In 4,545 elderly patients diagnosed with incident metastatic colon cancer, 2,504 (55%) received a biologics-containing regimen. Older age, African-American race, comorbidities, and lower income were associated with reduced likelihood of receiving biologics therapy. Bevacizumab was most frequently used in both first- and second-line treatment, cetuximab was the second most prescribed, and panitumumab use was mostly limited to third-line treatment.
Keywords: Biologics, Elderly, Medicare, Metastatic, Colon, Cancer
Abstract
Background.
We explored biologics receipt in metastatic colon cancer.
Methods.
We used Surveillance, Epidemiology, and End Results-Medicare data of 4,545 elderly patients diagnosed with incident metastatic colon cancer from 2003 to 2009, treated with chemotherapy and/or biologics, and followed up through 2010.
Results.
A total of 2,504 (55%) patients received a biologics-containing regimen. Treatment with biologics fluctuated between 46% and 63% of first-line regimens and 67% and 73% of second-line regimens. Bevacizumab accounted for 95% of first-line and 68% of second-line biologics use. Cetuximab accounted for 33% of second-line and 48% of third-line use. Panitumumab accounted for 5% of second-line and 27% of third-line use. The adjusted odds of biologics receipt decreased rapidly with age, resulting in a threefold difference between the youngest and the oldest study participants in the sample (odds ratio [OR] 0.35, p < .01). African Americans (OR 0.77, p = .03) and patients with Charlson Comorbidity Index of 1 (OR 0.83, p = .02) or >1 (OR 0.75, p < .01) were considerably less likely to receive biologics therapy. Medicare state buy-in was associated with 2% lower odds of receiving biologics (OR 0.98, p = .04).
Conclusion.
After controlling for sociodemographic and clinical differences, age, race, comorbidities, and low income had a statistically significantly negative effect on the likelihood of receiving biologics among treated patients. Use of biologics varied over time, across the treatment continuum, and by chemotherapy regimen. Bevacizumab was most frequently used in both first- and second-line treatment. Cetuximab was the second most prescribed biologic. Panitumumab use was mostly limited to third-line treatment.
Implications for Practice:
It is well-known that patients in the “real world” receive cancer treatments that do not reflect the strict treatment protocols of clinical trials. This is particularly true for complex and elderly patients with metastatic disease, who are frequently underrepresented in clinical trials. Although this article does not provide any additional evidence about the effectiveness of one treatment regimen or treatment sequence over another, it enhances our understanding of oncology practice outside of the clinical trial setting and provides useful information for future health services and health economics research in metastatic colon cancer.
Introduction
Patients with metastatic colon cancer disease may be treated with one or more chemotherapy and biologic targeted therapy drugs. The list of approved drugs during our study period includes the chemotherapy drugs fluorouracil (and capecitabine) (5FU), leucovorin (LV), oxaliplatin (OX), and irinotecan (IRI) and the biologic drugs bevacizumab (BEV), cetuximab (CET), and panitumumab (PAN).
BEV is an inhibitor of the vascular endothelial growth factor receptor. It was approved for first-line use in 2004 and for second-line use in 2006 [1, 2]. In comparison, CET and PAN, which work by inhibiting epidermal growth factor receptor (EGFR), were approved in 2004 and 2006, respectively, as single or adjunctive agents in patients who had failed or were intolerant to specific chemotherapies [2–4].
In 2006 and 2007, the scientific community became increasingly aware that certain KRAS mutations decreased the treatment response of EGFR inhibitors [5–11]. As a result, the U.S. Food and Drug Administration (FDA) modified the labels of CET and PAN in 2009 to indicate that these drugs were no longer recommended for the treatment of tumors with KRAS mutations in codon 12 or 13, which occur in 39%–43% of metastatic colon cancer patients [12, 13].
Colon cancer drugs are typically combined into a treatment regimen. When one regimen becomes ineffective or results in significant toxicities, patients may discontinue treatment or continue with another set of drugs. Thus, patients may receive multiple lines of treatment [14, 15].
This article examines the use of biologic targeted-therapy drugs among elderly patients with metastatic colon cancer disease. Because elderly patients are often underenrolled in clinical trials [16], we used historical claims-based data to find the factors associated with biologics receipt outside of the clinical trial setting and document how these drugs are used along the treatment continuum.
Materials and Methods
Data
This study used Surveillance, Epidemiology, and End Results (SEER)-Medicare data. The SEER program provides demographic and clinical information for all incident cancer cases in 17 geographic regions in the U.S. Medicare, the primary health care provider for the elderly in the U.S., collects information about covered health care services from time of enrollment until death. The linkage of individuals in the SEER cancer registry data to their Medicare claims is performed by the National Cancer Institute and the Centers for Medicare and Medicaid Services.
Population
The study population consisted of elderly (aged >65 years) SEER-Medicare enrollees diagnosed with incident metastatic colon cancer from 2003 to 2009. Tumor location was identified using the site recode variable in SEER-Medicare. Only people with site recode of 15 to 23 (15 = cecum, 16 = appendix, 17 = ascending colon, 18 = hepatic flexure, 19 = transverse colon, 20 = splenic flexure, 21 = descending colon, 22 = sigmoid colon, 23 = large intestine) were included in the study.
Study Sample
We included only patients who were treated with chemotherapy or biologics during the observation period. Furthermore, only patients with complete Medicare coverage (i.e., with both Medicare parts A and B) were included to minimize bias caused by unobserved claims data.
Patients enrolled in a managed care plan in the year before cancer diagnosis were excluded, because we did not have data about their health care utilization. People diagnosed postmortem with metastatic colon cancer were also excluded.
The remaining 4,545 patients were followed up until December 31, 2010. Subjects were censored from the study upon loss of complete Medicare coverage or upon enrollment in a managed care plan.
Treatment Detection
Receipt of specific chemotherapy or biologic drugs was assessed from claims in the period after cancer diagnosis using Healthcare Common Procedure Coding System (HCPCS) codes and National Drug Code (NDC) codes. All claim types, including Part D claims for outpatient drug coverage, were used to detect chemotherapy and biologic treatment. For the purposes of this study, we did not distinguish between capecitabine and its intravenous equivalent, fluorouracil.
Treatment Classification
The claims-based algorithm of Bikov et al. [17] was used to identify and classify treatment lines into the following five categories: (a) 5-FU/LV-based (5-FU/LV ± biologics); (b) OX-based (OX ± 5-FU/LV ± biologics); (c) IRI-based (IRI ± 5-FU/LV ± biologics); (d) IROX-based (IRI + OX ± 5-FU/LV ± biologics); and (e) biologics only.
Statistical Analysis
We used univariate descriptive analyses to examine the sociodemographic and clinical characteristics of our cohort. Using descriptive bivariate analyses, we compared prevalence rates of biologics receipt across patient groups defined by age, race/ethnicity, gender, marital status, Medicare state buy-in assistance (a proxy indicator for low income), urban living area, Charlson Comorbidity Index (CCI), myocardial infarction, chronic heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, chronic renal failure, peripheral vascular disease, and diabetes mellitus. In addition, the unadjusted rates of biologics use in first- and second-line therapy were stratified by age group. Pearson χ2 and t tests were used to compare frequency distributions of categorical and continuous variables, respectively.
We conducted multivariable logistic analysis to assess the adjusted effect of sociodemographic and clinical factors on receipt of biologic therapy. Our full model included the following independent variables: age, race/ethnicity, gender, marital status, Medicare state buy-in assistance, urban living area, CCI, year of cancer diagnosis, and SEER cancer registry. An alternative model included variables for myocardial infarction, chronic heart failure, cerebrovascular disease, chronic obstructive pulmonary disease, chronic renal failure, peripheral vascular disease, and diabetes mellitus in place of CCI. Our sensitivity analysis consisted of running multiple logistic regressions, each with a different subset of the independent variables.
Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, http://www.sas.com/en_us/home.html), and p < .05 was considered statistically significant.
IRB Protocol
The approved Institutional Review Board protocol number at the University of Maryland, Baltimore, was HP-00049426.
Results
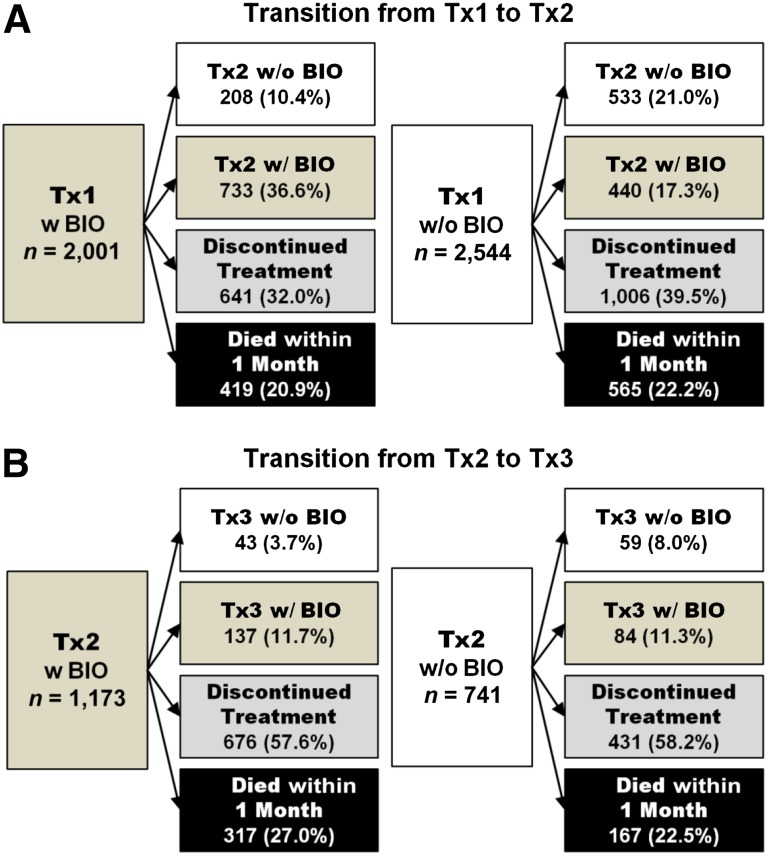
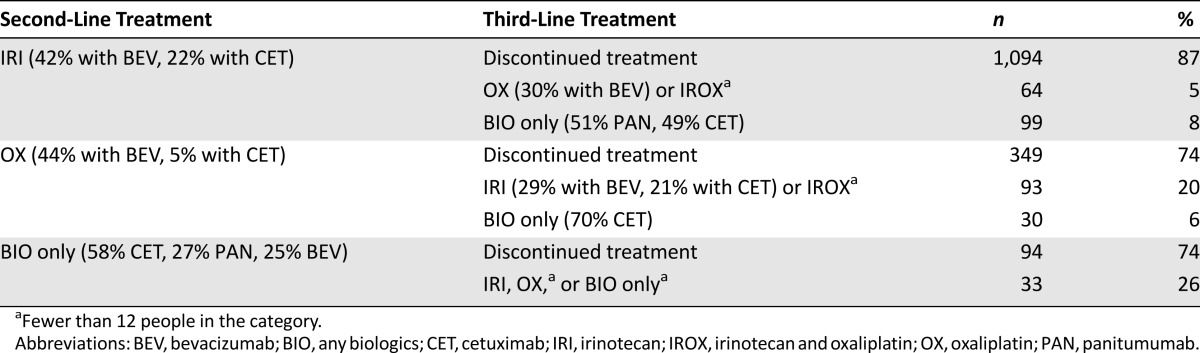
The study sample consisted of 4,545 elderly patients who received one or more lines of treatment after being diagnosed with incident metastatic colon cancer. Overall, 2,504 (55%) study participants were treated with biologics during the observation period. Of these, 2,001 (80%) began cancer treatment with a biologics-containing regimen; the remainder began with a chemotherapy-only regimen and did not start targeted therapy until their second (440 [18%]) or third (63 [1%]) line of treatment (Fig. 1).
Figure 1.
Utilization of biologics along the treatment continuum.
Abbreviations: BIO, biologics; Tx1, first-line treatment; Tx2, second-line treatment; Tx3, third-line treatment; w/, with; w/o, without.
Biologics Receipt Over Time
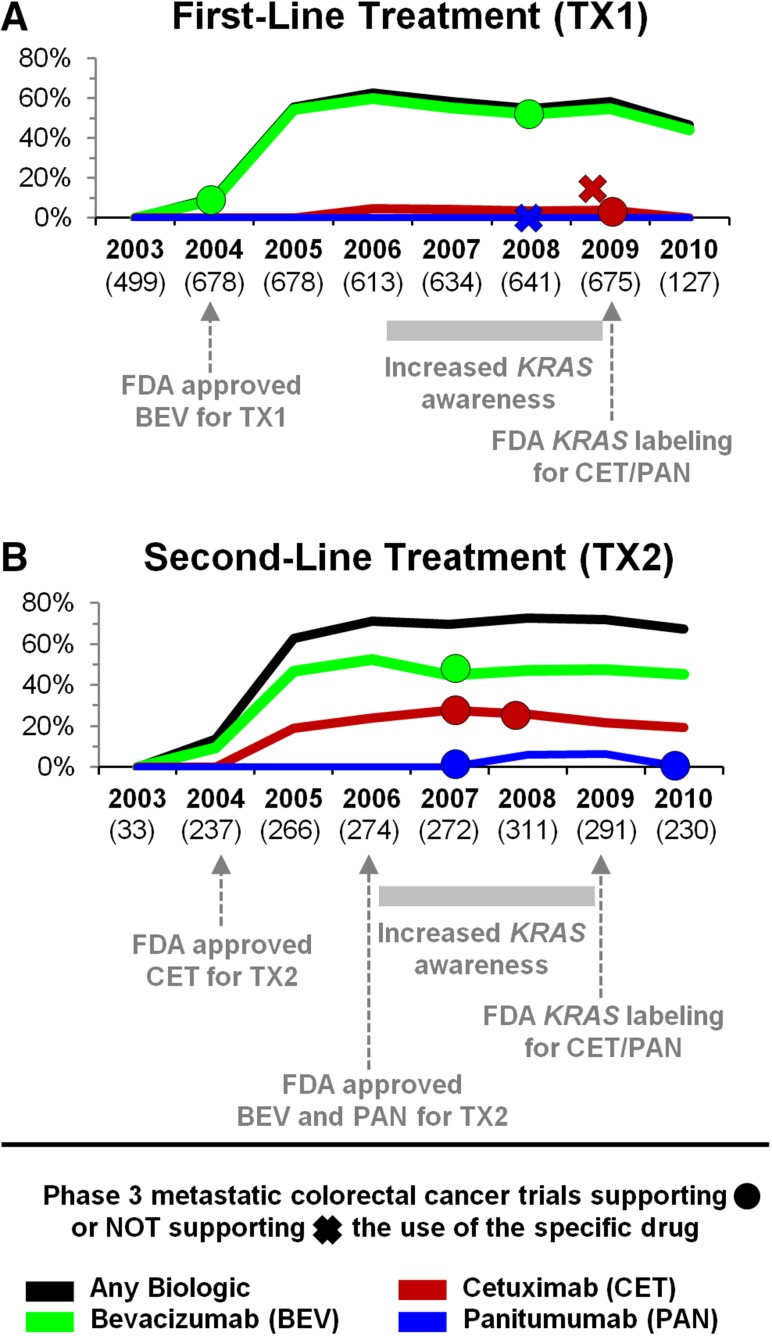
Initiation rates of targeted therapy varied over time. In 2005, a year after the first biologic drugs for metastatic colon cancer were approved, 55% of first- and 63% of second-line regimens included biologics. Rates continued to rise, peaking at 63% of first-line treatments in 2006 and 73% of second-line treatments in 2008. BEV use peaked in 2006, when it was included in 60% of first-line and 53% of second-line treatments. CET use peaked at 28% of second-line treatments in the following year. In 2010, the last year of our observation period, as many as 46% of first-line regimens and 67% of second-line regimens included biologics (Fig. 2).
Figure 2.
Utilization of biologics between 2003 and 2010, by year.
Abbreviation: FDA, U.S. Food and Drug Administration; TX1, first-line treatment; TX2, second-line treatment.
Biologics Receipt in First-Line Treatment
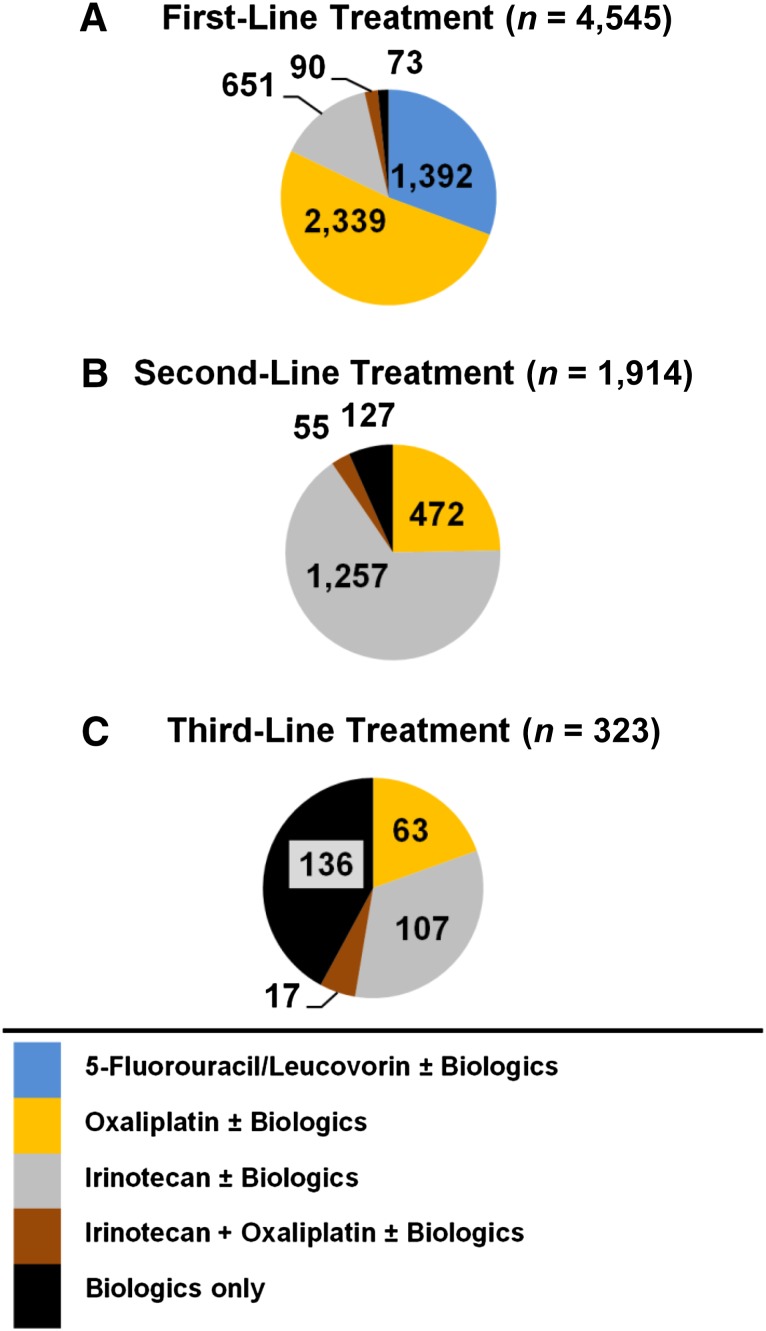
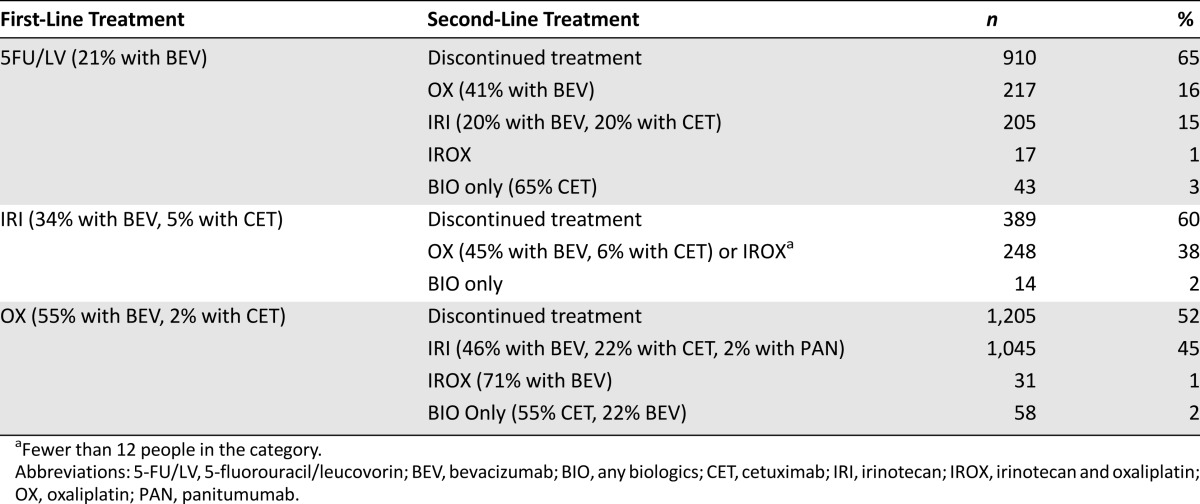
With the exception of 73 study participants who received only targeted therapy, almost everyone began cancer treatment with a chemotherapy regimen (Fig. 3A). The three most common regimens were OX-based (51%), 5FU/LV-based (31%), and IRI-based (14%). As many as 1,928 (43%) patients received both chemotherapy and biologics. BEV, which accounted for virtually all biologics use in first line, was included in 55% of OX-based regimens, 34% of IRI-based regimens, and only 21% of 5FU/LV-based regimens (Table 1). Meanwhile, CET, which accounted for only 3% of first-line regimens, was prescribed more frequently with IRI (5%) compared with OX (2%).
Figure 3.
Distribution of regimens by treatment line.
Table 1.
Most common first-line treatment regimens and corresponding next treatments
Biologics Receipt in Second-Line Treatment
Approximately two of five treated patients (1,914 [42%]) eventually discontinued their initial treatment and continued with a new treatment regimen (Fig. 3B). The remaining 2,631 (58%) patients stopped treatment altogether, of whom 984 died within a month (Fig. 1).
The transition from first- to second-line treatment was typically characterized by the replacement of one backbone chemotherapy drug with another (Table 1). Most patients received an IRI-based (66%) or OX-based (25%) second-line regimen (Fig. 3B). Use of targeted therapy in second line increased by 17 percentage points compared with first line. Overall, 1,173 (61% of those who progressed to second line) received biologics.
Table 2 shows the relative utilization of BEV, CET, and PAN as part of the three most common second-line treatments. BEV, which was included in 39% of second-line regimens, continued to be the most frequently prescribed biologic. It was added to 44% of OX-based and 42% of IRI-based treatments. CET was used in 20% of second-line regimens. This dramatic increase was driven by the popularity of IRI-based regimens, which were four times more likely to include CET compared with OX-based regimens. PAN saw little use (3% of second-line regimens) and was mostly limited to regimens consisting of biologics only, which accounted for 127 (7%) of second-line treatments.
Table 2.
Most common second-line treatment regimens and corresponding next treatments
Biologics Receipt in Third-Line Treatment
Only 323 patients (or 7% of our sample) eventually progressed to third-line treatment. Of these, 221 (68%) were treated with biologics, and 136 (42%) received biologics as stand-alone therapy. The remainder were treated with an IRI-based (107 [33%]), OX-based (63 [20%]), or IROX-based (17 [5%]) regimen (Fig. 3C). CET, which was included in 32% of regimens, was the most commonly used targeted therapy in third line. Meanwhile, the share of PAN increased to 19% of third-line regimens, whereas that of BEV decreased to 18%.
Factors Associated With Biologics Receipt
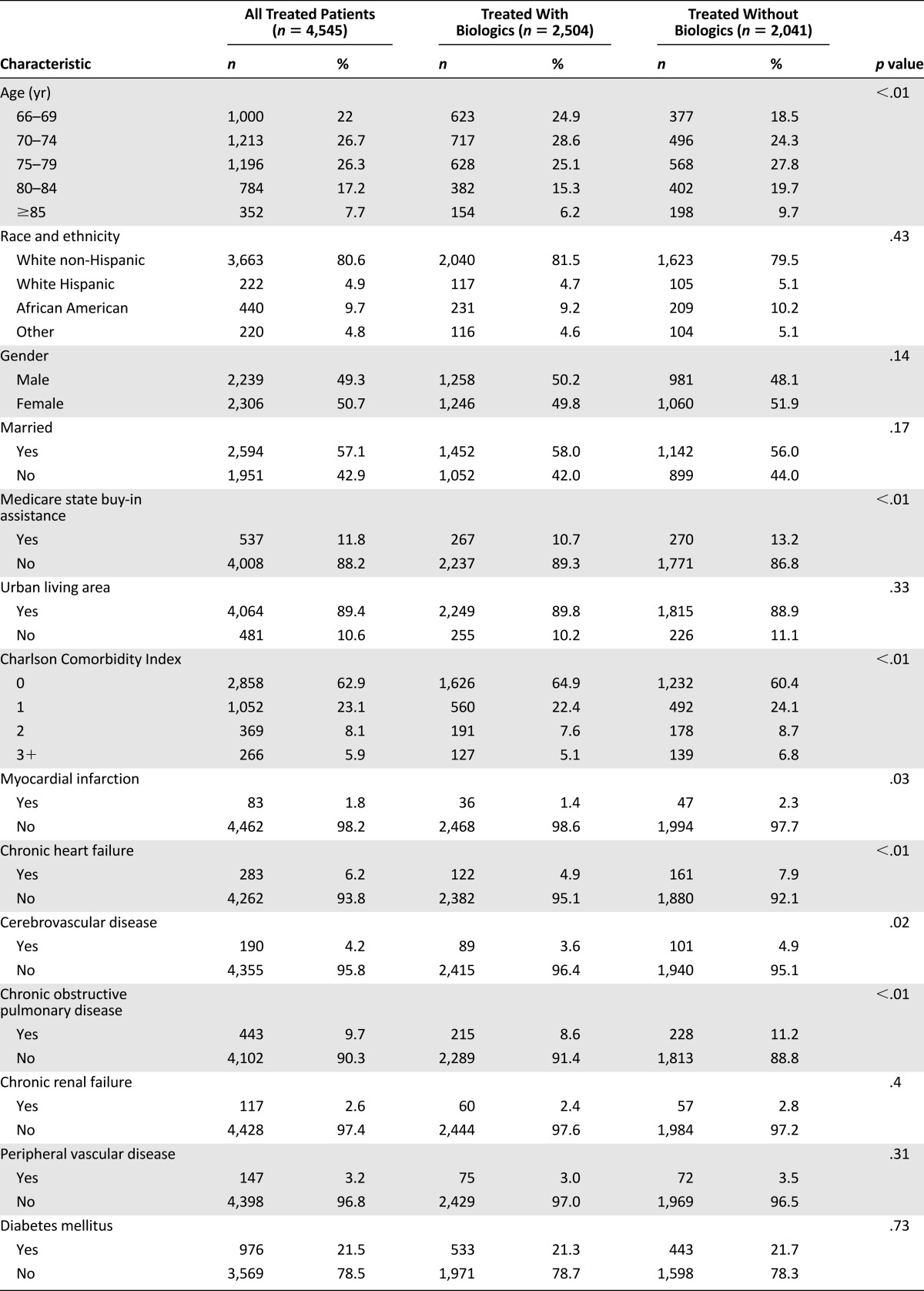
Patients treated with a biologics-containing first-line regimen were 1.2 years younger on average compared with those treated with chemotherapy alone (mean [SD], 74.5 [6.0] vs. 75.7 [6.2] years, p < .01). However, there was no statistically significant age difference in second line (73.9 [5.7] vs. 74.4 [5.8] years, p = .09).
Although rates of first-line biologics use declined consistently with age, the sharpest decline occurred at ∼75 years of age (age group 66–69, 50%; 70–74, 48%; 75–79, 41%; 80–84, 38%; ≥85, 38%; p < .01). In comparison, rates of second-line biologics use did not vary with age (age group 66–69, 65%; 70–74, 63%; 75–79, 57%; 80–84, 61%; ≥85, 57%; p = .12).
People treated with targeted therapy lived in census tracts with higher median household income compared with those treated with chemotherapy alone (mean [SD], $52,051 [$24,246] vs. $50,463 [$23,604], p = .03). In addition, we documented that Medicare state buy-in assistance was less common in patients treated with biologics compared with those treated with chemotherapy alone (11% vs. 13%, p < .01) (Table 3).
Table 3.
Unadjusted rates of biologics treatment by patient characteristics
Table 3 shows that patients treated with biologics and patients treated with chemotherapy alone had similar makeup with regard to race, gender, marital status, and urban/rural living area. However, they differed with regard to certain comorbidities. Compared with those treated with chemotherapy alone, patients treated with biologics had lower CCI (p < .01) and were less likely to have a history of myocardial infarction (1% vs. 2%, p = .03), chronic heart failure (5% vs. 8%, p < .01), cerebrovascular disease (4% vs. 5%, p = .02), and chronic obstructive pulmonary disease (9% vs. 11%, p < .01). There were no statistically significant differences in prior chronic renal failure, peripheral vascular disease, or diabetes mellitus.
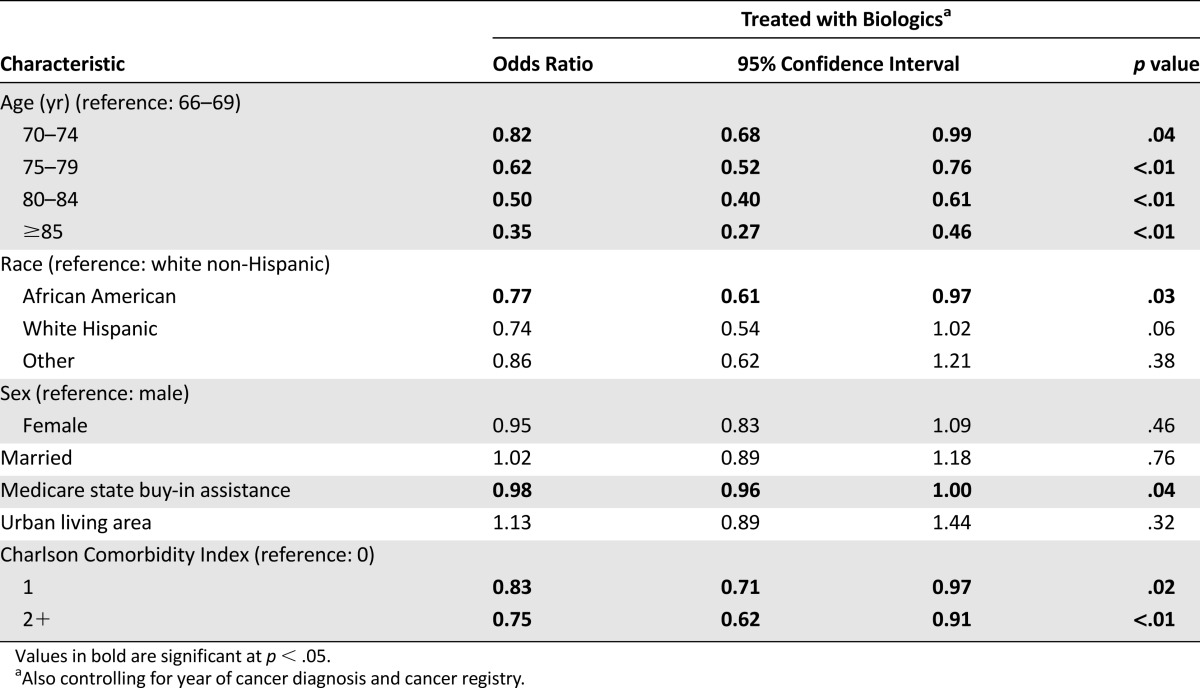
Table 4 shows the results from our full logistic model, which estimated how the odds of biologics receipt changed across patient groups after adjusting for sociodemographic and clinical factors. We found that African Americans (odds ratio [OR] 0.77, p = .03), as well as patients with CCI of 1 (OR 0.83, p = .02) or >1 (OR 0.75, p < .01) were considerably less likely to receive biologics therapy. Furthermore, the odds of being treated with biologics decreased rapidly with age, resulting in a threefold difference between the youngest (66–69 age group) and the oldest (≥75 age group) patients in our sample (OR 0.35, p < .01). Study participants with Medicare state buy-in had 2% lower odds of receiving biologics (OR 0.98, p = .04). Our sensitivity analyses produced qualitatively similar results.
Table 4.
Adjusted rates of biologics treatment by patient characteristics
Discussion
After FDA approval, biologics use in the study sample increased sharply to 63% of first-line treatments and 73% of second-line treatments. After this initial uptake in use, rates of biologics receipt remained relatively steady for the remainder of the observation period. Bevacizumab was the most frequently prescribed biologic in both first- and second-line treatment. Cetuximab and panitumumab were used mostly in second- or later-line therapies, and cetuximab was the dominant biologic in third-line treatment.
The observed higher utilization of bevacizumab compared with cetuximab or panitumumab is consistent with the clinical strategy of providing first-line bevacizumab in patients with low risk for atherothrombotic complications; it is also consistent with the strategy of reserving EGFR inhibitors, which offer good response rates at the expense of greater toxicity, for second- or later-line treatment in all but exceptionally fit elderly patients [15].
Our population-based findings are consistent with previous reports by Zafar et al. [18] and Hess et al. [19], who used medical records data to answer questions about the use of biologics in metastatic colon cancer patients. Zafar et al. examined data of patients with mean age of 58 who were diagnosed with metastatic colorectal cancer from 2003 to 2006 and found that bevacizumab was used in 74% of patients [18]. Hess et al. looked at 2004–2008 data of metastatic colorectal cancer patients with median age of 62 and found that bevacizumab use was highest in first-line treatment, but as treatment progressed beyond first line, cetuximab use increased [19].
Although the utilization of second-line biologics, as a group, remained steady, cetuximab receipt declined considerably in the second half of the observation period. At its peak in 2007, roughly one of three patients (28%) initiated second-line treatment with a cetuximab-containing regimen. In comparison, only one of five (19%) patients was given cetuximab in 2010. We believe that this decline was caused by increased KRAS testing.
Our multivariable results showed that older patients and patients with comorbidities were less likely to be treated with biologics. Low income also had a negative effect on the odds of receiving biologics. Finally, we observed significant disparities in access to biologic therapy across racial groups.
The strengths of the current study included using population-based claims data of elderly patients, who are often under-represented in clinical trials, and the use of a claims-based algorithm to identify lines of treatment, which allowed us to document how biologics use changed along the treatment continuum.
The primary limitation of our study was using older data (despite being the latest SEER-Medicare data available to researchers). A second limitation was the lack of KRAS and other test results, which are not reported on claims. A third limitation was that patients who started treatment with cetuximab (or panitumumab), and then stopped because of their KRAS test results, were still identified as cetuximab (or panitumumab) users.
Future research could assess the utilization patterns and rates of targeted therapies and genetic testing in commercial and more current datasets.
Conclusion
One of two elderly (age ≥66 years) SEER-Medicare patients in our study received targeted therapy as part of treatment for metastatic colon cancer disease. Initiation of biologics varied from year to year, fluctuating between 46% and 63% of first-line treatment regimens and 67% and 73% of second-line treatment regimens.
Bevacizumab accounted for virtually all biologics use in first line, and 68% in second line. One of three targeted therapy treatments in second line included cetuximab, which was the most frequently used biologic in third line (48% of biologics use). Panitumumab saw little use in second-line treatment, and it was used by 27% of targeted therapy patients in third line.
Biologics were most commonly received with oxaliplatin in first-, irinotecan in second-, and by themselves in third-line treatment. Treatment progression from first to second line was primarily driven by the addition or change in chemotherapy drugs, whereas the progression to third-line treatment was primarily driven by discontinuation of chemotherapy.
After controlling for sociodemographic and clinical differences, we found that age, race/ethnicity, low income, and history of chronic heart failure had a statistically significant negative effect on the likelihood of receiving biologics among treated metastatic colon cancer patients.
Acknowledgments
Bayer Healthcare Pharmaceuticals, Inc., provided funding for this project and the article processing charges. No medical writing assistance was received during the preparation of this manuscript. B. Seal is currently affiliated with Takeda Global Health Outcomes, Boston, Massachusetts, USA. The Pharmaceutical Research Computing Center housed in the Pharmaceutical Health Services Research Department at the University of Maryland School of Pharmacy was instrumental for the construction of the analytical data used in the analysis. The authors are especially grateful to Christine Franey and James Gardner from the Pharmaceutical Research Computing Center, as well as Jan Sieluk and Daisuke Goto, for their personal contribution to this study. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute, the Office of Research, Development, and Information, CMS, Information Management Services (IMS), Inc., and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute, and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Author Contributions
Conception/Design: Kaloyan A. Bikov, C. Daniel Mullins, Anna Hung, Brian Seal, Eberechukwu Onukwugha, Nader Hanna
Collection and/or assembly of data: Kaloyan A. Bikov, C. Daniel Mullins
Data analysis and interpretation: Kaloyan A. Bikov, C. Daniel Mullins, Anna Hung, Brian Seal, Eberechukwu Onukwugha, Nader Hanna
Manuscript writing: Kaloyan A. Bikov, C. Daniel Mullins, Anna Hung, Brian Seal, Eberechukwu Onukwugha, Nader Hanna
Final approval of manuscript: Kaloyan A. Bikov, C. Daniel Mullins, Anna Hung, Brian Seal, Eberechukwu Onukwugha, Nader Hanna
Disclosures
C. Daniel Mullins: Amgen, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Mundipharma, Novartis, Novo Nordisk, Pfizer (C/A), Amgen, Bayer, Pfizer (RF); Brian Seal: Bayer (E, OI); Eberechukwu Onukwugha: Astra Zeneca (C/A), Bayer Healthcare Pharmaceuticals, Amgen (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Genentech. Avastin (bevacizumab) prescribing information. Available at www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed August 3, 2014.
- 2.U.S. Food and Drug Administration. Drugs@FDA. Available at www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed August 3, 2014.
- 3.Bristol-Myers Squibb. Erbitux (cetuximab) prescribing information. Available at packageinserts.bms.com/pi/pi_erbitux.pdf. Accessed August 3, 2014.
- 4.Amgen. Vectibix (panitumumab) prescribing information. Available at pi.amgen.com/united_states/vectibix/vectibix_pi.pdf. Accessed August 3, 2014.
- 5.Gatzemeier U, Heller A, Foernzler D, et al. Exploratory analyses EGFR, kRAS mutations and other molecular markers in tumors of NSCLC patients (pts) treated with chemotherapy +/− erlotinib (TALENT) J Clin Oncol. 2005;23(suppl 16S):7028. [Google Scholar]
- 6.Kris MG, Sandler A, Miller VA, et al. EGFR and KRAS mutations in patients with bronchioloalveolar carcinoma treated with erlotinib in a phase II multicenter trial. J Clin Oncol. 2005;23(suppl 16S):7029. [Google Scholar]
- 7.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 8.Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 9.Di Fiore F, Le Pessot F, Lamy A, et al. KRAS mutation is highly predictive of cetuximab resistance in metastatic colorectal cancer. J Clin Oncol. 2007;25(suppl 18S):10502. [Google Scholar]
- 10.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 11.Finocchiaro G, Cappuzzo F, Janne PA, et al. EGFR, HER2 and KRAS as predictive factors for cetuximab sensitivity in colorectal cancer. J Clin Oncol. 2007;25(suppl 18S):4021. [Google Scholar]
- 12.Neumann J, Zeindl-Eberhart E, Kirchner T, et al. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract. 2009;205:858–862. doi: 10.1016/j.prp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Colon Cancer. Version 3.2014. Available at www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed June 10, 2014.
- 15.Sanoff HK, Goldberg RM. How we treat metastatic colon cancer in older adults. J Geriatr Oncol. 2013;4:295–301. doi: 10.1016/j.jgo.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 16.McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol. 2014;32:2570–2580. doi: 10.1200/JCO.2014.55.1960. [DOI] [PubMed] [Google Scholar]
- 17.Bikov KA, Mullins CD, Seal B, et al. Algorithm for identifying chemotherapy/biological regimens for metastatic colon cancer in SEER-Medicare. Med Care. 2013 doi: 10.1097/MLR.0b013e31828fad9f. [DOI] [PubMed] [Google Scholar]
- 18.Zafar SY, Marcello JE, Wheeler JL, et al. Longitudinal patterns of chemotherapy use in metastatic colorectal cancer. J Oncol Pract. 2009;5:228–233. doi: 10.1200/JOP.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess GP, Wang PF, Quach D, et al. Systemic therapy for metastatic colorectal cancer: Patterns of chemotherapy and biologic therapy use in US medical oncology practice. J Oncol Pract. 2010;6:301–307. doi: 10.1200/JOP.2010.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]