Abstract
Previous research has connected a specific pattern of beta oscillatory activity to proper motor execution, but no study to date has directly examined how resting beta levels affect motor-related beta oscillatory activity in the motor cortex. Understanding this relationship is imperative to determining the basic mechanisms of motor control, as well as the impact of pathological beta oscillations on movement execution. In the current study, we used magnetoencephalography (MEG) and a complex movement paradigm to quantify resting beta activity and movement-related beta oscillations in the context of healthy aging. We chose healthy aging as a model because preliminary evidence suggests that beta activity is elevated in older adults, and thus by examining older and younger adults we were able to naturally vary resting beta levels. To this end, healthy younger and older participants were recorded during motor performance and at rest. Using beamforming, we imaged the peri-movement beta event-related desynchronization (ERD) and extracted virtual sensors from the peak voxels, which enabled absolute and relative beta power to be assessed. Interestingly, absolute beta power during the pre-movement baseline was much stronger in older relative to younger adults, and older adults also exhibited proportionally large beta desynchronization (ERD) responses during motor planning and execution compared to younger adults. Crucially, we found a significant relationship between spontaneous (resting) beta power and beta ERD magnitude in both primary motor cortices, above and beyond the effects of age. A similar link was found between beta ERD magnitude and movement duration. These findings suggest a direct linkage between beta reduction during movement and spontaneous activity in the motor cortex, such that as spontaneous beta power increases, a greater reduction in beta activity is required to execute movement. We propose that, on an individual level, the primary motor cortices have an absolute threshold of beta power that must be reached in order to move, and that an inability to suppress beta power to this threshold results in an increase in movement duration.
Keywords: MEG, precentral gyrus, oscillations, motor network, motor control
1. Introduction
Proper movement execution is served by a specific pattern of oscillatory activity throughout the sensorimotor network. Basically, prior to movement, there is a strong event-related desynchronization (ERD) in the beta (14–30 Hz) band, commonly termed the peri-movement beta ERD, which is sustained throughout movement and dissipates shortly after movement termination (Cheyne et al., 2006; Gaetz et al., 2010; Heinrichs-Graham and Wilson, 2015; Heinrichs-Graham et al., 2014b; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Wilson et al., 2014; 2010; 2011b). After this beta ERD, there is a robust resynchronization in the beta band, called the post-movement beta rebound (PMBR), that overshoots baseline levels and is sustained for about 2.0 s after movement termination (Cheyne et al., 2006; Gaetz et al., 2010; Heinrichs-Graham et al., 2014b; Jurkiewicz et al., 2006; Ohara et al., 2000; Parkes et al., 2006; Szurhaj et al., 2003; Wilson et al., 2010; 2011b). The peri-movement beta ERD has been associated with movement planning and execution, while the PMBR is thought to be related to movement termination and inhibition processes (for a review, see (Cheyne, 2013)). Finally, there is a strong, transient gamma (60–90 Hz) synchronization that occurs shortly after movement onset and lasts about 50 to 250 ms (Cheyne et al., 2008; Dalal et al., 2008; Hall et al., 2011; Muthukumaraswamy, 2010; 2013; Wilson et al., 2010). These responses are at least somewhat spatially distinct, and are most often localized to the precentral and postcentral gyri bilaterally (stronger contralateral to movement), premotor cortices, parietal cortices, and supplementary motor area (Cheyne et al., 2006; 2008; Gaetz et al., 2010; Heinrichs-Graham and Wilson, 2015; Heinrichs-Graham et al., 2014b; Jurkiewicz et al., 2006; Muthukumaraswamy, 2010; Parkes et al., 2006; Wilson et al., 2010; 2011b).
Understanding the unique pattern of beta activity prior to, during, and after movement is a fundamental step in determining the basic mechanisms of motor control in humans. Further, a multitude of studies examining the neurophysiology of movement disorders, such as Parkinson’s disease (Brown, 2007; Cassidy et al., 2002; Heinrichs-Graham et al., 2014a; Heinrichs-Graham et al., 2014b; Little and Brown, 2014; Pollok et al., 2012; Weinberger et al., 2006), cerebral palsy (Kurz et al., 2014), Tourette syndrome (Franzkowiak et al., 2010; Niccolai et al., 2015; Tinaz et al., 2014), dystonia (Hinkley et al., 2012), and stroke (Rossiter et al., 2014a; Shiner et al., 2015; Wilson et al., 2011a) have shown aberrant sensorimotor beta power at rest and/or during movement. These beta aberrations are often correlated with symptom severity, which suggests that the degree of motor impairment is closely tied to beta activity in the motor cortices. Importantly, movement-related beta oscillatory activity is almost always expressed as a percent power change relative to the baseline, which is generally defined as a 0.5 to 1.0 s period of time that occurs 1.5 to 3.0 s prior to movement onset. Given this practice, abnormal resting beta activity would directly affect motor-related beta activity, as the baseline or “starting place” would be altered. This biasing could potentially mask beta ERD effects related to the baseline, depending on whether the amplitude of the beta ERD or the absolute beta level is the most critical parameter for motor performance. In short, the source of beta aberrations is not entirely clear and no study to date has directly investigated the relationship between resting and movement-related beta activity, in health or disease. Such data is imperative to understanding the basic mechanisms that serve motor control, as well as the impact of pathological beta oscillations on proper movement execution.
Recent work from our lab provides intriguing evidence as to the relationship between resting and task-related motor beta oscillations (Wilson et al., 2014). Briefly, we examined how circadian rhythms (e.g., time of day effects) affected movement-related beta oscillatory activity. Four participants were recorded at rest and during performance of a simple right-hand finger tapping task in the morning, midday, and afternoon for three consecutive days. We found that the amplitude of the peri-movement beta ERD significantly increased as a function of time of day in a number of motor regions, including the primary motor cortices bilaterally, left premotor cortex, and left supplementary area (Wilson et al., 2014). Resting beta levels also increased in these same brain regions as a function of time of day. Interestingly, the PMBR only increased in the SMA as a function of time of day; there were no time of day differences in PMBR amplitude in any other brain region. While this study had a relatively small sample size, it suggests, at least tentatively, that the beta ERD response is closely related to the local level of resting beta activity; essentially, as resting beta levels go up, beta ERD amplitude also goes up. An indirectly related MEG study from Rossiter and colleagues (Rossiter et al., 2014b) examined the effects of age on sensorimotor cortical beta rhythms during rest and a controlled-force grip task. They found a significant positive relationship between age and resting beta amplitude in the left primary motor cortex, such that the older the participant, the more elevated the resting beta amplitude. Further, they found a significant correlation between age and peri-movement beta ERD amplitude during movement execution in the ipsilateral primary motor cortex, with increased age being associated with greater beta suppression (i.e., stronger decrease relative to baseline). While the relationship between resting and movement-related beta activity was not directly probed in this study, the pattern of results again suggests that the beta ERD during movement is closely tied to the spontaneous beta level in the sensorimotor cortices, and also provides new evidence that resting and movement-related beta levels may be modulated by healthy aging. As such, it appears that healthy aging may be a useful, naturalistic model by which to study the association between resting beta activity and movement-related beta oscillatory activity.
In the current study, we used a complex motor sequence paradigm to study the relationship between spontaneous beta activity and movement-related beta oscillations in the context of healthy aging. The goal of this study was two-fold. First, using healthy aging as a model, we aimed to identify whether spontaneous (i.e., resting) beta activity in the motor cortex modulates movement-related beta oscillatory activity. Secondly, we sought to evaluate the effects of healthy aging on motor performance and peri-movement beta oscillatory activity. To this end, we utilized high-density MEG to record healthy younger and older participants at rest and while they performed sequences of finger movements. The peri-movement beta ERD response was imaged using beamforming, and the effects of aging on absolute and relative beta power were assessed. We hypothesized that beta ERD dynamics would be tightly yoked to spontaneous beta power. Further, we hypothesized that healthy older adults would have elevated beta power at rest, and as such would have an elevated beta ERD response during movement.
2. Methods
2.1 Subject Selection
We studied 16 healthy younger males (mean age: 28.31 (SD: 5.44) years) and 17 healthy older males (mean age: 65.41 (SD: 7.09) years), all of whom were recruited from the local community. We focused on males in this study due to several recent reports of sex differences in the aging brain (Scheinost et al., 2015; Shaw et al., 2016). Exclusionary criteria included any medical illness affecting CNS function, neurological or psychiatric disorder, history of head trauma, current substance abuse, and the MEG Laboratory’s standard exclusion criteria (e.g., dental braces, metal implants, battery operated implants, and/or any type of ferromagnetic implanted material). After complete description of the study was given to participants, written informed consent was obtained following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, which approved the study protocol.
2.2 Experimental Paradigm and Stimuli
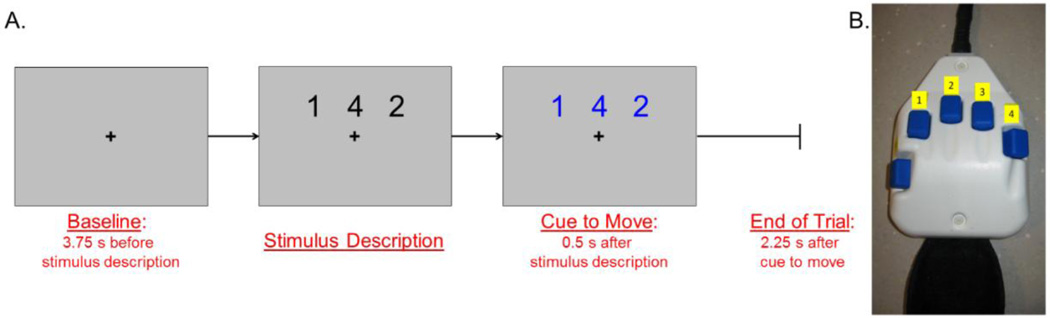
During MEG recording, participants were seated in a nonmagnetic chair within the magnetically-shielded room. Each participant rested their right hand on a custom-made five-finger button pad (see Figure 1b) while fixating on a crosshair presented centrally. This response pad was connected such that each button sent a unique signal (i.e., TTL pulse/trigger code) to the MEG system acquisition computer, and thus behavioral responses were temporally synced with the MEG data. This allowed accuracy, reaction times, and movement durations (in ms) to be computed offline. In order to create a sufficient baseline, participants initially fixated on a crosshair for 3.75 s before the beginning of each trial (Figure 1a). After this baseline period, a series of three numbers, each corresponding to a finger on the hand (Figure 1b), was presented on the screen in black for 0.5 s. After 0.5 s, the numbers changed color, signaling the participant to tap the fingers corresponding to the motor plan sequentially. The participant was given 2.25 s to complete the motor plan and return to rest. Then, the numbers disappeared and only the fixation crosshair remained. This series of slides constituted one trial; Figure 1a depicts the total time course of a single trial. A total of 160 trials were completed for this task. Participants also completed a six minute block of eyes-closed rest during each MEG session. Total MEG recording time was ~22 minutes per session (including both tasks).
Figure 1. Motor task paradigm.
A) Participants fixated on a crosshair for 3.75 s. After this baseline period, a series of three numbers (each corresponding to a digit on the finger) appeared on the screen in black and after 0.5 s the numbers changed color cueing the participant to move. The participant then had 2.25 s to complete the motor plan and return to rest. B) The button pad used during this task. Each button on the pad corresponded to a specific finger; the thumb was not used for task performance.
2.3 MEG Data Acquisition & Coregistration with Structural MRI
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (v2.2; Elekta), MEG data from each subject were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006; Taulu et al., 2005). For motion correction, the position of the head throughout the recording was aligned to the individual’s head position when the recording was initiated. Each participant’s MEG data were coregistered with high-resolution structural T1-weighted MRI data, prior to the application of source space analyses (i.e., beamforming), using BESA MRI (Version 2.0). These anatomic images were acquired with a Philips Achieva 3T X-series scanner using an eight-channel head coil and a 3D fast field echo sequence with the following parameters: TR: 8.09 ms; TE: 3.7 ms; field of view: 24 cm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm; sense factor: 1.5. The structural volumes were aligned parallel to the anterior and posterior commissures and transformed into standardized space. After beamformer analysis, each subject’s functional images were also transformed into standardized space using the transform applied to the structural MRI volume and spatially resampled.
2.4 MEG Preprocessing, Time-Frequency Transformation, & Sensor-Level Statistics
Cardio-artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 5.8 s duration, with 0.0 s defined as movement onset (i.e., first button press) and the baseline defined as the −1.8 to −1.2 s time window (i.e., before movement onset; Figure 1a). Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. Artifact-free epochs were then transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms; (Papp and Ktonas, 1977)) and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized by dividing the power value of each time-frequency bin by the respective bin’s baseline power, which was calculated as the mean power during the −1.8 to −1.2 s time period.
The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, time-frequency windows that corresponded to events of a priori interest (e.g., the peri-movement beta ERD) and contained a statistically significant oscillatory event across all participants were subjected to the beamforming analysis.
2.5 MEG Imaging & Virtual Sensor Extraction
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001; Van Veen et al., 1997), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images were derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged pre-stimulus noise period of equal duration and bandwidth (Hillebrand et al., 2005). MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.0) software. Normalized source power was computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Beamformer images per time-frequency window of interest were then averaged across all participants, and coordinates corresponding to the peak responses were identified. We then extracted virtual sensors for the peak voxel of these responses, which corresponded to the left and right precentral gyri. To create the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded two time series for each coordinate in source space and we used the time series with the maximal response for our analyses (Gross et al., 2001). Note that this virtual sensor extraction was done per participant, once the coordinates of interest (i.e., one per cluster) were known. Virtual sensors were extracted from the eyes-closed rest data using the same coordinates and computational method. Once these virtual sensors were extracted, absolute beta activity values during rest, and absolute and relative beta activity values during movement were computed and subjected to statistical analyses.
3. Results
3.1 Behavioral Results
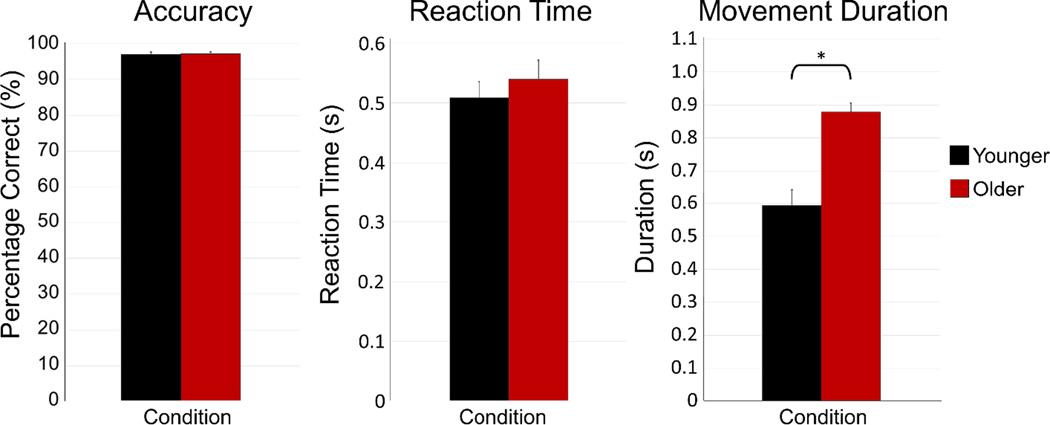
All participants were able to successfully complete the motor task, with an average accuracy of over 96% across both groups. There was no significant difference in accuracy between older and younger participants, t(31) = 0.237, p = .814, nor was there a difference in reaction time (i.e., time between the cue to move and first button press) between groups, t(31) = 0.712, p = .482. However, there was a difference in movement duration (i.e., how long it took to complete the tapping sequence) between groups, t(31) = 4.945, p < .001, with older participants executing sequences significantly slower than younger participants. Mean movement duration for the younger and older groups were 593.00 (SD: 207.72) ms and 878.97 (SD: 113.81) ms, respectively. Behavioral results are shown in Figure 2. To control for behavioral differences between groups, MEG data analyses were synced with movement onset in each trial and all analyses were focused on the time window preceding and during the early stages of movement execution (i.e., before movement termination in either group). Only correct trials were used for analysis. Following artifact rejection, the average number of trials used in the analysis was 131.44 (SD: 5.97) for younger adults and 130.24 (SD: 5.91) for older adults. This difference in number of trials used per group was not significant, t(31) = 0.581, p = .565.
Figure 2. Motor task behavioral results.
There were no group differences in accuracy (percent correct) or reaction time (in s) between age groups (both p’s > 0.48). However, there was a significant difference in movement duration (in s), with older participants taking significantly longer to complete sequences than younger participants (p < .001). See legend for color descriptions. Error bars denote the standard error of the mean (SEM). * = p < 0.05
3.2 MEG Sensor-level Results
Sensor level spectrograms were statistically examined using nonparametric permutation testing to derive the precise time-frequency bins for follow up beamforming analyses. The results showed significant (p < 0.001; corrected) beta ERD in a subset of gradiometers near the left and right sensorimotor cortices in each group, which extended from 16–26 Hz from approximately 0.5 s before movement onset until about 0.3 s after movement offset (0.0 s = movement onset). A significant alpha (8–12 Hz) ERD response was also found during this time period. In addition, a significant resynchronization (i.e., PMBR) in the same 16–26 Hz band was detected during the 0.8 to 1.4 s time window in roughly the same set of gradiometers near the left sensorimotor cortices (p < 0.001; corrected). These neural responses correspond closely to the peri-movement beta ERD, alpha ERD, and PMBR responses identified in many previous studies.
The time window corresponding to the maximum beta ERD response (−0.2 to 0.4 s, 0.0 s = movement onset) was imaged, using beamforming and a window of equal bandwidth and duration from the baseline period, to derive the spatial location of significant beta ERD activity for subsequent virtual-sensor analysis. The time window corresponding to the maximum alpha ERD response (−0.2 to 0.4 s, 0.0 s = movement onset) was also imaged to derive the spatial location of significant alpha ERD activity for virtual sensor analysis. Of note, we did not directly probe the PMBR response because it is tightly yoked to the termination of movement. Since there were significant differences in movement duration between groups, any potential alteration in the PMBR response would be confounded by performance differences. Thus, any neurophysiological changes in the PMBR would not be interpretable.
3.3 Virtual Sensor Results
3.3.1 Beta activity during movement
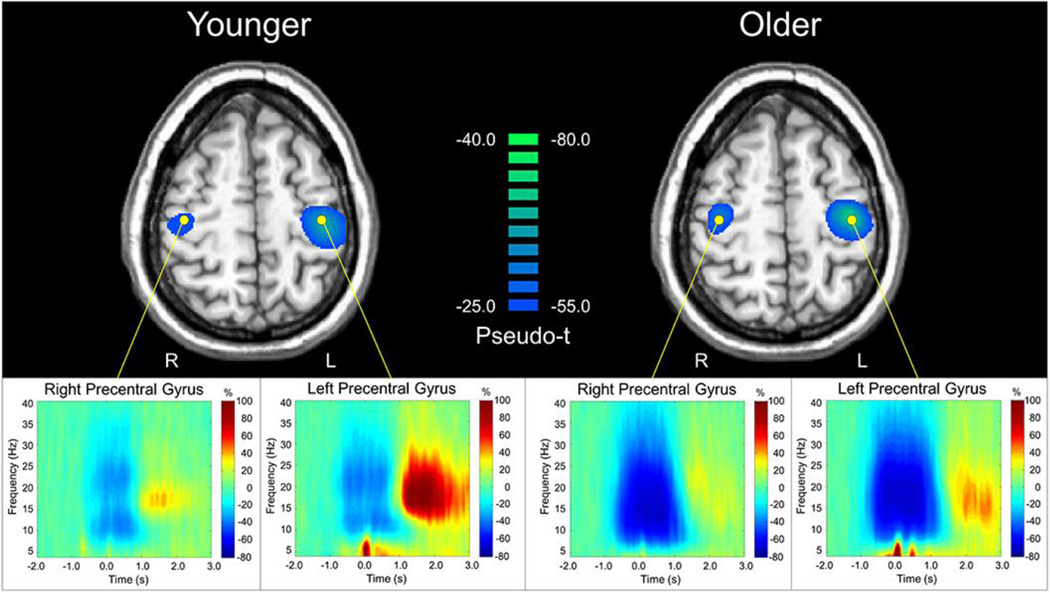
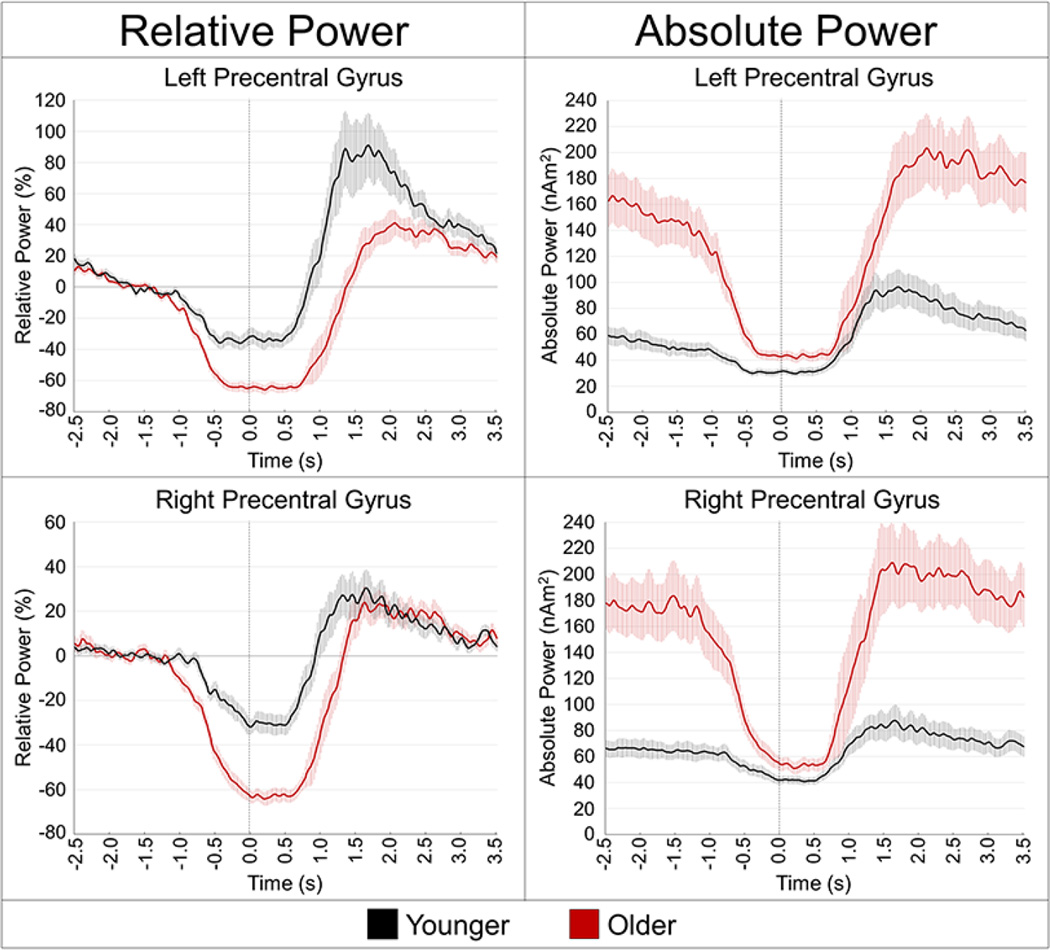
The peak peri-movement beta ERD locations were within the left and right precentral gyri, and are shown with group-averaged virtual sensor spectrograms in Figure 3. Group differences in peri-movement beta ERD power were assessed in the left and right precentral gyri individually, using the relative mean power during the −0.2 to 0.4 s time period, expressed as a percentage of the baseline, and the absolute beta level. The relative mean analyses showed significantly stronger beta ERD power in older adults compared to younger adults for the left, t(31) = 5.183, p < .001 and right precentral gyri, t(31) = 6.111, p < .001. During this time period, the older group showed greater beta ERD power, almost double on average, compared to the younger group. The results were similar for absolute beta power during this period, as older participants had greater absolute beta power in the left precentral gyrus, t(31) = 2.657, p = .012, and marginally greater absolute beta in the right precentral gyrus, t(31) = 1.895, p = .067. The time courses of relative and absolute beta power during movement are shown in Figure 4.
Figure 3. Identification of peri-movement beta ERD and peak voxel extraction.
Group mean beamformer images (pseudo-t; see color bar) of beta activity prior to and during movement (−0.2 to 0.4 s, 16–26 Hz) for each age group are shown in the top panel, with the peak voxel locations used for the virtual sensor analysis identified with a yellow dot. Note that a different pseudo-t scale is used in each group. Peak voxel time-frequency spectrograms are shown on the bottom panel. Time (in s) is denoted on the x-axis, with 0.0 s defined as movement onset. Frequency (in Hz) is shown on the y-axis. All signal power data (bottom panel) is expressed as the percent difference from baseline, with the color legend shown to the right.
Figure 4. Absolute and relative temporal evolution of the beta ERD response.
Voxel time series were extracted from the peak voxels of the left precentral gyrus (top panel) and right precentral gyrus (bottom panel) to more precisely examine the dynamics of the beta ERD in younger (black line) and older adults (red line). Time (in s, movement onset = 0.0 s) is denoted on the x-axis, while power is shown on the y-axis. The left panel shows each response as percentage relative to baseline (−1.8 to −1.2 s), while the right panel shows the absolute power (in nAm2). Note that older participants had much higher beta levels during the baseline period, but beta levels were reduced in the aging cortex to levels near that of younger participants just before movement onset. The shaded area around each line denotes the standard error of the mean (SEM).
3.3.2 Resting beta power & pre-movement baseline beta activity
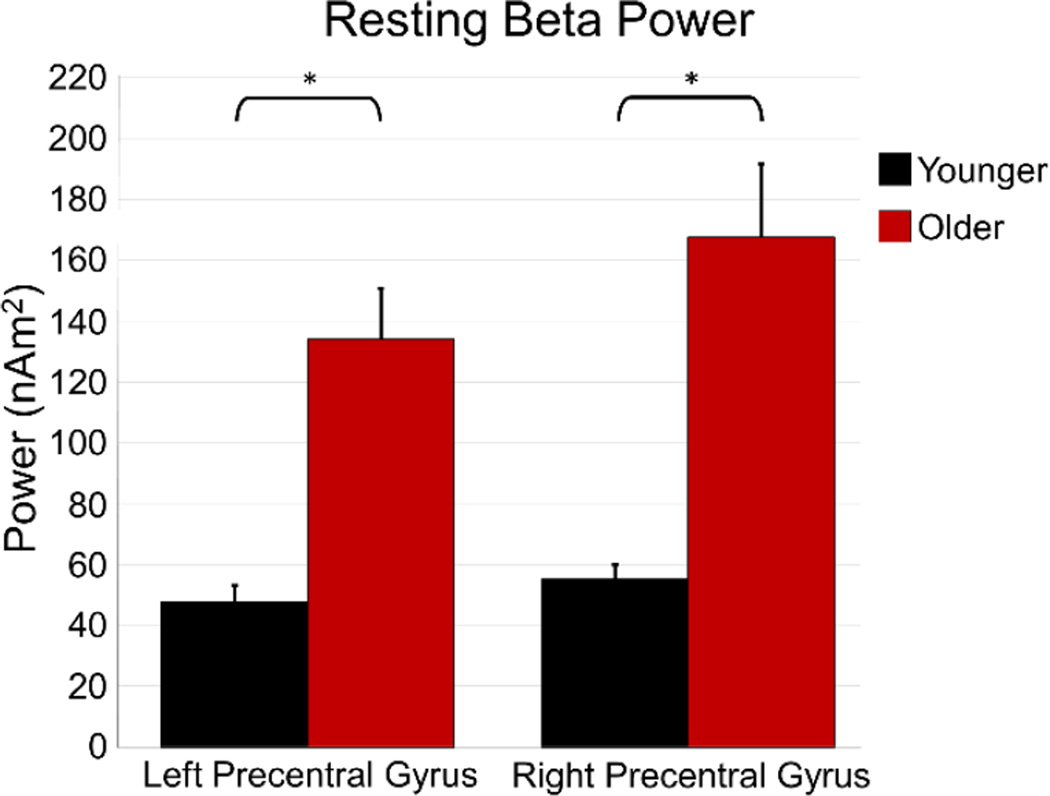
Analysis of beta power during rest also showed a significant effect of age, with older participants having higher beta power in the left primary motor cortex, t(31) = 4.674, p < .001, as well the right primary motor cortex, t(31) = 4.228, p < .001 compared to younger participants (Figure 5). In addition, we computed absolute beta power levels during the baseline period of the motor task (−1.8 to −1.2 s before movement onset) and found the same pattern of results in both the left precentral gyrus, t(31) = 5.261, p < .001, and right precentral gyrus, t(31) = 4.675, p < .001 (Figure 4).
Figure 5. Resting beta power in the motor cortices.
Resting beta power during eyes-closed rest was extracted from the voxels of the left and right precentral gyri that were identified in the motor task. Average power (in nAm2) is shown on the y-axis, while region is identified on the x-axis. The younger group is shown in black, while the older group is shown in red. As shown, resting beta activity was much stronger in older participants. Error bars denote SEM. * = p < 0.001
In order to investigate whether baseline levels during the movement task were artificially high due to unequal contamination by the PMBR in either hemisphere, we compared baseline beta power with resting beta power using a mixed-model ANOVA, with task as a within-subjects factor and group as a between-subjects factor. In the left precentral gyrus, there was a significant main effect of group, F(1,31) = 25.559, p < .001. The main effect of task was not significant, F(1,31) = 0.936, p = .341, nor was the group-by-task interaction, F(2,31) = 0.498, p = .486. In the right precentral gyrus, there was also a significant main effect of group, F(1,31) = 19.277, p < .001, but again no significant main effect of task, F(1, 31) = 2.332, p = .137, or group-by-task interaction, F(2, 31) = 0.040, p = .842. In sum, beta power at rest and baseline beta power during the movement task were not significantly different in either brain region; thus, we are confident that differences in baseline power between groups during the movement task were not due to contamination by the PMBR.
3.3.3 Alpha activity during movement and rest
In order to determine whether differences extended beyond the beta frequency, exploratory analyses were performed on alpha activity. Group differences in peri-movement alpha power were assessed in the left and right precentral gyri individually in the same way as described above. The relative mean analyses showed significantly higher alpha ERD power in older adults compared to younger adults for the left, t(31) = 3.140, p = .003 and right precentral gyri, t(31) = 4.143, p < .001. However, there were no significant differences between groups in absolute alpha power during rest or movement (all p’s < .10), although visual inspection of the alpha virtual sensors showed a somewhat similar pattern as that found in the beta frequency. These time courses are shown in Supplemental Figure S1.
3.3.4 Correlations between measures
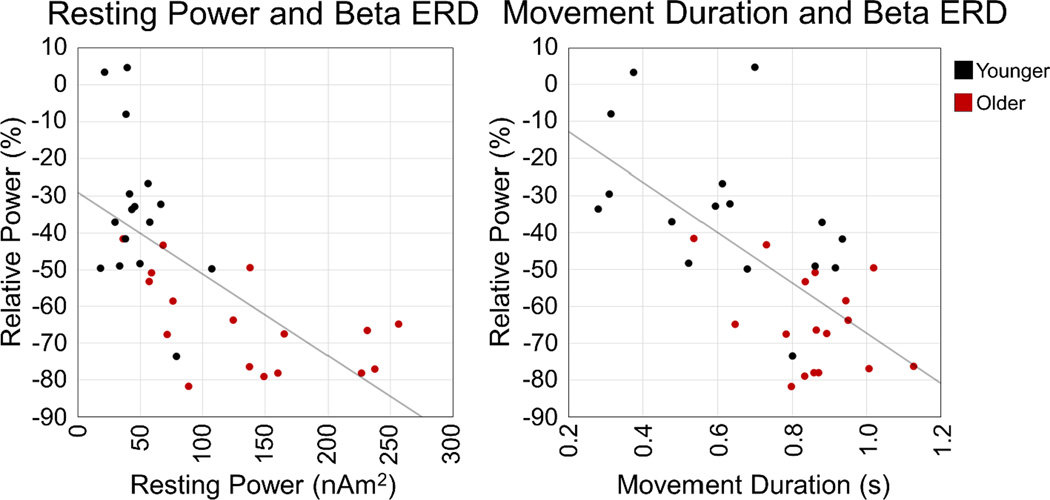
Correcting for age, there was a significant correlation between resting beta power and relative beta ERD power in the left precentral gyrus, r(30) = −.404, p = .022, as well as the right precentral gyrus, r(30) = −.539, p = .001, such that the greater the resting beta power, the stronger (i.e., more negative) the relative beta ERD. There was also a significant correlation between movement duration and beta ERD power in the left precentral gyrus after controlling for age, r(30) = −.361, p = .043. Basically, the greater the beta ERD power (i.e., more negative relative to baseline) contralateral to movement, the longer the movement. No other correlations were significant. These results are shown in Figure 6.
Figure 6. Correlations with beta ERD power.
On the left panel, the left precentral gyrus beta ERD power (% relative to baseline) is shown on the y-axis, with the region’s resting beta power (in nAm2) on the x-axis. There was a significant correlation between beta ERD power and resting beta power in both the left and right precentral gyri (not shown), such that the greater the resting power, the greater the beta ERD power relative to baseline, controlling for age. On the right panel, left precentral gyrus beta ERD power is denoted on the y-axis, while movement duration (in s) is shown on the x-axis. There was also a significant relationship between beta ERD power and movement duration in the left precentral gyrus, such that the larger the beta ERD power, the longer the movement duration, controlling for age. Younger adults are shown in black, while older adults are shown in red.
4. Discussion
In the current study, we investigated the relationship between peri-movement beta ERD activity and motor cortical resting beta levels in the context of healthy aging. Consistent with prior literature, we found increased resting beta power, as well as increased peri-movement beta ERD in the primary motor cortices bilaterally in older adults compared to younger adults. Interestingly, a comparison of absolute beta levels during the peri-movement beta ERD period showed that, despite a much larger reduction in beta power relative to baseline levels, older adults still had significantly higher absolute beta power during this time period than younger adults in the left precentral gyrus, and marginally in the right precentral gyrus. Furthermore, we found a significant correlation between resting beta power and beta ERD power in both the left and right precentral gyri, as well as beta ERD power in the left precentral gyrus and movement duration, above and beyond the effects of age. Below we discuss the implications of this work in understanding the functionality of beta oscillations in the motor cortex.
Older participants exhibited significantly stronger spontaneous (resting) beta power in the primary motor cortices. Elevated activity in these regions has been previously shown using fMRI (for a review, see (Li et al., 2015)), and thus our study adds further support and provides an important cross-modal link. Interestingly, older adults also had stronger beta desynchronizations (relative response) during movement compared to younger adults, although absolute beta power during movement was still significantly higher in these older participants. This pattern of results suggests not only that healthy aging results in an increase in spontaneous sensorimotor beta activity, but also that there is a direct link between spontaneous beta oscillations and peri-movement beta ERD levels in these regions. Rossiter and colleagues (2014b) found a correlation between age and peri-movement beta ERD, as well as resting beta levels, such that as age increased, resting beta amplitude in the contralateral (left) motor cortex increased and movement-related beta desynchronization became more negative in the ipsilateral motor cortex (relative to baseline) during isometric force. However, they did not directly analyze the relationship between these two variables. Regrettably, this study and the current study cannot be directly compared, in part because of the difference in movement parameters and the fact that age was treated as a continuous variable in the Rossiter et al. (2014b) study. Nonetheless, the current study directly compared beta activity in the motor cortices of younger and older adults, and found a strikingly similar pattern, which provides further evidence that healthy aging is related to an elevation of spontaneous beta levels in the motor cortex, as well as increased beta desynchronization during movement. The current study also augments the work of Rossiter et al. by showing that absolute beta levels during movement remain elevated in older participants, despite the significantly stronger beta ERD, and this appears to be related to parameters of the performed movement.
Interestingly, controlling for age, there was a negative relationship between peri-movement beta ERD power and movement duration, as well as a relationship between spontaneous beta power and beta ERD power, which indicates that the magnitude of the beta ERD relative to absolute baseline beta levels is important to successful movement completion. Taken together, our results suggest that the motor cortex may have an absolute beta threshold that must be reached in order to execute proper movements. Basically, the greater the spontaneous beta level in the motor cortex, the stronger the motor cortex beta suppression must be in order to reach a sufficient threshold to move. In turn, it is possible that the greater the reduction in beta activity required within the motor cortex, the less efficient the system, and consequently the longer it takes the participant to complete a movement. As mentioned in the introduction, our lab recently probed the effects of circadian rhythm (i.e., time of day effects) on movement-related beta oscillatory activity and resting beta levels in the sensorimotor network, and found that the peri-movement beta ERD significantly increased as a function of time of day in a number of regions throughout the sensorimotor network, including the primary motor cortices bilaterally (Wilson et al., 2014). Resting beta levels also increased in these regions as a function of time of day, similar to the current study. Given that beta ERD power is calculated relative to baseline levels, the increase in peri-movement beta desynchronization found in the Wilson et al. (2014) study could reflect the motor cortex returning to the same (absolute) beta level during movement execution, as resting beta was increasing across the day. This same hypothesis also applies to the significant differences in movement duration in the current study, as older adults had significantly higher absolute beta power during movement compared to younger adults, despite a much larger relative beta power (ERD) decrease. It is possible that, due to the significant increase in spontaneous beta power in the motor cortex with age, beta activity may not be suppressed to the necessary threshold for movement execution in the aging cortex (as suggested by the significantly higher absolute beta levels during movement in older adults compared to younger adults), and this may contribute to older participants taking longer to perform the movement. While our findings support this intriguing hypothesis, further studies are certainly necessary to fully vet this idea.
Mechanistically, a wealth of literature points to the role of the inhibitory neurotransmitter γ-aminobutryic acid (GABA) in modulating beta and gamma oscillatory power in the motor cortices (Gaetz et al., 2011; Hall et al., 2011; Jensen et al., 2005; Muthukumaraswamy et al., 2013). In general, with increased GABA level (either endogenously, or through pharmacological manipulation), studies show an increase in overall beta power in the sensorimotor cortices, as well as increases in motor-related oscillatory activity, although there is disagreement on whether the PMBR is also affected (Gaetz et al., 2011; Hall et al., 2011; Muthukumaraswamy et al., 2013). For example, Muthukumaraswamy et al. (2013) utilized MEG and a blinded, placebo-controlled, crossover design to study the effects of tiagabine, a GABA reuptake inhibitor, on movement-related oscillatory activity. They found that increased GABA concentration led to increased resting beta power, as well as increased beta desynchronization during movement (Muthukumaraswamy et al., 2013). A similar increase in spontaneous beta power, as well as enhanced peri-movement beta ERD (i.e., more negative relative to baseline), was also shown after administration of the GABA agonist diazepam in an earlier study (Hall et al., 2011). Though we were unable to directly determine the role of GABA in our results, the pattern exhibited in our older adults compared to younger adults resembled that of the drug relative to the placebo condition in the aforementioned studies, such that older adults had significantly greater resting beta power, as well as increased beta ERD power during movement compared to younger adults. Thus, it is intuitive that the increase in baseline beta activity and peri-movement beta ERD power are a result of increased GABA in older adults. However, a close but alternative interpretation that aligns with the current study, as well as prior work, is that the relative change in peri-movement beta ERD power (i.e., more negative) is a result of the elevation in baseline beta activity, and not directly due to differences in GABA itself. In other words, we suggest that resting beta activity in the motor cortices is related to GABA concentration, but that elevated beta ERD power is simply a byproduct, as the stronger resting beta activity must be suppressed further to reach the absolute threshold needed to execute a movement. Importantly, this interpretation is somewhat speculative and future work should directly compare the relationship between sensorimotor beta power during rest and movement-related beta power under differing GABA concentration levels to more fully address this hypothesis.
In conclusion, this study was the first to identify significant differences in motor-related beta oscillations between healthy younger and older adults. We found significantly higher spontaneous beta power, as well as significantly larger beta ERD (i.e., more negative relative to baseline) responses in the primary motor cortices of older adults compared to younger adults. However, despite having a much larger beta ERD relative to baseline, absolute beta power during movement was still significantly higher in older adults compared to younger adults. We also found a significant relationship between spontaneous beta power and relative beta ERD power, as well as relative beta ERD power and movement duration, above and beyond the effects of age. These findings suggest a direct relationship between beta reduction during movement and spontaneous activity in the motor cortex, such that as spontaneous beta power increases, there must be a greater reduction of beta power in the motor cortex in order to properly move. We propose that, on an individual level, the primary motor cortices may have an absolute threshold of beta power that must be reached in order to adequately execute a movement, and that an inability to reduce beta power to this threshold results in an increase in movement duration. Recent MEG studies of movement disorders such as cerebral palsy (Kurz et al., 2014), Parkinson’s disease (Heinrichs-Graham et al., 2014b; Pollok et al., 2012), and stroke (Rossiter et al., 2014a; Shiner et al., 2015; Wilson et al., 2011a) have demonstrated aberrant movement-related beta oscillatory activity within these populations. Future work should examine the relationship between resting beta activity and movement-related beta oscillations in the context of these movement disorders. Future studies should also probe how baseline beta activity is related to PMBR power and, as mention above, how GABA mediates these processes directly or indirectly by modulating baseline beta activity.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 MH103220 (TWW), NSF grant #1539067 (TWW), the Shoemaker Prize from the University of Nebraska Foundation (TWW), a Kinman-Oldfield Award for Neurodegenerative Research from the University of Nebraska Foundation (TWW), and a grant from the Nebraska Banker’s Association (TWW). The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, Brown P. Movement-related changes in synchronization in the human basal ganglia. Brain. 2002;125:1235–1246. doi: 10.1093/brain/awf135. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42:332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Cheyne DO. MEG studies of sensorimotor rhythms: a review. Exp Neurol. 2013;245:27–39. doi: 10.1016/j.expneurol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, Berger MS, Knight RT, Barbaro NM, Kirsch HE, Nagarajan SS. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: A basis for exact inference. Stat Sci. 2004;19:676–685. [Google Scholar]
- Franzkowiak S, Pollok B, Biermann-Ruben K, Sudmeyer M, Paszek J, Jonas M, Thomalla G, Baumer T, Orth M, Munchau A, Schnitzler A. Altered pattern of motor cortical activation-inhibition during voluntary movements in Tourette syndrome. Mov Disord. 2010;25:1960–1966. doi: 10.1002/mds.23186. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SD, Stanford IM, Yamawaki N, McAllister CJ, Ronnqvist KC, Woodhall GL, Furlong PL. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56:1506–1510. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Kurz MJ, Becker KM, Santamaria PM, Gendelman HE, Wilson TW. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson’s disease: a pharmaco-magnetoencephalography study. J Neurophysiol. 2014a;112:1739–1747. doi: 10.1152/jn.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Coding complexity in the human motor circuit. Hum Brain Mapp. 2015 doi: 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb Cortex. 2014b;24:2669–2678. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley LB, Dolberg R, Honma S, Findlay A, Byl NN, Nagarajan SS. Aberrant Oscillatory Activity during Simple Movement in Task-Specific Focal Hand Dystonia. Front Neurol. 2012;3:165. doi: 10.3389/fneur.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol. 2014;56:1072–1077. doi: 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Hou XH, Liu HH, Yue CL, Lu GM, Zuo XN. Putting age-related task activation into large-scale brain networks: A meta-analysis of 114 fMRI studies on healthy aging. Neurosci Biobehav Rev. 2015;57:156–174. doi: 10.1016/j.neubiorev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Little S, Brown P. The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat Disord 20 Suppl. 2014;1:S44–S48. doi: 10.1016/S1353-8020(13)70013-0. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JF, Wilson SJ, Nutt DJ, Lingford-Hughes A, Singh KD, Hamandi K. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage. 2013;66:36–41. doi: 10.1016/j.neuroimage.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Niccolai V, van Dijk H, Franzkowiak S, Finis J, Sudmeyer M, Jonas M, Thomalla G, Siebner HR, Muller-Vahl K, Munchau A, Schnitzler A, Biermann-Ruben K. Increased beta rhythm as an indicator of inhibitory mechanisms in tourette syndrome. Mov Disord. 2015 doi: 10.1002/mds.26454. [DOI] [PubMed] [Google Scholar]
- Ohara S, Ikeda A, Kunieda T, Yazawa S, Baba K, Nagamine T, Taki W, Hashimoto N, Mihara T, Shibasaki H. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain. 2000;123(Pt 6):1203–1215. doi: 10.1093/brain/123.6.1203. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Parkes LM, Bastiaansen MC, Norris DG. Combining EEG and fMRI to investigate the post-movement beta rebound. Neuroimage. 2006;29:685–696. doi: 10.1016/j.neuroimage.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Sudmeyer M. Motor-cortical oscillations in early stages of Parkinson’s disease. J Physiol. 2012;590:3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HE, Boudrias MH, Ward NS. Do movement-related beta oscillations change after stroke? J Neurophysiol. 2014a;112:2053–2058. doi: 10.1152/jn.00345.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage. 2014b;91:360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 2015;36:1524–1535. doi: 10.1002/hbm.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ME, Sachdev PS, Anstey KJ, Cherbuin N. Age-related cortical thinning in cognitively healthy individuals in their 60s: the PATH Through Life study. Neurobiol Aging. 2016;39:202–209. doi: 10.1016/j.neurobiolaging.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Shiner CT, Tang H, Johnson BW, McNulty PA. Cortical beta oscillations and motor thresholds differ across the spectrum of post-stroke motor impairment, a preliminary MEG and TMS study. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.037. [DOI] [PubMed] [Google Scholar]
- Szurhaj W, Derambure P, Labyt E, Cassim F, Bourriez JL, Isnard J, Guieu JD, Mauguiere F. Basic mechanisms of central rhythms reactivity to preparation and execution of a voluntary movement: a stereoelectroencephalographic study. Clin Neurophysiol. 2003;114:107–119. doi: 10.1016/s1388-2457(02)00333-4. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Trans Signal Process. 2005;53:3359–3372. [Google Scholar]
- Tinaz S, Belluscio BA, Malone P, van der Veen JW, Hallett M, Horovitz SG. Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Hum Brain Mapp. 2014;35:5834–5846. doi: 10.1002/hbm.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Fleischer A, Archer D, Hayasaka S, Sawaki L. Oscillatory MEG motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil Neural Repair. 2011a;25:188–193. doi: 10.1177/1545968310378511. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 2014;102P2:531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 2011b;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.