SUMMARY
REM sleep behavior disorder (RBD) is a disorder in which patients exhibit increased muscle tone and exaggerated myoclonic twitching during REM sleep. In addition, violent movements of the limbs, and complex behaviors that can sometimes appear to involve the enactment of dreams, are associated with RBD. These behaviors are widely thought to result from a dysfunction involving atonia-producing neural circuitry in the brainstem, thereby unmasking cortically generated dreams. Here we scrutinize the assumptions that led to this interpretation of RBD. In particular, we challenge the assumption that motor cortex produces twitches during REM sleep, thus calling into question the related assumption that motor cortex is primarily responsible for all of the pathological movements of RBD. Moreover, motor cortex is not even necessary to produce complex behavior; for example, stimulation of some brainstem structures can produce defensive and aggressive behaviors in rats and monkeys that are striking similar to those reported in human patients with RBD. Accordingly, we suggest an interpretation of RBD that focuses increased attention on the brainstem as a source of the pathological movements and that considers sensory feedback from moving limbs as an important influence on the content of dream mentation.
Keywords: Myoclonic twitching, development, muscle atonia, REM sleep without atonia, rapid eye movements, sensorimotor integration, corollary discharge, motor cortex, superior colliculus, brainstem
INTRODUCTION
REM sleep behavior disorder (RBD) is a parasomnia characterized by the pathological expression of overt and often violent motor behaviors during REM sleep [1,2]. What makes these behaviors pathological is that REM sleep is normally accompanied by muscle atonia punctuated by brief, jerky, myoclonic twitches throughout the body. Both of these aspects of motor system functioning—atonia and twitching—are severely disrupted in RBD. Perhaps most remarkably, in patients diagnosed exclusively with RBD, the motor system during wakefulness seems unaffected. Thus, RBD appears to result from the selective breakdown of REM sleep circuitry, especially that in the brainstem [3]. Although RBD is often associated with other neurological disorders, the majority of cases are diagnosed as “idiopathic”; however, many of these cases are actually associated with a progressive neurodegenerative process that culminates, late in life, with the onset of Parkinson disease (PD) and other α-synucleinopathies [4–7].
By the time RBD was officially classified as a human disorder in 1986 [8], a similar phenomenon—“REM sleep without atonia”—had already been studied extensively in cats with brainstem lesions [9]. These lesioned animals exhibited many signs of REM sleep as they also engaged in various rudimentary and complex behaviors—from alternating leg movements to standing and walking, to orienting toward, searching for, and attacking invisible prey. Naturally, the latter dream-like behaviors were the ones that grabbed the most attention. Videos produced at the time showed cats violently striking at non-existent objects in the environment. Noting that REM sleep in humans is “associated with intense dreams,” Morrison wrote that “it would be tempting to conclude that by [producing brainstem lesions] we are enabled to witness the animal acting out its dreams... however, there are good reasons to think this is not a full account of what happens in the episodes of REM sleep without atonia” (p. 98). Instead, he concluded that two neural systems are disrupted in REM sleep without atonia when animals engage in complex behavior: one responsible for controlling muscle tone and another associated with increased motor drive.
Nonetheless, contemporary accounts have coalesced around the idea that the pathological movements of RBD are the direct result of the loss of muscle atonia during REM sleep, thereby “unmasking” overt behavior reflective of cortically generated dreams [1,2,10]. For example, Mahowald and Schenck state that, in RBD, “somatic muscle atonia, one of the defining features of REM sleep, is absent, permitting the acting out of dream mentation, often with violent or injurious results” (p. 1283) [1]. Elsewhere they are more direct, stating that the absence of atonia in REM sleep “is alone sufficient to generate RBD” (p. 469) [11].
Clearly, the causal path from unobservable dream mentation to overt behavior is exceedingly difficult to demonstrate experimentally. Mahowald and Schenck [11] seem to acknowledge this difficulty when they write of lesioned cats “’acting out dreams’ (or ‘dreaming out acts’),” thereby displaying some agnosticism with respect to the direction of causation. Moreover, Boeve and colleagues write that the “two phenomena of ‘acting out one’s dreams’ and ‘dreaming around one’s actions’ are not mutually exclusive, and could be working in concert” (p. 2779) [4]. Within the literature, however, such statements are rare.
In contrast with the difficulty of inferring a causal relationship between dreams and behavior, numerous studies have used stimuli delivered during REM sleep to demonstrate that external events are often incorporated into dreams; depending on the stimulus, dream incorporation rates can vary from 9–87% (see [12]). Therefore, it is possible that sensory feedback from the increased and often-violent limb movements of RBD are similarly incorporated into dreams [4,13]. It is also possible, as suggested by Fantini et al. [14], that the increased aggressive content of RBD-related dreams and their associated vigorous motor behaviors are caused by a hyperactive neural mechanism that independently causes both. Regardless, these alternative interpretations are overshadowed by the power and ubiquity of “dream enactment” as the favored interpretation of the movements of RBD.
In considering the validity of this favored interpretation, it is important to put the full range of RBD-related behavior in perspective. Specifically, the majority (66–83%) of motor events in RBD have been described as minor, elementary, or jerky limb movements [2,15]. In other words, most of the movements of RBD resemble normal or, perhaps, exaggerated twitches. Beyond twitching, 13–31% of movements are designated as complex and only 1.8% are described as “scenic, including apparent ‘acting out’ of dream content” (p. 678) [2].
Videos of RBD patients engaging in the most elaborate of behaviors in their sleep (e.g., smoking an imaginary cigarette; see [16]) reasonably suggest the engagement of “higher” brain structures like motor cortex. However, because these elaborate behaviors are so exceedingly rare in relation to the other forms of pathological movement, they do not form a strong foundation upon which to build a model of RBD.
Indeed, we argue here that the perspective that one adopts to explain twitching in normal REM sleep shapes how one thinks about what goes wrong in RBD. If twitches are perceived as products of motor cortex, then it is a small step to thinking that all RBD-related motor activity is cortically controlled. However, if, as we will argue, twitches are generated within the brainstem without cortical involvement, then one is led to consider different models for explaining RBD-related movements. Moreover, although the complex movements of RBD are often attributed to cortical mechanisms, we will provide evidence that complex behaviors—including behaviors that bear a striking resemblance to those described in RBD patients—can be produced by experimental activation of brainstem neural circuits. Finally, contrary to the view that cortically generated dreams are exclusively responsible for RBD-related motor activity, we will suggest that at least some dream content entails incorporation of sensory feedback from moving limbs. In sum, we argue for a perspective that moves away from a strictly corticocentric, hierarchical model of RBD-related movements toward one that considers the dynamic contributions of multiple factors acting at multiple levels of the neuraxis.
A BRIEF TOUR OF SOME COMMON ASSUMPTIONS
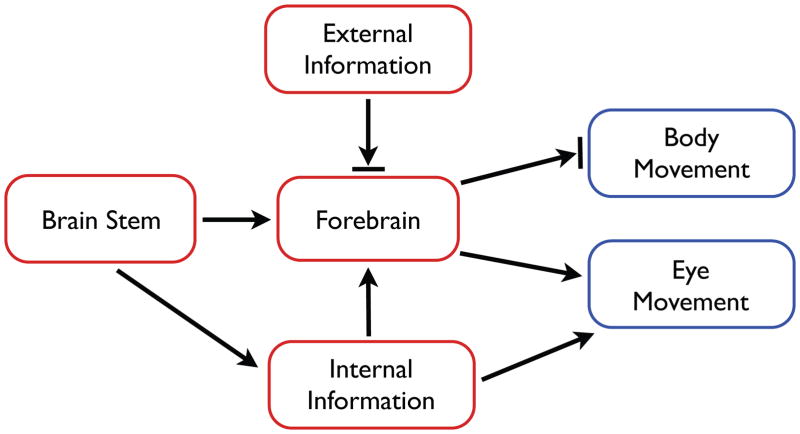
There are several key assumptions that have shaped and continue to shape thinking about the neural processes involved in REM sleep and RBD. These assumptions relate to the neural sources of twitching and the role of motor cortex, the processing of sensory input during sleep, and the necessity of motor cortex to produce complex behavior. Figure 1 provides a good starting point for this discussion because it neatly illustrates these assumptions in visual form. The model presented in this figure is redrawn from Hobson and McCarley’s seminal paper introducing the activation-synthesis hypothesis of dreaming [17]. Their hypothesis was that dreams result from the synthesis within the cerebral cortex of “partially random” brainstem activity interacting with previously stored information.
Figure 1.
Hobson and McCarley’s [17] model of the processes involved in the generation of dreams and the control of movement in REM sleep.
First, note that Figure 1 correctly indicates that during REM sleep the forebrain is activated by the brainstem. However, the figure also indicates that ‘external information’ is blocked from the forebrain, consistent with evidence that sensory thresholds are increased during sleep (e.g., [18–20]) but inconsistent with evidence demonstrating the incorporation of sensory stimuli into dreams [12]. Second, despite being a defining feature of REM sleep, Figure 1 makes no explicit reference to twitching. Instead, as indicated by the blocked arrow, the figure indicates the suppression of “body movement” during REM sleep, which is intended to indicate the suppression of overt, high-amplitude behaviors, such as those exhibited during wake. This intention is clear when the authors suggest that twitches are replaced by complex movements in cats with brainstem lesions that produce REM sleep without atonia (p. 1337).
Finally, note the absence of a bar at the end of the arrow linking the forebrain to eye movements. This absence denotes the presence, in REM sleep, of the rapid eye movements (REMs) that help to define this stage of sleep. This is one indication that the authors consider eye movements during sleep to be qualitatively distinct from other body movements. However, if REMs are simply twitches of the extraocular muscles [15, 16] and are, like limb twitches, produced within the brainstem, then the asymmetric treatment in Figure 1 of “eye movement” and “body movement” is unwarranted. To bring these two categories of movement into proper alignment, Figure 1 should be modified to combine “eye movement” and “body movement” into a single element: myoclonic twitches.
Thus, Figure 1 represents the assumption that twitches, like wake movements, are generated by the forebrain—especially motor cortex—and would be expressed as complex movements were it not for their blockade by a mechanism that produces muscle atonia. It bears noting that this model of twitch production was not universally accepted in 1977; in fact, a decade earlier Roffwarg and colleagues [21] suggested that during REM sleep, the brainstem sends ascending signals to the cortex and descending signals to motoneurons, the latter being responsible for REM-related motor events. Nonetheless, the assumption that twitches are the jetsam of dreams has proven a resilient one (see [13]).
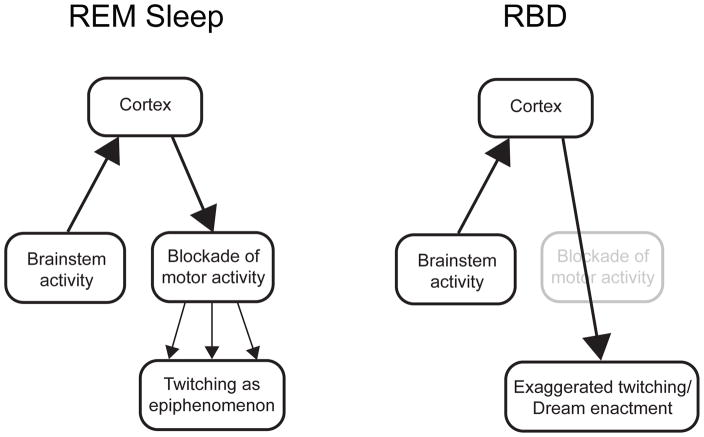
Figure 2 illustrates more clearly several key assumptions described above, but also incorporates the hypothesis that twitches are by-products of a dreaming cortex (Figure 2, left). According to this hypothesis, REM-related neural circuits in the brainstem activate the cerebral cortex to produce dreams, and atonia-producing circuits in the brainstem prevent the cortically generated dreams from being acted out. But because this inhibitory blockade is imperfect, remnants of motor outflow harmlessly leak through to produce epiphenomenal twitches. By extension, RBD is understood as a dysfunction of the atonia-producing circuitry (Figure 2, right). Accordingly, during REM sleep twitching increases in quantity and intensity and, occasionally, dream enactment results. As already noted, these models of normal REM sleep and RBD comport with numerous contemporaneous statements in the literature. However, as described in the next section, there are good reasons to question the validity of these models.
Figure 2.
Conventional hierarchical models of the relationship between cortically generated dreams and behavior in normal REM sleep (left) and RBD (right). In normal REM sleep, cortical motor outflow is incompletely suppressed in the medulla, resulting in the production of twitches as by-products. In RBD, the medullary blockade is diminished or lost, allowing dream-related motor activity to be expressed with greater fidelity. In the extreme, dreams are ‘enacted.’
CAUSES AND CONSEQUENCES OF MYOCLONIC TWITCHING
Sleep in early infancy is a process of change across several dimensions: New sleep components (e.g., cortical delta activity) emerge and coalesce with already-existing sleep components, initially fragmented bouts consolidate, homeostatic mechanisms are refined, and circadian rhythmicity develops (for reviews, see [22,23]). However, infant and adult sleep are also similar in many important ways, especially with regard to motor behavior and its neural control [24]. Specifically, as in adults, the wake movements of infant rats are produced against a background of high muscle tone and twitches are produced against a background of atonia. Bouts of twitching comprise conspicuous movements of the forelimbs, hindlimbs, and tail, as well as whiskers [25] and eyes [26]. Also as in adults (e.g., [27]), muscle atonia in week-old rats depends on active inhibition by neurons in the ventral medulla [28]. Chemical lesions in this area produce REM sleep without atonia in infant [28] and adult [29] rats. REM sleep without atonia in pups can also be produced by mesopontine lesions of the subcoeruleus region or nucleus pontis oralis [24]; importantly, these regions are implicated in the dysregulation of atonia in patients with RBD (see [3]).
Brainstem sources of twitching and the role of motor cortex
In order to understand the causes of the pathological movements of RBD, it is necessary to understand the neural mechanisms that normally produce twitches in REM sleep. In the 1960s, Villablanca recorded sleep and wakefulness in young cats after decerebration, a method that physically separates the forebrain from the brainstem [30]. He reported that twitching in the decerebrated cats were indistinguishable from that in intact cats. Around the same time, Marchiafava and Pompeiano [31] showed that twitching in adult cats is unaffected by transection of the pyramidal tract (which carries descending axons from motor cortex) or ablation of motor or sensory cortex. Although twitching was not quantified in these studies, they showed that the forebrain is not necessary for the production of twitching in adults.
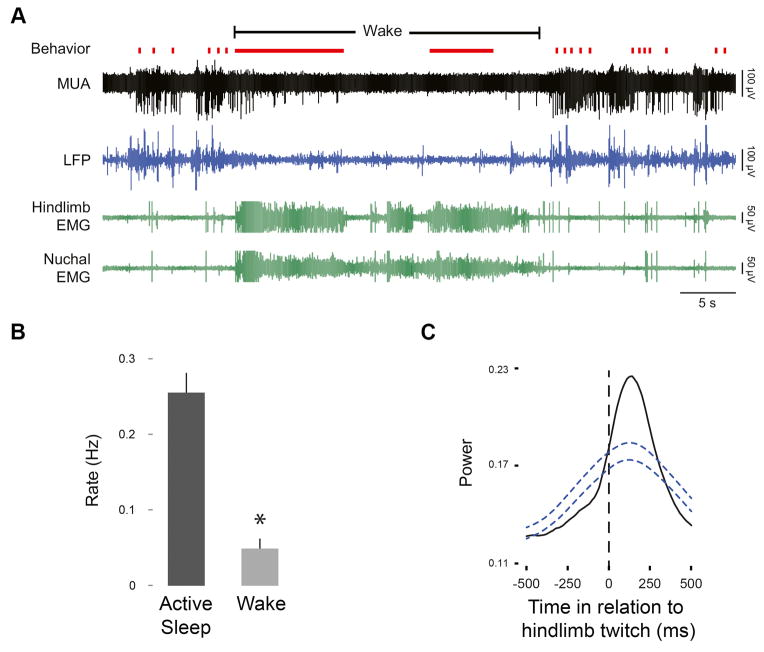
Several decades later, it was shown in week-old rats that complete transections just rostral to the mesopontine region yield rates of limb twitching that are quantitatively indistinguishable from intact controls [32]. Subsequently, a more refined and direct assessment of the contributions of motor cortex in infant rats led to the same conclusion [33]. It was found that the hindlimb region of motor cortex was highly active during REM sleep when the contralateral hindlimb was twitching, but went nearly silent when pups woke up and vigorously moved the hindlimb (Figure 3A); in fact, when quantified across subjects, cortical activity was approximately five times more active during REM sleep than wakefulness (Figure 3B). Moreover, when cortical activity increased during REM sleep, it did so after twitches (Figure 3C). Because the latencies—from twitch to peak neural activation—were greater than 100 ms, the conclusion is clear: Sensory feedback (i.e., reafference) from twitching limbs was driving activity in motor cortex.
Figure 3.
Sleep-wake behavior and motor cortex activity in P8 rats. (A) Representative recording in a P8 rat from the hindlimb region of motor cortex (MUA, multiunit activity; LFP, local field potential) across the sleep-wake cycle. Hindlimb and nuchal EMG activity and behavior are also shown. Behaviors are coded as twitches (red ticks) and wake movements (solid red lines). Note the prominent cortical activity during bouts of twitching and the near-total cortical silence during periods of wake-related movement. Mean rates of spindle bursts during active (REM) sleep and wake (B) and waveform average of spindle bursts in relation to hindlimb twitches (C) recorded from the hindlimb region of motor cortex. Rates of spindle bursts were significantly higher during active sleep (* P < 0.05). The waveform average of cortical activity increased significantly after hindlimb twitches with a peak latency of at least 100 ms. Blue dashed lines denote upper and lower confidence bands (P < 0.05). Similar results were found at P4 and P12. Adapted from [33].
Although it may seem incongruous for motor cortex to process sensory input, the motor functions of primary motor cortex develop relatively late in cats and other mammals [34,35]. Without a fully functioning motor cortex, young animals rely on earlier developing brainstem motor structures to control behavior [36]. Importantly, sensory processing in motor cortex persists throughout life, as evidenced by a large literature documenting this function [37]. All together, we are led once again to brainstem motor structures—not the motor cortex—as the source of twitching.
The red nucleus (RN) is one such brainstem structure that has long been implicated in twitching in adult cats [38,39] and, more recently, adult mice [40]. In cats, RN activity increased during periods of leg twitching as well as REMs and electrolytic lesions of the RN immediately reduced twitching [38,39]. And as additional evidence against a twitch-generating role for motor cortex, RN activity increased with twitching even after motor cortex was ablated.
RN recordings in week-old rats confirmed an important role for this nucleus in twitching [41]. In stark contrast with motor cortex at this age (see Figure 3), RN neurons fired bursts of action potentials just before wake-related limb movements and sleep-related limb twitches. In addition, RN neurons responded to reafference from twitching, suggesting that it is a site of sensorimotor integration—both producing twitches and processing their sensory consequences. This observation supports the recent demonstration in cats that a motor map develops early in the RN, before the map in motor cortex [36].
The RN is important for twitching, but it is not of singular importance. In week-old rats, unilateral pharmacological inactivation of the RN reduced twitching immediately but by only 50% [41]. And in adult cats, lesions of the red nucleus reduced twitching, but only transiently as twitching returned within several days [38,39]. Thus, there appear to be redundant or complementary brainstem areas that combine with the RN to produce twitching. Such areas may include structures that give rise to reticulospinal projections [42], including perhaps a recently identified area in the lateral pontine tegmentum of adult rats; this area contains non-cholinergic neurons that fire phasically during REM sleep [43]. Thus, although elegant studies in adult rats have begun to reveal the neurophysiological mechanisms responsible for driving brainstem motor neurons during twitching [44,45], more work is needed to identify the complete circuit that generates twitching.
Are rapid eye movements special?
Recall from the discussion of Figure 1 that limb twitches and REMs have traditionally been interpreted in qualitatively different ways. Whereas twitches have been typically viewed as remnants of dreams that result from incomplete blockade, REMs have been typically viewed as direct, unfiltered products of dreams. According to this view, under pathological conditions like RBD, twitches can be transformed into dream enactment, whereas under normal conditions REMs reflect an individual literally scanning her dream imagery. There are many reasons to doubt the “scanning hypothesis” of dreaming—including evidence that congenitally blind humans have REMs without visual dream imagery and that cats with lesions of the visual cortex continue to exhibit REMs (see [2,16]). We focus here on the notion, mentioned earlier, that REMs and limb twitching can be brought into functional alignment simply by considering them as different manifestations of the same fundamental phenomenon, that is, brainstem-generated twitching of skeletal muscles.
To explore this issue, recordings were made directly from the extraocular muscles of infant rats [26]. At postnatal day (P) 3, because the eye cannot yet move independently within its socket, eye movements were not detected. Nonetheless, spikes were clearly evident in the extraocular electromyogram (EMG) that were indistinguishable from twitch-related spikes recorded from the nuchal muscle. By P15, the age of eye opening, identical EMG spikes occurred, but they were now followed by clear REMs. Thus, extraocular muscle twitches precede REMs in development, and REMs—when they emerge—are homologous with other limb twitches.
The data just described are consistent with Chase and Morales’s [46] view that REMs do not reflect the “directed visualization of the dream experience” (p. 1198). Additional support for this perspective comes from studies of cortical electroencephalogram (EEG) activity in relation to wake-related eye movements (i.e., saccades) and REMs in human adults [47,48]. For example, in recordings from parieto-occipital cortex [47], wake-related saccades were immediately preceded by a so-called readiness potential (“presaccadic negativity”). In contrast, no such potential was observed immediately preceding REMs, suggesting that REMs are not generated in response to activity in visual cortex. Moreover, EEG activity changed in the period immediately after saccades or REMs (although in a different location within parieto-occipital cortex), indicative of post-eye-movement sensory processing. Similar post-REM increases in activity were observed using intracranial electroencephalography in the medial temporal lobe, an area associated with visual processing [49]. The parallels between increased post-REM cortical activity in human adults and increased post-twitch cortical activity in infant rats (see Figure 3C) are intriguing and deserve more attention.
Twitches as contributors to sensorimotor development
Although there has long been interest in the possible functional significance of twitches (see [50,51]), it seems that interest in their function was diminished by the persistent view that they are mere by-products of dreams. However, given that limbs twitches are generated within the brainstem (not motor cortex), that they are produced abundantly (especially in early development), and that they effectively activate neural circuits throughout the brain [52], old questions about the relations between dreams and twitching give way to new ones about why infants twitch more than adults and what twitching specifically affords the developing nervous system.
It was not until 2004 that twitching was first clearly implicated in the development of spinal [53] and cortical [54] circuits. Subsequent research has revealed the extent to which reafference from twitching limbs triggers activity throughout the brain (e.g., [25,33,55–57]), thereby expanding the range of opportunities for twitching to affect the development and refinement of neural circuits. Moreover, high-speed videographic analyses of twitching in developing rats and mice have demonstrated age-related changes in the spatiotemporal structure of twitching and the contributions of sensory experience [58,59].
As shown in Figure 3B, whereas sensory reafference from twitching limbs reliably activates motor cortex, even the most vigorous wake-related movements reliably fail to do so [33]. To make sense of this puzzling phenomenon, Sokoloff and colleagues hypothesized that twitches—uniquely among self-generated movements—are processed as if they lack corollary discharge [60]. Briefly, a corollary discharge is a copy of a motor signal that does not produce movement per se, but rather contributes to the process by which we distinguish between active (i.e., self-produced) and passive (i.e., other-produced) movements (see [61]). Accordingly, whereas wake-related movements are accompanied by a corollary discharge that gates reafferent proprioceptive signals arising from moving limbs, reafference arising from twitching limbs is allowed to cascade through the brain without interference. In other words, even though twitches are self-produced, they are processed as if they are other-produced. A subsequent study in infant rats garnered substantial support for this hypothesis [33]. From a functional standpoint, this feature of sensorimotor processing makes good sense if twitching, as suspected, drives activity-dependent development of sensorimotor circuits: To do otherwise would be to block the very activity upon which activity-dependent mechanisms depend.
All together, this work moves twitching far beyond its earlier characterization, depicted in Figure 2, as a mere by-product of a dreaming cerebral cortex. Twitching, instead, can now be viewed as a spatiotemporally complex behavior with unique sensorimotor properties. In effect, twitching represents a distinct class of movement. And as a distinct class of movement, twitching may have its own distinct pathology that is expressed in RBD.
CORTICAL AND SUBCORTICAL CONTRIBUTIONS TO COMPLEX MOVEMENTS AND DREAM ENACTMENT
How should we conceptualize the neural mechanisms responsible for the diverse motor behaviors of RBD, from twitching to complex movement to “dream enactment”? Should we assume that because some RBD movements are elaborate and learned, and therefore classified as likely products of motor cortex, that most RBD movements should be similarly classified? We think not. Instead, we aim to build on what is known about the neural sources of normal twitching and seek to understand how the neurological processes that trigger RBD might also give rise to novel but pathological neural circuits that make complex movements during REM sleep possible.
As should now be clear, our view is that motor cortex—through at least 12 days of age in rats [33] and in adult cats [30,31]—is not necessary for the production of limb twitches. Nor is there any direct evidence of which we are aware that motor cortex plays any role in the generation of twitches. Also, contrary to the widespread assumption that sleep entails isolation from sensory input (see Figure 1), the cerebral cortex during REM sleep is activated in response to limb and eye movements. Evidence for reafferent activation is clear in infant rats with respect to limb and whisker twitches [25,33,54,56] and in premature human infants with respect to hand twitches [62], and is suggestive in human adults with respect to REMs [47–49]. But again, in contrast with copious evidence detailing the role of the brainstem in the generation of twitches [30,32,38,40,41], there is no such evidence detailing a similar role for motor cortex.
As with twitches, it is often assumed that the movements of RBD are produced by motor cortex (e.g., [10,63]1). Such statements seem to align with detailed analyses of the complex movements in RBD patients [2,63,64]. For example, responding in part to the suggestion that RBD-related movements result from activation of brainstem and spinal central pattern generators [65], De Cock and colleagues [63] countered with the observation that RBD movements are “elaborated, complex, non-stereotyped, with learned speeches (e.g. political speech, lectures) and songs…, suggesting they result from the same cortical mechanisms as awake complex activities” (p. 455) [63]. De Cock and colleagues note further that the tremors characteristic of PD—which result from dysfunctional processing in the basal ganglia—cease entirely during REM sleep in RBD patients, suggesting that the basal ganglia are by-passed in REM sleep. Consequently, it may be that RBD movements—which are often jerky and rough—“result from the expression of the primary motor cortex [without] the filtering, smoothing control of the basal ganglia” (p. 456).
In the aggregate, we see in these statements the lingering legacy of the assumption that twitches are by-products of a dreaming cortex. But as detailed above, there is little support for this assumption. Our view is that the phenomenology of normal REM sleep twitching can be adequately explained without any reliance on cortical mechanisms. Specifically, the jerky nature of twitches and many RBD movements is a consequence of the bursty and discrete firing of the brainstem premotor nuclei that produce them [41]. Consequently, there may be no need to posit a mechanism whereby the basal ganglia are bypassed during REM sleep. As for the cessation of tremor during REM sleep in patients with PD, this may simply result from the predominance of the brainstem in motor outflow during REM sleep—thereby bypassing the forebrain entirely. If this perspective has merit, we are led to consider different hypotheses concerning the links between normal twitching and pathological RBD movements. If we grant the brainstem control over twitching, the next question concerns the extent to which the cortex is required to explain RBD movements.
Brainstem contributions to complex behavior and their relevance for rbd
Because the cerebral cortex is routinely considered the organizing center for complex movements, the sufficiency of the brainstem to produce such movements is often overlooked. Indeed, although the primate neurophysiologist Michael Graziano has focused extensively on the role of the primate motor cortex in the organization of ethologically relevant complex movements [66], he is careful to point out that the brainstem and even spinal cord can produce them, too [67].
For our present purposes, perhaps the most relevant observations come from studies in which the midbrain of adult animals is experimentally activated [68–70]. For example, pharmacological activation within the so-called mesencephalic locomotor region (MLR), including the cuneiform nucleus, produces running or escape responses [71,72]. Nearby structures within this region have also been implicated in exploratory behaviors [72]. Moreover, stimulation of the periaqueductal gray and superior colliculus produce various behaviors, including defensive responses [68,69]. For the sake of brevity, we focus our discussion below on the superior colliculus.
In rats [68], pharmacological disinhibition of the superior colliculus elicits a range of responses. Specifically, “stimuli that in normal circumstances appeared neutral or interesting (and provoked orienting and investigation) seemed to become threatening and dangerous, and produced just the kinds of responses that naturally dangerous stimuli produce” (p. 139). The authors continue: “Some of these movements closely mimic pursuit of a moving object, for example prey…. Together these data suggest that the [superior colliculus] has access to a large family of defense-related ‘fight-or-flight’ reactions, and is capable of producing a fully integrated response when appropriately triggered” (p. 141).
The parallels between these descriptions of behavior after collicular activation and the violent and aggressive behaviors—and matching dream reports—of RBD patients is striking: “Patients with RBD commonly report dreams in which they are attacked by animals or unfamiliar people and they would either fight back in self-defense or attempt to flee. Fear and anger are the most common associated emotions” (p 1010) [14]; “[M]ost behaviors observed during RBD are violent and mimic fight and defense behaviors” (p. 682)[2]; these behaviors include grabbing, punching, kicking, slapping, arm flailing, and jumping out of bed [2,14]. Furthermore, a questionnaire study of RBD patients with PD [73] found that “the most common associated dream was fighting or fleeing in response to danger (91%), whereas pleasant activity was reported in 20% [of] patients and daily activity in 22%” (p. 678) [2].
Whereas early work suggested that the primate superior colliculus does not organize defensive reactions [68], recent work in freely moving rhesus and pigtail macaques indicates otherwise [70]. Indeed, after pharmacological disinhibition of the superior colliculus, macaques exhibited many of the same responses as rats, including “defense-like responses that were both passive (i.e., cowering) and active (i.e., escape-like behavior, attack of objects), as well as an increase in defensive vocalization” (p. 153). Also as in rats, these movements engaged the whole body. For example, cowering “involved an ipsilaterally directed contraction of the trunk concurrent with a contralaterally directed eye gaze and head turning” (p. 154). As the authors noted, these observations suggest that the role of the superior colliculus in organizing behavioral responses to threat is conserved across species, including humans.
In addition to its role in the production of the behaviors described above, the superior colliculus also contains multiple visual, auditory, and sensorimotor maps that contribute to the implementation of orienting responses comprising movements of the body, head, whiskers, pinnae, and eyes [74]. This aspect of collicular functioning is relevant to RBD in light of evidence that the directionality of REMs and other RBD-related head and limb movements are often coherently organized, thus giving the impression of goal-oriented dream behavior [75]. The superior colliculus is a potential contributor to this coordinated and seemingly goal-directed aspect of behavior in RBD.
Finally, cortical stimulation experiments provide a useful contrast with the brainstem stimulation experiments described above. Specifically, using a long-duration electrical stimulation protocol, Graziano and colleagues mapped motor cortex in a way that reveals ethologically relevant complex movements (see [66]). Using this protocol, they identified eight distinct clusters of movements, only one of which comprised defensive movements and none of which could be described as aggressive. The remaining seven movements included such behaviors as hand-to-mouth movements, grasping, chewing, and exploratory gaze shifts. In other words, whereas stimulation of midbrain structures like the superior colliculus routinely evoke defensive and aggressive behaviors, stimulation of motor cortex rarely does so. We conclude from this that the majority of complex movements of RBD are more parsimoniously attributed to brainstem than cortical mechanisms.
A MULTICAUSAL, MULTILEVEL APPROACH TO RBD
Whereas RBD is primarily characterized by violent dreams and behaviors, it should be stressed that nonviolent behaviors also occur, although much less frequently [76]. Because these behaviors include such learned and culturally specific activities as smoking, singing, dancing, kissing, bicycling, and clapping (as at a show), it has been argued that RBD-related movements cannot be ‘primitive’ and, therefore, do not arise from subcortical mechanisms (which are, rightly or wrongly, often associated with “primitive” behaviors). Building further from these observations, Arnulf [2] argues that “because patients are able to speak with coherent syntax during RBD, we suggest that most behaviors are generated by the motor cortex and temporal lobe for language….” (p. 682–683).
In light of the evidence reviewed here, it seems unlikely to us that the motor cortex—which is normally not involved in the production of twitches—would abruptly ‘take control’ of movements during REM sleep in patients with RBD. Instead, we envision a more fluid and probabilistic relationship among (i) twitch-production brainstem mechanisms, (ii) nearby neural circuits that are capable of producing complex movements (e.g., superior colliculus) and that might be disinhibited during REM sleep in RBD patients, and (iii) forebrain structures that include motor cortex.
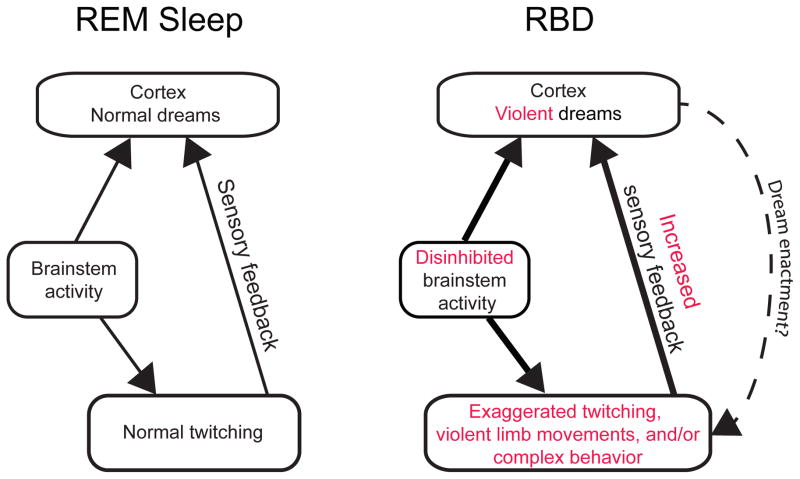
With this in mind, we propose a new approach to understanding the pathological motor control of RBD (see Figure 4). First, damage to the sublaterodorsal tegmental nucleus (SLD), a key structure in the regulation of REM sleep, may be a primary cause of the abnormal motor behavior of RBD [3,4]. According to one model of RBD generation [3], the SLD’s excitatory input to the gigantocellular reticular nucleus (Gi) is reduced, resulting in the loss of muscle atonia during REM sleep. This loss of atonia represents one component of RBD; however, as Morrison pointed out many years ago [9], loss of atonia alone is not sufficient to produce increased behavioral activation (see also [77]).
Figure 4.
Alternative models (see Figure 2) of the relationship between brainstem and cortical activity in normal REM sleep (left) and RBD (right). For simplicity, the mechanisms associated with muscle atonia are not depicted here. In normal REM sleep, brainstem activity provides parallel ascending activation of the cortex and descending activation of the skeletal muscles, the latter producing twitching. Sensory feedback (i.e., reafference) from twitching limbs provides another source of stimulation to the cortex. In RBD, degeneration of sleep-related neural circuits may lead to disinhibition of brainstem structures that control motor behavior (e.g., red nucleus, superior colliculus), resulting in exaggerated twitching, violent limb movements, and/or complex behavior, with concomitant effects on dream mentation. Motor cortex activity may occasionally be engaged, especially during “dream enactment” (dashed line).
Second, given that the Gi exerts an inhibitory influence over the RN [78], and given the RN’s role in the production of twitches and wake-related movements (e.g., [41,79]), one can imagine how exaggerated twitching and more complex ballistic movements could be disinhibited through this process (see [80]). However, the RN and related premotor structures have not, to our knowledge, been explicitly implicated in the production of defensive or aggressive behaviors. Conversely, as discussed above, experimental activation of midbrain structures can produce a variety of behaviors that are strikingly similar to those that occur in RBD. Therefore, we propose further that RBD entails disinhibition of neural circuits that contribute to the production of defensive and aggressive behaviors, including but not limited to the superior colliculus. In support of this proposal, cholinergic neurons in the laterodorsal tegmental nucleus and the pedunculopontine tegmental nucleus, which are vulnerable to degeneration in the α-synucleinopathies [81,82], project to the superior colliculus [83,84] and may modulate its activity [85].
If increased activation of the superior colliculus and/or other brainstem structures does indeed occur in RBD, aggression-filled and agitated dreams could be produced in one or both of the following ways: First, reafferent sensations arising from defensive and violent limb movements could be incorporated into dreams; and second, there could be direct ascending activation from the brainstem to the cerebral cortex. Then, over the course of a bout of REM sleep, recurrent loops of activation among the limbs, spinal cord, brainstem, and forebrain (including motor cortex) could be differentially engaged. In short, contrary to the notion of a dreaming cortex sitting atop a neural hierarchy, we posit that the distinct dream mentation of RBD arises from the dynamic and multilevel engagement of neural circuits throughout the neuraxis.
CONCLUSIONS AND FUTURE DIRECTIONS
The brain of an RBD patient is not the same as any other with only a piece missing here or there: It is a brain that is organized—and is organizing—differently, especially in those cases of RBD associated with progressive neurodegeneration. We should expect that such reorganization of neural circuits would be expressed as changes at multiple levels of the neuraxis, with important consequences for behavior and dream mentation.
With that in mind, we need to bridge the gap between our understanding of twitching and sensorimotor processing in infant rats and in human adults with and without RBD. Indeed, in healthy humans, we still know relatively little about the quantity and patterning of twitching across the lifespan, about the variability within and between individuals, and about how twitching differs across muscle groups (but see [86–88]; such normative data are critical if we wish to accurately identify abnormal movements in RBD [87]. Moreover, in future studies in healthy humans and those with RBD, kinematic analyses could help to objectively distinguish between twitches and other types of movements. Finally, in contrast with what is known in infant rats [33], we know little about the sensory consequences of twitching in human adults.
Therefore, resolving outstanding issues regarding the neural control of the motor system during REM sleep—and what is changed in RBD—will require precise measures of limb and muscle activity and topographically related neural structures (e.g., sensory and motor cortex). In such studies, it will be critical to distinguish between neural activity that precedes movement (indicative or motor outflow) and activity that follows movement (indicative of sensory reafference). Our prediction is that cortical and subcortical components of the motor system will be differentially engaged depending on the nature of the movement observed—whether normal twitching, exaggerated twitching, complex movement, or ‘dream enactment.’
It is not our view that the motor cortex is never involved in RBD-related motor behavior. Rather, we contend that—in part due to outmoded views of the relations between dreams and twitching under normal conditions, and in part due to a belief that complex behavior necessarily entails cortical involvement—the motor cortex has been too-readily recruited to explain all aspects of pathological behavior in RBD. Moreover, there has been insufficient attention paid to the contributions of reafference from moving limbs to neural activity and the content of dreams. Ultimately, the continued dearth of information concerning the causes and neural consequences of twitching is hampering our ability to gain a full understanding of the sensorimotor system in sleep under normal and pathological conditions.
Practice points.
RBD is a parasomnia characterized by increased phasic motor activity during REM sleep, often accompanied by intense and even violent behaviors along with reports of similarly intense dreams.
RBD-related behaviors (i.e., exaggerated twitching, complex behavior) are commonly assumed to result from a diminution or loss of atonia-producing mechanisms in the brainstem that, under normal conditions, prevent the ‘acting out’ of cortically generated dreams.
Although it is also commonly assumed that myoclonic twitches are produced by motor cortex, there is no evidence that this is the case in either infants or adults.
In contrast, there is substantial evidence that twitches are produced by brainstem structures, including the red nucleus.
Research in infants also indicates that reafference from twitching limbs is a prominent activator of neural activity across the neuraxis, including sensory and motor cortex; in adults with RBD, such reafference could play an important role in driving dream mentation.
Brainstem structures may be disinhibited in RBD to produce exaggerated twitching and complex movements.
A multilevel, multicausal approach to understanding RBD is more likely to be successful than one that relies on a model of neural organization in which the cerebral cortex sits atop the neural hierarchy.
Research agenda.
To gain a more thorough understanding of the quantity and patterning of twitching—including twitching in different muscle groups—in humans across the lifespan.
To compare twitch- and wake-related sensory and motor cortex activity in normal humans and patients with RBD; in such studies, it will be critical to distinguish between neural activity that precedes movement (motor) and activity that follows movement (sensory).
To determine whether sensory and motor cortex activity in RBD changes in relation to the intensity and complexity of movement.
To determine the brainstem neural circuits involved in complex movements in animals with REM sleep without atonia.
Precise assessment of sensorimotor processing in relation to RBD-related movements should yield valuable new insights into the neural processes involved and how they change over time.
Acknowledgments
MSB thanks Isabelle Arnulf for the invitation to speak and Jean Krieger and Michael Vitiello for the invitation to prepare an article for this journal. The authors also thank Markus Schmidt, Alex Tiriac, and Karen Adolph for many helpful comments. Preparation of this article was supported in part by a grant from NIH (R37-HD081168) to MSB.
Abbreviations
- EEG
electroencephalogram
- EMG
electromyogram
- GABA
gamma-aminobutyric acid
- Gi
gigantocellular reticular nucleus
- P
postnatal day
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
- REM
rapid eye movement
- RN
red nucleus
- SLD
sublaterodorsal tegmental nucleus
Footnotes
The authors report no conflicts of interest.
This article arose from an address presented by MSB at the 2015 meeting of the Société Française de Recherche et Médecine du Sommeil (SFRMS) in Lille, France.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahowald M, Schenck C. Insights from studying human sleep disorders. Nature. 2005;437:1279–85. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- *2.Arnulf I. REM sleep behavior disorder: Motor manifestations and pathophysiology. Mov Disord. 2012;27:677–89. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- *3.Peever J, Luppi PH, Montplaisir J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014;37:279–88. doi: 10.1016/j.tins.2014.02.009. [DOI] [PubMed] [Google Scholar]
- *4.Boeve B, Silber M, Saper C, Ferman T, Dickson D, Parisi J, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;11:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 5.Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–9. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fantini M, Ferini-Strambi L, Montplaisir J. Idiopathic REM sleep behavior disorder: toward a better nosologic definition. Neurology. 2005;64:780–6. doi: 10.1212/01.WNL.0000152878.79429.00. [DOI] [PubMed] [Google Scholar]
- 7.Schenck C, Bundlie S, Mahowald M. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388–93. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 8.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- *9.Morrison A. A window on the sleeping brain. Scientific American. 1983;248:94–102. doi: 10.1038/scientificamerican0483-94. [DOI] [PubMed] [Google Scholar]
- 10.Luppi PH, Clément O, Sapin E, Gervasoni D, Peyron C, Léger L, et al. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15:153–63. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Mahowald MW, Schenck CH. REM sleep without atonia--from cats to humans. Arch Ital Biol. 2004;142:469–78. [PubMed] [Google Scholar]
- 12.Schredl M, Atanasova D, Hörmann K, Maurer JT, Hummel T, Stuck BA. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. J Sleep Res. 2009;18:285–90. doi: 10.1111/j.1365-2869.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg MS. Beyond dreams: Do sleep-related movements contribute to brain development? Front Neurol. 2010;1:140. doi: 10.3389/fneur.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantini M, Corona A, Clerici S, Ferini-Strambi L. Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology. 2005;65:1010–5. doi: 10.1212/01.wnl.0000179346.39655.e0. [DOI] [PubMed] [Google Scholar]
- 15.Frauscher B, Gschliesser V, Brandauer E, Ulmer H, Peralta CM, Müller J, et al. Video analysis of motor events in REM sleep behavior disorder. Mov Disord. 2007;22:1464–70. doi: 10.1002/mds.21561. [DOI] [PubMed] [Google Scholar]
- 16.Leclair-Visonneau L, Oudiette D, Gaymard B, Leu-Semenescu S, Arnulf I. Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain. 2010;133:1737–46. doi: 10.1093/brain/awq110. [DOI] [PubMed] [Google Scholar]
- 17.Hobson JA, McCarley RW. The brain as a dream state generator: an activation-synthesis hypothesis of the dream process. Am J Psychiatry. 1977;134:1335–48. doi: 10.1176/ajp.134.12.1335. [DOI] [PubMed] [Google Scholar]
- 18.Baust W, Berlucchi G, Moruzzi G. Changes in the auditory input in wakefulness and during the synchronized and desynchronized stages of sleep. Arch Ital Biol. 1964;102:657–74. [PubMed] [Google Scholar]
- 19.Seelke AMH, Blumberg MS. Sniffing in infant rats during sleep and wakefulness. Behav Neurosci. 2004;118:267–73. doi: 10.1037/0735-7044.118.2.267. [DOI] [PubMed] [Google Scholar]
- 20.Taepavarapruk N, McErlane S, Soja P. State-related inhibition by GABA and glycine of transmission in Clarke’s column. J Neurosci. 2002;22:5777–88. doi: 10.1523/JNEUROSCI.22-13-05777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–19. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg MS, Gall AJ, Todd WD. The development of sleep–wake rhythms and the search for elemental circuits in the infant brain. Behav Neurosci. 2014;128 doi: 10.1037/a0035891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blumberg MS, Seelke AMH. The form and function of infant sleep: From muscle to neocortex. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 391–423. [Google Scholar]
- 24.Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AMH, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biol. 2005;3:e143. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS. Rapid whisker movements in sleeping newborn rats. Curr Biol. 2012;22:2075–80. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep. Eur J Neurosci. 2005;22:911–20. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai Y, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–6. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neurosci. 2005;130:275–83. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neurosci Lett. 1989;98:159–65. doi: 10.1016/0304-3940(89)90503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villablanca J. Behavioral and polygraphic study of "sleep" and “wakefulness” in chronic decerebrate cats. Electroencephalogr Clin Neurophysiol. 1966;21:562–77. doi: 10.1016/0013-4694(66)90175-1. [DOI] [PubMed] [Google Scholar]
- 31.Marchiafava P, Pompeiano O. Pyramidal influences on spinal cord during desynchronized sleep. Archs Ital Biol. 1964;102:500–29. [PubMed] [Google Scholar]
- 32.Kreider J, Blumberg MS. Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats. Brain Res. 2000;872:149–59. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- *33.Tiriac A, Del Rio-Bermudez C, Blumberg MS. Self-generated movements with “unexpected” sensory consequences. Curr Biol. 2014;24:2136–41. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J Neurophysiol. 2000;84:2582–94. doi: 10.1152/jn.2000.84.5.2582. [DOI] [PubMed] [Google Scholar]
- 35.Martin JH. The corticospinal system: From development to motor control. Neuroscientist. 2005;11:161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 36.Williams PTJA, Kim S, Martin JH. Postnatal maturation of the red nucleus motor map depends on rubrospinal connections with forelimb motor pools. J Neurosci. 2014;34:4432–41. doi: 10.1523/JNEUROSCI.5332-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Hatsopoulos NG, Suminski AJ. Sensing with the motor cortex. 2011;72:477–87. doi: 10.1016/j.neuron.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gassel M, Marchiafava P, Pompeiano O. Activity of the red nucleus during deep desynchronized sleep in the unrestrained cat. Archs Ital Biol. 1965;103:369–96. [PubMed] [Google Scholar]
- 39.Gassel M, Marchiafava P, Pompeiano O. Rubrospinal influences during desynchronized sleep. Nature. 1966;209:1218–20. doi: 10.1038/2091218a0. [DOI] [PubMed] [Google Scholar]
- 40.Li DW, Peever JH. Pharmacogenetic stimulation of the red nucleus influences muscle tone during rapid eye movement (REM) sleep in mice. Annual Meeting of the Associated Professional Sleep Societies; Minneapolis, MN. 2014. [Google Scholar]
- *41.Del Rio-Bermudez C, Sokoloff G, Blumberg MS. Sensorimotor processing in the newborn rat red nucleus during active sleep. J Neurosci. 2015;35:8322–32. doi: 10.1523/JNEUROSCI.0564-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gassel MM, Marchiafava PL, Pompeiano O. Phasic changes in muscular activity during desynchronized sleep in unrestrained cats. An analysis of the pattern and organization of myoclonic twitches. Arch Ital Biol. 1964;102:449–70. [PubMed] [Google Scholar]
- 43.Thankachan S, Fuller PM, Lu J. Movement- and behavioral state-dependent activity of pontine reticulospinal neurons. Neurosci. 2012;221:125–39. doi: 10.1016/j.neuroscience.2012.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks PL, Peever JH. Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–45. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgess C, Lai D, Siegel JM, Peever J. An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle. J Neurosci. 2008;28:4649–60. doi: 10.1523/JNEUROSCI.0334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chase M, Morales F. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–8. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa K, Nittono H, Hori T. Topography of the lambda-like response in rapid eye movement sleep: A current source-density analysis. Sleep and Biological Rhythms. 2003;1:153–4. doi: 10.1046/j.1446-9235.2003.00029.x. [DOI] [Google Scholar]
- 48.Ogawa K, Nittono H, Hori T. Brain potentials associated with the onset and offset of rapid eye movement (REM) during REM sleep. Psychiatry and Clinical Neurosciences. 2002;56:259–60. doi: 10.1046/j.1440-1819.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- 49.Andrillon T, Cirelli C, Tononi G, Fried I, Nir Y. Single-neuron activity and eye movements during human REM sleep and awake vision. Nature Communications. 2015;6:1–10. doi: 10.1038/ncomms8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blumberg MS, Lucas DE. A developmental and component analysis of active sleep. Dev Psychobiol. 1996;29:1–22. doi: 10.1002/(SICI)1098-2302(199601)29:1<1::AID-DEV1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 51.Corner M. Sleep and the beginnings of behavior in the animal kingdom—Studies of ultradian motility cycles in early life. Prog Neurobiol. 1977;8:279–95. doi: 10.1016/0301-0082(77)90008-9. [DOI] [PubMed] [Google Scholar]
- *52.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–7. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersson P, Waldenström A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–5. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- 54.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–61. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 55.Sokoloff G, Plumeau AM, Mukherjee D, Blumberg MS. Twitch-related and rhythmic activation of the developing cerebellar cortex. J Neurophys. 2015;114:1746–56. doi: 10.1152/jn.00284.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohns EJ, Blumberg MS. Neocortical activation of the hippocampus during sleep in newborn rats. J Neurosci. 2010;30:3438–49. doi: 10.1523/JNEUROSCI.4832-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McVea DA, Mohajerani MH, Murphy TH. Voltage-sensitive dye imaging reveals dynamic spatiotemporal properties of cortical activity after spontaneous muscle twitches in the newborn rat. J Neurosci. 2012;32:10982–94. doi: 10.1523/JNEUROSCI.1322-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blumberg MS, Coleman CM, Gerth AI, McMurray B. Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr Biol. 2013;23:2100–9. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blumberg MS, Coleman CM, Sokoloff G, Weiner JA, Fritzsch B, McMurray B. Development of twitching in sleeping infant mice depends on sensory experience. Curr Biol. 2015;25:656–62. doi: 10.1016/j.cub.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokoloff G, Uitermarkt BD, Blumberg MS. REM sleep twitches rouse nascent cerebellar circuits: Implications for sensorimotor development. Dev Neurobiol. 2015;75:1140–53. doi: 10.1002/dneu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–94. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- 63.De Cock V, Vidailhet M, Leu S, Texeira A, Apartis E, Elbaz A, et al. Restoration of normal motor control in Parkinson’s disease during REM sleep. Brain. 2007;130:450–6. doi: 10.1093/brain/awl363. [DOI] [PubMed] [Google Scholar]
- 64.Oudiette D, Leu-Semenescu S, Roze E, Vidailhet M, De Cock VC, Golmard J-L, et al. A motor signature of REM sleep behavior disorder. Mov Disord. 2011;27:428–31. doi: 10.1002/mds.24044. [DOI] [PubMed] [Google Scholar]
- 65.Tassinari CA, Rubboli G, Gardella E, Cantalupo G, Calandra-Buonaura G, Vedovello M, et al. Central pattern generators for a common semiology in fronto-limbic seizures and in parasomnias. A neuroethologic approach. Neurol Sci. 2005;26(Suppl 3):s225–32. doi: 10.1007/s10072-005-0492-8. [DOI] [PubMed] [Google Scholar]
- *66.Graziano MSA, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron. 2007;56:239–51. doi: 10.1016/j.neuron.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Graziano MSA. Ethologically relevant movements mapped on the motor cortex. In: Ghazanfar AA, Platt MJ, editors. Primate Neuroethology. New York: 2010. pp. 454–70. [Google Scholar]
- 68.Dean P, Redgrave P, Westby G. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–47. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- 69.Schenberg LC, Póvoa RMF, Costa ALP, Caldellas AV, Tufik S, Bittencourt AS. Functional specializations within the tectum defense systems of the rat. Neurosci Biobehav Rev. 2005;29:1279–98. doi: 10.1016/j.neubiorev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- *70.DesJardin JT, Holmes AL, Forcelli PA, Cole CE, Gale JT, Wellman LL, et al. Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. J Neurosci. 2013;33:150–5. doi: 10.1523/JNEUROSCI.2924-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell IJ, Redgrave P, Dean P. Plasticity of behavioural response to repeated injection of glutamate in cuneiform area of rat. Brain Res. 1988;460:394–7. doi: 10.1016/0006-8993(88)90389-7. [DOI] [PubMed] [Google Scholar]
- 72.Grillner S, Georgopoulos AP, Jordan LM. Selection and initiation of motor behavior. 1997. pp. 3–19. [Google Scholar]
- 73.Scaglione C, Vignatelli L, Plazzi G, Marchese R, Negrotti A, Rizzo G, et al. REM sleep behaviour disorder in Parkinson’s disease: a questionnaire-based study. Neurol Sci. 2005;25:316–21. doi: 10.1007/s10072-004-0364-7. [DOI] [PubMed] [Google Scholar]
- 74.Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev. 1986;66:118–71. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- 75.Arnulf I. The “scanning hypothesis” of rapid eye movements during REM sleep: a review of the evidence. Arch Ital Biol. 2011;149:367–82. doi: 10.4449/aib.v149i4.1246. [DOI] [PubMed] [Google Scholar]
- 76.Oudiette D, De Cock VC, Lavault S, Leu S, Vidailhet M, Arnulf I. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology. 2009;72:551–7. doi: 10.1212/01.wnl.0000341936.78678.3a. [DOI] [PubMed] [Google Scholar]
- 77.McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12:182–92. doi: 10.1007/s11910-012-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Cessation of activity in red nucleus neurons during stimulation of the medial medulla in decerebrate rats. J Physiol (Lond) 2002;545:997–1006. doi: 10.1113/jphysiol.2002.028985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morris R, Vallester KK, Newton SS, Kearsley AP, Whishaw IQ. The differential contributions of the parvocellular and the magnocellular subdivisions of the red nucleus to skilled reaching in the rat. Neurosci. 2015;295:48–57. doi: 10.1016/j.neuroscience.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 80.Li DW. Master’s thesis. University of Toronto; 2015. Role for the red nucleus in motor control during REM sleep. [Google Scholar]
- 81.Dugger BN, Murray ME, Boeve BF, Parisi JE, Benarroch EE, Ferman TJ, et al. Neuropathological analysis of brainstem cholinergic and catecholaminergic nuclei in relation to rapid eye movement (REM) sleep behaviour disorder. Neuropathology and Applied Neurobiology. 2012;38:142–52. doi: 10.1111/j.1365-2990.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmeichel AM, Buchhalter LC, Low PA, Parisi JE, Boeve BW, Sandroni P, et al. Mesopontine cholinergic neuron involvement in Lewy body dementia and multiple system atrophy. Neurology. 2008;70:368–73. doi: 10.1212/01.wnl.0000298691.71637.96. [DOI] [PubMed] [Google Scholar]
- 83.Beninato M, Spencer RF. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol. 1986;253:525–38. doi: 10.1002/cne.902530409. [DOI] [PubMed] [Google Scholar]
- 84.Krauthamer GM, Grunwerg BS, Krein H. Putative cholinergic neurons of the pedunculopontine tegmental nucleus projecting to the superior colliculus consist of sensory responsive and unresponsive populations which are functionally distinct from other mesopontine neurons. Neurosci. 1995;69:507–17. doi: 10.1016/0306-4522(95)00265-k. [DOI] [PubMed] [Google Scholar]
- 85.Cohen JD, Castro-Alamancos MA. Behavioral state dependency of neural activity and sensory (whisker) responses in superior colliculus. J Neurophysiol. 2010;104:1661–72. doi: 10.1152/jn.00340.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frauscher B, Iranzo A, Hogl B, Casanova-Molla J, Salamero M, Gschliesser V, et al. Quantification of electromyographic activity during REM sleep in multiple muscles in REM sleep behavior disorder. Sleep. 2008;31:724–31. doi: 10.1093/sleep/31.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frauscher B, Gabelia D, Mitterling T, Biermayr M, Bregler D, Ehrmann L, et al. Motor events during healthy sleep: A quantitative polysomnographic study. Sleep. 2014:1–13. doi: 10.5665/sleep.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stefani A, Gabelia D, Mitterling T, Poewe W, Högl B, Frauscher B. A prospective video-polysomnographic analysis of movements during physiological sleep in 100 healthy sleepers. Sleep. 2015;38:1479–87. doi: 10.5665/sleep.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]