ABSTRACT
Inside the cell, vital processes such as cell division and intracellular transport are driven by the concerted action of different molecular motor proteins. In C. elegans chemosensory cilia, 2 kinesin-2 family motor proteins, kinesin-II and OSM-3, team up to drive intraflagellar transport (IFT) in the anterograde direction, from base to tip, whereas IFT dynein hitchhikes toward the tip and subsequently drives IFT in the opposite, retrograde direction, thereby recycling both kinesins. While it is evident that at least a retrograde and an anterograde motor are necessary to drive IFT, it has remained puzzling why 2 same-polarity kinesins are employed. Recently, we addressed this question by combining advanced genome-engineering tools with ultrasensitive, quantitative fluorescence microscopy to study IFT with single-molecule sensitivity.1,2 Using this combination of approaches, we uncovered a differentiation in kinesin-2 function, in which the slower kinesin-II operates as an ‘importer’, loading IFT trains into the cilium before gradually handing them over to the faster OSM-3. OSM-3 subsequently acts as a long-range ‘transporter’, driving the IFT trains toward the tip. The two kinesin-2 motors combine their unique motility properties to achieve something neither motor can achieve on its own; that is to optimize the amount of cargo inside the cilium. In this commentary, we provide detailed insight into the rationale behind our research approach and comment on our recent findings. Moreover, we discuss the role of IFT dynein and provide an outlook on future studies.
Keywords: fluorescence microscopy, IFT, kinesin, motor cooperation, motor proteins, sensory cilia
Introduction
All eukaryotic organisms face a challenge: their cells are too large for thermal-energy driven diffusion to effectively distribute organelles, vesicles, RNA and other components.3 This problem is particularly severe in the long extensions of neurons, which can be meters long in larger mammals. To solve this problem, eukaryotic organisms have evolved intricate intracellular transport systems, consisting of motor proteins, cargo and tracks.3 The tracks are formed by microtubules and actin filaments, which are part of the cytoskeleton. The motor proteins are specialized proteins that are able to use these tracks as intracellular highways by converting chemical energy in the form of ATP to mechanical work. Three large superfamilies of motor proteins have evolved: dyneins,4 myosins5 and kinesins.6 The myosins step along actin filaments, whereas dyneins and kinesins use microtubules as tracks. Both microtubules and actin filaments are polarized, enabling unidirectional transport: kinesins generally move in the plus-end direction along microtubules, whereas dyneins move in the opposite direction. The myosin superfamily contains motors that can move in either direction along actin. Over the past 3 decades, structural and functional studies of the motor proteins have taught us in great detail how they operate on the molecular level. In vitro single-molecule motility assays using optical tweezers or fluorescence microscopy have allowed detailed measurement of velocities, step sizes, processivity and force generation.7 It has become clear, however, that understanding the behavior of a single motor protein is not sufficient to fully grasp intracellular transport. The main reason is that, in vivo, in many cases, multiple motors with distinct motility properties team up to move the same cargo.8 This results in a complex interplay between motor proteins, which is also affected by cargo rigidity and by the properties and complexity of the tracks. Furthermore, the motors, tracks and cargo are under tight regulatory control, adding to the complexity. A complete understanding of intracellular transport therefore requires a systems approach, taking into account all players. Specific perturbations can then be made in order to determine how these proteins affect each other and how their cooperative behavior drives the system as a whole. In our laboratory we have embarked on such an approach by using intraflagellar transport (IFT) as a model for intracellular transport and motor-protein cooperation.9 IFT is a particularly appropriate transport mechanism, since it takes place in specific organelles, flagella or cilia, and makes use of a specific machinery that includes motor proteins with different properties. IFT is essential for the assembly, maintenance and function of both motile (e.g. flagella of sperm cells or the cilia in the human trachea that transport mucus) and primary (or sensory) cilia (e.g., the connection between photoreceptor rod and cell body, or cilia that are responsible for olfaction or taste). Malfunctioning of cilia is the underlying cause of many different human diseases, including Bardet-Biedl Syndrome and polycystic kidney disease.10-12 Studying IFT therefore does not only contribute to a deeper understanding of intracellular transport but could also increase our insight into ciliopathies.
Intraflagellar transport in C. elegans
The chemosensory cilia in the C. elegans amphid and phasmid channels have become important models to study IFT, for several reasons.13 First, there are the general benefits of C. elegans as a model organism: fast reproduction, well-known genetics, well-developed transgenesis techniques, biobanks with numerous mutants and its transparency that renders it very suitable for fluorescence microscopy. In addition, the C. elegans chemosensory cilia show a particularly intriguing level of complexity: the ciliary axoneme has a bipartite structure with a middle segment composed of microtubule doublets from which a distal segment, composed of 9 microtubule singlets, protrudes.14 In addition, in these cilia, IFT is driven by the interplay between 3 different motor proteins. As demonstrated by early experiments in the Scholey laboratory, anterograde transport is driven by 2 kinesin-2 family members, heterotrimeric kinesin-II (consisting of 3 subunits KLP-11, KLP-20 and KAP-1)15,16 and homodimeric OSM-3.17,18 Retrograde transport is driven by IFT dynein, a cilium-specific dynein. 19
Snow and co-workers subsequently demonstrated that kinesin-II and OSM-3 do not work independently while transporting cargo but cooperate.20 The key evidence for this was that both kinesin-2 motors and cargoes move at the same velocity of ∼0.7 μm/s along the microtubule doublets of the middle segment, while IFT velocity increases to ∼1.2 μm/s in the absence of kinesin-II and decreases to ∼0.5 μm/s in the absence of OSM-3. An interesting redundancy in the IFT system was also uncovered: both kinesin-2 motors are capable of building the middle segment on their own, whereas OSM-3 is essential for the formation of the distal segment. These observations raised the question why the slower kinesin-II is employed when OSM-3 is capable of building the middle and distal segment by itself. Further insights into kinesin-2 cooperation came from a phenotypic screen for ciliary mutants copying the osm-3 phenotype.21 Among the candidates, bbs-8, a gene associated with Bardet-Biedl syndrome (BBS), was identified. IFT assays on worm strains lacking bbs-8 (as well as bbs-7) function, showed that kinesin-II and OSM-3 were moving along the middle segment at their individual velocities of ∼0.5 μm/s and ∼1.2 μm/s, respectively. The same was true for the IFT particle subcomplexes; the IFT-A subcomplex, known to be associated with kinesin-II, moved at ∼0.5 μm/s while the IFT-B subcomplex, known to be associated with OSM-3, moved at ∼1.2 μm/s. This suggested that the BBS-protein complex stabilizes the interaction between IFT particle subcomplex A and B, linking kinesin-II to OSM-3 and resulting in an intermediate velocity of ∼0.7 μm/s for the IFT train. While this study provided insight into the functional coordination of IFT, it was not clear why the combined activity of slow and fast operating motors would result in an intermediate velocity for the motor ensemble as a whole. A series of in vitro microtubule-gliding assays using mixtures of purified C. elegans kinesin-2 motors in combination with mathematical modeling addressed this question.22 In these gliding assays, microtubules were moved by different ratios of coverslip-bound OSM-3 and kinesin-II and microtubule velocities were measured. The velocity-versus-motor ratio plots were compared with different models for motor cooperation. The authors concluded that the in vitro data could be well described by 2 different models. In one model, the transport is driven by the independent, alternating activity of kinesin-II and OSM-3; in the other model both motors are active at the same time and pull on each another, affecting each other's motility properties.
Studying IFT with single-molecule sensitivity
But what is the functional significance of this intriguing kinesin-2 motor cooperation? Why is the slower kinesin-II employed? In Prevo et al.,1 we addressed this question using improved imaging tools and a different approach to generate transgenic animals. We made use of MosSCI (Mos-1 mediated single-copy insertion) to stably integrate single-copy transgenes encoding for fluorescently labeled IFT proteins in the genome.23 A key advantage of this approach over traditional methods is that expression levels are comparable to wild-type expression levels.23 For our experiments we crossed transgenic animals with animals carrying the specific null allele to create animals only expressing fluorescent versions of the protein of interest. We studied the phasmid cilia in the tails of these transgenic animals using ultrasensitive quantitative fluorescence microscopy, employing wide-field EMCCD-based detection and laser-epi illumination.24 This approach offers far less optical sectioning capability than the traditionally used spinning-disk confocal microscopes; however, sectioning is not required since the IFT proteins are almost exclusively expressed in the very thin chemosensory cilia, (in the C. elegans tail), which are almost entirely located in a single image plane. We chose to image IFT in the tail-located phasmid cilia rather than the head-located amphid cilia for 3 reasons. First, there are only 2 cilia per phasmid channel compared to 10 in each amphid channel, substantially simplifying interpretation. Second, the autofluorescence background is significantly lower in the tail of C. elegans than in the head, increasing sensitivity. Third, animals frequently twitch and move upon excitation with laser light, which is detrimental to imaging quality. Twitching occurred far more frequently in the head than in the tail. Together, this combination of microscopy and labeling strategy allowed for the visualization and quantification of the distribution and dynamics of, on the one hand, whole IFT-protein ensembles, and on the other, the behavior of single IFT proteins in the phasmid cilia, deep inside the living worm.
Using this approach, we obtained fluorescence image sequences from individual worms, initially focusing on illumination conditions minimizing photo bleaching such that bulk IFT behavior could be analyzed. We started our analysis of these image sequences by time averaging to obtain steady-state distributions of key IFT components along the cilia. While most IFT components were quite evenly distributed over the cilia (with a substantial enrichment close to the base), the 2 kinesin-2 motors were distributed in an almost mirrored way. Kinesin-II was highly enriched at base and transition zone and disappeared within micrometers in the proximal segment, beyond the transition zone (more gradual and earlier than observed before). OSM-3, on the other hand, was only marginally present at base and transition zone, while its density gradually increased in the proximal segment, reaching a plateau in the distal segment.
To obtain further insight in the molecular basis of this remarkable difference in motor distributions, we next focused on the dynamics of IFT. Image sequences showed many, partially overlapping clusters of IFT components moving together along the cilia in both directions. The clusters were too dense to be tracked with single-particle tracking analysis methods. We thus analyzed the image sequences using kymography, a powerful technique to visualize dynamics along linear tracks.25 We used a newly developed, open-source (http://www.nat.vu.nl/~erwinp/downloads.html) quantitative kymograph analysis tool to automatically extract parameters such as position-dependent velocities of IFT trains and number of IFT proteins in trains.
A new view on same-polarity motor cooperation in IFT
Using these kymography tools, we found that the kinesin-2 motor composition of an IFT train changes dramatically along the cilium. Anterograde trains contain many tens of kinesin-II motors close to the base and transition zone and move slowly at a pace set by kinesin-II, while only few OSM-3 motors are present. In the distal segment, trains contain many OSM-3 motors and no kinesin-II and move at a high velocity governed by OSM-3. In between the transition zone and the distal segment, a region of a couple of micrometers can be identified, the “handover zone,” where the kinesin-2 motor ratio on IFT trains gradually changes, concomitant with an increase in velocity. This data allowed extraction of the dependency between OSM-3 motor fraction and train velocity, which showed an intriguing non-linear increase. A model in which kinesin-II is outcompeted tenfold by OSM-3 fitted the data well. This is distinct from what had been observed in in vitro microtubule gliding assays,22 possibly reflecting differences in mechanical connection between the motors in vivo and in vitro, different regulatory mechanisms, or differences in microtubule track or geometry.
When IFT trains arrive at the ciliary tip, they can revert direction, the minus-end motor IFT dynein taking over from the plus-end kinesins. From the changes in direction of IFT trains in our kymographs we could infer that this indeed happens, although we have not yet visualized IFT dynein directly. What we did observe is retrograde-moving IFT trains recycling both kinesin-2 motors to the base. Taken together, these results suggest that IFT trains remain relatively intact while moving from base to tip or from tip to base. The number and ratio of kinesin-2 motors bound to trains, however, changes dramatically along the way.
In order to shed further light on the molecular basis of these changes in train composition, we imaged individual motor proteins using controlled photoactivation of individual paGFP labels or controlled photobleaching of eGFP labels. In the single-molecule trajectories we saw that kinesin-II undergoes turnarounds along the whole middle segment and that OSM-3 undergoes turnarounds along the full length of the axoneme, whereas the IFT particle subcomplexes only turn around at the ciliary tip. Kinesin-II and OSM-3 switch direction by releasing from an anterograde moving train and subsequently binding to a retrograde moving train driven by IFT dynein and vice versa. These results show that while IFT particle trains cycle between base and tip, the 2 kinesin-2 motors make their own cycles. Kinesin-II cycles back and forth between base and proximal segment, while OSM-3 mostly cycles between transition zone and tip. Ultimately, this IFT dynamics ensures that kinesin-II concentration is enhanced around the base and the transition zone, and that OSM-3 is enriched in the distal segment, with an intriguing handover zone along the proximal segment where neither motor has a dominant presence.
Finally, careful analysis of worm strains with specific mutations in IFT components provided additional evidence for a functional differentiation between the kinesin-2 motors. We discovered that kinesin-II functions as a loader and navigator of the IFT trains through the dense transition zone, which separates cilium from dendrite, and OSM-3 as a long-range transporter of trains (Fig. 1). The motility parameters of both motor proteins are quite different, OSM-3 being relatively fast ∼1.5 μm/s and processive (average run length ∼2 μm), and kinesin-II substantially slower ∼0.5 μm/s and less processive (average run length ∼0.2 μm) and might be optimized for their respective tasks.26 The lower processivity of kinesin-II and its ability to switch microtubule tracks might be beneficial for successful circumventing roadblocks on the tracks,27 e.g. provided by the Y-shaped linker proteins connecting microtubules with membrane in the transition zone. Together, this team of distinct kinesin-2 motor proteins appears to drive IFT more efficiently and regularly than one of the motors could do on its own.
Figure 1.
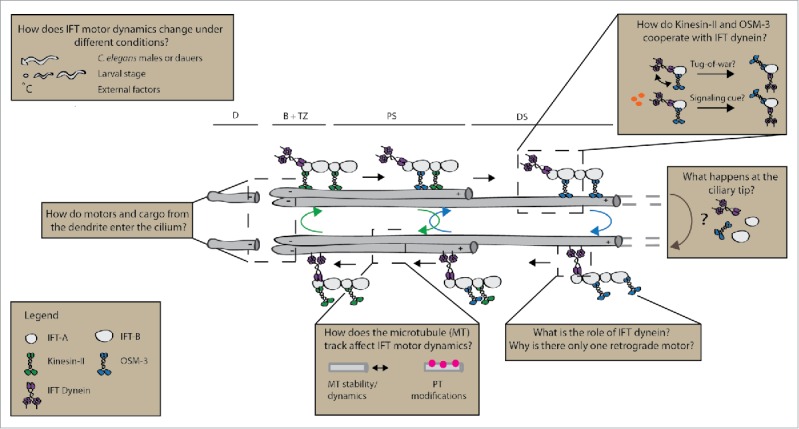
Sketch of IFT in the C. elegans phasmid chemosensory cilium. (B, base; TZ, transition zone; PS, proximal segment; DS, distal segment) and dendrite (D). Several avenues for future research are highlighted: the influence of external factors (e.g., temperature) on IFT; opposite-polarity motor cooperation; ciliary tip dynamics; the role of IFT dynein; microtubule track complexity and the interplay between the dendrite and cilium. PT: Posttranslational Modification.
Outlook
These new insights into kinesin-2 motor cooperation in IFT in C. elegans raise important new questions for future research (Fig. 1). One of the key findings is the undocking and docking of the kinesin-2 motors in the handover zone, a specific region in the proximal segment extending about 3 µm from the transition zone. It is not clear how this is regulated: is detachment of motors a random process along the whole cilium, or is it position-specific, regulated by positional cues such as the different ciliary structures (e.g. the doublet and singlet microtubule tracks, the track extremities or Y-shaped linkers)? Further experiments involving mutant animals might shed light on this. Another important question concerns the exact nature of the mechanical cooperation of the 2 kinesin-2 motors connected to one cargo. Because of their velocity differences they will exert force on each other, which likely has an effect on their motility parameters. It is unclear whether and how this affects the chemical or mechanical transitions within individual motors, or results in enhanced detachment of motors from the microtubule track. In vitro experiments on well-controlled motor assemblies employing optical tweezers or fluorescence microscopy might provide further insight.28
Other key questions concern the role of IFT dynein, the driver of retrograde transport. How IFT dynein is able to carry out both long-range transport in the proximal and distal segment, as well as navigate the transition zone, remains an open question. In this respect, it is interesting to note that in contrast to the regular 8 nm steps taken by kinesin motors, recent single-molecule studies have shown that cytoplasmic dynein 1 can take larger steps up to 32 nm, can step sideways and even backward.29-32 Additionally, it can change its motility parameters by recruiting regulators such as NudE and Lis1.33,34 It could be that this versatile stepping behavior of dynein allows the motor to change its motility properties when required, moving at full speed along the distal and proximal segment, before slowing down in the transition zone. In vivo and vitro motility assays on IFT dynein will be required to demonstrate whether IFT dynein has similar properties and versatility to cytoplasmic dynein.
Another intriguing question is how kinesin-2 driven anterograde transport is coordinated with IFT-dynein driven retrograde transport. It appears that IFT trains travel in one go from base to tip and from tip to base. This might suggest that the activity of the different directionality motors is tightly regulated and that ‘tug-of-war’ mechanical competition between kinesins and IFT-dynein does not play a role. A crucial aspect in this respect is to understand what exactly happens at the ciliary tip where IFT trains reverse direction. EM tomography analysis of IFT trains in Chlamydomonas reinhardtii showed that anterograde trains are smaller than retrograde trains, substantiating the hypothesis that remodeling or reassembly of IFT trains occurs at the tip.35 Further high-resolution in vivo imaging of IFT dynamics with a particular focus on the ciliary tip will be required.
In addition, little is known about the interplay between cilium ultrastructure (in particular at base and transition zone) and transport properties. How do IFT trains assemble at the base and what is the exact mechanism of the slowdown of transport we observed in the transition zone? Moreover, the exact architecture and dynamics of the microtubule tracks might play important roles in regulation IFT. It could be that certain motors have a preference for microtubule doublets or singlets. Furthermore, specific tubulin isoforms and post-translational modifications might also affect motility parameters.36,37
Furthermore, it is not very well known how IFT connects to transport in the dendrites. It appears that dendritic cargo is disassembled at the ciliary base where it is prepared for IFT. It would be interesting to investigate the connection between these transport mechanisms, for example by specifically blocking or inhibiting one of them and measuring the effect on the other.
Finally, many unanswered questions remain about the connection between IFT and chemosensation, the biological function of the cilia. It is not known to what extent proteins involved in chemosensation are in fact transported by IFT. We have shown that the flux of IFT particles transported is about halved in animals lacking functional kinesin-II. It is unclear whether such defect in IFT has an effect on chemosensation. This could be tested in behavioral assays. 38,39
Conclusion
How groups of molecular motors work together to drive transport remains an intriguing question. The quantitative approach presented in Prevo et al.1 can readily be applied to study the dynamics of opposite-polarity motor cooperation, such as between IFT dynein, OSM-3 and kinesin-II in the cilium or at the ciliary tip. Altogether, it is clear that such quantitative studies of the IFT system will shed more light on the system parameters of this particular intracellular transport mechanism, in particular with respect to motor cooperation and regulation. It is very likely that similar regulation and cooperation mechanisms are used to optimize other intracellular transport processes as well.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank our collaborators on this project: Pierre Mangeol, Felix Oswald and Jonathan M. Scholey.
Funding
This work was financially supported by the Netherlands Organization for Scientific Research (NWO) Vici grant and ALW Open Program grant.
References
- [1].Prevo B, Mangeol P, Oswald F, Scholey JM, Peterman EJ. Functional differentiation of cooperating kinesin-2 motors orchestrates cargo import and transport in C. elegans cilia. Nat Cell Biol 2015; 17:1536-45; PMID:26523365; http://dx.doi.org/ 10.1038/ncb3263 [DOI] [PubMed] [Google Scholar]
- [2].O'Hagan R, Barr MM. A motor relay on ciliary tracks. Nat Cell Biol 2015; 17:1517-9; PMID:26612573; http://dx.doi.org/ 10.1038/ncb3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Howard J. Mechanics of Motor Proteins and the Cytoskeleton Sunderland, MA: Sinauer Associates, 2001. [Google Scholar]
- [4].Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol 2013; 14:713-26; PMID:24064538; http://dx.doi.org/ 10.1038/nrm3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hartman MA, Spudich JA. The myosin superfamily at a glance. J Cell Sci 2012; 125:1627-32; PMID:22566666; http://dx.doi.org/ 10.1242/jcs.094300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 2009; 10:682-96; PMID:19773780; http://dx.doi.org/ 10.1038/nrm2774 [DOI] [PubMed] [Google Scholar]
- [7].Prevo B, Acar S, Kruijssen DLH, Peterman EJG. Single-Molecule Spectroscopy of Motor Proteins. Encyclopedia of Analytical Chemistry: John Wiley & Sons, Ltd, 2014; 1-25 [Google Scholar]
- [8].Mallik R, Rai AK, Barak P, Rai A, Kunwar A. Teamwork in microtubule motors. Trends Cell Biol 2013; 23:575-82; PMID:23877011; http://dx.doi.org/ 10.1016/j.tcb.2013.06.003 [DOI] [PubMed] [Google Scholar]
- [9].Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Topics Dev Biol 2008; 85:23-61; PMID:19147001; http://dx.doi.org/ 10.1016/S0070-2153(08)00802-8 [DOI] [PubMed] [Google Scholar]
- [10].Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al.. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet 2007; 39:727-9; PMID:17468754; http://dx.doi.org/ 10.1038/ng2038 [DOI] [PubMed] [Google Scholar]
- [11].Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, et al.. Mutations in IFT172 cause isolated retinal degeneration and Bardet-Biedl syndrome. Hum Mol Genet 2015; 24:230-42; PMID:25168386; http://dx.doi.org/ 10.1093/hmg/ddu441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Ann Rev Gen Hum Genet 2006; 7:125-48; PMID:16722803; http://dx.doi.org/ 10.1146/annurev.genom.7.080505.115610 [DOI] [PubMed] [Google Scholar]
- [13].Inglis PN, Ou G, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. WormBook 2007:1-22; PMID:18050505; http://dx.doi.org/10.1895/wormbook.1.126.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR. The molecular architecture of axonemes revealed by cryoelectron tomography. Science (New York, NY) 2006; 313:944-8; http://dx.doi.org/ 10.1126/science.1128618 [DOI] [PubMed] [Google Scholar]
- [15].Orozco JT, Wedaman KP, Signor D, Brown H, Rose L, Scholey JM. Movement of motor and cargo along cilia. Nature 1999; 398:674; PMID:10227290; http://dx.doi.org/ 10.1038/19448 [DOI] [PubMed] [Google Scholar]
- [16].Scholey JM. Intraflagellar transport. Ann Rev Cell Dev Biol 2003; 19:423-43; PMID:14570576; http://dx.doi.org/ 10.1146/annurev.cellbio.19.111401.091318 [DOI] [PubMed] [Google Scholar]
- [17].Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 1986; 117:456-87; PMID:2428682; http://dx.doi.org/ 10.1016/0012-1606(86)90314-3 [DOI] [PubMed] [Google Scholar]
- [18].Signor D, Wedaman KP, Rose LS, Scholey JM. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol Biol Cell 1999; 10:345-60; PMID:9950681; http://dx.doi.org/ 10.1091/mbc.10.2.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol 1999; 147:519-30; PMID:10545497; http://dx.doi.org/ 10.1083/jcb.147.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol 2004; 6:1109-13; PMID:15489852; http://dx.doi.org/ 10.1038/ncb1186 [DOI] [PubMed] [Google Scholar]
- [21].Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature 2005; 436:583-7; PMID:16049494; http://dx.doi.org/ 10.1038/nature03818 [DOI] [PubMed] [Google Scholar]
- [22].Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol 2006; 174:1035-45; PMID:17000880; http://dx.doi.org/ 10.1083/jcb.200606003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, Grunnet M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet 2008; 40:1375-83; PMID:18953339; http://dx.doi.org/ 10.1038/ng.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van den Wildenberg SM, Prevo B, Peterman EJ. A brief introduction to single-molecule fluorescence methods. Methods Mol Biol 2011; 783:81-99; http://dx.doi.org/ 10.1007/978-1-61779-282-3_5 [DOI] [PubMed] [Google Scholar]
- [25].Brust-Mascher I, Ou G, Scholey JM. Measuring rates of intraflagellar transport along Caenorhabditis elegans sensory cilia using fluorescence microscopy. Methods Enzymol 2013; 524:285-304; PMID:23498746; http://dx.doi.org/ 10.1016/B978-0-12-397945-2.00016-0 [DOI] [PubMed] [Google Scholar]
- [26].Scholey JM. Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Ann Rev Cell Dev Biol 2013; 29:443-69; PMID:23750925; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122335 [DOI] [PubMed] [Google Scholar]
- [27].Schroeder HW 3rd, Hendricks AG, Ikeda K, Shuman H, Rodionov V, Ikebe M, Goldman YE, Holzbaur EL. Force-dependent detachment of kinesin-2 biases track switching at cytoskeletal filament intersections. Biophys J 2012; 103:48-58; PMID:22828331; http://dx.doi.org/ 10.1016/j.bpj.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Derr ND, Goodman BS, Jungmann R, Leschziner AE, Shih WM, Reck-Peterson SL. Tug-of-war in motor protein ensembles revealed with a programmable DNA origami scaffold. Science (New York, NY) 2012; 338:662-5; http://dx.doi.org/ 10.1126/science.1226734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Single-molecule analysis of dynein processivity and stepping behavior. Cell 2006; 126:335-48; PMID:16873064; http://dx.doi.org/ 10.1016/j.cell.2006.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DeWitt MA, Chang AY, Combs PA, Yildiz A. Cytoplasmic dynein moves through uncoordinated stepping of the AAA+ ring domains. Science (New York, NY) 2012; 335:221-5; http://dx.doi.org/ 10.1126/science.1215804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qiu W, Derr ND, Goodman BS, Villa E, Wu D, Shih W, Reck-Peterson SL. Dynein achieves processive motion using both stochastic and coordinated stepping. Nat Struct Mol Biol 2012; 19:193-200; PMID:22231401; http://dx.doi.org/ 10.1038/nsmb.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell 2007; 131:952-65; PMID:18045537; http://dx.doi.org/ 10.1016/j.cell.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vallee RB, McKenney RJ, Ori-McKenney KM. Multiple modes of cytoplasmic dynein regulation. Nat Cell Biol 2012; 14:224-30; PMID:22373868; http://dx.doi.org/ 10.1038/ncb2420 [DOI] [PubMed] [Google Scholar]
- [34].Cianfrocco MA, DeSantis ME, Leschziner AE, Reck-Peterson SL. Mechanism and Regulation of Cytoplasmic Dynein. Ann Rev Cell Dev Biol 2015; 31:83-108; PMID:26436706; http://dx.doi.org/ 10.1146/annurev-cellbio-100814-125438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol 2009; 187:135-48; PMID:19805633; http://dx.doi.org/ 10.1083/jcb.200905103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol 2011; 13:790-8; PMID:21642982; http://dx.doi.org/ 10.1038/ncb2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 2014; 16:335-44; PMID:24633327; http://dx.doi.org/ 10.1038/ncb2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hukema RK, Rademakers S, Jansen G. Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learning & memory (Cold Spring Harbor, NY) 2008; 15:829-36; http://dx.doi.org/ 10.1101/lm.994408 [DOI] [PubMed] [Google Scholar]
- [39].Stephens GJ, Johnson-Kerner B, Bialek W, Ryu WS. Dimensionality and dynamics in the behavior of C. elegans. PLoS Comput Biol 2008; 4:e1000028; PMID:18389066; http://dx.doi.org/ 10.1371/journal.pcbi.1000028 [DOI] [PMC free article] [PubMed] [Google Scholar]


