in systolic and diastolic blood pressure, suggesting that renin-angiotensin-aldosterone system (RAAS) inhibition may have greater benefits over and above blood pressure control in diabetes.
The impact of the RAAS inhibition approach was studied in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE), which involved 1,195 individuals with diabetes, hypertension, and signs of left ventricular hypertrophy to receive the angiotensin receptor blocker (ARB) losartan- or atenolol-based treatment.252 Both drug groups achieved similar blood pressure control, but the losartan-treated group had a 24% reduction in relative risk of the cardiovascular events (fatal and non-fatal myocardial infarction or stroke), after a mean follow-up of 4.7 years.
In contrast, in the Irbesartan Diabetic Nephropathy Trial (IDNT), a global multicenter trial of 1,715 adults with type 2 diabetes, diabetic nephropathy and hypertension, the use of irbesartan versus amlodipine or placebo in addition to conventional antihypertensive therapy did not confer a reduction in the composite cardiovascular endpoint.253 Other trials examining the use of ACE inhibition concomitant with angiotensin receptor blockers (ARB) have instead found increased risk for adverse events.254 Thus, there is not a uniform finding of unique cardiovascular benefit over and above blood pressure control of various strategies for RAAS inhibition in diabetes. However, consistent with the current AHA/ADA guidelines,220 RAAS blockade with an ACE inhibitor or ARB should be used first- in the treatment of hypertension in diabetes. Because there is evidence that this combination can lead to more adverse events, the opinion of the authors agrees with current guidelines by the ADA that recommend avoiding combined therapy with ACE inhibition and ARBs simultaneously.219
The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial was a global, multicenter study conducted in 11,140 patients with type 2 diabetes randomized to fixed combination of the ACE inhibitor, perindopril, and the indoline diuretic, indapamide, or matching placebo in addition to current therapy.255 Patients were also randomized in a factorial design to standard versus intensive glucose-lowering therapy with a goal HbA1c of less than 6.5%. Approximately one-third of subjects had macrovascular disease at baseline. The initial trial had a mean follow-up of 4.3 years, and the mean achieved blood pressure was 139.3 mmHg systolic and 78.7 mmHg diastolic. The relative risk for the primary endpoint (a composite of major macrovascular and microvascular events), was reduced by 9% (HR 0.91, 95% CI 0.83–1.00, P=0.04), which reflected a 1.3% absolute risk reduction. The nearly 6 year long-term follow up of ADVANCE confirmed a sustained but attenuated benefit in reduction of all-cause and cardiovascular mortality.256
Blood Pressure Goals
The intensity of systolic blood pressure lowering was examined in the ACCORD-BP study, which included 4,733 participants with type 2 diabetes at high risk for cardiovascular events randomized to intensive blood pressure lowering therapy targeting a systolic blood pressure below 120 mmHg, or standard therapy targeting a systolic blood pressure below 140 mmHg.257 The mean achieved systolic blood pressure was 119.3 mmHg in the intensive group and 133.5 mmHg in the standard group. There was no statistical difference between groups in the primary composite outcome of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes but there was a trend towards benefit after a mean follow-up of 4.7 years. However, there were more serious adverse events in the intensive compared with the standard therapy group (3.3% versus 1.3% of events attributed to blood pressure medications, P<0.001) including hypotension, bradycardia or arrhythmia, and hyperkalemia. Importantly, the mean estimated glomerular filtration rate was lower, and the serum creatinine was higher in the intensive group despite a lower prevalence of macroalbuminuria in the intensive group.
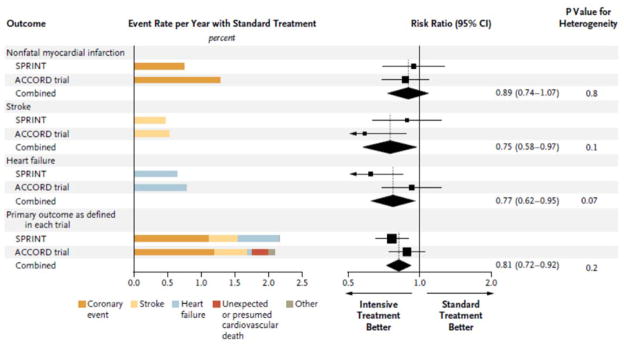
In contrast, results of the Systolic Blood Pressure Intervention Trial (SPRINT) support the benefits of intensive blood pressure control in the broad hypertensive population. Unfortunately this trial excluded patients with diabetes and thus the findings cannot be directly extrapolated to persons with diabetes.258 The trial does provide evidence supporting a much lower blood pressure goal than is represented in current guidelines and highlights an area of potential controversy. The study involved 9,361 participants with systolic blood pressure between 130–180 mmHg and an increased risk of cardiovascular events. Patients were randomized (but not fully blinded) to a systolic blood pressure target below 140 mmHg (standard treatment) or below 120 mmHg (intensive treatment). The antihypertensive regimen titration algorithm was similar to that used in the ACCORD-BP trial. The primary composite cardiovascular outcome (myocardial infarction, other acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes) was significantly reduced with a hazard ratio of 0.75 with intensive treatment (95% CI 0.64–0.89, P<0.001) with participants followed over a mean of 3.26 years. In general, the SPRINT cohort was older than ACCORD (28% of subjects were age 75 or older, mean age 68 years compared with 62 years in ACCORD-BP), and included individuals with chronic kidney disease. There was more syncope and hypotension but not more falls in the intensive treatment group, and there was a higher rate of acute kidney injury and acute renal failure in the intensive treatment group. At this time, it is unclear why there is greater benefit in the primary outcome in SPRINT versus only a trend for benefit in the ACCORD-BP but in fact the point estimates for the primary outcome and components of the primary showed similar trends in both trials. Outcomes data for SPRINT versus ACCORD are compared in Figure 3.259
Figure 3. Cardiovascular outcomes in two recent blood pressure lowering trials in patients with and without baseline diabetes.
Outcomes data for blood pressure-lowering trials in a high-risk population without diabetes: SPRINT (Systolic Blood Pressure Intervention Trial, n=9,361) and in a high risk population with diabetes: ACCORD (Action to Control Cardiovascular Risk in Diabetes, n=4,733). SPRINT was conducted in patients without Diabetes and ACCORD in patients with diabetes. Although reduction in individual outcomes did not reach statistical significance in ACCORD except for stroke, tendencies for benefits are similar and combining ACCORD with SPRINT demonstrated reduction in primary outcome and individual components with intensive treatment. Reprinted with permission from Perkovic V, Rodgers A. N Engl J Med. 2015;373(22):2175–8.
Several meta-analyses provide insight into the collective clinical experience of lowering blood pressure in diabetes. One such meta-analysis identified 40 trials with 100,354 participants performed either exclusively in type 2 diabetes or included trial population subgroups with diabetes.260 In diabetes every 10 mmHg of systolic blood pressure lowering has an associated 13% reduction in all-cause mortality, 11% reduction in cardiovascular events and 27% reduction in stroke events. This meta-analysis also reveals substantial benefit in lowering blood pressure on reducing microvascular events. Importantly, antihypertensive drug class does not affect the overall results; rather it is the blood pressure lowering per se that confers clinical benefit. However, there are differences between particular drugs and individual cardiovascular endpoints. For example, diuretics are associated with a 17% lower relative risk for heart failure (mainly driven by ALLHAT), and ARBs are associated with a lower relative risk of heart failure and mortality (mainly driven by LIFE in which ARBs were compared to beta-blockers). In contrast, calcium channel blockers are associated with a higher relative risk of heart failure but a lower risk of stroke, while beta-blockers appeared to be associated with a higher relative risk of stroke. What is important in putting individual antihypertensive drugs in perspective is that blood pressure lowering by any drug class is associated with cardiovascular benefit in diabetes, but in individual patients, tailoring drug selection to address a particular cardiovascular concern (such as risk of heart failure versus risk of stroke) may be necessary.
In all, only three randomized controlled trials have examined the effect of standard versus more intensive blood pressure lowering in individuals with diabetes.249,257,261–263 While the ACCORD-BP trial257 focused on systolic blood pressure, the others targeted control of diastolic blood pressure. A recent meta-analysis including these three trials of a total of 7312 adult participants with type 2 diabetes and hypertension followed for 2–5 years.264 Intensive reduction of systolic blood pressure to 130 mmHg or lower or diastolic blood pressure to 80 mmHg or lower was associated with a 35% decrease in risk for stroke (RR 0.65, 95% CI 0.48–0.86) and a trend for reduced mortality (RR 0.76, 95% CI 0.55–1.05) as compared with a standard systolic blood pressure target of 140–160 mmHg and diastolic blood pressure target of 85–100 mmHg. However, these results are somewhat inconclusive due to the very different trial designs and blood pressure targets. The total sample size and number of events may not have been sufficient to detect significant benefit for all cardiovascular endpoints. On a cautionary note, ACCORD-BP demonstrated a significant increase in serious adverse events with intensive blood pressure lowering, as described above.
The 2016 Standards of Medical Care by the ADA,219 the 2015 AHA/ADA Scientific Statement Update,220 and the most recent American Heart Association guidelines for cardiovascular risk reduction132 recommend a blood pressure goal of below 140 mmHg for systolic blood pressure and below 90 mmHg for diastolic blood pressure. Initial antihypertensive therapy should include an angiotensin-converting enzyme inhibitor (ACEI), or angiotensin receptor blocker (ARB) if the ACEI is not tolerated, for renal protection. If the blood pressure is not at target, the next choice could include a thiazide diuretic or calcium channel blocker. In the context of the recently-published SPRINT results in patients without diabetes, it is the opinion of the authors that it would be reasonable to also consider targeting blood pressure below 120/80 in individuals with diabetes, especially in the presence of renal disease (elevated urine albumin excretion or chronic kidney disease) or increased risk for stroke, while exercising caution in patients with symptoms of hypotension or requiring multiple agents to achieve this target (Table 1).
Table 1. Suggested treatment prioritization to reduce ASCVD risk in type 2 diabetes.
Author-recommended approach to cardiovascular risk reduction in type 2 diabetes. Recommendations for aggressive blood pressure control and limitation of aspirin to patients with established coronary artery disease do not necessarily agree with guideline recommendations but do reflect the authors’ assessment of the current literature.
| Primary prevention | Secondary prevention (Coronary/carotid diseaseb) | ||
|---|---|---|---|
| Moderate ASCVD riska | High ASCVD risk | ||
| Provide or reinforce lifestyle interventions:c diet, exercise, weight loss, and smoking cessation | Continue emphasis on lifestyle interventionsc | ||
| Vascular territory | |||
| Macrovascular and microvascular |
|
||
| Microvascular |
|
|
|
Diabetes per se confers an increased risk of ASCVD, so the vast majority of diabetes patients without ASCVD fall into the moderate or high ASCVD risk category; treatment should be individualized and a few patients may fall into a lower risk category, but all patients should undergo lifestyle intervention. Special caution should be used in the elderly.
Coronary or carotid disease refers to a clinical history of an acute ischemic event (acute coronary syndrome or ischemic stroke) or coronary revascularization.
Randomized trials of lifestyle interventions of diet, exercise and weight loss in diabetes have not shown a reduction in CV events.265 However lifestyle modification and weight loss help improve CV risk factors and result in other positive health outcomes.
Additional lipid-lowering therapies could include fibrates in persons with diabetes and elevated triglyceride and low HDL cholesterol levels.
The AHA/ADA Guidelines132,219 recommend BP <140/90 mmHg but consider targeting <120/80 mmHg if tolerated,258 especially in renal disease or increased stroke risk. Cardiorenal disease includes elevated urine albumin excretion or chronic kidney disease.
The AHA/ADA Guidelines132,219 recommend low-dose aspirin for those with 10-year CVD risk of ≥10% without increased risk of bleeding as well as those at intermediate risk (10-year CVD risk 5–10%) but the evidence is Level B and C, and data to support this are controversial.
A period of good glycemic control (HbA1c of 7% vs 7.9%) in patients with newly-diagnosed type 2 diabetes led to a reduction in MI and all-cause mortality after 20 years41
ASCVD = atherosclerotic cardiovascular disease, BP = blood pressure, CV = cardiovascular, CVD = cardiovascular disease, HbA1c = hemoglobin A1c
Antithrombotic medications
Platelet activation and atherothrombosis play key roles in acute coronary syndromes, cerebrovascular events, and the formation and progression of atherosclerotic plaques.266 The benefits of aspirin in patients with acute or previous vascular disease were first assessed in clinical trials published over 20 years ago involving approximately 100,000 patients in placebo-controlled trials.267 Meta-analyses of these trials clearly established benefit of aspirin for secondary prevention in patients at high risk due to established cardiovascular disease,268 defined as patients with an acute or prior history of myocardial infarction, a past history of stroke or transient ischemic attack and patients with stable or unstable angina, vascular surgery, angioplasty, and peripheral artery disease (but not just multiple risk factors). In these patients aspirin was associated with a 27% odds reduction in MACE events. However, in low risk patients (primary prevention) aspirin had a non-significant 10% reduction in MACE events.
In early meta-analyses from 1994, diabetes was included as a high risk group that demonstrated cardiovascular risk reduction with antiplatelet therapy with regimens consisting predominantly of ticlopidine, dipyridamole and sulphinpyrazone.267 In these early trials in diabetes, only 2 of 10 studies evaluated aspirin (with or without dipyridamole). In those 2 studies of aspirin in diabetes, there were 399 cardiovascular events on the aspirin regimen and 414 on control. In 2002, an update from the antiplatelet trialists’ also included diabetes as a high risk subgroup.269 This update included 4961 patients with diabetes from 9 trials and demonstrated that antiplatelet therapy was associated with only a 7% proportional reduction in serious vascular events in those with diabetes, as compared with a 25% reduction overall, but wider confidence limits do not exclude benefit for this group. Thus antiplatelet therapy in diabetes, particularly with the use of aspirin, may be less effective at cardiovascular risk prevention than in patients without diabetes. It should also be noted that these trials were performed at a time when intensive management of LDL-cholesterol and blood pressure were not well established.
Therefore, while the benefit of aspirin in secondary prevention is established, benefit of aspirin in primary prevention of ASCVD in individuals with diabetes is less clear.219, 270 There are three recent published clinical trials of aspirin therapy for primary prevention of cardiovascular disease in diabetes: the Early Treatment Diabetic Retinopathy Study (ETDRS),271 Prevention of Progression of Arterial Disease and Diabetes (POPADAD),272 and Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD).273 ETDRS compared aspirin 650 mg daily versus placebo in 3,711 patients with diabetes (type 1 or type 2 diabetes) and retinopathy, and showed patients receiving aspirin had a numeric decrease in the risk of nonfatal or fatal myocardial infarction; however, the confidence interval crossed 1 (RR 0.85, 95% CI 0.73–1.00). POPADAD was a multicenter study of 1,276 adults with type 1 or type 2 diabetes with subclinical cardiovascular disease defined as a reduced ankle-brachial index in the lower extremity (Figure 4). This study employed a 2 × 2 factorial design with aspirin 100 mg daily and/or an antioxidant capsule. No effect of aspirin (or antioxidant capsule) on the composite primary cardiovascular endpoints (hazard ratio 0.98) was found after a median follow-up period of 6.7 years. JPAD included 2539 patients with type 2 diabetes without known ASCVD in a multicenter study in Japan, randomized to receive 81–100 mg aspirin daily or no aspirin (placebos were not allowed in physician-directed studies at that time) and followed for 4.37 years (Figure 4). The lower rate of the primary endpoint on aspirin treatment did not reach statistical significance (5.4% of subjects in the aspirin group reached the primary endpoint while 6.7% of subjects did so in the non-aspirin group, P=0.16). Importantly, the primary outcome event rate was quite low, and the investigators did not feel that their study was powered to detect a difference between treatment groups. Subsequently, two subgroup analyses of JPAD have been published which need to be interpreted with caution give the overall negative trial. These subgroups had even fewer events but suggested possible benefit of aspirin therapy to reduce cerebrovascular events in patients with type 2 diabetes and poorly controlled blood pressure274 or high C-reactive protein.275 An important study limitation is that the initial trial that did not reach statistical significance; hence as subgroup analyses of a negative trial, these present intriguing hypothesis-generating conclusions which must be considered with caution in clinical practice. Several meta-analyses examining the effect of aspirin for primary prevention of ASCVD in diabetes have also been published276–278 as well as a meta-analysis performed in the general population.267 Intriguingly, early data suggest that twice daily dosing may increase the beneficial effect,279,280 and support further study. However, the mechanism for potential greater benefit with twice daily aspirin dosing remains unclear as aspirin covalently modifies cyclooxygenase-1, leading to inhibition of platelet aggregation. As platelets are anucleated cells they cannot synthesize new cyclooxygenase-1, so antiplatelet effects extend over the duration of the platelet lifespan. Taken together, the recent evidence for aspirin in diabetes without baseline clinical cardiovascular disease has largely been neutral (Figure 4), and recommendations for aspirin use must be tempered by increased risk for bleeding.
Figure 4. Comparison of data from contemporary trials for aspirin in primary prevention of ASCVD in diabetes.
JPAD (Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes, n=2,539) and POPADAD (Prevention of Progression of Arterial Disease and Diabetes, n=1,276). Although there was a trend toward reduction in ischemic outcomes with aspirin therapy for primary prevention in diabetes in both trials (except for CHD or stroke death in POPADAD), there were very few events, highlighting an area of potential controversy in ASCVD risk reduction.
The 2016 ADA Standards of Medical Care for Diabetes recommendations regarding aspirin therapy are consistent with the American Heart Association and American College of Cardiology Foundation statement on aspirin for primary prevention of cardiovascular events in people with diabetes, suggesting that low-dose aspirin should be considered in patients with increased CV risk (10-year risk >10%) and for those at intermediate risk, but not for those at low risk for ASCVD (10-year risk <5%).219, 278 Unfortunately, there is no direct clinical trial evidence to support these risk-based treatment recommendations creating an area of controversy in primary prevention for patients with diabetes. This is in contrast to the clear benefit as outlined in the guidelines for secondary prevention in diabetes which recommend aspirin therapy (75–162 mg daily) in individuals with diabetes and a history of ASCVD. Additionally, dual antiplatelet therapy is reasonable up to 1 year after an acute coronary syndrome. Based on the Dual Antiplatelet Therapy study, in which almost one-third of patients had diabetes, extending dual antiplatelet therapy beyond 1 year after implantation of a drug-eluting stent in patients who have tolerated dual antiplatelet therapy without recurrent ischemic events or bleeding may be considered to reduce major adverse cardiac and cerebrovascular events and stent thrombosis.281
Lifestyle
An intensive program including counseling about medical nutrition therapy, physical activity, and behavior change, with ongoing support and frequent follow-up are needed for lifestyle management of weight, cardiovascular risk factors, and glycemia itself in diabetes.219,282 Weight loss, a healthy eating pattern, and increasing physical activity are also effective for reducing cardiovascular risk factors in individuals without diabetes,219, 283,284 and are effective for prevention of diabetes.205 Lifestyle interventions should be recommended in all patients with diabetes, patients at risk for ASCVD, and patients with known ASCVD.
The Look AHEAD trial is the seminal study of lifestyle intervention in type 2 diabetes treatment, conducted as a multi-center, U.S.-only trial of 5145 obese or overweight patients with type 2 diabetes randomized to receive intensive lifestyle intervention consisting of frequent visits, calorie restriction, and physical activity versus a control group who received only standard diabetes education and support.265 The primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina, with a maximum follow-up planned for 13.5 years. Despite greater weight loss, reduction in HbA1c, and improvements in fitness and cardiovascular risk factors in the intervention group, there was no difference in the primary outcome between treatment arms (HR 0.95; 95% CI 0.83 to 1.09; p=0.51), resulting in early trial termination for futility at a median follow-up of 9.6 years.265 The narrow 95% CI for the primary outcome was consistent with the conclusion that the absence of between-group differences was not due to lack of a sufficient number of primary endpoint events.285
More than half of adults in the U.S. with diabetes are obese,286 while more than 75% are overweight.178 One of the cornerstones for lifestyle management of ASCVD consists of weight loss for individuals who are obese or overweight.283 Weight loss results in increased HDL cholesterol levels,287,288, decreased triglycerides,287–289 and decreased blood pressure.287,290 A modest degree of weight loss (5–7% of body weight) is recommended with similar clinical recommendations for individuals with or without diabetes.291 Another parallel goal is to prevent weight gain, and certain glucose-lowering medications are more likely to result in weight gain (insulin, sulfonylureas and thiazolidinediones), while others are either weight neutral (dipeptidyl peptidase 4 [DPP4] inhibitors, or gliptins), and some result in weight loss (metformin, glucagon-like peptide [GLP]-1 receptor agonists and sodium-glucose cotransporter [SGLT]2 inhibitors, or gliflozins).292 Interestingly, the use of insulin does not preclude weight loss,285 and short- and long-term weight loss can occur with adherence to treatments. The most effective strategies for weight loss in diabetes include a Mediterranean diet287 and an intensive program combining diet and physical activity.265 Participants in the intensive lifestyle arm of the Look AHEAD trial maintained a weight loss of 6% of body weight at 10 years as compared with 3.5% in the standard lifestyle group.265 Strategies that were associated with decreased body mass index (BMI) in the Look AHEAD study included self-weighing on a weekly basis, eating breakfast regularly, and reducing the intake of fast foods, increasing physical activity, decreasing portion sizes, and meal replacements.293
Recommendations for healthy eating should be individually tailored according to caloric requirements, personal and cultural food preferences, type of diabetes, prescribed medications, and comorbid medical conditions. General recommendations include reducing the intake of saturated fat (to 5–6% of total calories), eliminating trans-fat, lowering sodium intake (to below 2300 mg/day or even lower, to less than 1500 mg/day), and increasing intake of dietary fiber.282 A Mediterranean-style diet high in monounsaturated fatty acids (MUFAs) is a recommended alternative to consuming a higher carbohydrate low fat diet in order to control glycemia and cardiovascular risk factors.282 Although there have been many clinical trials examining low versus high carbohydrate diets in patients with insulin resistance and/or diabetes,288,290,294–298 the long-term cardiovascular effects of diets low in carbohydrates have not been examined adequately,282 and an optimal combination of macronutrients cannot be recommended for type 2 diabetes to prevent ASCVD. In order to help control lipids and blood pressure, a dietary pattern that focuses on the intake of vegetables, moderate amounts of fruit and whole grains, poultry, fish, low-fat dairy, legumes, non-tropical vegetable oils and nuts, and limits the intake of sweets, sugar-sweetened beverages and red meats is recommended.283 The Dietary Approaches to Stop Hypertension (DASH) diet,299,300 the ADA recommendations for medical nutrition therapy,282 and the American Heart Association/American College of Cardiology lifestyle management guidelines283 can all be used to guide dietary recommendations. Alcohol intake in patients with diabetes can increase the risk for delayed hypoglycemia and blunt symptom recognition, so patient education regarding management strategies is needed.
Physical inactivity is associated with increased risk of mortality.301 A number of epidemiologic studies show regular physical activity reduces cardiovascular and total mortality in patients with diabetes or prediabetes.302–304 Moderate or high levels of physical activity were associated with a reduced risk of total and cardiovascular mortality, independent of BMI, blood pressure, total cholesterol and smoking, in a cohort of 3708 individuals with type 2 diabetes in Finland followed prospectively for a mean of 18.7 years.305 Even being physically active only occasionally (less than once a week) reduces the risk of all-cause mortality by 28%, while exercising once a week confers a 40% lower risk of mortality compared with being completely sedentary.306 However, individuals with type 2 diabetes may have more barriers to exercise than people without diabetes that limit successful implementation of a physical activity prescription, including presence of comorbidities, risk of hypoglycemia related to glucose-lowering medications, presence of microvascular complications such as visual impairment, peripheral and autonomic neuropathy and even decreased functional exercise capacity which can increase the discomfort associated with initiating a bout of physical activity.307 It should also be noted that no clinical trials have demonstrated that physical activity per se reduces cardiovascular events in diabetes.
Despite the fact that the Look AHEAD study did not demonstrate a reduction in cardiovascular outcomes for intensive lifestyle intervention as compared with standard advice, there were a number of other benefits including increased physical fitness308, reduction in diabetic renal disease,309 and remission of diabetes310 which have positive impacts on cardiovascular risk, and reduction of glucose-lowering medications310 and depression,311–313 which both have direct impact on quality of life. Every attempt must be made to promote and reinforce lifestyle management recommendations and to refer individuals with diabetes to specialists if hypoglycemia or fear of hypoglycemia present a barrier to implementing lifestyle interventions.
Glycemic control
Although patients with type 2 diabetes may be able to achieve glycemic targets without use of glucose-lowering medications, this is often possible only soon after diagnosis when the degree of hyperglycemia is mild to moderate and/or significant modifiable aspects of lifestyle are contributing to the patient’s disordered glucose metabolism (e.g. excess weight, a dietary pattern high in excess calories and refined carbohydrates and low in viscous fiber, little to no physical activity). Diabetes is a progressive, chronic condition, and while there are multiple pathophysiologic mechanisms contributing to hyperglycemia in diabetes,314 progressive beta-cell failure is a key aspect of the natural history of type 2 diabetes,315–317 with gradually worsening hyperglycemia and rising HbA1c.199,318 Eighty-five percent of individuals with established diabetes take glucose-lowering medications, making up 17.7 million people in the U.S.319 Since ASCVD in the form of myocardial infarction and stroke accounts for up to 80% of mortality in type 2 diabetes,320 medications taken to manage hyperglycemia must not adversely impact cardiovascular risk factors or increase ASCVD, and ideally would ameliorate or reverse atherogenesis and lower cardiovascular risk. However, many of the medications available to treat hyperglycemia in diabetes have no effect on cardiovascular risk or may have a theoretical concern for exacerbating cardiovascular risk factors and/or cardiovascular disease itself.321–328 While lowering glucose concentrations will ameliorate immediate symptoms of hyperglycemia (polyuria, polydipsia, and weight loss) and longer term microvascular disease, there is less evidence for targeting HbA1c to lower cardiovascular risk.
Intensive glycemic control reduces relative risk of nonfatal myocardial infarction and coronary heart disease events by about 15%, although there is no effect of intensive glycemic control on all-cause mortality in meta-analysis of the multiple randomized clinical trials targeting different intensity of glycemic control.329 Most studies are of relatively short duration, and long-term follow-up of both the Diabetes Control and Complications Trial (DCCT)330 and United Kingdom Prospective Diabetes Study (UKPDS)41 show reduced risk for cardiovascular events emerge over time in those with recently diagnosed type 1 or type 2 diabetes respectively who were previously randomized to intensive glycemic control. However, these effects take years to manifest and few studies extend observations over the extended timeframe which may be necessary to realize benefit of glucose lowering per se. Therefore reasonable glycemic control, targeting hemoglobin A1c (HbA1c) below 7% may be appropriate for those with ASCVD or multiple risk factors but long life expectancy, while higher targets, above 7% but below levels associated with signs and symptoms of hyperglycemia, are appropriate for those with more limited life expectancy or in whom lower targets cannot be achieved safely.
The UKPDS follow-up study provides the strongest evidence to date for glucose control and reduction in cardiovascular events and mortality in type 2 diabetes.41 UKPDS enrolled patients with newly-diagnosed type 2 diabetes who were randomized to intensive versus standard glycemic control and followed for 10 years, with prolonged follow-up study over an additional 10 years of observation without further intervention, during which time participants returned to their primary care physicians for clinical care. The initial separation of HbA1c of 0.9% (mean HbA1c 7.0% in the intensive group and 7.9% in the conventional treatment group) was lost early in the follow-up observation study, during which the average HbA1c in the two groups converged. Although not seen initially over the first 10 years when glycemic differences were achieved,199 a 15% relative risk reductions for myocardial infarction and 13% risk reductions for death from any cause emerged over time, despite loss of between group differences in HbA1c over the first year of prolonged observation. As similar latent benefits have been seen in type 1 diabetes in the context of the DCCT330 a “legacy effect” or “metabolic memory” has been postulated, with persistence of benefits of initial good glycemic control (and conversely, persistent adverse effects of poor glycemic control).331,332 It has been proposed that the long period of time required to see an effect of glycemic control on cardiovascular risk may be partially explained by improved adherence of all study participants to other aspects of cardiovascular risk factor reduction, such as use of statins and ACEI diluting effects of glycemic control per se on cardiovascular endpoints.41 Reductions in cardiovascular risk has not been observed in subsequent large, randomized controlled trials of intensive glucose-lowering in type 2 diabetes over shorter duration in patients with more advanced cardiovascular disease. It remains uncertain whether absence of benefit is due to inclusion of patients with advanced disease beyond a period of reversibility, short trial duration for effect to manifest, safety of glucose lowering with current available interventions, or absence of effect of glucose lowering per se. Studies have not revealed improvement in primary cardiovascular endpoints with lowering of HbA1c below 6.5–7%333–335 and even raise concern about increasing cardiovascular risk, as seen in ACCORD.333 Meta-analyses of trials that include ACCORD, ADVANCE and the Veterans Affairs Diabetes Trial (VADT) support glucose-lowering as beneficial for reducing composite cardiovascular endpoints and nonfatal myocardial infarction, but there was no effect on total mortality which warrants further investigation.329,336–338 Evaluating these potential benefits of intensive glycemic control in low-risk diabetes is challenging due to low event rates needed to achieve adequate statistical power. It should be noted that ACCORD was designed to define the most effective combinatorial treatment of risk factors in type 2 diabetes (glycemia, blood pressure, lipids) to maximally reduce cardiovascular risk.339 The trial was stopped prematurely due to the unexpected finding of increased all-cause mortality (primarily cardiovascular) (HR 1.21; 95%CI, 1.02 to 1.44), although without difference in the rate of the primary outcome (a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) (P=0.13) and fewer nonfatal myocardial infarctions. The increase in cardiovascular death was driven in part by congestive heart failure, in the setting of fewer nonfatal myocardial infarctions in the intensive treatment group. Neither ADVANCE nor VADT demonstrated an increased mortality or composite cardiovascular risk with intensive glycemic control as defined by HbA1c below 7%.334,335
Several explanations may account for discrepant findings in UKPDS and ACCORD, ADVANCE, and VADT.24,247 Patients in UKPDS had newly-diagnosed diabetes, whereas patients with type 2 diabetes enrolled in the other three trials had diagnosed diabetes for a mean duration of 7.9–11.5 years. A high proportion of latter trial participants had established coronary artery or macrovascular disease at baseline (32–40%), which was an intentional aspect of trial design in ACCORD, ADVANCE and VADT. Average achieved HbA1c ranged from 6.4–6.9% in the intensive treatment groups in ACCORD, ADVANCE, and VADT, but was higher in UKPDS. As anticipated, there were fewer cardiovascular events in ACCORD subjects without prior cardiovascular disease or with baseline HbA1c below 8%. However, only the subset of participants with baseline HbA1c above 8.5% in the intensive treatment group was found to be at higher risk for mortality. Besides higher baseline HbA1c, increased mortality was associated with history of neuropathy and higher HbA1c on treatment, with higher mortality occurring in the subset of patients randomized to intensive control but unable to achieve target glycemia.340–343 A higher average on-treatment HbA1c was also a strong predictor of mortality regardless of treatment group.343 There was a linear increase in risk for mortality with increasing HbA1c across the range of 6% to 9% in the intensive-treatment group, while there was a U-shaped relationship in the standard-treatment group, with lowest hazard rates of all-cause mortality in the intensive control group achieving lowest glycemic targets but at HbA1c of 7–8% for standard-of-care,343 suggesting factors associated with inability to achieve glycemic targets influence mortality, including diverse factors such as more severe underlying disease, inability to adhere to lifestyle and/or pharmacologic regimens, or drug interactions with use of polypharmacy in attempt to achieve goals. Although individuals with severe hypoglycemia in ADVANCE had a higher risk for death, the higher incidence of severe hypoglycemia in the intensive treatment group was not associated with higher risk for cardiovascular endpoints.334 Furthermore, although there were more instances of severe hypoglycemia in the intensive treatment group in ACCORD, lower rather than higher mortality was observed for those with severe hypoglycemia in this group.344
The VADT follow-up study345 demonstrated that an expanded MACE endpoint was reduced significantly in the intensive treatment group; however, cardiovascular death was not, tempering enthusiasm for the results and consistent with prior meta-analyses showing that intensive glycemic control reduces relative risk of nonfatal MI and coronary heart disease events but without an effect on mortality. Subgroup analysis suggests that persons without macrovascular disease at baseline may benefit the most, but these are not necessarily the patients who were enrolled in many of the CV outcome trials.
Insulin-sparing versus insulin-provisional strategies
Epidemiologic studies demonstrate a higher prevalence of cardiovascular disease in patients treated with insulin.326,346 While this has raised concern about the safety of insulin, this concern has not been born out in multiple studies104,347 and may be the result of indication bias, since insulin tends to be used more in patients with more severe diabetes of longer duration, which coincides with factors that increase cardiovascular risk. BARI-2D examined insulin provisional versus insulin sparing (sensitization) therapies in 2368 patients with type 2 diabetes and heart disease undergoing prompt revascularization with intensive medical therapy as compared with intensive medical therapy alone.104 There was no difference in survival or freedom from major adverse cardiovascular events in groups randomized to receive insulin or a sulfonylurea versus metformin or a thiazolidinedione. Likewise, insulin glargine had a neutral effect on cardiovascular outcomes when used to target normal fasting plasma glucose levels for more than 6 years, in patients with cardiovascular risk factors plus impaired fasting glucose, impaired glucose tolerance, or type 2 diabetes.347 Furthermore, randomized controlled trials utilizing insulin therapy as part of the glycemic lowering strategy have not shown an increase in cardiovascular risk with insulin.199,348 However, it is plausible there could be differences in potential cardiovascular risks for different forms of insulin; the most recent example was a delay in approval of insulin degludec by the Food and Drug Administration because of initial concerns about a signal for increased risk of major adverse cardiovascular events, although the upper limit of risk if present must be small as the FDA has approved degludec based on interim data while the definitive cardiovascular outcome trial is ongoing (NCT01959529).
Hemoglobin A1c as a surrogate endpoint
When the Diabetes Control and Complications Trial (DCCT)348 showed substantial decreases in microvascular complications with intensive glycemic control in type 1 diabetes, and similar reductions in risk for development and/or progression of microvascular complications with intensive glycemic control were observed in type 2 diabetes,199,349 HbA1c, as a measure of average glycemia over time became recognized as a surrogate endpoint intended to substitute for the clinical endpoint of microvascular disease. Importantly, an appropriate surrogate endpoint should be highly associated with the disease and disease severity such that a treatment effect on a surrogate biomarker should be directly correlated with clinical benefit, while lack of treatment on a surrogate endpoint should be associated with no clinical benefit. In DCCT, although differences in HbA1c between intensive and standard treatment groups explain virtually all of the differences in risk of retinopathy between groups, total glycemic exposure (HbA1C and duration of diabetes) accounts for only 11% of the variation in retinopathy risk in the complete cohort. Other factors, such as genetics, environment, and non-glycemic risk factors, including dyslipidemia and blood pressure, presumably account for the remaining 89% variability in risk of retinopathy complications in patients with diabetes independent of HbA1c.350 Therefore, HbA1c is only one indicator of risk for microvascular complications of diabetes, and whether HbA1c is a valid surrogate endpoint for macrovascular disease risk remains uncertain. Investigation into new risk markers that account for factors not captured by HbA1c that may impact risk of complications59,351,352 and risk prediction models including these new putative factors is needed.353
Bariatric surgery
Bariatric surgery induces substantial and sustained weight loss354 and remission or improvement in type 2 diabetes in 40–85% of patients, hypertension in 28–75% of patients, and dyslipidemia in 70–90% of patients.355,356 Rates of improvement in obesity comorbidities vary based on both the specific procedure performed and underlying patient characteristics. Together, these metabolic improvements may lead to reduced ASCVD risk and event rates. Indeed these observational cohort studies suggest bariatric surgery versus usual care may be associated with reduced number of cardiovascular deaths and lower incidence of cardiovascular events in obese adults,357,358 as well as reduced incidence of myocardial infarction in obese individuals with type 2 diabetes.359 Total mortality rates appear 30–40% lower in patients who have had bariatric surgery for obesity management354,357 and may be more prominent in those with diabetes at the time of surgery,360 although survival benefit effects may take years to manifest. While key observational cohort controlled studies demonstrate mortality benefit, these data originate from non-randomized trials and thus must be interpreted with extreme caution. Randomized controlled clinical trials comparing effectiveness of bariatric surgery to non-surgical medical management of type 2 diabetes are shorter in duration and have small numbers of participants but replicate observational studies for improvement in obesity and diabetes related comorbidities.361–365 Remission of type 2 diabetes may not be sustained and relapse requiring additional pharmacologic therapy may become necessary. It remains uncertain whether long periods of metabolic improvement following bariatric surgery will improve cardiovascular and/or mortality outcomes, as suggested by longer term follow-up for the UKPDS,41 Steno-2,366 and the Swedish Obesity Subjects (SOS) study.358 However, it is reasonable for surgically-appropriate obese patients with type 2 diabetes to consider bariatric intervention, as associated surgical risks have decreased in the setting of more stringent standards and the formation of Bariatric Centers of Excellence, and with the reduction of short-term surgical risk due to laparoscopic versus open procedures.367,368 Thus, for patients with diabetes and BMI above 35 kg/m2 who have undergone appropriate medical and psychologic evaluation, who understand the risks, lifestyle changes, and monitoring involved with bariatric surgery, and have medical, social and psychologic support, bariatric surgery can be recommended when performed by an experienced bariatric surgeon at a bariatric center of excellence. Interestingly, higher baseline fasting insulin, a proxy for insulin resistance, rather than higher BMI best predicts groups most likely to realize lower incident rates for type 2 diabetes and cardiovascular risk among patients without diabetes undergoing bariatric surgery.358
Cardiovascular risk of diabetes drugs
The UKPDS showed a cardiovascular benefit for metformin, albeit in a relatively small number of individuals.103 Although concerns have been raised regarding increased cardiovascular risk with sulfonylureas in epidemiologic studies, this has not been consistent, and intensive glycemic control using sulfonylureas in UKPDS did not demonstrate increased risk.199 Interestingly, over longer term observation, cardiovascular benefits were realized in the intensive group originally receiving sulfonylureas.
In 2008 the FDA issued a Guidance for Industry requiring that the approval of all new antidiabetic drugs rule out an unacceptable level of excess cardiovascular risk. From 1995 to this time, HbA1c was the sole efficacy endpoint for approval of anti-diabetes therapies. Randomized trials of new glycemic lowering agents were typically 6 months in duration or less, with open label extension; the majority of patients enrolled in the trials were diabetes drug naïve, or had short duration of disease; cardiovascular disease and renal disease were often exclusionary; the safety databases had between 3000 to 5000 patients exposed (the majority less than one year), with longer-term experience uncontrolled; there were sparse cardiovascular events and no central, blinded adjudication process for adverse experiences to facilitate meta-analysis across enabling trials. Thus, experience in the pre-approval trials for new diabetes drugs differed substantially from the way drugs might be used in the broader population, especially relevant for patients with diabetes and cardiovascular disease, which co-occur with high prevalence. FDA concerns regarding diabetes drug development were furthered in part by a meta-analysis of several small trials suggesting excess risk with the drug rosiglitazone.369 Notably the Rosiglitazone Evaluated for Cardiovascular Outcomes in oral agent combination therapy for type 2 Diabetes (RECORD) trial 370 did not substantiate these concerns, even following re-adjudication of the cardiovascular events within the RECORD trial.371 Furthermore, the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) did not show increased ASCVD risk with pioglitazone.102
For these reasons, in 2008 the FDA established a new guidance for industry for approval of diabetes therapeutic agents which remains in effect for all new diabetes drugs, including recently approved agents in multiple novel classes of glucagon-like peptide (GLP)-1 receptor agonists, dipeptidyl peptidase (DPP)-4 inhibitors, and sodium glucose cotransporter-2 (SGLT2) inhibitors, which have become available since implementation of the guidance process. The cardiovascular effects of glucose-lowering drugs have been reviewed recently by Ferrannini and DeFronzo.372 Recent cardiovascular outcome trials have shown a cardiovascular benefit with empagliflozin, an SGLT2 inhibitor373 and have been reported for liraglutide,374 a GLP-1 receptor agonist, although publication of trial data for the latter is still pending. No glucose lowering agent studied under the guidance document has shown increased risk of major adverse cardiac events. Interesting and unexpected findings have come from these cardiovascular outcome trials. The DPP-4 inhibitor saxagliptin appears to modestly increase risk for hospitalization for heart failure without effect on major adverse cardiovascular events (see below section: Management of diabetes in heart failure).375
SGLT1 and SGLT2 are the two sodium glucose cotransporters responsible for glucose reabsorption in the proximal tubule of the kidney.376 Inhibition of SGLT2 results in increased glucose transport to the distal nephron with reduction in renal hyperfiltration377 and lowering of the serum glucose threshold for renal reabsorption of glucose leading to excretion of 80–100 grams of glucose per day.376 Three drugs in this class have been approved by the FDA for the treatment of diabetes: dapagliflozin, canagliflozin, and empagliflozin. Several other drugs in this class are in development. These drugs lower HbA1c by 0.5–1.2% and do not cause hypoglycemia except when used with insulin provisional therapies (insulin or sulfonylureas).378 The SGLT2 inhibitors lower blood pressure without increasing heart rate, increase HDL- and LDL-cholesterol levels, and induce modest weight loss. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG) study enrolled 7020 patients with type 2 diabetes at high risk for cardiovascular events, and randomized participants to receive empagliflozin 10 mg versus 25 mg versus placebo.373 The primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, and the key secondary outcome was hospitalization for heart failure. The study was stopped early, with a mean observation time of 3.1 years. Empagliflozin resulted in a lower risk of the primary outcome (HR 0.86, 95% CI 0.74–0.99, P=0.04 for superiority), lower risk of cardiovascular death and all-cause mortality. Potential mechanisms for the reduction in cardiovascular risk found with empagliflozin include combined reductions in blood pressure, body weight (including visceral adiposity), albuminuria, glucose, arterial stiffness, sympathetic nervous system activation, oxidative stress, uric acid, diuresis and improvement in cardiac function.379 Other potential mechanisms remain speculative including potential secondary effects on SGLT1380 and effects on mineralocorticoid metabolism.377 Whether this beneficial effect on cardiovascular outcomes is a class effect or a drug effect is unknown, and results of other cardiovascular outcome trials with SGLT2 inhibitors will not be available until 2017 for canagliflozin [CANagliflozin cardioVascular Assessment Study (CANVAS), NCT01032629] and 2018 for dapagliflozin [Dapagliflozin Effect on CardiovascuLAR Events (DECLARE)-TIMI58, NCT01730534]. Until then, current evidence supports use of empagliflozin may reduce cardiovascular risk. Because of the beneficial effects on factors that play a role in cardiovascular risk such as weight, visceral fat, blood pressure, arterial stiffness, and albuminuria, the SGLT2 inhibitors may be preferred over other drug classes, although until the additional cardiovascular outcome trials are completed, this approach warrants continued caution. Furthermore, as with any other medical therapy, each individual patient’s comorbidities and complications must be considered prior to initiating SGLT2 inhibitor therapy. Potential adverse effects of SGLT2 inhibitor therapy include genitourinary infections, hypovolemia, “euglycemic” diabetic ketoacidosis, and skeletal fractures.
Importantly, these cardiovascular outcome trials conducted under the 2008 FDA Guidance are designed to evaluate safety of use of diabetes agents in patients with diabetes. Study populations enrolled are at especially high risk for or with underlying ASCVD, as this population is necessary to accrue sufficient events in a short period of time for study conduct. These studies do evaluate cardiotoxicity over short to medium term administration, but they do not address impact of early diabetes intervention, impact of long duration glycemic control, nor the merit of lowering glucose per se, as other anti-diabetes medications outside of the class under investigation are adjusted according to individual glucose goals, permitting assessment of possible drug-specific effects by minimizing potential confounding from differential glucose control.
Management of diabetes in the setting of ASCVD
Since type 2 diabetes confers an increased risk of ASCVD, the vast majority of diabetes patients without ASCVD fall into the moderate or high ASCVD risk category. Though some patients may fall into a lower risk category, all patients should undergo lifestyle intervention. Diabetes therapies should otherwise be selected on an individual basis, with special caution used when treating older patients. In the setting of known ASCVD, we recommend a treatment strategy that primarily focuses on cardiovascular risk reduction (Table 1). Regarding glycemic control, a goal HbA1c 7.0% or less is recommended by the authors if multiple diabetes drugs are tolerated, and polypharmacy does not diminish intensity of cardiovascular risk management; however if this is not the case, a less intensive goal of HbA1c 7.5% or below is recommended. For patients at high risk of ASCVD, an HbA1c 7.0% or below would be reasonable, if this can be achieved with minimal hypoglycemia. For patients without known ASCVD who are at moderate risk, an aggressive goal of HbA1c 6.5% or below will substantially reduce the risk for microvascular complications and may contribute to eventual reduction in ASCVD.41
Multiple factors must be considered in the selection of diabetes medications for patients with ASCVD. The strong data supporting the effect of glucose-lowering to reduce microvascular disease which has a direct impact on cardiovascular risk (especially albuminuria) and eventual long-term reduction in ASCVD over 20 years (from the UKPDS follow-up study) must be balanced against potential acute effects of hypoglycemic episodes, increased risk of heart failure (thiazolidinediones and potentially select DPP-4 inhibitors) and weight gain (sulfonylureas, insulin), other side effects, and cost. Upon a background of lifestyle interventions, the first-line pharmacologic therapy for glucose-lowering should be metformin, if renal function is adequate (Table 2),381–386 and noting recently revised 2016 FDA guidance for renal safety of metformin.387 eGFR should be assessed before initiation of metformin and at least annually for patients using metformin and more frequently for patients at increased risk for renal impairment; starting metformin is generally not recommended for those with eGFR between 30–45 ml/min/1.73m2 but may be continued in this range for those already on the drug when the risk-benefit supports ongoing use; for those with or developing eGFR below 30 ml/min/1.73m2 metformin should be discontinued. In addition to those with heart failure, liver disease, alcoholism, treatment with metformin should be discontinued at the time of iodinated contrast imaging and resumed if renal function is stable when reassessed after 48 hours.
Table 2.
Glucose-lowering therapy: Key clinical considerations
| Lifestyle: Medical Nutritional Therapy consisting of healthy food choices and portion control Exercise as tolerated at least 30 minutes five times weekly | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-insulin add-on therapies to
metformin*, or Monotherapy when metformin is
contraindicated Optimal selection of diabetes medications should be made on an individual basis after consideration of multiple factors such as HbA1c lowering, risk for hypoglycemia, effect on weight and comorbidities, side effects, complexity of regimen, cost, and/or preference such as willingness to use injectable therapies. |
||||||||||
| Class | Biguanide *Metformin is first choice unless contraindicated |
Glucagon-like peptide (GLP)-1 Receptor Agonist | Sodium-glucose cotransporter (SGLT) 2 Inhibitor | Dipeptidyl Peptidase (DPP) 4 Inhibitor | Insulin Secretagogue (sulfonylurea, meglitinide, or D-phenylalanine derivative) | Thiazolidinedione (TZD) | α-Glucosidase Inhibitor | Bile Acid Sequestrant | Centrally Acting Agent | Amylin Analog *Only for use with Insulin* |
| Estimated HbA1c reduction | 1% | 0.8–1.5% | 0.5–0.6% | 0.75% | 0.75–1.25% | 1.0–1.25% | 0.5–1.0% | 0.3–0.9% | 0.4–0.8% | 0.5–0.6% |
| Agents | metformin | exenatide liraglutide albiglutide dulaglutide |
canagliflozin dapagliflozin empaglifozin |
alogliptin linagliptin saxagliptin sitagliptin |
glipizide glyburide glimepiride repaglinide nateglinide |
pioglitazone rosiglitazone |
acarbose miglitol |
colesevelam | Bromocriptine mesylate | Pramlintide |
| Route | oral | Injectable | oral | oral | oral | oral | oral | oral | oral | injectable |
| Actions | Decreases hepatic glucose production and intestinal absorption of glucose; improves insulin sensitivity by increasing peripheral glucose uptake and utilization | Increases glucose-dependent insulin secretion, lowers glucagon secretion, slows gastric emptying, and promotes satiety | Lowers the renal threshold for glucose reabsorption to increase urinary glucose excretion | Blocks inactivation of incretin hormones to increase insulin release and decrease glucagon in a glucose-dependent manner | Stimulates insulin release from pancreatic β cells. | Activates PPARγ, increases peripheral glucose utilization, and decreases hepatic glucose production | Reduces absorption of dietary carbohydrate | Uncertain mechanism of glucose lowering; binds bile acids in intestine, impeding their reabsorption and increasing LDL-C clearance | Uncertain mechanism of glucose lowering with increased insulin sensitivity and glucose disposal | Slows gastric emptying, reduces glucagon secretion, and increases satiety |
| Side Effects | Diarrhea, nausea/vomiting, flatulence, vitamin B12 deficiency, lactic acidosis | Nausea, diarrhea, possible pancreatitis | Polyuria, urinary frequency, hypovolemia, genital and urinary tract infections, hyperkalemia. Reported diabetic ketoacidosis with serum glucose as low as 200 mg/dl | Occasional gastrointestinal discomfort, upper respiratory tract complaints | Hypoglycemia | Weight gain, edema, congestive heart failure in patients with underlying disease | Gastrointestinal discomfort, flatulence, diarrhea, elevated transaminases | Gastrointestinal discomfort, Reduces gastric absorption of some drugs | Gastrointestinal discomfort, headache | Gastrointestinal discomfort, headache |
| Contraindications | Chronic kidney disease with eGFR<30 mL/min; Acidosis; Alcohol excess; severe congestive heart failure; hypovolemia. If IV contrast to be used, hold on day of study and restart 48 hours after IV contrast if eGFR>45 mL/min | History of pancreatitis. personal or family history of medullary thyroid cancer or MEN2. Do not use with DPP4 inhibitors. | Severe renal disease with eGFR<30 mL/min, use with caution in patients with bladder cancer | History of pancreatitis. Do not use with GLP-1 receptor agonists. | Severe liver or renal disease | Severe heart disease at risk for CHF, NYHA Class III or IV heart failure, liver disease. Caution in patients with macular edema | Chronic intestinal disorders, moderate to severe renal impairment (creatinine >2 mg/dl), caution in cirrhosis | Serum triglyceride >500 mg/dl; History of hypertriglyceridemia-related pancreatitis, Bowel obstruction | Lactating women, syncopal migraines or use of ergot class of medications | Gastroparesis. Do not use with α-glucosidase inhibitors, may delay absorption of concomitant medications |
| Comments | Modest weight loss. Reduced cardiovascular event rates (UKPDS). | Weight loss. Decrease in MACE seen with liraglutide (LEADER). No increase in cardiovascular risk or hospitalizations for heart failure in high risk patients with lixisenatide (ELIXA). Others under evaluation. | Reduced blood pressure, weight loss. Improved cardiovascular and total mortality, and decreased hospitalization for heart failure in high risk patients seen with empagliflozin (EMPA-REG). Others under evaluation. | Weight neutral. No increase in cardiovascular risk compared to other diabetes agents in high risk patients (SAVOR-TIMI53, EXAMINE, TECOS). May increase hospitalization for heart failure, but not seen with sitagliptin (TECOS). | Long duration follow-up of glycemic lowering using SFU is associated with reductions in cardiovascular risk (UKPDS). | Increased risk for bone fractures. Increased fluid retention. Rosiglitazone is not inferior for cardiovascular outcomes (RECORD). Pioglitazone is neutral to beneficial for composite cardiovascular outcomes (PROACTIVE) | May reduce cardiovascular risk in patients with impaired glucose tolerance (STOP-NIDDM). | Lowers LDL-C. | Reduced cardiovascular events in FDA enabling trials Cycloset™ Safety Trials. | |
eGFR, estimated glomerular filtration rate; FDA, Food and Drug Administration; IV, intravenous; LDL-C, low density lipoprotein cholesterol; MEN2, Multiple endocrine neoplasia type 2; NYHA, New York Heart Association; PPARγ, Peroxisome proliferator-activated receptor gamma; SFU, sulfonylurea.
Studies: Cycloset Safety trials;381 ELIXA, Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With Lixisenatide;382 EMPA-REG, (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients;373 EXAMINE trial, EXamination of cArdiovascular outcoMes with alogliptIN versus standard of care;383 LEADER trial, Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results.384 PROACTIVE, PROspective pioglitAzone Clinical Trial In macroVascular Events;380 RECORD, Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes;370 SAVOR-TIMI53, Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53;375 STOP-NIDDM, Stop Non-Insulin Dependent Diabetes Mellitus;385 TECOS, Trial Evaluating Cardiovascular Outcomes With Sitagliptin;386 UKPDS, United Kingdom Prospective Diabetes Study.41,103
In light of the EMPA-REG trial results with empagliflozin373 and early reports on the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results - A Long Term Evaluation (LEADER) trial with liraglutide reducing major adverse cardiovascular event rates,374 the next drug to be considered in a patient with persistent hyperglycemia despite adherence to lifestyle and metformin might include either these specific drugs or SGLT2 inhibitors or GLP-1 receptor agonists class agents. Although only top-line results are available for LEADER, details supporting these findings are anticipated shortly. Interestingly a neutral cardiovascular profile was seen in the Evaluation of LIXisenatide in Acute Coronary Syndrome (ELIXA) trial with lixisenatide,382 currently approved by the European Medicines Agency, and additional review of the data is needed to help determine if key aspects of study design underlie potential differences in findings between trials or if differences between class agents may be important. DPP-4 inhibitors are an option except possibly in individuals with heart failure, as discussed in the next section on heart failure.386 Prioritization for sulfonylureas is lower in the setting of ASCVD because of the risk for hypoglycemia, although with careful monitoring and in selected patients, these agents are a reasonable choice, especially in those with higher baseline HbA1c in whom hypoglycemia is less likely to occur. Insulin is often needed to achieve glycemic targets and has not been shown to increase ASCVD risk,347 although it carries a high risk of hypoglycemia and increases renal sodium retention.388–390 The BARI-2D trial showed that an insulin sparing strategy did not confer a cardiovascular benefit, but because of the aforementioned concerns, a basic paradigm of insulin sparing (reduction in insulin dose or discontinuation of insulin preferentially before reducing or stopping other diabetes medications) is generally recommended, recognizing that insulin provision therapy is frequently necessary with longer duration of disease and may be preferred when multiple agents are required to maintain glycemic targets.
For patients requiring multiple agents to achieve their individualized HbA1c goal, a strategy for prioritizing management of diabetes in the setting of CV risk reduction is needed. A suggested approach by the authors is outlined in Table 1. An overview of diabetes treatment including available classes of glucose-lowering agents, magnitude of HbA1c lowering, mechanisms of action, side effects, and key clinical considerations is included in Table 2. Published randomized controlled trials of cardiovascular outcomes for glucose-lowering agents are outlined in Figure 5.391–396
Figure 5. Randomized, controlled, cardiovascular outcome trials of glucose-lowering drugs or strategies in people with type 2 diabetes.
ACS=acute coronary syndrome; ASCVD=atherosclerotic cardiovascular disease including myocardial infarction or ischemic stroke; CV risk=increase risk for cardiovascular disease based on risk factors, but not ischemic ASCVD; HR=hazard ratio; MACE=major adverse cardiovascular event: cardiovascular mortality, myocardial infarction, stroke; RRR=relative risk reduction; SFU=sulfonylurea. T2DM=type 2 diabetes mellitus; MACE=cardiovascular mortality, myocardial infarction, stroke.
Studies: ACCORD, Action to Control Cardiovascular Risk in Diabetes;333 ACCORDION, ACCORD Follow-on study;391 ADDITION, Intensive Treatment in People With Screen Detected Diabetes in Primary Care;392 ADVANCE, Action in Diabetes and Vascular Disease Preterax and Diamicron MR Controlled Evaluation;334 BARI 2D, Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes;104 DIGAMI2, Diabetes Mellitus Insulin Glucose Infusion in Acute Myocardial Infarction;393 ELIXA, Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With Lixisenatide;382 EMPA-REG OUTCOME, (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; 373 EXAMINE trial, EXamination of cArdiovascular outcoMes with alogliptIN versus standard of care;383 HEART2D, Hyperglycemia and its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in patients with Type 2 Diabetes;394 Look AHEAD, Action for Health in Diabetes;284 ORIGIN, Outcome Reduction With Initial Glargine Intervention;347 PROactive, PROspective pioglitAzone Clinical Trial In macroVascular Events;102 RECORD, Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes;370 SAVOR-TIMI53, Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53;395 Steno-2, Multifactorial Intervention in Type 2 Diabetes at the Steno Diabetes Center;176,366 TECOS, Trial Evaluating Cardiovascular Outcomes With Sitagliptin; 386 UKPDS, United Kingdom Prospective Diabetes Study;41,103,199 VADT, Veterans Affairs Diabetes Trial.335,345
Reference: Data taken from Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383:2008–2011.396
Epidemiology of diabetes and heart failure
Heart failure has been called “the frequent, forgotten, and often fatal complication of diabetes.”397 Risk for heart failure increases 2.4-fold in men and 5-fold in women with compared to those without diabetes, as seen in the Framingham Heart Study.398 Conversely, diabetes is an important predictor of heart failure, independent of concomitant hypertension or coronary artery disease.398 Subsequent studies confirm higher prevalence of heart failure, incident diagnosis of new heart failure in patients without baseline heart failure, and risk of heart failure hospitalization or death among patients with compared to those without diabetes.399–403 Heart failure is the second most common manifestation of cardiovascular disease after peripheral arterial disease, as demonstrated in the largest cohort study of almost 1.9 million patients with type 2 diabetes followed for a median of 5.5 years.404 Multiple factors, including age, ischemic heart disease, and peripheral artery disease, as well as diabetes-specific risk factors, such as poor glycemic control (higher HbA1c) and insulin resistance, have been associated with heart failure in patients with diabetes.36, 401–403, 405–407 Accelerated atherosclerosis of diabetes may be diffuse and severe. Consequently, ischemic cardiomyopathy is a major cause of heart failure in the diabetes population. Even in the absence of epicardial coronary artery disease, microvascular disease, characterized by arterial thickening and fibrosis, as well as endothelial and vasomotor dysfunction, can increase risk of heart failure in diabetes. Hypertension is another common comorbid condition in diabetes causing left ventricular hypertrophy and contributing to the development of heart failure. 408
The relationship between diabetes and heart failure appears bi-directional. There is increased risk of heart failure in diabetes, and heart failure is a risk factor for diabetes.409,410 Increased rates of diabetes occur among patients with heart failure: 42% of 48,612 patients hospitalized with heart failure in the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry and 40% of hospitalized patients with low ejection fraction in the Evalve® Cardiovascular Valve Repair (MitraClip®) System Endovascular Valve Edge-to-Edge REpair STudy (EVEREST) had diabetes.411,412 The presence of diabetes in hospitalized heart failure patients is associated with worse outcomes, including longer hospital stay, heart failure-related rehospitalization, and greater risk of cardiovascular mortality.411,413–415 Among stable outpatients with heart failure, diabetes remains an independent predictor of heart failure hospitalization and cardiovascular mortality after adjusting for left ventricular ejection fraction.416 Even in the absence of overt diabetes, up to two-thirds of heart failure patients exhibit insulin resistance,417 which not only increases the risk of subsequent diabetes but also independently predicts poor prognosis in patients with heart failure. 418
In 1954, Lundbaek made the initial observation that myocardial dysfunction was common in elderly patients with diabetes, and he termed this phenomenon “diabetic cardiopathy”.419,420 Almost two decades later, Rubler reported postmortem findings from four patients with diabetes and heart failure in the absence of significant epicardial coronary atherosclerosis, valvular, congenital, or hypertensive heart disease, or alcoholism.421 He described myocardial hypertrophy, fibrosis, and microvascular wall thickening and proposed a diabetes-related cardiomyopathy caused by abnormal myocardial metabolism or myocardial microangiopathy. These observations stimulated basic and clinical studies examining phenotypes and pathophysiologic mechanisms of diabetes-related cardiomyopathy. Despite efforts to understand diabetic cardiomyopathy, there is ongoing controversy surrounding its unique pathophysiology, and it remains a vaguely-defined condition only briefly acknowledged in contemporary clinical practice guidelines.246, 422 Furthermore, although diabetes is now accepted as an independent cause of cardiomyopathy, severity of diabetes-related myocardial abnormalities may be amplified by comorbid conditions which increase the risk of ventricular hypertrophy and/or susceptibility to myocardial ischemia, ultimately increasing the risk for heart failure.
Diabetic cardiomyopathy- clinical phenotype
The natural history of diabetic cardiomyopathy remains incompletely understood. Experimental animal models of diabetes and cardiac imaging-based human studies demonstrate both diastolic and systolic dysfunction.423–432 Traditionally, diabetic cardiomyopathy has been characterized as a progressive disease beginning with subtle, early features of impaired diastolic dysfunction followed by overt diastolic dysfunction, with or without ventricular hypertrophy. Thus, diastolic dysfunction is the hallmark characteristic of diabetic cardiomyopathy, with systolic dysfunction representing the final stage of progressive disease.430–432 Depressed pressure development and decay rates and prolonged cardiac relaxation times occur as early as 2–3 weeks after streptozotocin-induced diabetes in rats, prior to left ventricular remodeling, supporting this diastolic-dysfunction hypothesis.428,429 Human studies similarly demonstrate early abnormalities in diastolic function, including reduced peak myocardial systolic and early diastolic velocities, seen in 27–70% of asymptomatic patients with diabetes without overt diastolic dysfunction or LV hypertrophy,423–427 as well as greater LV mass, wall thickness, and arterial stiffness (independent of blood pressure and body mass index) in diabetes patients.433–435 Clinically, patients with diabetic cardiomyopathy and diastolic dysfunction initially present with a restrictive phenotype as heart failure with preserved ejection fraction (HFpEF). 436–438
However, diabetic cardiomyopathy can also present with systolic dysfunction and a dilated phenotype as heart failure with reduced ejection fraction (HFrEF). In animal models, diabetes has been associated with systolic dysfunction,439,440 with longer duration of diabetes necessary for development of reduced ejection fraction.441 These findings are paralleled by human studies in which diabetes has been associated with a significant increase in the odds of idiopathic dilated cardiomyopathy (odds ratio 1.75; 95% confidence interval 1.71, 1.79).442 Additionally, left ventricular systolic dysfunction can be induced with exercise in asymptomatic patients with diabetes and normal resting ejection fraction,443,444 perhaps representing reduced cardiac reserve and an early, preclinical phase of systolic dysfunction. It is also hypothesized that subtle impairments in longitudinal systolic function may be missed due to the traditional focus on radial myocardial contraction and more sensitive techniques, such as strain and myocardial tissue Doppler velocity, may better detect early systolic abnormalities in patients with diabetes.445,446 Taken together, these data suggest a spectrum of diabetic cardiomyopathy. 432
Recently, Seferovic and Paulus proposed a new paradigm in which restrictive and dilated manifestations of diabetic cardiomyopathy are two distinct clinical phenotypes rather than successive stages.447 This conceptual shift is based on multiple lines of evidence. Normal age-related cardiac remodeling is characterized by decreasing left ventricular dimensions and increasing fractional shortening, a pattern attenuated but not reversed by diabetes.448 Furthermore, development of symptoms in hypertensive patients with HFpEF is associated with a reduction, not dilatation, of the left ventricle.449 In the general HFpEF population, progression to a dilated phenotype is uncommon; when it occurs, it is often related to myocardial infarction or older age but not diabetes.450 Finally, differential mechanisms of left ventricular remodeling specific to HFpEF versus HFrEF have been proposed.451 Thus, diastolic dysfunction may not be a precursor to systolic dysfunction; rather, specific mechanisms with selective involvement of endothelial cells versus myocytes in diabetes-related metabolic and cellular disturbances, may account for the independent evolution of two distinct forms of diabetic cardiomyopathy.447
Pathophysiologic mechanisms of heart failure in diabetes
The pathogenesis of diabetic cardiomyopathy is complex and multifactorial. Although hyperglycemia and insulin resistance are the main physiologic disturbances in diabetes, multiple mechanisms, such as derangements in cellular metabolism, function, and structure, autonomic neuropathy, and neurohormonal dysregulation are believed to play a role in the associated cardiomyopathy (Figure 6). Diabetic cardiomyopathy can be primarily traced to multiple processes: oxidative stress,452 hyperglycemia,432,453,454 hyperinsulinemia,453,454 or hyperlipidemia,453,454 but no consensus exists regarding a unifying pathophysiologic hypothesis. A general overview of major contributing mechanisms is discussed herein, although the reader is referred to comprehensive reviews focused solely on diabetes-related heart failure for additional information.431,447,453–458
Figure 6. Pathophysiologic mechanisms of heart failure in diabetes.
Hyperglycemia, insulin resistance, and hyperinsulinemia, the main physiologic disturbances in diabetes, contribute to atherosclerotic cardiovascular disease (ASCVD), hypertension, and multiple derangements of cellular metabolism, function, and structure, as well as activation of the renin-angiotensin-aldosterone system (RAAS). The different cardiomyopathies that result from these processes clinically present as heart failure in diabetes. AGE, advanced glycation end-product; FFA, free fatty acids.
Metabolic perturbations
Major energy sources for cardiac metabolism include glucose and free fatty acids. Free fatty acids are preferentially utilized in the fasting state, whereas glucose is the substrate of choice in the post-prandial state or under conditions of stress or ischemia. Healthy cardiomyocytes are able to switch between these sources to accommodate different physiologic conditions. In patients with diabetes, insulin resistance and hyperglycemia cause down regulation of myocardial glucose transporters (primarily GLUT4 in adults), reduced glucose oxidation, and an increase in fatty acid oxidation and levels of free fatty acids.431,458 A higher oxygen requirement of fatty acid oxidation compared with glucose metabolism leads to relative cardiac ischemia, resulting in an accumulation of lactate and impaired calcium homeostasis and myocyte contraction. Additionally, elevated levels of free fatty acids cause lipid accumulation in cardiomyocytes and lipotoxicity, which manifests as contractile dysfunction and eventual cardiomyocyte apoptosis. 431
Functional alterations
Impaired calcium handling in cardiomyocytes is a key feature of diabetic cardiomyopathy. In the normal state, excitation-contraction coupling of cardiomyocytes is mediated by several intracellular calcium transporters, including the ryanodine receptor, and relaxation occurs via the ejection of calcium from the cell through the sarcoplasmic reticulum calcium pump 2a (SERCA2a), the sodium-calcium exchanger, and the plasma membrane calcium ATPase.459 Animal models of diabetes have altered expression, activity, and function of all of these transporters.458 Reduced SERCA2a activity and consequent calcium overload in the cytosol can cause impaired relaxation as well as altered calcium sensitivity of proteins involved in regulation of the cardiac actomysin system and shifting of cardiac myosin heavy chain isoforms from V1 to V3, which can lead to reduced contractile force.456,459
Mitochondrial dysfunction and increased oxidative stress contribute to the pathogenesis of diabetic cardiomyopathy. Proposed mechanisms for diabetes-related mitochondrial dysfunction include fatty acid-induced mitochondrial uncoupling leading to increased myocardial oxygen consumption and reduced cardiac efficiency, impaired mitochondrial calcium handing, and mitochondrial proteomic remodeling via post-translational modifications of mitochondrial proteins.459–461 Hyperglycemia can cause mitochondrial production of superoxide in endothelial cells.447 Although increased production of reactive oxygen species occurs mainly in mitochondria, cytosolic systems, such as NAPDH oxidase, can also generate reactive oxygen species and increase oxidative stress, potentially contributing to diabetic cardiomyopathy via accelerated apoptosis, cardiac hypertrophy, fibrosis, subcellular remodeling, and impaired calcium handling. 455,462,463
Structural changes
Cardiomyocyte hypertrophy is common in diabetic cardiomyopathy and may result from insulin resistance and cell growth in response to hyperinsulinemia, as occurs in type 2 diabetes.104,347, 431 This observation has led to concern about the use of insulin but has not been born out in clinical trials. In contrast, patients with type 1 diabetes more frequently exhibit hyperglycemia-related myocardial fibrosis rather than hypertrophy.431,454 Fibrosis may be related to the formation of AGEs,459 comprised of cross-linked collagen which may deposit in arterial walls, myocardium, and endothelial cells.458 Higher serum levels of AGEs are associated with greater ventricular isovolumetric relaxation times, a marker of diastolic dysfunction, and greater arterial stiffness.431 Deposition of AGEs in arterioles may also result in microvascular remodeling and angiopathy characterized by capillary basement membrane thickening and formation of microaneurysms, leading to subsequent impairment of nitric oxide production.456,461 Subsequent endothelial dysfunction from decreased nitric oxide bioavailability and endothelial damage from high glucose exposure may explain the reduced coronary blood flow reserve and myocardial hypertrophy observed in diabetic cardiomyopathy. 462
Cardiac autonomic neuropathy
Under normal conditions, sympathetic stimulation causes vasodilation of coronary resistance vessels and improves left ventricular contraction and left ventricular relaxation rates.458 Cardiac autonomic neuropathy in diabetes is characterized by sympathetic denervation, depletion of myocardial catecholamine stores, and functional impairment in cardiac sympathetic nerve fibers.454,458 Each of these processes may contribute to left ventricular systolic and diastolic dysfunction and reduced 8-year survival among patients with cardiac autonomic neuropathy compared with those with normal autonomic function (77% versus 97%, respectively). 464
Neurohormonal activation
Neurohormonal abnormalities are present in both diabetes and heart failure. There is early activation of RAAS in diabetes,454 leading to overproduction of angiotensin II and supporting the blood pressure trials discussed above. Excess angiotensin II and aldosterone production induce cardiac hypertrophy and fibrosis via collagen deposition and fibroblast proliferation, as well as oxidative damage and cellular apoptosis.459 In addition, angiotensin II may cause myocardial ischemia through calcium overloading in cardiomyocytes.461 These effects of angiotensin II and aldosterone, which clinically manifest as diastolic dysfunction, are intensified by hyperglycemia and compounded by renin stimulation related to sympathetic nervous system over-activity observed in heart failure. 456
Management of heart failure in diabetes
Multiple pharmacologic and device therapies have proven benefits and are recommended for the treatment of chronic heart failure.422,465 In chronic heart failure trials, up to 30% of participants generally have comorbid diabetes,466 and subgroup analyses have not demonstrated significant differences in treatment effects among diabetes patients.456 Despite previous concern regarding beta-blocker use in diabetes due to potential masking of hypoglycemia and adverse effects on insulin resistance, current literature supports use of beta-blockers in the treatment of heart failure among diabetes patients.422,456,465,467 Similarly, beneficial effects of angiotensin-converting enzyme inhibitors, angiotensin-receptor blockade, mineralocorticoid-receptor antagonism, and cardiac resynchronization therapy are demonstrated among heart failure patients with diabetes.401,468–471 Although use of diuretic medications has been associated with impaired glucose tolerance potentially related to hypokalemia and/or visceral fat deposition,472,473 these drugs are often necessary to treat stable and acutely decompensated heart failure patients; no studies have examined differential effects of these medications according to diabetes status. Based on available data, management of heart failure patients with diabetes should follow standard treatment guidelines for the general heart failure population.465,474
Management of diabetes in heart failure
While heart failure management is not different in patients with diabetes, special consideration should be taken in glycemic management as effects on heart failure differ among agents. Studies to date suggest that SGLT2 medications appear to be safe from a cardiovascular perspective,475,476 and empagliflozin reduces the relative risk for heart failure hospitalization by 35% (HR 0.65, 95% CI 0.50, 0.85) compared with placebo in high-risk cardiovascular patients with type 2 diabetes.373 Possible mechanisms for this observation include osmotic diuresis and reduced arterial stiffness caused by SLGT-2 inhibition leading to lower plasma volume and blood pressure, as well as altering tubular-glomerular feedback mechanisms,377 or indirect effect on SGLT1,380 the transporter primarily responsible for intestinal glucose absorption.477 As with cardiovascular mortality effects, whether this heart failure benefit is a class effect is not yet known and will be evaluated in the ongoing studies Canagliflozin Cardiovascular Assessment Study (NCT01032629) and Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (NCT01730534).
Potential cardiovascular effects of incretin-based diabetes treatments in patients with heart failure are not well-understood. Incretin mimetics improve glycemia by increasing glucose-dependent insulin secretion, lowering glucagon, inhibiting gluconeogenesis, and increase insulin sensitivity, while promoting weight loss. In endothelial cells and myocytes, the incretin hormone GLP-1 may have cardioprotective effects, including vasodilatory and anti-inflammatory properties, independent of its anti-hyperglycemic action.478 GLP-1 also has been shown to reduce blood pressure via an atrial natriuretic pathway.479 Prior data suggested that GLP-1 is involved in maintaining normal cardiac structure and function, as evidenced by the increased thickness, impaired contractility, and elevated end diastolic pressure of the left ventricle, as well as diastolic dysfunction and reduced cardiac reserve observed in knockout mice deficient in the GLP-1 receptor.480 However, this hypothesis has been challenged recently by studies in which experimental murine models of myocardial infarction and heart failure have not been affected by lack of GLP-1 receptor expression.481 The cardiovascular outcome trial with the GLP-1 receptor agonist, lixisenatide which is not yet available in the US, showed no significant between-group differences in the rate of hospitalization for heart failure when compared to placebo and standard of care in persons with type 2 diabetes who had previous myocardial infarction or who had been recently hospitalized for unstable angina. Small studies in humans, some of which were non-randomized and not placebo-controlled, have examined the effects of exenatide or GLP-1 on cardiac function in heart failure patients with mixed results: only two showed an association between GLP-1 infusion with improvement in left ventricular function.482,483 These findings suggest GLP-1 receptor agonists can be used safely in patients with heart failure, although additional studies using are ongoing. Recently, the Effect of Exenatide Compared with Insulin Glargine on Cardiac Function and Metabolism of Type 2 Diabetic Patients with Congestive Heart Failure: A Randomized Comparator-controlled Trial (NCT00766857) comparing the effect of exenatide versus insulin glargine on left ventricular ejection fraction was completed, but results have not yet been published. Further insight will come from the LEADER trial with liraglutide, and the Exenatide Study of Cardiovascular Event Lowering Trial (NCT01144338), a placebo-controlled, randomized study of ~14,000 patients that will examine the effects of exenatide on cardiovascular outcomes, including heart failure hospitalizations. To date, available data suggest safe use of GLP-1 receptor agonists in patients with heart failure.
Three large randomized cardiovascular outcome trials of DPP-4 inhibitors compared to placebo have now been reported, and effects on heart failure are inconsistent. In the Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS)386 in 14,671 persons with type 2 diabetes and established preexisting vascular disease, hospitalization for heart failure rates were similar in sitaglipitin compared with placebo groups, 3.1% sitagliptin versus 3.1% placebo: hazard ratio 1.00 (95%CI: 0.83–1.20). The hazard ratio for hospitalization for heart failure was similar in the sitagliptin-treated group in those with baseline history of heart failure, although absolute rates were higher. In contrast, the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)-Thrombolysis in Myocardial Infarction (TIMI) 53 trial,395 which randomized 16,492 participants with established cardiovascular disease or increased cardiovascular risk, demonstrated an unanticipated increased risk of heart failure in the saxagliptin-treated group: event rate 3.5% in saxigliptin versus 2.8% in placebo; hazard ratio 1.27 (95% CI: 1.07–1.51), in the setting of non-inferiority for the primary cardiovascular outcome (cardiovascular death, myocardial infarction, stroke). In this trial, heart failure was a prespecified individual clinical endpoint within the major secondary composite cardiovascular outcome. Increased hospitalization for heart failure was primarily observed in those with highest baseline N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) concentration.484 The smaller, shorter trial EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE (EXAMINE)383 with randomization of 5,380 higher risk acute coronary syndrome patients demonstrated a non-statistically significant trend with similar point estimate seen in the SAVOR-TIMI 53 trial of saxagliptin towards increased heart failure in the alogliptin-treated group: event rate 3.9% alogliptin versus 3.3% placebo; hazard rate 1.19 (95% CI: 0.89–1.58), also in the setting of non-inferiority for the primary cardiovascular outcome (cardiovascular death, myocardial infarction, stroke). Hospitalization for heart failure was not increased in the alogliptin-treated group in those with history of heart failure or higher baseline B-type natriuretic peptide (BNP) concentration.485
Increased risk of hospitalization for heart failure was not consistent across SAVOR-TIMI 53, EXAMINE, and TECOS. These were not head-to-head comparisons of the three DPP-4 inhibitors, and caution should be applied when comparing agents. There were differences in patient illness severities, comorbidities, and concomitant therapies, sample size and duration of follow-up, and the potential for altered attentiveness and therapeutic practices due to baseline differences, or variation in definition or acquisition of heart failure events across trials. It is possible there are intrinsic pharmacologic differences between the DPP-4 inhibitors in specificities on and/or off target. However, it is also possible heart failure is a class effect, as a test for heterogeneity for hospitalization for congestive heart failure across trials was not significant. There are plausible mechanisms for increased heart failure with DPP-4 inhibition. DPP-4 cleaves additional protein substrates beyond the incretins GLP-1 and glucose-dependent insulinotropic peptide, and multiple DPP-4 substrates have been identified which may influence the cardiovascular system, including natiuretic peptide which is inactivated by DPP4 and provides a plausible mechanism for altered fluid balance.486 Alternatively, in preclinical models, diabetic mice treated with the potent DPP-4 inhibitor MK-0626 exhibit modest cardiac hypertrophy, impairment of cardiac function, and dysregulated expression of genes and proteins controlling inflammation and cardiac fibrosis.487 A recent multinational multicenter observational study of use of incretin based drugs and heart failure involving approximately 1.5 million persons finds no association of incretin based therapies with heart failure as compared with combinations of oral antidiabetes drugs, although both DPP-4 inhibitors and GLP-1 receptor agonists were combined in this analysis and comparative risk of specific agents was not possible.488 As heart failure is common in patients with type 2 diabetes, providers must select therapeutic agents for the totality of their risk benefit, and in the setting of non-inferiority for major adverse cardiovascular events, watch for signs and symptoms of heart failure in their patients.
Several diabetes medications should be used with caution in patients with concomitant heart failure. Thiazolidinediones increase insulin sensitivity by activating the peroxisome proliferator-activated receptor (PPAR) gamma. In meta-analyses of randomized clinical trials, patients treated with thiazolidinediones versus placebo had an increased risk of heart failure (OR up to 2.1, 95% CI 1.08–4.08).489–491 Observational data suggest the risk for heart failure appears higher with rosiglitazone than pioglitazone.492 Although not approved for clinical use, aleglitazar, a dual PPAR alpha and gamma agonist, also increased the risk of heart failure by 22% in the Aleglitazar Acute Coronary Syndrome and Type 2 Diabetes Mellitus (AleCardio) trial.493 Thiazolidinediones have not been shown to have adverse effects on ventricular remodeling or ventricular contractility and function.494 Instead, thiazolidinedione-related heart failure may be due to fluid retention from PPAR-gamma stimulation of sodium reabsorption in renal epithelial sodium channels.495–497 In contrast with the setting of HFrEF, volume retention from thiazolidinediones is typically early, not progressive, and responsive to withdrawal of therapy.495,496 Thiazolidinediones have an FDA-issued black box warning and are contraindicated in patients with New York Heart Association class III–IV heart failure.,498 They should be used cautiously in patients with class I–II heart failure with close monitoring for fluid retention. 465,474
Insulin therapy can also stimulate sodium retention and has been associated with edema.499 In vitro and animal studies have shown that insulin increases the activity of renal sodium transporters, though clinical studies suggest the main effect is in the distal tubule via the same epithelial sodium channel stimulate by thiazolidinediones.500 Consequently, the risk of edema is greater when insulin and thiazolidinediones are given together (5% with single therapy versus 13–16% in combination).500 Insulin use in heart failure patients has been associated with worse prognosis, including increased all-cause mortality, cardiovascular mortality, and heart failure hospitalizations.489,501,502 This relationship may be confounded by the use of insulin later in the course of type 2 diabetes, and increased heart failure was not seen in the Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial, where insulin glargine was used earlier in disease, and a trend toward decreased risk of heart failure hospitalization was observed.347
Other diabetes drug classes with potentially deleterious effects in heart failure include biguanides and sulfonylureas. Metformin is a first-line treatment for diabetes. However, the FDA issued a black box warning against the use of metformin in heart failure patients requiring pharmacologic management due to a risk of lactic acidosis.503 The risk of lactic acidosis is small and related to decreased drug clearance in the setting of hypoperfusion or renal dysfunction, both of which may be worsened in acute heart failure exacerbations. Although metformin-related lactic acidosis has been reported,504 observational data suggest that metformin use in patients with stable chronic heart failure is associated with a mortality benefit without excess risk of lactic acidosis.505 Metformin may thus be used with caution in heart failure patients with stable renal perfusion and an adequate glomerular filtration rate. The data for sulfonylureas are also mixed and report variable associations between increased risk of heart failure and first- and second-generation sulfonylureas, but may be used when need for glycemic lowering outweighs these uncertain risk.478,500,505
Role of glycemic control
Hyperglycemia in diabetes is associated with increased risk of heart failure. A secondary analysis from the UKPDS showed an adjusted rate of heart failure of 2.3 events/100 person-years among patients with HbA1c levels of <6% compared to 11.9 among those with HbA1c levels >10%.30 Among 48,858 patients with predominantly type 2 diabetes, each 1% increase in HbA1c is associated with an 8% increased relative risk of heart failure;9 a similar relationship has been observed among 20,985 patients with type 1 diabetes (30% increased relative risk of heart failure per 1% increase in HbA1c).506
Despite a consistent association between hyperglycemia and heart failure, no causal relationship has been proven. Studies have failed to consistently demonstrate a relationship between blood glucose or insulin levels and left ventricular function.507,508 While the ORIGIN trial targeting normal fasting plasma glucose levels for more than 6 years showed a trend toward decreased risk of heart failure hospitalization in the insulin glargine group,347 most trials have failed to show a relationship between strict glycemic control and reductions in heart failure in diabetes.211,334,336 The optimal glycemic level in diabetes patients with heart failure thus remains unclear and warrants further investigation.
Considerations for the management of concurrent heart failure and diabetes
The prevalence of patients with concomitant heart failure and diabetes is high, and providers should be aware of both restrictive and dilated forms of diabetic cardiomyopathy. While standard heart failure guidelines for the general population should be followed when treating heart failure in patients with diabetes, the converse is not true: careful selection of medications is necessary in the management of diabetes in heart failure patients In particular, inhibition of SGLT2 with empagliflozin appears associated with a reduction in heart failure hospitalizations, making this a preferred therapy if otherwise tolerated, while thiazolidinediones should be avoided in patients with severe heart failure symptoms and used with caution in mildly symptomatic patients. Metformin may be used cautiously in heart failure patients with stable renal function and adequate renal perfusion. To date, there has not been any clear signal for heart failure benefit with incretin-based treatment, and there may even be an excess risk for heart failure hospitalization related to some DPP-4 inhibitors. Thus, heart failure risk differs across diabetes therapeutic classes, and additional prospective study is required to fully understand potential drug effects in this unique population.
What is on the horizon?
A number of new approaches for glucose lowering are under development and may provide agents with dual benefit for diabetes and the diabetic heart. Additionally, drugs that are developed for cardiovascular risk reduction may also lower glycemia. In the meantime, cardiovascular outcome trials will provide useful information on cardiovascular safety of use of diabetes drugs in patients with established heart disease or at high risk for events and additional exposure information that may reveal previously unrecognized positive or negative effects. These trials may help to better address the question of the impact of specific glucose-lowering drugs and pharmacologic class agents on the diabetic heart.
Conclusions
The long-term treatment of diabetes is challenging due to diverse goals: to address metabolic derangements and to reduce risks for both micro- and macrovascular adverse outcomes. Management of hyperglycemia has resulted in substantial reductions in risks for retinopathy with associated preservation of vision, and nephropathy with prevention of end-stage renal disease when combined with aggressive blood pressure control. Progress in prevention and amelioration of these microvascular complications has conversely resulted in a shift in the major causes of long-term morbidity and mortality in diabetes, which now consists of cardiovascular risk with associated ischemic heart disease, ischemic stroke, peripheral artery disease, and congestive heart failure. Diabetes clearly exacerbates mechanisms of atherosclerosis and heart failure. Unfortunately, these mechanisms are not adequately fully modulated or addressed by focusing solely on optimal glycemic control. Fortunately, aggressive management of cardiovascular risk factors, particularly lowering of LDL-cholesterol concentration and blood pressure along with glycemic management, provide substantial improvements in cardiovascular outcomes. Therefore, we prioritize recommendations for management of cardiovascular and heart failure risk to focus on the most effective therapies, as summarized in Table 1.
Multiple areas of further investigation remain. Potential cardiovascular benefits versus risks of new glucose-lowering agents and timing of (early and/or prolonged) glucose-targeting interventions are incompletely understood. Evidence for a more effective antiplatelet regimen than aspirin in moderate to high risk patients with diabetes without ischemic heart disease is necessary. Recommended blood pressure goals are also not fully evidence-based, and the role of new potent lipid lowering therapies (PCSK9 inhibitors) and lipid lowering drugs that target triglycerides and HDL cholesterol needs further study. Another unaddressed issue is the ultimate risk-benefit ratio for polypharmacy that occurs when physicians add multiple drugs to achieve an optimal level of glycemic control and cardiovascular risk management. These are pragmatic concerns wherein the use of multiple medications can result in less overall medication compliance and increased risk of drug-drug interactions. Although the overall management of diabetes has improved substantially over the past 2 decades, there is a large unmet need for cardiovascular prevention.
Supplementary Material
Acknowledgments
We are grateful for collaborations and conversations with colleagues over the years regarding this and related topics. We thank Patrick Lane for his assistance draftingthe figures, and Craig Wesselman for drafting Figure 4. ABG acknowledges the enrichment core of the Joslin Diabetes Research Center P30 DK036836. We also thank the Circulation editors and peer reviewers for their critical appraisal and comments, and the staff for their assistance with preparation of this manuscript.
Footnotes
Disclosures
Dr. Hiatt receives grant support from the AstraZeneca, Bayer, GSK, and Janssen, and he serves as a consultant for Kowa. The other authors have no relationships to report.
References
- 1.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013;34:2444–2452. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Cardiovascular diseases (CVDs) Fact sheet No 317. [Accessed December 17, 2015];Internet. 2015 Jan; Available at: URL: http://www.who.int/mediacentre/factsheets/fs317/en/
- 4.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 6.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Levine S. Angina pectoris: Some clinical considerations. JAMA. 1922;79:928–933. [Google Scholar]
- 8.Joslin E. Arteriosclerosis and diabetes. Annals of Clinical Medicine. 1927;5:1061. [Google Scholar]
- 9.Marble A. Coronary artery disease in the diabetic. Diabetes. 1955;4:290–297. doi: 10.2337/diab.4.4.290. [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 11.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–148. doi: 10.1111/j.1464-5491.2008.02640.x. [DOI] [PubMed] [Google Scholar]
- 12.Howard BV, Best LG, Galloway JM, Howard WJ, Jones K, Lee ET, Ratner RE, Resnick HE, Devereux RB. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29:391–397. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 14.Schramm TK, Gislason GH, Kober L, Rasmussen S, Rasmussen JN, Abildstrom SZ, Hansen ML, Folke F, Buch P, Madsen M, Vaag A, Torp-Pedersen C. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117:1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 15.Schraer CD, Adler AI, Mayer AM, Halderson KR, Trimble BA. Diabetes complications and mortality among Alaska Natives: 8 years of observation. Diabetes Care. 1997;20:314–321. doi: 10.2337/diacare.20.3.314. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93:1317–1542. doi: 10.1152/physrev.00004.2012. [DOI] [PubMed] [Google Scholar]
- 19.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52:255–262. doi: 10.1016/j.jacc.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bornfeldt KE. 2013 Russell Ross memorial lecture in vascular biology: cellular and molecular mechanisms of diabetes mellitus-accelerated atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:705–714. doi: 10.1161/ATVBAHA.113.301928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reusch JE, Wang CC. Cardiovascular disease in diabetes: where does glucose fit in? J Clin Endocrinol Metab. 2011;96:2367–2376. doi: 10.1210/jc.2010-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Suppl 1):S13–S22. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 27.Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with Type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–1301. doi: 10.1111/j.1464-5491.2008.02581.x. [DOI] [PubMed] [Google Scholar]
- 28.Gerstein HC, Pogue J, Mann JF, Lonn E, Dagenais GR, McQueen M, Yusuf S. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48:1749–1755. doi: 10.1007/s00125-005-1858-4. [DOI] [PubMed] [Google Scholar]
- 29.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 30.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, Eliasson B. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J Intern Med. 2010;268:471–482. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 33.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di AE, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seshasai SR, Kaptoge S, Thompson A, Di AE, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med. 2005;165:1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 37.Mazzone T. Intensive glucose lowering and cardiovascular disease prevention in diabetes: reconciling the recent clinical trial data. Circulation. 2010;122:2201–2211. doi: 10.1161/CIRCULATIONAHA.109.913350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 39.Kuusisto J, Mykkanen L, Pyorala K, Laakso M. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes. 1994;43:960–967. doi: 10.2337/diab.43.8.960. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez BL, Lau N, Burchfiel CM, Abbott RD, Sharp DS, Yano K, Curb JD. Glucose intolerance and 23-year risk of coronary heart disease and total mortality: the Honolulu Heart Program. Diabetes Care. 1999;22:1262–1265. doi: 10.2337/diacare.22.8.1262. [DOI] [PubMed] [Google Scholar]
- 41.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 42.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da RR, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211–1218. doi: 10.1161/01.cir.0000027569.76671.a8. [DOI] [PubMed] [Google Scholar]
- 43.Perkins JM, Joy NG, Tate DB, Davis SN. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab. 2015;309:E168–E176. doi: 10.1152/ajpendo.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: lack of direct growth-promoting effects of high glucose levels. Diabetes. 2001;50:851–860. doi: 10.2337/diabetes.50.4.851. [DOI] [PubMed] [Google Scholar]
- 45.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reaven P, Merat S, Casanada F, Sutphin M, Palinski W. Effect of streptozotocin-induced hyperglycemia on lipid profiles, formation of advanced glycation endproducts in lesions, and extent of atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:2250–2256. doi: 10.1161/01.atv.17.10.2250. [DOI] [PubMed] [Google Scholar]
- 47.Galkina EV, Butcher M, Keller SR, Goff M, Bruce A, Pei H, Sarembock IJ, Sanders JM, Nagelin MH, Srinivasan S, Kulkarni RN, Hedrick CC, Lattanzio FA, Dobrian AD, Nadler JL, Ley K. Accelerated atherosclerosis in Apoe−/− mice heterozygous for the insulin receptor and the insulin receptor substrate-1. Arterioscler Thromb Vasc Biol. 2012;32:247–256. doi: 10.1161/ATVBAHA.111.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerrity RG, Natarajan R, Nadler JL, Kimsey T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes. 2001;50:1654–1665. doi: 10.2337/diabetes.50.7.1654. [DOI] [PubMed] [Google Scholar]
- 50.McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN, Chait A, O’Brien KD. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem. 2007;55:1149–1157. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 52.Smolock AR, Mishra G, Eguchi K, Eguchi S, Scalia R. Protein kinase C upregulates intercellular adhesion molecule-1 and leukocyte-endothelium interactions in hyperglycemia via activation of endothelial expressed calpain. Arterioscler Thromb Vasc Biol. 2011;31:289–296. doi: 10.1161/ATVBAHA.110.217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vedantham S, Thiagarajan D, Ananthakrishnan R, Wang L, Rosario R, Zou YS, Goldberg I, Yan SF, Schmidt AM, Ramasamy R. Aldose reductase drives hyperacetylation of Egr-1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes. 2014;63:761–774. doi: 10.2337/db13-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 55.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan SF, Ramasamy R, Schmidt AM. Receptor for AGE (RAGE) and its ligands-cast into leading roles in diabetes and the inflammatory response. J Mol Med (Berl) 2009;87:235–247. doi: 10.1007/s00109-009-0439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng XL, Yuan SG, Peng DQ. Phenotype-specific inhibition of the vascular smooth muscle cell cycle by high glucose treatment. Diabetologia. 2007;50:881–890. doi: 10.1007/s00125-006-0543-6. [DOI] [PubMed] [Google Scholar]
- 58.Nishizawa T, Bornfeldt KE. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann Med. 2012;44:555–563. doi: 10.3109/07853890.2011.585346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 61.Hanley AJ, Williams K, Gonzalez C, D’Agostino RB, Jr, Wagenknecht LE, Stern MP, Haffner SM. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 62.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 63.Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Gemma L, Santi L, Bonadonna RC, Muggeo M. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med. 2004;21:52–58. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 64.Howard G, O’Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, Selby JV, Saad MF, Savage P, Bergman R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 65.Ferrannini E, Balkau B, Coppack SW, Dekker JM, Mari A, Nolan J, Walker M, Natali A, Beck-Nielsen H. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab. 2007;92:2885–2892. doi: 10.1210/jc.2007-0334. [DOI] [PubMed] [Google Scholar]
- 66.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 67.Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the atherosclerosis risk in communities study. Diabetes. 2002;51:3069–3076. doi: 10.2337/diabetes.51.10.3069. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:2013–2018. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 69.Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care. 2009;32:361–366. doi: 10.2337/dc08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 71.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277:1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 72.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16:1931–1942. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- 73.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 74.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007;28:463–491. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 75.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289:H813–H822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 76.Potenza MA, Addabbo F, Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab. 2009;297:E568–E577. doi: 10.1152/ajpendo.00297.2009. [DOI] [PubMed] [Google Scholar]
- 77.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes. 2003;52:2562–2569. doi: 10.2337/diabetes.52.10.2562. [DOI] [PubMed] [Google Scholar]
- 79.Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53:2735–2740. doi: 10.2337/diabetes.53.11.2735. [DOI] [PubMed] [Google Scholar]
- 80.Wang CC, Sharma G, Draznin B. Early growth response gene-1 expression in vascular smooth muscle cells effects of insulin and oxidant stress. Am J Hypertens. 2006;19:366–372. doi: 10.1016/j.amjhyper.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 81.Reusch JE, Draznin BB. Atherosclerosis in diabetes and insulin resistance. Diabetes Obes Metab. 2007;9:455–463. doi: 10.1111/j.1463-1326.2006.00620.x. [DOI] [PubMed] [Google Scholar]
- 82.Shulman GI. Cellular mechanisms of insulin resistance in humans. Am J Cardiol. 1999;84:3J–10J. doi: 10.1016/s0002-9149(99)00350-1. [DOI] [PubMed] [Google Scholar]
- 83.Cheatham B, Kahn CR. Insulin action and the insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 84.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 85.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zecchin HG, Bezerra RM, Carvalheira JB, Carvalho-Filho MA, Metze K, Franchini KG, Saad MJ. Insulin signalling pathways in aorta and muscle from two animal models of insulin resistance--the obese middle-aged and the spontaneously hypertensive rats. Diabetologia. 2003;46:479–491. doi: 10.1007/s00125-003-1073-0. [DOI] [PubMed] [Google Scholar]
- 88.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of Endothelial Constitutive Nitric Oxide Synthase Gene Expression in Endothelial Cells and In Vivo : A Specific Vascular Action of Insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 89.Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–196. doi: 10.1080/07853890701854702. [DOI] [PubMed] [Google Scholar]
- 90.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 93.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 95.Raines EW, Ross R. Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J. 1993;69:S30–S37. doi: 10.1136/hrt.69.1_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red EA, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li SL, Reddy MA, Cai Q, Meng L, Yuan H, Lanting L, Natarajan R. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55:2611–2619. doi: 10.2337/db06-0164. [DOI] [PubMed] [Google Scholar]
- 98.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–E174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1850–H1858. doi: 10.1152/ajpheart.01199.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doronzo G, Russo I, Mattiello L, Riganti C, Anfossi G, Trovati M. Insulin activates hypoxia-inducible factor-1alpha in human and rat vascular smooth muscle cells via phosphatidylinositol-3 kinase and mitogen-activated protein kinase pathways: impairment in insulin resistance owing to defects in insulin signalling. Diabetologia. 2006;49:1049–1063. doi: 10.1007/s00125-006-0156-0. [DOI] [PubMed] [Google Scholar]
- 102.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 103.UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 104.Bari 2D Study Group. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.American Diabetes Association. 8. Cardiovascular Disease and Risk Management. Diabetes Care. 2016;39(Suppl 1):S60–S71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 106.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP, Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Parhofer KG. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab J. 2015;39:353–362. doi: 10.4093/dmj.2015.39.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109:1112–1117. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 109.Rabbani N, Godfrey L, Xue M, Shaheen F, Geoffrion M, Milne R, Thornalley PJ. Glycation of LDL by methylglyoxal increases arterial atherogenicity: a possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes. 2011;60:1973–1980. doi: 10.2337/db11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J. 1985;110:1100–1107. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 111.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 112.Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63:1469–1479. doi: 10.1016/j.metabol.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 113.Lorenzo C, Hartnett S, Hanley AJ, Rewers MJ, Wagenknecht LE, Karter AJ, Haffner SM. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2013;98:1622–1630. doi: 10.1210/jc.2012-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clin Chim Acta. 2006;368:1–19. doi: 10.1016/j.cca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 115.Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50:315–321. doi: 10.2337/diabetes.50.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rachek LI. Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci. 2014;121:267–292. doi: 10.1016/B978-0-12-800101-1.00008-9. [DOI] [PubMed] [Google Scholar]
- 117.Oliver MF. Fatty acids and the risk of death during acute myocardial ischaemia. Clin Sci (Lond) 2015;128:349–355. doi: 10.1042/CS20140404. [DOI] [PubMed] [Google Scholar]
- 118.Xie W, Zhai Z, Yang Y, Kuang T, Wang C. Free fatty acids inhibit TM-EPCR expression through JNK pathway: an implication for the development of the prothrombotic state in metabolic syndrome. J Thromb Thrombolysis. 2012;34:468–474. doi: 10.1007/s11239-012-0793-8. [DOI] [PubMed] [Google Scholar]
- 119.Barter PJ, Rye KA, Tardif JC, Waters DD, Boekholdt SM, Breazna A, Kastelein JJ. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation. 2011;124:555–562. doi: 10.1161/CIRCULATIONAHA.111.018259. [DOI] [PubMed] [Google Scholar]
- 120.Drew BG, Duffy SJ, Formosa MF, Natoli AK, Henstridge DC, Penfold SA, Thomas WG, Mukhamedova N, de Courten B, Forbes JM, Yap FY, Kaye DM, van Hall G, Febbraio MA, Kemp BE, Sviridov D, Steinberg GR, Kingwell BA. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 121.Drew BG, Rye KA, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8:237–245. doi: 10.1038/nrendo.2011.235. [DOI] [PubMed] [Google Scholar]
- 122.Mortensen SP, Boushel R. High-density lipoprotein: a new therapeutic target for glucose intolerance? Circulation. 2013;128:2349–2350. doi: 10.1161/CIRCULATIONAHA.113.006345. [DOI] [PubMed] [Google Scholar]
- 123.Apro J, Tietge UJ, Dikkers A, Parini P, Angelin B, Rudling M. Impaired Cholesterol Efflux Capacity of High-Density Lipoprotein Isolated From Interstitial Fluid in Type 2 Diabetes Mellitus. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.307385. pii: ATVBAHA.116.307385. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 125.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 126.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 127.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 128.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 129.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 130.Rana JS, Boekholdt SM, Ridker PM, Jukema JW, Luben R, Bingham SA, Day NE, Wareham NJ, Kastelein JJ, Khaw KT. Differential leucocyte count and the risk of future coronary artery disease in healthy men and women: the EPIC-Norfolk Prospective Population Study. J Intern Med. 2007;262:678–689. doi: 10.1111/j.1365-2796.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 131.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 133.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Satoh M, Tabuchi T, Itoh T, Nakamura M. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin Sci (Lond) 2014;126:233–241. doi: 10.1042/CS20130043. [DOI] [PubMed] [Google Scholar]
- 135.Cuaz-Perolin C, Billiet L, Bauge E, Copin C, Scott-Algara D, Genze F, Buchele B, Syrovets T, Simmet T, Rouis M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:272–277. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- 136.Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2014;114:32–40. doi: 10.1161/CIRCRESAHA.113.302089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ye X, Jiang X, Guo W, Clark K, Gao Z. Overexpression of NF-kappaB p65 in macrophages ameliorates atherosclerosis in apoE-knockout mice. Am J Physiol Endocrinol Metab. 2013;305:E1375–E1383. doi: 10.1152/ajpendo.00307.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 141.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 142.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 143.Moreira DM, Lueneberg ME, da Silva RL, Fattah T, Mascia Gottschall CA. Rationale and design of the TETHYS trial: the effects of methotrexate therapy on myocardial infarction with ST-segment elevation. Cardiology. 2013;126:167–170. doi: 10.1159/000351972. [DOI] [PubMed] [Google Scholar]
- 144.Erhayiem B, Pavitt S, Baxter P, Andrews J, Greenwood JP, Buch MH, Plein S. Coronary Artery Disease Evaluation in Rheumatoid Arthritis (CADERA): study protocol for a randomized controlled trial. Trials. 2014;15:436. doi: 10.1186/1745-6215-15-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Micha R, Imamura F, Wyler von BM, Solomon DH, Hernan MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116:531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Di ME, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond) 2015;129:199–216. doi: 10.1042/CS20150093. [DOI] [PubMed] [Google Scholar]
- 149.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 150.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 151.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 152.Ceriello A, Esposito K, Piconi L, Ihnat M, Thorpe J, Testa R, Bonfigli AR, Giugliano D. Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res Clin Pract. 2008;82:262–267. doi: 10.1016/j.diabres.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 153.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140–146. doi: 10.1016/j.numecd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 154.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 155.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20:295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kobayashi T, Nogami T, Taguchi K, Matsumoto T, Kamata K. Diabetic state, high plasma insulin and angiotensin II combine to augment endothelin-1-induced vasoconstriction via ETA receptors and ERK. Br J Pharmacol. 2008;155:974–983. doi: 10.1038/bjp.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sarafidis PA, Bakris GL. Insulin and Endothelin: An Interplay Contributing to Hypertension Development? J Clin Endocrinol Metab. 2007;92:379–385. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 158.Montero D, Walther G, Perez-Martin A, Vicente-Salar N, Roche E, Vinet A. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia. 2013;56:2122–2133. doi: 10.1007/s00125-013-2974-1. [DOI] [PubMed] [Google Scholar]
- 159.Duncan ER, Walker SJ, Ezzat VA, Wheatcroft SB, Li JM, Shah AM, Kearney MT. Accelerated endothelial dysfunction in mild prediabetic insulin resistance: the early role of reactive oxygen species. Am J Physiol Endocrinol Metab. 2007;293:E1311–E1319. doi: 10.1152/ajpendo.00299.2007. [DOI] [PubMed] [Google Scholar]
- 160.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D’Agostino R, Liau CS, Mas JL, Rother J, Smith SC, Jr, Salette G, Contant CF, Massaro JM, Steg PG. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 161.Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55:202–208. [PubMed] [Google Scholar]
- 162.Osende JI, Badimon JJ, Fuster V, Herson P, Rabito P, Vidhun R, Zaman A, Rodriguez OJ, Lev EI, Rauch U, Heflt G, Fallon JT, Crandall JP. Blood thrombogenicity in type 2 diabetes mellitus patients is associated with glycemic control. J Am Coll Cardiol. 2001;38:1307–1312. doi: 10.1016/s0735-1097(01)01555-8. [DOI] [PubMed] [Google Scholar]
- 163.Kyrgios I, Maggana I, Giza S, Stergidou D, Mouzaki K, Kotanidou EP, Papadakis E, Galli-Tsinopoulou A. Suboptimal glycaemic control enhances the risk of impaired prothrombotic state in youths with type 1 diabetes mellitus. Diab Vasc Dis Res. 2014;11:208–216. doi: 10.1177/1479164114528821. [DOI] [PubMed] [Google Scholar]
- 164.Kim HK, Kim JE, Park SH, Kim YI, Nam-Goong IS, Kim ES. High coagulation factor levels and low protein C levels contribute to enhanced thrombin generation in patients with diabetes who do not have macrovascular complications. J Diabetes Complications. 2014;28:365–369. doi: 10.1016/j.jdiacomp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 165.Cavender MA, Scirica BM, Bonaca MP, Angiolillo DJ, Dalby AJ, Dellborg M, Morais J, Murphy SA, Ophuis TO, Tendera M, Braunwald E, Morrow DA. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation. 2015;131:1047–1053. doi: 10.1161/CIRCULATIONAHA.114.013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 167.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 168.Elkeles RS, Godsland IF, Feher MD, Rubens MB, Roughton M, Nugara F, Humphries SE, Richmond W, Flather MD. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29:2244–2251. doi: 10.1093/eurheartj/ehn279. [DOI] [PubMed] [Google Scholar]
- 169.Hadamitzky M, Hein F, Meyer T, Bischoff B, Martinoff S, Schomig A, Hausleiter J. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care. 2010;33:1358–1363. doi: 10.2337/dc09-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hoff JA, Quinn L, Sevrukov A, Lipton RB, Daviglus M, Garside DB, Ajmere NK, Gandhi S, Kondos GT. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol. 2003;41:1008–1012. doi: 10.1016/s0735-1097(02)02975-3. [DOI] [PubMed] [Google Scholar]
- 171.Huang CL, Wu IH, Wu YW, Hwang JJ, Wang SS, Chen WJ, Lee WJ, Yang WS. Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS One. 2014;9:e90201. doi: 10.1371/journal.pone.0090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang Z, Jiang Y, Liu N, Ren L, Zhu Y, An Y, Chen D. Advanced glycation end-product Nepsilon-carboxymethyl-Lysine accelerates progression of atherosclerotic calcification in diabetes. Atherosclerosis. 2012;221:387–396. doi: 10.1016/j.atherosclerosis.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 173.Pugliese G, Iacobini C, Blasetti FC, Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238:220–230. doi: 10.1016/j.atherosclerosis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 174.Maser RE, Lenhard MJ, Sneider MB, Pohlig RT. Osteoprotegerin is a Better Serum Biomarker of Coronary Artery Calcification than Osteocalcin in Type 2 Diabetes. Endocr Pract. 2015;21:14–22. doi: 10.4158/EP14229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Al-Aly Z. Arterial calcification: a tumor necrosis factor-alpha mediated vascular Wnt-opathy. Transl Res. 2008;151:233–239. doi: 10.1016/j.trsl.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 176.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 177.Bittner V, Bertolet M, Barraza FR, Farkouh ME, Goldberg S, Ramanathan KB, Redmon JB, Sperling L, Rutter MK. Comprehensive Cardiovascular Risk Factor Control Improves Survival: The BARI 2D Trial. J Am Coll Cardiol. 2015;66:765–773. doi: 10.1016/j.jacc.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of Goals in U.S. Diabetes Care, 1999–2010. N Engl J Med. 2013;368:1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 179.Yilmaz A, Reiss C, Weng A, Cicha I, Stumpf C, Steinkasserer A, Daniel WG, Garlichs CD. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79:529–538. doi: 10.1189/jlb.0205064. [DOI] [PubMed] [Google Scholar]
- 180.Moutzouri E, Tellis CC, Rousouli K, Liberopoulos EN, Milionis HJ, Elisaf MS, Tselepis AD. Effect of simvastatin or its combination with ezetimibe on Toll-like receptor expression and lipopolysaccharide - induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis. 2012;225:381–387. doi: 10.1016/j.atherosclerosis.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 181.Kinlay S, Schwartz GG, Olsson AG, Rifai N, Sasiela WJ, Szarek M, Ganz P, Libby P. Effect of atorvastatin on risk of recurrent cardiovascular events after an acute coronary syndrome associated with high soluble CD40 ligand in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study. Circulation. 2004;110:386–391. doi: 10.1161/01.CIR.0000136588.62638.5E. [DOI] [PubMed] [Google Scholar]
- 182.Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, Nicolosi GL, Porcu M, Cosmi F, Stefanelli S, Tognoni G, Tavazzi L. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009;30:2327–2336. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 183.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 185.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, Waters D. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 186.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 187.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924–1929. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 189.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 190.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 192.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d’Emden M, Whiting M, Ehnholm C, Laakso M. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 193.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64(Suppl 2):43–60. doi: 10.2165/00003495-200464002-00005. [DOI] [PubMed] [Google Scholar]
- 194.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 195.Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–362. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- 196.Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, Wactawski-Wende J, Manson JE, Qiao Y, Liu S, Merriam PA, Rahilly-Tierny C, Thomas F, Berger JS, Ockene JK, Curb JD, Ma Y. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172:144–152. doi: 10.1001/archinternmed.2011.625. [DOI] [PubMed] [Google Scholar]
- 197.Centers for Disease Control and Prevention. Crude and Age-Adjusted Percentage of Adults Aged 18 Years or Older with Diagnosed Diabetes Reporting Visual Impairment, United States, 1997–2011. Sep 21, 2012. 2-4-2016. Ref Type: Serial (Book, Monograph) [Google Scholar]
- 198.Centers for Disease Control and Prevention. Crude and Age-Adjusted Incidence of End-Stage Renal Disease Related to Diabetes Mellitus (ESRD-DM) per 100,000 Diabetic Population, United States, 1980–2008. Oct 15, 2014. 2-4-2016. Ref Type: Online Source. [Google Scholar]
- 199.UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 200.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kostapanos MS, Liamis GL, Milionis HJ, Elisaf MS. Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol. 2010;8:612–631. doi: 10.2174/157016110792006879. [DOI] [PubMed] [Google Scholar]
- 202.Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49:1881–1892. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 203.Thongtang N, Ai M, Otokozawa S, Himbergen TV, Asztalos BF, Nakajima K, Stein E, Jones PH, Schaefer EJ. Effects of maximal atorvastatin and rosuvastatin treatment on markers of glucose homeostasis and inflammation. Am J Cardiol. 2011;107:387–392. doi: 10.1016/j.amjcard.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sattar NA, Ginsberg H, Ray K, Chapman MJ, Arca M, Averna M, Betteridge DJ, Bhatnagar D, Bilianou E, Carmena R, Ceska R, Corsini A, Erbel R, Flynn PD, Garcia-Moll X, Gumprecht J, Ishibashi S, Jambart S, Kastelein JJ, Maher V, da Silva PM, Masana L, Odawara M, Pedersen TR, Rotella CM, Salti I, Teramoto T, Tokgozoglu L, Toth PP, Valensi P, Verges B. The use of statins in people at risk of developing diabetes mellitus: evidence and guidance for clinical practice. Atheroscler Suppl. 2014;15:1–15. doi: 10.1016/j.atherosclerosissup.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 205.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.American Diabetes Association. 7. Approaches to Glycemic Treatment. Diabetes Care. 2016;39(Suppl 1):S52–S59. doi: 10.2337/dc16-S010. [DOI] [PubMed] [Google Scholar]
- 207.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 208.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 209.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De LP, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 210.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bezafibrate Infarction Prevention (BIP) study. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 213.Goldfine AB, Kaul S, Hiatt WR. Fibrates in the treatment of dyslipidemias--time for a reassessment. N Engl J Med. 2011;365:481–484. doi: 10.1056/NEJMp1106688. [DOI] [PubMed] [Google Scholar]
- 214.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 215.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 216.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 217.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 218.Khera AV, Cuchel M, Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.American Diabetes Association. 8. Cardiovascular Disease and Risk Management. Diabetes Care. 2016;39(Suppl 1):S60–S71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 220.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J, Inzucchi SE, Kosiborod M, Nelson RG, Patel MJ, Pignone M, Quinn L, Schauer PR, Selvin E, Vafiadis DK. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Circulation. 2015;132:691–718. doi: 10.1161/CIR.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 222.Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, Grefhorst A, Staels B, Krempf M. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:6211–6218. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 223.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–2842. doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 224.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 225.Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007;4:214–225. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- 226.Naoumova RP, Tosi I, Patel D, Neuwirth C, Horswell SD, Marais AD, van HC, Soutar AK. Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: long-term follow-up and treatment response. Arterioscler Thromb Vasc Biol. 2005;25:2654–2660. doi: 10.1161/01.ATV.0000190668.94752.ab. [DOI] [PubMed] [Google Scholar]
- 227.Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51:2714–2721. doi: 10.1194/jlr.M008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 229.Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S. Alirocumab as Add-On to Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized Trial. J Clin Endocrinol Metab. 2015;100:3140–3148. doi: 10.1210/jc.2015-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J. 2015;169:906–915. doi: 10.1016/j.ahj.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 231.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El SM, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 232.Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara-Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 233.Roth EM, McKenney JM. ODYSSEY MONO: effect of alirocumab 75 mg subcutaneously every 2 weeks as monotherapy versus ezetimibe over 24 weeks. Future Cardiol. 2015;11:27–37. doi: 10.2217/fca.14.82. [DOI] [PubMed] [Google Scholar]
- 234.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 235.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Mahaffey KW, Moryusef A, Pordy R, Roe MT, Rorick T, Sasiela WJ, Shirodaria C, Szarek M, Tamby JF, Tricoci P, White H, Zeiher A, Steg PG. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168:682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 236.Moriarty PM, Jacobson TA, Bruckert E, Thompson PD, Guyton JR, Baccara-Dinet MT, Gipe D. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 2014;8:554–561. doi: 10.1016/j.jacl.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 237.Colhoun HM, Robinson JG, Farnier M, Cariou B, Blom D, Kereiakes DJ, Lorenzato C, Pordy R, Chaudhari U. Efficacy and safety of alirocumab, a fully human PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC Cardiovasc Disord. 2014;14:121. doi: 10.1186/1471-2261-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Robinson JG, Colhoun HM, Bays HE, Jones PH, Du Y, Hanotin C, Donahue S. Efficacy and safety of alirocumab as add-on therapy in high-cardiovascular-risk patients with hypercholesterolemia not adequately controlled with atorvastatin (20 or 40 mg) or rosuvastatin (10 or 20 mg): design and rationale of the ODYSSEY OPTIONS Studies. Clin Cardiol. 2014;37:597–604. doi: 10.1002/clc.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kastelein JJ, Robinson JG, Farnier M, Krempf M, Langslet G, Lorenzato C, Gipe DA, Baccara-Dinet MT. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia not adequately controlled with current lipid-lowering therapy: design and rationale of the ODYSSEY FH studies. Cardiovasc Drugs Ther. 2014;28:281–289. doi: 10.1007/s10557-014-6523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 241.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 242.Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 243.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 244.Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 245.Keenan NL, Rosendorf KA. Prevalence of hypertension and controlled hypertension - United States, 2005–2008. MMWR Surveill Summ. 2011;60(Suppl):94–97. [PubMed] [Google Scholar]
- 246.Authors/Task Force M; Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JLE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG S.C. Committee for Practice Guidelines. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 247.Wang CC, Reusch JE. Diabetes and cardiovascular disease: changing the focus from glycemic control to improving long-term survival. Am J Cardiol. 2012;110:58B–68B. doi: 10.1016/j.amjcard.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 249.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 250.Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–652. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 251.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- 252.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de FU, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 253.Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Parikh CR, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–549. doi: 10.7326/0003-4819-138-7-200304010-00010. [DOI] [PubMed] [Google Scholar]
- 254.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 255.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 256.Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, Cooper ME, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Lisheng L, Mancia G, Marre M, Matthews DR, Mogensen CE, Perkovic V, Poulter N, Rodgers A, Williams B, MacMahon S, Patel A, Woodward M. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 257.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Perkovic V, Rodgers A. Redefining Blood-Pressure Targets--SPRINT Starts the Marathon. N Engl J Med. 2015;373:2175–2178. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 260.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 261.Estacio RO, Coll JR, Tran ZV, Schrier RW. Effect of intensive blood pressure control with valsartan on urinary albumin excretion in normotensive patients with type 2 diabetes. Am J Hypertens. 2006;19:1241–1248. doi: 10.1016/j.amjhyper.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 262.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 263.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl 2):B54–B64. [PubMed] [Google Scholar]
- 264.McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, Tonelli M, Leiter LA, Klarenbach SW, Manns BJ. Intensive and Standard Blood Pressure Targets in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis. Arch Intern Med. 2012;172:1296–1303. doi: 10.1001/archinternmed.2012.3147. [DOI] [PubMed] [Google Scholar]
- 265.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 267.Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy--I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 268.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Antiplatelet Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Perk J, De BG, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 271.ETDRS Investigators. Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. ETDRS Investigators. JAMA. 1992;268:1292–1300. doi: 10.1001/jama.1992.03490100090033. [DOI] [PubMed] [Google Scholar]
- 272.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O’Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 274.Soejima H, Ogawa H, Morimoto T, Nakayama M, Okada S, Uemura S, Kanauchi M, Doi N, Sakuma M, Jinnouchi H, Sugiyama S, Waki M, Saito Y. Aspirin reduces cerebrovascular events in type 2 diabetic patients with poorly controlled blood pressure. Subanalysis from the JPAD trial. Circ J. 2012;76:1526–1532. doi: 10.1253/circj.cj-11-1033. [DOI] [PubMed] [Google Scholar]
- 275.Soejima H, Ogawa H, Morimoto T, Nakayama M, Okada S, Sakuma M, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Waki M, Saito Y. Aspirin possibly reduces cerebrovascular events in type 2 diabetic patients with higher C-reactive protein level: subanalysis from the JPAD trial. J Cardiol. 2013;62:165–170. doi: 10.1016/j.jjcc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 276.De BG, Sacco M, Strippoli GF, Pellegrini F, Graziano G, Tognoni G, Nicolucci A. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ. 2009;339:b4531. doi: 10.1136/bmj.b4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, Wang K, Zou Y, Ge J. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2010;87:211–218. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 278.Pignone M, Alberts MJ, Colwell JA, Cushman M, Inzucchi SE, Mukherjee D, Rosenson RS, Williams CD, Wilson PW, Kirkman MS. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395–1402. doi: 10.2337/dc10-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bethel MA, Harrison P, Sourij H, Sun Y, Tucker L, Kennedy I, White S, Hill L, Oulhaj A, Coleman RL, Holman RR. Randomized controlled trial comparing impact on platelet reactivity of twice-daily with once-daily aspirin in people with Type 2 diabetes. Diabet Med. 2015;33:224–230. doi: 10.1111/dme.12828. [DOI] [PubMed] [Google Scholar]
- 280.Dillinger JG, Drissa A, Sideris G, Bal dit SC, Voicu S, Manzo SS, Logeart D, Drouet L, Henry P. Biological efficacy of twice daily aspirin in type 2 diabetic patients with coronary artery disease. Am Heart J. 2012;164:600–606. doi: 10.1016/j.ahj.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 281.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR, Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver LP, Rinaldi MJ, Massaro JM. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, Yancy WS., Jr Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–3842. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston MN, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 284.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 285.Wing RR. Implications of Look AHEAD for clinical trials and clinical practice. Diabetes Obes Metab. 2014;16:1183–1191. doi: 10.1111/dom.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg. 2011;21:351–355. doi: 10.1007/s11695-010-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Esposito K, Maiorino MI, Ciotola M, Di PC, Scognamiglio P, Gicchino M, Petrizzo M, Saccomanno F, Beneduce F, Ceriello A, Giugliano D. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151:306–314. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 288.Larsen RN, Mann NJ, Maclean E, Shaw JE. The effect of high-protein, low-carbohydrate diets in the treatment of type 2 diabetes: a 12 month randomised controlled trial. Diabetologia. 2011;54:731–740. doi: 10.1007/s00125-010-2027-y. [DOI] [PubMed] [Google Scholar]
- 289.Li TY, Brennan AM, Wedick NM, Mantzoros C, Rifai N, Hu FB. Regular consumption of nuts is associated with a lower risk of cardiovascular disease in women with type 2 diabetes. J Nutr. 2009;139:1333–1338. doi: 10.3945/jn.108.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Brehm BJ, Lattin BL, Summer SS, Boback JA, Gilchrist GM, Jandacek RJ, D’Alessio DA. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care. 2009;32:215–220. doi: 10.2337/dc08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 293.Raynor HA, Jeffery RW, Ruggiero AM, Clark JM, Delahanty LM. Weight loss strategies associated with BMI in overweight adults with type 2 diabetes at entry into the Look AHEAD (Action for Health in Diabetes) trial. Diabetes Care. 2008;31:1299–1304. doi: 10.2337/dc07-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bradley U, Spence M, Courtney CH, McKinley MC, Ennis CN, McCance DR, McEneny J, Bell PM, Young IS, Hunter SJ. Low-fat versus low-carbohydrate weight reduction diets: effects on weight loss, insulin resistance, and cardiovascular risk: a randomized control trial. Diabetes. 2009;58:2741–2748. doi: 10.2337/db09-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER., III The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care. 2013;36:1132–1137. doi: 10.2337/dc12-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gumbiner B, Low CC, Reaven PD. Effects of a monounsaturated fatty acid-enriched hypocaloric diet on cardiovascular risk factors in obese patients with type 2 diabetes. Diabetes Care. 1998;21:9–15. doi: 10.2337/diacare.21.1.9. [DOI] [PubMed] [Google Scholar]
- 297.McLaughlin T, Carter S, Lamendola C, Abbasi F, Yee G, Schaaf P, Basina M, Reaven G. Effects of moderate variations in macronutrient composition on weight loss and reduction in cardiovascular disease risk in obese, insulin-resistant adults. Am J Clin Nutr. 2006;84:813–821. doi: 10.1093/ajcn/84.4.813. [DOI] [PubMed] [Google Scholar]
- 298.Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, Yancy WS, Jr, Brinkworth GD. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr. 2015;102:780–790. doi: 10.3945/ajcn.115.112581. [DOI] [PubMed] [Google Scholar]
- 299.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 300.Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons-Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, Karanja N. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 301.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 302.Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men with Type 2 diabetes/impaired glucose tolerance: evidence from the Whitehall study. Diabet Med. 2002;19:580–588. doi: 10.1046/j.1464-5491.2002.00748.x. [DOI] [PubMed] [Google Scholar]
- 303.Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, Willett WC, Manson JE. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 304.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107:2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 305.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005;28:799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 306.Sundquist K, Qvist J, Sundquist J, Johansson SE. Frequent and occasional physical activity in the elderly: a 12-year follow-up study of mortality. Am J Prev Med. 2004;27:22–27. doi: 10.1016/j.amepre.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 307.Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999;22:1640–1646. doi: 10.2337/diacare.22.10.1640. [DOI] [PubMed] [Google Scholar]
- 308.Jakicic JM, Egan CM, Fabricatore AN, Gaussoin SA, Glasser SP, Hesson LA, Knowler WC, Lang W, Regensteiner JG, Ribisl PM, Ryan DH. Four-year change in cardiorespiratory fitness and influence on glycemic control in adults with type 2 diabetes in a randomized trial: the Look AHEAD Trial. Diabetes Care. 2013;36:1297–1303. doi: 10.2337/dc12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:801–809. doi: 10.1016/S2213-8587(14)70156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE, Pi-Sunyer FX, Wing RR, Bertoni AG. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–2496. doi: 10.1001/jama.2012.67929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Rubin RR, Wadden TA, Bahnson JL, Blackburn GL, Brancati FL, Bray GA, Coday M, Crow SJ, Curtis JM, Dutton G, Egan C, Evans M, Ewing L, Faulconbridge L, Foreyt J, Gaussoin SA, Gregg EW, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Knowler WC, Lang W, Lewis CE, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Rejeski WJ, Rosenthal RH, Ruelas V, Toledo K, Van DB, Vitolins M, Williamson D, Wing RR, Yanovski SZ, Zhang P. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37:1544–1553. doi: 10.2337/dc13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rubin RR, Peyrot M, Gaussoin SA, Espeland MA, Williamson D, Faulconbridge LF, Wadden TA, Ewing L, Safford M, Evans-Hudnall G, Wing RR, Knowler WC. Four-year analysis of cardiovascular disease risk factors, depression symptoms, and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetes Care. 2013;36:1088–1094. doi: 10.2337/dc12-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Rubin RR, Gaussoin SA, Peyrot M, DiLillo V, Miller K, Wadden TA, West DS, Wing RR, Knowler WC. Cardiovascular disease risk factors, depression symptoms and antidepressant medicine use in the Look AHEAD (Action for Health in Diabetes) clinical trial of weight loss in diabetes. Diabetologia. 2010;53:1581–1589. doi: 10.1007/s00125-010-1765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Knowler WC, Pettitt DJ, Saad MF, Bennett PH. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev. 1990;6:1–27. doi: 10.1002/dmr.5610060101. [DOI] [PubMed] [Google Scholar]
- 317.Leahy JL. Natural history of beta-cell dysfunction in NIDDM. Diabetes Care. 1990;13:992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- 318.Schultz TA, Lewis SB, Davis JL, Rost CR, Bliziotes MM. Effect of sulfonylurea therapy and plasma glucose levels on hemoglobin A1c in type II diabetes mellitus. Am J Med. 1981;70:373–378. doi: 10.1016/0002-9343(81)90774-9. [DOI] [PubMed] [Google Scholar]
- 319.Centers for Disease Control and Prevention. Age-Adjusted Percentage of Adults with Diabetes Using Diabetes Medication, by Type of Medication, United States, 1997–2011. Nov 20, 2012. 2-4-2016. Ref Type: Online Source. [Google Scholar]
- 320.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 321.Freed MI, Ratner R, Marcovina SM, Kreider MM, Biswas N, Cohen BR, Brunzell JD. Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abnormalities in type 2 diabetes mellitus. Am J Cardiol. 2002;90:947–952. doi: 10.1016/s0002-9149(02)02659-0. [DOI] [PubMed] [Google Scholar]
- 322.Del PS, Pulizzi N. The place of sulfonylureas in the therapy for type 2 diabetes mellitus. Metabolism. 2006;55:S20–S27. doi: 10.1016/j.metabol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 323.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 324.van Wijk JP, de Koning EJ, Martens EP, Rabelink TJ. Thiazolidinediones and blood lipids in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2003;23:1744–1749. doi: 10.1161/01.ATV.0000090521.25968.4D. [DOI] [PubMed] [Google Scholar]
- 325.Schernthaner G, Chilton RJ. Cardiovascular risk and thiazolidinediones--what do meta-analyses really tell us? Diabetes Obes Metab. 2010;12:1023–1035. doi: 10.1111/j.1463-1326.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- 326.Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013;98:668–677. doi: 10.1210/jc.2012-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Eurich DT, Simpson S, Senthilselvan A, Asche CV, Sandhu-Minhas JK, McAlister FA. Comparative safety and effectiveness of sitagliptin in patients with type 2 diabetes: retrospective population based cohort study. BMJ. 2013;346:f2267. doi: 10.1136/bmj.f2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Holden SE, Currie CJ. Mortality risk with sulphonylureas compared to metformin. Diabetes Obes Metab. 2014;16:885–890. doi: 10.1111/dom.12280. [DOI] [PubMed] [Google Scholar]
- 329.Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 330.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med. 2008;359:1618–1620. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 332.Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep. 2010;12:432–439. doi: 10.1007/s11883-010-0128-1. [DOI] [PubMed] [Google Scholar]
- 333.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Advance Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 335.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD the VADTI. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 336.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 337.Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2009;19:604–612. doi: 10.1016/j.numecd.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 338.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- 339.Genuth S, Ismail-Beigi F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab. 2012;97:41–48. doi: 10.1210/jc.2011-1679. [DOI] [PubMed] [Google Scholar]
- 340.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Calles-Escandon J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA, Failor A, Hamilton B. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:721–727. doi: 10.2337/dc09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O’Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 345.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 346.Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care. 2011;34:77–83. doi: 10.2337/dc10-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Origin Trial Investigators. Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 348.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 349.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–B29. [PubMed] [Google Scholar]
- 350.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57:995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 351.Bibra H, Siegmund T, Ceriello A, Volozhyna M, Schumm-Draeger PM. Optimized postprandial glucose control is associated with improved cardiac/vascular function - comparison of three insulin regimens in well-controlled type 2 diabetes. Horm Metab Res. 2009;41:109–115. doi: 10.1055/s-0028-1112136. [DOI] [PubMed] [Google Scholar]
- 352.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707–1708. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 353.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303:2291–2292. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 354.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 355.Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150:94–103. doi: 10.7326/0003-4819-150-2-200901200-00007. [DOI] [PubMed] [Google Scholar]
- 356.Puzziferri N, Roshek TB, III, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 358.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 359.Romeo S, Maglio C, Burza MA, Pirazzi C, Sjoholm K, Jacobson P, Svensson PA, Peltonen M, Sjostrom L, Carlsson LM. Cardiovascular events after bariatric surgery in obese subjects with type 2 diabetes. Diabetes Care. 2012;35:2613–2617. doi: 10.2337/dc12-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van SL, Yancy WS, Jr, Eid G, Weidenbacher H, Maciejewski ML. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 361.Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, Toledo FG, Jakicic JM. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149:707–715. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mingrone G, Panunzi S, De GA, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 363.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ding SA, Simonson DC, Wewalka M, Halperin F, Foster K, Goebel-Fabbri A, Hamdy O, Clancy K, Lautz D, Vernon A, Goldfine AB. Adjustable Gastric Band Surgery or Medical Management in Patients With Type 2 Diabetes: A Randomized Clinical Trial. J Clin Endocrinol Metab. 2015;100:2546–2556. doi: 10.1210/jc.2015-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, Hamdy O, Abrahamson M, Clancy K, Foster K, Lautz D, Vernon A, Goldfine AB. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149:716–726. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 367.Arterburn D, Schauer DP, Wise RE, Gersin KS, Fischer DR, Selwyn CA, Jr, Erisman A, Tsevat J. Change in predicted 10-year cardiovascular risk following laparoscopic Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19:184–189. doi: 10.1007/s11695-008-9534-7. [DOI] [PubMed] [Google Scholar]
- 368.Rubino F, Kaplan LM, Schauer PR, Cummings DE. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405. doi: 10.1097/SLA.0b013e3181be34e7. [DOI] [PubMed] [Google Scholar]
- 369.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 370.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 371.Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs--insights from the rosiglitazone experience. N Engl J Med. 2013;369:1285–1287. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 372.Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36:2288–2296. doi: 10.1093/eurheartj/ehv239. [DOI] [PubMed] [Google Scholar]
- 373.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE Empa-Reg Outcome Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 374.Novo Nordisk. Victoza significantly reduces the risk of major adverse cardiovascular events in the LEADER trial. Mar 4, 2016. 4-7-2016. Ref Type: Online Source. [Google Scholar]
- 375.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 376.Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-Glucose Cotransporter Inhibitors: Effects on Renal and Intestinal Glucose Transport: From Bench to Bedside. Diabetes Care. 2015;38:2344–2353. doi: 10.2337/dc15-0642. [DOI] [PubMed] [Google Scholar]
- 377.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von EM. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 378.Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2015;309:F889–F900. doi: 10.1152/ajprenal.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, Espadero RM, Woerle HJ, Broedl UC, Johansen OE. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014;306:F188–F193. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc. 2012;1:e002279. doi: 10.1161/JAHA.112.002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 383.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 384.Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, Pocock S, Steinberg WM, Bergenstal RM, Mann JF, Ravn LS, Frandsen KB, Moses AC, Buse JB. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823–830. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 385.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 386.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 387.U.S. Food and Drug Administration. [Accessed 4-25-2016];FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2016 Apr 8; http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm.
- 388.Norgaard K, Jensen T, Skott P, Thorsteinsson B, Bruun NE, Giese J, Feldt-Rasmussen B. Effects of insulin on renal haemodynamics and sodium handling in normal subjects. Scand J Clin Lab Invest. 1991;51:367–376. doi: 10.1080/00365519109091628. [DOI] [PubMed] [Google Scholar]
- 389.Norgaard K, Feldt-Rasmussen B. Sodium retention and insulin treatment in insulin-dependent diabetes mellitus. Acta Diabetol. 1994;31:19–25. doi: 10.1007/BF00580755. [DOI] [PubMed] [Google Scholar]
- 390.Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 1997;92:51–58. doi: 10.1042/cs0920051. [DOI] [PubMed] [Google Scholar]
- 391.ACCORD Study Group Writing Committee. 9-Year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016 doi: 10.2337/dc15-2283. pii: dc152283. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, Sharp SJ, Simmons RK, van den Donk M, Wareham NJ, Lauritzen T. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378:156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenstrom A. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 394.Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32:381–386. doi: 10.2337/dc08-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 396.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383:2008–2017. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 397.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 398.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 399.Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, Goff D, Leenen F, Mohiuddin S, Papademetriou V, Proschan M, Ellsworth A, Golden J, Colon P, Crow R Antihypertensive, Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research G. Role of diuretics in the prevention of heart failure: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation. 2006;113:2201–2210. doi: 10.1161/CIRCULATIONAHA.105.544031. [DOI] [PubMed] [Google Scholar]
- 400.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 401.Carr AA, Kowey PR, Devereux RB, Brenner BM, Dahlof B, Ibsen H, Lindholm LH, Lyle PA, Snapinn SM, Zhang Z, Edelman JM, Shahinfar S. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. Am J Cardiol. 2005;96:1530–1536. doi: 10.1016/j.amjcard.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 402.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 403.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24:1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 404.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Erqou S, Lee CT, Suffoletto M, Echouffo-Tcheugui JB, de Boer RA, van Melle JP, Adler AI. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. European journal of heart failure. 2013;15:185–193. doi: 10.1093/eurjhf/hfs156. [DOI] [PubMed] [Google Scholar]
- 406.Barzilay JI, Kronmal RA, Gottdiener JS, Smith NL, Burke GL, Tracy R, Savage PJ, Carlson M. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2236–2241. doi: 10.1016/j.jacc.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 407.Iribarren C, Karter AJ, Go AS, Ferrara A, Liu JY, Sidney S, Selby JV. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–2673. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 408.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Guglin M, Villafranca A, Morrison A. Cardiogenic diabetes. Heart Fail Rev. 2014;19:595–602. doi: 10.1007/s10741-013-9412-9. [DOI] [PubMed] [Google Scholar]
- 410.Guglin M, Lynch K, Krischer J. Heart failure as a risk factor for diabetes mellitus. Cardiology. 2014;129:84–92. doi: 10.1159/000363282. [DOI] [PubMed] [Google Scholar]
- 411.Sarma S, Mentz RJ, Kwasny MJ, Fought AJ, Huffman M, Subacius H, Nodari S, Konstam M, Swedberg K, Maggioni AP, Zannad F, Bonow RO, Gheorghiade M Everest investigators. Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. European journal of heart failure. 2013;15:194–202. doi: 10.1093/eurjhf/hfs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, Gheorghiade M, O’Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;154:e1–e8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 413.Tribouilloy C, Rusinaru D, Mahjoub H, Tartiere JM, Kesri-Tartiere L, Godard S, Peltier M. Prognostic impact of diabetes mellitus in patients with heart failure and preserved ejection fraction: a prospective five-year study. Heart. 2008;94:1450–1455. doi: 10.1136/hrt.2007.128769. [DOI] [PubMed] [Google Scholar]
- 414.Gustafsson I, Brendorp B, Seibaek M, Burchardt H, Hildebrandt P, Kober L, Torp-Pedersen C Danish Investigatord of A, Mortality on Dofetilde Study G. Influence of diabetes and diabetes-gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol. 2004;43:771–777. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 415.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996;77:1017–1020. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 416.Romero SP, Garcia-Egido A, Escobar MA, Andrey JL, Corzo R, Perez V, Garcia-Domiguez GJ, Gomez F. Impact of new-onset diabetes mellitus and glycemic control on the prognosis of heart failure patients: a propensity-matched study in the community. International journal of cardiology. 2013;167:1206–1216. doi: 10.1016/j.ijcard.2012.03.134. [DOI] [PubMed] [Google Scholar]
- 417.Wong AK, AlZadjali MA, Choy AM, Lang CC. Insulin resistance: a potential new target for therapy in patients with heart failure. Cardiovascular therapeutics. 2008;26:203–213. doi: 10.1111/j.1755-5922.2008.00053.x. [DOI] [PubMed] [Google Scholar]
- 418.Doehner W, Rauchhaus M, Ponikowski P, Godsland IF, von Haehling S, Okonko DO, Leyva F, Proudler AJ, Coats AJ, Anker SD. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019–1026. doi: 10.1016/j.jacc.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 419.Lundbaek K. Diabetic angiopathy: a specific vascular disease. Lancet. 1954;266:377–379. doi: 10.1016/s0140-6736(54)90924-1. [DOI] [PubMed] [Google Scholar]
- 420.Lundbaek K. Is there a diabetic cardiopathy? Stuttgart: Schattauer; 1969. Pathogenetishe Faktoren des Myokardinfarkts. [Google Scholar]
- 421.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 422.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 423.Ofstad AP, Urheim S, Dalen H, Orvik E, Birkeland KI, Gullestad LW, Fagerland M, Johansen OE, Aakhus S. Identification of a definite diabetic cardiomyopathy in type 2 diabetes by comprehensive echocardiographic evaluation: A cross-sectional comparison with non-diabetic weight-matched controls. J Diabetes. 2015;7:779–790. doi: 10.1111/1753-0407.12239. [DOI] [PubMed] [Google Scholar]
- 424.Paillole C, Dahan M, Paycha F, Solal AC, Passa P, Gourgon R. Prevalence and significance of left ventricular filling abnormalities determined by Doppler echocardiography in young type I (insulin-dependent) diabetic patients. Am J Cardiol. 1989;64:1010–1016. doi: 10.1016/0002-9149(89)90799-6. [DOI] [PubMed] [Google Scholar]
- 425.Zarich SW, Arbuckle BE, Cohen LR, Roberts M, Nesto RW. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J Am Coll Cardiol. 1988;12:114–120. doi: 10.1016/0735-1097(88)90364-6. [DOI] [PubMed] [Google Scholar]
- 426.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, Romijn JA, de Roos A, Radder JK. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 427.Fang ZY, Najos-Valencia O, Leano R, Marwick TH. Patients with early diabetic heart disease demonstrate a normal myocardial response to dobutamine. J Am Coll Cardiol. 2003;42:446–453. doi: 10.1016/s0735-1097(03)00654-5. [DOI] [PubMed] [Google Scholar]
- 428.Litwin SE, Raya TE, Anderson PG, Daugherty S, Goldman S. Abnormal cardiac function in the streptozotocin-diabetic rat. Changes in active and passive properties of the left ventricle. J Clin Invest. 1990;86:481–488. doi: 10.1172/JCI114734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Borges GR, de Oliveira M, Salgado HC, Fazan R., Jr Myocardial performance in conscious streptozotocin diabetic rats. Cardiovasc Diabetol. 2006;5:26. doi: 10.1186/1475-2840-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 431.Yilmaz S, Canpolat U, Aydogdu S, Abboud HE. Diabetic Cardiomyopathy; Summary of 41 Years. Korean Circ J. 2015;45:266–272. doi: 10.4070/kcj.2015.45.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Maisch B, Alter P, Pankuweit S. Diabetic cardiomyopathy--fact or fiction? Herz. 2011;36:102–115. doi: 10.1007/s00059-011-3429-4. [DOI] [PubMed] [Google Scholar]
- 433.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68:85–89. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 434.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 436.Garg N, Senthilkumar A, Nusair MB, Goyal N, Garg RK, Alpert MA. Heart failure with a normal left ventricular ejection fraction: epidemiology, pathophysiology, diagnosis and management. The American journal of the medical sciences. 2013;346:129–136. doi: 10.1097/MAJ.0b013e31828c586e. [DOI] [PubMed] [Google Scholar]
- 437.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 438.Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de las Fuentes L, Joseph SM, Vader J, Hernandez AF, Redfield MM. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64:541–549. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Joffe II, Travers KE, Perreault-Micale CL, Hampton T, Katz SE, Morgan JP, Douglas PS. Abnormal cardiac function in the streptozotocin-induced non-insulin-dependent diabetic rat: noninvasive assessment with doppler echocardiography and contribution of the nitric oxide pathway. J Am Coll Cardiol. 1999;34:2111–2119. doi: 10.1016/s0735-1097(99)00436-2. [DOI] [PubMed] [Google Scholar]
- 440.Hoit BD, Castro C, Bultron G, Knight S, Matlib MA. Noninvasive evaluation of cardiac dysfunction by echocardiography in streptozotocin-induced diabetic rats. Journal of cardiac failure. 1999;5:324–333. doi: 10.1016/s1071-9164(99)91337-4. [DOI] [PubMed] [Google Scholar]
- 441.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–H982. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 442.Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26:2791–2795. doi: 10.2337/diacare.26.10.2791. [DOI] [PubMed] [Google Scholar]
- 443.Mustonen JN, Uusitupa MI, Laakso M, Vanninen E, Lansimies E, Kuikka JT, Pyorala K. Left ventricular systolic function in middle-aged patients with diabetes mellitus. Am J Cardiol. 1994;73:1202–1208. doi: 10.1016/0002-9149(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 444.Mildenberger RR, Bar-Shlomo B, Druck MN, Jablonsky G, Morch JE, Hilton JD, Kenshole AB, Forbath N, McLaughlin PR. Clinically unrecognized ventricular dysfunction in young diabetic patients. J Am Coll Cardiol. 1984;4:234–238. doi: 10.1016/s0735-1097(84)80207-7. [DOI] [PubMed] [Google Scholar]
- 445.Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41:611–617. doi: 10.1016/s0735-1097(02)02869-3. [DOI] [PubMed] [Google Scholar]
- 446.Petrie MC, Caruana L, Berry C, McMurray JJ. “Diastolic heart failure” or heart failure caused by subtle left ventricular systolic dysfunction? Heart. 2002:87. doi: 10.1136/heart.87.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Seferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–1727. 1727a–1727c. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- 448.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circulation Heart failure. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 452.Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40:239–247. doi: 10.1016/s0008-6363(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 453.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 454.Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF. The diabetic cardiomyopathy. Acta Diabetol. 2011;48:173–181. doi: 10.1007/s00592-010-0180-x. [DOI] [PubMed] [Google Scholar]
- 455.Joshi M, Kotha SR, Malireddy S, Selvaraju V, Satoskar AR, Palesty A, McFadden DW, Parinandi NL, Maulik N. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem. 2014;386:233–249. doi: 10.1007/s11010-013-1861-x. [DOI] [PubMed] [Google Scholar]
- 456.Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, Gheorghiade M, Fonarow GC. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 457.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Falcao-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 459.Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J Diabetes. 2015;6:943–960. doi: 10.4239/wjd.v6.i7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010;88:229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Zhang X, Chen C. A new insight of mechanisms, diagnosis and treatment of diabetic cardiomyopathy. Endocrine. 2012;41:398–409. doi: 10.1007/s12020-012-9623-1. [DOI] [PubMed] [Google Scholar]
- 462.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, Tsao PS. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int J Mol Sci. 2015;16:25234–25263. doi: 10.3390/ijms161025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Givertz MM, Sawyer DB, Colucci WS. Antioxidants and myocardial contractility: illuminating the “Dark Side” of beta-adrenergic receptor activation? Circulation. 2001;103:782–783. doi: 10.1161/01.cir.103.6.782. [DOI] [PubMed] [Google Scholar]
- 465.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 466.Krum H, Gilbert RE. Demographics and concomitant disorders in heart failure. Lancet. 2003;362:147–158. doi: 10.1016/S0140-6736(03)13869-X. [DOI] [PubMed] [Google Scholar]
- 467.Haas SJ, Vos T, Gilbert RE, Krum H. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J. 2003;146:848–853. doi: 10.1016/S0002-8703(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 468.Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, van Veldhuisen DJ, Zannad F, Krum H, Mukherjee R, Vincent J Ephesus Investigators. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–431. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 469.Martin DT, McNitt S, Nesto RW, Rutter MK, Moss AJ. Cardiac resynchronization therapy reduces the risk of cardiac events in patients with diabetes enrolled in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT) Circulation Heart failure. 2011;4:332–338. doi: 10.1161/CIRCHEARTFAILURE.110.959510. [DOI] [PubMed] [Google Scholar]
- 470.Shekelle PG, Rich MW, Morton SC, Atkinson CS, Tu W, Maglione M, Rhodes S, Barrett M, Fonarow GC, Greenberg B, Heidenreich PA, Knabel T, Konstam MA, Steimle A, Warner Stevenson L. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–1538. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 471.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K Charm Investigators, Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 472.Eriksson JW, Jansson PA, Carlberg B, Hagg A, Kurland L, Svensson MK, Ahlstrom H, Strom C, Lonn L, Ojbrandt K, Johansson L, Lind L. Hydrochlorothiazide, but not Candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of Candesartan (MEDICA) Study. Hypertension. 2008;52:1030–1037. doi: 10.1161/HYPERTENSIONAHA.108.119404. [DOI] [PubMed] [Google Scholar]
- 473.Ramsay LE, Yeo WW, Jackson PR. Diabetes, impaired glucose tolerance and insulin resistance with diuretics. Eur Heart J. 1992;13(Suppl G):71. doi: 10.1093/eurheartj/13.suppl_g.68. [DOI] [PubMed] [Google Scholar]
- 474.Writing Committee M; Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 475.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Annals of internal medicine. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 476.Dziuba J, Alperin P, Racketa J, Iloeje U, Goswami D, Hardy E, Perlstein I, Grossman HL, Cohen M. Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab. 2014;16:628–635. doi: 10.1111/dom.12261. [DOI] [PubMed] [Google Scholar]
- 477.Riggs K, Ali H, Taegtmeyer H, Gutierrez AD. The Use of SGLT-2 Inhibitors in Type 2 Diabetes and Heart Failure. Metab Syndr Relat Disord. 2015;13:292–297. doi: 10.1089/met.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Zhong J, Goud A, Rajagopalan S. Glycemia Lowering and Risk for Heart Failure: Recent Evidence from Studies of Dipeptidyl Peptidase Inhibition. Circulation Heart failure. 2015;8:819–825. doi: 10.1161/CIRCHEARTFAILURE.114.001967. [DOI] [PubMed] [Google Scholar]
- 479.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 480.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 481.Ussher JR, Baggio LL, Campbell JE, Mulvihill EE, Kim M, Kabir MG, Cao X, Baranek BM, Stoffers DA, Seeley RJ, Drucker DJ. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab. 2014;3:507–517. doi: 10.1016/j.molmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Myat A, Redwood SR, Gersh BJ, Yellon DM, Marber MS. Diabetes, incretin hormones and cardioprotection. Heart. 2014;100:1550–1561. doi: 10.1136/heartjnl-2012-303242. [DOI] [PubMed] [Google Scholar]
- 483.Mikhail N. Effects of incretin-based therapy in patients with heart failure and myocardial infarction. Endocrine. 2014;47:21–28. doi: 10.1007/s12020-014-0175-4. [DOI] [PubMed] [Google Scholar]
- 484.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 485.Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Lam H, White WB. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 486.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Mulvihill EE, Varin EM, Ussher JR, Campbell JE, Bang KW, Abdullah T, Baggio LL, Drucker DJ. Inhibition of Dipeptidyl Peptidase-4 impairs ventricular function and promotes cardiac fibrosis in high fat-fed diabetic mice. Diabetes. 2016;65:742–754. doi: 10.2337/db15-1224. [DOI] [PubMed] [Google Scholar]
- 488.Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, Hu N, Paterson JM, Targownik L, Turin TC, Udell JA, Ernst P. A Multicenter Observational Study of Incretin-based Drugs and Heart Failure. N Engl J Med. 2016;374:1145–1154. doi: 10.1056/NEJMoa1506115. [DOI] [PubMed] [Google Scholar]
- 489.Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, Johnson JA. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 491.Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care. 2007;30:2148–2153. doi: 10.2337/dc07-0141. [DOI] [PubMed] [Google Scholar]
- 492.Varas-Lorenzo C, Margulis AV, Pladevall M, Riera-Guardia N, Calingaert B, Hazell L, Romio S, Perez-Gutthann S. The risk of heart failure associated with the use of noninsulin blood glucose-lowering drugs: systematic review and meta-analysis of published observational studies. BMC cardiovascular disorders. 2014;14:129. doi: 10.1186/1471-2261-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Lincoff AM, Tardif JC, Schwartz GG, Nicholls SJ, Ryden L, Neal B, Malmberg K, Wedel H, Buse JB, Henry RR, Weichert A, Cannata R, Svensson A, Volz D, Grobbee DE, AleCardio I. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311:1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 494.Fonarow GC. Approach to the management of diabetic patients with heart failure: role of thiazolidinediones. Am Heart J. 2004;148:551–558. doi: 10.1016/j.ahj.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 495.Karalliedde J, Buckingham R, Starkie M, Lorand D, Stewart M, Viberti G Rosiglitazone Fluid Retention Study G. Effect of various diuretic treatments on rosiglitazone-induced fluid retention. J Am Soc Nephrol. 2006;17:3482–3490. doi: 10.1681/ASN.2006060606. [DOI] [PubMed] [Google Scholar]
- 496.Wang CH, Weisel RD, Liu PP, Fedak PW, Verma S. Glitazones and heart failure: critical appraisal for the clinician. Circulation. 2003;107:1350–1354. doi: 10.1161/01.cir.0000054675.30348.9a. [DOI] [PubMed] [Google Scholar]
- 497.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 498.Food and Drug Administration. [Accessed 2-4-2016];Safety Alerts for Human Medical Products: Thiazolidinediones [ACTOS (pioglitazone HCl); AVANDIA (rosliglitazone maleate)] 2002 Apr; http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM170872.pdf.
- 499.Kalambokis GN, Tsatsoulis AA, Tsianos EV. The edematogenic properties of insulin. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;44:575–590. [PubMed] [Google Scholar]
- 500.Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet. 2015;385:2107–2117. doi: 10.1016/S0140-6736(14)61402-1. [DOI] [PubMed] [Google Scholar]
- 501.Smooke S, Horwich TB, Fonarow GC. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am Heart J. 2005;149:168–174. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 502.Murcia AM, Hennekens CH, Lamas GA, Jimenez-Navarro M, Rouleau JL, Flaker GC, Goldman S, Skali H, Braunwald E, Pfeffer MA. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Archives of internal medicine. 2004;164:2273–2279. doi: 10.1001/archinte.164.20.2273. [DOI] [PubMed] [Google Scholar]
- 503.Food and Drug Administration. [Accessed December 17, 2015];Glucophage drug label. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/20357S019_Glucophage_prntlbl.pdf.
- 504.Fisman EZ, Tenenbaum A. Antidiabetic treatment with gliptins: focus on cardiovascular effects and outcomes. Cardiovasc Diabetol. 2015;14:129. doi: 10.1186/s12933-015-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circulation Heart failure. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 506.Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet. 2011;378:140–146. doi: 10.1016/S0140-6736(11)60471-6. [DOI] [PubMed] [Google Scholar]
- 507.Olsen MH, Hjerkinn E, Wachtell K, Hoieggen A, Bella JN, Nesbitt SD, Fossum E, Kjeldsen SE, Julius S, Ibsen H. Are left ventricular mass, geometry and function related to vascular changes and/or insulin resistance in long-standing hypertension? ICARUS: a LIFE substudy. J Hum Hypertens. 2003;17:305–311. doi: 10.1038/sj.jhh.1001545. [DOI] [PubMed] [Google Scholar]
- 508.Nielsen R, Norrelund H, Kampmann U, Botker HE, Moller N, Wiggers H. Effect of acute hyperglycemia on left ventricular contractile function in diabetic patients with and without heart failure: two randomized cross-over studies. PLoS One. 2013;8:e53247. doi: 10.1371/journal.pone.0053247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.