Abstract
We present the package fslr, a set of R functions that interface with FSL (FMRIB Software Library), a commonly-used open-source software package for processing and analyzing neuroimaging data. The fslr package performs operations on ‘nifti’ image objects in R using command-line functions from FSL, and returns R objects back to the user. fslr allows users to develop image processing and analysis pipelines based on FSL functionality while interfacing with the functionality provided by R. We present an example of the analysis of structural magnetic resonance images, which demonstrates how R users can leverage the functionality of FSL without switching to shell commands.
Introduction
FSL (FMRIB Software Library) is a commonly-used software for processing and analyzing neuroimaging data (Jenkinson et al., 2012). This software provides open-source, command-line tools and a graphical user interface (GUI) for image processing tasks such as image smoothing, brain extraction (Smith, 2002), bias-field correction, segmentation (Zhang et al., 2001), and registration (Jenkinson and Smith, 2001; Jenkinson et al., 2002). Many of these functions are used extensively in medical imaging pipelines. According to a recent survey paper by Carp (2012), 13.9% of published neuroimaging studies used FSL.
There exist a number of R packages for reading and manipulating image data, including AnalyzeFMRI (Bordier et al., 2011), RNiftyReg (Clayden, 2015), and fmri (Tabelow and Polzehl, 2011) (see the Medical Imaging CRAN task view http://CRAN.R-project.org/view=MedicalImaging for more information). Although these packages are useful for performing image analysis, much of the fundamental functionality that FSL and other imaging software provide is not currently implemented in R. In particular, this includes algorithms for performing slice-time correction, motion correction, brain extraction, tissue-class segmentation, bias-field correction, co-registration, and normalization. This lack of functionality is currently hindering R users from performing complete analysis of image data within R. Instead of re-implementing FSL functions in R, we propose a user-friendly interface between R and FSL that preserves all the functionality of FSL, while retaining the advantages of using R. This will allow R users to implement complete imaging pipelines without necessarily learning software-specific syntax.
The fslr package relies heavily on the oro.nifti (Whitcher et al., 2011) package implementation of images (referred to as ‘nifti’ objects) that are in the Neuroimaging Informatics Technology Initiative (NIfTI) format, as well as other common image formats such as ANALYZE. oro.nifti also provides useful functions for plotting and manipulating images. fslr expands on the oro.nifti package by providing additional functions for manipulation of ‘nifti’ objects.
fslr workflow
The general workflow for most fslr functions that interface with FSL is as follows:
Filename or ‘nifti’ object is passed to fslr function.
FSL command is created within fslr function and executed using the system command.
Output is written to disk and/or read into R and returned from the function.
From the user's perspective, the input/output process is all within R. The advantage of this approach is that the user can read in an image, do manipulations of the ‘nifti’ object using standard syntax for arrays, and pass this object into the fslr function without using FSL-specific syntax written in a shell language. Also, one can perform image operations using FSL, perform operations on the ‘nifti’ object in R that would be more difficult using FSL, and then perform additional operations using FSL by passing that object to another fslr command. Thus, users can create complete pipelines for the analysis of imaging data by accessing FSL through fslr.
fslr setup
To use fslr, a working installation of FSL is required. The following code was run using FSL version 5.0.0. fslr must also have the path of FSL specified. If using R from a shell environment, and the FSLDIR environment variable is set (which can be done when installing FSL), fslr will use this as the path to FSL. If using R through a graphical user interface (GUI) such as RStudio (RStudio, Boston, MA), environmental variables and paths are not explicitly exported. Therefore, FSLDIR is not set, and the path to FSL can be specified using options(fsl.path = “/path/to/fsl”).
fslr also requires an output type for the format of images returned from FSL. Some fslr functions produce intermediate files that the user may want removed after the command is executed. When the filename argument is passed to a fslr command, the extension of the file does not need to be specified, but simply the prefix. When the command is executed, the FSL command appends an extension, and to remove this file, using the R command file.remove, the extension for the file is required. If working in a shell environment, fslr will use the environment variable for output type: FSLOUTPUTTYPE. If working in a GUI, the default is given by NIFTI_GZ, which returns compressed NIfTI images, ending in “.nii.gz”. This can be changed by setting the fsl.outputtype option. See http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/fsl/formats.html for a description of FSL output types.
The R code below is all that is needed to load the fslr package, set the path to FSL, and specify the output type for files, respectively.
library(fslr) options(fsl.path = “/usr/local/fsl”) options(fsl.outputtype = “NIFTI_GZ”)
Image preprocessing with fslr
We present a complete analysis of structural magnetic resonance imaging (MRI) data performed using fslr and R. Images were obtained from a patient with multiple sclerosis (MS) at 2 different visits (Sweeney et al., 2013), located at bit.ly/FSL_Data. At each visit, the image modalities obtained were T1-weighted (T1), T2-weighted (T2), fluid-attenuated inversion recovery (FLAIR), and proton density (PD). In this example we will perform a MRI bias-field correction using FAST (FMRIB's Automated Segmentation Tool; Zhang et al. 2001), co-register scans within visits to the T1 image of that visit, and register T1 images between visits. Once these operations have been performed, one can take within-modality difference images to see the changes between visits. We will also register all images to a common stereotaxic template, as this is common in population-based analyses.
Bias-field correction
MRI images typically exhibit good contrast between soft tissue classes, but intensity inhomogeneities in the radio frequency (RF) field can cause differences in the ranges of tissue types at different spatial locations. These inhomogeneities can cause problems with algorithms based on histograms, quantiles, or raw intensities (Zhang et al., 2001). Therefore, correction for image inhomogeneities is a crucial step in many analyses. FSL implements the bias-field correction from Guillemaud and Brady (1997) in its FAST segmentation pipeline (Zhang et al., 2001).
The fsl_biascorrect command from fslr will create the corrected images. We pass in the filename in the file argument, any additional options for FSL in the opts argument, such as -v for verbose diagnostic outputs, and the output filename in the outfile argument, which is our inhomogeneity-corrected image.
fsl_biascorrect(file = “01-Baseline_T1.nii.gz”, outfile = “01-Baseline_T1_FSL_BiasCorrect”, opts = “-v”)
We can observe the difference in voxel values from the baseline T1 image compared to the bias-corrected version in Figure 1. In Panel A we display the T1 image, and in Panel B we display the bias-corrected T1 image. The T1 image is brighter in the middle of the image, while the bias-corrected image is more uniform in white matter (brighter regions). As this difference may be hard to distinguish visually, we present the scatterplot of these images in Figure 1C, using the ggplot2 package (Wickham, 2009). Note, both scales are in arbitrary units (a.u.).
Figure 1. Results of inhomogeneity correction.
We present the original T1 image (A), bias-corrected T1 image (B), and the scatterplot of the sampled values comparing the values from the T1 image to the bias-corrected values (C). We see in Panel C for values in the low range of the data (< 10), the T1 values and bias-corrected T1 values, on average, fall along the diagonal (blue line), which is further illustrated in Panel D, which plots values < 40. Values > 10 for the original T1 image are lower than the bias-corrected T1 values shown by a generalized additive model (GAM) smoother (pink line, Panel C).
The blue line in Figure 1C represents the 45° diagonal line, where the original and bias-corrected image intensities are equal (X = Y), and the pink line represents a generalized additive model (GAM) (Hastie and Tibshirani, 1990) scatterplot smoother estimate obtained using the mgcv package (Wood, 2011). We see that for values in the low range of the data (< 10), the T1 values and bias-corrected T1 values, on average, fall along the diagonal, but in the higher range the bias-corrected values are lower.
Within-visit co-registration
All subsequent steps will be performed on the bias-corrected images. We will first co-register the images within each separate visit to the T1 image from that visit. This operation overlays the images on one another and allows us to investigate joint distributions of voxel intensities from different image modalities. This is performed using FMRIB's Linear Image Registration Tool (FLIRT; Jenkinson and Smith 2001; Jenkinson et al. 2002). As the images are from the same individual, we may assume that the overall shape of the brain has not changed, but each scan may have undergone a translation and/or rotation in space. Therefore, we will use a rigid-body transformation, with 6 degrees of freedom (dof).
The fslr command flirt calls the FSL command flirt, taking the input image (infile) and the reference image that serves as a template (reffile). Any additional options for FLIRT can be passed using the opts argument. We will use the defaults (i.e. trilinear interpolation) and the -v option for diagnostic messages to be printed. Since we are doing a rigid-body transformation, we set the degrees of freedom (dof) to 6. Here we present the code for registering the baseline T2 image to the baseline T1 image; we will subsequently repeat this process for the baseline FLAIR and PD images and for the follow-up scans.
flirt(reffile = “01-Baseline_T1_FSL_BiasCorrect”, infile = “01-Baseline_T2_FSL_BiasCorrect”, omat = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1.mat”, dof = 6, outfile = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1”, opts = “-v”)
The resulting image transformation is stored using the file name passed to the omat (output matrix) argument. This matrix can be used to transform other images, that were in the same space as the input image, to the reference image space. The fslr package will currently only return ‘nifti’ objects, and not a list of objects, such as the output image, transformation matrix, etc. Thus, any transformation files that are needed after the command is executed must be specified.
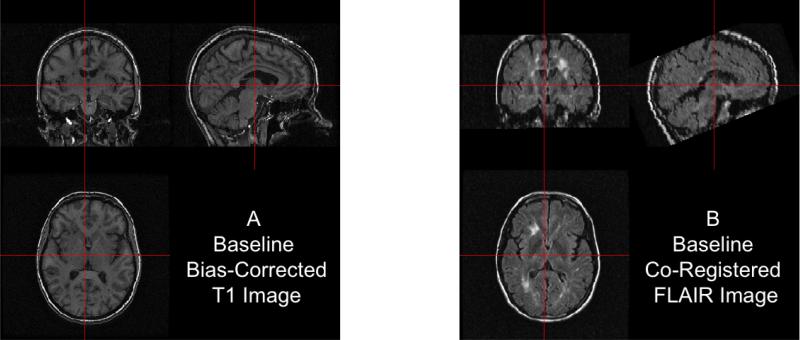
After co-registration, one could compare images of different modalities at the same voxels, such as T1 versus FLAIR images, which is presented in Figure 2. The images are presented at the same cross section for the baseline T1 (Panel 2A) and FLAIR (Panel 2B) images. The same brain areas are presented in each modality, indicating adequate registration.
Figure 2. Results of within-visit co-registration.
We present the bias-corrected T1 image (A) and the co-registered bias-corrected FLAIR image (B).
In the previous example, we presented a rigid-body transformation, using the default parameters. flirt has options for different cost functions to optimize over, interpolation operators to estimate voxel intensity, and additional degrees of freedom for performing affine transformations. These options can be passed to the FSL flirt command using the opts argument in the fslr flirt function.
Note that each fslr function has a corresponding help function, which is the fslr command appended with .help(), which prints out the FSL help page for that function. For example, users can see which options can be changed in the FSL flirt command by executing the flirt.help() function. Additional non-linear registration techniques are presented in Section “Registration to the MNI template”.
Between-visit co-registration
Though across-modality comparisons can be achieved by performing within-visit co-registration, across-visit registration is required for assessing within-modality differences between longitudinal scans. To compute difference images, we co-register follow-up images to the baseline images within each modality. Similar to the within-visit co-registration, we use a rigid-body transformation. We will register the T1 images from baseline and follow-up, and apply this transformation to the co-registered-to-T1 images from above (see Figure 3 for illustration).
Figure 3. Between-visit registration process.
First, we registered all scans within a visit (baseline or follow-up) to the T1 image. We then registered the follow-up T1 image to the baseline T1 image and applied the transformation to the follow-up T2, FLAIR, and PD images previously co-registered to the follow-up T1 image.
Though this registration involves two interpolations of the data and may not be optimal for within-modality comparisons, we have already obtained the co-registered-to-T1 images in Section “Within-visit co-registration” and must perform only one additional registration. This operation also demonstrates how to apply transformation matrices in fslr. Here we register the follow-up T1 image to the baseline T1 image, again using a rigid-body transformation (6 dof):
flirt(reffile = “01-Baseline_T1_FSL_BiasCorrect”, infile = “01-Followup_T1_FSL_BiasCorrect”, omat = “01-Followup_T1_FSL_BiasCorrect_rigid_to_BaseT1.mat”, dof = 6, outfile = “01-Followup_T1_FSL_BiasCorrect_rigid_to_BaseT1”, opts = “-v”)
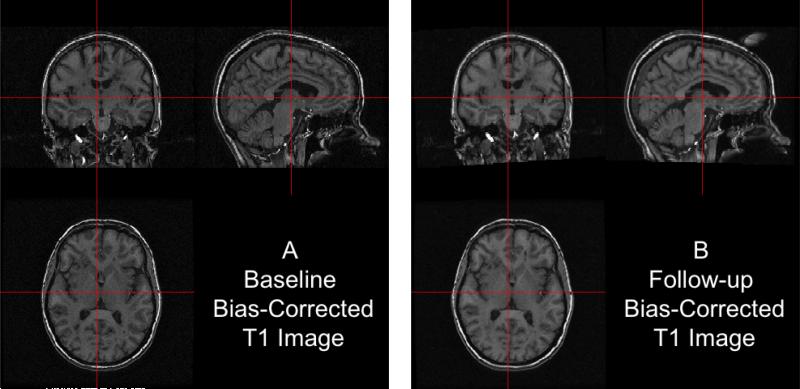
Now, both T1 images are aligned in the space of the baseline T1 image. We present the results in Figure 4: the bias-corrected baseline T1 image in Panel A and the co-registered bias-corrected follow-up T1 in Panel B. The images displayed at the same cross section correspond to the same brain area, indicating a good registration.
Figure 4. Results from FLIRT.
The bias-corrected baseline T1 is presented in (A) and the registered bias-corrected follow-up T1 is presented in (B), each displayed at the same intersection. We observe that the observed images correspond to the same brain area, indicating a good registration.
Using the flirt_apply function from fslr, we can apply the transformation matrix to the T2, PD, and FLAIR images from the follow-up visit, previously co-registered to the T1 from follow-up, to align them to the baseline T1 image space. The code below aligns the follow-up T2 image, previously registered to the follow-up T1 image, to the baseline T1 image:
flirt_apply(reffile = “01-Baseline_T1_FSL_BiasCorrect”, # register to this infile = “01-Followup_T2_FSL_BiasCorrect_rigid_to_T1”, # reg to Followup T1 initmat = “01-Followup_T1_FSL_BiasCorrect_rigid_to_BaseT1.mat”, #transform outfile = “01-Followup_T2_FSL_BiasCorrect_rigid_to_BaseT1” # output file )
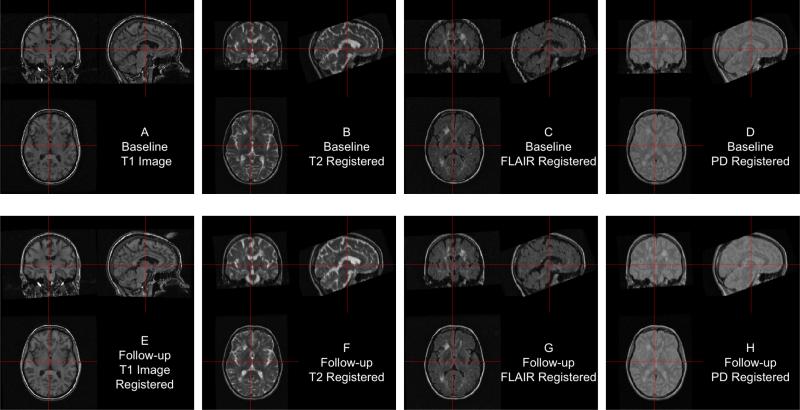
In Figure 5, we display each image after FLIRT has been applied. Each image is in the baseline T1 image space, displayed at the same cross section. Each panel shows the same brain areas across modalities, indicating adequate registration. We see that some areas of the brain are cropped from the field of view, which may be problematic if relevant brain areas are removed. We have registered all images with the skull and extracranial tissue included. A better method may be to perform registration on brain tissues only, in which case we must perform brain extraction.
Figure 5. Between-visit registration results.
The complete set of acquired images, first co-registered within visit to the T1 image of that visit, then registered to the baseline T1 image using the follow-up T1 to baseline T1 transformation matrix. All registrations performed rigid-body transformations.
Brain extraction
The process of extracting brain tissue from the acquired image, referred to as brain extraction or skull stripping, is a crucial step in many analyses. We will perform brain extraction using FSL's brain extraction tool (BET; Smith 2002; Jenkinson et al. 2005) using parameters recommended by Popescu et al. (2012), which were derived from patients with MS. No other published R package on CRAN (e.g. AnalyzeFMRI, RNiftyReg, or fmri) has brain extraction functionality for brain imaging. Other neuroimaging software provide brain extraction, such as AFNI (Cox, 1996), SPM (Ashburner and Friston, 2005), and Freesurfer (Fischl, 2012), and multi-atlas label fusion techniques (Doshi et al., 2013).
fslbet(infile = “01-Baseline_T1”, outfile = “01-Baseline_T1_FSL_BiasCorrect_Brain”, opts = “-B -f 0.1 -v”, # from Popescu et al. betcmd = “bet”, intern = FALSE)
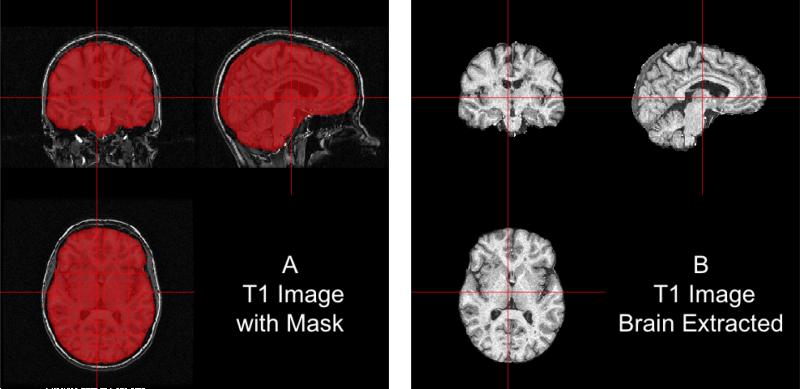
We ran BET on the non-corrected T1 image as the -B option performs inhomogeneity correction from FAST as part of the procedure. The option -f 0.1 denotes the fractional intensity (FI) parameter in BET: it varies between 0 and 1 and determines the location of the edge of the segmented brain image; smaller values correspond to larger brain masks. In Figure 6, the bias-corrected T1 image is shown with the brain mask overlaid in red (Panel A) and the resulting masked brain (Panel B). We see that the brain extraction performed well, not including any areas of the skull or the neck while not discarding brain tissue. Towards the back of the brain, some areas of the subarachnoid space remain, which may be unacceptable for certain analyses, such as estimation of the volume of brain tissue.
Figure 6. Results from BET.
In (A), we show the bias-corrected T1 image with the mask from BET overlaid in red. In (B), we display the extracted brain. We see that the brain extraction performed well, not including any areas of the skull or the neck while not discarding large areas of the brain.
Note that fslbet writes both a file containing the brain-extracted image and another image containing the binary brain mask. As all other images are registered to the baseline T1 space, we can use this mask to extract the brain from other images, such as the baseline T2 image, using the fslr function fslmask. In this example, we mask the registered-to-T1, bias-corrected T2 image with the binary brain mask, save the image to the filename specified in outfile, and also set the retimg option to TRUE, indicating the fslmask command to return the image. The returned object is a ‘nifti’ object, assigned to the R object mask:
mask <- fslmask(file = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1”, mask = “01-Baseline_T1_FSL_BiasCorrect_Brain_mask”, outfile = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1_Brain”, retimg = TRUE)
We now have all images in the same stereotaxic space with the brain extracted.
Registration to the MNI template
In many studies, information is aggregated across a population of images from different participants. For the information to have the same interpretation spatially across participants, images from all participants need to be aligned in the same stereotaxic space (“template” space), requiring registration to a standard template image. A frequently used set of templates are provided by MNI (Montreal Neurological Institute). We have registered the baseline T1 image to the MNI T1 template (Grabner et al., 2006), included with FSL. As an individual's brain does not necessarily have the same size as the template, it is not appropriate to use rigid-body transformations. Instead, non-linear transformations are needed. As these are patients with MS and have lesions, different non-linear registrations to template space can have a large impact on the outcome of analysis (see Eloyan et al. 2014 for discussion).
We will first register the baseline T1 image to the T1 template using an affine registration, which can perform scaling and shearing operations in addition to translation and rotation. Although an affine transformation has more degrees of freedom than a rigid transformation, it may not provide a registration sufficient for analysis. We will then use FNIRT (FMRIB's Nonlinear Image Registration Tool) to achieve better overlap of local brain structures (Jenkinson et al., 2012; Andersson et al., 2007). As we are concerned with good overlap only in brain structures, and not in areas such as the skull, we will register the brain-extracted brain images to the brain-only template. The fslr function fnirt_with_affine will register using flirt with an affine transformation and then non-linearly register this image to the template using fnirt. If this affine transformation is not executed before fnirt, the image will not achieve the necessary scaling into the template space.
fnirt_with_affine(infile = “01-Baseline_T1_FSL_BiasCorrect_Brain”, reffile = file.path(fsldir(), “data”, “standard”, “MNI152_T1_1mm_brain”), flirt.omat = “01-Baseline_T1_FSL_BiasCorrect_Brain_affine_toMNI.mat”, flirt.outfile = “01-Baseline_T1_FSL_BiasCorrect_Brain_affine_toMNI”, outfile = “01-Baseline_T1_FSL_BiasCorrect_Brain_toMNI”)
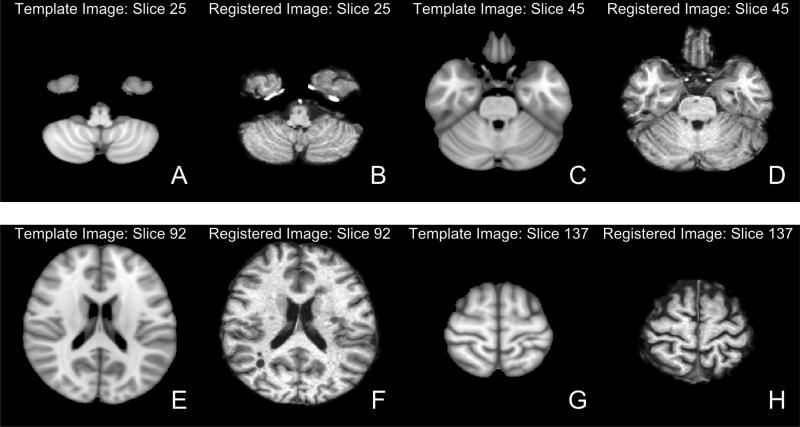
The results of the registration can be seen in Figure 7. Each panel represents a different axial slice (z = 25, 45, 92, or 137) in the template space of the template image (A, C, E, G) or the registered T1 image (B, D, F, H). Each slice shows the registered T1 image has similar brain structure represented in the same area as the template image, indicating good registration.
Figure 7. Results from FNIRT.
We present different axial slices of the template (A, C, E, G) and the registered T1 image (B, D, F, H). The slices represented are 25 (A, B), 45 (C, D), 92 (E, F) and 137 (G, H). We note that areas of the brain coincide between the template and registered image.
Applying transformations to co-registered data
Since all the data is represented in the same image space, we can apply the estimated affine transformation and non-linear warping coefficient field to each image to represent that image in template space. The affine transformation must be applied with flirt_apply and the warping coefficient using fsl_applywarp, which calls the FSL applywarp function.
Here we present the application of the transformations to the baseline T2 image, previously registered to the baseline T1.
flirt_apply(infile = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1_Brain”, reffile = file.path(fsldir(), “data”, “standard”, “MNI152_T1_1mm_brain”), initmat = “01-Baseline_T1_FSL_BiasCorrect_Brain_affine_toMNI.mat”, outfile = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1_Brain_toMNI”) fsl_applywarp(infile = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1_Brain_toMNI”, reffile = file.path(fsldir(), “data”, “standard”, “MNI152_T1_1mm_brain”), warpfile = “01-Baseline_T1_FSL_BiasCorrect_Brain_affine_toMNI_warpcoef”, outfile = “01-Baseline_T2_FSL_BiasCorrect_rigid_to_T1_Brain_toMNI”)
These two operations can also be performed in a single call to the fslr fnirt_with_affine_apply function.
With multiple participants, this process yields a multi-person, multi-modal, longitudinal imaging dataset that can be used for analyses.
Conclusion
The neuroimaging community has developed a large collection of tools for image processing and analysis. R has a number of packages to perform operations on images; EBImage is one good example (Pau et al., 2010). Much of the fundamental functionality of neuroimage processing is not currently available in R, such as brain extraction and tissue segmentation. We present fslr to provide R users functions for image processing and analysis that are based on FSL, an established image processing and analysis software suite. Interfacing R with existing, powerful software provides users with thoroughly-tested software and an additional community of users, which would not be available if the functions were rewritten in R. fslr should be easy to use for any standard R user; the workflow allows R users to manipulate array-like ‘nifti’ objects, pass them to fslr functions, which return ‘nifti’ objects. Moreover, as FSL and R are open-source and free, this software is readily available to all users.
There has been an increasing popularity of similar interfacing of tools within the Python community such as Nipype (Gorgolewski et al. 2011; https://qa.debian.org/popcon.php?package=nipype). As many users of R may not have experience with Python or bash scripting, we believe fslr provides a lower threshold for use in the R community. Other packages provide R users additional neuroimaging processing functionality such as AnalyzeFMRI, RNiftyReg, and fmri.
For example, other inhomogeneity correction methods exist, such as the popular N3 (Sled et al., 1998) and N4 (Tustison et al., 2010), methods which are not implemented in fslr. ANTsR (Avants et al., 2015) is an R package that interfaces with the ANTs (advanced normalization tools) software suite (Avants et al., 2011). ANTs has implementations of these correction methods, an increased set of registration techniques, and other methods for image processing. Other packages such as this, along with fslr, can create a diverse set of tools for neuroimaging within R, building on preexisting and widely-accepted software.
Most importantly, as fslr is based on the R framework, all the benefits of using R are available, such as dynamic documents, reproducible reports, customized figures, and state-of-the-art statistical methods. These benefits provide unique functionality compared to other software packages for neuroimaging.
All data and code processed here is located at https://github.com/muschellij2/FSLR_Data.
Supplementary Material
Glossary of acronyms
- MRI
Magnetic Resonance Imaging/Image
- PD
Proton Density
- FLAIR
Fluid-Attenuated Inversion Recovery
- MS
Multiple Sclerosis
- FMRIB
Functional MRI of the Brain Group
- MNI
Montreal Neurological Institute
- FSL
FMRIB Software Library
- FAST
FMRIB's Automated Segmentation Tool
- FLIRT
FMRIB's Linear Image Registration Tool
- BET
Brain Extraction Tool
- FNIRT
FMRIB's Nonlinear Image Registration Tool
Contributor Information
John Muschelli, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21231, USA, jmuschel@jhsph.edu.
Elizabeth Sweeney, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21231, USA; Special Volunteer Translational Neuroradiology Unit, Neuroimmunology Branch, National Institute of Neurological Disease and Stroke, National Institute of Health, Bethesda, MD 20892, USA, emsweene@jhsph.edu.
Martin Lindquist, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21231, USA, mlindqui@jhsph.edu.
Ciprian Crainiceanu, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21231, USA, ccrainic@jhsph.edu.
Bibliography
- Andersson JL, Jenkinson M, Smith S. FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. Non-linear registration, aka Spatial normalisation. URL http://fmrib.medsci.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf. [p169] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [p167] [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011 Feb;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [p170] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Kandel BM, Duda JT, Cook PA, Tustison NJ. ANTsR: An R Package Providing ANTs Features in R. R package version 0.3.1. 2015 URL http://stnava.github.io/ANTsR/ [p170]
- Bordier C, Dojat M, de Micheaux PL. Temporal and spatial independent component analysis for fMRI data sets embedded in the AnalyzeFMRI R package. Journal of Statistical Software. 2011;44(9):1–24. URL http://www.jstatsoft.org/v44/i09/. [p163] [Google Scholar]
- Carp J. The secret lives of experiments: Methods reporting in the fMRI literature. NeuroImage. 2012 Oct;63(1):289–300. doi: 10.1016/j.neuroimage.2012.07.004. [p163] [DOI] [PubMed] [Google Scholar]
- Clayden J. RNiftyReg: Medical Image Registration Using the NiftyReg Library. R package version 1.1.3, based on original code by Marc Modat and Pankaj Daga. 2015 URL http://CRAN.R-project.org/package=RNiftyReg [p163]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroim-ages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [p167] [DOI] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-atlas skull-stripping. Academic Radiology. 2013 Dec;20(12):1566–1576. doi: 10.1016/j.acra.2013.09.010. [p167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloyan A, Shou H, Shinohara RT, Sweeney EM, Nebel MB, Cuzzocreo JL, Calabresi PA, Reich DS, Lindquist MA, Crainiceanu CM. Health effects of lesion localization in multiple sclerosis: Spatial registration and confounding adjustment. PLoS ONE. 2014 Sep;9(9):e107263. doi: 10.1371/journal.pone.0107263. [p169] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [p167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS. Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Frontiers in Neuroinformatics. 2011;5(13):170. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: An application to the hippocampus in older adults. In: Hutchison D, Kanade T, Kittler J, Kleinberg JM, Mattern F, Mitchell JC, Naor M, Nierstrasz O, Rangan CP, Steffen B, Sudan M, Terzopoulos D, Tygar D, Vardi MY, Weikum G, Larsen R, Nielsen M, Sporring J, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2006. Vol. 4191. Springer Berlin Heidelberg; Berlin, Heidelberg: 2006. pp. 58–66. [p169] [DOI] [PubMed] [Google Scholar]
- Guillemaud R, Brady M. Estimating the bias field of MR images. IEEE Transactions on Medical Imaging. 1997;16(3):238–251. doi: 10.1109/42.585758. [p164] [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized Additive Models. Vol. 43. CRC Press; 1990. [p164] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [p163, 165] [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [p163, 165] [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, Smith S. BET2: MR-based estimation of brain, skull and scalp surfaces. Eleventh Annual Meeting of the Organization for Human Brain Mapping. 2005;17:167. URL http://mickaelpechaud.free.fr/these/HBM05.pdf. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012 Aug;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [p163, 169] [DOI] [PubMed] [Google Scholar]
- Pau G, Fuchs F, Sklyar O, Boutros M, Huber W. EBImage—an R package for image processing with applications to cellular phenotypes. Bioinformatics. 2010;26(7):979–981. doi: 10.1093/bioinformatics/btq046. [p170] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu V, Battaglini M, Hoogstrate WS, Verfaillie SCJ, Sluimer IC, van Schijndel RA, van Dijk BW, Cover KS, Knol DL, Jenkinson M, Barkhof F, de Stefano N, Vrenken H. Optimizing parameter choice for FSL-Brain Extraction Tool (BET) on 3d T1 images in multiple sclerosis. NeuroImage. 2012 Jul;61(4):1484–1494. doi: 10.1016/j.neuroimage.2012.03.074. [p167] [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [p170] [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002 Nov;17(3):143–155. doi: 10.1002/hbm.10062. [p163, 167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney EM, Shinohara RT, Shea CD, Reich DS, Crainiceanu CM. Automatic lesion incidence estimation and detection in multiple sclerosis using multisequence longitudinal MRI. American Journal of Neuroradiology. 2013 Jan;34(1):68–73. doi: 10.3174/ajnr.A3172. [p164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabelow K, Polzehl J. Statistical parametric maps for functional MRI experiments in R: The package fmri. Journal of Statistical Software. 2011;44(11):1–21. URL http://www.jstatsoft.org/v44/i11/. [p163] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4itk: Improved N3 bias correction. IEEE Transactions on Medical Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [p170] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcher B, Schmid VJ, Thornton A. Working with the DICOM and NIfTI data standards in R. Journal of Statistical Software. 2011;44(6):1–28. URL http://www.jstatsoft.org/v44/i06/. [p163] [Google Scholar]
- Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; 2009. URL http://had.co.nz/ ggplot2/book. [p164] [Google Scholar]
- Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semi-parametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2011;73(1):3–36. [p164] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [p163, 164] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.