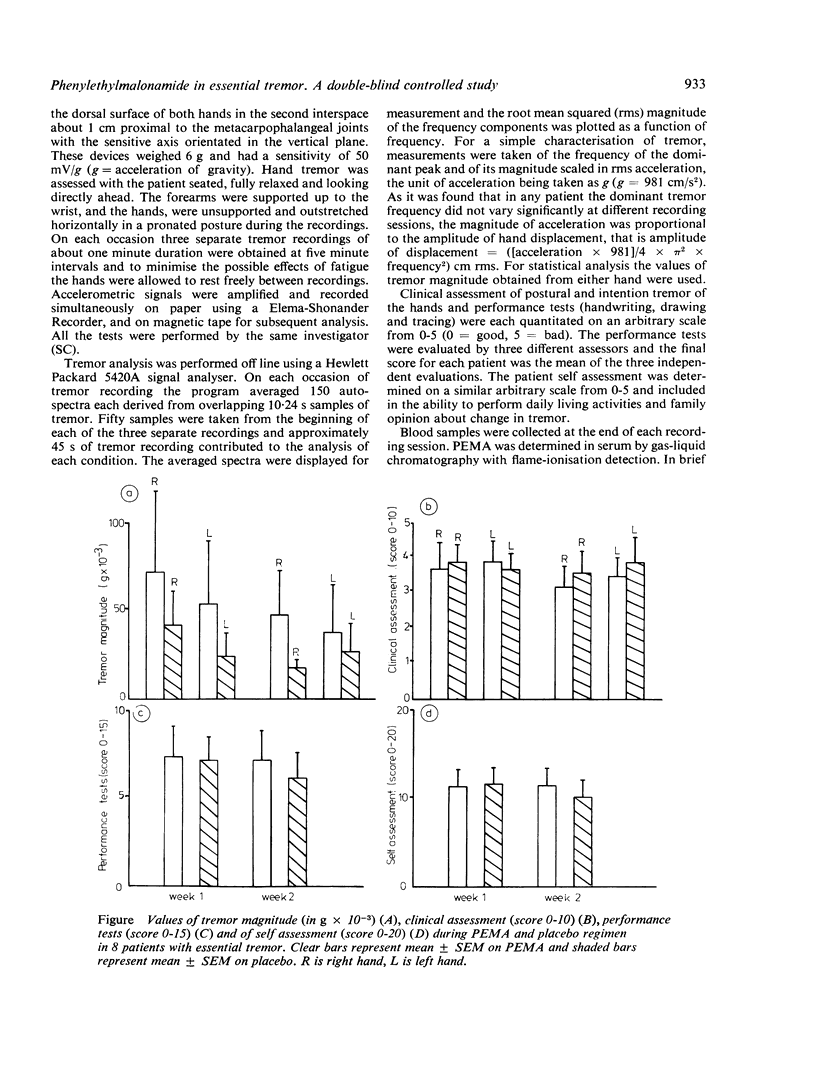
Abstract
A randomised double-blind placebo-controlled trial of phenylethylmalonamide, the major metabolite of primidone was performed in eight patients with essential tremor. Phenylethylmalonamide was given in a daily dose of 400 mg for one week and 800 mg for a second week. The compound had no statistically significant effect on the amplitude of tremor assessed by an accelerometric method, tests of performance, clinical evaluation and patient self assessment. No side effects occurred. Serum levels of phenylethylmalonamide on a daily dose of 400 mg were 11-27 micrograms/ml and on 800 mg daily were 16-48.5 micrograms/ml.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- O'Brien M. D., Upton A. R., Toseland P. A. Benign familial tremor treated with primidone. Br Med J (Clin Res Ed) 1981 Jan 17;282(6259):178–180. doi: 10.1136/bmj.282.6259.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen P. S., Paulson O. B., Steiness E., Jansen E. C. Essential tremor treated with propranolol: lack of correlation between clinical effect and plasma propranolol levels. Ann Neurol. 1981 Jan;9(1):53–57. doi: 10.1002/ana.410090110. [DOI] [PubMed] [Google Scholar]


