Summary
The ventral pallidum (VP) is posited to contribute to reward-seeking by conveying upstream signals from the nucleus accumbens (NAc). Yet, very little is known about how VP neuron responses contribute to behavioral responses to incentive cues. Here, we recorded activity of VP neurons in a cue-driven reward-seeking task previously shown to require neural activity in the NAc. We find that VP neurons encode both learned cue value and subsequent reward seeking, and that activity in VP neurons is required for robust cue-elicited reward seeking. Surprisingly, the onset of VP neuron responses occurs at a shorter latency than cue-elicited responses in NAc neurons. This suggests that this VP encoding is not a passive response to signals generated in the NAc, and, that VP neurons integrate sensory and motivation-related information received directly from other mesocorticolimbic inputs.
eToC
Richard et al. demonstrate phasic responses to incentive stimuli in ventral pallidum that predict the likelihood and vigor of reward-seeking. Ventral pallidal responses occur in parallel with signals in nucleus accumbens, contrary to canonical views of basal ganglia information processing.
Introduction
The ventral pallidum (VP) is a critical output structure of the ventral basal ganglia (Heimer et al., 1982), linking signals from the nucleus accumbens (NAc) to downstream motor nuclei (Heimer et al., 1987; Leung and Balleine, 2015; Mogenson and Yang, 1991). The canonical view of VP is that it is a relay for NAc “indirect” pathway signals (Gittis et al., 2014), which in the dorsal striatum are primarily carried by D2-dopamine receptor-expressing medium spiny neurons (D2 MSNs) (Gerfen and Surmeier, 2011). Yet, emerging research suggests that this segregation of D1 “direct” and D2 “indirect” output pathways does not apply to the ventral basal ganglia (Humphries and Prescott, 2010; Zhou et al., 2003), where similar proportions of D1 and D2 MSNs project from NAc to VP (Lu et al., 1998; Tripathi et al., 2010) and are equally likely to target VP neurons that project to the thalamus (Kupchik et al., 2015).
Both the NAc and VP are implicated in the promotion of reward-seeking actions by reward cues (Ambroggi et al., 2011; du Hoffmann and Nicola, 2014; Leung and Balleine, 2013; Mahler et al., 2014; Nicola et al., 2005; Perry and McNally, 2013; Yun et al., 2004). In a discriminative stimulus (DS) task, cue-evoked responses in NAc neurons encode cue value (Ambroggi et al., 2008, 2011) and both the probability and vigor of reward-seeking actions (McGinty et al., 2013; Nicola et al., 2004). While VP neurons encode the value of primary rewards (Itoga et al., 2015; Tindell et al., 2006) and Pavlovian reward cues (Smith et al., 2011; Tindell et al., 2005, 2009) the relationship between VP cue responses and cue-induced reward seeking actions has not been studied. Here, we report that, like NAc neurons (Ambroggi et al., 2011; Yun et al., 2004), VP neurons are required for optimal performance of a DS task. We then investigated how VP neurons encode a DS, how this compares to NAc responses, and whether VP neuron responses predict the vigor of subsequent reward seeking actions.
Results
The ventral pallidum is necessary for behavioral performance in the DS task
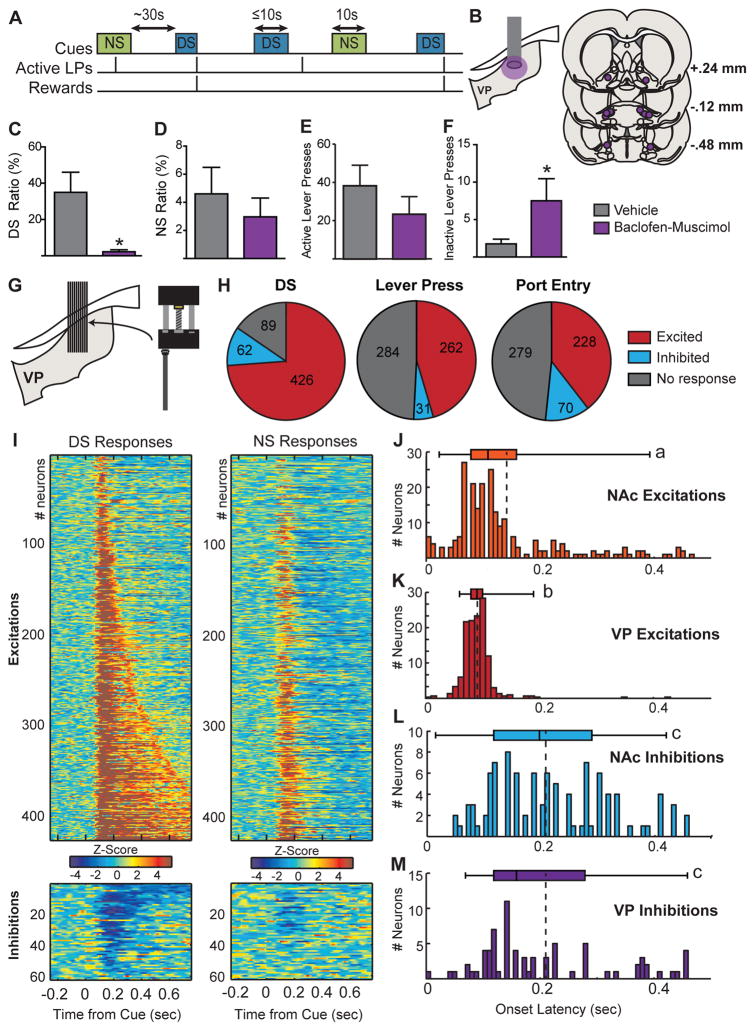
Rats were trained to perform a DS task, in which presses on the “active” lever during the DS (an auditory cue lasting up to 10s) resulted in delivery of liquid sucrose (10%). Presses made in the absence of the DS or during presentations of an alternative auditory cue (the non-rewarded stimulus; NS) had no programmed consequences (Figure 1A). First, we investigated whether activity in VP neurons is necessary for performance of the task, by infusing a low-dose mixture of the GABAA agonist muscimol and the GABAB agonist baclofen into the VP of rats trained to respond to at least 60% of DS presentations. Inactivation reduced the probability of a response to the DS (t(6) = 3.087, p = .0215; Fig. 1C), but had no effect on total active lever presses (t(6) = 0.9702, p = .36; Figure 1E) or on active presses during the intertrial interval (t(5)=0.095, p = 0.92) or during the NS (t(6) = 0.6689, p = 0.5284; Figure 1D). VP inactivation slightly but significantly enhanced the low number of inactive lever presses (t(6) = 2.542, p = 0.044; Figure 1F). Because 3/7 rats made zero DS responses following VP inhibition, the effect on latency could not be reliably assessed. Overall, these results establish a role for VP activity in performance in the DS task, but not what information about the DS is encoded by VP neuronal activity. To address this question, we recorded the activity of VP neurons during performance of the task.
Figure 1. Schematic of the DS task, pharmacological inactivation, recording histology and result summary.
A, The discriminative stimulus (DS) and control cue (NS) are two auditory cues, lasting up to 10 s, presented on a variable-interval schedule with an average interval of 30s. Presses on the active lever during the DS terminate the DS and result in delivery of a 10% sucrose reward. Presses during the NS are recorded, but have no programmed consequences. B, Histological reconstruction of microinjection placements in VP. Inactivation of VP with baclofen and muscimol reduced the percentage of DS presentation that animals responded to (DS Ratio, C), but had no effect on the percentage of NS presentations that animals responded to (NS ratio, D), or on total active lever presses during the 2hr session (E). VP inactivation increased inactive lever presses (F). G, Targeted electrode placement in VP. H, Pie chart showing the proportion of neurons (labels indicate number of units) for which we initially detected an increase (excited, red) or decrease (inhibited, blue) in firing following the DS (left), after the first port entry following reward delivery (middle), or surrounded the lever press during the DS (right). I, Heat maps of responses to the DS (left) and NS (right) split into neurons in which we detected an initial excitation following the DS (top) and neurons in which we detected an inhibition (bottom). Each line represents the PSTH of an individual neuron, normalized (z-score) and color-coded. Within each category, neurons are sorted by duration of response to the DS. Histograms depict the distribution of onset latencies for NAc excitations (J), VP excitations (K), NAc inhibitions (L), and VP inhibitions (M) elicited by the DS. Boxplots depict the 5–95 percentile range (whiskers), the interquartile range (boxes) and median (bands); dotted lines indicate the median. Letters indicate statistically significant differences (p < 0.05) using Dunn’s multiple comparison test.
Ventral pallidum neurons respond to a learned appetitive cue at short latencies that precede NAc responses
Rats were trained until they made active lever presses on > 80% of DS trials and < 20% of NS trials and were implanted with drivable multielectrode arrays aimed at VP. Following retraining we recorded from 577 neurons throughout the rostrocaudal extent of VP (6 rats, 45 sessions; Figures 1G and S1A). Basal firing rates ranged from 0.2 to 18.7 Hz (interquartile range: 2.9–6.7; median = 4.7) and were not correlated with the magnitude of cue responses, or their encoding of behavioral responses. While many VP neurons responded to multiple task events (Figure 1H and S2), we focused our primary analysis on explicit cue responses. Using our first response detection method, searching for post-cue bins in which firing rate is outside the 99% confidence interval of the baseline firing rate (10s pre-cue), we found that the majority of VP neurons are excited by the DS (426/577, 73.8%; Figure 1H and I), whereas a smaller proportion are inhibited (62/577, 10.7%) and/or exhibit both response types (23/577, 4%). This large proportion of DS responsive neurons in the VP (more than 70%) was surprising, given the much smaller proportion observed in the NAc (15–20% excited, 10–15% inhibited; Ambroggi et al., 2011). We expected the onset of VP responses to the DS to reflect upstream responses in NAc and thus to occur at longer latency. However, we observed VP DS excitations at short latencies (Figure 1K) similar to those we previously reported for DS excitations in NAc (Figure 1J). To compare the latencies of DS-evoked responses in the VP and NAc, we reanalyzed previous NAc recordings from rats performing the identical task (Ambroggi et al., 2011), using the same onset detection criteria. Rats in both cohorts received identical training, in the same operant boxes, and were trained to identical criteria. VP excitations to the DS had a shorter onset latency (median = 90ms) than either NAc excitations (median = 110ms; t(642)=7.06, p < 0.0001) or inhibitions (median = 200ms; t(533)=14.5, p < 0.0001; Figure 1L). This suggests that short latency DS-evoked excitations in VP neurons are not driven by phasic NAc responses to the cue. In contrast, VP inhibitions to the DS (median = 170ms; Figure 1M) are detected after NAc excitations (t(295)=4.708, p < 0.0001) and at a similar latency to NAc inhibitions (t(186) = 0.051, p = .96).
What is encoded by changes in firing of VP neurons evoked by the DS?
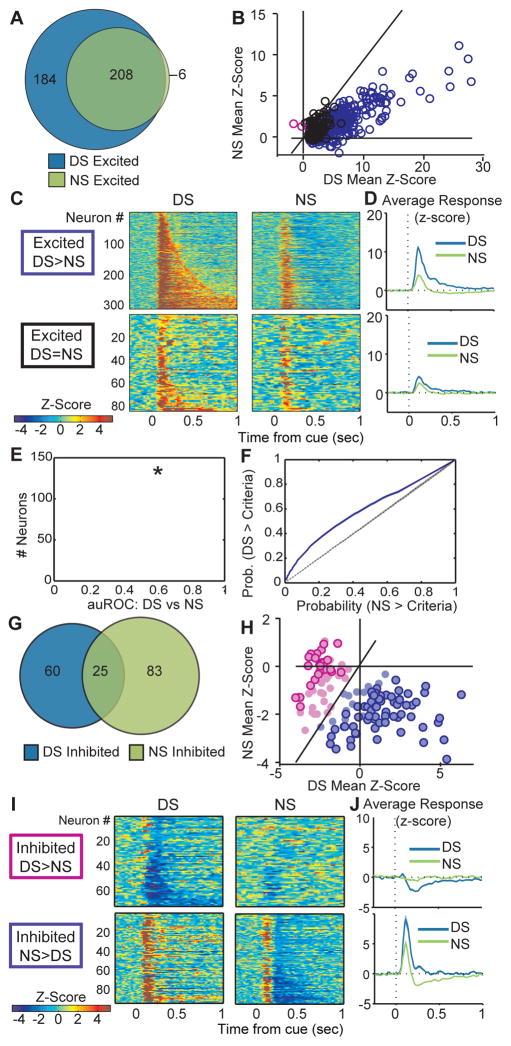
We next assessed whether the changes in VP neuron firing, which predominantly precede those observed “upstream” in the NAc, occurred differentially to the DS versus the NS. Because the onset of inhibitions occurred at a longer latency (Figure 1M), we conducted this analysis on firing during time windows based on these onsets (90 – 300ms for excitations, 200 – 300ms for inhibitions). While the majority of neurons recorded in VP were significantly excited by the DS (392/577, 73.5%; Figure 2A) about half of these neurons were also excited by the NS (214/392, 54.9%; Figure 2A). The magnitude of NS responses was significantly smaller than to the DS (t(391)=29.26, p < .001) and the majority of individual neurons were more excited by the DS than the NS (Figure 2B–D; 54% of total neurons). To assess the predictive ability of VP neuron firing rates, we ran receiver operating characteristic (ROC) analysis (Figure 2E and F) to assess detection of the DS (versus baseline), the NS, and cue identity (DS versus NS), calculating the area under the ROC curve (auROC) for each neuron for each time window. We found that the population auROC distribution was significantly different from a control distribution (mean control auROC=0.497) for the detection of the DS (t(576)=12.1026, p <0.001), and identification of the DS versus the NS (t(576)=20.58, p <0.001; Figure 2E), but not for detection of the NS (t(576)=1.3297, p = 0.18). This provides additional evidence that firing in the VP neural population is predictive of the occurrence of the DS and of the identity of the DS versus the NS.
Figure 2. VP neurons are more excited by the DS than the NS.
A, Venn diagram showing that of the 392 neurons excited by the DS, 214 are also excited by the NS. B, Scatterplot of normalized (as z-score) responses to the NS versus the DS, showing neurons that repond significantly more to the DS (purple) or the NS (pink). C, Heat maps of responses to the DS (left) and NS (middle) split into neurons that respond significantly more to the DS than the NS (top), and neurons that do not respond significantly differently to the DS versus the NS (bottom). Each line represents the PSTH of an individual neurons, normalized and color-coded. Within each category, neurons are sorted by response to the NS. D, Average responses to the DS (blue) and NS (green) as grouped in C. E, Histogram of the area under the receiver-operating characteristic (auROC) curve, for the analysis of firing in the DS and NS analysis windows; the population average is indicated in red, and the dotted line shows the average control auROC (* = p<0.001). D, Average ROC curve for the whole population for predicting the DS versus NS (purple line; shading depicts standard error) and the control analysis comparing two baseline windows (dotted black line). G, Venn diagram showing neurons inhibited by the DS (85), and NS (108) are only semi-overlapping. H, Scatterplot of normalized (as z-score) responses to the NS versus the DS, showing neurons that are significantly more inhibited following the DS (dark pink outline) or the NS (dark purple outline). I, Heat maps of responses to the DS (top) and NS (bottom) split into neurons that are more inhibited by the DS than the NS (left; pink centers in H), or more inhibited by the NS than the DS (right; purple centers in H). Each line represents the PSTH of an individual neurons, normalized and color-coded. Within each category, neurons are sorted by response to the NS. J, Average responses to the DS (blue) and NS (green) as grouped n I.
In comparison to the DS-excited population, a small proportion of VP neurons were inhibited by the DS (85/577, 14.7%). A slightly larger proportion of neurons were inhibited by the NS (108/577, 18.7%; X2 =7.299, p<.001; Figure 2G), many of which were excited by the DS (76/108 neurons, 70%), and exhibited a brief excitation to the NS prior to the inhibition (53/108, 90–300ms post-DS; Figure 2I and J). Inhibitions selective to the DS were generally not preceded by excitations (Figure 2I and J). We hypothesize that these NS inhibitions contribute to inhibiting inappropriate responding.
Ventral pallidum DS excitations predict latency to make an instrumental response
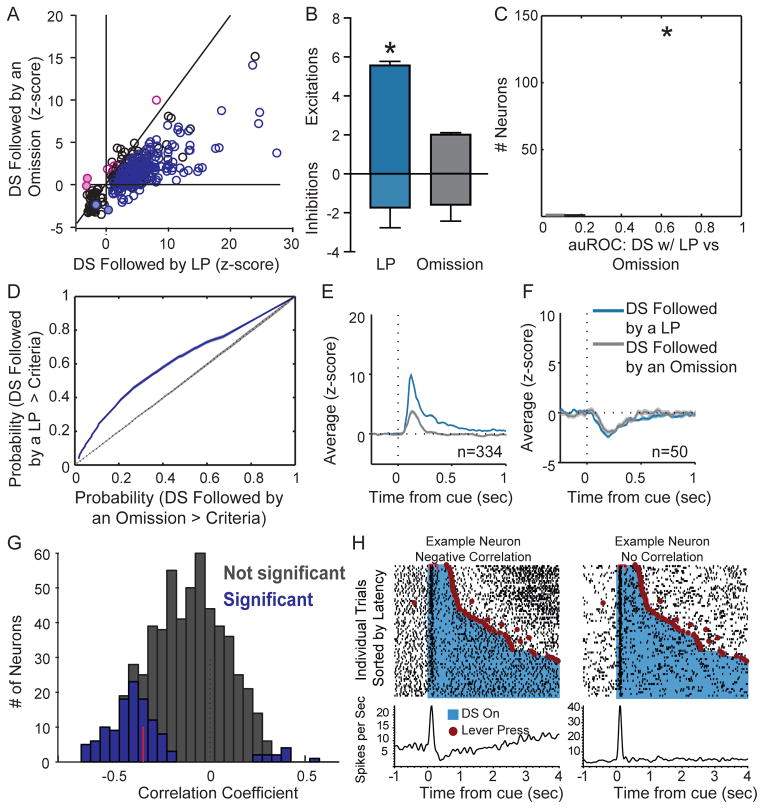
Beyond representing the predictive value of the DS, VP neurons responded differentially depending on the speed and likelihood of a subsequent reward-seeking response. This suggests that VP neurons not only encode the incentive value of the DS but promote responding to it. We analyzed DS trials based on whether they were followed by an active lever press or not (Figure 3A), and found that, as a group, DS-excited neurons are significantly more excited when the DS is followed by reward-seeking (t(333)=17.27, p < .001; Figure 3B and E). Further, of the 334 DS-excited neurons from sessions with >10 omitted trials, more than half (188/334, 56.3%) had significantly greater DS excitations on trials with a lever press, though even on omitted trials VP responses to the DS are still greater than responses to the NS (t(331) = −3.57, p <.001). In contrast to the excitations, the DS inhibitions were not significantly different in magnitude based on behavioral response (Figure 3B and F; t(49)=−0.49, p = 0.63), suggesting that inhibitions encode something more static than incentive motivation. Consistent with this, inhibitions to the DS were stronger than to the NS, even when the DS was not followed by a response (t(49)=−6.97, p <.0001). While DS inhibitions do not encode response likelihood, it is possible that DS inhibitions in a subset of VP neurons are necessary for DS-induced reward seeking. We also assessed firing to the DS on trials with and without a lever press using ROC curve analysis, and found that the auROC distribution was significantly greater than the control distribution (Figure 3C; t(576)=19.49, p <0.001) and that the majority of VP neurons had auROCs greater than 95% of control auROCs (333 neurons, 57.7%), further suggesting that VP neuron excitations encode the likelihood of reward seeking.
Figure 3. VP neuron excitations to the DS depend on whether the DS is followed by a lever press, and on the subsequent latency to respond.
A, Scatterplot of normalized (as z-score) responses to the DS when it is followed by a response versus when it is not, showing neurons that are significantly more excited when the DS is followed by a response (purple) than when it is not (pink). B, Average (+SEM) modulation after the DS is stronger for excited neurons (top) when the animal makes a subsequent lever press, but not for inhibited neurons (bottom). C, Histogram of auROCs, for the analysis of DS-related firing on trials with and without a lever press; the population average is indicated in red, and the dotted line shows the average control auROC (* = p<0.001). D, Average ROC curve for the whole population for the DS with lever press versus omission analysis (purple line; shading depicts standard error) and the control analysis (dotted black line). Average responses to the DS with a response (blue) and without (grey), split into neurons that are significanlty excited by the DS (E, left, n = 334), and those that are inhibited by the DS (F, right, n = 50). Only sessions in which at least 10 DS presentations were not followed by a response were included in this analysis. G, Distribution of Spearman rank correlation coefficients relating firing (0 – 300 ms after DS onset) to the animal’s latency to press the lever on a trial-by-trial basis. Bars shaded in dark grey show neurons with significant correlations, red line shows median correlation coefficient of the significant neurons. H, Example DS-excited neurons with either a significant negative correlation between firing rate and latency (left) or no significant correlation (right). Individual trials are sorted by latency between the onset of the DS (blue) the time of the lever press (red circle). Rasters and correponding histograms show firing aligned to DS onset.
Finally, we examined whether the DS response on a given trial is related to the latency of the animal to respond on that trial, by running Spearman rank correlations on individual neurons (Figure 3G–H and S3). The firing rate of 156 (27%) neurons following the DS significantly predicted the animal’s latency to make a lever press. In most of these neurons (n=144), the DS-evoked firing rate was inversely correlated with latency to lever press. Even when we restricted our analysis to the inhibition analysis window, few neurons had positive correlations (200–300ms post-DS; 12/577 neurons, 2.08%; Figure S3C). These findings indicate that VP neuron DS excitations encode the motivational strength of the reward predictive cue, and may play a causal role in the vigor of approach to the lever after the cue.
Optogenetic inhibition of VP during the DS increases omissions and latency to respond
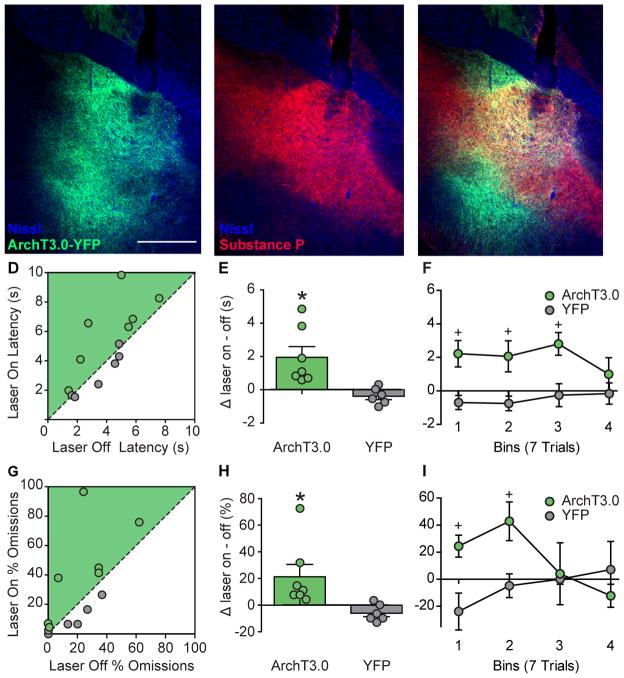
Based on our electrophysiological data, we hypothesized that inhibition of VP during the cue period would slow and/or reduce the likelihood of DS-induced responses. We targeted expression of ArchT3.0-eYFP or eYFP alone to VP, implanted an optic fiber over VP (Figure 4A–C) and trained rats in the DS task until they responded on >80% of DS trials and <20% of NS trials. On the test day, we illuminated VP with green light (~15mW) for the full duration of the DS during 50% of DS presentations (Figure S4A). We found that bilateral photoinhibition of VP during the DS significantly increased latency to lever press (Figure 4D and E; laser X virus: F(1,11)=10.615, p = 0.008). Additionally, photoinhibition of VP increased the number of omissions (Figure 4G and H; laser X virus: F(1,11)=6.672, p = 0.025). That inhibition of VP during the DS acutely increases both latency to respond to the DS, as well as the likelihood of a response omission, indicates that VP activity during the cue actively promotes DS-elicited reward seeking behavior.
Figure 4. Optogenetic inhibition of VP increases DS response latency and omissions.
A, Example of ArchT virus expression (green) in VP with fiber placement (Dapi in blue; 5x). B, Example of substance P immunohistochemsitry (red) for demarcating the borders of the VP. C, Merged photo of substance P (red) and ArchT virus expression (green) in VP. Scatterplots of average latency (D) and % of omissions (proportion of trials with no response, G) on laser trials versus no laser trials. Individual data points and mean (+/− SEM) for the difference between laser and no laser trials for latency (E) and % of omissions (H). Data split into 4 bins of 7 trials each to demonstrate the time course of the difference between laser and no laser trials for latency (F) and % omissions (I) in ArchT3.0 (green) and YFP control rats (grey). * = p<0.05 laser versus no laser, + = p<0.05 ArchT3.0 versus YFP.
Discussion
Here, we demonstrate that the majority of VP neurons are robustly excited by a cue signaling the availability of reward. The onsets of DS-evoked excitations are detected at a shorter latency than either excitations or inhibitions of NAc neurons in the same task, suggesting VP DS excitations are driven primarily by inputs other than NAc MSNs. That these VP neuron excitations promote reward seeking is supported by three lines of evidence: First, DS excitations are greater when followed by a reward-seeking response. Second, the firing rate of many neurons following the DS is significantly inversely correlated with the animal’s latency to respond. Finally, optogenetic inhibition of VP neurons during DS presentations increases the latency of reward-seeking actions following the DS, as well as the probability of omissions. Together these findings demonstrate that activity in VP neurons is a critical mechanism by which incentive stimuli generate reward-seeking behavior.
Is VP a simple relay of upstream signals originating primarily in NAc?
Much interest in the role of VP in reward-seeking stems from its proposed role as a major downstream target of the NAc. Previous work has demonstrated that NAc neurons encode cue value (Ambroggi et al., 2008, 2011; Day et al., 2006) as well as the vigor of reward-seeking actions (McGinty et al., 2013; Nicola et al., 2004), and we had expected that VP activity would reflect upstream encoding in NAc. However, our data are not consistent with this idea. First, we find that reward-predictive cues are more widely represented in the VP than in the NAc, which could be driven by NAc neurons if they had an extensive axonal arborization within the VP. Yet, anatomical data suggests this is not the case (Tripathi et al., 2010). Second, the onset latencies of most VP excitations to the DS are too short to be evoked by cue-evoked phasic inhibitions or phasic excitations in the NAc. Therefore, the earliest components of the excitatory DS responses in many VP neurons, which predict the latency to initiate a reward-seeking response, are not driven or gated by transient changes in NAc neuron firing.
NAc inputs to VP may play an important role in cue-elicited reward seeking that is independent of the timing of cue-evoked responses in NAc neurons, as suggested by work using either tonic inhibition or “disconnection” of this pathway (Leung and Balleine, 2013; Stefanik et al., 2013; but see Khoo et al., 2015). Inputs from NAc to VP may also be important for inhibitions observed following both the DS and the NS, which occur at a sufficient latency (Hakan et al., 1992; Lavin and Grace, 1996; Mogenson and Nielsen, 1983; Yang and Mogenson, 1985). Approximately 17% of NAc core neurons and 12% of NAc shell neurons are excited by the DS (Ambroggi et al., 2011); these neurons could mediate the inhibitions observed downstream in VP. Yet, the magnitude of VP neuron inhibitions evoked by the DS does not differ depending on whether the animal makes a subsequent lever press. Overall, our results are not consistent with the hypothesis that phasic encoding of the incentive strength of the DS by VP neurons is driven by upstream phasic encoding in NAc.
Given this, what is the origin of the phasic signals to the VP that drive, or that could be integrated to drive, VP encoding of DS response vigor? VP neurons receive inputs from a variety of “limbic” areas including the prefrontal, insular, and orbital cortices, and basolateral amygdala (Kelley et al., 1982; Maslowski-Cobuzzi and Napier, 1994; Reep and Winans, 1982), basal ganglia structures including the ventral tegmental area (VTA) and substantia nigra (Heimer et al., 1991; Martinez-Murillo et al., 1988; Maurice et al., 1997), as well as inputs from the lateral hypothalamus (Grove, 1988), midline thalamic nuclei, subthalamic nucleus (Fuller et al., 1987) and parabrachial nucleus (Saper and Loewy, 1980). One promising candidate region projecting to VP, which has been implicated in the DS task and shown to influence NAc encoding of incentive stimuli and reward-seeking vigor is the basolateral amygdala (Ambroggi et al., 2008; Ishikawa et al., 2008a; Jones et al., 2010; Záborszky et al., 1984). Prefrontal cortex is also implicated in the DS task (Ishikawa et al., 2008b) as well as other forms of cue-induced reward seeking (Capriles et al., 2003; Peters et al., 2009; Stefanik et al., 2012). Phasic inputs from these regions may be integrated with more tonic signals from NAc to generate incentive salience encoding. Future studies are required to determine what is specifically encoded by these inputs, and which VP efferent targets are critical for the generation of motivational vigor in response to cues.
Supplementary Material
Highlights.
Cue responses in ventral pallidum precede “upstream” responses in nucleus accumbens
Ventral pallidal neurons selectively respond to a cue predicting reward availability
Cue responses are predictive of both the likelihood and latency of reward-seeking
Ventral pallidal photoinhibition reduces reward-seeking likelihood and vigor
Acknowledgments
This work was supported by National Institutes of Health grants AA022290 (JMR) and AA014925 (PHJ), and by funds provided by the State of California for medical research on alcohol and substance abuse. FA is supported by the CNRS (ATIP-Avenir program). The authors thank Deanna Acs and Catriona Miller for technical assistance.
Footnotes
Author Contributions
Conceptualization, JMR, FA and HLF; Investigation, JMR; Formal Analysis, JMR and FA; Resources, HLF and PHJ; Writing – Original Draft, JMR and HLF; Writing – Review & Editing, JMR, FA, PHJ, HLF; Visualization, JMR and FA; Funding Acquisition, JMR, PHJ, HLF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of Nucleus Accumbens Core and Shell in Incentive-Cue Responding and Behavioral Inhibition. J Neurosci. 2011;31:6820–6830. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacol. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci. 2006;23:1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamatergic aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM, Schmidt R. New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci. 2014;34:15178–15183. doi: 10.1523/JNEUROSCI.3252-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988;277:315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Hakan RL, Berg GI, Henriksen SJ. Electrophysiological evidence for reciprocal connectivity between the nucleus accumbens septi and ventral pallidal region. Brain Res. 1992;581:344–350. doi: 10.1016/0006-8993(92)90730-w. [DOI] [PubMed] [Google Scholar]
- Heimer L, Switzer RD, Van Hoesen GW. Ventral striatum and ventral pallidum. Trends Neurosci. 1982;5:83–87. [Google Scholar]
- Heimer L, Zaborszky L, Zahm DS, Alheid GF. The ventral striatopallidothalamic projection: I. The striatopallidal link originating in the striatal parts of the olfactory tubercle. J Comp Neurol. 1987;255:571–591. doi: 10.1002/cne.902550409. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008a;155:573–584. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008b;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoga CA, Berridge KC, Aldridge JW. Ventral Pallidal Coding of a Learned Taste Aversion. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Wheeler RA, Carelli RM. The basolateral amygdala differentially regulates conditioned neural responses within the nucleus accumbens core and shell. Neuroscience. 2010;169:1186–1198. doi: 10.1016/j.neuroscience.2010.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AEE, Domesick VBB, Nauta WJHJ. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Khoo AT, Gibson GD, Prasad AA, McNally GP. Role of the striatopallidal pathway in renewal and reacquisition of alcohol seeking. Behav Neurosci. 2015;129:2–7. doi: 10.1037/bne0000036. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015 doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Grace AA. Physiological properties of rat ventral pallidal neurons recorded intracellularly in vivo. J Neurophysiol. 1996;75:1432–1443. doi: 10.1152/jn.1996.75.4.1432. [DOI] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. J Neurosci. 2013;33:13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. Ventral pallidal projections to mediodorsal thalamus and ventral tegmental area play distinct roles in outcome-specific Pavlovian-instrumental transfer. J Neurosci. 2015;35:4953–4964. doi: 10.1523/JNEUROSCI.4837-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Murillo R, Semenenko F, Cuello AC. The origin of tyrosine hydroxylase-immunoreactive fibers in the regions of the nucleus basalis magnocellularis of the rat. Brain Res. 1988;451:227–236. doi: 10.1016/0006-8993(88)90767-6. [DOI] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, Napier TC. Activation of dopaminergic neurons modulates ventral pallidal responses evoked by Amygdala stimulation. Neuroscience. 1994;62:1103–1119. doi: 10.1016/0306-4522(94)90347-6. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Position of the ventral pallidum in the rat prefrontal cortex-basal ganglia circuit. Neuroscience. 1997;80:523–534. doi: 10.1016/s0306-4522(97)00002-x. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78:910–922. doi: 10.1016/j.neuron.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Res Bull. 1983;11:309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-Evoked Firing of Nucleus Accumbens Neurons Encodes Motivational Significance During a Discriminative Stimulus Task. J Neurophysiol. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–1033. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- Perry CJ, McNally GP. A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking. Eur J Neurosci. 2013;38:2762–2773. doi: 10.1111/ejn.12283. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Winans SS. Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus auratus. Neuroscience. 1982;7:2609–2635. doi: 10.1016/0306-4522(82)90087-2. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2012;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci. 2013;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “Wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Cebrián C, Mengual E. Axonal branching patterns of nucleus accumbens neurons in the rat. J Comp Neurol. 2010;518:4649–4673. doi: 10.1002/cne.22484. [DOI] [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. An electrophysiological study of the neural projections from the hippocampus to the ventral pallidum and the subpallidal areas by way of the nucleus accumbens. Neuroscience. 1985;15:1015–1024. doi: 10.1016/0306-4522(85)90250-7. [DOI] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Léránth C, Heimer L. Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neurosci Lett. 1984;52:219–225. doi: 10.1016/0304-3940(84)90165-4. [DOI] [PubMed] [Google Scholar]
- Zhou L, Furuta T, Kaneko T. Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle. Neuroscience. 2003;120:783–798. doi: 10.1016/s0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.