Abstract
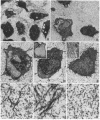
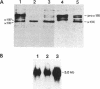
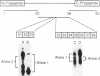
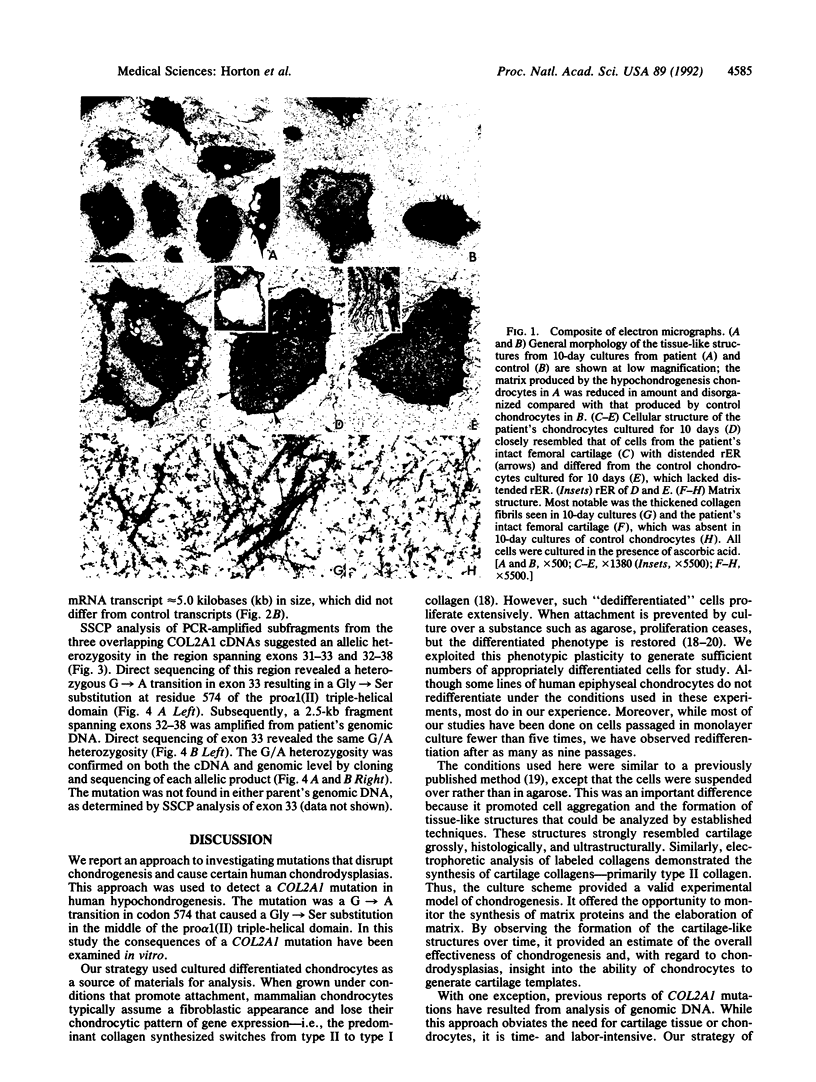
A subtle mutation in the type II collagen gene COL2A1 was detected in a case of human hypochondrogenesis by using a chondrocyte culture system and PCR-cDNA scanning analysis. Chondrocytes obtained from cartilage biopsies were dedifferentiated and expanded in monolayer culture and then redifferentiated by culture over agarose. Single-strand conformation polymorphism and direct sequencing analysis identified a G----A transition, resulting in a glycine substitution at amino acid 574 of the pro alpha 1(II) collagen triple-helical domain. Morphologic assessment of cartilage-like structures produced in culture and electrophoretic analysis of collagens synthesized by the cultured chondrocytes suggested that the glycine substitution interferes with conversion of type II procollagen to collagen, impairs intracellular transport and secretion of the molecule, and disrupts collagen fibril assembly. This experimental approach has broad implications for the investigation of human chondrodysplasias as well as human chondrocyte biology.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulthouse A. L., Beck M., Griffey E., Sanford J., Arden K., Machado M. A., Horton W. A. Expression of the human chondrocyte phenotype in vitro. In Vitro Cell Dev Biol. 1989 Jul;25(7):659–668. doi: 10.1007/BF02623638. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Chan D., Cole W. G. Low basal transcription of genes for tissue-specific collagens by fibroblasts and lymphoblastoid cells. Application to the characterization of a glycine 997 to serine substitution in alpha 1(II) collagen chains of a patient with spondyloepiphyseal dysplasia. J Biol Chem. 1991 Jul 5;266(19):12487–12494. [PubMed] [Google Scholar]
- Engel J., Prockop D. J. The zipper-like folding of collagen triple helices and the effects of mutations that disrupt the zipper. Annu Rev Biophys Biophys Chem. 1991;20:137–152. doi: 10.1146/annurev.bb.20.060191.001033. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Upton M. P., Shapiro F. D., Wilkinson R. H., Vawter G. F. Nonexpression of cartilage type II collagen in a case of Langer-Saldino achondrogenesis. Am J Hum Genet. 1986 Jul;39(1):52–67. [PMC free article] [PubMed] [Google Scholar]
- Godfrey M., Hollister D. W. Type II achondrogenesis-hypochondrogenesis: identification of abnormal type II collagen. Am J Hum Genet. 1988 Dec;43(6):904–913. [PMC free article] [PubMed] [Google Scholar]
- Godfrey M., Keene D. R., Blank E., Hori H., Sakai L. Y., Sherwin L. A., Hollister D. W. Type II achondrogenesis-hypochondrogenesis: morphologic and immunohistopathologic studies. Am J Hum Genet. 1988 Dec;43(6):894–903. [PMC free article] [PubMed] [Google Scholar]
- Horton W. A., Campbell D., Machado M. A., Chou J. Type II collagen screening in the human chondrodysplasias. Am J Med Genet. 1989 Dec;34(4):579–583. doi: 10.1002/ajmg.1320340425. [DOI] [PubMed] [Google Scholar]
- Horton W. A., Machado M. M. Extracellular matrix alterations during endochondral ossification in humans. J Orthop Res. 1988;6(6):793–803. doi: 10.1002/jor.1100060603. [DOI] [PubMed] [Google Scholar]
- Lee B., D'Alessio M., Ramirez F. Modifications in the organization and expression of collagen genes associated with skeletal disorders. Crit Rev Eukaryot Gene Expr. 1991;1(3):173–187. [PubMed] [Google Scholar]
- Lee B., D'Alessio M., Vissing H., Ramirez F., Steinmann B., Superti-Furga A. Characterization of a large deletion associated with a polymorphic block of repeated dinucleotides in the type III procollagen gene (COL3A1) of a patient with Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1991 Mar;48(3):511–517. [PMC free article] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L. W., Bautista J., James P. L., Rimoin D. L. Type II collagen defects in the chondrodysplasias. I. Spondyloepiphyseal dysplasias. Am J Hum Genet. 1989 Jul;45(1):5–15. [PMC free article] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Baldwin C. T., Constantinou C. D. Mutations in type I procollagen genes that cause osteogenesis imperfecta. Adv Hum Genet. 1990;19:105–132. doi: 10.1007/978-1-4757-9065-8_2. [DOI] [PubMed] [Google Scholar]
- Rimoin D. L. The chondrodystrophies. Adv Hum Genet. 1975;5:1–118. doi: 10.1007/978-1-4615-9068-2_1. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Horton W. A., Rimoin D. L. Morphologic studies in the skeletal dysplasias. Am J Pathol. 1979 Sep;96(3):813–870. [PMC free article] [PubMed] [Google Scholar]
- Spranger J. Bone dysplasia 'families'. Pathol Immunopathol Res. 1988;7(1-2):76–80. doi: 10.1159/000157098. [DOI] [PubMed] [Google Scholar]
- Stanescu V., Stanescu R., Maroteaux P. Pathogenic mechanisms in osteochondrodysplasias. J Bone Joint Surg Am. 1984 Jul;66(6):817–836. doi: 10.2106/00004623-198466060-00002. [DOI] [PubMed] [Google Scholar]
- Su M. W., Lee B., Ramirez F., Machado M., Horton W. Nucleotide sequence of the full length cDNA encoding for human type II procollagen. Nucleic Acids Res. 1989 Nov 25;17(22):9473–9473. doi: 10.1093/nar/17.22.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacchetti C., Quarto R., Nitsch L., Hartmann D. J., Cancedda R. In vitro morphogenesis of chick embryo hypertrophic cartilage. J Cell Biol. 1987 Aug;105(2):999–1006. doi: 10.1083/jcb.105.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- Wenstrup R. J., Willing M. C., Starman B. J., Byers P. H. Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet. 1990 May;46(5):975–982. [PMC free article] [PubMed] [Google Scholar]