Abstract
The female genital tract (FGT) provides a means of entry to pathogens, including HIV, yet immune cell populations at this barrier between host and environment are not well defined. We initiated a study of healthy women to characterize resident T cell populations in the lower FGT from lavage and patient-matched peripheral blood to investigate potential mechanisms of HIV sexual transmission. Surprisingly, we observed FGT CD4 T cell populations were primarily CCR7hi, consistent with a central memory (TCM) or recirculating memory T cell phenotype. In addition, roughly half of these CCR7hi CD4 T cells expressed CD69, consistent with resident memory T cells (TRM), while the remaining CCR7hi CD4 T cells lacked CD69 expression, consistent with recirculating memory CD4 T cells that traffic between peripheral tissues and lymphoid sites. HIV susceptibility markers CCR5 and CD38 were increased on FGT CCR7hi CD4 T cells compared to blood, yet migration to the lymphoid homing chemokines CCL19 and CCL21 was maintained. Infection with GFP-HIV showed that FGT CCR7hi memory CD4 T cells are susceptible HIV targets, and productive infection of CCR7hi memory T cells did not alter chemotaxis to CCL19 and CCL21. Variations of resident CCR7hi FGT CD4 T cell populations were detected during the luteal phase of the menstrual cycle and longitudinal analysis showed the frequency of this population positively correlated to progesterone levels. These data provide evidence women may acquire HIV through local infection of migratory CCR7hi CD4 T cells and progesterone levels predict opportunities for HIV to access these novel target cells.
Keywords: Human, Mucosa, HIV, T cells, Cell Trafficking
INTRODUCTION
Human immunodeficiency virus (HIV) infection is one of the gravest health challenges worldwide with an estimated 35 million people currently infected (1). Sexual intercourse is the predominant mode of HIV acquisition in women and accounts for an estimated 1 million new infections annually (1), yet the mechanisms by which HIV penetrates the female genital tract (FGT) mucosa to establish a systemic infection are not fully understood. Potential routes of genital infection are CCR5 dependent and are thought to involve Langerhans cells (LCs) and/or CCR5+ CD4 T cells at the superficial vaginal mucosa (2). T cell characterization data show increased CCR5 expression on CD4 T cells in the vagina (3) and cervix (4). Nonhuman primate and human explant models show CD4 T cells in the FGT are directly and productively infected following virus inoculation (5, 6) and virus transmission of Simian Immunodeficiency Virus (SIV) in both rectal and vaginal mucosal tissues are proportional the number of CCR5+ CD4+ T cells at these sites (7). Recently, a study using cervical CD4 T cells showed that HIV preferentially targets populations of CD4 T cells with increased expression of the HIV co receptor CCR5 in combination with the activation marker CD69, and mucosal integrins α4β7 and α4β1(8). Together, these studies demonstrate that FGT CD4 T cells express HIV susceptibility markers and are direct targets of HIV infection. However, it is unclear whether the population of CD4 T cells at the surface of the genital tract can directly contribute to establishment of a systemic HIV infection. Furthermore, there is currently limited information on the CD4 T cell subsets present in the FGT of healthy women, the impact of sex hormones on the composition of the FGT CD4 T cell population, and whether distinct FGT CD4 T cell subsets may have differential contributions to HIV transmission.
The FGT is an immune-restricted site where T cell trafficking is tightly regulated (9, 10). Immune-restricted tissues primarily contain memory T cells of an effector memory phenotype (TEM; CCR7lo, CD45RAlo), which recent studies have shown are mostly comprised of long-lived, non-circulating tissue-resident memory cells (TRM: CD103+ CD69+) that provide tissue-specific protection from invading pathogens (11, 12). In murine models, resident populations of CD4 T cells in the FGT predominantly comprise a TEM phenotype expressing CD69 but with little to no CD103 expression in comparison to FGT CD8 TRM (13, 14). Interestingly, the protective functions of TRM cells at mucosal sites can be mediated by cells embedded in the tissue as well as cells located on the luminal side of the epithelium (15–18). For example, in the lung CD8 TRM have differential effector functions based on whether they are localized to the airway lumen or the underlying tissue, and both populations contribute to optimal immune protection (19). Therefore, to obtain a complete picture of the resident T cell population at mucosal sites it is important to characterize immune cells on both the apical and basal side of the epithelium.
Though CCR7hi T cells are not characteristically found in immune restricted tissue, recirculating memory T cells are a recently identified subset of CCR7 expressing CD69− memory CD4 T cells detected in cutaneous tissue under steady-state conditions (20). These recirculating CD4 T cells migrate from peripheral tissue into draining lymph nodes in a CCR7-dependent fashion and are thought to function in immune surveillance. Even though the identification of recirculating memory CD4 T cells in humans is relatively recent (21), prior mechanistic studies in murine models have shown that CD4 T cells located in the lumen of mucosal tissues can traverse the epithelium and migrate to lymph nodes (22–24). Importantly, this process required expression of CCR7, as CCR7-deficient CD4 T cells failed to migrate out of the lumen (20). Thus, memory CD4 T cells located on the apical surface of the mucosal epithelium have the potential to migrate across the epithelium and re-enter the lymphatics in a CCR7-dependent manner.
To date, investigations of tissue resident immune cell populations at the human FGT mucosal surface are complicated by collection procedures that sample small surface areas that may not represent areas most susceptible to HIV infection (25, 26), moreover such sampling has the potential to introduce blood-borne leukocytes into resident cell samples (27). Notably, these methods would sample both luminal T cells as well as T cells embedded in the underlying tissue, whereas isolation of FGT immune cells by lavage allows for the specific examination of the FGT T cell population located on the apical surface of the epithelium. We developed an atraumatic enhanced lavage-enrichment method to characterize local T cell populations at the apical surface of the mucosal epithelium in the lower FGT for the purpose of better understanding T cell-mediated susceptibility to HIV infection from sexual exposure. Using this method we determined the majority of FGT memory CD4 T cells (CD69+/− CD45RAlo) in healthy women express the lymphoid homing chemokine receptor CCR7, and approximately half of the CCR7hi CD4 T cells did not express the residency marker CD69. In addition, FGT CCR7hi CD4 T cells expressed the HIV susceptibility markers CCR5 and CD38, and display chemotaxis to CCL19 and CCL21. Using a CCR5-tropic GFP-HIV reporter we further show that CCR7hi FGT CD4 T cells are targets of infection and separate migration studies of productively infected CCR7-expressing T cells show no altered chemotaxis to CCL19 and CCL21. These data suggest CCR7hi memory CD4 T cells resident in the genital tract could be infected at the earliest stages of HIV transmission and serve as a mechanism for viral entry and dissemination to lymphoid tissues. In addition, the sex hormones estradiol and progesterone that regulate the menstrual cycle also impact the immune environment in the FGT and some evidence suggest these hormones may influence HIV sexual transmission in the FGT, though the mechanism of this increased susceptibility are unclear (28, 29). In correlation to these emerging studies, we found this T cell population more concentrated during the luteal phase of the menstrual cycle and upon longitudinal analysis the frequency of FGT CCR7hi CD4 T cells positively correlated to blood progesterone levels. Our data identify an unexpected recirculating memory CD4 T cell population at the surface of the FGT and suggest a novel mechanism of HIV transmission and establishment of systemic infection through the migration of infected, recirculating FGT CCR7hi CD4 T cells into underlying lymphoid tissues. Furthermore, our data show these target cells are more accessible to HIV during the luteal phase of the menstrual cycle.
MATERIALS & METHODS
Specimen collection and processing
Paired blood and CVL (cervicovaginal lavage) specimens were collected from women participating in a study of oral maraviroc pharmacokinetics in HIV-negative volunteers (Atlanta, Georgia) at visits without detectable maraviroc. Additional CVL only specimens for the FGT T cell characterizations were obtained from a convenience sample of women attending a normal well-woman visit at Emory Clinic and used in some experiments where only CVL cells were analyzed. Protocols were approved by the Emory University and Centers for Disease Control and Prevention Institutional Review Boards and the Grady Research Oversight Committee. All participants provided informed consent.
Menstrual cycle follicular (<10 days following start of previous menses) and luteal phase (>18 days following start of previous menses) were determined according to self-report. Progesterone and estradiol were measured from blood samples using the estradiol, progesterone Milliplex kit for Luminex (EMD Millipore, Billerica, MA) according to manufacturer’s instructions.
Blood was collected in 8 mL sodium citrate-containing CPT tubes (BD Biosciences, Franklin Lakes, New Jersey) and separated into plasma and peripheral blood mononuclear cells (PBMC) by centrifugation. During a speculum examination, CVL specimens were collected by directing 2 series of 10 mL of phosphate-buffered saline toward the endocervix and vaginal walls as per the protocol described by the Microbicide Trials Network (www.mtnstopshiv.org/node/773). Briefly, 1/3 PBS directed at cervical os and endocervix, 1/3 PBS directed at right vaginal wall, and 1/3 PBS directed at left vaginal wall were then allowed to pool into the posterior fornix then aspirated into a 15mL conical containing 0.5mL of human AB serum (Cellgro, Manassas, Virginia). The CVL was transported in a 15 mL conical tube containing 0.5 mL human AB serum (Cellgro, Manassas, Virginia). CVL was centrifuged into cell-free supernatant and cellular fractions at 300 × g for 10 minutes. CVL cell fractions were enriched for leukocytes by centrifugation through a 40%/80% Percoll (GE healthcare, Pittsburgh, Pennsylvania) gradient at 900 × g for 20 minutes at room temperature without brake. Enriched CVL leukocytes were collected at the gradient interface and passed through a 70 μm cell strainer. Leukocytes were washed with cRPMI (RPMI containing 10% fetal bovine serum, penicillin and streptomycin) and stained for cell surface markers as indicated below. Separation of PBMC and CVL cell fractions was performed within 4 hours of sample collection. CVL samples were measured for semen using the ABACard p30 Antigen Detection Test (Abacus Diagnostics, West Hill, California) to identify samples containing cells from a male sexual partner that might affect immune characterization.
Sexually Transmitted Infection Testing
DNA was extracted from DrySwab® (Lakewood Biochemical Company) using the Qiagen DNA Mini kit to amplify targets from Gonorrhea, Chlamydia, Syphilis Herpes simplex virus types 1 and 2, PID, Candida, and HIV using real-time duplex PCR and Qiagen Rotor-Gene Q and 6000 real-time PCR instruments. Qiagen Rotor-Gene Q Series software was used to analyze data.
Chemotaxis assays
Chemotactic activity to CCL19 and CCL21 was measured using a standard migration assay as previously described (30). In brief, CCL19 (600ng/mL) and CCL21 (600ng/mL) chemokines (R&D Systems, Minneapolis, Minnesota) were added to the lower chamber of 24-well transwell plates (5.0 μm pore size, Corning, Tewksbury, Massachusetts) and incubated 20 minutes at 37°C. Cells (1 × 106) were added to the upper chamber and incubated 3 hours at 37°C. Lower chamber contents were analyzed by flow cytometry as indicated using TRUCOUNT tubes (BD Biosciences, San Jose, California) to calculate the absolute number of cells. Chemotactic index was calculated as the number of cells migrating in response to indicated chemokines divided by the number of cells migrating in response to media lacking additional chemokines.
Sample staining and flow cytometry
All cells were stained for viability using the Zombie Fixable Viability Kit (Biolegend), incubated with anti-CD16/32 Fc-block (BioXcell), and stained with the indicated antibodies: CD19 APC, CD3 APC-H7 or BV450, CD4 AF700, CD8 V500, CCR7 PE-CF594, CD69 BV785, CCR5 PE, CD103 FITC, (BD Biosciences) CD38 PE-Cy7, CD11c BV711, CD14 BV650, CD45RA BV605 (Biolegend). Stained samples were run on an LSRII flow cytometer, data acquired using FACS DIVA software (BD Biosciences) and analyzed using FlowJo software (TreeStar, Inc., Ashland, Oregon).
HIV infection of T cells
PBMC from healthy donors were infected overnight with 2×105 TCID50 of CCR5-tropic subtype C HIV (NG201530, AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Bethesda, Maryland) and p24 levels were measured as previously described (31). PBMC collected from healthy blood donors were incubated in cRPMI-10 with 20 units/mL IL-2 (Roche), then activated with Cell Stimulation Cocktail (eBioscience, San Diego, California) overnight. Cells were washed in cRPMI, rested for 4 days, and incubated in cRPMI with 20 units/mL IL-2. Cells (2 × 106) were incubated overnight with 2× 105 TCID50 of CCR5-tropic subtype C HIV (NG201530, AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Bethesda, Maryland). Infected cells were washed with cRPMI and incubated with cRPMI containing 20 units/mL IL-2 for 4 days. Cells were assayed for chemotactic index using the migration assay described above. Cells were stained with the following antibodies: CD19 APC, CD3 APC-H7, CD4 AF700, CD8 V500, (BD biosciences), CD45RA BV605 (Biolegend). For intracellular staining, cells were permeabilized with Cytofix/Cytoperm kit (BD Biosciences) prior to staining with p24 PE (KC57-RD1 Beckman Coulter, Indianapolis, Indiana).
For infection of CVL from healthy women, 2–10×106 enriched CVL cells were infected for 1 hour with an R5-tropic GFP-HIV (a gift from Thorsten Mempel, Harvard Medical School) (32), washed, and cultured for 48hours in complete media supplemented with 20 units/mL IL-2. CVL cells were stained as described above with the indicated antibodies. Cells were assessed for GFP expression on an LSRII flow cytometer (BD) and data analyzed as described above.
Statistics
Statistical analysis was performed with Prism 5 software (Graphpad). Unless otherwise noted an unpaired two-tailed Student’s t test was used to determine significance. ns not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
RESULTS
The lower FGT mucosal surface is an immune restricted site with a majority CCR7hi CD4 memory T cell population
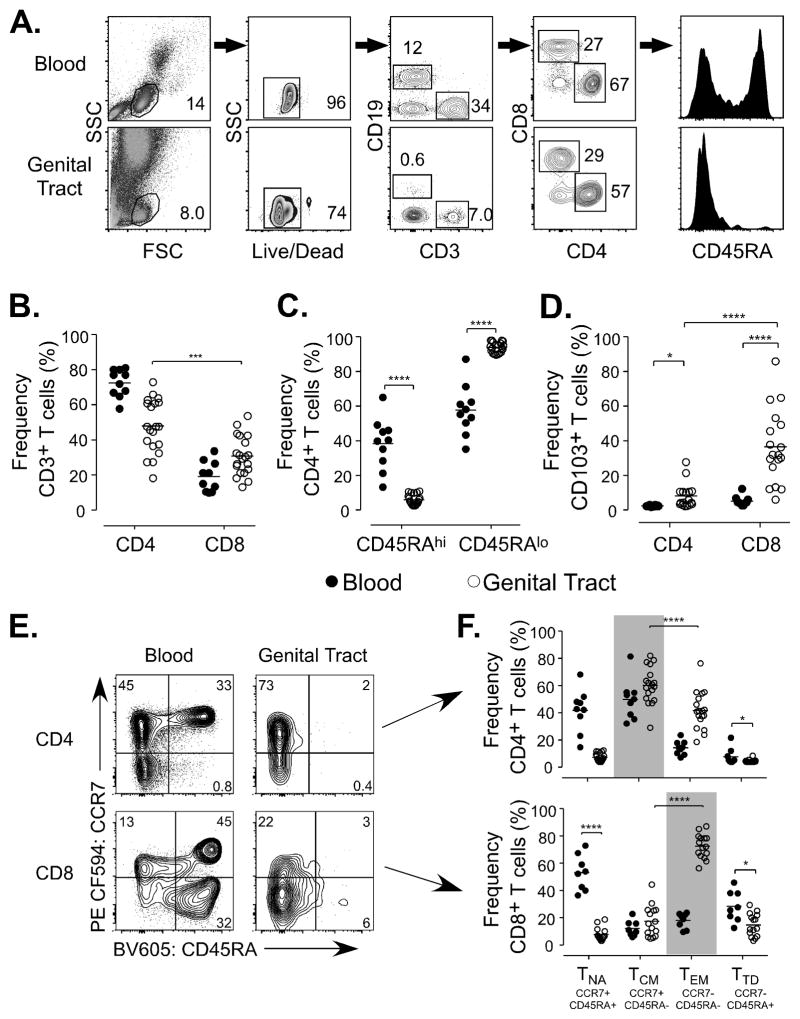
To investigate how T cells at the FGT mucosal surface may influence HIV acquisition we initiated a study of pre-menopausal healthy women to perform atraumatic broad surface area sampling of the lower FGT. Participants were enrolled and screened for the purpose of collecting genital lavage and matched blood samples. Using standard CVL collection procedures we optimized an enhanced lavage and enrichment technique to increase leukocyte yields while minimizing tissue trauma. To determine whether lavage samples provided characterizations representative of an immune restricted environment, we implemented three criteria to confirm method validity; i) a low proportion of cells from blood circulation (<3% CD19+ B cells detected among lymphocytes)(33) (Fig. 1A), ii) the absence of naïve T cells (Fig. 1C, 1F), and iii) an increased frequency of the mucosal residence marker CD103 on T cells compared to matched peripheral blood samples (CD4 p=0.0181, CD8 p=<0.0001) (Fig. 1D) (34). A description of the CVL samples used in the characterizations in Figures 1–3 is provided in Supplemental Table I.
Figure 1.
(A) Representative stain illustrating the gating strategy for FGT T cell characterizations. (B) CD4 and CD8 frequency of CD3 population from blood and FGT samples. (C) CD45RA frequency of CD4 T cell populations from blood and FGT samples. (D) CD103 expression of CD4 and CD8 T cell populations from blood and FGT samples. (E) Representative stain of CCR7 and CD45RA T cell populations on either CD4 T cells (top panels) or CD8 T cells (lower panels) from blood (left panels) or the FGT (right panels). (F) CD45RA and CCR7 population frequency in CD4 and CD8 T cells from blood and FGT. Labeled, Naïve T cells (TNA) CD45RAhi CCR7hi, Central Memory T cells (TCM) CD45RAlo CCR7hi, Effector Memory T cells (TEM) CD45RAlo CCR7lo, and Terminally Differentiated T cells (TTD) CD45RAhi CCR7lo.
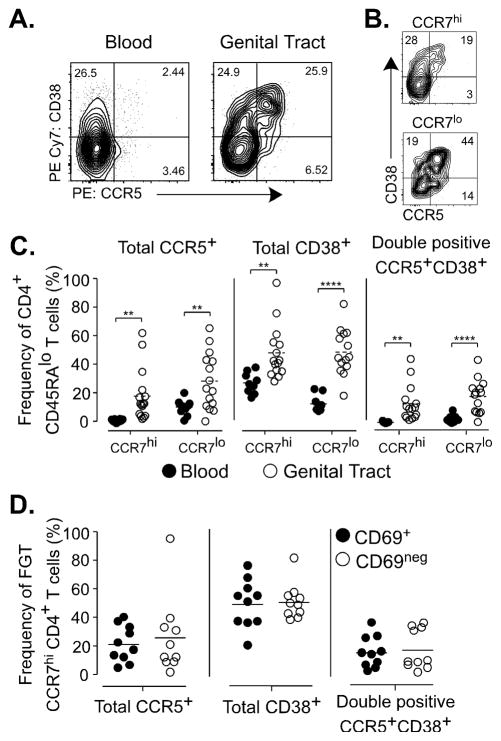
Figure 3.
(A) Representative stain of CCR5 and CD38 expression on memory CD4 T cells from blood (left panel) or FGT (right panel). (B) Representative stain of CCR5 and CD38 on FGT CD4 T cells gated by CCR7 expression. (C) CD4 memory T cells gated by CCR7 expression and measured for expression of CCR5, CD38. (D) FGT CCR7hi CD4 T cells gated by CD69 expression and measured for expression of CCR5 and CD38.
Initial characterizations found the predominant T cell population at the FGT mucosal surface was memory CD4 cells (CD45RAlo) (CD4 p=0.0002, CD45RAlo p=<0.0001) (Fig 1B, 1C). We further measured the frequency of CD45RA and CCR7, to distinguish naïve and terminally differentiated cells (TTD), as well as central (TCM) and effector memory (TEM) subsets (Fig. 1E, 1F) (12, 35). Notably, though previously characterized mucosal sites contain a predominant TEM population, the primary population of FGT T cells was CCR7hi CD4 memory cells (p=<0.0001), consistent with a TCM phenotype (CD45RAlo CCR7hi). FGT CD8 T cells, in contrast, expressed a predominant TEM phenotype (CD45RAhi CCR7lo) (p=<0.0001). TTD (CD45RAhi CCR7lo) cells were reduced within the CD4 (p=0.279) and CD8 (p=0.0313) T cell populations (Fig. 1F). These results show that human mucosal T cell populations in the lower FGT are primarily memory CD4 T cells expressing CCR7, a chemokine receptor important for T cell trafficking to the lymphatics from peripheral tissue sites (20).
FGT CCR7hi CD4 T cells populations express increased CD69 expression yet display chemotaxis to CCL19 and CCL21
The expression of CCR7 on human CD4 T cells in the lower FGT is distinct from characterization studies using human hysterectomy tissue (36) as well as previous studies in mice (14). To better characterize FGT CD4 T cells enriched from the mucosal surface, we measured expression of the tissue retention markers CD69 and CD103 in comparison to CCR7 expression (Figure 2A–C). CD69 interferes with sphingosine-1-phosphate receptor function and CD103 binds E-cadherin to facilitate adherence to the epithelium (37, 38). CCR7hi CD4 T cells expressed a greater range in CD69 frequency (10–93.8) compared to CCR7lo CD4 T cells (30–93.3) though the average percentage of CD69+ cells was similar between CCR7hi and CCR7lo CD4 T cell subsets (mean 54.2 and 60.2, respectively) and no significant differences in CD69 expression were detected (Figure 2B). CD103 expression was detected with a greater frequency on FGT CCR7lo CD4 T cells compared to CCR7hi CD4 T cells (mean 22.8 and 2.3) (p=0.0005) (Figure 2C). These data show that CD4 T cells in the FGT comprise CCR7hi CD69+/− populations consistent with both CD4 TRM (CD69+) and recirculating memory CD4 T cells (CD69−).
Figure 2.
(A) Representative stain of CCR7 and CD45RA expression on memory CD4 T cells from the FGT (left panel). Cells were gated by CCR7 expression and measured for the expression on CD69 and CD103 (right panel). (B,C) FGT CD4 T cells were gated by CCR7 expression and measured for CD69 (B) or CD103 expression expression (D) CD4 T cell chemotactic function to CCL19 and CCL21 (gray bar n=8). (E) FGT CD4 T cell migration to CCL19/21 shown by change in % of CD4 T cells that migrated in complete media compared to CD4 T cells that migrated to CCL19/21 chemokines (n=7). (D, E) Dotted line represent chemotactic index of 1.
The expression of CD69 on T cells at peripheral sites relates to long-term tissue retention (37), yet the detection of CCR7 on FGT CD4 T cell populations indicates these cells may be able to exit the tissue via the lymph in response to CCR7 chemokines. To determine whether CCR7 expression on FGT CD4 T cell populations would enable migratory function and trafficking into afferent lymphatics, we investigated chemotaxis to CCR7-binding chemokines CCL19 and CCL21 by transwell assay (39). In all FGT samples tested (n=8), CD4 T cells exhibited migration to CCR7-binding chemokines (p=0.0259) (Fig. 2D). Notably, although the chemotactic patterns of FGT CD4 T cells displayed variation among participant samples, there was a significant increase the frequency of migrated cells comparing the media control (average 1.9 ± 2.7 s.d.) with CCL19+CCL21 (average 11.6 ± 11.9 s.d.) (Fig. 2E). These data show that FGT CCR7hi CD4 T cells are comprised of both CD69+ and CD69− memory subsets and these populations can migrate to CCR7 chemokines. Thus, the combination of migratory ability displayed by FGT CD4 T cell populations in addition to phenotypic analysis support that a subset of these cells are consistent with recirculating memory CD4 T cells localized to the FGT.
FGT CCR7hi CD4 T cells express HIV susceptibility markers
Due to their localization at the surface of the genital tract, it was possible that these CCR7hi memory CD4 T cells would also be early targets of HIV infection from sexual transmission. To investigate evidence for HIV susceptibility in CCR7hi CD4 T cell populations in the FGT, we analyzed FGT CD4 T cells for expression of the HIV co-receptor CCR5 and HIV susceptibility marker CD38 (40) based on CCR7 expression. As shown (Fig. 3A–C) by comparing CCR5 and CD38 expression on FGT and blood CD4 T cells, both CCR7hi and CCR7lo FGT CD4 T cell populations exhibited increased expression. Similarly, CCR5 and CD38 co-expression was increased on CCR7hi (p=0.0017) and CCR7lo (p=0.002) FGT CD4 T cells compared to blood suggesting that both FGT CD4 T cell subsets, including FGT CCR7hi CD4 T cells, would be susceptible targets for HIV infection. To distinguish whether FGT CCR7hi CD4 T cells may exhibit different patterns of HIV susceptibility markers based on a resident or recirculating T cell phenotype we also measured CCR5 and CD38 expression on both the CD69+ and CD69− subsets (Figure 3D). Despite the differences in CD69 expression, these subsets of CCR7hi CD4 T cells express similar levels of CCR5 and CD38. Thus, based on the surface expression of HIV susceptibility markers both CD69+ and CD69− CCR7hi CD4 T cells in the FGT are likely permissive to HIV infection.
FGT CCR7hi memory CD4 T cells are susceptible to HIV infection
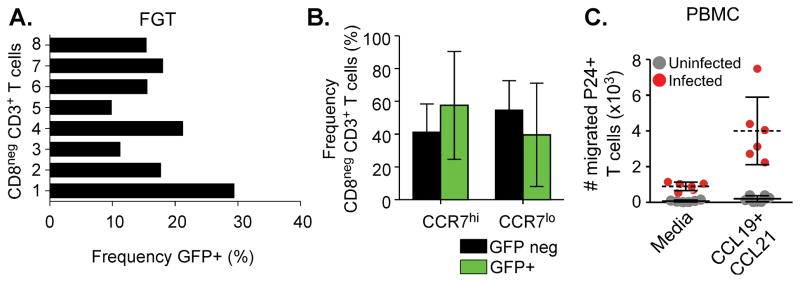
CD4 TEM may be selectively infected by HIV due to increased expression of CCR5 in comparison to CD4 TCM cells (35, 41). FGT CD4 T cells comprise both CCR7hi and CCR7lo CD4 T cells with increased HIV susceptibility markers including CCR5 in comparison to similar populations from blood, but whether HIV preferentially targets FGT CD4 T cells based upon CCR7 expression is unknown. To test HIV infection of FGT CD4 T cell populations based upon CCR7 expression, we infected CVL cells from eight individual subjects with HIV-GFP and measured for infection in CCR7hi and CCR7lo CD4 T cells following 48 hours of cell culture (Fig. 4A and 4B). The gating strategy used for the identification of infected cells is shown in Supplemental Figure 1. We detected GFP signal with a greater frequency in CCR7hi (mean frequency of infected CD3+ CD8− T cells: 56.1) compared to CCR7lo FGT CD4 T cells populations (mean frequency of infected CD3+ CD8− T cells: 43.57), though this difference was not significant (Figure 4B). These data indicate that both CCR7hi and CCR7lo FGT CD4 T cells are equally susceptible to HIV infection.
Figure 4.
(A, B) Unstimulated CVL cells infected with R5-tropic GFP-HIV ex-vivo (2–10×106 enriched CVL cells) were measured for GFP signal following 48 hours of cell culture. (A) GFP frequency measured from gated FGT CD3+ CD8− T cells (frequency range: 9.83–29.4, mean: 17.3). (B) GFP positive FGT T cells were measured for CCR7 expression. GFP positive CCR7hi CD3+ CD8− T cells (mean frequency: 56, range:6–89%) and CCR7lo CD3+ CD8− T cells (mean frequency: 43.57, range:16–85) are compared to the CCR7 frequency of GFP negative FGT CD3+ CD8− T cell populations (C) PBMC infected with CCR5 tropic subtype C HIV then tested for CCL19/21 chemotaxis. HIV infected T cells (red circles) measured by intracellular p24 expression in comparison to uninfected T cells (gray circles). Absolute number of p24 positive T cells migrated to CCL19/21 or complete media.
CCR7 expression on infected T cells support virus trafficking to lymphoid homing chemokines
HIV-infected TCM can directly disseminate virus within lymph nodes upon subcutaneous transfer in humanized mice (32). However, HIV viral proteins have been shown to interfere with lymph homing signaling pathways in infected cells (42). FGT memory CD4 T cells co-express CCR7 and markers of HIV susceptibility, but whether these cells could support virus trafficking into lymph nodes via CCR7-mediated chemotaxis is unknown. To determine whether CCR7hi CD4 T cells exhibited trafficking to lymph homing chemokines after infection, we infected blood-derived CD4 T cells with a clinically derived R5-tropic subtype C HIV isolate and measured migration of infected TCM to CCL19 and CCL21. We observed that HIV-infected CD4 T cells migrated to CCL19 and CCL21 (p=0.0025) (Fig. 4C) and did not exhibit impaired chemotaxis (Supplementary Fig. 2). These results support the concept that expression of CCR7 on FGT memory CD4 T cells enables HIV to utilize these cells for direct trafficking and dissemination of virus into secondary lymphoid tissue.
Frequencies of FGT CCR7hi memory CD4 T cells associate with the luteal phase of the menstrual cycle and correlate with blood progesterone levels
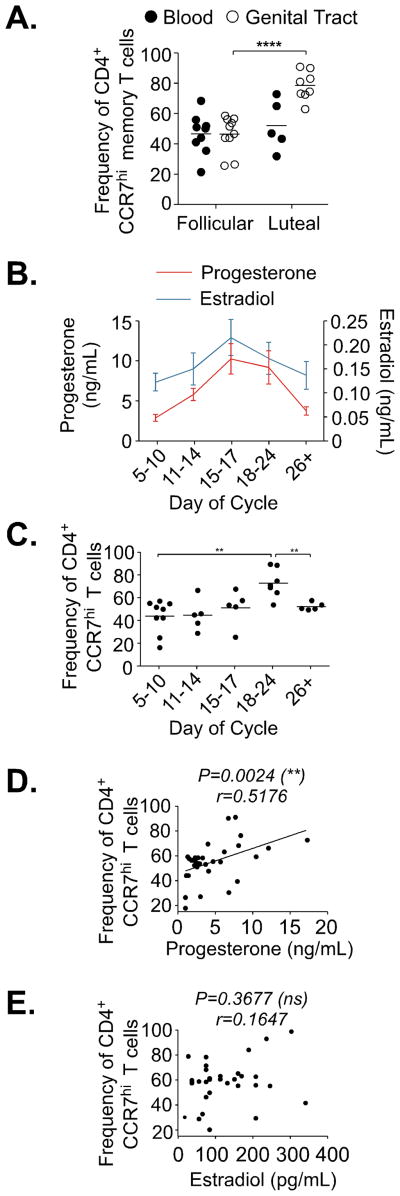
Previous studies using nonhuman primate models and evidence from humans suggest the luteal phase of the menstrual cycle is associated with increased HIV susceptibility (28, 43, 44). To explore whether the phase of the menstrual cycle may associate with changes in the FGT memory CD4 T cell population, we compared lavage samples collected at the follicular phase (4–10 days following start of menses) or luteal phase of the menstrual cycle (18–26 days following start of menses) for any differences in T cell characteristics. By analyzing T cell populations based upon self-reported phase of the menstrual cycle, we found the frequency of FGT CCR7hi memory CD4 T cells increased at time points corresponding to the luteal phase (p=<0.0001) (Fig. 5A), though the average frequency of CCR7hi memory CD4 T cell populations in blood remained similar between time points.
Figure 5.
(A) Frequency of CCR7hi CD4 T cells from FGT compared to matched blood measured according to self-reported menstrual cycle phase. (B) Average blood plasma levels of Progesterone and Estradiol shown at indicated timepoints throughout menstrual cycle. Day of cycle represent range estimated from self-reported start of menses. (C) Frequency of CCR7hi CD4 T cells from the FGT measured at indicated timepoints progesterone and estradiol were measured. (D) Progesterone levels compared to FGT CCR7hi CD4 T cell frequency. Statistics calculated by Spearman correlation (Pearson correlation also performed and show significance, r=0.4079 and p=0.0032). (E) Estradiol levels compared to FGT CCR7hi CD4 T cell frequency. Statistics calculated by Spearman correlation (Pearson correlation also performed and show no significance r=0.3006 and p=0.2111).
To more directly examine the association of FGT CCR7hi memory CD4 T cells with the menstrual cycle, we followed a subset of healthy participants longitudinally, collecting blood and lavage samples at indicated time points throughout the cycle and measured estradiol and progesterone from blood plasma to accurately define menstrual cycle phase (45) (Fig. 5B). The frequency of FGT CCR7hi memory CD4 T cells was highest at time points with increased plasma progesterone levels, corresponding to the luteal phase of the menstrual cycle (p=0.0017) (Fig. 5C). As progesterone levels declined, the frequency of FGT CCR7hi memory CD4 T cells decreased into ranges detected during the follicular phase (p=0.0059). By directly comparing CCR7 expression on FGT memory CD4 T cells, a significant positive correlation was observed with progesterone concentrations (p=0.0024) (Fig. 5D). No correlations were detected upon comparing FGT CCR7hi memory CD4 T cell frequencies and estradiol concentrations (Fig. 5E). Notably, no change in the HIV susceptibility markers CCR5 and CD38 was detected on FGT CD4 T cells based upon phase of the menstrual cycle (Supplementary Fig. 3A and 3B). Increased CCR7 frequency in correlation to plasma progesterone levels was not related to a change in total CD4 T cells detected (Supplementary Fig. 3C).
DISCUSSION
Understanding how HIV can penetrate mucosal surfaces and establish a systemic infection is needed to advance promising biomedical interventions. Estimated HIV sexual transmission rates through the cervical-vaginal mucosa of women are relatively low compared to other exposure routes (46) and are often a result of a single founder variant that may be specific to CD4 T cells (47, 48). Mucosal barriers involve a wide variety of immune mediators in order to adequately defend against infection, including resident memory T cell populations that provide a superior and tissue-specific immune response to invading pathogens (49–53), hence their maintenance at these sites is vital. Previous work involving adoptive transfers studies in mice found that the expression of CCR7 on CD4 T cells was sufficient to enable them to migrate from mucosal barriers into afferent lymphatics (23, 24), demonstrating that not all memory T cells present at mucosal sites permanently reside in the tissue. Furthermore, the subsequent identification of recirculating memory CD4 T cells in cutaneous tissues in humans demonstrated an even greater complexity to the maintenance and homeostasis of tissue-resident memory CD4 T cells at peripheral sites (21). Together, these data suggest that the memory CD4 T cell pool resident in mucosal tissues at any one time is a dynamic population comprised of both stationary and migratory cells that maintain local immune surveillance.
Our study is the first to identify a novel population of CCR7hi memory CD4 T cells consistent with both CD4 TRM and recirculating memory CD4 T cell phenotypes in the FGT mucosa of healthy women. Delineating resident and recirculating FGT memory CD4 T cells based on the residency marker CD69, we showed that both of these subsets are enriched for the expression of the HIV susceptibility markers CCR5 and CD38 and thus are likely targets of HIV infection. It should be noted, however, that while CD69 can contribute to tissue residency it is not a definitive marker for tissue-resident T cells, and it is possible that the dynamic regulation of CD69 expression in the tissue lessens its utility as a marker to distinguish resident and recirculating memory CD4 T cells (37, 54). Nevertheless, we demonstrated that CCR7hi memory CD4 T cells isolated from CVL were susceptible to HIV infection and that CCR7hi memory CD4 T cells retained their ability to migrate to CCR7 ligands after HIV infection. Several immune cell types, including Langerhans cells and myeloid cells, have been implicated in transporting HIV across the mucosal epithelium (5, 55–57). Our data suggest that in addition to these mechanisms, heterosexual HIV transmission may also occur by infection of recirculating CCR7hi memory CD4 T cells at the luminal surface of the FGT that traffic across the epithelium to lymphoid tissues where a systemic infection can be established.
How these FGT CCR7hi CD4 T cells may function in immune protection remains to be understood, as do the signals that direct their migration into this site. One interesting finding from our data is the difference between FGT CD4 and CD8 T cells in CCR7 and CD103 expression. Significantly fewer FGT memory CD8 T cells expressed CCR7 compared to CD4 T cells, while the frequency of FGT memory CD8 T cells that expressed CD103 was significantly greater. Together, these data suggest that FGT CD8 memory T cells may be more likely to remain resident in the tissue, whereas the FGT memory CD4 population may be comprised of both tissue resident and recirculating cells. The changing frequency of FGT CD4 T cells over the course of the menstrual cycle suggests that as recirculating FGT CCR7hi CD4 T cells migrate from the tissue to lymphoid sites, their numbers must be maintained by an influx of new cells into the FGT. One possibility is that CCR7 mediates both the influx and efflux of recirculating FGT CCR7hi CD4 T cells, as constitutive expression of the CCR7 chemokines CCL19 and CCL21 in human endometrium has been reported (58). However, we think it unlikely that CCR7-mediated signals draw recirculating cells into the FGT as we and others do not observe any naïve CD4 T cells in the FGT, which would also migrate to CCR7 chemokines. More recently, it has been shown that CCR5-mediated signals are necessary for sustaining resident memory CD4 T cells in the FGT (13). Although it is unclear whether CCR5 directs the migration of recirculating CCR7hi CD4 T cells to the FGT under homeostatic conditions, it is an intriguing possibility considering that this potential mechanism may increase the number of permissive HIV targets at the surface of the FGT. We are currently investing the role of CCR5 and other migratory signals in directing the recruitment of recirculating CCR7hi memory CD4 T cells to the FGT.
In correlation to emerging studies that suggest a role of sex hormones in sexual transmission of HIV (28), we found an increased frequency of CCR7hi cells among the FGT CD4 T cell population during the luteal phase of the menstrual cycle that positively correlated with blood progesterone levels. The exact roles by which progesterone alters HIV susceptibility are still debated, and include a thinning of the epithelial layers of the vaginal wall, changes in the vaginal microbiota, and increased expression of CCR5 on CD4 T cells (59, 60). If future studies demonstrate that CCR5-mediated signals are required for the migration of recirculating memory CD4 T cell into the FGT, increased CCR5 expression due to higher progesterone levels during the luteal phase of the menstrual cycle may explain the increased frequency of CCR7hi CD4 T cells we observed. Alternatively, the increased frequency of these cells on the surface of the FGT may result from the thinning of the vaginal epithelium and more direct access to the luminal surface. Regardless of the mechanisms involved, the observed increase in recirculating HIV target cells on the surface of the FGT associated with high blood progesterone levels should be considered when evaluating mechanisms of potential increased risk during the use of progesterone-based hormonal contraceptives.
In summary, our detection of a previously unrecognized population of recirculating CCR7hi memory CD4 T cells at the surface of the lower FGT that express HIV susceptibility markers and are permissive to infection suggests a mechanism of systemic virus dissemination that could begin with HIV infection of CCR7hi CD4 T cells in the lumen of the FGT. Taken together, these data emphasize the need to further define resident immune cell populations at the site of pathogen exposure to better inform the design of more effective biomedical intervention and vaccination strategies.
Supplementary Material
Acknowledgments
Financial support: Provided by the United States Centers for Disease Control and Prevention, funds from Emory University, and in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (KLR2TR000455 and UL1TR000454). This work was supported by K23HD078153 (L.B.H.), K23AI114407 (A.N.S.), HL122559 (J.E.K), and facilitated by the Center for AIDS Research at Emory University (P30AI050409).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention or the Department of Health and Human Services. The authors thank the study participants and colleagues who contributed to study implementation: We also acknowledge the support of the Atlanta Clinical and Translational Research Institute Clinical Research Network (Grady site), the KL2-Mentored Clinical and Translational Research Program, the Grady Infectious Diseases Program, and the Emory CFAR Clinical Core. We also thank Michelle Owen, Kelly Curtis, and Krystin Ambrose in the CDC Division of HIV/AIDS Prevention for assistance with hormone assays.
Abbreviations
- FGT
female genital tract
- TTD
terminally differentiated T cells
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.NAIDS. AIDS by the Numbers. Geneva: 2013. [Google Scholar]
- 2.Xu H, Wang X, Veazey RS. Mucosal immunology of HIV infection. Immunol Rev. 2013;254:10–33. doi: 10.1111/imr.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. The Journal of infectious diseases. 2003;187:769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 4.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, Kaul R. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. Journal of immunology. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 5.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 7.Pandrea I, Parrish NF, Raehtz K, Gaufin T, Barbian HJ, Ma D, Kristoff J, Gautam R, Zhong F, Haret-Richter GS, Trichel A, Shaw GM, Hahn BH, Apetrei C. Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites. Journal of virology. 2012;86:4158–4168. doi: 10.1128/JVI.07141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joag VR, McKinnon LR, Liu J, Kidane ST, Yudin MH, Nyanga B, Kimwaki S, Besel KE, Obila JO, Huibner S, Oyugi JO, Arthos J, Anzala O, Kimani J, Ostrowski MA, Toronto HIVRG, Kaul R. Identification of preferential CD4 T-cell targets for HIV infection in the cervix. Mucosal immunology. 2015 doi: 10.1038/mi.2015.28. [DOI] [PubMed] [Google Scholar]
- 9.Cauley LS, Lefrancois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 2013;6:14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends in immunology. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iijima N, Iwasaki A. Tissue instruction for migration and retention of TRM cells. Trends in immunology. 2015;36:556–564. doi: 10.1016/j.it.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath CN, Shaler CR, Jeyanathan M, Zganiacz A, Xing Z. Mechanisms of delayed anti-tuberculosis protection in the lung of parenteral BCG-vaccinated hosts: a critical role of airway luminal T cells. Mucosal immunology. 2012;5:420–431. doi: 10.1038/mi.2012.19. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys. Journal of virology. 2011;85:11007–11015. doi: 10.1128/JVI.05346-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slutter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity. 2013;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. The Journal of clinical investigation. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. Journal of immunology. 2015;195:203–209. doi: 10.4049/jimmunol.1402975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. Journal of immunology. 2013;190:970–976. doi: 10.4049/jimmunol.1202805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, Clark RA. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Science translational medicine. 2015;7:279ra239. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemoto Y, Kanai T, Shinohara T, Ito T, Nakamura T, Okamoto R, Tsuchiya K, Lipp M, Eishi Y, Watanabe M. Luminal CD4(+) T cells penetrate gut epithelial monolayers and egress from lamina propria to blood circulation. Gastroenterology. 2011;141:2130–2139. e2111. doi: 10.1053/j.gastro.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nature immunology. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 24.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature immunology. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smedley J, Turkbey B, Bernardo ML, Del Prete GQ, Estes JD, Griffiths GL, Kobayashi H, Choyke PL, Lifson JD, Keele BF. Tracking the luminal exposure and lymphatic drainage pathways of intravaginal and intrarectal inocula used in nonhuman primate models of HIV transmission. PLoS One. 2014;9:e92830. doi: 10.1371/journal.pone.0092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, Rothwangl KB, Veazey RS, Hope TJ. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS pathogens. 2014;10:e1004440. doi: 10.1371/journal.ppat.1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinnon LR, Hughes SM, De Rosa SC, Martinson JA, Plants J, Brady KE, Gumbi PP, Adams DJ, Vojtech L, Galloway CG, Fialkow M, Lentz G, Gao D, Shu Z, Nyanga B, Izulla P, Kimani J, Kimwaki S, Bere A, Moodie Z, Landay AL, Passmore JA, Kaul R, Novak RM, McElrath MJ, Hladik F. Optimizing viable leukocyte sampling from the female genital tract for clinical trials: an international multi-site study. PLoS One. 2014;9:e85675. doi: 10.1371/journal.pone.0085675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nature reviews Immunology. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women’s risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15:181–189. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascola JR, Louder MK, Winter C, Prabhakara R, De Rosa SC, Douek DC, Hill BJ, Gabuzda D, Roederer M. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. Journal of virology. 2002;76:4810–4821. doi: 10.1128/JVI.76.10.4810-4821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, Brameier M, Walter L, Rogers K, Mayne AE, Dunbar P, Villinger T, Little D, Parslow TG, Santangelo PJ, Villinger F, Fauci AS, Ansari AA. Targeting alpha4beta7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat Med. 2014;20:1397–1400. doi: 10.1038/nm.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nature immunology. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR. Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal immunology. 2014;7:1375–1385. doi: 10.1038/mi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. Journal of immunology. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 38.Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 40.Chattopadhyay PK, Roederer M. Good cell, bad cell: flow cytometry reveals T-cell subsets important in HIV disease. Cytometry A. 2010;77:614–622. doi: 10.1002/cyto.a.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nie C, Sato K, Misawa N, Kitayama H, Fujino H, Hiramatsu H, Heike T, Nakahata T, Tanaka Y, Ito M, Koyanagi Y. Selective infection of CD4+ effector memory T lymphocytes leads to preferential depletion of memory T lymphocytes in R5 HIV-1-infected humanized NOD/SCID/IL-2Rgammanull mice. Virology. 2009;394:64–72. doi: 10.1016/j.virol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Vassena L, Giuliani E, Koppensteiner H, Bolduan S, Schindler M, Doria M. HIV-1 Nef and Vpu Interfere with L-Selectin (CD62L) Cell Surface Expression To Inhibit Adhesion and Signaling in Infected CD4+ T Lymphocytes. Journal of virology. 2015;89:5687–5700. doi: 10.1128/JVI.00611-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kersh EN, Henning T, Vishwanathan SA, Morris M, Butler K, Adams DR, Guenthner P, Srinivasan P, Smith J, Radzio J, Garcia-Lerma JG, Dobard C, Heneine W, McNicholl J. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol. 2014 doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. Aids. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheth AN, Evans-Strickfaden T, Haaland R, Martin A, Gatcliffe C, Adesoye A, Omondi MW, Lupo LD, Danavall D, Easley K, Chen CY, Pau CP, Hart C, Ofotokun I. HIV-1 Genital Shedding is Suppressed in the Setting of High Genital Antiretroviral Drug Concentrations Throughout the Menstrual Cycle. The Journal of infectious diseases. 2014;210:736–744. doi: 10.1093/infdis/jiu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. Aids. 2014;28:1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, Manigart O, Mulenga J, Keele BF, Shaw GM, Hahn BH, Allen SA, Derdeyn CA, Hunter E. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS pathogens. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nature reviews Immunology. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 50.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nature immunology. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 51.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nature immunology. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Frontiers in immunology. 2014;5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. Aids. 2014;28:2175–2187. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganor Y, Zhou Z, Bodo J, Tudor D, Leibowitch J, Mathez D, Schmitt A, Vacher-Lavenu MC, Revol M, Bomsel M. The adult penile urethra is a novel entry site for HIV-1 that preferentially targets resident urethral macrophages. Mucosal immunology. 2013;6:776–786. doi: 10.1038/mi.2012.116. [DOI] [PubMed] [Google Scholar]
- 57.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. The Journal of experimental medicine. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deruaz M, Luster AD. Chemokine-mediated immune responses in the female genital tract mucosa. Immunol Cell Biol. 2015;93:347–354. doi: 10.1038/icb.2015.20. [DOI] [PubMed] [Google Scholar]
- 59.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocrine reviews. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prakash M, Kapembwa MS, Gotch F, Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. Journal of reproductive immunology. 2002;54:117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.