Abstract
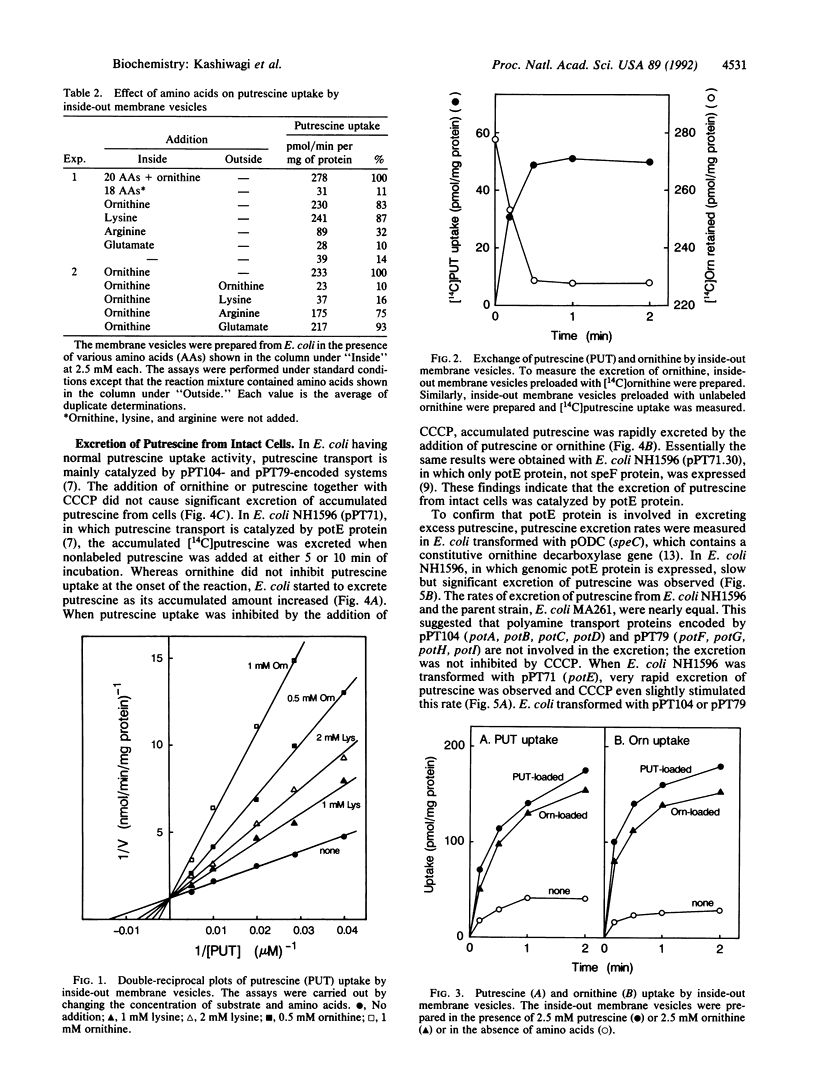
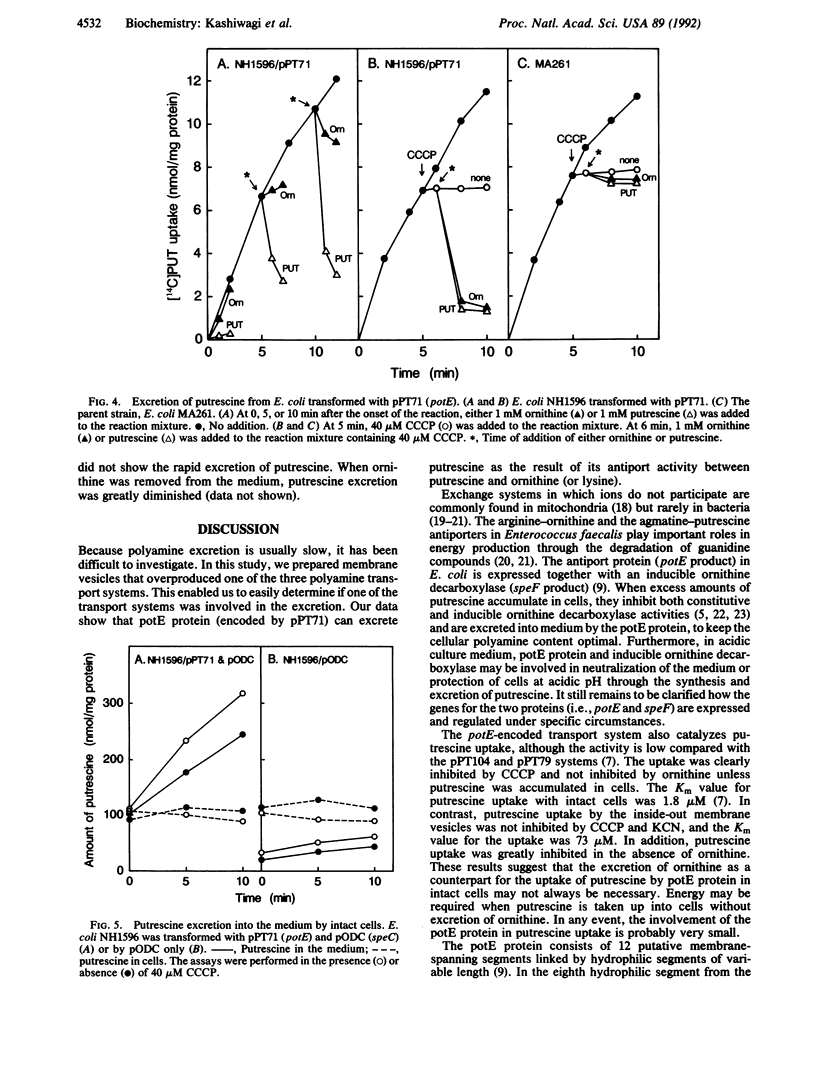
Excretion of putrescine from Escherichia coli was assessed by measuring its uptake into inside-out membrane vesicles. The vesicles were prepared from wild-type E. coli or E. coli transformed with plasmids containing one of the three polyamine transport systems. The results indicate that excretion of putrescine is catalyzed by the putrescine transport protein, encoded by the potE gene located at 16 min on the E. coli chromosome. Loading of ornithine (or lysine) inside the vesicles was essential for the uptake of putrescine, indicating that the protein exchanges putrescine and ornithine (or lysine) by an antiport mechanism. The Km and Vmax values for the putrescine uptake by inside-out membrane vesicles were 73 microM and 0.82 nmol/min per mg of protein, respectively. The antiport protein (potE protein) also catalyzed putrescine-putrescine and ornithine-ornithine exchange. The transport activity was not disturbed by inhibitors of energy production such as KCN and carbonyl cyanide m-chlorophenylhydrazone. When intact E. coli was used instead of the inside-out membrane vesicles, excretion of putrescine was also catalyzed by the antiport protein in the presence of ornithine in the medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L. Uptake, intracellular binding, and excretion of polyamines during growth of Neurospora crassa. Arch Biochem Biophys. 1989 Jun;271(2):315–322. doi: 10.1016/0003-9861(89)90281-6. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., Smid E. J., Konings W. N. Transport of diamines by Enterococcus faecalis is mediated by an agmatine-putrescine antiporter. J Bacteriol. 1988 Oct;170(10):4522–4527. doi: 10.1128/jb.170.10.4522-4527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuchi T., Kashiwagi K., Kobayashi H., Igarashi K. Characteristics of the gene for a spermidine and putrescine transport system that maps at 15 min on the Escherichia coli chromosome. J Biol Chem. 1991 Nov 5;266(31):20928–20933. [PubMed] [Google Scholar]
- Houng H. S., Lynn A. R., Rosen B. P. ATP-driven calcium transport in membrane vesicles of Streptococcus sanguis. J Bacteriol. 1986 Nov;168(2):1040–1044. doi: 10.1128/jb.168.2.1040-1044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Hamasaki H., Miura A., Kakegawa T., Hirose S., Matsuzaki S. Formation of a compensatory polyamine by Escherichia coli polyamine-requiring mutants during growth in the absence of polyamines. J Bacteriol. 1986 Apr;166(1):128–134. doi: 10.1128/jb.166.1.128-134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Sugawara K., Izumi I., Nagayama C., Hirose S. Effect of polyamines of polyphenylalanine synthesis by Escherichia coli and rat-liver ribosomes. Eur J Biochem. 1974 Oct 2;48(2):495–502. doi: 10.1111/j.1432-1033.1974.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Kashiwagi K., Watanabe S., Kameji T., Hayashi S., Igarashi K. Influence of the 5'-untranslated region of ornithine decarboxylase mRNA and spermidine on ornithine decarboxylase synthesis. J Biol Chem. 1990 Aug 5;265(22):13036–13041. [PubMed] [Google Scholar]
- Kashiwagi K., Hosokawa N., Furuchi T., Kobayashi H., Sasakawa C., Yoshikawa M., Igarashi K. Isolation of polyamine transport-deficient mutants of Escherichia coli and cloning of the genes for polyamine transport proteins. J Biol Chem. 1990 Dec 5;265(34):20893–20897. [PubMed] [Google Scholar]
- Kashiwagi K., Igarashi K. Adjustment of polyamine contents in Escherichia coli. J Bacteriol. 1988 Jul;170(7):3131–3135. doi: 10.1128/jb.170.7.3131-3135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K., Suzuki T., Suzuki F., Furuchi T., Kobayashi H., Igarashi K. Coexistence of the genes for putrescine transport protein and ornithine decarboxylase at 16 min on Escherichia coli chromosome. J Biol Chem. 1991 Nov 5;266(31):20922–20927. [PubMed] [Google Scholar]
- Kashiwagi K., Yamaguchi Y., Sakai Y., Kobayashi H., Igarashi K. Identification of the polyamine-induced protein as a periplasmic oligopeptide binding protein. J Biol Chem. 1990 May 25;265(15):8387–8391. [PubMed] [Google Scholar]
- Krause D. C., Winkler H. H., Wood D. O. Cloning and expression of the Rickettsia prowazekii ADP/ATP translocator in Escherichia coli. Proc Natl Acad Sci U S A. 1985 May;82(9):3015–3019. doi: 10.1073/pnas.82.9.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Linderoth N., Morris D. R. Structural specificity of the triamines sym-homospermidine and aminopropylcadaverine in stimulating growth of spermidine auxotrophs of Escherichia coli. Biochem Biophys Res Commun. 1983 Dec 16;117(2):616–622. doi: 10.1016/0006-291x(83)91245-7. [DOI] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Williamson L. R., Plano G. V., Winkler H. H., Krause D. C., Wood D. O. Nucleotide sequence of the Rickettsia prowazekii ATP/ADP translocase-encoding gene. Gene. 1989 Aug 15;80(2):269–278. doi: 10.1016/0378-1119(89)90291-6. [DOI] [PubMed] [Google Scholar]


