Abstract
Chemical signals arising from body secretions and excretions communicate information about health status as have been reported in a range of animal models of disease. A potential common pathway for diseases to alter chemical signals is via activation of immune function—which is known to be intimately involved in modulation of chemical signals in several species. Based on our prior findings that both immunization and inflammation alter volatile body odors, we hypothesized that injury accompanied by inflammation might correspondingly modify the volatile metabolome to create a signature endophenotype. In particular, we investigated alteration of the volatile metabolome as a result of traumatic brain injury. Here, we demonstrate that mice could be trained in a behavioral assay to discriminate mouse models subjected to lateral fluid percussion injury from appropriate surgical sham controls on the basis of volatile urinary metabolites. Chemical analyses of the urine samples similarly demonstrated that brain injury altered urine volatile profiles. Behavioral and chemical analyses further indicated that alteration of the volatile metabolome induced by brain injury and alteration resulting from lipopolysaccharide-associated inflammation were not synonymous. Monitoring of alterations in the volatile metabolome may be a useful tool for rapid brain trauma diagnosis and for monitoring recovery.
Key words: animal model, bioassay, chemical analysis, diagnosis, inflammation, traumatic brain injury, urine
Introduction
Information about an animal’s gender, age, diet, reproductive status, individual identity, and health can be encoded in chemical signals made up of small volatile molecules (Penn and Potts 1998; Johnston 2003; Brennan and Kendrick 2006). These metabolites, contained in the volatile metabolome, are considered a highly evolved form of communication that provides critical information to conspecifics regulating behavior and physiology. Because odors associated with disease can be detected by other animals and/or by instrumental analyses, volatile metabolites are an intriguing target for novel diagnostics (Shirasu and Touhara 2011). Volatile metabolomic approaches have previously been employed for human health studies of autoimmune diseases (Kang et al. 2015), lung cancer (Matsumura et al. 2010; Rudnicka et al. 2014), tuberculosis (Syhre et al. 2009), and gastrointestinal disorders (Probert et al. 2009) among others. However, alteration of the volatile metabolome resulting from bodily injury has not been previously studied and represents an interesting and novel area for research.
Mild to moderate TBI is exceedingly common but presents significant challenges for diagnosis and treatment (Hoffer 2015). Potential diagnostic techniques include clinical evaluations, monitoring changes in visual function, and brain imaging approaches. A promising approach involves discovery of objective biomarkers that could be used to diagnose patients and to monitor their recovery. The most widely investigated prospective sources of such biomarkers are proteins or protein fragments present in CSF or blood. Several potential candidates have been identified but have yet to be implemented (Siman et al. 2013; Zetterberg et al. 2013). Cerebrospinal fluid (and to a lesser degree blood or serum) may not be easily accessible, so additional bodily fluids such as saliva, sweat, tears, or urine could have advantages as rich sources for biomarkers. However, it has been argued that these fluids are unlikely to yield reliable biomarkers because molecular changes in these fluids are not clearly linked to TBI (Zetterberg and Blennow 2015). Nevertheless, it is our contention that it is premature to dismiss such fluids as biomarker sources on purely theoretic grounds.
It is well established that acute treatment with lipopolysaccharide (LPS), a potent immune activator and promoter of inflammation, results in a change in mouse urine odor (Arakawa et al. 2010; Boillat et al. 2015). Because inflammatory processes are nearly universally detected in neurological disorders (Raghupathi 2004; Lozano et al. 2015; Rathbone et al. 2015), we hypothesized that mild to moderate traumatic brain injury (TBI) would likewise alter the volatile metabolome and that urine may similarly serve as a valuable source of these odorants. The major goal of this work was to test the hypothesis that TBI alters urinary odorants in a mouse model. Two independent assays were employed to investigate volatile metabolites arising from experimentally induced TBI: discrimination tasks with trained biosensor animals and gas chromatographic headspace analyses. Toward an understanding of the mechanisms involved in alterations of the volatile metabolome, comparisons were made between LPS-induced inflammation and experimental TBI.
Materials and methods
Subjects
Inbred male C57BL/6 mice were obtained commercially (Jackson Laboratories) or bred in-house to be used as urine donors and trained biosensors. Urine was obtained from donor mice by gentle abdominal pressure (Yamazaki et al. 1983) and urine samples were individually stored at −20 °C prior to use in behavioral and chemical assays (Figure 1).
Figure 1.
Graphical representation of the experiment methods. Urine collected from mice subjected to either LFPI or sham surgery was presented to trained mice (Experiment 1) as well as subjected to chemical analyses (Experiment 2). Urine collected from mice subjected to i.p. treatment with LPS or PBS was similarly used in bioassay (Experiment 3) and chemical assay (Experiment 4).
Mouse fluid percussion brain injury
A lateral fluid percussion injury (LFPI) protocol was performed in Children’s Hospital of Philadelphia (CHOP) facilities over 2 days to produce TBI models following established procedures (Witgen et al. 2005; Cole et al. 2010). On the first day, the animal was anesthetized using a combination of ketamine (2.6mg/kg) and xylazine (0.16mg/kg) and placed in a mouse stereotactic frame (Stoelting). Under 0.7–3.5× magnification, the scalp was incised and reflected and a craniectomy was performed with a trephine (3mm outer diameter) over the right parietal area between the bregma anteriorly, the lambda posteriorly, the sagittal suture medially, and the lateral cranial ridge laterally. The dura remained intact throughout the procedure. A rigid Luer-loc needle hub (3mm inside diameter) was secured to the skull over the opening with cyanoacrylate and dental acrylic. The skull sutures were sealed with cyanoacrylate during this process to ensure that the fluid bolus from the injury remained within the cranial cavity. The hub was capped and the scalp was sutured closed. The mouse was placed on a heating pad and returned to the home cage once ambulatory.
On the second day, the animal was anesthetized with isofluorane (2% oxygen in 500ml/minute) via nose cone. The needle hub was filled with isotonic sterile saline and a 32cm piece of high-pressure tubing from the LFPI device (Department of Biomedical Engineering, Virginia Commonwealth University, Richmond, VA) was attached via the Luer-loc fitting. The animal was then immediately placed on its left side as a 20ms pulse of saline was delivered onto the dura before sensitivity to stimulation returned. A pressure gauge attached to an oscilloscope was used to ensure delivered pressures between 1.8 and 2.1 atm. Immediately after injury, the hub was removed from the skull and the animal placed in a supine position. The animal was reanesthetized with isoflurane for scalp closure. Sham animals received all of the above, with the exception of the fluid pulse. Following completion of the procedure, the animal was returned to a heating pad until ambulatory and then returned to the home cage. Urine collections began on day 1 of recovery and continued for up to 15 days.
As applied in this study, LFPI was designed to produce a mild to moderate, nonpenetrating (dura is not breached) brain injury. The righting time reflex (the length of time after the injury until the animal spontaneously righted itself) was used as an acute neurological assessment of the severity of the injury (Morehead et al. 1994). Mice subjected to sham surgery were retained in the study if the righting time was less than 20s. Mice subjected to LFPI were included in the study if the righting time indicated mild to moderate brain injury. Animals indicating minor brain injury (less than 250s) or severe brain injury (greater than 350s) were excluded from the study (Witgen et al. 2005).
LPS-induced inflammation
The LPS solution was prepared in 0.01M phosphate-buffered saline (PBS) at a concentration of 0.20mg/mL for i.p. delivery of 300 µL (60 µg per mouse or ~ 2mg/Kg). Control donors received matching volume i.p. injections of the 0.01M PBS solution.
Behavioral assay
For each experiment in a Y-maze, urines collected from mice subjected to experimental treatment or control were randomly assigned for use in either training or generalization sessions. In this way, urines presented in critical generalization trials were derived from donor mice not used in rewarded training trials which ensured that trained animals responded to treatment (LPS or LFPI) and not chemosensory information learned about individual identities during rewarded training sessions. The Y-maze was used as previously described (Yamazaki et al. 2002). The procedure, developed in our laboratory, ensures blind testing and controls for many factors that could influence subject responses. Air is conducted through 2 odor chambers, each containing urine samples exposed in Petri dishes, to the 2 arms of the maze. Training sessions (a series of 35–50 two-choice trials) consisted of pair-wise offerings of urine from control and treated donors. In these daily sessions, water-restricted mice were rewarded with a drop of water for choosing the maze arm associated with the treatment (LPS or LFPI donor urine) offered in comparison to controls (PBS or sham donor urine). Successful discrimination during the training sessions was indicated by 80% concordance over multiple consecutive sessions.
A typical generalization session with a trained mouse began with a series of rewarded trials using training stimuli. Generalization trials were initiated after 6 consecutive (or 8 of 9) correct rewarded trials. If the mouse did not achieve required performance in rewarded trials, the session was terminated and reestablished the next day. A continuing session consisted of 5 extinction (unrewarded trials using training stimuli) and 5 generalization (unrewarded trials using novel stimuli) trials offered alternatively with 2 repeating rewarded trials. The identities of the generalization stimuli were blind to the operator, which was possible because no reward was provided in association with these odor sources (i.e., double blind design).Urine samples offered in unrewarded generalization trials were derived from novel subjects (urine from these donors was not used during training), thus ensuring that the trained animals respond to the treatment (LPS or LFPI) and did not learn identities of individual donors during rewarded training sessions.
Responses of trained individual mice were subjected to chi-square tests of independence in SAS using Fisher’s Exact Test for small cell sizes (PROC FREQ). When responses did not differ among individuals in a biosensor panel, cumulative responses of the full panel were calculated and subjected to statistical tests of binomial proportion using the continuity correction for low incidence events (Carriere 2001). Panel responses for each generalization test were compared with the null hypothesis of proportion >0.5. No comparisons were made among the different generalization trials.
Chemical assay
Urine samples (25 or 50 µL) were fortified with 800ng l-Carvone by addition of 10 µL of an aqueous fortification solution to each sample. Vials containing no urine samples (carvone blanks) were also fortified with carvone and analyzed. The aqueous fortification solution was prepared weekly from a concentrated carvone solution in ethanol. Samples were subjected to headspace analysis using a HT3 dynamic headspace analyzer (Teledyne Tekmar, Mason, OH, USA) outfitted with Supelco Trap K Vocarb 3000 trap (Sigma–Aldrich Co). During headspace collection, samples were maintained at 40 °C, swept with helium for 10min (flow rate of 75mL/min), and the volatiles collected on the thermal desorption trap. Trap contents were desorbed at 260 °C directly into a Thermo Scientific ISQ single-quadrapole gas chromatograph-mass spectrometer (GC-MS; Thermo Scientific) equipped with a 30 m × 0.25mm id Stabiliwax-DA fused-silica capillary column (Restek). The GC oven program consisted of an initial temperature of 40 °C (held for 3.0min) followed by a ramp of 7.0 °C/min to a final temperature of 230 °C (held for 6.0min). The mass spectrometer was operated in scan mode from 33 to 400 m/z.
Chemometric analyses
Baseline correction, noise elimination, and peak alignment of the chromatographic data were achieved using Metalign (Lommen 2009) and the MSClust tool was used to perform mass spectral extraction for generation of selected ion chromatogram peak responses (Tikunov et al. 2012). Each resultant data set, consisting of a single response for each chromatographic peak detected in the headspace, was subjected to principal components analysis (PCA) using Unscrambler (CAMO Software) to visually identify outliers exhibiting undue influence or leverage in residual plots. Donor means were calculated for all peak responses according to the specified urine collection periods (see Experiment descriptions below).
For statistical analysis, a subset (Model set) of the treated and control mice were selected for constructing a model that differentiated treatment from control. A different subset of treated and control mice (3–4 mice in each group—the Test set) then served to evaluate the model’s success in classifying Test set mice into the appropriate treatment or control group. Importantly, data from the test set were not used to create the model. Assignment into unique Model and Test sets not only follows standard statistical practices, but also paralleled behavioral testing where mice were trained with urines from one subset of donors and then tested in unrewarded generalization trials with urines collected from donors that had not been used in training.
Linear discriminant analysis (LDA) models were then constructed from response data of the Model set using stepwise selection to identify peak responses which significantly contributed to the discrimination task using SAS (PROC STEPDISC). Predictors identified during model building were evaluated to verify the peak(s) were associated with urine samples and not artifactual (present in carvone blanks) by inspection of original chromatographic data. Valid predictors were employed in discriminant analyses using PROC DISCRIM with cross-validation. Predictors were also subjected to pair-wise t-tests to determine if they increased or decreased in response to treatment using the false discovery rate controlling procedure for multiple tests (Benjamini and Hochberg 1995). The resulting model was then used to predict the treatment of donors assigned to the Test set.
Experiment 1
Mice subjected to LFPI (n = 20) or sham surgery (n = 19) were transported to Monell for urine collection. Samples were collected daily up to 15 days postsurgery. Samples from all donors were separated into 2 groups based on collection day (days 1–10; or days 11–15 postsurgery). For bioassay in the Y-maze, urine samples were pooled according to donor and collection day grouping. A panel of 5 biosensors was trained in the Y-maze to discriminate urines from LFPI from urines of sham donors on the basis of volatile metabolites. Rewarded training trials were conducted using day 1–10 urines from 17 (of 20) LFPI and 16 (of 19) sham donors. Urines collected from 3 LFPI and 3 sham donors were withheld from rewarded training trials so as to ensure these samples would be novel when presented in unrewarded generalization trials. Generalization trials were conducted in 2 consecutive sessions. The first session included unrewarded generalization trials with novel donor urines collected days 11–15 followed by a second session with novel donor urines collected days 1–10.
Experiment 2
There was insufficient urine available from 2 LFPI and 2 sham donors used in Experiment 1 to be used for chemical analyses. Three replicate urine samples (50 µL) from each of the available 18 LFPI and 17 sham donors representing days 2–10 postsurgery were subjected to chemical analysis and chromatographic data processing. Assignment to the statistical Model and Test sets followed the assignments made for training and generalization in Experiment 1, except urine was not available from one LFPI donor used for generalization trials. Thus, 1 donor was randomly chosen to ensure that the Test set consisted of 3 donors from each treatment group. All remaining donors were assigned to the Model set.
Peak responses were scaled to the internal standard and mean peak responses were calculated for all subjects. Peak response data from the Model set of donors (15 LFPI and 14 sham) were used to construct a LDA model for LFPI-associated urine volatiles and used as the calibration data. Mean peak response data from 6 donors withheld from the model (Test set) were subjected to the LDA for classification as members of either the LFPI or sham surgery treatment group.
Chemical predictors identified by model building were further subjected to multivariate analysis of variance using PROC GLM in SAS using data from all samples (no subject means were calculated). The appropriate scaled peak responses from the LDA model (the repeated measures variable was termed “volatiles”) were employed in the analysis with treatment (LFPI or sham) the between-subjects effect and postsurgical urine collection day the covariate.
Experiment 3
Forty urine donors unique to Experiment 3 were used for training biosensors to the urine odor of LPS-induced inflammation. Urine samples were collected from each of 20 LPS-treated and 20 control (PBS) donor mice beginning 10 days posttreatment. Five donors from each treatment were randomly assigned for use in generalization trials, the remainder being available for rewarded training trials. Twelve additional mice subjected to either LFPI (n = 7) or sham (n = 5) surgeries were used as urine donors for generalization trials in the Y-maze. Urine samples were collected from these donors in the CHOP laboratory on days 2–13 following surgery and the urines were transported to Monell were they were combined (by donor) into week 1 and week 2 samples.
Bioassays were conducted with a new panel of 6 biosensor mice (different from Experiment 1) trained to discriminate LPS urine odor from control urine odor. For training, urines from 15 LPS and 15 control donors collected days 10–14 posttreatment were used in rewarded trials. Upon successful training (greater than 80% correct responding to LPS urine), unrewarded generalization trials were conducted with urines collected on days 11, 12, 13, 14, and 24 posttreatment from 5 LPS and 5 PBS donors. The final generalization trials were conducted in sessions using week 1 and week 2 urines collected from LFPI and sham donors to evaluate if urines collected from LFPI-treated mice were perceptually similar to urines from LPS-treated mice.
Experiment 4
Urine samples were collected from 40 donors (in addition to Experiment 3 donors) treated with either LPS or PBS (controls). Four donors from each treatment were randomly assigned to the Test set. Three urine samples (25 µL) from each donor collected 10–14 days posttreatment were subjected to headspace analysis. Mean peak responses (scaled to the internal standard) from 16 LPS-treated and 16 control (PBS-treated only) donors in the Model set were used to build a LDA model for LPS-associated urine volatiles. Data from the analyses of 4 LPS-treated and 4 control donors (Test set donors withheld from model building) were used for testing model predictions.
Results
Experiment 1
A panel of 5 biosensors was trained in a Y-maze to discriminate urines of LFPI donors from urines of sham donors on the basis of volatile metabolites. Responses to test stimuli did not differ among the 5 trained biosensor mice (P = 0.551). Biosensors successfully discriminated urines from LFPI and sham donors and generalized this response to urines from novel donors collected days 1–10 (P = 0.027) and days 11–15 (P < 0.0001; Figure 2). This result demonstrates that the learned response was a consequence of donor treatment and not individual identities of the donors. The difference between LFPI and sham odor signatures was robust and evident for at least 2 weeks postinjury.
Figure 2.
Mean responses (with 95% confidence intervals) of trained biosensor selection of urines associated with LFPI in Experiment 1. Labels indicate P < 0.05 (*) and P < 0.0001 (**) for the 1-tailed test of the hypothesis that identification rate >50% in generalization trials. Odor recognition of urine collected days 1–10 postinjury in training (black bar) was generalized to urine from novel donors collected up to 15 days postinjury. Generalization trials with urine collected days 11–15 posttreatment were conducted first.
Experiment 2
Urine samples from LFPI and sham donors (from Experiment 1) were subjected to chemical analysis and chromatographic data processing. Chemometric analyses verified that urine volatiles were altered by LFPI relative to sham surgery alone. Urine donors could be accurately classified as receiving LFPI or sham surgery by LDA employing peak responses of 5 tentatively identified urine volatiles: exo-brevicomin, β-Farnesene, o-toluidine, dimethyl sulfone, and formanilide (Table 1). Eleven (73%) LFPI and 13 (93%) sham donors were correctly classified with a cross-validation error rate of 16.9%. Further evaluation indicated that o-toluidine peak responses increased as a result of LFPI (P = 0.027). β-Farnesene responses were also greater in LFPI-treated donors whereas responses of exo-brevicomin, dimethyl sulfone, and formanilide were greater in the urines of sham donors; but these differences were not statistically significant. When data from Test set (3 LFPI donors and 3 sham donors withheld from model building) were subject to the LDA model, all of the LFPI and 2 of the sham donors were correctly classified. Multivariate analysis of variance indicated that LDA peak responses did not vary according to day over the period of days 2–10 postsurgery. As expected from LDA model selection, treatment was significant (P = 0.0020) as was treatment × day (P = 0.0057); however, day was not (P = 0.8630). Among within-subjects effects, volatiles × day was not significant (P = 0.5355); whereas all other effects were significant (P < 0.006). These results indicate the volatile profile associated with LFPI was consistent over these days.
Table 1.
Biological relevance of compounds impacted by treatment (significant pair-wise comparisons vs. control) and/or employed in LDA models of classification for experiments with LFPI or LPS
| Metabolite | LDAa | Pair-wiseb | Comments |
|---|---|---|---|
| β-Farnesene | LFPI | Mouse pheromone (Novotny 2003) | |
| Elevated in mouse urine by predator exposure (Zhang et al. 2008) | |||
| o-Toluidine | LFPI | LFPI | Diet-based mouse urine volatile (Kwak et al. 2008) |
| Dimethyl sulfone | LFPI | Genetically based mouse urine volatile (Kwak et al. 2008) | |
| Linked to microbiome (He and Slupsky 2014) | |||
| Formanilide | LFPI | Known mouse urine volatile (Schwende et al. 1986) | |
| exo-Brevicomin | LFPI | LPS | Reduced form of mouse pheromone (Novotny 2003) |
| LPS | Increased in urine of tumor-bearing mice (Hanai et al. 2012) | ||
| 6-hydroxy-6-methyl-3-heptanone | LPS | Mouse pheromone (Novotny 2003) | |
| Methyl (methylthio)methyl disulfide | LPS | Genetically based mouse urine volatile (Kwak et al. 2008) | |
| Benzaldehyde | LPS | Genetically based mouse urine volatile (Kwak et al. 2008) |
Metabolite identifications are tentative.
aCompounds employed in linear discriminant analysis models for discrimination of treatment condition from appropriate controls using urine volatiles.
bCompounds found to be in significantly greater abundance in urines derived from treatment condition versus appropriate controls.
Experiment 3
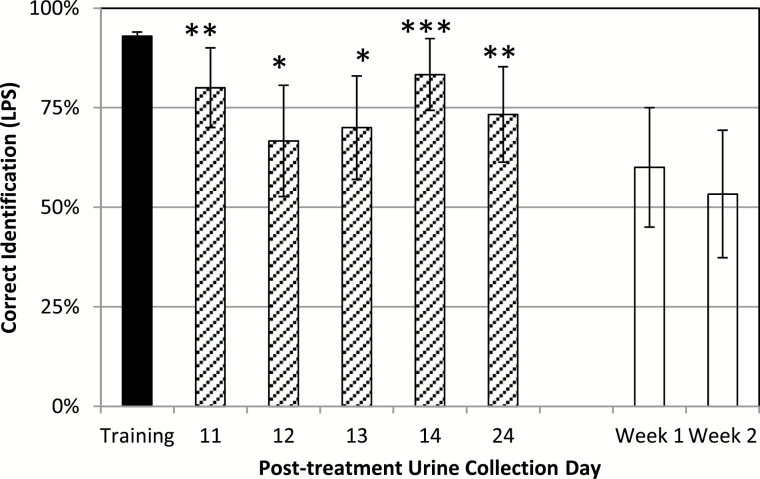
A new panel of 6 biosensor mice (different from Experiment 1) was successfully trained to discriminate between urines derived from LPS and control (PBS) treated donors; confirming that i.p. treatment with LPS altered urine volatiles (Figure 3). Responses among trained biosensors did not differ (P = 0.96). The learned response to LPS odor was generalized to urines from novel LPS-treated donors collected 11 (P = 0.0005), 12 (P = 0.0339), 13 (P = 0.0142), 14 (P < 0.0001), and 24 (P = 0.0053) days posttreatment. However, the LPS-trained biosensors did not generalize the trained response to urine from LFPI donors. When presented in the Y-maze in comparison to urines from sham donors, urines from LFPI donors were selected 18 of 30 times (60%; P = 0.2733) for week 1 urine samples; whereas LFPI donors were selected 16 of 30 times (53%; P = 0.715) for week 2. This lack of generalization indicates that odors from LPS- and LFPI-treated mice were different.
Figure 3.
Mean responses (with 95% confidence intervals) of trained biosensor selection of urines associated with LPS in Experiment 3. Labels indicate P < 0.05 (*), P < 0.001 (**), and P < 0.0001 (***) for the 1-tailed test of the hypothesis that identification rate >50% in generalization trials. Odor recognition of urine in rewarded training trials (black bar) was generalized to urine collected 11, 12, 13, 14, and 24 days (diagonal bars) after LPS administration from novel donors in unrewarded generalization trials. However, the response to LPS was not generalized to odor of LFPI urine collected 1 or 2 weeks postinjury (white bars).
Experiment 4
Urine samples collected from donors treated with either LPS or PBS were subjected to headspace analysis. Chemometric analyses verified that urine volatiles were altered by LPS, but differently than LFPI. An LDA model was constructed with the peak responses of 4 tentatively identified compounds: exo-brevicomin, benzaldehyde, 6-hydroxy-6-methyl-3-heptanone (HMH), and methyl methyl(thiomethyl) disulfide (Table 1). By comparison, this LDA model shared only one volatile metabolite response with the LFPI model in Experiment 2: exo-brevicomin. The model correctly classified 81% (13 of 16) of LPS donors and 94% (15 of 16) of control donors with a cross-validation error rate of 12.5%. Peak responses of exo-brevicomin were greater in urines of LPS-treated donors (P = 0.00012). Among the other predictors, benzaldehyde and methyl methyl(thiomethyl) disulfide responses were also increased by LPS treatment (whereas HMH responses were reduced), but these differences were not statistically significant. When predictions were made for the donors from the Test set, 3 of 4 donors were correctly predicted for both LPS and control treatments.
Discussion
Taken together, Experiments 1 and 2 demonstrate that LFPI induces a distinct odor and odorant change in an animal model that can be used to distinguish individuals with LFPI-induced brain injury from those receiving sham surgeries in the absence of brain injury. As employed in our study, LFPI produces hippocampal-dependent anterograde and retrograde cognitive impairment as demonstrated by decreased freezing in contextual fear conditioning studies (Witgen et al. 2005; Lifshitz et al. 2007; Cole et al. 2010). Cognitive functions are recovered approximately 1 month after injury. Furthermore, the degree of experimental injury in our models produces an inability to maintain wakefulness, including a significant decrease in theta oscillations during rapid eye movement sleep (Lim et al. 2013). In addition, LFPI leads to a significant reduction (30–35%) in the number of neurons in all subregions of the ipsilateral hippocampus (CA1, CA3, hilus, and dentate gyrus) as measured using unbiased stereology (Witgen et al. 2005).
We chose a single “dose” of LFPI based on past experience with this technique. Thus, we have no data on how varying pulse pressures to alter intensity of the injury would impact the odor change. Presumably some minimal force would not cause a measurable odor change relative to sham surgery. It could be interesting and important in future work to compare the degree to which the behavioral and physiological effects of LFPI correlate with odor changes. Such quantitative evaluations could be important in gauging the diagnostic value of odor monitoring of brain injury.
Importantly, the use of sham surgical controls for comparison to LFPI demonstrated that the source of these odors was not related to surgery (e.g., anesthetics, analgesics, or trauma associated with craniotomy) or surgical site inflammation. Two complimentary methods were employed to demonstrate odor change resulting from LFPI treatment. Behavioral assays were first conducted to determine whether an odor signal was present. Olfaction is perhaps the most sensitive instrument to identify the presence of a reliable signal. For example, in our laboratories we have trained mice to accurately discriminate body odor variability among fetal odortype (Beauchamp et al. 1994, 1995), disease (Yamazaki et al. 2002; Matsumura et al. 2010), age (Osada et al. 2003), diet (Kwak et al. 2008), and immunization (Kimball et al. 2014). Research by other investigators has successfully demonstrated the utility, reliability, and sensitivity of in vivo mouse olfactory training (Bodyak and Slotnick 1999; Rinberg et al. 2006; Slotnick 2007; Sorwell et al. 2008).
Behavioral assays demonstrated that urine odors differed between LFPI and sham donors. An interesting question is whether sham surgery is itself capable of altering odor in the mouse model and, if so, for what duration. That is, would it be possible to differentiate sham-surgical mice from unaltered mice based on odor (trained biosensor) and/or urinary volatile profiles (chemical analysis)? Because the primary purpose of the current study was to investigate the possibility of brain injury-induced change in body odor, we did not test this possibility. Nevertheless, it would be interesting and perhaps important in the future to determine the extent to which various forms of physical trauma, including that resembling the sham surgical procedures employed here, can independently induce an odor change. To our knowledge, such studies have never been published.
Available evidence suggests that body odor signals are comprised of complex mixtures and experimental analyses of these odors (and alterations therein) should focus on recognition of odorant patterns (see review by Kwak et al. 2010). Trained mice have been shown to be extremely adept at recognizing patterns, or “combinatorial odor codes” even when these patterns could possibly be masked by other sources of variation. For example, urine-based signals of individuality are not obscured by dietary changes that elicit profound alterations in urinary volatiles (Kwak et al. 2008). Accordingly, in the current study behavioral assays were followed by chemometric analyses to determine if patterns of odorants indicative of treatment (e.g., LFPI, LPS) could be identified. Specifically, LDA was employed to model alterations (both up and down regulation) of urinary volatiles for the purpose of discriminating treatments (LFPI and sham). Variations of feature selection, such as PCA, machine learning, and LDA are commonly used for metabolomic investigations or “fingerprinting” (Dicke et al. 1990; Ruijschop et al. 2009).
Behavioral and chemometric discrimination tasks are rarely performed in tandem as applied in this study. However, 2 recent studies combined chromatographic analyses with trained mice (Matsumura et al. 2010) and dogs (Rudnicka et al. 2014) in studies of volatile biomarkers for lung cancer. The authors concluded that the 2 independent assays yielded similar classification sensitivity. Further work is required to determine whether the odorants identified in chemometric models and the volatiles used by trained animals in behavioral assays are synonymous, both in previous studies as well as the experiments described here. Nevertheless, it is unnecessary to demonstrate congruence in order to conclude with certainty that both brain injury and LPS administration alter the volatile metabolome.
Both chemometric and behavioral assays suggest that odor changes caused by LFPI differed significantly from LPS-induced inflammation. Only one volatile compound was shared between the LFPI and LPS classification models developed in Experiments 2 and 4 (Table 1). The lack of discrimination by LPS-trained biosensors to LFPI urines (in comparison to sham odors) in Experiment 3 further suggests that the volatile metabolites contributing to the odor of LFPI were distinct from those induced by LPS-induced inflammation. Interestingly, LFPI- and LPS-induced odor changes persisted for at least 2 weeks following treatment. Future work will be directed at determining the specific time course of LFPI-induced odor changes.
Mechanisms underlying changes in body odor caused by pathogen or physical insult are poorly understood and the specificity of associated odor changes has rarely been explored (Alves et al. 2010; Arakawa et al. 2010; Kimball et al. 2013). However, it is unlikely that odor changes were directly related to changes in inflammatory cytokines. Immune markers such as interleukins IL-6, IL-10, and tumor necrosis factor (TNF-α) are elicited by treatment with LPS (Vodovotz et al. 2006). Not coincidentally, many of these same inflammatory markers are rapidly elevated and detected within hours after neurological injury (Buttram et al. 2007; Chiaretti et al. 2008; Kirchhoff et al. 2008); including animal model studies (Ziebell and Morganti-Kossmann 2010). However, many inflammatory cytokine levels commonly return to baseline after 24h following LPS administration in mice (Vodovotz et al. 2006). For example, following treatment with 4mg/kg LPS (twice the dose used in this study), IL-1β is no longer observed after 12h in the mouse model, whereas IL-6 levels are approaching baseline after 48h (Thomson et al. 2014). Furthermore, TNF-α was not evident at 6h following this dose of LPS. However, increased transcription of a number of interferon-stimulated genes (ISGs) was observed in the brain and peripheral blood leucocytes (PBL) in the first 6h following treatment and persisted for at least 48h (Thomson et al. 2014). It is also known that IL-10 expression is observed for several days following LPS-induced sepsis, however, the dose required for such a response may be several times larger than employed here (Howard et al. 1993).
Major histocompatibility complex (MHC; H-2 in mice) genes and genes coding for major urinary proteins (MUPs) are involved in odor signaling in mice and other species (Yamazaki et al. 1990; Beauchamp et al. 2000; Hurst and Beynon 2004). The treatments used in the current studies could in principle alter MHC- and/or MUP-regulated signaling by altering expression of odorants (MHC) or by influencing protein binding of odorants (MUPs) coded for by these gene families. Arguing against a role for MHC mediation of LFPI- or LPS-induced odor changes are recent finding in our lab suggesting that aspects of adaptive immunity, where MHC is centrally involved, may not participate in odor changes caused by elicitation of the immune system (Kimball et al. 2014). Additional studies would be required to specifically determine the roles MHC and/or MUPs might play in brain injury-induced odor changes.
Gut bacteria may have been involved in alteration of urine volatiles by acting upon changes in endogenous substrates resulting from inflammation. For example, endogenous gut bacteria were thought to play a significant role in the alteration of fecal odors following experimental infection of ducks with an avian influenza by acting on infection-caused increases of cellular pyruvate (Kimball et al. 2013). Conversely, host metabolic substrates may have remained constant whereas the gut microbiota was altered via inflammatory processes. Alteration of gut bacteria populations (i.e., dysbiosis) can be caused by intestinal inflammation (Faber and Baumler 2014; Cullen et al. 2015). Imbalances among dominant groups of microbiota have been linked to metabolic diseases such as obesity and diabetes (Burcelin et al. 2009). These same diseases possess distinct, yet undetermined, chronic inflammatory components—despite the lack of inflammatory pathophysiology (Tabas and Glass 2013). These metabolic exchanges between host and microbiome generate a “cometabolome” resulting from cometabolism of substrates (Nicholson et al. 2005). As a result, metabolomic profiling of urine as a tool for diagnosis of metabolic disorders has been known for some time because changes in the gut microbiome strongly impact urinary metabolites (Nicholson et al. 2005). The data presented here suggest that volatile metabolites associated with various forms of inflammatory conditions may also be a rich area for further investigation.
Finally, a significant problem with diagnosing mild to moderate TBI is the lack of rapid and robust noninvasive biomarkers (Zetterberg et al. 2013). Currently, the best tools for a noninvasive assessment of mild to moderate TBI are cognitive performance tests—requiring a priori baseline measures for each individual. Recently, promising leads in the search for protein-based biomarkers from CSF and blood have been identified (Siman et al. 2013; Zetterberg et al. 2013; Zetterberg and Blennow 2015). Based on the studies described here, we suggest that changes in volatile profiles of other emanations that are easily attainable, such as urine, sweat, or breath (Kim et al. 2012), may provide additional or complimentary information useful for diagnosis and warrant further investigation. In particular, it will be important to determine the time course for onset of the volatile changes and their persistence as well as how they relate to recovery in animal models and in human patients. Volatile signatures of TBI may not stand alone as a solitary diagnostic tool, but could be a rapid first step in a process of differential diagnosis that could be followed up with additional, more invasive, assessments.
Ethical approval
All experiments received prior approval by the Institutional Animal Care and Use Committees of both the Monell Center (#1153) and Children’s Hospital of Philadelphia (#2013-6-694).
Funding
This work was supported in part by US Army Telemedicine and Advanced Technology Research Center grant W81XWH-12-2-0081, EDMS 5584, “Odor signals of immune activation and CNS inflammation” (G.K.B., M.O.). Additional support was provided by NIH grants R37 HD59288 and RO1 NS069629 (A.S.C.) and the Knut and Alice Wallenberg Foundation (2012.0141; J.N.L.).
Acknowledgments
Data analysis and manuscript review comments of Drs Nicholas Beauchamp, Danielle Reed, Colin Smith, Patrick Millet, and Dan Wesson were greatly appreciated. Mention of specific products does not constitute endorsement by any institution.
References
- Alves GJ, Vismari L, Lazzarini R, Merusse JL, Palermo-Neto J. 2010. Odor cues from tumor-bearing mice induces neuroimmune changes. Behav Brain Res. 214(2):357–367. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Deak T. 2010. Sickness-related odor communication signals as determinants of social behavior in rat: a role for inflammatory processes. Horm Behav. 57(3):330–341. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Curran M, Yamazaki K. 2000. MHC-mediated fetal odourtypes expressed by pregnant females influence male associative behaviour. Anim Behav. 60(3):289–295. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Katahira K, Yamazaki K, Mennella JA, Bard J, Boyse EA. 1995. Evidence suggesting that the odortypes of pregnant women are a compound of maternal and fetal odortypes. Proc Natl Acad Sci U S A. 92(7):2617–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Yamazaki K, Curran M, Bard J, Boyse EA. 1994. Fetal H-2 odortypes are evident in the urine of pregnant female mice. Immunogenetics. 39(2):109–113. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B. 57:289–300. [Google Scholar]
- Bodyak N, Slotnick B. 1999. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 24(6):637–645. [DOI] [PubMed] [Google Scholar]
- Boillat M, Challet L, Rossier D, Kan C, Carleton A, Rodriguez I. 2015. The vomeronasal system mediates sick conspecific avoidance. Curr Biol. 25(2):251–255. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Kendrick KM. 2006. Mammalian social odours: attraction and individual recognition. Phil Trans R Soc B. 361:2061–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcelin R, Luche E, Serino M, Amar J. 2009. The gut microbiota ecology: a new opportunity for the treatment of metabolic diseases? Front Biosci. 14:5107–5117. [DOI] [PubMed] [Google Scholar]
- Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM. 2007. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 24(11):1707–1717. [DOI] [PubMed] [Google Scholar]
- Carriere KC. 2001. How good is a normal approximation for rates and proportions of low incidence events? Commun Stat Simul Comput. 30:327–337. [Google Scholar]
- Chiaretti A, Antonelli A, Mastrangelo A, Pezzotti P, Tortorolo L, Tosi F, Genovese O. 2008. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotrauma. 25(3):225–234. [DOI] [PubMed] [Google Scholar]
- Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. 2010. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 107(1):366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. 2015. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science. 347:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M, Van Beek TA, Posthumus MA, Ben Dom N, Van Bokhoven H, De Groot A. 1990. Isolation and identification of volatile kairomone that affects acarine predatorprey interactions. Involvement of host plant in its production. J Chem Ecol. 16(2):381–396. [DOI] [PubMed] [Google Scholar]
- Faber F, Baumler AJ. 2014. The impact of intestinal inflammation on the nutritional environment of the gut microbiota. Immunol Lett. 162:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai Y, Shimono K, Oka H, Baba Y, Yamazaki K, Beauchamp GK. 2012. Analysis of volatile organic compounds released from human lung cancer cells and from the urine of tumor-bearing mice. Cancer Cell Int. 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Slupsky CM. 2014. Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism. J Prot Res. 13:5281–5292. [DOI] [PubMed] [Google Scholar]
- Hoffer ME. 2015. Mild traumatic brain injury: neurosensory effects. Curr Opin Neurol. 28(1):74–77. [DOI] [PubMed] [Google Scholar]
- Howard M, Muchamuel T, Andrade S, Menon S. 1993. Interleukin-10 protects mice from lethal endotoxemia. J Exp Med. 177:1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ. 2004. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 26(12):1288–1298. [DOI] [PubMed] [Google Scholar]
- Johnston RE. 2003. Chemical communication in rodents: from pheromones to individual recognition. J Mammal. 84:1141–1162. [Google Scholar]
- Kang J, Zhu L, Lu J, Zhang X. 2015. Application of metabolomics in autoimmune diseases: insight into biomarkers and pathology. J Neuroimmunol. 279:25–32. [DOI] [PubMed] [Google Scholar]
- Kim KH, Jahan SA, Kabir E. 2012. A review of breath analysis for diagnosis of human health. Trends Anal Chem. 33:1–8. [Google Scholar]
- Kimball BA, Opiekun M, Yamazaki K, Beauchamp GK. 2014. Immunization alters body odor. Physiol Behav. 128:80–85. [DOI] [PubMed] [Google Scholar]
- Kimball BA, Yamazaki K, Kohler D, Bowen RA, Muth JP, Opiekun M, Beauchamp GK. 2013. Avian influenza infection alters fecal odor in mallards. PLoS One. 8(10):e75411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C, Buhmann S, Bogner V, Stegmaier J, Leidel BA, Braunstein V, Mutschler W, Biberthaler P. 2008. Cerebrospinal IL-10 concentration is elevated in non-survivors as compared to survivors after severe traumatic brain injury. Eur J Med Res. 13(10):464–468. [PubMed] [Google Scholar]
- Kwak J, Willse A, Matsumura K, Curran Opiekun M, Yi W, Preti G, Yamazaki K, Beauchamp GK. 2008. Genetically-based olfactory signatures persist despite dietary variation. PLoS One. 3(10):e3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J, Willse A, Preti G, Yamazaki K, Beauchamp GK. 2010. In search of the chemical basis for MHC odourtypes. Proc R Soc B. 277:2417–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz J, Witgen BM, Grady MS. 2007. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Behav Brain Res. 177(2):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Elkind J, Xiong GX, Galante R, Zhu JX, Zhang L, Lian J, Rodin J, Kuzma NN, Pack AI, et al. 2013. Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci Trans Med. 5:215ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen A. 2009. MetAlign: interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal Chem. 81(8):3079–3086. [DOI] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV. 2015. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. 11:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Opiekun M, Oka H, Vachani A, Albelda SM, Yamazaki K, Beauchamp GK. 2010. Urinary volatile compounds as biomarkers for lung cancer: a proof of principle study using odor signatures in mouse models of lung cancer. PLoS One. 5(1):e8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead M, Bartus RT, Dean RL, Miotke JA, Murphy S, Sall J, Goldman H. 1994. Histopathologic consequences of moderate concussion in an animal model: correlations with duration of unconsciousness. J Neurotrauma. 11(6):657–667. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Wilson ID. 2005. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 3(5):431–438. [DOI] [PubMed] [Google Scholar]
- Novotny MV. 2003. Pheromones, binding proteins and receptor responses in rodents. Biochem Soc Trans. 31(Pt 1):117–122. [DOI] [PubMed] [Google Scholar]
- Osada K, Yamazaki K, Curran M, Bard J, Smith BPC, Beauchamp GK. 2003. The scent of age. Proc R Soc B. 270:929–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn D, Potts WK. 1998. Chemical signals and parasite-mediated sexual selection. Trends Ecol Evol. 13:391–396. [DOI] [PubMed] [Google Scholar]
- Probert CS, Ahmed I, Khalid T, Johnson E, Smith S, Ratcliffe N. 2009. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J Gastrointestin Liver Dis. 18(3):337–343. [PubMed] [Google Scholar]
- Raghupathi R. 2004. Cell death mechanisms following traumatic brain. Brain Pathol. 14:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone AT, Tharmaradinam S, Jiang S, Rathbone MP, Kumbhare DA. 2015. A review of the neuro- and systemic inflammatory responses in post concussion symptoms: introduction of the “post-inflammatory brain syndrome” PIBS. Brain Behav Immun. 46:1–16. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. 2006. Speed-accuracy tradeoff in olfaction. Neuron. 51:351–358. [DOI] [PubMed] [Google Scholar]
- Rudnicka J, Walczak M, Kowalkowski T, Jezierski T, Buszewski B. 2014. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography-mass spectrometry versus trained dogs. Sensor Actuat B Chem. 202:615–621. [Google Scholar]
- Ruijschop RM, Burgering MJ, Jacobs MA, Boelrijk AE. 2009. Retro-nasal aroma release depends on both subject and product differences: a link to food intake regulation? Chem Senses. 34(5):395–403. [DOI] [PubMed] [Google Scholar]
- Schwende FJ, Wiesler D, Jorgenson JW, Carmack M, Novotny M. 1986. Urinary volatile constituents of the house mouse, Mus musculus, and their endocrine dependency. J Chem Ecol. 12(1):277–296. [DOI] [PubMed] [Google Scholar]
- Shirasu M, Touhara K. 2011. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 150(3):257–266. [DOI] [PubMed] [Google Scholar]
- Siman R, Giovannone N, Hanten G, Wilde EA, McCauley SR, Hunter JV, Li X, Levin HS, Smith DH. 2013. Evidence that the blood biomarker SNTF predicts brain imaging changes and persistent cognitive dysfunction in mild TBI patients. Front Neurol. 4:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick B. 2007. Response accuracy and odor sampling time in mice trained to discriminate between enantiomers of carvone and those of terpinen-4-ol. Chem Senses. 32(7):721–725. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Wesson DW, Baum MJ. 2008. Sexually dimorphic enhancement by estradiol of male urinary odor detection thresholds in mice. Behav Neurosci. 122(4):788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syhre M, Manning L, Phuanukoonnon S, Harino P, Chambers ST. 2009. The scent of Mycobacterium tuberculosis–part II breath. Tuberculosis (Edinb). 89(4):263–266. [DOI] [PubMed] [Google Scholar]
- Tabas I, Glass CK. 2013. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 339:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, McColl A, Cavanagh J, Graham GJ. 2014. Peripheral inflammation is associated with remote global gene expression changes in the brain. J Neuroinflammation. 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikunov YM, Laptenok S, Hall RD, Bovy A, de Vos RC. 2012. MSClust: a tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics. 8(4):714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodovotz Y, Chow CC, Bartels J, Lagoa C, Prince JM, Levy RM, Kumar R, Day J, Rubin J, Constantine G, et al. 2006. In silico models of acute inflammation in animals. Shock. 26:235–244. [DOI] [PubMed] [Google Scholar]
- Witgen BM, Lifshitz J, Smith ML, Schwarzbach E, Liang SL, Grady MS, Cohen AS. 2005. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience. 133(1):1–15. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Egorov IK, Bard J, Thomas L, Boyse EA. 1983. Sensory distinction between H-2b and H-2bm1 mutant mice. Proc Natl Acad Sci U S A. 80(18):5685–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Beauchamp GK, Imai Y, Bard J, Phelan SP, Thomas L, Boyse EA. 1990. Odor types determined by the major histocompatibility complex in germfree mice. Proc Natl Acad Sci U S A. 87(21):8413–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Boyse EA, Bard J, Curran M, Kim D, Ross SR, Beauchamp GK. 2002. Presence of mouse mammary tumor virus alters the body odor of mice. Proc Nat Acad Sci U S A. 99:5612–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Blennow K. 2015. Fluid markers of traumatic brain injury. Mol Cell Neurosci. 66:99–102. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Smith DH, Blennow K. 2013. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 9(4):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Sun LX, Bruce KE, Novotny MV. 2008. Chronic exposure of cat odor enhances aggression, urinary attractiveness and sex pheromones of mice. J Ethol. 26:279–286. [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. 2010. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 7(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]