Abstract
To identify genetic effects modulating blood stage replication of the malarial parasite, we phenotyped a group of 25 inbred mouse strains for susceptibility to Plasmodium chabaudi chabaudi AS infection (peak parasitemia, survival). A broad spectrum of responses was observed, with strains such as C57BL/6J being the most resistant (low parasitemia, 100% survival), and strains such as NZW/LacJ and C3HeB/FeJ being extremely susceptible (very high parasitemia and uniform lethality). A number of strains showed intermediate phenotypes and gender specific effects, suggestive of rich genetic diversity in response to malaria in inbred strains. An F2 progeny were generated from SM/J (susceptible) and C57BL/6J (resistant) parental strains, and was phenotyped for susceptibility to P. chabaudi chabaudi AS. A whole genome scan in these animals identified the Char1 locus (LOD=7.40) on chromosome 9 as a key regulator of parasite density and pointed to a conserved 0.4Mb haplotype at Char1 that segregates with susceptibility/resistance to infection. In addition, a second locus was detected in [SM/J x C57BL/6J] F2 mice on the X chromosome (LOD=4.26), which was given the temporary designation Char11. These studies identify a conserved role of Char1 in regulating response to malaria in inbred mouse strains, and provide a prioritized 0.4Mb interval for the search of positional candidates.
Keywords: Plasmodium chabaudi, malaria, inbred mouse strains, QTL, haplotype mapping
INTRODUCTION
Malaria, caused by infection with Plasmodium parasites, is responsible for an estimated 250 million clinical cases and close to 1 million deaths annually. It is endemic in over 100 countries and half of the world’s population lives at risk of developing malaria (http://www.who.int/en). Despite extensive research efforts, the malaria global health problem has been exacerbated by a widespread resistance to anti-malarial drugs in the Plasmodium parasite and to pyrethroid insecticides in the Anopheles mosquito vector. In addition, attempts to develop an efficacious vaccine remain, so far, unsuccessful1–3.
In regions of endemic disease, host genetic polymorphisms influencing disease incidence and severity have been well documented with strong evidences of selective pressure by the parasite on the human gene pool4; 5. The vast majority of these genetic variations affect the erythroid system, an important replication niche for the parasite, and genes encoding key molecules of the innate or adaptive immune processes. For instance, erythrocyte polymorphisms causing sickle cell anemia, G6PD deficiencies, α and β thalassemias, Duffy negativity, band 3 ovalocytosis have been associated with protection against malaria6. Finally, numerous case-control studies have detected association between onset or severity of cerebral malaria (CM) and genes involved in host inflammatory (TNFα, IL-12, IFNγ, NOS2A, LTA, IRF1) and immune (HLA, FCGR2A) responses4; 5; 7. Parallel family-based genome-wide linkage analyses in nuclear families from Cameroon, Burkina Faso and Senegal have independently detected a strong effect of a cytokine cluster on 5q31-q33, which regulates the level of blood parasitemia8–11. In the Senegalese families, additional genetic effects of Chr. 5p and 13q (number of clinical episodes), and 12 (prevalence of asymptomatic infections) were detected12. Also, a genome-wide linkage analysis of “mild malaria phenotypes” in families from rural Ghana detected a major locus on 10p15.3 (PFFE-1) (malaria fever episodes), with additional contributions (suggestive linkages) from Chr. 13q and 1p36 (blood parasitemia)9. These studies have shown that the genetic component of susceptibility to malaria in humans is complex. Reduced penetrance, variable expressivity, and a wide disease spectrum associated with variations in parasite-encoded virulence determinants, together with variable diagnostic criteria, make it difficult to decipher and map single gene effects in human populations13.
Such complex genetic traits can be dissected in inbred and recombinant strains of mice, where single gene effects have been fixed by inbreeding and can be identified by positional cloning. In mouse, blood-stage malaria (merozoite replication in RBCs) can be studied in the Plasmodium chabaudi chabaudi AS infection model14, whereas cerebral malaria (CM) can be induced by infection with P. berghei ANKA15. The symptoms and pathology, including anemia, associated with blood-stage P. chabaudi chabaudi AS infection in mice, resemble those associated with P. falciparum malaria in humans16; 17. Inbred strains differ in their degree of susceptibility to blood stage malaria induced by infection with P. chabaudi chabaudi AS. Resistant strains such as C57BL/6J (B6) display low parasitemia with moderate anemia and 100% survival18. They develop marked splenomegaly, robust erythropoiesis, as well as local proliferation and accumulation of dendritic cells, NK cells, macrophages, and T and B lymphocytes. An early type 1 immune response, dependent on IL-12 and IFN-γ-occurs with development of strong immunity to challenge infection17; 19. On the other hand, susceptible strains, such as A/J (A), develop a severe disease associated with high parasitemia, severe anemia, and with the majority of the mice (~75%) succumbing to disease by 9–12 days post infection. In A/J mice, there is a defect in both splenic erythropoiesis and early type 1, IL-12- and IFN-γ-dependent immune responses. Studies in backcross and F2 mice, as well as in AXB/BXA recombinant inbred lines showed that the genetic control of the A vs. B6 inter-strain difference is multigenic18; 20. Additional quantitative trait locus (QTL) mapping by whole genome scan in crosses between A and B6 by our group21 and between susceptible SJL, C3H and resistant B6 by others22; 23 mapped 2 major Chabaudi resistance loci on chromosomes 9 (Char1) and 8 (Char2) that control peak parasitemia and survival. A third H-2 linked locus on Chr. 17, (Char3, LOD=5.0) regulates parasite clearance immediately after the peak of infection24. Also, 4 additional weaker gene effects (LOD<2.0) mapping to Chrs. 5 (Char5, Char6), 17 (Char7 related to Char3), and 11 (Char8 syntenic with human 5q31-q33) were detected in F11 advanced intercross lines (from A and B6 parents), as modulating blood stage replication25; 26. The genes responsible for these effects have not been identified.
We have used a set of 36 AcB/BcA recombinant congenic mouse lines derived from C57BL/6J (resistant) and A/J (susceptible) progenitors to identfy novel gene effects that regulate susceptibility to P. chabaudi chabaudi AS27. We detected 2 discordant strains (AcB55, AcB61) that were highly resistant to infection (low parasitemia, 100% survival) despite presence of A/J-derived susceptibility alleles at Char1 and Char2. Resistance in these strains is determined by a 2-locus system Char4 (Chr. 3), and Char9 (Chr. 10)28. The Char4 protective effect is caused by homozygosity for a mutation (PklrI90N) in the RBC form of pyruvate kinase (Pklr)29; 30. We subsequently showed that loss of PKLR function reduces replication of P. falciparum in human erythrocytes ex vivo31; 32. Char9-associated susceptibility to P. chabaudi chabaudi AS infection is caused by a loss of pantetheinase enzyme activity33. Treatment of mice with the pantetheinase metabolite cysteamine (Cys) can reduce blood-stage replication of P. chabaudi chabaudi AS and can strongly potentiate the anti-malarial activity of artemisinin type drugs34; 35. Finally, we identified AcB62 as a discordant strain, being PK-deficient (PklrI90N), but yet susceptible to P. chabaudi chabaudi AS infection (high parasitemia), suggesting the presence of a locus (designated Char10) modifying the protective effect of PK-deficiency36.
RESULTS
Phenotypic responses of inbred strains to P. chabaudi chabaudi AS infection
To explore the effect of allelic diversity in the laboratory mouse gene pool on response to blood-stage malaria, we surveyed 25 of the 40 Tier 1–3 inbred strains of the Mouse Phenome project37 for susceptibility to blood stage malaria. These strains were selected to represent the different ancestry of the laboratory mouse (phylogenetic differences) and maximize representation and sampling of the allelic pool present in the wild. Between 5 and 20 animals from each inbred strains were challenged intraperitoneally with 106 P. chabaudi chabaudi AS parasitized erythrocytes (pRBC), and progress of infection was monitored over the course of 3 weeks. In these animals, blood parasitemia was measured daily on thin blood smears, and parasitemia at the peak of infection was determined. Survival from infection was also monitored daily. The strains tested, the number of animals tested, the peak parasitemia and the survival rates are summarized in Table 1. We observed a variety of responses to P. chabaudi chabaudi AS infection, ranging from resistant to highly susceptible. We also noted a general gender effect on peak parasitemia, which tended to be lower in females than males, and in some of the strains this gender effect was concomitant to differential rates of recovery and survival. Strains such as SM/J, KK/HIJ, NOD/LtJ, MRL/MpJ, CAST/EiJ, SJL/J, SWR/J, C3H/HeJ, NZW/LacJ, NU/J, C3HEB/FeJ were found to be highly susceptible with very high peak parasitemia (> 50%) and uniform lethality in both genders (except for NOD/LtJ, SJL/J and NU/J for which only one gender was tested). A second group of strains showed an intermediate phenotype. A/J, CBA/J, PL/J, and BALB/cByJ displayed high parasitemia and significant lethality, but a significant fraction of the mice (up to 60% in some strains) recovered from infection despite high peak parasitemia levels. In addition, strains such as BALB/cJ, DBA/2J, AKR/J and BTBRT+tf/J presented a striking gender effect: all females survived the infection while all males succumbed. 129S1/SvlmJ, FVB/NJ, DBA/1LacJ, RBF/DnJ and NZB/BINJ were all found to be resistant with respect to overall survival (both in males and females), with peak parasitemia levels ranging from 41% (low) to 67% (high). Finally, C57BL/6J was found to be the most resistant inbred strain, with relatively low peak parasitemia values (31% to 43%), and uniform survival from infection. These results suggest a rich phenotypic diversity in the response of inbred strains to blood stage malaria.
Table 1. Phenotypic responses of 25 inbred strains to infection with P. chabaudi chabaudi AS.
Twenty five commonly used inbred strains from the Jackson Phenome Database were phenotyped for susceptibility to P. chabaudi chabaudi AS infection. Mice were infected i.p. with 106 parasitized RBC and parasitemia (expressed as percentage of parasitized RBC) and survival were assessed daily following infection. Mean peak parasitemia levels are indicated ± SD. Resistance or susceptibility status was based on overall survival.
| Strain | # of mice (M,F) | Peak Parasitemia (%pRBC) | Survival (%) | ||||
|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | ||||
|
| |||||||
| C57BL/6J | (5,5) | 43±4 | 31±7 | 100 | R | 100 | R |
| 129S1/SvlmJ | (4,4) | 56±2 | 41±4 | 100 | R | 100 | R |
| FVB/NJ | (5,5) | 61±5 | 48±4 | 80 | R | 100 | R |
| DBA/1LacJ | (0,5) | ND | 48±3 | ND | 100 | R | |
| RBF/DnJ | (5,0) | 56±4 | ND | 80 | R | ND | |
| NZB/BINJ | (5,0) | 67±6 | ND | 80 | R | ND | |
|
| |||||||
| BALB/cJ | (5,5) | 51±10 | 41±6 | 0 | S | 100 | R |
| DBA/2J | (4,4) | 58±6 | 43±3 | 0 | S | 100 | R |
| AKR/J | (5,5) | 61±4 | 51±2 | 0 | S | 100 | R |
| BTBRT+tf/J | (4,4) | 72±6 | 68±4 | 0 | S | 100 | R |
| CBA/J | (5,5) | 65±6 | 57±9 | 40 | I | 60 | I |
| PL/J | (5,0) | 49±7 | ND | 40 | I | ND | |
| BALB/cByJ | (4,4) | 54±9 | 69±4 | 50 | I | 0 | S |
| A/J | (10,10) | 60±8 | 52±7 | 0 | S | 50 | I |
|
| |||||||
| SM/J | (5,5) | 62±13 | 60±7 | 20 | S | 20 | S |
| KK/HIJ | (4,4) | 59±4 | 51±3 | 0 | S | 0 | S |
| NOD/LtJ | (5,0) | 63±5 | ND | 0 | S | ND | |
| MRL/MpJ | (5,5) | 63±5 | 61±3 | 0 | S | 0 | S |
| CAST/EiJ | (4,4) | 69±3 | 56±4 | 0 | S | 0 | S |
| SJL/J | (0,5) | ND | 57±4 | ND | 0 | S | |
| SWR/J | (5,5) | 70±1 | 70±4 | 0 | S | 0 | S |
| C3H/HeJ | (5,5) | 71±5 | 66±9 | 0 | S | 0 | S |
| NZW/LacJ | (5,5) | 72±4 | 68±5 | 0 | S | 0 | S |
| NU/J | (0,5) | ND | 73±3 | ND | 0 | S | |
| C3HeB/FeJ | (5,5) | 72±2 | 70±5 | 0 | S | 0 | S |
R = resistant; I = intermediate; S = susceptible; ND = not determined
To explore the genetic basis for inter-strain differences in response to P. chabaudi chabaudi AS, we used the strain distribution pattern (peak parasitemia), and high density SNP datasets available for the 25 strains (Mouse HapMap SNP data) to perform whole genome association mapping. We utilized a statistical method EMMA38 that corrects for population structure and genetic relatedness between inbred strains by estimating a kinship matrix. EMMA analysis failed to identify a significant locus that determines the differential susceptibility of inbred strains to blood stage malaria (Figure S1). This suggests a complex and multigenic control of susceptibility to P. chabaudi chabaudi AS in inbred mouse strains.
Genetic analysis of differential susceptibility of C57BL/6J and SM/J mice
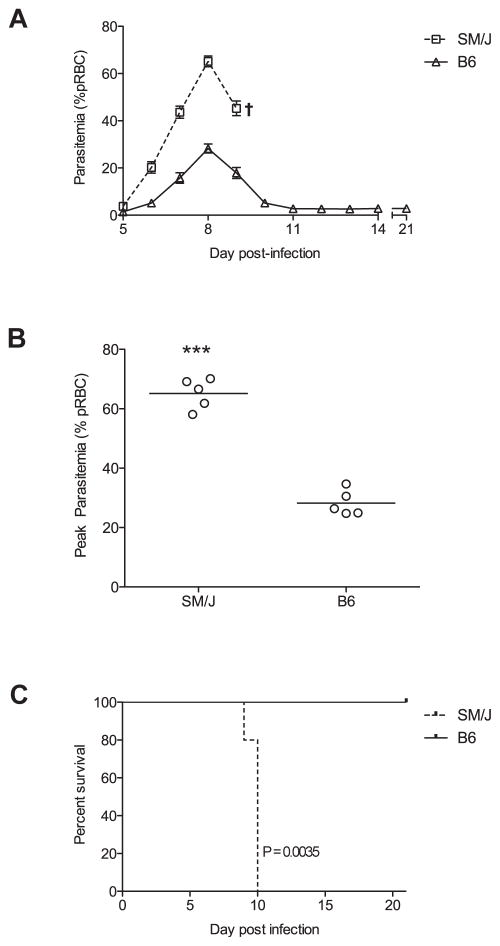
We turned to standard quantitative trait locus mapping to identify genetic effects regulating response to P. chabaudi chabaudi AS in these strains. We arbitrarily selected C57BL/6J and SM/J as representative resistant and susceptible strains for further genetic analyses. Differential susceptibility in these two strains is associated with differential rates of blood-stage replication of the parasite during the first week infection (Figure 1A). At day 8 parasitemia peaks in both strains but is very high in SM/J (~65%) and much lower in C57BL/6J (~28%) (Figure 1B). Subsequently, C57BL/6J mice recover from infection and clear the parasite, while SM/J succumb from hyperparasitemia and associated severe malaria-induced anemia by day 10 (Figure 1C).
Figure 1. Differential susceptibility of C57BL/6J and SM/J mice to P. chabaudi chabaudi AS infection.
Mice were infected i.v. with 105 parasitized RBC, and daily parasitemia (expressed as percentage of parasitized RBC) and survival were recorded. (A) Course of infection (blood parasitemia) in C57BL/6J and SM/J mice over a 21 day period. (B) Peak parasitemia levels are shown for strain C57BL/6J and SM/J. Each circle represents one mouse. Statistical significance (two-tailed Student’s t-test; compared to B6) is indicated by stars: ***P<0.0001. (C) Kaplan-Meier survival curve. SM/J is represented by a dashed line and C57BL/6J by a solid line. Survival curves were compared by a Log-rank test and the median survival of SM/J was found to be significantly different from C57BL/6J (P=0.0035).
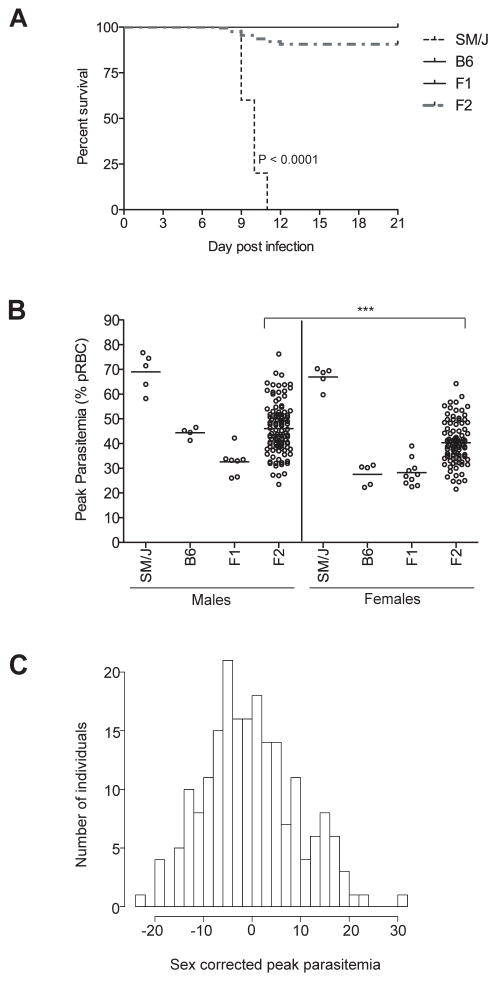
The genetic control of differential susceptibility of C57BL/6J and SM/J strains to P. chabaudi chabaudi AS, including the number and chromosomal positions of potential regulating loci, was studied by linkage analysis in informative crosses. For this, we generated 201 [SM/JxC57BL/6J] informative F2 mice and phenotyped them for susceptibility to P. chabaudi chabaudi AS using peak parasitemia as a phenotypic measure of susceptibility in individual animals. Control C57BL/6J, SM/J as well as [SM/JxC57BL/6J]F1 and [SM/JxC57BL/6J]F2 animals were infected with P. chabaudi chabaudi AS. Studies in [SM/JxC57BL/6J]F1 mice showed that they were very resistant to infection: they displayed very low parasitemia either lower (33%, males) or equal (28%, females) to that measured in the resistant C57BL/6J controls (44%, males; 28%, females) with no recorded mortality (Figure 2A and 2B). This result suggests that susceptibility in SM/J mice is completely recessive. In [SM/JxC57BL/6J]F2 animals, parasitemia ranged from the very high to very low extremes represented by the parental strains, with 10% of the F2 mice succumbing from infection. Overall parasitemia values were significantly lower in [SM/JxC57BL/6J]F2 females compared to males (Figure 2B). Hence, for genetic analysis in these mice, we regressed the peak parasitemia levels to gender specific means and transformed the data to a “sex-adjusted peak parasitemia” (Figure 2C). Once corrected for sex, F2 animals show a normal and continuous distribution (Figure 2C), amenable to QTL mapping.
Figure 2. Segregation analysis of susceptibility to P. chabaudi chabaudi AS infection in [SM/J x C57BL/6J]F1 and F2 mice.
17 F1 and 201 F2 animals as well as parental controls were infected i.v. with 105 parasitized RBC, and daily parasitemia (expressed as percentage of parasitized RBC) as well as survival from infection were recorded. (A) Kaplan-Meier survival curve. Survival curves were compared by a Log-rank test and the median survival of SM/J was found to be significantly different from C57BL/6J (P<0.0001). (B) The peak of parasitemia is shown separately for males and females. Each circle represents one mouse. Statistical significance between F2 males and females (two-tailed Mann-Whitney test) is indicated by stars: ***P<0.0001. (C) Distribution of peak parasitemia in the entire F2 population after regression of peak parasitemia levels to a gender-specific mean.
Genetic mapping studies in [SM/JxC57BL/6J]F2 mice
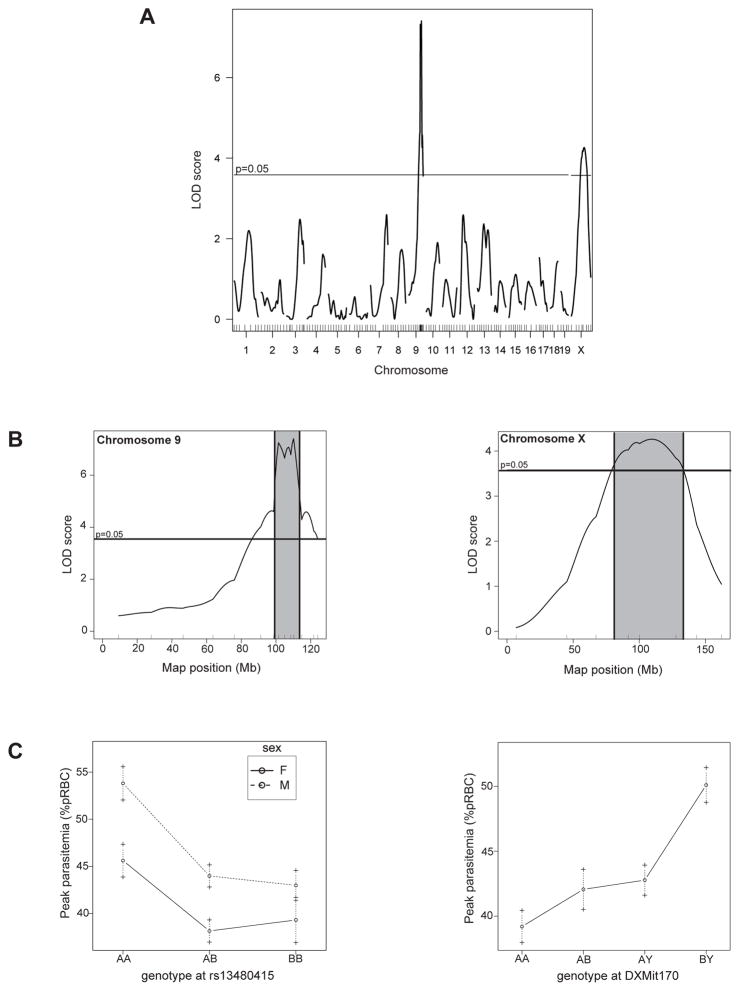
All 201 [SM/JxC57BL/6J]F2 mice were genotyped for 137 informative polymorphic markers (SNPs and microsatellites) distributed with an average of 10–15Mb across the mouse genome. Quantitative trait analysis was performed in R/qtl across all chromosomes, using sex-adjusted peak parasitemia as a quantitative trait. Genome-wide significance thresholds were determined by empirical permutation testing, consisting of 1000 tests for autosomes and 14297 tests for the X chromosome. This analysis identified a highly significant linkage on chromosome 9 (peak LOD score=7.40, peak marker=rs13480415) and a weaker linkage on chromosome X (peak LOD score=4.37, peak marker=DXMit170) (Figure 3A and 3B). The Bayesian 95% credible interval for the distal chromosome 9 locus was determined to be 101.4–111.4Mb (99% confidence interval= 100.4–112.4Mb) (Figure 3B). Strikingly, this region has previously shown to carry the Char1 locus by the group of S. Foote22, and shown to control susceptibility to infection with P. chabaudi adami DS in [C3H/HexC57BL/6] and [SJLxC57BL/6]F2 crosses, with C57BL/6 being resistant and C3H/He and SJL being susceptible. The group of Ohno et al., in 2001, mapped, in the same region, a locus they called Pymr in a [(NC/Jic×129/SvJ)×NC/Jic] cross where mice were challenged with P. yoelii 17XL39, with 129/SvJ being resistant and NC/Jic being susceptible. It is therefore extremely likely that the chromosome 9 locus we detected as influencing susceptibility to P. chabaudi chabaudi AS in [SM/JxC57BL/6J]F2 is in fact Char1. Allelic combination (Figure 3C) indicate that susceptibility alleles at Char1 (peak marker rs13480415) are inherited in a completely recessive fashion in male and female mice, consistent with the mode of inheritance detected by analysis of response of [SM/JxC57BL/6J]F1 mice (Figure 2A and 2B). The weaker gene effect on chromosome X (peak marker DXMit170) was given the temporary designation Char11 (LOD=4.26), and maps to a Bayesian 95% credible interval spanning positions 79–133Mb (Figure 3B). SM/J allele(s) at Char11 are associated with lower parasitemia compared to mice carrying the C57BL/6J allele(s) (Figure 3C), with the effect being stronger in males.
Figure 3. Genetic analysis of differential susceptibility to P. chabaudi chabaudi AS infection in resistant C57BL/6J and susceptible SM/J mice.
Whole-genome analysis was carried out in 201 [SMxC57BL/6]F2 mice infected i.v. with 105 parasitized RBC, using sex corrected peak parasitemia as a quantitative trait. Interval mapping was done using the R/qtl software package. (A) Results for all chromosomes are plotted. Significance thresholds revealed by permutation testing (1000 permutations for autosomes and 14297 permutations for the X chromosome) are indicated. (B) Enhanced view of chromosome 9 and X. Vertical lines and hatched area delimit the 1.5-LOD support interval. (C) Effect of Char1 and Char11 alleles (at peak marker) on peak parasitemia. Males and females are plotted separately in order to reveal any potential sex effect. A and B correspond to SM/J and C57BL/6J alleles respectively.
Overall our results point to Char1 as the major gene effect regulating differential susceptibility to blood stage malaria in C57BL/6J and SM/J mouse strains.
Haplotype analysis of Char1 in 129/SvJ, C57BL/6J, SM/J, SJL/J, C3H/HeJ and NC/Jic mouse strains
The gene(s) underlying the Char1 effect has not yet been identified, despite much effort. We carried out haplotype analysis of the Char1 region (100.4–112.4Mb) in inbred mouse strains that a) had been previously phenotyped for susceptibility to malaria, and b) where high density SNP maps are available to support this analysis. In addition, we selected strains in which resistance/susceptibility to P. chabaudi or P. yoelii infection had been linked previously22; 39 or here to Char1 alleles. We aimed to identify possible segments of the Char1 region that may segregate with resistance/susceptibility in these strains, and that may facilitate the delineation of a minimal physical interval for Char1. We used two recently available SNP lists. The first consisted of 28 000 SNPs for chromosome 9 only and was obtained from G. Churchill at the Jackson laboratory (personal communication). The second consisted of about 132 000 SNPs for the entire genome and was obtained from the Mouse Hapmap project. Genotypes for these SNPs were available for strains 129/SvJ, C57BL/6J, SM/J, SJL/J, and C3H/HeJ while we generated haplotype information for the NC/Jic by genomic DNA sequencing. In Figure 4, the C57BL/6J and 129/SvJ haplotypes (resistant strains) are fixed in grey, while discordant SNPs are labeled in black. We detected a unique bi-allelic haplotype block between positions 108.5 and 108.9 Mb, that was common to the two resistant strains (129/SvJ and C57BL/6J) but was different from susceptible strains (SM/J, SJL/J, C3H/HeJ and NC/Jic). Our findings suggest that the gene responsible for the Char1 effect may be contained within this sub-haplotype.
Figure 4. Co-segregation of Char1 haplotypes in 129x1/SvJ, C57BL/6J, SM/J, SJL/J, C3H/HeJ and NC/Jic inbred strains.
The position of the unique bi-allelic haplotype block common to C57BL/6J and 129/SvJ (resistant strains) but different from SM/J, SJL, C3H/He and NC/Jic (susceptible strains) is shown. SNP-based genotypes for C57BL/6J, 129/SvJ, SM/J, SJL/J and C3H/HeJ were obtained from the Mouse Hapmap project. The genotype of NC/Jic for the indicated SNPs was determined by genomic DNA sequencing. C57BL/6J alleles are depicted in grey. Alleles different from C57BL/6J alleles are depicted in black. Inbred strains were segregated into susceptible (S) and resistant (R) strains.
DISCUSSION
To explore the phenotypic diversity of inbred strains of mice for susceptibility to malaria, we surveyed a set of 25 phylogenetically distant inbred strains of mice selected from the Tier 1–3 strains sets from the Mouse Phenome Project37. These strains were infected with the murine malarial parasite P. chabaudi chabaudi AS and parasitemia was followed daily during infection and survival was monitored. Parasite density at the peak of infection and survival were used to estimate the degree of susceptibility to infection. These experiments produced several observations. First, there is great diversity in response to infection among inbred strains, covering a broad spectrum, ranging from highly resistant C57BL/6J (low parasitemia, no lethality) to highly susceptible strains such as SM/J, KK/HIJ, NOD/LtJ, MRL/MpJ, CAST/EiJ, SJL/J, SWR/J, C3H/HeJ, NZW/LacJ, NU/J, and C3HEB/FeJ (very high parasitemia and associated anemia, high lethality). Secondly, there was a striking gender effect, which was most visible in strains showing an intermediate level of susceptibility, with females showing a higher degree of resistance (lower parasitemia, increased survival). Such a gender effect had been previously detected in mice, and has been attributed to the major male sex hormone testosterone. Indeed, administration of testosterone results in increased female susceptibility while castration improves outcome in male mice40–42. Thirdly, haplotype association mapping by EMMA analysis failed to detect any single major locus contributing to resistance/susceptibility in the entire cohort of inbred strains phenotyped. This observation together with the broad spectrum of response to infection suggested that the genetic control of susceptibility to blood stage malaria in mouse is complex with a plurality of independent loci involved.
To initiate the search for novel loci regulating parasite replication and survival to infection, we selected C57BL/6J as the most resistant strain and SM/J as a susceptible parent and generated an informative F2 cross. These F2 mice were phenotyped for response to P. chabaudi chabaudi AS and linkage analysis was conducted by a whole genome scanning approach. This study detected a major genetic effect on chromosome 9, which co-localizes with the known Char1 locus22. Char1 was previously mapped by Foote et al. as regulating susceptibility to blood stage malaria (parasite density, survival) in two independent [C3H/HexC57BL/6] and [SJLxC57BL/6]F2 crosses (LOD=9.1 and 6.6), where mice were challenged with P. chabaudi adami DS22. Ohno et al. mapped in the same region the Pymr locus, which determines susceptibility of [(NC/Jic×129/SvJ)×NC/Jic] backcross mice to infection with P. yoelii 17XL39. Together, these results indicate that the Char1 locus is a major regulator of response to infection with different malarial parasites in unrelated inbred strains. Our linkage studies in [SM/JxC57BL/6J]F2 provided a 95% Bayesian credible interval of 101.4–111.4Mb (LOD=7.41; p value<0.01). In an effort to further reduce the size of the Char1 interval, we conducted haplotype analysis in 6 inbred strains for which the malaria-susceptibility phenotype had been established and had been demonstrated to be regulated by Char1 alleles in informative crosses. This analysis showed a lack of haplotype association except for a small region overlapping positions 108.5Mb–108.9Mb. This segment shows the same haplotype for resistant C57BL/6J and 129/SvJ mice, while showing the same and opposite haplotype for susceptible SM/J, SJL/J, C3H/HeJ and NC/Jic strains. Although this haplotype association has low power, it nevertheless suggests that the 13 genes contained within this region (Table S1) should be prioritized for the search of positional candidates for the Char1 effect.
Early response to P. chabaudi chabaudi AS infection involves a robust Th1 polarization of immune response mediated by IL12 and IFNγ production by mononuclear phagocytes and T lymphocytes/NK cells, respectively, and mice lacking IFNγ, IL12p40 or CD4+ T cells are susceptible to P. chabaudi chabaudi AS infection17; 19. Interestingly, susceptible SM/J mice were found to have significantly lower numbers of CD4+ T cells in the spleen than resistant C57BL/6J mice (Figure S2), suggesting that the gene(s) underlying Char1 could be immune-related. STAT1 is a signal transducer and activator of transcription that mediates signaling by interferons (IFN). In response to IFNγ, STAT1 is tyrosine and serine-phosphorylated. It then forms a homodimer called IFN-gamma-activated factor (GAF), migrates into the nucleus and binds IFNγ activated sequence (GAS) to drive the expression of target genes. Robertson et al.43 recently used chromatin immunoprecipitation and DNA sequencing to map functional STAT1 binding sites (and associated genes), which are induced by IFNγ treatment of human HeLa S3 cells. We investigated the Char1 region for the presence of positional candidates that may be bound to STAT1 in response to IFNγ. We found that there is a cluster of genes containing STAT1 binding site(s) in the large Char1 interval. More interestingly, nearly all of the 13 genes found in the small haplotype block (108.5–108.9Mb) contain STAT1 binding site(s) (Table S1). This suggests that genes in this 0.4Mb block play an important role in regulating response to infection through IFNγ dependent pathways. Additional experiments will be needed to test this hypothesis.
MATERIALS AND METHODS
Animals
Pathogen-free, 8–10 week old inbred mice (C57BL/6J, 129S1SvlmJ, FVB/NJ, DBA/1LacJ, RBF/DnJ, NZB/BINJ, BALB/cJ, DBA/2J, AKR/J, BTBRT+tf/J, CBA/J, PL/J, BALB/cByJ, A/J, SM/J, KK/HIJ, NOD/LtJ, MRL/MpJ, CAST/EiJ, SJL/J, SWR/J, C3H/HeJ, NZW/LacJ, NU/J, and C3HeB/FeJ) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). 201 [SM/JxC57BL/6J]F2 progeny were bred by systematic brother-sister mating of [SM/JxC57BL/6J]F1 progenitors. F1 and F2 mice were bred in house in the animal care facility of McGill University. All animal experiments were carried out according to Canadian Council on Animal Care guidelines and approved by the Animal Care Committee of McGill University.
Parasite and infection
A lactate dehydrogenase virus-free isolate of P. chabaudi chabaudi AS, originally obtained from Dr. Walliker (University of Edinburgh), was maintained by weekly passage in A/J mice by i.p. infection with 106 parasitized red blood cells (pRBC) suspended in 1 ml of pyrogen-free saline. Experimental mice were infected i.p. with 106 pRBC (inbred strain survey) or i.v. with 105 pRBC (following experiments). The percentage of pRBC was determined daily on thin blood smears stained with Diff-Quick (Dade Behring, Newark, DE, USA) on days 4 to 21 post-infection. Survival from infection was monitored twice daily and moribund animals were sacrificed.
Genotyping
Before infection, tail biopsies were obtained from all animals and genomic DNA was isolated by a standard procedure involving proteinase K treatment27. All 201 [SM/JxC57BL/6]F2 mice were genotyped for a total of 137 single nucleotide polymorphisms (SNP) and microsatellite markers. SNP genotyping was performed at the McGill University and Genome Quebec Innovation Centre (Montreal, QC, Canada) using Sequenome iPlex Gold technology and a custom panel of SNP distributed across the genome. Additional microsatellite markers obtained from the Mouse Genome Informatics Database (www.informatics.jax.org) were genotyped by a standard PCR-based method using (α-32P) dATP labeling and separation on denaturing 6% polyacrylamide gels.
Linkage analysis
All linkage analysis was performed with R/qtl using peak parasitemia for individual mice as a phenotype. QTL mapping was performed using EM maximum likelihood algorithm. Genome-wide significance was calculated by permutation testing (1 000 and 14297 tests for autosomes and chromosome X respectively).
EMMA scan
The detailed algorithm underlying the efficient mixed-model for association mapping has been previously published38. The EMMA algorithm is based on the mixed-model association where a kinship matrix accounting for genetic relatedness between inbred mouse strains is estimated and the n fitted to the vector of the phenotype, thereby reducing false positive rates. In order to assess significance, the Bonferroni multiple testing correction44 was computed. EMMA is publically available as an R package implementation.
Flow cytometry
Spleens from C57BL/6J and SM/J mice were harvested at day 7 post infection, and single cell suspensions were prepared following lysis of erythrocytes with NH4Cl (Sigma-Aldrich, St. Louis, MO). Cell viability was always >95% as determined by trypan blue exclusion (Invitrogen-Life technology). Cells (1 × 106) were treated with 0.5 μg of anti-CD16/CD32 monoclonal antibodies (e-Biosciences, Inc. San Diego, CA) prior to staining with 1μg of phycoerythrin (PE)-conjugated monoclonal antibody against mouse CD4 (clone GK1.5, rat IgG2b, eBiosciences). As negative control, cells were also stained with isotype-matched monoclonal antibody. Cells were acquired using a FACS Calibur and data was analyzed using CellQuest software (BD Biosciences) and FlowJo version 9.3.3.
Statistical analysis
An unpaired, two-tailed Student’s t-test was used to establish significance of differences in mean parasitemia levels (Figure 1B) and in CD4+ cells percentage (Figure S2) between different groups of mice. A two-tailed Mann-Whitney test was used to establish significance in parasitemia levels between different groups of mice (Figure 2B). Survival of mice was analyzed by a Log-rank test and survival fractions were compared using the χ2 statistic. These data were analyzed using GraphPad Prism 4.0 statistical software. P values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We would like to thank Dr. Molly Bogue, Director of the mouse Phenome Project at the Jackson Laboratory for providing mice, and Dr. Gary Churchill at the Jackson Laboratory for sharing a list of 28 000 SNPs from chromosome 9. We would like to thank Dr. Tamio Ohno at Nagoya University for providing DNA from NC/Jic mice, and further acknowledge Susan Gauthier for breeding and maintaining the mice. This work was supported by a CIHR Team Grant in Malaria (CTP 79842) and operating grant MOP-79343 (PG), FRSQ (AL) and CIHR (GM, AL) studentships.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Genes and Immunity website
References
- 1.Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118(4):1266–1276. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101(3):207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77(2):171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongfen SE, Laroque A, Berghout J, Gros P. Genetic and genomic analyses of host-pathogen interactions in malaria. Trends Parasitol. 2009;25(9):417–422. doi: 10.1016/j.pt.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Weatherall DJ. Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol. 2008;141(3):276–286. doi: 10.1111/j.1365-2141.2008.07085.x. [DOI] [PubMed] [Google Scholar]
- 7.Allison AC. Genetic control of resistance to human malaria. Curr Opin Immunol. 2009;21(5):499–505. doi: 10.1016/j.coi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Rihet P, Traore Y, Abel L, Aucan C, Traore-Leroux T, Fumoux F. Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31-q33. Am J Hum Genet. 1998;63(2):498–505. doi: 10.1086/301967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmann C, Evans JA, Konig IR, Kleensang A, Ruschendorf F, Lenzen J, et al. Genome-wide linkage analysis of malaria infection intensity and mild disease. PLoS Genet. 2007;3(3):e48. doi: 10.1371/journal.pgen.0030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flori L, Kumulungui B, Aucan C, Esnault C, Traore AS, Fumoux F, et al. Linkage and association between Plasmodium falciparum blood infection levels and chromosome 5q31-q33. Genes Immun. 2003;4(4):265–268. doi: 10.1038/sj.gene.6363960. [DOI] [PubMed] [Google Scholar]
- 11.Garcia A, Marquet S, Bucheton B, Hillaire D, Cot M, Fievet N, et al. Linkage analysis of blood Plasmodium falciparum levels: interest of the 5q31-q33 chromosome region. Am J Trop Med Hyg. 1998;58(6):705–709. doi: 10.4269/ajtmh.1998.58.705. [DOI] [PubMed] [Google Scholar]
- 12.Sakuntabhai A, Ndiaye R, Casademont I, Peerapittayamongkol C, Rogier C, Tortevoye P, et al. Genetic determination and linkage mapping of Plasmodium falciparum malaria related traits in Senegal. PLoS One. 2008;3(4):e2000. doi: 10.1371/journal.pone.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009;41(6):657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamb TJ, Brown DE, Potocnik AJ, Langhorne J. Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med. 2006;8(6):1–22. doi: 10.1017/S1462399406010581. [DOI] [PubMed] [Google Scholar]
- 15.Coltel N, Combes V, Hunt NH, Grau GE. Cerebral malaria -- a neurovascular pathology with many riddles still to be solved. Curr Neurovasc Res. 2004;1(2):91–110. doi: 10.2174/1567202043480116. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Seixas E, Langhorne J. Rodent malarias: the mouse as a model for understanding immune responses and pathology induced by the erythrocytic stages of the parasite. Med Microbiol Immunol. 2001;189(3):115–126. doi: 10.1007/s430-001-8017-8. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol. 2004;4(3):169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 18.Fortin A, Stevenson MM, Gros P. Complex genetic control of susceptibility to malaria in mice. Genes Immun. 2002;3(4):177–186. doi: 10.1038/sj.gene.6363841. [DOI] [PubMed] [Google Scholar]
- 19.Urban BC, Ing R, Stevenson MM. Early interactions between blood-stage plasmodium parasites and the immune system. Curr Top Microbiol Immunol. 2005;297:25–70. doi: 10.1007/3-540-29967-x_2. [DOI] [PubMed] [Google Scholar]
- 20.Fortin A, Stevenson MM, Gros P. Susceptibility to malaria as a complex trait: big pressure from a tiny creature. Hum Mol Genet. 2002;11(20):2469–2478. doi: 10.1093/hmg/11.20.2469. [DOI] [PubMed] [Google Scholar]
- 21.Fortin A, Belouchi A, Tam MF, Cardon L, Skamene E, Stevenson MM, et al. Genetic control of blood parasitaemia in mouse malaria maps to chromosome 8. Nat Genet. 1997;17(4):382–383. doi: 10.1038/ng1297-382. [DOI] [PubMed] [Google Scholar]
- 22.Foote SJ, Burt RA, Baldwin TM, Presente A, Roberts AW, Laural YL, et al. Mouse loci for malaria-induced mortality and the control of parasitaemia. Nat Genet. 1997;17(4):380–381. doi: 10.1038/ng1297-380. [DOI] [PubMed] [Google Scholar]
- 23.Lin E, Pappenfuss T, Tan RB, Senyschyn D, Bahlo M, Speed TP, et al. Mapping of the Plasmodium chabaudi resistance locus char2. Infect Immun. 2006;74(10):5814–5819. doi: 10.1128/IAI.01690-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burt RA, Baldwin TM, Marshall VM, Foote SJ. Temporal expression of an H2-linked locus in host response to mouse malaria. Immunogenetics. 1999;50(5–6):278–285. doi: 10.1007/s002510050603. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Valladares M, Naessens J, Gibson JP, Musoke AJ, Nagda S, Rihet P, et al. Confirmation and dissection of QTL controlling resistance to malaria in mice. Mamm Genome. 2004;15(5):390–398. doi: 10.1007/s00335-004-3042-4. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Valladares M, Rihet P, ole-MoiYoi OK, Iraqi FA. Mapping of a new quantitative trait locus for resistance to malaria in mice by a comparative mapping approach with human Chromosome 5q31-q33. Immunogenetics. 2004;56(2):115–117. doi: 10.1007/s00251-004-0667-0. [DOI] [PubMed] [Google Scholar]
- 27.Fortin A, Diez E, Rochefort D, Laroche L, Malo D, Rouleau GA, et al. Recombinant congenic strains derived from A/J and C57BL/6J: a tool for genetic dissection of complex traits. Genomics. 2001;74(1):21–35. doi: 10.1006/geno.2001.6528. [DOI] [PubMed] [Google Scholar]
- 28.Fortin A, Cardon LR, Tam M, Skamene E, Stevenson MM, Gros P. Identification of a new malaria susceptibility locus (Char4) in recombinant congenic strains of mice. Proc Natl Acad Sci U S A. 2001;98(19):10793–10798. doi: 10.1073/pnas.191288998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min-Oo G, Fortin A, Tam MF, Nantel A, Stevenson MM, Gros P. Pyruvate kinase deficiency in mice protects against malaria. Nat Genet. 2003;35(4):357–362. doi: 10.1038/ng1260. [DOI] [PubMed] [Google Scholar]
- 30.Min-Oo G, Tam M, Stevenson MM, Gros P. Pyruvate kinase deficiency: correlation between enzyme activity, extent of hemolytic anemia and protection against malaria in independent mouse mutants. Blood Cells Mol Dis. 2007;39(1):63–69. doi: 10.1016/j.bcmd.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Ayi K, Min-Oo G, Serghides L, Crockett M, Kirby-Allen M, Quirt I, et al. Pyruvate kinase deficiency and malaria. N Engl J Med. 2008;358(17):1805–1810. doi: 10.1056/NEJMoa072464. [DOI] [PubMed] [Google Scholar]
- 32.Ayi K, Liles WC, Gros P, Kain KC. Adenosine triphosphate depletion of erythrocytes simulates the phenotype associated with pyruvate kinase deficiency and confers protection against Plasmodium falciparum in vitro. J Infect Dis. 2009;200(8):1289–1299. doi: 10.1086/605843. [DOI] [PubMed] [Google Scholar]
- 33.Min-Oo G, Fortin A, Pitari G, Tam M, Stevenson MM, Gros P. Complex genetic control of susceptibility to malaria: positional cloning of the Char9 locus. J Exp Med. 2007;204(3):511–524. doi: 10.1084/jem.20061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min-Oo G, Ayi K, Bongfen SE, Tam M, Radovanovic I, Gauthier S, et al. Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium. Exp Parasitol. 2010;125(4):315–324. doi: 10.1016/j.exppara.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min-Oo G, Fortin A, Poulin JF, Gros P. Cysteamine, the molecule used to treat cystinosis, potentiates the antimalarial efficacy of artemisinin. Antimicrob Agents Chemother. 2010;54(8):3262–3270. doi: 10.1128/AAC.01719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min-Oo G, Willemetz A, Tam M, Canonne-Hergaux F, Stevenson MM, Gros P. Mapping of Char10, a novel malaria susceptibility locus on mouse chromosome 9. Genes Immun. 2010;11(2):113–123. doi: 10.1038/gene.2009.78. [DOI] [PubMed] [Google Scholar]
- 37.Paigen K, Eppig JT. A mouse phenome project. Mamm Genome. 2000;11(9):715–717. doi: 10.1007/s003350010152. [DOI] [PubMed] [Google Scholar]
- 38.Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, et al. Efficient control of population structure in model organism association mapping. Genetics. 2008;178(3):1709–1723. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohno T, Ishih A, Kohara Y, Yonekawa H, Terada M, Nishimura M. Chromosomal mapping of the host resistance locus to rodent malaria (Plasmodium yoelii) infection in mice. Immunogenetics. 2001;53(9):736–740. doi: 10.1007/s00251-001-0390-z. [DOI] [PubMed] [Google Scholar]
- 40.Wunderlich F, Marinovski P, Benten WP, Schmitt-Wrede HP, Mossmann H. Testosterone and other gonadal factor(s) restrict the efficacy of genes controlling resistance to Plasmodium chabaudi malaria. Parasite Immunol. 1991;13(4):357–367. doi: 10.1111/j.1365-3024.1991.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 41.Krucken J, Dkhil MA, Braun JV, Schroetel RM, El-Khadragy M, Carmeliet P, et al. Testosterone suppresses protective responses of the liver to blood-stage malaria. Infect Immun. 2005;73(1):436–443. doi: 10.1128/IAI.73.1.436-443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wunderlich F, Mossmann H, Helwig M, Schillinger G. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect Immun. 1988;56(9):2400–2406. doi: 10.1128/iai.56.9.2400-2406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson G, Hirst M, Bainbridge M, Bilenky M, Zhao Y, Zeng T, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4(8):651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 44.Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity. 2001;87(Pt 1):52–58. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.