Abstract
Reducing expression or inhibiting translocation of protein kinase C epsilon (PKCε) prolongs ethanol intoxication and decreases ethanol consumption in mice. However, we do not know if this phenotype is due to reduced PKCε kinase activity or to impairment of kinase-independent functions. In this study, we used a chemical-genetic strategy to determine whether a potent and highly selective inhibitor of PKCε catalytic activity reduces ethanol consumption. We generated ATP analog-specific PKCε (AS-PKCε) knock-in mice harboring a point mutation in the ATP binding site of PKCε that renders the mutant kinase highly sensitive to inhibition by 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine (1-NA-PP1). Systemically administered 1-NA-PP1 readily crossed the blood brain barrier and inhibited PKCε-mediated phosphorylation. 1-NA-PP1 reversibly reduced ethanol consumption by AS-PKCε mice but not by wild type mice lacking the AS-PKCε mutation. These results support the development of inhibitors of PKCε catalytic activity as a strategy to reduce ethanol consumption, and they demonstrate that the AS-PKCε mouse is a useful tool to study the role of PKCε in behavior.
Keywords: protein kinase C, alcohol, ethanol, 1-NA-PP1, AS-kinase
Chemical compounds: 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine
1. Introduction
Alcohol use disorder (AUD) is highly prevalent and incurs great cost to society (Rehm et al., 2009). Despite this fact, there are currently only three drugs approved in the U.S to treat AUD: Disulfuram, naltrexone, and acamprosate (Johnson, 2008). Although all are effective, disulfuram is only useful for short-term treatment of highly motivated patients in supervised settings, while naltrexone and acamprosate suffer from compliance issues and small effect sizes (Johnson, 2008). Hence, there is considerable need to develop novel therapies to combat alcoholism.
Studies from our laboratory suggest that protein kinase C epsilon (PKCε) is a target for development of drugs to reduce ethanol consumption. Previous studies found that Prkce−/− mice drink substantially less ethanol than wild type mice (Hodge et al., 1999) and show heightened aversion to ethanol (Newton and Messing, 2007), possibly because of impaired acute functional tolerance to its ataxic and hypnotic effects (Wallace et al., 2007). These behaviors do not result from developmental changes since inducible transgenic expression of PKCε in the amygdala and striatum restores normal sensitivity to intoxication and increases drinking in Prkce−/− mice to levels observed in wild type mice (Choi et al., 2002). Moreover, knockdown of PKCε in the amygdala by RNA interference (Lesscher et al., 2009) or inhibition by a peptide designed to block translocation of activated PKCε (Cozzoli et al., 2015) reduces ethanol consumption in adult wild type mice. PKCε may modulate ethanol intoxication and consumption through phosphorylation of at least 2 substrates: GABAA γ2 subunits at Ser-327 (Qi et al., 2007) and the N-ethylmaleimide sensitive factor at Ser-460 and Thr-461 (Chou et al., 2010). However, all of the evidence implicating PKCε in behavioral responses to ethanol is derived entirely from genetic or shRNA-mediated reductions in PKCε expression or use of a peptide translocation inhibitor. The effect of selective, pharmacological inhibition of PKCε kinase activity with a small molecule inhibitor has not been tested. Such studies are needed to determine if PKCε is a viable drug candidate for the treatment of alcohol use disorder.
Unfortunately, there are no compounds currently available to selectively inhibit the catalytic activity of PKCε. To circumvent this problem, we have used a chemical-genetic approach to study kinase inhibition by selective, cell-permeable, small molecule inhibitors. The strategy targets the ATP-binding pocket conserved in all kinases, replacing a bulky gatekeeper residue with an alanine or glycine to generate mutant alleles that can utilize ATP analogs in addition to ATP, and that are uniquely sensitive to novel kinase inhibitors, such as analogs of PP1 (Bishop et al., 2001). We have generated such an ATP analog-sensitive PKCε (AS-PKCε) carrying the mutation M486A and have used it successfully to probe PKCε function in cell lines (Durgan et al., 2008, Qi et al., 2007). Here, we report the generation of an AS-PKCε knock-in mouse to examine the effects of PKCε on behavior. Using the AS-kinase inhibitor 1-Naphthyl-PP1 (1-NA-PP1) and AS-PKCε mice, we found that selective inhibition of AS-PKCε prolongs the ataxic and hypnotic effects of ethanol and reduces ethanol consumption. These results are consistent with our previous findings in Prkce−/− mice (Choi et al., 2002, Hodge et al., 1999) and validate PKCε as a candidate for drug development, while demonstrating the utility of the AS-PKCε mouse as a useful tool for investigating the role of PKCε in behavior.
2. Materials and methods
2.1. Generation of AS-PKCε mice
Knock-in mice were generated by Caliper Discovery Alliances and Services (Hanover, MD). The Ensembl database was used to identify the BAC clone RP23-75J18 containing the genomic sequence of mouse chromosome 17 from nt # 86451480 to 86613962. This sequence includes the exon encoding Prkce M486. 5' arm (~1.9 kb) and 3' homology arms (~6.0 kb) were generated by PCR and cloned into the targeting vector pLoxNwCD, which contains a floxed neo expression cassette for positive selection and a DTA expression cassette for negative selection. The M486A mutation was introduced into the 5' arm by site-directed mutagenesis. The final vector was confirmed by restriction digestion and end sequencing analysis, and then linearized and electroporated into C57BL/6 ES cells. Approximately 192 ES clones that survived selection were screened using a 5' external probe and 4 clones were expanded. Southern analysis of the ES cell DNA using 5' external, 3' external and neo cassette probes identified three correctly aligned clones with a single neo insertion. Two were transfected with Cre recombinase and one was confirmed to be neo deleted by PCR. Presence of the mutation in that clone was confirmed by PCR and sequencing. These ES cells were injected into tyrosinase deficient blastocysts and transplanted into pseudo-pregnant mice. Germ line transmission of the mutation from chimeras was confirmed by PCR using the following primers; CAGCACGGAGTGATCTACAGGTATTCTC (forward primer) and CGGACACAAACAGCAGGTCAAATCT (reverse primer). Heterozygous mutant progeny were then intercrossed to generate homozygous AS-PKCε mice, which were subsequently intercrossed and maintained as an inbred line on a C57BL/6NTac background. Mice used for experiments were housed under a reverse light dark cycle (lights off at 10AM; lights on at 10PM). Only male mice were tested so that we could compare results with prior studies that used male Prkce−/− mice (Choi et al., 2002, Hodge et al., 1999). All procedures followed the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 2011) and were approved by the Institutional Animal Care and Use Committees of the Ernest Gallo Clinic and Research Center and the University of Texas at Austin. All efforts were made to minimize animal suffering and to reduce the number of animals used in experiments.
2.2. Administration of 1-NA-PP1
1-NA-PP1 was obtained from Dr. Kevin Shokat (UCSF) or from Tocris Biosciences (Bristol, UK). For ethanol, saccharin, and quinine consumption studies, we dissolved 1-NA-PP1 in 100% DMSO at 20 or 30mg/ml and then diluted it 20-fold in deionized water containing 10% Tween-80 with sonication. For studies using oral administration, we prepared 1-NA-PP1 as a 100mM stock solution in 100% DMSO by gentle heating and sonication. This stock was diluted to 500µM in water containing 1% cremophor-RH40 (Sigma-Aldrich, St. Louis, MO) and 2g/L sucralose (Sigma-Aldrich) to increase palatability. Control animals received an equivalent amount of DMSO vehicle in cremophor-sucralose-water. 1-NA-PP1 food pellets (1g/kg) were obtained from Research Diets (New Brunswick, NJ). Control food pellets contained an equivalent amount of vehicle (DMSO). To determine the effects of 1-NA-PP1 on protein phosphorylation, we dissolved 1-NA-PP1 in vehicle containing 5% DMSO and 20% Cremophor EL (Sigma-Aldrich).
2.3. Western blot analysis
Animals were sacrificed with CO2 asphyxiation or cervical dislocation and the amygdala and striatum were rapidly dissected on ice. Brain regions were homogenized with a glass Dounce homogenizer using 20 strokes in 0.1-0.5ml of ice-cold extraction buffer (25mM HEPES –pH7.8, 300mM NaCl, 1.5mM MgCl2, 1% Triton X-100, 0.1mM DTT) containing Phosphatase Inhibitor 1 and 3 (Sigma-Aldrich) and Protease inhibitor Complete™ (Roche Diagnostics USA, Indianapolis, IN.). Lysates were clarified by centrifugation at 10,621×g for 15 minutes at 4°C and then resolved by SDS-PAGE using 4–12% gradient gels (Invitrogen, Carlsbad, CA). Proteins were transferred to nitrocellulose membranes which were blocked with Tris buffered saline (TBS: 50mM Tris, pH 7.6, 150mM NaCl) containing 0.1%Tween-20 (TBS-T) and 5% BSA. The blots were incubated with anti-phospho-GABAA γ2-S(P)327 antibody (Qi et al., 2007) at 1:1000 dilution in 5% BSA overnight at 4°C. Blots were washed 3 times in TBS-T, 8 minutes per wash, incubated in horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA; 1:1000 in 5% nonfat dry milk) for 1h at room temperature, washed again, and visualized using the SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Fisher, Waltham, MA). Immunoreactive bands were quantified by densitometric scanning using Image J (Schneider et al., 2012). Blots were stripped and re-probed with total GABAA γ2 antibody (Alamone labs, Jerusalem, Israel; 1:1000 dilution) or GAPDH (Cell Signaling, Danvers, MA; 1:10,000 dilution).
2.4. Behavioral screen
We examined mice for morphological abnormalities, startle response, righting reflex, and body weight (Crawley, 2008). Strength was measured using the hanging wire test. Motor learning and coordination were assessed as in prior work (Lee et al., 2013) using a rotarod treadmill (AccuRotor Rota-Rod; Omnitech Electronics, Columbus, OH) that accelerated from 0 to 40 rpm in 5 min. Locomotor activity was recorded as the distance traveled in an open field chamber over 60 min (Hodge et al., 1999). Anxiety-like behavior was measured using an elevated plus maze as in previous work (Hodge et al., 2002). Thermal sensation was tested using a tail-flick apparatus (Columbus Instruments, Columbus, OH).
2.5. Ethanol, saccharin, and quinine consumption
Continuous access two-bottle choice drinking was performed as described previously (Lim et al., 2012). Once a stable level of drinking of 10% ethanol was achieved, mice were habituated to 3 intraperitoneal injections of vehicle. The effects of 1-NA-PP1 on drinking were studied using a within-subjects design, under which each animal received vehicle, 20, or 30mg/kg 1-NA-PP1 on different days. 1-NA-PP1 was administered 5-10 min before the onset of the dark cycle. The amount of ethanol and water consumed was monitored for 48 hours after injection. Ethanol preference was calculated by dividing the amount of ethanol-containing solution consumed by total fluid intake. Mice were allowed to recover for 1-2 days between doses of 1-NA-PP1.
Following the ethanol consumption procedure mice were tested for two-bottle choice saccharin (0.03% then 0.06%) and quinine (0.015, 0.03 and 0.06mM) consumption. Each concentration of saccharin was presented for 4 or 5 days. After 2 days of exposure to 0.06% saccharin, mice were habituated to 2 intraperitoneal injections of vehicle. The effect of 1-NA-PP1 on saccharin consumption was tested using a within-subjects design as outlined for the ethanol consumption study. Mice were then allowed to consume water containing increasing concentrations of quinine. After exposure to 0.06mM quinine for 2 days, mice were habituated to 2 vehicle injections and then administered 1-NA-PP1 in a within-subjects design.
2.6. Responses to acute ethanol administration
Ethanol-induced ataxia was evaluated as described (Wallace et al., 2007) with the rotarod treadmill set to a fixed speed of 6 rpm. The ethanol-induced loss of the righting reflex was examined as described in prior work (Choi et al., 2002, Hodge et al., 1999, Lee et al., 2014, Wallace et al., 2007).
2.7. Ethanol clearance
Mice were administered 4g/kg of ethanol intraperitoneally and the 20µl of blood were obtained via tail puncture at 30, 60, 90, 120, and 180 min post-injection. Blood samples were stored at −80°C, until BECs were determined using an NAD-ADH enzymatic assay (Carnicella et al., 2009).
2.8. Statistical Analysis
Data were analyzed using Prism 6.0e (GraphPad Software, La Jolla, CA). Data were expressed as mean ± SEM values and analyzed by two-tailed t-test or repeated measures ANOVA with post-hoc Dunnet’s multiple comparisons test as appropriate.
3. Results
3.1. Generation of AS-PKCε mice
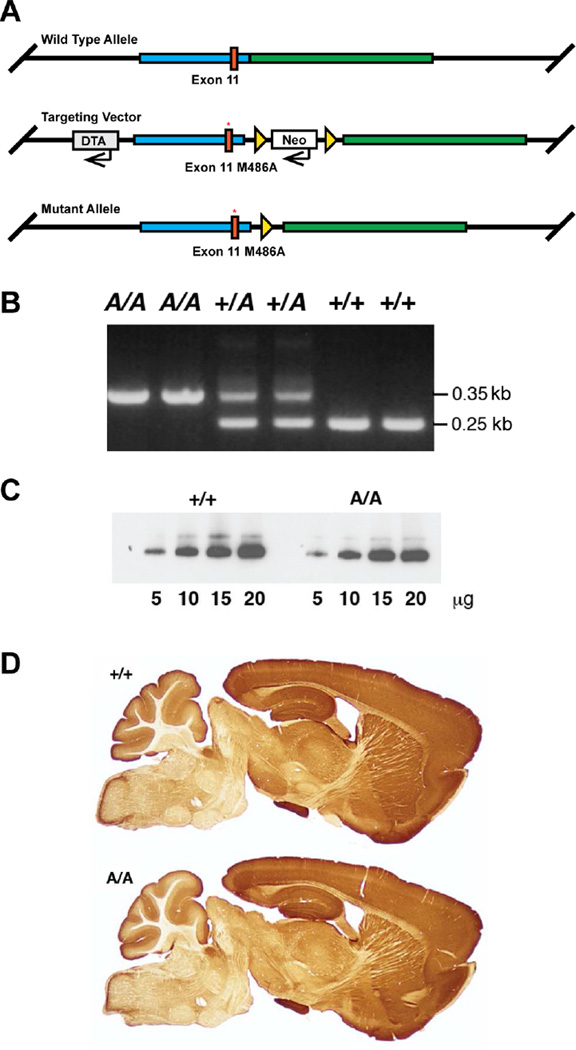
AS-PKCε mice were generated by altering the sequence of exon 11 in the Prkce gene to encode the M486A mutation (Fig. 1A and B). Homozygous AS-PKCε and wild type C57BL/6N mice showed similar abundance and pattern of PKCε immunoreactivity in the brain (Figs. 1C and D). AS-PKCε mice were like wild type littermates in appearance and home cage behavior, and showed a similar startle response and righting reflex. They were also like wild type animals in other behaviors including open field exploration, anxiety like-behavior on the elevated plus maze, hot plate tail-flick latency, ability to remain on an accelerating rotarod, and ability to hang suspended from a wire (Table 1). These results indicate that the PKCε M486A knock-in mutation did not disrupt development or baseline behavior.
Fig. 1.
Generation of AS-PKCε knock-in mice. (A) Schematic showing targeting strategy for generating the M486A mutation (red asterisk) in exon 11 of the mouse Prkce gene. DTA = diphtheria toxin A expression cassette for negative selection; Neo = neomycin expression cassette for positive selection. Triangles represent loxP sites for Cre-recombinase mediated excision of the Neo cassette in embryonic stem cell clones. (B) PCR of tail DNA demonstrated presence of mutant (A) and wild type (+) alleles. (C) Western blot analysis showed similar levels of PKCε immunoreactivity in AS-PKCε (A/A) and wild type (+/+) hippocampus. (D) The distribution of brain PKCε immunoreactivity was similar in AS-PKCε (A/A) and wild type (+/+) mice.
Table 1.
Baseline behaviors are similar in wild type and AS-PKCε mice
| Test (males, age P75-P165) | Wild type (n) | AS-PKCε (n) | P value |
|---|---|---|---|
| Weight (g) at age P90-P120 | 27.76 ± 0.92 (10) | 26.43 ± 0.56 (7) | 0.289 |
| Wire suspension fall latency (sec) | 111.7 ± 30.7 (10) | 74.3 ± 37.6 (7) | 0.450 |
| Accelerating rotarod fall latency (sec) | 56.1 ± 4.8 (19) | 64.1 ± 4.2 (7) | 0.350 |
| Open field total distance (cm) | 6292 ± 462.9 (8) | 5532 ± 733.5 (7) | 0.319 |
| Open field % time in center | 22.4 ± 3.9 (8) | 21.8 ± 4.6 (7) | 0.929 |
| Elevated plus maze (5 min) | |||
| % Open time | 41.0 ± 9.4 (10) | 38.3 ± 10.9 (7) | 0.858 |
| % Open entries | 43.9 ± 5.5 (10) | 43.4 ± 7.6 (7) | 0.959 |
| # Closed entries | 11.9 ± 1.1 (10) | 15.0 ± 2.0 (7) | 0.158 |
| Hot plate latency at 56°C (sec) | 5.80 ± 0.27 (8) | 6.01 ± 0.34 (7) | 0.640 |
3.2. Pharmacokinetics of 1-NA-PP1
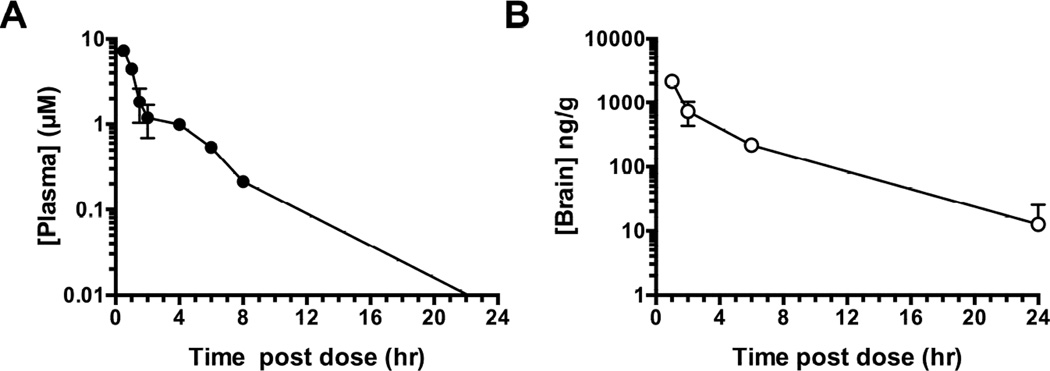
To determine the abundance and half-life of 1-NA-PP1 in plasma and brain, a pharmacokinetic study was performed following intraperitoneal administration of 30mg/kg 1-NA-PP1 in 5% DMSO and 10% Tween-80 to wild type C57BL/6J mice (Fig. 2A). Plasma levels of 1NA-PP1 reached 7.3 ± 0.43µM thirty minutes after injection and declined biphasically (R2= 0.94) with half-lives of 0.47 and 11.62 hours (Fig. 2A). Brain levels reached 2167 ± 85 ng/g (~6.8 ± 0.27µM) one hour after injection and declined in a single-phase (R2 = 0.93) with a half-life of 0.57 hours (Fig. 2B). These results indicate that 1-NA-PP1 enters the brain rapidly and efficiently after intraperitoneal administration and achieves concentrations predicted to inhibit AS-PKCε (Ki = 18.7nM) based on in vitro studies (Qi et al., 2007).
Fig. 2.
1-NA-PP1 pharmacokinetics after intraperitoneal injection of 30mg/kg 1-NA-PP1. Data shown are (A) plasma and (B) brain concentrations of 1-NA-PP1, with n = 3 for each data point.
Plasma and brain concentrations of 1-NA-PP1 were also determined following repeated oral administration. Wild type C57BL/6N mice were provided food pellets containing 1g/kg 1-NA-PP1 and water containing 500µM 1-NA-PP1 in 1% Cremophor-RH40 and 0.2% sucralose. Control animals were fed food and water containing the corresponding vehicles. Mice were sacrificed after 3 days and the concentration of 1-NA-PP1 was determined by LC-MS/MS. Oral administration of 1-NA-PP1 yielded a plasma concentration of 117 ± 23nM (n=5) and brain concentration of 140 ± 54ng/g protein (~ 441 ± 172nM; n=5). These results indicate that repeated administration of 1-NA-PP1 in food and water leads to levels of 1-NA-PP1 in the brain and plasma predicted to inhibit AS-PKCε (Qi et al., 2007).
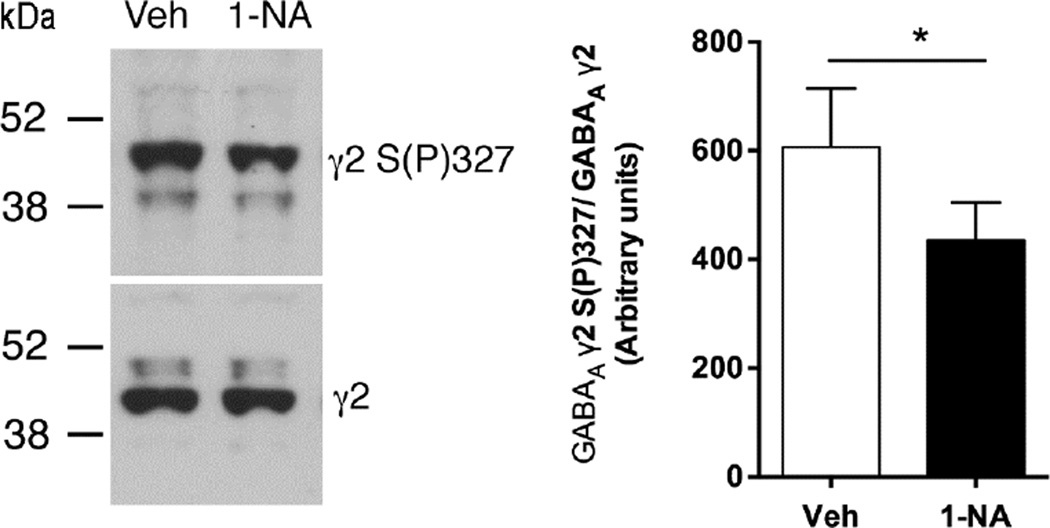
To determine whether systemic administration of 1-NA-PP1 inhibits AS-PKCε-mediated phosphorylation in the brain, we examined phosphorylation of the GABAA receptor γ2 subunit since we previously found that PKCε phosphorylates this subunit at S327 (Qi et al., 2007). We administered 1-NA-PP1 by intraperitoneal injection rather than orally in this experiment to better control the dosage relative to the timing of tissue collection. AS-PKCε mice were administered 25mg/kg 1-NA-PP1 or vehicle and sacrificed 1 hour later. Although we used a different vehicle (5%DMSO/20% Cremophor-EL) to dissolve 1-NA-PP1 for this experiment, pharmacokinetic analyses after intraperitoneal injection of 30mg/kg 1-NA-PP1 in this vehicle revealed plasma (6.47 ± 0.25µM; n = 2) and brain concentrations (2055 ± 455ng/g; ~4.43 ± 2.03µM; n = 2) similar to those observed for 1-NA-PP1 dissolved in 5%DMSO/10% Tween-80. Compared with vehicle-injected mice, there was a 33% reduction in γ2-S(P)327 phosphoimmunoreactivity in the striatum of 1-NA-PP1-treated mice (Fig. 3).
Fig. 3.
GABAA γ2 receptor subunit phosphorylation. Intraperitoneal injection of 25mg/kg 1-NA-PP1 decreased GABAA γ2-S(P)327 immunoreactivity compared with vehicle. Left panel shows representative western blots for anti GABAA γ2S(P)327 immunoreactivity (top) and total GABAA γ2 immunoreactivity (bottom) from the same vehicle (Veh)- and 1-NA-PP1 (1-NA)-treated samples. Right panel shows mean ± S.E.M. results from all animals. *P = 0.0175, t(8) = 2.98, two-tailed, unpaired t-test; n = 5 per group.
3.3. 1-NA-PP1 reduces ethanol consumption by AS-PKCε mice
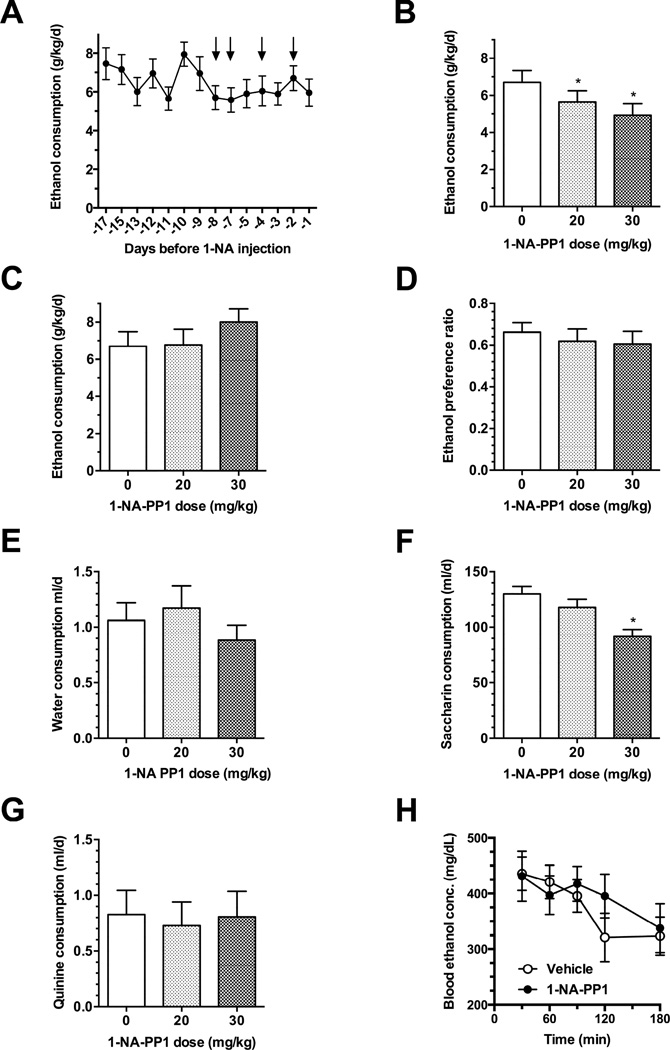
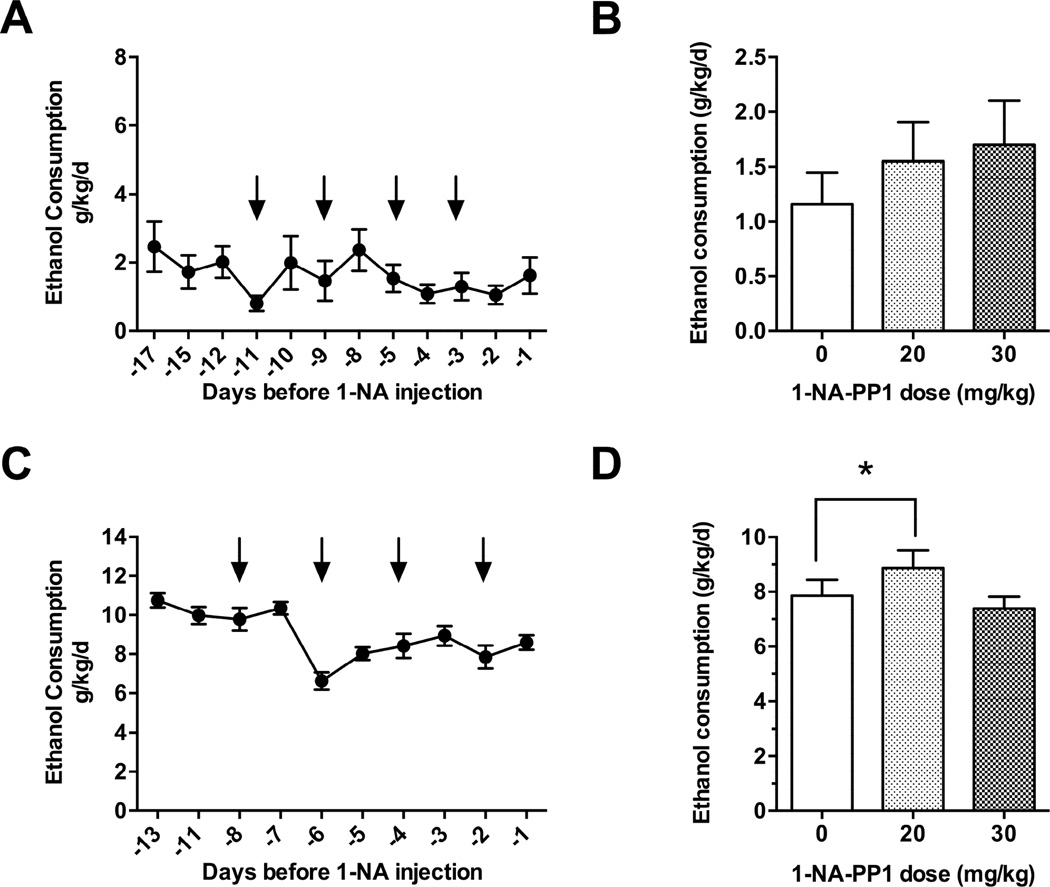
To determine whether 1-NA-PP1 alters ethanol consumption, we subjected AS-PKCε mice to a continuous access, two-bottle choice-drinking procedure whereby the ethanol concentration was escalated from 3% to 6%, and finally to 10% over 8 days. After mice were habituated to vehicle injections and had attained a stable level of drinking 10% ethanol for three consecutive drinking sessions [F(2, 34) = 1.474, P = 0.2433; Fig. 4A], they were administered 1-NA-PP1 using a within-subjects design in which all animals received vehicle or 1-NA-PP1 on different days. 1-NA-PP1 at 20 or 30mg/kg reduced ethanol consumption during the first 24 h [F(2, 34) = 10.69; P = 0.0003; Fig. 4B]. This effect was reversible since ethanol consumption was similar 48 h after treatment with vehicle or 1-NA-PP1 [F(2, 34) = 3.058; P = 0.0601; Fig. 4C]. 1-NA-PP1 did not significantly alter ethanol preference [F(2, 34) = 0.9508; P = 0.3965; Fig. 4D]. Although there was a trend towards reduced water consumption at 30mg/kg, this effect was not statistically significant [F(2, 34) = 1.722; P = 0.1940; Fig. 4E].
Fig. 4.
Ethanol consumption by AS-PKCε mice. (A) AS-PKCε mice were habituated to vehicle injections and allowed to achieve a stable baseline level of drinking. Arrows point to days when animals received vehicle injections. 1-NA-PP1 reduced ethanol consumption (B) and this effect was reversible since it was no longer present 48 hours after administration of 1-NA-PP1 (C). (D) 1-NA-PP1 did not alter preference for ethanol over water or water intake (E) 1-NA-PP1 (30 mg/kg) reduced saccharin intake (F), but not quinine (G) intake.(H) 1-NA-PP1 (30 mg/kg) did not alter ethanol clearance. *P < 0.05, Dunnett’s test; n = 18 per group (A-E), n = 14 per group (F and G), n = 7 per group (H).
To determine whether 1-NA-PP1 alters taste perception, we examined its effect on consumption of saccharin- and quinine-containing solutions. 1-NA-PP1 at 30 mg/kg significantly reduced saccharin consumption [F(2, 26) = 11.22; P = 0.0003; Fig. 4F], but did not alter the amount of quinine consumed [F(2, 26) = 0.099; P = 0.906; Fig. 4G]. These results suggest that at 30mg/kg, 1-NA-PP1 affects perception of sweet, but not bitter taste.
To examine the possibility that 1-NA-PP1 reduced ethanol intake by altering ethanol metabolism, we measured clearance of ethanol administered to AS-PKCε mice 4 hours after intraperitoneal injection of 30mg/kg 1-NA-PP1. Blood ethanol concentrations were measured every 30 minutes for 3 hours (Fig. 4H). 1-NA-PP1 did not significantly alter the time course of ethanol clearance [FTime (4, 40) = 6.423; P = 0.0004; FDrug (1, 10) = 0.867; P = 0.3738; FDrug×Time (4, 40) = 1.034; P = 0.4015].
Finally, to determine whether the effects of 1-NA-PP1 on ethanol consumption were specific for AS-PKCε, we also investigated whether 1-NA-PP1 had an effect in wild type C57BL/6NTac mice that were habituated to vehicle injections and had achieved a stable baseline level of ethanol consumption for three consecutive drinking sessions [F(2,14) = 1.263, P = 0.3132; Fig. 5A]. 1-NA-PP1 did not reduce ethanol intake [F (2, 12) = 0.64; P = 0.54] by C57BL/6NTac mice (Fig. 5B). However, these mice consumed much less ethanol than AS-PKCε mice, causing concern that our inability to observe an effect of 1-NA-PP1 could be do to a floor effect. Hence, we also examined ethanol consumption by C57BL/6J mice, which consume large amounts of ethanol in the continuous access paradigm (Hwa et al., 2011). Mice were habituated to vehicle injections and allowed to achieve a stable baseline level of ethanol consumption for three consecutive drinking sessions prior to administration of 1-NA-PP1 [F(2,18) = 2.162, P = 0.1441; Fig. 5C]. 1-NA-PP1 caused a small increase in ethanol intake by C57BL/6J mice at the 20mg/kg dose only [F (2, 18) = 6.41; P = 0.0079; Fig 5D]. These results demonstrate that 1-NA-PP1 does not reduce ethanol intake in two strains of wild type mice that lack the AS-PKCε mutation.
Fig. 5.
Ethanol consumption by wild type mice. (A) C57BL/6NTac mice were habituated to vehicle injections and stable baseline drinking was attained prior to administration of 1-NA-PP1. Arrows point to days when animal received vehicle injections. (B) Administration of 1-NA-PP1 did not significantly alter drinking by C57BL/6NTac mice (n = 7 per group). (A) C57BL/6J mice were habituated to vehicle injections and stable baseline drinking was attained prior to administration of 1-NA-PP1. Arrows point to sessions when animals received vehicle injections. (B) 1-NA-PP1 produced a small but significant increase in ethanol consumption in C57BL/6J mice at 20mg/kg, but not at 30mg/kg (n = 10 per group). *P < 0.05, Dunnett’s test.
3.4. 1-NA-PP1 prolongs ethanol intoxication in AS-PKCε mice
We previously found that Prkce−/− mice show prolonged signs of ethanol intoxication due to impaired acute functional tolerance to ethanol (Hodge et al., 1999, Wallace et al., 2007). Therefore, to determine if inhibiting PKCε alters ethanol intoxication, and to test whether oral administration of 1-NA-PP1 was effective in producing a phenotype, we fed AS-PKCε mice 1-NA-PP1 or control food and water for 11 days. On average, mice in the 1-NA-PP1 group consumed 3.00 ± 0.14g of 1-NA-PP1 food pellets/day, which was less than the amount consumed by the control group (3.65 ± 0.16g/day; P = 0.02). Mice in the 1-NA-PP1 group also consumed less water (2.00 ± 0.01ml) than mice in the control group (3.5 ± 0.25ml of control liquid /day; P < 0.0001). Nevertheless, despite these differences in food and water intake, body weights were similar in 1-NA-PP1-fed (25.5 ± 0.18g) and control-fed (25.8 ± 0.23g) animals.
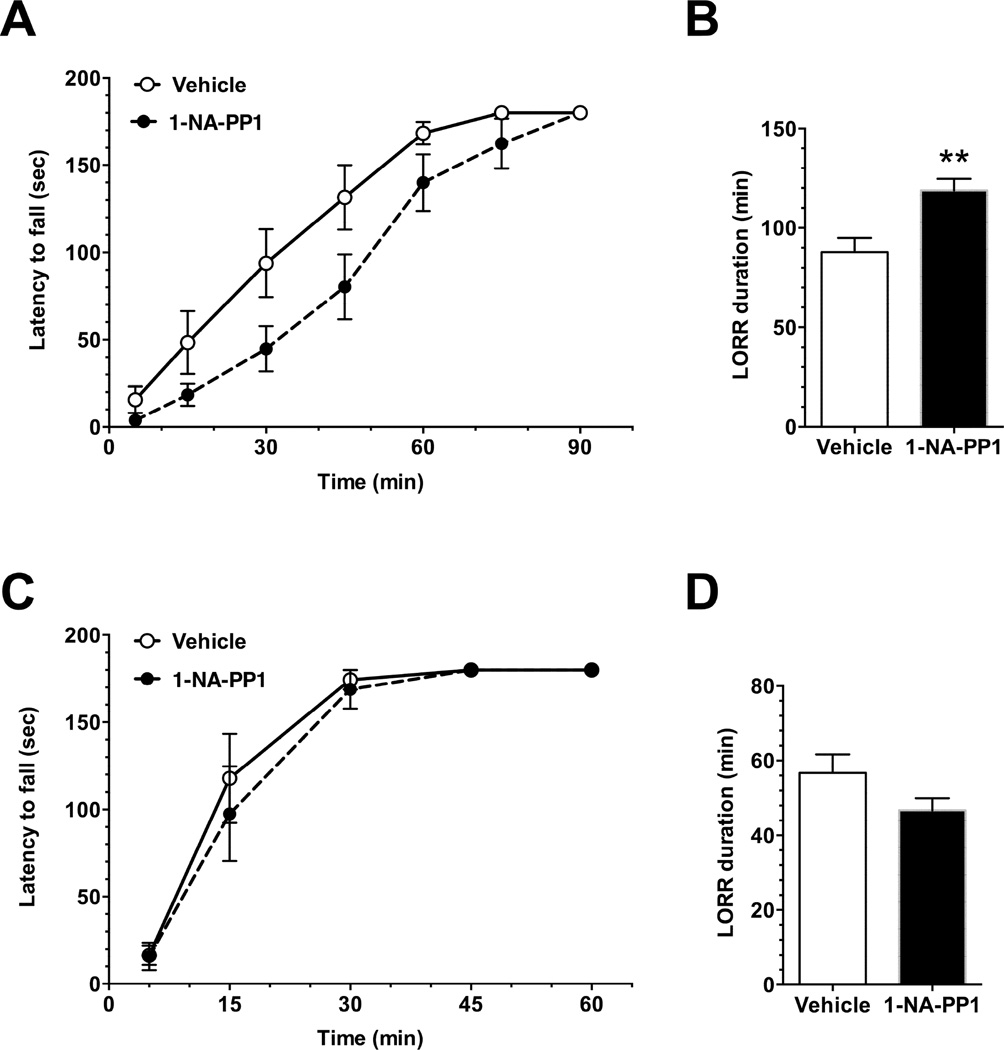
Three days after the start of the feeding protocol, mice were tested for ethanol-induced ataxia (Fig. 6A). 1-NA-PP1 impaired recovery from ataxia induced by 1.5g/kg ethanol [FTime(6, 132) = 84.60, P < 0.0001; FDrug(1, 22) = 5.572, P = 0.0275; FTime×Drug(6, 132) = 1.618, P = 0.147]. The same cohort was tested 2 days later for ethanol-induced loss of the righting reflex (LORR) after receiving 5 days of 1-NA-PP1 in food and water. A second cohort of mice underwent the same feeding protocol for 5 days but also received an i.p. injection of 25mg/kg 1-NA-PP1 thirty min before the test. We observed a similar effect of 1-NA-PP1 in both cohorts, and therefore combined data from both. 1-NA-PP1 significantly increased the duration of the LORR induced by 3.6g/kg ethanol (P = 0.0014, t49=3.392; Fig. 6B).
Fig. 6.
Ethanol-induced ataxia and LORR. In AS-PKCε mice, 30mg/kg 1-NA-PP1 prolonged recovery from ataxia induced by 1.5g/kg ethanol; n = 11 (vehicle), n = 13 (1-NA-PP1). (B) In AS-PKCε mice, 1-NA-PP1 also increased the duration of LORR induced by 3.6g/kg ethanol; n = 25 (vehicle), n = 26 (1-NA-PP1). (C) In wild type mice, 1-NA-PP1 did not alter recovery from ataxia induced by 1.5g/kg ethanol; n = 8 (vehicle), n = 7 (1-NA-PP1). (D) In wild type mice, 1-NA-PP1 also did not alter the duration of the LORR induced by 3.6g/kg ethanol (n = 8 per group). * P = 0.0014, t49 = 3.392, two-tailed, unpaired t-test.
To determine whether the effects of 1-NA-PP1 were specific for AS-PKCε, we tested the effects of 1-NA-PP1 in wild type C57BL/6NTac mice. Mice that were provided 1-NA-PP1 consumed similar amounts of food (4.15g ± 0.34g/day) and fluid (2.76 ± 0.3ml/day) as mice fed a control diet (4.14 ± 0.28g/day and 2.4 ± 0.21 ml/day). 1-NA-PP1 did not affect the duration of ataxia induced by 1.5g/kg ethanol [FTime(4,52) = 69.93, P < 0.0001; FDrug(1, 13) = 0.2997; P = 0.593; FTime×Drug(4,52) = 0.2612; P = 0.902; Fig. 6C]. Likewise, the durations of the LORR induced by 3.6g/kg ethanol were similar in mice administered the 1-NA-PP1 diet and the control diet (Fig. 6D). These results indicate that 1-NA-PP1 has no effect on ethanol-induced ataxia or LORR in wild type mice.
4. Discussion
In this study, we used a chemical-genetic strategy to determine whether a potent and highly selective inhibitor of PKCε could mimic phenotypes we have observed in PKCε knockout mice, namely reduced ethanol consumption and prolonged ethanol intoxication (Hodge et al., 1999). We generated a novel AS-PKCε mouse line harboring a point mutation in the ATP binding site rendering it highly sensitive to inhibition by nanomolar concentrations of the PP1 analog 1-NA-PP1. Systemically administered 1-NA-PP1 crossed the blood-brain barrier and reached high enough concentrations in the brain to inhibit AS-PKCε. 1-NA-PP1 prolonged the ataxic and hypnotic effects of ethanol and reduced ethanol consumption by AS-PKCε mice. These effects of 1-NA-PP1 were not observed in wild type mice lacking the AS-PKCε mutation. These results suggest that compounds that inhibit the catalytic activity of PKCε could be useful in reducing ethanol consumption.
Pharmacokinetic analyses indicated that 1-NA-PP1 is rapidly and readily detected in the plasma and brain after parenteral administration. We also detected significant amounts of 1-NA-PP1 in the brain after chronic oral administration. 1-NA-PP1 inhibited phosphorylation of the GABAA γ2 subunit at S327 in mouse striatum, indicating that 1-NA-PP1 is able to inhibit PKCε-mediated phosphorylation in vivo. We had previously found that GABAA γ2-S(P)327 immunoreactivity is reduced by 60 ±6% in the frontal cortex of Prkce−/− mice (Qi et al., 2007), and phosphatase treatment did not further reduce this residual immunoreactivity, indicating that the antibody also detects dephosphorylated protein. Therefore, a 60% reduction in GABAA γ2-S(P)327 immunoreactivity represents 100% reduction in phosphorylation at this site. Based on these results, we conclude that intraperitoneal administration of 25mg/kg 1-NA-PP1 reduced PKCε-mediated phosphorylation of GABAA γ2 in AS-PKCε mice by approximately 50%.
1-NA-PP1 reduced ethanol consumption in a reversible manner, without significantly reducing alcohol preference at either of the doses tested. Although water intake was not significantly altered, there was some variability in water intake that may have masked a significant reduction in ethanol preference. At the 30mg/kg dose, there was a trend towards reduced water consumption that was not statistically significant. Saccharin, but not quinine consumption, was significantly reduced at the 30mg/kg dose of 1-NA-PP1. This result is different from what was observed in Prkce−/− mice (Hodge et al., 1999), which showed no deficit in saccharin consumption. It is possible that at the 30mg/kg dose, 1-NA-PP1 reduced ethanol consumption by altering the perception of taste for sweet substances, or by effects on brain reward mechanisms or fluid intake. Of note, a reduction in saccharin and sucrose intake has been observed for naltrexone, which is FDA approved to treat alcohol use disorder (Czachowski and Delory, 2009, Ripley et al., 2015).
Baseline ethanol consumption by wild type C57BL/6NTac mice was much lower than by AS-PKCε mice even though both are on a C57BL/6NTac background. This difference in ethanol consumption could be due to differences in rearing environments and to genetic drift in our AS-PKCε colony from inbreeding. Hence, in addition to the C57BL/6NTac strain, we decided to examine the effects of 1-NA-PP1 on ethanol consumption in C57BL/6J mice, which display high intake and preference for alcohol. Importantly, 1-NA-PP1 did not reduce ethanol drinking in either strain of wild type mice, which both lack the AS-PKCε mutation, indicating that the effects of 1-NA-PP1 on ethanol consumption are specific for AS-PKCε.
Our previous molecular studies suggested that PKCε mediates its effects on ethanol-related behaviors by reducing inhibitory GABA neurotransmission through actions at GABAA receptors. We have identified two substrates of PKCε that could contribute to decreased GABAA receptor function: the GABAA γ2 subunit, which when phosphorylated at S327 shows a reduced response to the positive allosteric effects of benzodiazepines and ethanol (Qi et al., 2007), and the N-ethylmaleimide sensitive factor, which when phosphorylated at S460 and T461 reduces the number of cell surface GABAA receptors (Chou et al., 2010). It is likely that additional PKCε substrates play a role in regulating GABAA receptor function and behavioral responses to ethanol. The M486A mutation allows AS-PKCε to use bulky ATP analogs such as N6-benzyl-ATP as phosphate donors, while native kinases cannot use such ATP analogs (Bishop et al., 2001, Zhang et al., 2013). ATP analogs with a thiophosphate at the γ-phosphate position can generate a kinase-transferable tag, allowing use of a covalent capture-and-release method to purify tagged peptides from digests of protein mixtures (Hertz et al., 2010, Ultanir et al., 2012). Mass spectrometric analysis of these peptides reveals the identity of the corresponding proteins and the location of the phosphorylation sites. Use of this methodology with tissues from AS-PKCε mice could identify novel substrates of PKCε in the brain that regulate GABAA receptor function and behavioral responses to ethanol in an unbiased manner.
5. Conclusions
In summary, our results demonstrate that specific inhibition of PKCε reduces ethanol consumption and prolongs ethanol intoxication, confirming phenotypes we have observed previously using strategies that reduce PKCε expression in the brain. Our results strengthen the rationale for developing small molecule inhibitors of PKCε catalytic activity as therapeutics to decrease ethanol consumption. In addition, our findings demonstrate the utility of the AS-PKCε mouse as a tool for studying the role of PKCε in behavior and for identifying direct substrates of PKCε.
Highlights.
Novel knock-in AS-PKCε mice were generated with an ATP analog-specific gatekeeper mutation in the purine-binding site of PKCε.
Administration of the selective AS-kinase inhibitor 1-NA-PP1 to AS-PKCε mice reduced their ethanol consumption.
Administration of 1-NA-PP1 to AS-PKCε mice prolonged ethanol intoxication.
Selective inhibitors of PKCε catalytic activity may prove useful for decreasing ethanol consumption.
Acknowledgments
This work was supported by NIH grants AA13588 and AA017072, and by funds provided by the State of California for medical research for alcohol and substance abuse through UCSF to R.O.M. We thank Michael Cameron and the DMPK core at Scripps Florida and Yong Huang and the Drug Studies Unit, Analytical Division, UCSF College of Pharmacy for their work on the pharmacokinetics and tissue measurement of 1-NA-PP1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: R.O.M. is an inventor on U.S. Patent No. US 8,785,648 B1 entitled PKC-Epsilon Inhibitors, awarded July 22, 2014. None of the other authors of this manuscript have any financial conflicts to disclose.
References
- Bishop AC, Buzko O, Shokat KM. Magic bullets for protein kinases. Trends Cell Biol. 2001;11:167–172. doi: 10.1016/s0962-8924(01)01928-6. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, Janak PH, Ron D. GDNF is an endogenous negative regulator of ethanol-mediated reward and of ethanol consumption after a period of abstinence. Alcohol Clin Exp Res. 2009;33:1012–1024. doi: 10.1111/j.1530-0277.2009.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wang D, Dadgar J, Chang WS, Messing RO. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WH, Wang D, McMahon T, Qi ZH, Song M, Zhang C, Shokat KM, Messing RO. GABAA receptor trafficking is regulated by protein kinase C(epsilon) and the N-ethylmaleimide-sensitive factor. J Neurosci. 2010;30:13955–13965. doi: 10.1523/JNEUROSCI.0270-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Rostock C, Campbell RR, Wroten MG, McGregor H, Caruana AL, Miller BW, Hu JH, Wu Zhang P, Xiao B, Worley PF, Crabbe JC, Finn DA, Szumlinski KK. Protein kinase C epsilon activity in the nucleus accumbens and central nucleus of the amygdala mediates binge alcohol consumption. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan J, Cameron AJ, Saurin AT, Hanrahan S, Totty N, Messing RO, Parker PJ. The identification and characterization of novel PKCepsilon phosphorylation sites provide evidence for functional cross-talk within the PKC superfamily. Biochem J. 2008;411:319–331. doi: 10.1042/bj20071348. [DOI] [PubMed] [Google Scholar]
- Hertz NT, Wang BT, Allen JJ, Zhang C, Dar AC, Burlingame AL, Shokat KM. Chemical genetic approach for kinase-substrate mapping by covalent capture of thiophosphopeptides and analysis by mass spectrometry. Curr Protoc Chem Biol. 2010;2:15–36. doi: 10.1002/9780470559277.ch090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–419. doi: 10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Zou ME, Lim JP, Stecher J, McMahon T, Messing RO. Deletion of Prkcz increases intermittent ethanol consumption in mice. Alcohol Clin Exp Res. 2014;38:170–178. doi: 10.1111/acer.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HM, Wallace MJ, Zeng L, Wang V, Deitchman JK, McMahon T, Messing RO, Newton PM. Amygdala protein kinase C epsilon controls alcohol consumption. Genes Brain Behav. 2009;8:493–499. doi: 10.1111/j.1601-183X.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JP, Zou ME, Janak PH, Messing RO. Responses to ethanol in C57BL/6 versus C57BL/6 × 129 hybrid mice. Brain Behav. 2012;2:22–31. doi: 10.1002/brb3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Increased sensitivity to the aversive effects of ethanol in PKCepsilon null mice revealed by place conditioning. Behav Neurosci. 2007;121:439–442. doi: 10.1037/0735-7044.121.2.439. [DOI] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Sanchez-Roige S, Bullmore ET, Mugnaini M, Maltby K, Miller SR, Wille DR, Nathan P, Stephens DN. The novel mu-opioid antagonist, GSK1521498, reduces ethanol consumption in C57BL/6J mice. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultanir SK, Hertz NT, Li G, Ge WP, Burlingame AL, Pleasure SJ, Shokat KM, Jan LY, Jan YN. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 Uncovers their roles in dendrite arborization and spine development. Neuron. 2012;73:1127–1142. doi: 10.1016/j.neuron.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, Messing RO. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lopez MS, Dar AC, Ladow E, Finkbeiner S, Yun CH, Eck MJ, Shokat KM. Structure-guided inhibitor design expands the scope of analog-sensitive kinase technology. ACS Chem Biol. 2013;8:1931–1938. doi: 10.1021/cb400376p. [DOI] [PMC free article] [PubMed] [Google Scholar]