Summary
Human papillomavirus (HPV) is a worldwide public health problem, particularly in resource-limited countries. Fifteen high-risk genital HPV types are sexually transmitted and cause 5% of all cancers worldwide, primarily cervical, anogenital and oropharyngeal carcinomas. Skin HPV types are generally associated with benign disease, but a subset is linked to non-melanoma skin cancer. Licensed HPV vaccines based on virus-like particles (VLPs) derived from L1 major capsid antigen of key high risk HPVs are effective at preventing these infections but do not cover cutaneous types and are not therapeutic. Vaccines targeting L2 minor capsid antigen, some using capsid display, adjuvant and fusions with early HPV antigens or Toll-like receptor agonists, are in development to fill these gaps. Progress and challenges with L2-based vaccines are summarized.
Keywords: human papillomavirus, cervical cancer, oropharyngeal cancer, anogenital cancer, non-melanoma skin cancer, L2, capsid display, multimer, toll-like receptor agonist, adjuvant
The problem: Human Papillomaviruses and their associated diseases
Human papillomavirus (HPV) infection is ubiquitous and there are more than 180 known HPV genotypes. Papillomaviruses have a ~8Kb double stranded and covalent closed circular DNA genome [1]. HPV genotypes are classified taxonomically in to five genera (alpha, beta, gamma, mu, or nu) based upon L1 sequence homologies. The alpha HPV types are trophic for anogenital mucosa (i.e. `genital types') and typically transmitted via sexual intercourse, whereas the beta, gamma, mu and nu HPV preferentially infect skin (i.e. `cutaneous types') and are transmitted non-sexually, likely via fomites at sites of local epithelial micro-trauma [2]. The approximately forty genital HPV of the genus alpha are divided by oncogenicity into two groups: low-risk types (lrHPV such as genotypes −6,−11,−40,−42,−43,−44,−53,−54,−51,−72,−73,−81) which are isolated from benign genital warts, and 15 or so high-risk types (hr HPV, notably genotypes 16,−18,−31,−33,−35,−45,−51,−52,−56,−58,−59,−68) that are detected in cervical cancer [3]. Over 95% of cervical cancers are hrHPV+ leading to a consensus that persistent hrHPV infection is a necessary cause of cervical cancer [4]. HPV16 and HPV18 are associated with 50–60% and 20% of all cases of cervical cancer and have therefore been the focus of early vaccine and therapeutic development efforts [5]. Importantly, HPV16 is also associated with ~90% of all HPV+ anal, vaginal, vulval, penile and oropharyngeal cancers [6]. Indeed, hrHPV infection is responsible for 5% of all cancer cases worldwide (notably 10% of all cancers of women worldwide) [7]. This impact is driven by high cervical cancer incidence in developing countries (>85% of cases) that lack the resources and infrastructure for routine Pap screening for high grade cervical intraepithelial lesions (CIN2/3) and intervention to remove or ablate this precursor lesion. The incidence of HPV-associated cancer is elevated in immunocompromised patients, including those with concurrent HIV infection, and the disproportionate impact of HIV and lack of treatment has further contributed to the death toll of cervical cancer in developing countries [8]. Therefore a broad-spectrum preventive HPV vaccine that can be delivered globally has the potential to dramatically reduce future cases of cervical and other HPV-related cancers [7].
The genus beta HPV infect skin and the archetypal HPV5 and 8 were first isolated from papillomas of patients with the rare inherited syndrome epidermodysplasia verruciformis (EV) [9]. EV patients develop warts and macular lesions that persist, and by their 40s 30–60% develop multifocal carcinoma in situ or squamous cell cancer (SCC). Since 90% of SCC in EV patients have been reported contain HPV5/8, these types have been classified as potentially oncogenic [10]. Beta HPV may also be the causal agent for non-melanoma skin cancer (NMSC) in solid organ transplant recipients, of whom 90% develop skin warts and 40% develop NMSC within 15 years of their transplant [11]. The role of beta HPV in the development of NMSCs in other healthy patients is much more controversial. Dissection of their role in immune competent patients is complicated by the frequency of their detection in the absence of disease, a large number of genotypes, a different proposed mechanism of transformation to alpha HPVs in cervical cancer involving exposure to UV light, and the failure to detect beta HPV in most SCC cells requiring a hit-and-run type mechanism [9, 12].
Benign papillomas, notably hand and foot warts associated with some alpha (α2) species, gamma, mu, or nu HPV, are also associated with considerable morbidity and costs to the healthcare system associated with repeated destructive treatments [13] [14]. These lesions are especially common in childhood and although most regress in a year they can be recalcitrant and may even progress to squamous cell cancer in immunocompromised patients.
Licensed prophylactic HPV vaccines
The etiologic connection between HPV16 and HPV18 (and other common hrHPV) drove the development of prophylactic HPV vaccines using a cocktail of virus-like particles (VLPs) derived from the major capsid protein L1 of each of these types [15]. The first-generation vaccines to market were the bivalent product Cervarix (GlaxoSmithKline) containing L1 VLP of HPV16 and 18 adjuvanted with alum and monophosphoryl lipid A [16], and the alum-adjuvanted quadrivalent product Gardasil (Merck & Co.) which also includes L1 VLP of HPV 6 and 11 to protect against genital warts [17]. Although VLPs are a highly immunogenic antigen and provide almost complete prophylaxis against new infections with the HPV type from which it was derived, protection is largely type-restricted [18]. This limited breadth of protection drove the need for multi-type L1 VLP formulations to broaden protection among the 15 hrHPV contributing to cervical cancer, although this increases production costs. In 2014 the nonavalent L1 VLP vaccine Gardasil 9 was approved in the USA. Gardasil 9 targets 7 hrHPV types (−16, −18, −31,−33,−45,−52,−58) accounting for ~90% of cervical cancer cases, and HPV 6 and 11 that are present in ~90% of genital warts [19].
Remaining challenges and scope of review
The licensed vaccines are remarkably effective for protection against the targeted alpha HPV types associated with cervical cancer as well as other anogenital and orophayryngeal cancers. Although some less common hrHPV types may not be adequately covered, the primary challenge is global distribution of these vaccines. Major impediments to delivery in the countries that most need these vaccines are cost, which may be approached through local and/or generic production, bundling or negotiations by a global vaccine alliance such as GAVI (http://www.gavi.org/), the need for a cold chain, which may be mitigated through lyophilization or protectants, and the need for multiple immunizations, although promising results have been obtained with a single dose and certainly 2-dose regimens [20, 21].
Although there have been some hints of clinical activity using L1 VLP (without adjuvant) in patients with warts [22], there is no conclusive evidence that vaccination with either L1 or L2-based vaccines provides therapeutic activity in clearly established lesions; this likely reflects an absence of capsid antigen expression in the basal epithelial cells that harbor the virus, and the restriction of late protein expression to the uppermost layers of terminally differentiated cells [23]. This contrasts with the consistent expression of E6 and E7 in all HPV infected cells, and thus most therapeutic vaccination strategies target one or both, and less commonly other early viral antigens such as E1 or E2 [24]. Current VLP-based vaccine protection is limited to individuals naïve to virus and therefore the impact of the vaccine is reduced in the older adult population already exposed to HPV. Because of the long lead time for the development of cervical cancer, the impact of HPV vaccination on incidence has not been demonstrated yet (although likely soon given that the vaccine has been clinically available for a decade).
The licensed vaccines do not target the HPV types associated with cutaneous infections, including those linked with NMSC [12]. In addition, the coverage of types is not complete for cervical cancer or genital warts although the dominant types are covered by Gardasil 9.
While the licensed vaccines exclusively target L1 [25], numerous pre-clinical studies have also demonstrated the promise of minor capsid protein L2 as a protective antigen [26–29] and, while controversial, in some studies a therapeutic activity has also been proposed [30, 31]. In this review we describe the potential and status of candidate next-generation HPV vaccines based upon the minor capsid antigen L2 developed with the intent of addressing many of these issues including cost reduction, broader protection including non-genital HPV, fewer doses, stability at ambient temperature and therapeutic efficacy.
Clinical studies of vaccines that include L2
Several clinical studies with therapeutic intent have been performed using L2 as a fusion protein with early viral antigens. These studies have centered on two related fusion proteins expressed in E. coli and purified as filterable amorphous aggregates; HPV6 L2E7 termed TA-GW that was intended for the treatment of genital warts, and HPV16 L2E7E6 termed TA-CIN developed for the treatment of HPV16-related intraepithelial neoplasia. A phase I study in 42 men with genital warts examined three intramuscular (i.m.) doses of 0, 3, 30 or 300 μg TA-GW formulated on aluminum hydroxide, and also compared administration at 0, 1 and 4 weeks or 0, 4 and 8 weeks [32]. Vaccination was well tolerated and elicited dose-dependent and durable HPV-specific T cell and antibody responses. The 300 μg TA-GW dose and 0, 1, 4 week schedule were used for a phase IIa study in 27 patients with genital warts [33]. T cell proliferative and antibody responses were induced and 5/27 cleared their warts within 8 weeks (although it is not clear if this was related to vaccination). The remainder received conventional therapy thereafter, and none of the 13/27 that cleared their lesions recurred [33]. A phase IIb study in 320 subjects with HPV6 and/or 11-associated genital warts examined vaccination using HPV6 L2E7 formulated in ASO2A (which contains monophosphoryl lipid A (MPL) and saponin QS21 and an oil-in-water emulsion) versus placebo [34]. In one cohort, 191 patients received lesion ablation and vaccination with either HPV6 L2E7 in AS02A or placebo. However there was no difference in the recurrence rate at 6 months after treatment between the vaccine and placebo arms. A second cohort of 129 subjects was treated with podophyllotoxin application to the lesion and vaccination with either HPV6 L2E7 in AS02A or placebo. Unfortunately, again there was no significant difference in the clearance of anogenital warts between the vaccine and placebo groups at month 2 post treatment. However, a sub-analysis of subjects infected with HPV6 alone showed a positive trend toward clearance in the vaccine group [34].
The TA-CIN vaccine at doses of 26, 128 and 533 μg was administered intramuscularly without adjuvant on weeks 0, 4 and 8 in a placebo-controlled phase I study in 40 healthy volunteers [35]. The vaccine was well tolerated and induced dose-dependent HPV-specific T cell proliferative and antibody responses.
As an approach to boost early antigen-specific responses, a second study examined three monthly i.m. doses of TA-CIN followed by a heterologous boost by a single dermal scarification with a recombinant vaccinia virus expression HPV16/18 E6/E7 (termed TA-HPV) in 29 women with high grade anogenital intraepithelial neoplasia [36, 37]. The regimen was immunogenic but there was no clear correlation with the 6 observed clinical responses. In another study in 10 women with HPV16-associated high grade vulval intraepithelial neoplasia (VIN) the TA-HPV was administered prior to TA-CIN [38] [37]. This regimen was also immunogenic but again no direct correlation was seen with the 3 women with clinical responses.
A recent phase II trial in 19 women with high grade VIN examined TA-CIN vaccination administered intramuscularly without adjuvant three times at monthly intervals beginning 2 weeks after completion of an 8 week course of local imiquimod therapy [39]. Complete histologic regression was observed in 6/19 women after imiquimod therapy, rising to 12 by one year suggesting a slow response. However, there was no placebo control so the contribution of TA-CIN administration to clinical outcome is unclear. Interestingly, after completion of TA-CIN vaccination there were significant increases in lesional infiltration of both CD4+ and CD8+ T cell populations and systemic antigen-specific T cell proliferative responses; conversely non-responders exhibited significantly greater lesional infiltration by T regulatory cells [39]. Although not the intent of these trials, a later analysis demonstrated that TA-CIN vaccination elicited cross-neutralizing antibody responses in most patients. However, these antibody responses were weakly neutralizing compared to L1 VLP vaccination [40], although sufficient to confer protection by passive transfer in a mouse challenge model [41].
None of the clinical studies to date have examined L2 vaccination for prophylaxis. However, one phase I study examined the immunogenicity of a synthetic peptide comprising residues 108-120 of HPV16 L2 [42]. This peptide was initially identified as being recognized by a cross-neutralizing monoclonal antibody and found to be immunogenic in mice when administered intra-nasally [43, 44]. Thirteen volunteers were administered 0.1 mg (n=5), 0.5 mg (n=5) or placebo (n=3) without adjuvant at weeks 0, 4 and 12. None of the placebo or 0.1 mg dose cohorts developed a serologic response, but 4/5 patients vaccinated with 0.5 mg of peptide generated L2-specific and neutralizing antibody responses. These weak responses suggest that T help may be lacking, which is a common problem with peptide-based vaccines, and that an adjuvant and/or multivalent display may be required to elicit robust neutralizing antibody responses to protective epitopes of L2 [42].
Emergence of a pipeline of candidate HPV vaccines based on L2
The promise of L2 as a prophylactic antigen became apparent in the early 1990s based upon studies in animal papillomavirus challenge models [26]. Several groups prepared full length and polypeptides of L2 from recombinant E. coli and tested their ability to protect naïve animals from cottontail rabbit papillomavirus or bovine papillomavirus-1 or -4 challenge [27, 28, 45, 46]. These studies consistently demonstrated protection against homologous type viral challenge, although the titers of neutralizing antibodies elicited by vaccination were very low [47, 48]. However, L1 VLP vaccination could also elicit homologous type protection but with much higher titers of neutralizing antibodies, and thus this strategy became the focus for prophylactic studies [49, 50].
Further animal work narrowed down the protective epitopes of L2 and demonstrated the importance of neutralizing antibodies in protection [51]. As the epitopes recognized by these neutralizing antibodies were defined it became clear that several towards the amino terminus of L2 were well conserved amongst HPV types [52]. Such sequence conservation provides an opportunity to elicit a broadly protective response using only one or a few epitopes. Examples of such epitopes defined by broadly neutralizing monoclonal antibodies include residues 17-36 [51], 65–81 and 108-120 of HPV16 L2 [43, 53]. This contrasted the highly type-restricted response elicited by L1 VLPs [54]. However, it was also clear that L2 is subdominant to L1 [52], possibly because it is mostly buried below the capsid surface except during infection and/or spaced to far apart on the capsid surface to efficiently promote B cell receptor cross-linking and activation [55, 56]. The relatively weak immunogenicity of L2 likely reflects its inability to multimerize and assemble into a VLP, and is a concern when trying to achieve durable, preferably lifetime, protection. Much of the work in the development of a pipeline of L2-based vaccines has centered upon broadening the spectrum of protection by careful selection of the most cross-reactive epitopes, enhancing immunogenicity through a variety of strategies including capsid display, adjuvant selection or fusion with toll-like receptor (TLR) ligands, and incorporating therapeutic activity by fusion with early HPV antigens.
Multivalent Display of L2
Efforts to boost L2 immunogenicity through multivalent display have focused on utilizing various VLP or virus particle platforms, including HPV VLP, bacteriophage, adenovirus and adeno-associated virus type 2 VLP. This technique been shown to enhance antibody responses to target antigens because antigens that are displayed multivalently are typically highly immunogenic [57] [58] [56], and because VLP platforms serve as a rich source of linked T helper epitopes.
Display of L2 by L1 VLP
Several groups have generated chimeric L1 VLP displaying ~20mer conserved L2 neutralizing epitopes in surface displayed loops (presumed to be immunodominant L1 neutralizing epitopes) with the intent of maintaining the strong homologous type neutralizing antibody titers elicited by the remaining neutralizing epitopes of the L1-VLP scaffold while also enhancing the broadly neutralizing potential of L2. Varsani et al inserted L2 108-120 into HPV16 L1 between 81–93, 131–143, 174–186, 414–426 or 431–443 [59] and several constructs have been produced in plants [60]. It became clear that the L1 VLP scaffold is sensitive to the insertion of epitopes, and that the nature of the epitopes can also impact the structure. Kondo et al selected replacement of L1 430–433 with HPV16 L2 18–38, 56–75, or 96–115 and generated L1 VLP [61]. Building on their earlier work [62], Schellenbacher et al inserted many epitopes into L1's DE loop (notably between residues 136 and 137 of HPV16 L1) [63] and selected the HPV16 L2 17-36 insert (termed RG1-VLP), based upon evidence of increased cross-neutralization and cross-protection [64]. Vaccination with the chimeric RG1-VLPs displaying L2 amino acid (aa) 17-36 of HPV16 and formulated in Freund's adjuvant induced cross-neutralizing antibodies to genetically divergent hrHPV types, lrHPV, and even some beta-type HPV. Use of a human-compatible adjuvant, aluminum hydroxide and monophosphoryl lipid A (Alum-MPL), with RG1-VLP also induced cross-neutralizing antibodies (to HPV types 18, 45, 52, 58), but at 1- to 2-log lower, yet still protective, titers [63]. Immune serum was broadly cross-protective upon passive transfer into naïve mice and genital HPV challenge [64]. This RG1-VLP product is currently being prepared at cGMP by the National Cancer Institute (NCI)'s PREVENT program for early phase clinical development.
L2 display in other capsid platforms
Tobacco mosaic virus particles were explored by Palmer et al as a scaffold to display a protective L2 epitope (94–122). This approach was both immunogenic and protective in the cottontail rabbit papillomavirus (CRPV) challenge model [65].
Bacteriophage-based VLPs are an attractive display platform as they are capable of displaying various antigens, both peptide and non-peptide, and readily encapsulate single-stranded RNA, which serves as an adjuvant by activating toll-like receptors (TLRs) 7 and 8(although not required for strong immunogenicity). Chackerian and Peabody exploited this flexibility to display multiple L2-peptides on VLPs from bacteriophages such as PP7 or MS2 to enhance the antibody response and broaden protection [66, 67]. Notably, through National Institute of Allergy and Infectious Diseases (NIAID) they are producing cGMP MS2 VLPs displaying HPV16 L2 17-31 (see Figure 1) because N-terminal display on the coat protein further enhances cross-reactivity between HPV genotypes, likely reflecting the conformational flexibility afforded by this method of L2 display (i.e. tethered at one end only) [68]. Additionally, display of a consensus sequence from hrHPV L2 aa65-85 inserted in the AB-loop of bacteriophage-based VLPs greatly enhanced neutralizing antibody titers for HPV16,-18,-45,-58 and included protection against HPV31, suggesting this is another approach to broaden protection [69]. Even a single-dose of these bacteriophage VLPs have induced high-titer antibody responses and at least a year of protection in mice, although responses were clearly boosted by a second dose [70]. These phage VLPs can be readily produced in large quantities and easily purified. To address thermostability issues, they also demonstrated their bacteriophage VLPs maintained vaccine activity up to 7 months room temperature storage when spray dried with a dessico-protectant [71].
Figure 1.
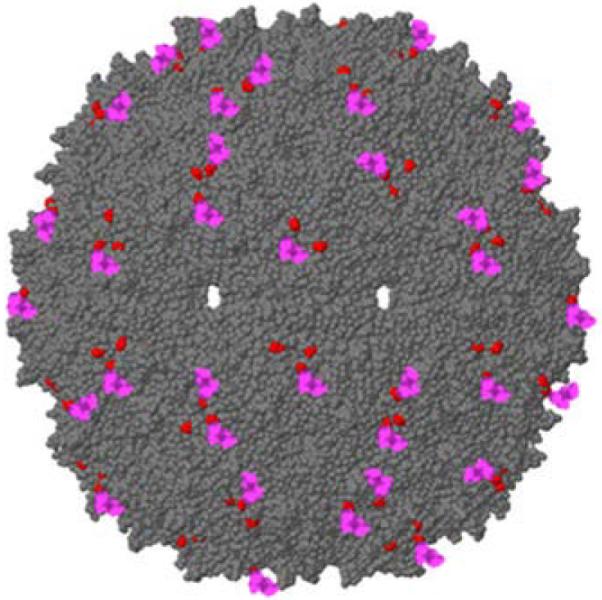
A RasMol-generated schematic of an MS2 VLP displaying a peptide representing HPV16 L2 (17-31). Red depicts the location of the N-terminus of the MS2 coat protein. L2 Peptides are displayed at the N-terminus of the viral coat protein (shown in red). In these recombinant VLPs, only 90 of the 180 coat proteins/VLP display the L2 peptide (shown in pink).
Adeno-associated virus 2 (AAV2)-based VLPs (AAVLPs) were also explored as a display model because of their simple one protein (VP3) capsid, inability to transfer accidentally packaged genes, and stability in varying pH and temperature. Nieto et al incorporated HPV16 and 31 L2 aa17-36 into AAVLPs in two distinct locations. In conjunction with montanide ISA51 adjuvant, high-titer cross-neutralizing antibodies against HPV18, -45, -52 -58 were detected in both C57BL/6 and Balb/c mice after only two-dosages [72]. Further mice administered lyophilized L2-displaying AAVLPs maintained an immunogenic response, allowing for non-cold chain distribution [72]. To determine whether a similar response was elicited when using a clinically relevant adjuvant, AAVLPs tested in conjunction with alum, alum and monophoryl lipid A (MPL), or RIBI adjuvant. Alum alone was sufficient to elicit long-term protection however, inclusion of MPL and RIBI adjuvants boosted antibody titers. Vaccinated rabbits demonstrated protection against HPV16,-31,-35,-39,-45,-58,-59 for up to a year [73].
There is considerable interest in achieving durable protection from a single vaccination without the use of needles. This might be achievable with L1 VLP, but for L2-based vaccines a live recombinant vector approach would likely be required. Live adenovirus vaccines have been used to confer long-term protection against adenovirus induced respiratory disease through asymptomatic self-replication and can be administered in a pill format. In an effort to develop a long-lasting single-dose HPV vaccine, a live recombinant adenovirus vaccine was designed such that HPV16 L2 aa12-41 were inserted into immunodominant neutralizing epitopes of the Ad5 hexon protein for surface display [74]. However, in mice these L2-recombinant adenoviruses only provided partial HPV16 protection without adjuvant, although this vaccine cannot replicate in this host. Combining the L2 recombinant Ad5 virus particles with alum and MPL adjuvants enhanced their immunogenicity in mice, inducing both low neutralizing antibody titers and protection against genital challenge with HPV16 and 73. However, the recombinant vaccine was unable to cross-protect against HPV56 infection which has some divergence in the 17-36 region of L2 [74]. This suggests the need to insert more L2 epitopes into the Ad5 capsid, and there are several promising sites for insertion into hexon. Thus, while capsid display of L2 epitopes is feasible using adenovirus, further optimization to enhance immunogenicity of L2 and testing in a replication competent host is required [74].
Display of HPV16 L2 on the surface of Lactobacillus casei via fusion with poly-γ-glutamic acid synthetase A (pgsA) has also been tested. Yoon et al showed that repeated delivery via oral intra gastric lavage to mice over 25 days induced broadly cross-neutralizing antibodies and evidence of protection against genital challenge [75].
Multimers of L2
Conformation of L1-derived neutralizing epitopes in the VLP vaccines is critical for protection and induction of neutralizing antibodies [25, 76]. However, L2 does not form a VLP [77]. Rather L2 has linear neutralizing epitopes (although some conformational nature has not been ruled out), meaning that L2-derived peptides can be used as the basis of vaccines. While peptide-based vaccines typically elicit lower titer responses, the use of peptides provides the opportunity to concatenate multiple L2-derived peptides without compromising the induction of neutralizing antibodies [78]. In order to boost immune response and further broaden protection, concatenated multitype L2 fusion protein vaccines were developed from known cross-protective L2 epitopes (e.g. residues 11-88) of several divergent HPV types. Of those tested, aa11-88×5 (containing L2 residues 11-88 of 5 divergent HPV) was highly expressed and immunogenic [78–80]. Vaccination with aa11-88×5 induced robust neutralizing antibody titers against all HPV types tested. This immune response suggested that multimeric L2 fusion proteins maybe a viable broad-protection vaccine approach, particularly if paired with an appropriate adjuvant such as alum and MPL [80].
Bacterial thioredoxin (Trx), known to stimulate T cell proliferation, has also been used as a novel multimeric L2 display platform. Trx-L2 constructs were designed to display 1 to 15 copies of HPV 16 L2 peptides that covered as much as aa1-120. Of the L2 epitopes tested, aa20-38 was shown to elicit the strongest immune response to not only HPV 16 but also protected against HPV 31,-45,-58 [81]. Of note, Trx-L2 in combination with human-use approved alum adjuvant was capable of inducing immunogenicity comparable to those induced when in combination with Freund-adjuvant [82]. However, neutralization was strongest in constructs including only 3 and 9 copies of L2, indicating there is a constraining capacity that could limit multimeric and multitype L2 display [81]. However, a trivalent archebacterial Trx-L2 vaccine incorporating 3 proteins displaying L2 20-38 of HPV16, 31 and 51 respectively shows a promising spectrum of protection and its heat stability offers a rapid method of purification and sterilization [83, 84].
Direct fusion of L2 with a TLR ligand
To boost the immunogenicity of L2, preclinical research has also focused on optimizing its combination with adjuvants. Although Freund's adjuvant often induces the strongest response its toxicity excludes it for human use. Semi-synthetic saponin GPI-0100, alum, alum-MPL and 1018 ISS adjuvants have also been shown to boost vaccine effects [78, 85]. However, rather than mixing the adjuvant with the antigen, direct coupling can be more efficacious. Thus attempts to boost L2 immunogenicity have also included fusing immunogenic L2 epitopes to toll-like receptor (TLR) ligands [86]. Fusion of conserved HPV16 L2 aa17-36 to T helper epitope (P25) and TLR2 ligand dipalmitoyl-S-glyceryl cysteine (P2C) allows it to function as a self-adjuvanting immunogen that is capable of generating neutralizing antibodies against not only HPV16 but also divergent types HPV5,-18,-45. Notably, needleless intranasal (i.n.) administration of the lipopeptide induced as potent immune response as subcutaneous (s.c.) injections thus providing a delivery advantage in resource-limited countries [86]. This approach is exciting because the vaccine can be generated through direct chemical synthesis. However, a limitation is the need to include a T helper epitope functional in all patients.
Alternatively, targeting TLR5 through fusion of HPV 16 L2 aa 11–200 to genetically modified bacterial flagellin (Fla) provided protection against HPV16 as well as HPV5,-18,-31,-45,-58 using very low doses of antigen. Consistent with previous multimeric L2 studies, fusion of aa11-88 of five HPV types further enhanced the breadth of neutralizing antibodies induced, and provided broad protection in both mucosal and cutaneous-targeting animal challenge models [87].
Designing HPV L2-based vaccines with therapeutic potential
VLP-based vaccines
Even as prophylactic vaccines improve and implementation slowly ramps up to decrease the population of newly infected individuals, there remains a need for a vaccine active in previously exposed individuals. One promising approach is the fusion of early HPV antigen(s) to L2 which is then co-assembled into chimeric VLP upon co-expression with L1 using recombinant baculovirus vectors in insect cells [88]. Greenstone et al showed that chimeric HPV16 L1/L2-E7 VLPs exhibit similar morphology and L1-specific immunogenicity as L1 VLP. In addition, vaccination with chimeric HPV16 L1/L2-E7 VLPs elicited prophylactic immunity against the HPV16 E6/E7 expressing syngeneic mouse tumor model TC-1 even in MHCII-deficient animals, although neither perforin nor β2-microglobulin deficient animals were protected. This suggests that chimeric VLP are an efficient approach to generate MHCI-dependent immunity against E7 and high titer HPV16 neutralizing antibodies [88].
Fusion proteins and adjuvant
The fusion protein vaccine TA-CIN induced dose-dependent HPV16-specific antibody and CD4 and CD8 T cell responses in early phase clinical trials without adjuvant [35]. However, there was little evidence of clinical impact [36–38]. However, when formulated with adjuvant, this vaccine was active for prophylaxis as well as treatment to contain growth in minimal TC-1 tumor burden environments [89]. In combination with adjuvant GPI-0100, there was a significantly increase in HPV-specific antibody titers as well as CD8 T cell responses, and TA-CIN/GPI-0100 treatment after cisplatin treatment was most effective in extending survival of mice bearing HPV16 tumors [85, 90]. Currently, the NCI's NExT program is preparing a combination of TA-CIN with adjuvant GP1-0100 for Phase 1 clinical trials.
Naked DNA-based vaccines
Fusion to heat shock proteins, including human calreticulin, is a strategy to enhance MHC class I presentation of antigens and boost antigen-specific CD8+ T cell responses, especially when delivered via DNA-based or viral vectors [91]. A naked DNA-based vaccine that expresses calreticulin (CRT) fused to HPV16 E6, E7, and L2 induces HPV16 neutralizing antibodies and CD8+ T cell responses when administered to mice through intramuscular (i.m.) injection with electroporation [92]. HIV+ patients have an increased risk to develop HPV associated disease, especially as their CD4+ T cell counts drop. Interestingly, hCRT-E6E7L2 vaccination maintained cellular (but not humoral) immune reactivity in CD4+ T cell-depleted mice and controlled TC-1 lung nodules in a hematogeneous spread model [93, 94]. The hCRT-E6E7L2 DNA vaccine is being prepared for clinical study by the NCI's PREVENT program and has potential as a therapeutic vaccine for both immune competent and compromised populations.
Expert commentary
The clearest advantage that L2 holds over L1-based vaccines is that its protective epitopes are relatively well-conserved across HPV types. Thus, vaccination with L2 has the ability to induce serum antibody that cross-neutralizes divergent HPV types, providing potential for broader protection [95]. While the licensed vaccines provide coverage against the major mucosal HPV infections associated with cervical cancer, it is impractical to generate L1 VLP for all remaining hrHPV. However, especially when vaccinating populations that do not have access to screening, it is very important to prevent infection by all hrHPV. In the absence of complete protection against hrHPV, screening must be continued. This poses problems of increased need for resources, and that the predictive value of current cytologic screening will likely be insufficient in vaccinated populations. Further, cutaneous HPVs, such as those associated with non-melanoma skin cancer and benign skin warts, are not targeted by the licensed vaccines and potentially can be addressed with L2-based immunogens.
L2 has limitations as an antigen. Notably L2 is relatively poorly immunogenic, likely in part a result of its inability to form a VLP. HPV VLP vaccines elicit potent and durable immunity, likely reflecting the repetitive and close-packed structure of their capsid. Many groups have sought to present the subdominant L2 antigen in the immunodominant neutralizing epitopes of L1 or other viral capsids, i.e. viral display, as an approach to generate a high titer and durable L2-specific neutralizing antibody response. These approaches have typically yielded durable L2-specific responses that, although not achieving the in vitro neutralization titer levels seen for type-specific L1 responses. Nevertheless, vaccines based on capsid display of L2 do provide broad immunity that appeared stable in animals for many months, although time points much beyond a year have not been explored. Similar immunity has also been achieved using L2 multimers in various formats, but selection of an appropriate adjuvant is important. While alum is helpful, the responses were boosted by addition of TLR agonists such as MPL. Clearly, clinical studies are needed to determine whether these approaches also provide broad-spectrum and durable immunity to natural exposure to HPV.
Global implementation remains a challenge for the licensed vaccines. While L1 VLP formulations must be highly multivalent to afford broad protection, the potential to achieve similar coverage with only a single L2 antigen could reduce both the complexity and cost of vaccine manufacture. An ability to achieve durable immunity with a single dose without needles would greatly facilitate global implementation. However this is likely to be a challenge for a L2-based recombinant vaccine unless it is live (although there is promising data for single dose HPV VLP). As such L2 display on live recombinant adenovirus and L. casei is being explored for potential single dose oral administration. However, live recombinant vaccines raise safety concerns especially for immunocompromised patients. VLP displaying L2 might be delivered orally or nasally as a potential alternative [96] [97] [98].
Vaccines based upon L1 and/or L2 have been intended for prophylactic use. However, with the advent of routine HPV DNA testing in cytologic specimens in several countries, there is considerable interest in developing combination preventive and therapeutic vaccines. These approaches generally utilize fusions of the capsid antigen and a viral early antigen, typically E6 and/or E7 because they are also expressed in HPV+ cancer cells. Much remains to be learned about the best approach to elicit a potent T cell response in infected patients, how it is effectively targeted to the site of infection and how best to overcome viral-driven mechanisms of immune escape. Finally, HPV+ cancer cells do not express the capsid antigens [99] and therefore are not typically targets for L2-based vaccines.
Five-year View
The next advances center on the early phase trials of several candidate HPV vaccines that contain L2. The manufacture of cGMP material is ongoing for the MS2 phase VLP displaying HPV16 L2 aa17-31, as is the production of HPV16 L1 VLP displaying HPV16 L2 aa17-36. Both candidate vaccines are designed for prophylactic application and will be tested for safety and immunogenicity with respect to neutralizing antibody titers to determine dosing for healthy volunteers.
In early studies, L2-specific neutralizing antibody titers were initially measured using Pseudovirion-Based Neutralization Assay (L1-PBNA). However, while this assay is highly sensitive for L1-specific neutralizing antibodies, it detects L2-specific neutralization poorly as it does not replicate several features of the infectious process that occurs in vivo (resulting in low sensitivity) [100, 101]. As a result, in infection models even low L2-specific titers were still protective against virus challenge. This suggests that passive transfer assays based on injection of immune sera into naïve animals and subsequent vaginal challenge with HPV pseudovirions can provide a robust readout of protective humoral responses [102]. However, such assays are cumbersome to qualify, low throughput and expensive. L2-based ELISA provide a sensitive detection of L2-specific antibodies but only a subset are neutralizing. However, Day et al developed an in vitro furin-dependent neutralization assay that better models the in vivo infectious process that is greatly more sensitive to L2-specific antibody-mediated neutralization [101]. A similar Furin-Cleaved Pseudovirion-Based Neutralization Assay (FC-PBNA) likewise increases sensitivity to L2 antibody responses while still maintaining the L1-specific sensitivity of L1-PBNA at higher throughput [103]. The WHO standard sera and Gardasil vaccinated patient sera showed similar L1 antibody responses in both assays. However, FC-PBNA was more sensitive to L2 antibodies than L1-PBNA in sera from L2-based TA-CIN vaccinated patients [41]. As the development of L2-based vaccines continue and potentially advance to clinical trials, use of FC-PBNA instead of original format L1-PBNA would likely provide a better indication of the immunogenicity of L2-based vaccines [104]. Further, two humanized L2-specific monoclonal antibodies have been developed to provide stable reagents for positive controls in such assays [105].
In addition to these two candidate prophylactic HPV vaccines, cGMP production of two combination preventive and therapeutic HPV vaccines is also underway; TA-CIN protein formulated in GPI-0100 adjuvant, and the naked DNA vector pNGVL4a-CRTE6E7L2. The safety and immunogenicity of these vaccines will also be assessed in early phase clinical trials, both delivered i.m.. Interestingly, the DNA vaccine will be tested using in vivo electroporation as an approach to enhance transduction and antigen expression [106]. These trials will be performed in patients with HPV16-associated disease, and the measurement of the immune response will be complicated by the need to measure the T cell responses to E6 and E7 (fused to L2 in both candidate vaccines) in addition to L2-specific antibodies. However, the vaccination of patients with HPV16-associated disease offers the opportunity of an early glimpse of efficacy in relatively small trials by following histology, viral load and symptoms.
Key Issues.
Multivalent L1 VLP vaccines have high production costs, require multiple doses and cold chain, lack therapeutic activity, and offer type-restricted protection.
Sequence conservation in protective epitopes within L2 provides provide potential for a simple pan-HPV vaccine.
In early phase clinical studies L2 vaccination induced low titer cross-neutralizing antibodies without use of adjuvant, but it is not clear what level is sufficient for protection.
L2 is poorly immunogenic compared to L1 and thus low-titer neutralizing antibodies raise questions about potential for sustained long-term protection.
Durable protection likely requires boosting L2 immunogenicity using multimerization, capsid display methods and/or adjuvant combinations.
New assays and reagents have been developed to better measure L2-specific neutralizing antibodies in vitro.
Combination preventive and therapeutic vaccines may be developed by fusing capsid antigen such as L2 and early viral proteins, notably E6 and E7.
Acknowledgments
Research reported in this publication was supported by US Public Health Service grants (grants.nih.gov) from the National Cancer Institute of the National Institutes of Health under awards numbered R01CA118790 and P50CA09825 to RBSR. This work was also supported, in part, by a cooperative agreement from the US National Institutes of Health (National Institute of Allergy and Infectious Diseases) establishing the Epidemiology and Prevention Interdisciplinary Center for Sexually Transmitted Diseases (U19 AI113187). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Under a licensing agreement between Sanofi-Aventis and the Johns Hopkins University, R Roden is entitled to a share of royalty received by the University on sales of products. The value of these products could be impacted by the outcome of the study described in this article. Under a licensing agreement between PaxVax, the NIH, and the Johns Hopkins University, R Roden is entitled to a share of royalty received by the University on sales of the vaccine described in this article. R Roden is a co-founder of and has an equity ownership interest in Papivax LLC. Also, he owns Papivax Biotech Inc. stock options and is a member of Papivax Biotech Inc.'s Scientific Advisory Board. Under a licensing agreement between Papivax Biotech, Inc. and the Johns Hopkins University, the University is entitled to royalties on an invention described in this article. Under an option agreement between PathoVax LLC and the Johns Hopkins University and University of Vienna Medical School, R Roden and C Schellenbacher are entitled to distributions of payments associated with an invention described in this publication. R Roden and C Schellenbacher also own equity in PathoVax LLC and are members of its scientific advisory board. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. B Chackerian is a co-founder of and has an equity ownership interest in Agilvax, and is entitled to a share of royalties received by the University of New Mexico on intellectual property licensed to Agilvax.
Footnotes
Financial and competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Bernard HU, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401(1):70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mammas IN, Sourvinos G, Spandidos DA. The paediatric story of human papillomavirus (Review) Oncology letters. 2014;8(2):502–506. doi: 10.3892/ol.2014.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]; *Defines key hrHPV types that need to be targetted by vaccination
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 8.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin-Drubin ME. Human papillomaviruses and non-melanoma skin cancer. Seminars in oncology. 2015;42(2):284–90. doi: 10.1053/j.seminoncol.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Majewski S, Jablonska S. Human papillomavirus-associated tumors of the skin and mucosa. Journal of the American Academy of Dermatology. 1997;36(5 Pt 1):659–85. doi: 10.1016/s0190-9622(97)80315-5. quiz 686–8. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients--where do we stand today? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(11):2192–8. doi: 10.1111/j.1600-6143.2008.02386.x. [DOI] [PubMed] [Google Scholar]
- 12.Quint KD, Genders RE, de Koning MN, et al. Human Beta-papillomavirus infection and keratinocyte carcinomas. The Journal of pathology. 2015;235(2):342–54. doi: 10.1002/path.4425. [DOI] [PubMed] [Google Scholar]
- 13.Miller DJ, Strauch RJ. Management of Cutaneous Warts of the Hand. The Journal of hand surgery. 2015;40(11):2274–6. doi: 10.1016/j.jhsa.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Bellew SG, Quartarolo N, Janniger CK. Childhood warts: an update. Cutis. 2004;73(6):379–84. [PubMed] [Google Scholar]
- 15.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(9447):1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 17.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 18.Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12(10):781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 19.Vesikari T, Brodszki N, van Damme P, et al. A Randomized, Double-Blind, Phase III Study of the Immunogenicity and Safety of a 9-Valent Human Papillomavirus L1 Virus-Like Particle Vaccine (V503) Versus Gardasil(R) in 9–15-Year-Old Girls. The Pediatric infectious disease journal. 2015;34(9):992–8. doi: 10.1097/INF.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 20.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. The Lancet. Oncology. 2015;16(7):775–86. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. The Lancet. Oncology. 2015 doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardine D, Lu J, Pang J, et al. A randomized trial of immunotherapy for persistent genital warts. Human vaccines & immunotherapeutics. 2012;8(5):623–9. doi: 10.4161/hv.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doorbar J, Quint W, Banks L, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 24.Han R, Reed CA, Cladel NM, et al. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: protective immunity induced by gene gun-mediated intracutaneous delivery but not by intramuscular injection. Vaccine. 2000;18(26):2937–44. doi: 10.1016/s0264-410x(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 25.Kirnbauer R, Booy F, Cheng N, et al. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89(24):12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campo MS, Roden RB. Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol. 2010;84(3):1214–20. doi: 10.1128/JVI.01927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandrachud LM, Grindlay GJ, McGarvie GM, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211(1):204–8. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- 28.Christensen ND, Kreider JW, Kan NC, et al. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181(2):572–9. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- 29.Pilacinski WP, Glassman DL, Glassman KF, et al. Immunization against bovine papillomavirus infection. Ciba Foundation symposium. 1986;120:136–56. doi: 10.1002/9780470513309.ch10. [DOI] [PubMed] [Google Scholar]
- 30.Jarrett WF, Smith KT, O'Neil BW, et al. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- 31.Hitzeroth II, Passmore JA, Shephard E, et al. Immunogenicity of an HPV-16 L2 DNA vaccine. Vaccine. 2009;27(46):6432–4. doi: 10.1016/j.vaccine.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson HS, Davies ML, Holding FP, et al. Phase I safety and antigenicity of TA-GW: a recombinant HPV6 L2E7 vaccine for the treatment of genital warts. Vaccine. 1999;17(1):40–9. doi: 10.1016/s0264-410x(98)00146-7. [DOI] [PubMed] [Google Scholar]
- 33.Lacey CJ, Thompson HS, Monteiro EF, et al. Phase IIa safety and immunogenicity of a therapeutic vaccine, TA-GW, in persons with genital warts. J Infect Dis. 1999;179(3):612–8. doi: 10.1086/314616. [DOI] [PubMed] [Google Scholar]
- 34.Vandepapeliere P, Barrasso R, Meijer CJ, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192(12):2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 35.de Jong A, O'Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20(29–30):3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 36.Smyth LJ, Van Poelgeest MI, Davidson EJ, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004;10(9):2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 37.Fiander AN, Tristram AJ, Davidson EJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16(3):1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 38.Davidson EJ, Faulkner RL, Sehr P, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22(21–22):2722–9. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 39.Daayana S, Elkord E, Winters U, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102(7):1129–36. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Describes use of HPV16 L2E7E6 vaccination in imiquimod-treated VIN patients
- 40.Gambhira R, Gravitt PE, Bossis I, et al. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res. 2006;66(23):11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]; **Demonstrates that L2-based vaccination of patients can induce cross-neutralizing antibodies
- 41.Wang JW, Jagu S, Wang C, et al. Measurement of neutralizing serum antibodies of patients vaccinated with human papillomavirus L1 or L2-based immunogens using furin-cleaved HPV Pseudovirions. PLoS ONE. 2014;9(7):e101576. doi: 10.1371/journal.pone.0101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawana K, Yasugi T, Kanda T, et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine. 2003;21(27–30):4256–60. doi: 10.1016/s0264-410x(03)00454-7. [DOI] [PubMed] [Google Scholar]; *Demonstrates limited immunogenicity of an L2 peptide in patients
- 43.Kawana K, Yoshikawa H, Taketani Y, et al. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73(7):6188–90. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawana K, Kawana Y, Yoshikawa H, et al. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine. 2001;19(11–12):1496–502. doi: 10.1016/s0264-410x(00)00367-4. [DOI] [PubMed] [Google Scholar]
- 45.Lin YL, Borenstein LA, Selvakumar R, et al. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187(2):612–9. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 46.Campo MS, O'Neil BW, Grindlay GJ, et al. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology. 1997;234(2):261–6. doi: 10.1006/viro.1997.8649. [DOI] [PubMed] [Google Scholar]
- 47.Gambhira R, Jagu S, Karanam B, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N-terminus of HPV16 minor capsid antigen L2. J Virol. 2007;81(21):13927–13931. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaukroger JM, Chandrachud LM, O'Neil BW, et al. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J Gen Virol. 1996;77(Pt 7):1577–83. doi: 10.1099/0022-1317-77-7-1577. [DOI] [PubMed] [Google Scholar]
- 49.Kirnbauer R, Chandrachud LM, O'Neil BW, et al. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219(1):37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 50.Suzich JA, Ghim SJ, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92(25):11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gambhira R, Karanam B, Jagu S, et al. A protective and broadly cross-neutralizing epitope of Human Papillomavirus L2. J Virol. 2007;81(24):13927–31. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roden RB, Yutzy WHt, Fallon R, et al. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]; **Describes the potential for cross-neutralization using L2-specific antibodies, and their limited immunogenicity
- 53.Rubio I, Seitz H, Canali E, et al. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology. 2011;409(2):348–59. doi: 10.1016/j.virol.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 54.Roden RB, Greenstone HL, Kirnbauer R, et al. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. Journal of virology. 1996;70(9):5875–83. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buck CB, Cheng N, Thompson CD, et al. Arrangement of L2 within the papillomavirus capsid. J Virol. 2008;82(11):5190–7. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chackerian B, Lenz P, Lowy DR, et al. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002;169(11):6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- 57.Bachmann MF, Rohrer UH, Kundig TM, et al. The influence of antigen organization on B cell responsiveness. Science. 1993;262(5138):1448–51. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 58.Roost HP, Bachmann MF, Haag A, et al. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity [see comments] Proc Natl Acad Sci U S A. 1995;92(5):1257–61. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varsani A, Williamson AL, de Villiers D, et al. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. J Virol. 2003;77(15):8386–93. doi: 10.1128/JVI.77.15.8386-8393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pineo CB, Hitzeroth II, Rybicki EP. Immunogenic assessment of plant-produced human papillomavirus type 16 L1/L2 chimaeras. Plant biotechnology journal. 2013;11(8):964–75. doi: 10.1111/pbi.12089. [DOI] [PubMed] [Google Scholar]
- 61.Kondo K, Ochi H, Matsumoto T, et al. Modification of human papillomavirus-like particle vaccine by insertion of the cross-reactive L2-epitopes. J Med Virol. 2008;80(5):841–6. doi: 10.1002/jmv.21124. [DOI] [PubMed] [Google Scholar]
- 62.Slupetzky K, Gambhira R, Culp TD, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol. 2009;83(19):10085–95. doi: 10.1128/JVI.01088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schellenbacher C, Kwak K, Fink D, et al. Efficacy of RG1-VLP Vaccination against Infections with Genital and Cutaneous Human Papillomaviruses. The Journal of investigative dermatology. 2013 doi: 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]; **An L2-display technology that is under development for clinical testing
- 65.Palmer KE, Benko A, Doucette SA, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006;24(26):5516–25. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 66.Caldeira Jdo C, Medford A, Kines RC, et al. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 28(27):4384–93. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tumban E, Peabody J, Peabody DS, et al. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS ONE. 2011;6(8):e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tumban E, Peabody J, Tyler M, et al. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS ONE. 2012;7(11):e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Another L2-display technology under development for early clinical trials
- 69.Tyler M, Tumban E, Dziduszko A, et al. Immunization with a consensus epitope from human papillomavirus L2 induces antibodies that are broadly neutralizing. Vaccine. 2014;32(34):4267–74. doi: 10.1016/j.vaccine.2014.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tumban E, Peabody J, Peabody DS, et al. A universal virus-like particle-based vaccine for human papillomavirus: longevity of protection and role of endogenous and exogenous adjuvants. Vaccine. 2013;31(41):4647–54. doi: 10.1016/j.vaccine.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tumban E, Muttil P, Escobar CA, et al. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine. 2015;33(29):3346–53. doi: 10.1016/j.vaccine.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nieto K, Weghofer M, Sehr P, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS ONE. 2012;7(6):e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jagu S, Karanam B, Wang JW, et al. Durable immunity to oncogenic human papillomaviruses elicited by adjuvanted recombinant Adeno-associated virus-like particle immunogen displaying L2 17-36 epitopes. Vaccine. 2015;33(42):5553–63. doi: 10.1016/j.vaccine.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu WH, Alkutkar T, Karanam B, et al. Capsid display of a conserved human papillomavirus L2 peptide in the adenovirus 5 hexon protein: a candidate prophylactic hpv vaccine approach. Virology journal. 2015;12:140. doi: 10.1186/s12985-015-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon SW, Lee TY, Kim SJ, et al. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine. 2012;30(22):3286–94. doi: 10.1016/j.vaccine.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Breitburd F, Kirnbauer R, Hubbert NL, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirnbauer R, Taub J, Greenstone H, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67(12):6929–36. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jagu S, Karanam B, Gambhira R, et al. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101(11):782–92. doi: 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jagu S, Kwak K, Schiller JT, et al. Phylogenetic considerations in designing a broadly protective multimeric L2 vaccine. J Virol. 2013;87(11):6127–36. doi: 10.1128/JVI.03218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jagu S, Kwak K, Karanam B, et al. Optimization of multimeric human papillomavirus L2 vaccines. PLoS ONE. 2013;8(1):e55538. doi: 10.1371/journal.pone.0055538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubio I, Bolchi A, Moretto N, et al. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20 -- 38) peptide displayed on bacterial thioredoxin. Vaccine. 2009;27(13):1949–56. doi: 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 82.Seitz H, Dantheny T, Burkart F, et al. Influence of oxidation and multimerization on the immunogenicity of a thioredoxin-l2 prophylactic papillomavirus vaccine. Clinical and vaccine immunology : CVI. 2013;20(7):1061–9. doi: 10.1128/CVI.00195-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canali E, Bolchi A, Spagnoli G, et al. A high-performance thioredoxin-based scaffold for peptide immunogen construction: proof-of-concept testing with a human papillomavirus epitope. Scientific reports. 2014;4:4729. doi: 10.1038/srep04729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seitz H, Ribeiro-Muller L, Canali E, et al. Robust In Vitro and In Vivo Neutralization against Multiple High-Risk HPV Types Induced by a Thermostable Thioredoxin-L2 Vaccine. Cancer prevention research. 2015;8(10):932–41. doi: 10.1158/1940-6207.CAPR-15-0164. [DOI] [PubMed] [Google Scholar]
- 85.Karanam B, Gambhira R, Peng S, et al. Vaccination with HPV16 L2E6E7 fusion protein in GPI-0100 adjuvant elicits protective humoral and cell-mediated immunity. Vaccine. 2009;27(7):1040–9. doi: 10.1016/j.vaccine.2008.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]; **A candidate prophylactic and therapeutic vaccine under development for clinical testing
- 86.Alphs HH, Gambhira R, Karanam B, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A. 2008;105(15):5850–5. doi: 10.1073/pnas.0800868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalnin K, Tibbitts T, Yan Y, et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine. 2014;32(28):3540–7. doi: 10.1016/j.vaccine.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greenstone HL, Nieland JD, de Visser KE, et al. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci U S A. 1998;95(4):1800–5. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Burg SH, Kwappenberg KM, O'Neill T, et al. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine. 2001;19(27):3652–60. doi: 10.1016/s0264-410x(01)00086-x. [DOI] [PubMed] [Google Scholar]
- 90.Peng S, Wang JW, Karanam B, et al. Sequential cisplatin therapy and vaccination with HPV16 E6E7L2 fusion protein in saponin adjuvant GPI-0100 for the treatment of a model HPV16+ cancer. PLoS One. 2015;10(1):e116389. doi: 10.1371/journal.pone.0116389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng WF, Hung CF, Chai CY, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108(5):669–78. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim D, Gambhira R, Karanam B, et al. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine. 2008;26(3):351–60. doi: 10.1016/j.vaccine.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng S, Song L, Knoff J, et al. Control of HPV-associated tumors by innovative therapeutic HPV DNA vaccine in the absence of CD4+ T cells. Cell & bioscience. 2014;4(1):11. doi: 10.1186/2045-3701-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang JW, Jiang R, Peng S, et al. Immunologic Control of Mus musculus Papillomavirus Type 1. PLoS pathogens. 2015;11(10):e1005243. doi: 10.1371/journal.ppat.1005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang JW, Hung CF, Huh WK, et al. Immunoprevention of human papillomavirus-associated malignancies. Cancer prevention research. 2015;8(2):95–104. doi: 10.1158/1940-6207.CAPR-14-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balmelli C, Roden R, Potts A, et al. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions [In Process Citation] J Virol. 1998;72(10):8220–9. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nardelli-Haefliger D, Lurati F, Wirthner D, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23(28):3634–41. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 98.Gerber S, Lane C, Brown DM, et al. Human papillomavirus virus-like particles are efficient oral immunogens when coadministered with Escherichia coli heat-labile enterotoxin mutant R192G or CpG DNA. Journal of virology. 2001;75(10):4752–60. doi: 10.1128/JVI.75.10.4752-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin Z, Yemelyanova AV, Gambhira R, et al. Expression pattern and subcellular localization of human papillomavirus minor capsid protein L2. The American journal of pathology. 2009;174(1):136–43. doi: 10.2353/ajpath.2009.080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260–70. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Day PM, Pang YY, Kines RC, et al. An HPV in vitro neutralization assay that recapitulates the in vivo process of infection provides a sensitive measure of L2 infection-inhibiting antibodies. Clin Vaccine Immunol. 2012 doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 103.Wang JW, Jagu S, Kwak K, et al. Preparation and properties of a papillomavirus infectious intermediate and its utility for neutralization studies. Virology. 2014;449:304–16. doi: 10.1016/j.virol.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang JW, Matsui K, Pan Y, et al. Production of Furin-Cleaved Papillomavirus Pseudovirions and Their Use for In Vitro Neutralization Assays of L1- or L2-Specific Antibodies. Current protocols in microbiology. 2015;38:14B 5 1–14B 5 26. doi: 10.1002/9780471729259.mc14b05s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang JW, Jagu S, Wu WH, et al. Seroepidemiology of Human Papillomavirus 16 (HPV16) L2 and Generation of L2-Specific Human Chimeric Monoclonal Antibodies. Clinical and vaccine immunology : CVI. 2015;22(7):806–16. doi: 10.1128/CVI.00799-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim TJ, Jin HT, Hur SY, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nature communications. 2014;5:5317. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]


