Abstract
Endogenous opioid signaling in ventral cortico-striatal-pallidal circuitry is implicated in elevated alcohol consumption and relapse to alcohol seeking. Mu-opioid receptor activation in the medial shell of the nucleus accumbens (NAc), a region implicated in multiple aspects of reward processing, elevates alcohol consumption while NAc opioid antagonists reduce it. However, the precise nature of the increases in alcohol consumption, and the effects of mu-opioid agonists on alcohol seeking and relapse are not clear. Here, we tested the effects of the mu-opioid agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) in rat NAc shell on lick microstructure in a free-drinking test, alcohol seeking during operant self-administration, extinction learning and expression, and cue-reinforced reinstatement of alcohol seeking. DAMGO enhanced the number, but not the size of drinking bouts. DAMGO also enhanced operant alcohol self-administration and cue-induced reinstatement, but did not affect extinction learning or elicit reinstatement in the absence of cues. Our results suggest that mu-opioid agonism in NAc shell elevates alcohol consumption, seeking and conditioned reinforcement primarily by enhancing the incentive motivational properties of alcohol and alcohol-paired cues, rather than by modulating palatability, satiety, or reinforcement.
Keywords: alcohol, nucleus accumbens, mu-opioid receptor, self-administration, reinstatement, extinction
1. Introduction
There is growing evidence that release of endogenous opioids by alcohol consumption promotes further consumption and/or relapse following abstinence. Alcohol administration changes gene expression, production and release of endogenous opioids, including pro- and met-enkephalin and/or β-endorphin (Chang et al., 2010; Lam et al., 2010; Marinelli et al., 2005; Méndez et al., 2010, 2006; Mitchell et al., 2012; Oliva et al., 2008; Olive et al., 2001). Of the four classes of the opioid receptor family (mu, delta, kappa and nociceptin), the weight of evidence indicates that mu opioid receptor (MOR; alternatively known as MOP) agonists exert the most robust effect on alcohol consumption. In humans, alcohol consumption promotes release of endogenous opioids that bind to MOR (Mitchell et al., 2012), and MOR availability correlates with alcohol craving (Heinz et al., 2005). Furthermore, in rodents MOR agonists increase alcohol but not water consumption, both systemically (Sabino et al., 2007), or following infusion directly into the nucleus accumbens (NAc) (Zhang and Kelley, 2002).
Here, we investigated which aspects of alcohol seeking and consumption are potentiated by NAc mu-opioid agonism. NAc MOR activation affects many aspects of reward-processing, producing reductions in sensory-specific satiety (Katsuura et al., 2011; Woolley et al., 2007b) and enhancements of palatability or ‘liking’ of preferred or high-value foods (Kelley et al., 2002; Taha et al., 2006; Woolley et al., 2006; Zhang et al., 1998). These effects are reported predominantly when MOR agonists are locally infused into in the NAc shell subregion (Castro and Berridge, 2014; Peciña and Berridge, 2005, 2000; Taha et al., 2009; Wassum et al., 2009). NAc opioid signaling may also affect extinction learning, which may utilize brain mechanisms involved in satiety, as both extinction and satiety involve reductions in reward seeking related to decreases in reward value (Millan et al., 2011). Alcohol-induced opioid release may also directly increase the motivation to consume alcohol (Herz, 1997) or enhance the “positively-reinforcing” effect of alcohol (Swift, 1995). Knowing whether MOR stimulation of NAc can directly enhance the palatability or motivational properties of alcohol, and/or reinforces alcohol drinking through repeated pairings is critical for understanding how opioids promote drinking. To examine these possibilities we tested the effects of NAc MOR activation on lick microstructure in a free-drinking test, in operant self-administration, in extinction learning and expression, and in a “cue-induced” reinstatement test, in rats consuming and seeking low-to-moderate amounts of alcohol.
2. Materials and Methods
2.1. Subjects
Male Long Evans rats (Harlan Laboratories; total n = 79), weighing 250–275 grams upon arrival were housed individually in a temperature-controlled colony room (21 °C) on a 12-hour reversed (lights off at 10:00 AM) or semi-reversed (lights off at 3:00 PM) light cycle, with ad libitum access to both food and water. All behavioral experiments were performed during the first four hours of the dark portion of the cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for Care and Use of Laboratory Animals (NIH).
2.2. Research Design
At 7 days after arrival, all rats were first habituated to the taste and pharmacological effects of ethanol by giving them 24-hr continuous access to 10% ethanol (v/v) in the home cage for 8 weeks. Body weights and liquid volumes consumed were recorded Monday, Wednesday and Friday, and the average g/kg/day was calculated. Mean +/− SEM ethanol intake over the final two weeks of pre-exposure was calculated at 2.24 +/− .16 g/kg. A small number of rats (n=3) that did not reliably consume greater than 0.2 g/kg/day during the final two weeks of pre-exposure were excluded from further testing. Following pre-exposure rats were designated for testing in free drinking (n=29), operant self-administration (n=11), extinction learning (n=18), extinction retention (n=12) or cue-induced reinstatement (n=21). Rats underwent a maximum of five microinjection test days. Where possible, given this constraint (as well at cannulae patency), rats were tested in two adjacent experimental phases to compare the effects of MOR agonism on different measures of alcohol reward. At least half of the rats included in each experimental phase were naïve to DAMGO microinjection prior to that phase, and rats that received DAMGO during any tests of extinction learning or retention were not tested in any subsequent testing phase (see Table 1 for final rat allocation). We observed no significant differences between naïve rats and those exposed to DAMGO during a previous test phase.
Table 1.
Allocation of rats to experimental phases.
| Experimental Phase | # Rats |
|---|---|
| Free Drinking (15 min delay) and Self-Administration | 6 |
| Free Drinking (15 min delay) Only | 3 |
| Free Drinking: 15 versus 60 min delay | 11 |
| Free Drinking: Effects of Delayed Opioid Antagonism | 5 |
| Free Drinking Anatomical Controls | 4 |
| Self-Administration and Extinction Learning | 5 |
| Extinction Learning Only | 7 |
| Extinction Learning and Retention | 2 |
| Extinction Retention Only | 6 |
| Extinction Learning/Retention and Reinstatement | 4 |
| Reinstatement Only | 17 |
| Rats excluded after pre-exposure | 3 |
| Rats excluded based on localization of injector tips | 6 |
| Total | 79 |
2.3. Surgery
Rats designated for the free drinking tests (n=29) underwent surgery immediately after the end of the pre-exposure phase. All other rats underwent surgery after three weeks of self-administration training. Under isoflurane anesthesia, rats received preemptive analgesia in the form of carprofen (5 mg/kg) and local injection of lidocaine and were placed in the stereotaxic apparatus with the mouth bar set to 5.0 mm above intra-aural zero, so that cannulae could be angled to avoid puncturing the lateral ventricles. Rats received bilateral 23-gauge stainless steel guide cannulae aimed at the following coordinates corresponding to 2 mm above NAc shell: anteroposterior (AP) +3.1 mm, mediolateral (ML) +/− 1.0 mm, dorsoventral (DV) – 5.7 mm (mouth bar at 5.0mm). A group of anatomical controls received cannulae aimed at the septum and dorsal peduncular cortex (n=4), to control for diffusion up the cannulae tract. Cannulae were anchored to the skull using surgical screws and dental cement and stainless steel obturators (28 gauge) were inserted to prevent occlusion. Rats were treated with penicillin (intramuscularly, 1 mg/kg) and topical antibiotics, and were allowed to recover for at least 7 days before retraining or testing.
2.4. Drugs and Microinjections
To test the effect of mu-opioid agonism on alcohol drinking and seeking behaviors we infused the mu agonist, D-Ala2,N-Me-Ph4,Glyol5-enkephalin (DAMGO, Sigma), at a dose of 0.15 μg in .3 μl saline; this is a concentration previously shown to elevate alcohol consumption when injected in NAc (Zhang and Kelley, 2002). On microinjection test days, DAMGO or vehicle was bilaterally infused at a speed of 0.3 μl per minute through stainless steel injectors (29 gauge, Plastics One) extending 2mm beyond the end of the guide cannulae, which were attached to Hamilton syringes via PE-20 tubing. Injectors were left in place for 1-min, then removed, obturators replaced, and then testing commenced according to the specific delays described below. In one experiment intended to test the effects of opioid antagonism, rats received intraperitoneal injections of 4 mg/kg naloxone (Sigma) dissolved in saline, or an equivalent volume of saline vehicle. Habituation injections of saline vehicle were given at least 3 days prior to the first test injection.
2.5. Free Drinking Testing and Lick Analysis
Rats were habituated to drinking 10% ethanol in operant boxes during 2-hr, daily sessions beginning approximately 30-min after lights off. Ethanol was consumed from a lick spout equipped with an attached photobeam lickometer, which allowed licks to be time-stamped and stored for off-line analysis. Ethanol was available continuously throughout the 2-hr free drinking sessions. Rats underwent habituation for at least 5 days, or until total licks per session did not deviate by more than 15% for two consecutive days. In the first experiment, rats (n=19; 15 NAc shell and 4 anatomical controls) received vehicle or DAMGO infusion 15-min prior to being placed in the operant boxes. After at least two intervening baseline sessions, rats were tested with the opposite drug injection. In order to test the importance of continuous ethanol access for the delayed increases in drinking that we observed with DAMGO we tested a semi-overlapping group of rats (total n=11; new n=5) for the effects of vehicle and DAMGO under similar conditions, but with a 60-min delay between the end of the infusion and entry into the operant chamber for drinking. A separate group of rats (n=5) was tested for the importance of ongoing opioid receptor stimulation in the delayed increase in ethanol drinking we observed; these rats received vehicle or DAMGO microinjections into NAc shell 60-min prior to the test, and received a systemic naloxone (2 mg/kg; Sigma) or vehicle injection 45-min post-microinjection (15-min prior to the test). Total amount consumed (g/kg) was calculated based on liquid consumed during the test; these data were missing from one rat in the 15 minute delay experiment. Lick data were analyzed for total licks, as well as for the size (licks per bout) and number of bouts of licking behavior, which have been suggested to be altered selectively by changing the palatability of the solution versus altering the animal’s physiological motivational state, respectively (Davis and Perez, 1993; Spector et al., 1998; Taha et al., 2009). That is, increases in consumption induced by food deprivation or specific nutrient deficiency tend to manifest as increases in the number of bouts (Davis and Perez, 1993; Markison et al., 2000), whereas changes in consumption as the result of alterations in palatability (such as caused by increasing the sweetness or fat content of a solution, or the addition of quinine) generally result in changes in the bout size (Katsuura et al., 2011; Spector and St. John, 1998; Spector et al., 1998). Based on prior studies (Spector et al., 1998; Wassum et al., 2009) we defined two different types of bouts: bursts (bouts consisting of at least three licks separated by interlick-intervals [ILIs] of at least .25-sec) and clusters (bouts of licking separated by ILIs of at least .5-sec). We also assessed the organization of drinking on a longer time scale, in the forms of meals (bouts of licking separated by ILIs of at least 10-min) which has been used as a measure of satiety (Katsuura et al., 2011). Rats (n=6) that only received 2 microinjections during the free drinking test (at the initial delay time) continued on to self-administration training and testing.
2.6. Operant Self-Administration Training and Testing
All rats designated for self-administration, extinction or reinstatement testing (n=47) were trained to self-administer ethanol for 28 days. Rats were placed in chambers outfitted with two retractable levers flanking a reward receptacle or lick spout equipped with photobeam lickometer. Responses on the active lever triggered a 5 sec auditory cue (an intermittent tone), simultaneous illumination of a cue light above the reward receptacle, and either delivery of 0.2 ml of 10% ethanol to the reward receptacle (n=41; rats not tested in free drinking) or 5 sec of ethanol availability from the lick spout (n=6; rats previously used in free drinking experiment). In rats receiving alcohol from the lick spout, the first lick following the cue presentations turned off the cue and initiated 5 sec of ethanol availability, during which each lick triggered delivery of approximately 10 μl of solution. The separate lick spout group was included to allow assessment of lick rate/microstructure during the self-administration tests, in the event that we observed changes in lick structure consistent with palatability in the free drinking test. Responses on the inactive lever resulted in no programmed events in either group. Rats were first trained daily to press for ethanol on a fixed ratio (FR)-1 schedule, in two overnight (14-hr) sessions and five subsequent 2-hr sessions, beginning approximately 30-min into the dark phase. Rats then received 14 training sessions on an FR3, before undergoing surgery if they had not already done so. Recovery was followed by 7 additional days of FR-3 training. Rats designated for self-administration testing (n=11; n=6 previously tested in free drinking) received vehicle or DAMGO injections 15-min prior to the self-administration test. Following at least two days of re-baseline testing, rats were tested following the opposite injection condition. All rats that had not been tested in the free drinking phase (n=41) continued on to the extinction phase.
2.7. Extinction Learning and Retention
All rats designated for extinction or reinstatement testing (n=41) underwent extinction training following the completion of self-administration training. During extinction training, presses on the active lever no longer resulted in presentations of the auditory cue or the cue light, or ethanol delivery. Rats underwent daily 2 hr extinction sessions for at least 8 days, and until they made 15 or fewer presses on the active lever on 2 sequential days (up to 11 sessions). In order to test the effects of mu-opioid agonism on extinction learning, subsets of rats received either DAMGO (n = 9) or vehicle (n = 9) injections prior to the first two extinction sessions, while the remaining rats (n=23) received no extinction infusions. Following these test days all rats completed extinction with no further injections. In order to test whether NAc shell DAMGO would interfere with “retention” of extinction (i.e. whether DAMGO would reinstate ethanol seeking on its own) a separate subset of rats (n=12; including n=6 rats that received vehicle during extinction, balanced in the two groups) received injections of vehicle (n=6) or DAMGO (n=6) prior to an additional 2-hr extinction session. Rats (n=4) that received vehicle during the extinction learning and/or retention tests whose cannulae remained patent were tested again in the cue-induced reinstatement test.
2.8. Reinstatement for an Alcohol Cue
Following self-administration and extinction training, rats (n=2, including n=4 previously vehicle infused) received either vehicle (n=13) or DAMGO (n=8) 15-min prior to undergoing a “cue-induced” reinstatement test. During the reinstatement sessions, presses on the active lever resulted in presentation of the auditory cue and cue light for 5 sec, but no ethanol availability. Lever presses for cue presentations are used as a measure of reinstatement (Ciccocioppo et al., 2002; Nie and Janak, 2003; Shaham et al., 2003), but are also a measure of the conditioned reinforcement properties of the ethanol-paired cues (Smith et al., 1977). In order to isolate the conditioned reinforcement properties of the discrete alcohol-paired cue and its interactions with MOR agonism, we did not utilize an alcohol drop in the receptacle, an olfactory discriminative stimulus, or sucrose-fading, which are frequently used to achieve enhanced cue-induced reinstatement (Katner et al., 1999; Lê and Shaham, 2002; Nie and Janak, 2003; Wouda et al., 2011).
2.9. Histology
Following the completion of behavioral testing, rats were anesthetized with an overdose of sodium pentobarbital, and perfused transcardially with 0.9% saline followed by 10% formalin. Brains were removed, post-fixed in 10% formalin (1 day) followed by 25% sucrose (2 days), sectioned at 60 μm and stained with cresyl violet in order to verify placement of injector tips in NAc shell. Rats were excluded if injector tips were not situated in the NAc shell (n=6).
2.10. Statistical Analysis
The effects of DAMGO on alcohol consumption or seeking behaviors was assessed using paired-samples t-tests (free drinking, self-administration), independent-sample t-tests (cue-induced reinstatement, extinction learning day 3, extinction retention) or mixed 1×2 ANOVAs (extinction learning days 1 and 2). To assess the effects of DAMGO on free drinking at different delays, we analyzed the data using linear mixed models, to account for a combination of paired and independent data (a subset of rats were tested at both delays). Depending on the best-fitting covariance model (Verbeke and Molenberghs, 2009), the degrees of freedom may be a non-integer value. To assess the effects of systemic naloxone on DAMGO-induced drinking, we used a repeated measures 2×2 ANOVA and pairwise comparisons with Sidak corrections. To assess the relationship between DAMGO-induced changes in total licks and lick microstructure measures we ran a Pearson r correlation.
3. Results
3.1. NAc MOR agonism increases free consumption of 10% ethanol
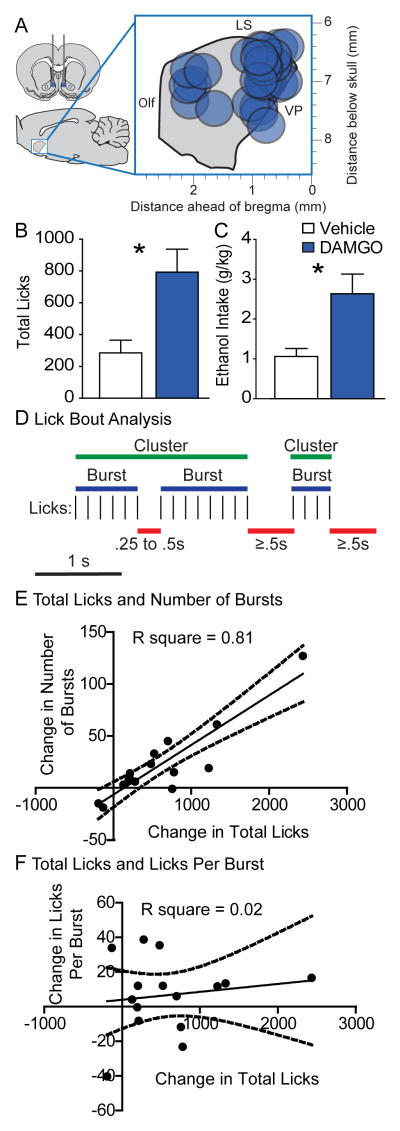
As expected, infusion of the MOR agonist, DAMGO, into NAc (Figure 1A) increased consumption of 10% ethanol during the free drinking test (Figure 1C; t(13)=3.043, p = 0.0094), more than tripling total licks during the 2-hr session (Figure 1B; t(14) = −3.42, p = 0.004). To further understand the nature of this effect, we examined lick microstructure (Figure 1B). Increases in consumption induced by food deprivation or specific nutrient deficiency manifest as increases in the number of bouts (Davis and Perez, 1993; Markison et al., 2000), whereas changes in consumption resulting from alterations in palatability (such as caused by increasing the sweetness or fat content of a solution, or the addition of quinine) generally result in bout size changes (Katsuura et al., 2011; Spector and St. John, 1998; Spector et al., 1998). We found that DAMGO increased the number of “bursts” (bouts of licking with no ILI greater than .25 sec; t(14) = −2.378 p = .032) and the number of “clusters” (bouts of licking with no ILI greater than .5 sec; t(14) = −2.619, p = .020), but had no effect on the number of licks per burst (i.e. burst size; t(14) = −1.203, p = .249) or licks per cluster (t(14) = −.367, p = .719). The DAMGO-induced change in total licks was significantly correlated with the change in number of bursts (r(15) = 0.8999, p < 0.0001), but not with the change in the size of bursts (licks per burst, r(15) = 0.1423, p = 0.61; Figure 1C and D). We found the same relationship between the change in drinking and the change in number of lick clusters: the total change in licks was correlated with the change in the number of clusters (r(15) = .7884, p < 0.001), but not the size (licks per cluster, r(15) = .2133, p = 0.44). These correlations suggest that DAMGO-induced increases in alcohol drinking are more related to change in the appetitive state of the animal than to changes in the perception of alcohol’s palatability. Because ‘liking’ reactions to sucrose are increased when DAMGO is injected in the rostrodorsal quadrant of shell (Peciña and Berridge, 2005), we separately analyzed the lick structure of just those rats with injection sites in rostrodorsal NAc shell (n=4). Even in these rats, DAMGO failed to enhance the size of lick bursts (t(3)=1.40, p = 0.25) or lick clusters (t(3)=0.23, p = .83), and there was no correlation between the total change in licks and the size of bursts (r(4) = −0.62, p = 0.38) or clusters (r(4) = −0.12, p = 0.88). To control for possible diffusion up the cannulae track, we assessed the effects of DAMGO at anatomical control placements in the septum and dorsal peduncular cortex (n=4). We found that DAMGO infusion in these structures failed to enhance drinking (t(3) = 1.135, p = .339).
Figure 1. Changes in microstructure of licks for 10% ethanol induced by NAc shell DAMGO.
Histological reconstruction of microinjection placements for free drinking experiments in the sagittal plane (A): placements were located throughout the rostrocaudal extent of NAc shell. DAMGO (blue) enhanced both total licks for ethanol (B) and ethanol intake (g/kg, C) above vehicle levels (white). Example (D) of lick patterns that would be classified as bursts (blue) or clusters (green), based on the length of the interlick interval (ILI; red). Scatterplots and regression lines show that the change in total licks (n=15) induced by DAMGO (DAMGO – Vehicle) was correlated with the change in the number of bursts (E), but not the change in licks per burst (F). Dotted lines indicate the 95% confidence interval. Bar graph data presented are mean +/− SEM. Olf = Olfactory nucleus, LS = lateral septum, VP = ventral pallidum.
3.2. DAMGO induced increase in consumption occurs after a prolonged delay
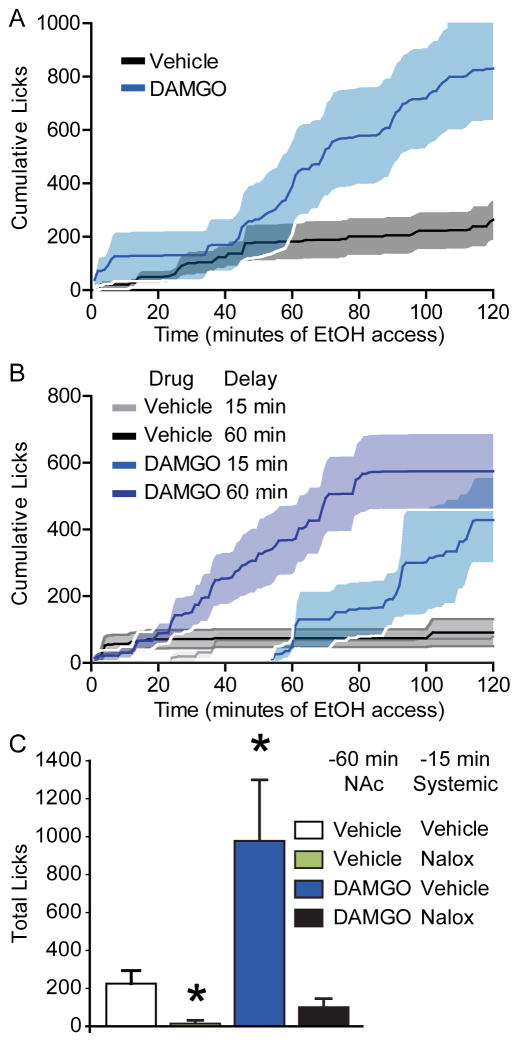
A notable feature of the DAMGO-induced increase in drinking is that, as reported previously (Zhang and Kelley, 2002) it was delayed until the second half of the 2-hr drinking session: the average cumulative licks of alcohol following DAMGO infusion did not differ from vehicle until approximately 60-min into the session (75-min post-DAMGO; Figure 2A), and did not become significantly different until 90-min into the session (115 post-DAMGO, t(14) = −2.312, p = .036). If DAMGO acts by increasing the magnitude of “reinforcement” produced by alcohol (Clissold and Pratt, 2014) its main effect in this behavioral paradigm may be that, similar to its action on palatable food consumption, it counteracts the development of satiety (Woolley et al., 2007a). Potentially consistent with a decrease in satiety, we found a significant increase in meal size (t(14) = 2.74, p = 0.016), which was significantly correlated with the increase in total licks (r(15) = 0.71, p = 0.003). For this reason, we elected to compare the effects of DAMGO on alcohol drinking using a 60-min delay (versus the 15-min delay used in the original test). If DAMGO acts to increase drinking by either a) increasing the learned value of alcohol during alcohol drinking in the first half of the session and/or b) reducing alcohol satiety late in the session, then rats would be expected to increase drinking after a similar delay in the session (i.e. after a similar amount of re-experience with the alcohol). Yet, when rats were tested 60-min after DAMGO infusion, they began to increase their drinking above vehicle levels much earlier in the testing session. DAMGO-induced alcohol drinking began to rise above vehicle levels by approximately 20-min into the testing session (Figure 2B) and was significantly different from vehicle levels at 40-min into the session (t(10) = −2.512, p = .031). This approximates the post-infusion time course of DAMGO-induced drinking that we found in the original drinking test (75 – 110 minutes post-infusion), indicating that the delay in the increase in drinking does not depend upon experience with alcohol. This is consistent with enhanced motivation to consume alcohol that does not depend upon reversal of satiety or enhanced reinforcing value of alcohol consumed. The longer delay before test also had no effect on the structure of DAMGO-induced alcohol drinking, including total licks (F(1,9.8) = 3.112, p = .109), number of bursts (F(1,9.18) = .542, p = .480), licks per burst (F(1,16.777) = .101, p = .755), number of clusters (F(1,8.345) = .107, p = .752), or licks per cluster (F(1,16.881) = 0.70, p = .795). The pattern of changes in lick microstructure when rats were tested after a 60-min delay was similar to changes we observed after 15-min. The total change in licks was significantly correlated with the number of bursts (r(11) = 0.835, p = 0.0014) or clusters (r(11) = .8070, p = 0.0027), but not the size of bursts (r(11) = 0.107, p = .75) or clusters (r(11) = .087, p = .799). Overall, rats that spent 60-min after the DAMGO infusion without access to alcohol still increased their drinking of alcohol on the same time scale and with the same changes in lick microstructure, except that increases in total licks were no longer correlated with the increase in meal size (r(11) = 0.4914, p = 0.13).
Figure 2. Changes in consumption of 10% ethanol induced by NAc shell DAMGO.
Cumulative licks (A) over time following DAMGO (blue) or vehicle (grey) infusion (n=15); shaded areas show SEM. Introducing a 60-minute delay (B) following the injection prior to ethanol access did not change the post-injection time at which DAMGO increase cumulative licks (15 min delay, n=15; 60 min delay n=10). Systemic naloxone (Nalox; C) prevented DAMGO-induced increases in drinking and reduced drinking overall (n=5), *=versus vehicle, p<0.05.
Given that the delay in DAMGO potentiation in drinking was not dependent on alcohol access, it is likely due to a delayed pharmacological effect of mu-opioid agonism, either directly in NAc or via circuit-level interactions. An alternative explanation is that dropping levels of opioid agonist (e.g. as in withdrawal), which may be found at prolonged delays after DAMGO infusion, may be required for DAMGO-induced increases in drinking. Based on this alternative hypothesis one would predict that precipitation of this drop in opioid stimulation levels (e.g. with an opioid antagonist) would enhance the effect of administered DAMGO on drinking. To test this possibility we examined the effects of systemic naloxone injections, given 45-min after the DAMGO infusion and 15-min before the drinking test. We found that systemic naloxone following DAMGO did not enhance DAMGO-induced drinking; instead, it prevented DAMGO-induced increases in drinking (Figure 2C; DAMGO x naloxone, F(1,4) = 9.985, p = 0.040) and reduced drinking overall (main effect of naloxone: F(1,4) = 11.766, p = .027). This indicates that delayed DAMGO-induced drinking is not driven by falling concentrations of DAMGO and instead requires prolonged opioid receptor stimulation.
3.3. NAc DAMGO enhances operant self-administration of alcohol
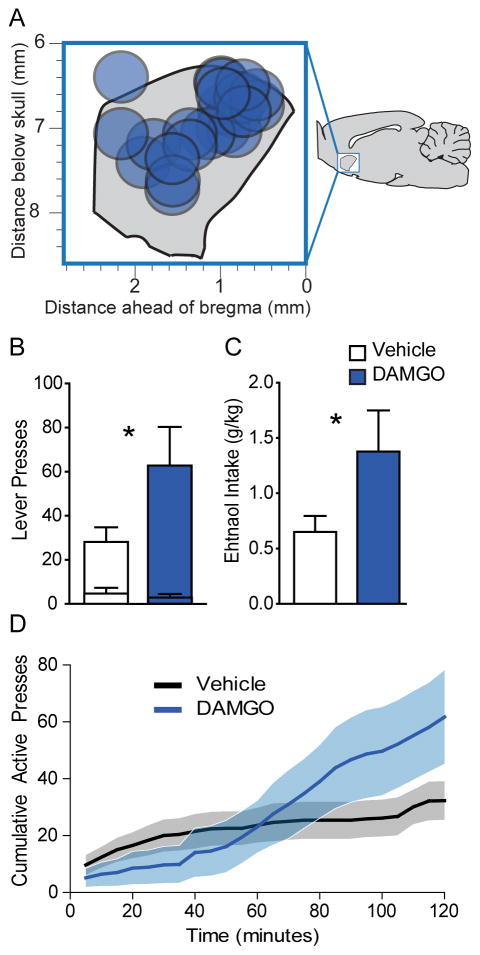
In order to test the effects of NAc DAMGO on operant responding for alcohol, we trained rats to self-administer alcohol. After 30-days of training, we tested the effects of NAc DAMGO on alcohol self-administration on a fixed-ratio 3 schedule (Figure 3A). DAMGO enhanced active lever presses for alcohol (t(10) = 2.255, p = 0.0478), but had no effect on presses of the inactive lever (t(10) = 0.75, p = .47; Figure 3B). Total ethanol intake was also enhanced by DAMGO prior to the self-administration session, approximately doubling from 0.65 g/kg to 1.38 g/kg (t(10)=2.262, p = 0.0472; Figure 3C). Similar to the free drinking test, DAMGO selectively enhanced operant alcohol seeking in the second hour of the 2-hr session (hour 1, t(10) = 0.06, p = .95; hour 1, t(10) = 3.094, p = 0.01; Figure 3D). The initial suppressive effects of DAMGO appeared even stronger in the operant self-administration test (Figure 3D), perhaps due to the greater motor requirements of the self-administration test. This relates to the hypothesis that the biphasic effects of DAMGO may be due to short-lived suppression of locomotor activity (Babbini and Davis, 1972; Bakshi and Kelley, 1993; Zhang and Kelley, 2002, 1997). In the subset of rats that consumed alcohol from the lickometer spout, we did not observe a change in lick rate, during the alcohol availability periods (t(5) = 1.871, p = 0.12). Because we found no interaction between the effects of DAMGO and the alcohol delivery method on operant responding for alcohol (F(1,9) = 0.33, p = 0.577), the data are presented together.
Figure 3. NAc shell DAMGO enhances operant self-administration of 10% ethanol.
Histological reconstruction of microinjection placements from self-administration experiment in the sagittal plane (A). DAMGO (blue) significantly enhances total (B) active lever presses (inner bars = inactive) as well as total ethanol consumption (C; g/kg) during the self-administration test, *= DAMGO versus vehicle, p<0.05. Cumulative active lever presses (D) during the self-administration test following vehicle (black) or DAMGO (blue) infusion (n=11). Data presented as mean +/− SEM.
3.4. NAc DAMGO has no effect on extinction learning
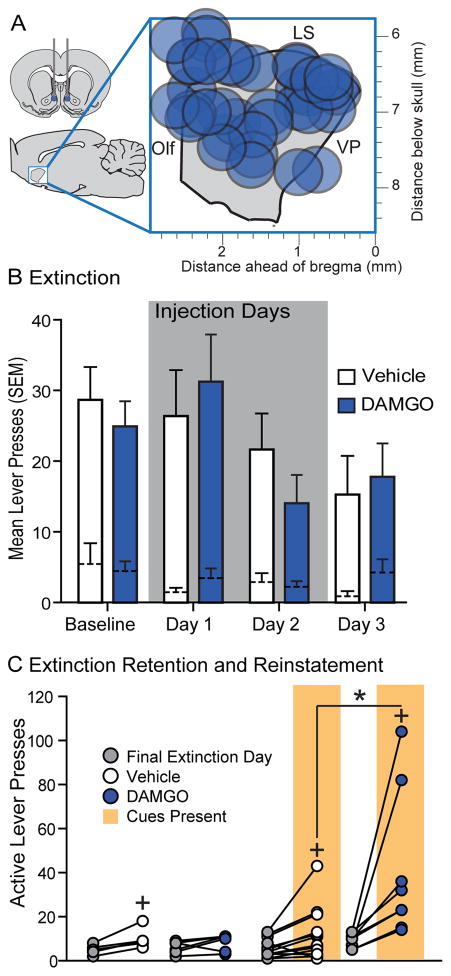
Following self-administration training and testing, rats underwent extinction training for up to 10-days, or until they made fewer than 15 active lever presses on two successive extinction sessions. During extinction learning, presses on the active lever no longer resulted in presentation of the auditory cue or alcohol. As expected, rats gradually reduced their responding on the active lever across extinction days (F(7,147) = 8.813, p < .001). To test the effects of NAc DAMGO we infused separate groups of rats with vehicle or DAMGO 15-min prior to the first two extinction sessions and assessed these groups off-drug on a third extinction day (Figure 4A). Infusion of DAMGO had no effect on the significant reduction in active lever presses across these three extinction sessions (Figure 4B; main effect of DAMGO, F(1,14) = 0.02, p = 0.888; extinction day x DAMGO, F(3,42) = 0.70, p = 0.5572; main effect of extinction, days 1–3, F(3,42) = 3.54, p = 0.0226). Additionally, active lever presses were not different between the two groups when we directly compared them on the first extinction session sans drug (t(14)=.343, p = .736). These results suggest, that at least at early stages of extinction, mu-opioid agonism in the NAc does not impact learning.
Figure 4. NAc shell DAMGO has no effect on extinction learning or retention, but enhances cue-induced reinstatement.
Histological reconstruction of microinjection placements from the extinction learning and reinstatement experiments in the sagittal plane (A). DAMGO (blue) had no acute effect on lever presses during extinction learning (B: vehicle, n=9; DAMGO, n=9). During reinstatement testing (C) NAc shell DAMGO (blue) had no effect on lever presses made in the absence of cues or ethanol (left; vehicle, n = 6; DAMGO, n = 6), but DAMGO (n=8) did increase lever presses for contingent presentations of an alcohol paired cue above levels observed following vehicle infusion (vehicle, n=13). *=DAMGO versus vehicle, p<0.05, +=reinstatement versus extinction, p<0.05; Olf = Olfactory nucleus, LS = lateral septum, VP = ventral pallidum.
3.5. DAMGO potentiates cue-reinforced alcohol seeking, but not reinstatement of operant responding on its own
Because NAc DAMGO potentiated operant self-administration of alcohol, as well as free drinking of alcohol, we hypothesized that NAc DAMGO would also potentiate cue-induced reinstatement of alcohol seeking. Following extinction training, rats received infusions of either vehicle or DAMGO 15-min prior to a reinstatement test, in which presses on the active lever now produced presentations of the auditory cue, but not alcohol. Following vehicle infusions, cue presentations produced mild, but significant reinstatement of active lever presses in comparison to the final day of extinction (Figure 4C; extinction versus reinstatement in vehicle rats alone: t(12)=2.440, p = .031). As expected, NAc DAMGO significantly potentiated cue-reinforced responding in the reinstatement test (Figure 4C; interaction of drug condition and extinction versus reinstatement: F(1,19) = 7.77, p = 0.0117). Surprisingly, we did not observe a significant effect of NAc DAMGO on port entries during reinstatement (t(16)=1.484, p = .157). In a separate group of rats we tested whether NAc DAMGO reinstates alcohol seeking in the absence of explicit sensory cues. NAc DAMGO had no significant effect on lever presses during the reinstatement test in the absence of cues (t(10) = .653, p = .528) nor on port entries (t(10) = 1.351, p = .207). While NAc DAMGO potentiates alcohol seeking driven by contingently presented cues, it is insufficient to generate reinstatement-like behavior on its own (Figure 4C).
Discussion
Here, we demonstrate that infusion of the MOR selective agonist DAMGO into NAc elevates alcohol drinking by increasing the number, but not duration, of licking bouts. NAc DAMGO also elevates operant responding for alcohol and potentiates extinguished operant responding for an alcohol paired sensory cue, but does not alter extinction learning or retention.
Implications for the role of palatability or ‘liking’ in mu-opioid induced increases in drinking
Given previous reports of the effects of NAc DAMGO or naloxone on ‘liking’ reactions in a taste reactivity taste (Castro and Berridge, 2014; Peciña and Berridge, 2005), and on other measures of the palatability of sucrose or fatty tastants (Katsuura et al., 2011; Wassum et al., 2009; Woolley et al., 2006; Zhang et al., 1998), it is somewhat surprising that we did not see lick microstructure changes typically associated with increases in palatability (i.e. an increase in bout size) for alcohol. Since DAMGO-induced increases in ‘liking’ generally occur following microinjection into a fairly restricted neuroanatomical zone in the rostrodorsal portion of the NAc shell (Castro and Berridge, 2014; Peciña and Berridge, 2005) we assessed the effects of DAMGO in this NAc region on bout size, but also found no effect. Our findings are also somewhat surprising in light of previous work by Kiefer and others (Coonfield et al., 2004, 2002; Ferraro 3rd et al., 2002; Hill and Kiefer, 1997) showing that systemic opioid antagonism reduces ingestive reactions to alcohol even more so than for sucrose. Importantly, opioid antagonists were given systemically in these studies and it is likely that opioid antagonists exert additional effects on palatability when administered outside of the NAc, for example, in the ventral pallidum (Smith and Berridge, 2007, 2005), where mu-opioid agonism enhances ‘liking’ for sucrose. Furthermore, the ingestive reactions measured in these alcohol studies include mouth movements, which in some taste reactivity studies are classified as neutral/consummatory and in some cases are not included in hedonic reaction scores (Castro and Berridge, 2014; Leeb et al., 1991; Pelloux et al., 2014; Richard and Berridge, 2011). It is also possible that if we had assessed palatability using a taste reactivity test, rather than lickometer analysis we would have found a different result. Changes in MOR levels in the NAc due to alcohol exposure may account for the lack of altered palatability or other effects reported here: mu-opioid receptor gene expression is reported to increase after 2 and 4 months of moderate alcohol pre-exposure (similar to the levels seen here) (Jonsson et al., 2014), though decreases in MOR expression (Saland et al., 2005) or no change (Shah et al., 1997) have been reported with other sets of exposure parameters. Finally, DAMGO’s lack of effect on palatability-related measures of alcohol consumption may be related in part to the taste of alcohol functioning as a conditioned reinforcer, rather than as a primary reinforcer, or to its mixed hedonic and aversive taste qualities (Bice et al., 1992). Thus, while NAc DAMGO can increase consumption of a variety of tastants, it appears to do so by selectively modulating aspects of lick microstructure differently for rewards like sucrose and fats versus alcohol.
Implications for the role of NAc DAMGO in “positive reinforcement” and satiety
Our results suggest that the role of NAc shell MOR in promoting alcohol seeking and consumption is not primarily related to an effect on learning or satiety during the test session. First, the delayed increase in consumption induced by DAMGO occurs even without continued access to alcohol during the delay. Furthermore, NAc MOR agonism does not interfere with extinction learning – suggesting that NAc DAMGO does not substitute for the reinforcing effects of alcohol during extinction trials and that changes in MOR agonist levels in NAc are not involved in computing the outcome value of instrumental actions. Furthermore, alcohol (sensory or caloric) satiety cannot explain the changes we observed in responding for alcohol-paired cues during cue-induced reinstatement, as rats received no alcohol during this test. While we observed an increase in meal duration when rats were tested at a 15 minute delay after DAMGO infusion, consistent with a reduction in satiety, we did not see a significant increase in meal duration when rats were tested at the 60 minute time point, despite seeing a similar increase in overall consumption, total licks, and number of licking bursts. Thus, while NAc MOR agonism can exert some sensory satiety-related effects, it exerts more consistent effects related to the incentive motivational properties of alcohol and alcohol cues, as illustrated by the increased initiation of alcohol drinking bouts, increased operant responding for alcohol and the increase in conditioned reinforcement.
NAc MOR in motivational properties of alcohol and alcohol cues
The DAMGO-induced behavioral changes we observed across multiple tests were consistent with the reported role of NAc MORs in appetitive motivation. NAc DAMGO increased the number of bouts of licking, a change in microstructure typically seen following inductions of hunger or specific physiological need (Davis and Perez, 1993; Markison et al., 2000), rather than changes in the orosensory properties of the stimulus. Additionally, NAc DAMGO increased lever pressing for alcohol-paired cues in a cue-induced reinstatement test, suggesting that DAMGO potentiates the reinforcing properties of conditioned incentive cues. Previous studies with food reward have reported no increase in conditioned reinforcement by NAc DAMGO in a classic conditioned reinforcement paradigm (Cunningham and Kelley, 1992) and a decrease in “cue-induced” reinstatement (Guy et al., 2011). These results may be due to a combination of the biphasic nature of DAMGO effects in NAc and the relatively brief nature of the tests in these cases (45 and 20 minutes, respectively). Further experiments are required to determine whether NAc DAMGO can potentiate other properties of Pavlovian alcohol cues (Milton and Everitt, 2010), including their ability to non-contingently elicit motivational states, such as in Pavlovian-to-instrumental transfer (Corbit and Janak, 2007), or to attract attention and approach (Krank et al., 2008). Importantly, for cues predicting sucrose, DAMGO infusions throughout the NAc, including in the shell, enhance Pavlovian-instrumentral transfer (Peciña and Berridge, 2013), suggesting the incentive motivational effects of MOR activation are not limited to alcohol. Given that similar incentive effects are reported in the core, the delayed incentive motivational effects may have been due in part to diffusion to this subregion. Here, we specifically demonstrate that NAc DAMGO enhances the conditioned reinforcement properties of alcohol cues (i.e. responding for contingent cue presentations), yet NAc MOR signaling may also promote relapse by potentiating other conditioned cue elicited effects, including their ability to non-contingently elicit motivational states, such as in context-induced reinstatement.
Role of NAc MOR in reinstatement of drug seeking
We are aware of one other study investigating the role of NAc MOR in reinstatement of alcohol seeking. Perry and McNally (2013) found that infusions of a MOR selective antagonist in NAc shell blocked context-induced reinstatement (“renewal”) of alcohol seeking, but did not affect reinstatement induced by a small priming dose of alcohol. Intracerebroventricular infusions of a MOR selective antagonist also attenuate context-induced reinstatement of alcohol seeking, but have no effect on cue-induced reinstatement in a model that included one non-contingent cue presentation, followed by contingent cue presentations (Marinelli et al., 2009). Many studies investigating opioids in reinstatement of alcohol seeking have used mixed procedures in which animals are re-exposed to both discrete and contextual cues, such as odors presented throughout the self-administration session. Under these conditions, reinstatement is reduced by systemic naltrexone (Ciccocioppo et al., 2003; Williams and Schimmel, 2008), as well as systemic injections of the delta-opioid receptor antagonist naltrindole and the irreversible MOR antagonist naloxonazine (Ciccocioppo et al., 2002). It is difficult to conclude from these studies whether the observed reinstatement is primarily driven by discrete or contextual cues, but we should note that systemic naltrexone reduces context-induced reinstatement of alcohol seeking, in the absence of discrete alcohol-paired cues (Burattini et al., 2006), consistent with Perry and McNally’s finding for MORs in NAc shell (2013). Many of these studies may include contextual or discriminatory cues because, as least for alcohol self-administration, reinstatement elicited by discrete cues is often less robust than reinstatement induced by contexts (Tsiang and Janak, 2006). Consistent with this finding, we only observed robust reinstatement of alcohol seeking for contingent presentations of the discrete alcohol cue in animals that had received MOR agonism in NAc shell, whereas reinstatement to these same cues in rats that received vehicle infusions was weaker. Importantly, we observed no reinstatement of alcohol seeking elicited by infusions of DAMGO alone, unless cues were also presented contingent on the animal’s behavior. This suggests that while endogenous MOR signaling in the NAc is required for renewal of operant responding for alcohol by contexts (as in Perry and McNally, 2013), exogenous activation of MOR signaling by DAMGO in a previously extinguished context is insufficient to renew the incentive motivational properties of that context.
Conclusions
In humans, opioid antagonism reduces craving for alcohol (Chick et al., 2000), heavy drinking days (Monti et al., 2001), and relapse to heavy drinking (Guardia et al., 2002; Herz, 1997), but also produces aversive side effects that affect compliance (Bouza et al., 2004). Importantly, NAc MORs may contribute to separable aspects of alcohol reward via modulation of distinct output pathways and neuronal populations. For instance, outputs to ventral pallidum appear selectively involved in NAc shell DAMGO enhancement of ‘liking’, but not consumption: naloxone in ventral pallidum blocks NAc DAMGO induced increases in ‘liking’, but not eating (Smith and Berridge, 2007), and ipsilateral lesions of ventral pallidum fail to prevent sucrose consumption elicited by NAc DAMGO (Taha et al., 2009). NAc DAMGO-induced increases in consumption require activity in a distributed brain network including the ventral tegmental area, the lateral hypothalamus and the dorsomedial hypothalamus (Will et al., 2003; Zhang and Kelley, 2000). Here, we demonstrate that NAc DAMGO elevates alcohol seeking and consumption without impacting learning or affect-related measures. This finding suggests that the neural circuits underlying opioid-induced changes in the motivation to consume alcohol can be dissociated from the affective consequences of alcohol consumption, and future treatment strategies should aim to selectively target the downstream mediators of MOR-induced increases in motivation to drink.
Highlights.
NAc shell mu-opioid agonism with DAMGO enhances alcohol consumption by increasing the number, but not size of lick bouts
NAc shell DAMGO potentiates operant self-administration of alcohol and alcohol-paired cues during cue-induced reinstatement
NAc shell DAMGO has no effect on acquisition or expression of extinction of operant responding for alcohol
Acknowledgments
We thank Mark Laubach for sharing code for analyzing bouts of licking, Annika Rose and Catriona Miller for technical support, and Jennifer Mitchell and Patricia Janak for feedback on the manuscript. This work was supported by NIH grant no AA022290 to JMR and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jocelyn M Richard, Email: jocelyn.richard@jhu.edu.
Howard L Fields, Email: howard.fields@ucsf.edu.
References
- Babbini M, Davis WM. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol. 1972;46:213–24. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding Induced By Opioid Stimulation of the Ventral Striatum - Role of Opiate Receptor Subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. [PubMed] [Google Scholar]
- Bice PJ, Kiefer SW, Elder NB. Evaluating the palatability of alcohol for rats with measures of taste reactivity, consumption, and lick rate. Alcohol. 1992;9:381–7. doi: 10.1016/0741-8329(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Magro A, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139:877–87. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34:4239–50. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin Exp Res. 2010;34:761–70. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35:587–93. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–15. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–9. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Hill KG, Kaczmarek HJ, Ferraro FM, 3rd, Kiefer SW. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol. 2002;26:43–47. doi: 10.1016/s0741-8329(01)00180-x. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Kiefer SW, Ferraro FM, Sinclair JD. Ethanol palatability and consumption by high ethanol-drinking rats: manipulation of the opioid system with naltrexone. Behav Neurosci. 2004;118:1089–96. doi: 10.1037/0735-7044.118.5.1089. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol Clin Exp Res. 2007;31:766–74. doi: 10.1111/j.1530-0277.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Opiate Infusion Into Nucleus-Accumbens - Contrasting Effects On Motor-Activity and Responding For Conditioned Reward. Brain Res. 1992;588:104–114. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Ferraro FM, 3rd, Hill KG, Kaczmarek HJ, Coonfield DL, Kiefer SW. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol. 2002;27:107–114. doi: 10.1016/s0741-8329(02)00220-3. [DOI] [PubMed] [Google Scholar]
- Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramírez M, Mengual I, Gonzalvo B, Segura L, Trujols J, Casas M. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26:1381–7. doi: 10.1097/01.ALC.0000030561.15921.A9. [DOI] [PubMed] [Google Scholar]
- Guy EG, Choi E, Pratt WE. Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues. Behav Brain Res. 2011;219:265–72. doi: 10.1016/j.bbr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Heinz A, Reimold M, Wrase J, Hermann D, Croissant B, Mundle G, Dohmen BM, Braus DHFH, Schumann G, Machulla HJJ, Bares R, Mann K. Correlation of stable elevations in striatal {micro}-opioid receptor availability in detoxified alcoholic patients with alcohol craving: a positron emission tomography study using carbon 11-labeled carfentanil. Arch Gen Psychiatry. 2005;62:57–64. doi: 10.1001/archpsyc.62.1.57. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hill KG, Kiefer SW. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcohol Clin Exp Res. 1997;21:637–641. [PubMed] [Google Scholar]
- Jonsson S, Ericson M, Söderpalm B. Modest long-term ethanol consumption affects expression of neurotransmitter receptor genes in the rat nucleus accumbens. Alcohol Clin Exp Res. 2014;38:722–9. doi: 10.1111/acer.12307. [DOI] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–9. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Katsuura Y, Heckmann JA, Taha SA. mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am J Physiol Regul Integr Comp Physiol. 2011;301:R244–54. doi: 10.1152/ajpregu.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Krank MD, O’Neill S, Squarey K, Jacob J. Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 2008;196:397–405. doi: 10.1007/s00213-007-0971-0. [DOI] [PubMed] [Google Scholar]
- Lam MP, Nurmi H, Rouvinen N, Kiianmaa K, Gianoulakis C. Effects of acute ethanol on beta-endorphin release in the nucleus accumbens of selectively bred lines of alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology (Berl) 2010;208:121–30. doi: 10.1007/s00213-009-1733-y. [DOI] [PubMed] [Google Scholar]
- Lê A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–56. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Leeb K, Parker L, Eikelboom R. Effects of pimozide on the hedonic properties of sucrose: analysis by the taste reactivity test. Pharmacol Biochem Behav. 1991;39:895–901. doi: 10.1016/0091-3057(91)90050-c. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29:1821–8. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Lê AD. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci. 2009;30:671–8. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markison S, Thompson BL, Smith JC, Spector AC. Time course and pattern of compensatory ingestive behavioral adjustments to lysine deficiency in rats. J Nutr. 2000;130:1320–8. doi: 10.1093/jn/130.5.1320. [DOI] [PubMed] [Google Scholar]
- Méndez M, Barbosa-Luna IG, Pérez-Luna JM, Cupo A, Oikawa J, Mendez M, Perez-Luna JM. Effects of acute ethanol administration on methionine-enkephalin expression and release in regions of the rat brain. Neuropeptides. 2010;44:413–420. doi: 10.1016/j.npep.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Méndez M, Morales-Mulia M, Mendez M. Ethanol exposure differentially alters pro-enkephalin mRNA expression in regions of the mesocorticolimbic system. Psychopharmacol. 2006;189:117–124. doi: 10.1007/s00213-006-0503-3. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–62. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–19. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25:1634–47. [PubMed] [Google Scholar]
- Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168:222–8. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- Oliva JM, Ortiz S, Pérez-Rial S, Manzanares J. Time dependent alterations on tyrosine hydroxylase, opioid and cannabinoid CB1 receptor gene expressions after acute ethanol administration in the rat brain. Eur Neuropsychopharmacol. 2008;18:373–82. doi: 10.1016/j.euroneuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37:1529–40. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Meffre J, Giorla E, Baunez C. The subthalamic nucleus keeps you high on emotion: behavioral consequences of its inactivation. Front Behav Neurosci. 2014;8:414. doi: 10.3389/fnbeh.2014.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, McNally GP. μ-Opioid receptors in the nucleus accumbens shell mediate context-induced reinstatement (renewal) but not primed reinstatement of extinguished alcohol seeking. Behav Neurosci. 2013;127:535–43. doi: 10.1037/a0032981. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. Eur J Neurosci. 2011;33:736–47. doi: 10.1111/j.1460-9568.2010.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP. 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2007;192:537–46. doi: 10.1007/s00213-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Saland LC, Hastings CM, Abeyta A, Chavez JB. Chronic ethanol modulates delta and mu-opioid receptor expression in rat CNS: immunohistochemical analysis with quantitiative confocal microscopy. Neurosci Lett. 2005;381:163–8. doi: 10.1016/j.neulet.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Shah S, Duttaroy A, Sehba F, Chen B, Philippe J, Monderson T, Lau-Cam C, Carroll J, Yoburn BC. The effect of ethanol drinking on opioid analgesia and receptors in mice. Alcohol. 1997;14:361–6. doi: 10.1016/s0741-8329(96)00184-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SG, Werner TE, Davis WM. Alcohol-associated conditioned reinforcement. Psychopharmacology (Berl) 1977;53:223–226. doi: 10.1007/BF00492355. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–94. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1687–1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- Swift RM. Effect of naltrexone on human alcohol consumption. J Clin Psychiatry. 1995;56(Suppl 7):24–9. [PubMed] [Google Scholar]
- Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009;161:718–733. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, Fields HL. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006;24:1220–6. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–8. doi: 10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer; New York: 2009. [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, Balleine BW. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci U S A. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Schimmel JS. Effect of naltrexone during extinction of alcohol-reinforced responding and during repeated cue-conditioned reinstatement sessions in a cue exposure style treatment. Alcohol. 2008;42:553–63. doi: 10.1016/j.alcohol.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–17. doi: 10.1016/j.neuroscience.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Kim B, Fields HL. Opposing effects of intra-nucleus accumbens mu and kappa opioid agonists on sensory specific satiety. Neuroscience. 2007a;146:1445–52. doi: 10.1016/j.neuroscience.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Taha SA, Fields HL. Nucleus accumbens opioid signaling conditions short-term flavor preferences. Neuroscience. 2007b;146:19–30. doi: 10.1016/j.neuroscience.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer ANM, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacol. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–14. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–77. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]