Abstract
In developed countries, specific metabolites have been associated with obesity and metabolic diseases, e.g. type 2 diabetes. It is unknown whether a similar profile persists across populations of African-origin, at increased risk for obesity and related diseases. In a cross-sectional study of normal-weight and obese black women (33.3 ± 6.3 years) from the US (N = 69, 65 % obese), South Africa (SA, N = 97, 49 % obese) and Ghana (N = 82, 33 % obese) serum metabolite profiles were characterized via gas chromatography-time of flight/mass spectrometry. In US and SA women, BMI correlated with branched-chain and aromatic amino acids, as well as dopamine and aminoadipic acid. The relationship between BMI and lipid metabolites differed by site; BMI correlated positively with palmitoleic acid (16:1) in the US; negatively with stearic acid (18:0) in SA, and positively with arachidonic acid (20:4) in Ghana. BMI was also positively associated with sugar-related metabolites in the US; i.e. uric acid, and mannitol, and with glucosamine, glucoronic acid and mannitol in SA. While we identified a common amino acid metabolite profile associated with obesity in black women from the US and SA, we also found site-specific obesity-related metabolites suggesting that the local environment is a key moderator of obesity.
Keywords: Obesity, African-origin, Amino acid profile
1 Introduction
The worldwide prevalence of overweight and obesity has risen substantially in the past three decades, but is influenced by region (Ng et al. 2014). In developed countries, the obesity prevalence rate over the last 8 years has slowed, while in developing countries, where almost two-thirds of the world's population resides, it continues to increase (Ng et al. 2014). Obesity is associated with increased risk for non-communicable diseases including diabetes, and cardiovascular disease, and which are greater in populations of African-origin than white populations (Golden et al. 2012).
Genetic factors alone cannot explain the rapid increases in obesity and associated disease risk in populations of African-origin (Corona et al. 2013). Rather, obesity is multi-factorial, and involves a complex interaction between genetic, environmental and lifestyle factors. An understanding of this interaction is essential to address the problem of obesity and associated diseases in this high-risk population, and can be explored by measuring changes in intermediary metabolism associated with obesity. This can be achieved by using metabolomics, a comprehensive metabolite profiling approach. Due to its location downstream of the genome, it provides a direct link between biochemical pathways and observed phenotypes (Xie et al. 2012).
Via the use of metabolomics, independent studies have presented a potential link between key intermediates in lipid, protein and carbohydrate metabolism and obesity, and related metabolic diseases such as type 2 diabetes, cardiovascular disease and certain cancers (Newgard et al. 2009; Wang et al. 2011; Newgard 2012; Xie et al. 2012; Cheng et al. 2012; Moore et al. 2014). Most of these studies were however undertaken in developed countries, and predominantly focused on white populations. It is currently not known whether a similar metabolite profile will be observed in populations residing in developing countries, whose habitual diet and daily physical activity (PA) levels vary markedly from those in developed countries (Dugas et al. 2011; Luke et al. 2014). An obesity-related metabolite profile, common to black populations at different levels of transition might provide an early therapeutic target for populations at an increased risk of obesity-related diseases such as type 2 diabetes.
We are uniquely placed to explore the metabolite profiles associated with obesity in black populations using the Modeling the Epidemiologic Transition Study (METS) cohort, a longitudinal international study examining the relationships between lifestyle factors and obesity, type 2 diabetes and cardiovascular disease risk (Luke et al. 2012). Therefore, the primary aim of the study was to identify metabolites and/or metabolite profiles associated with obesity in normal-weight and obese black women spanning the epidemiologic transition (Ghana, South Africa and the US). Secondly, we examined whether the metabolite profile associated with obesity was common between countries whose level of transition, and hence lifestyle behaviors (i.e. diet and physical activity) differs.
2 Materials and methods
2.1 Participant selection
Previously, 2500 adults (24-45 years), predominantly of African descent, were enrolled in METS between January 2010 and December 2011 and tested on a yearly basis (Luke et al. 2012). For the current study, stored blood samples from 248 normal-weight (BMI < 25 kg/m2) and obese (BMI ≥ 30 kg/m2) women from three sites, Ghana, South Africa (SA), and US, representing countries spanning the epidemiologic transition, based on the Human Development Index (HDI, (2014). Ghana's HDI is 0.541 (low middle), SA is 0.619 (middle), and the US index is 0.910 (very high). Participants were included in the current study after the exclusion of overweight women (i.e. BMI ≥ 25 and BMI < 30 kg/m2) and then matched between sites by using space-filling design in a principal component analysis model calculated from 2010 METS descriptors including, PA, BMI, age and body fat (Thysell et al. 2012). Due to the lack of normal-weight women in the US cohort and obese women in the Ghanaian cohort, the proportion of normal-weight and obese women in these sites were not equal. The final sample included 24 normal-weight and 44 obese US women, 48 normal-weight and 47 obese SA women, and 52 normal-weight and 26 obese Ghanaian women. The study protocols were approved by the Institutional Review Board of Loyola University Chicago, IL, USA; the Committee on Human Research Publication and Ethics of Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; and the Health Sciences Faculty Research Ethics Committee of the University of Cape Town, SA. Written informed consent was obtained from all participants.
2.2 Body composition, lifestyle and biochemical measurements
All measurements were made at outpatient clinics located in the respective communities. Weight and height were measured and body composition was estimated by bio-electrical impedance analysis (BIA, model 101Q; RJL Systems, Clinton Township, MI). Fat-free mass and fat mass were estimated from measured resistance by using an equation validated against isotope dilution in the METS cohorts (Luke et al. 1997).
Physical activity (PA) and sedentary time was assessed using the Actical accelerometer (Phillips Respironics, Bend, OR, USA). Participant files were included for analysis if they contained four or more valid days, i.e. 10 or more hours of wear time. Sedentary, light, moderate and vigorous activity levels were defined using published cut-points (Wong et al. 2011) and moderate and vigorous activity were summed to create the variable moderate vigorous physical activity (MVPA).
Dietary Intake was estimated using two 24-h recalls (Carriquiry 2003), at least 7 days apart (Steyn et al. 2011). The Nutrient Data System for Research (NDSR; University of Minneapolis, MN, USA) (Carriquiry 2003) was used to calculate total energy intake and macronutrient composition (i.e., % kJ from fat, carbohydrate and protein).
Participants were requested to fast from 8 pm in the evening prior to the clinic examination, during which fasting capillary glucose concentrations were determined using finger stick (Accu-check Aviva, Roche). In addition, blood samples were drawn for the subsequent analysis of serum lipids (total cholesterol, HDL-cholesterol and triglycerides), high sensitivity c-reactive protein (hsCRP) and metabolites. Serum total cholesterol, HDL-cholesterol and triglycerides were measured by enzymatic colorimetric assays using a Hitachi 917 instrument (Roche, IN, USA). LDL-cholesterol was calculated using the Friedewald equation (Friedewald et al. 1972).
2.3 Metabolomics analysis
A run order design was constructed to minimize bias from sample preparation and analysis when comparing samples between sites and BMI groups (Jonsson et al. 2015). The samples were analyzed using gas chromatography-time of flight/mass spectrometry (GC-TOF/MS). Prior to GC-TOF/MS analysis, a MeOH-H2O extraction followed by a two-step derivatization procedure of serum metabolites was performed (A et al. 2005). The samples were then injected in splitless mode by a CTC Combi Pal autosampler (CTC Analytics AG, Zwingen, Switzerland)) into an Agilent 6890 gas chromatograph equipped with a 10 m × 0.18 mm i.d. fused silica capillary column with a chemically bonded 0.18 μm DB 5-MS stationary phase (J&W Scientific, Folsom, CA). The column effluent was introduced into the ion source of a Pegasus III time-of-flight mass spectrometer, GC-TOF/MS (Leco Corp., St Joseph, MI). To monitor instrument specificity and sensitivity, quality control (QC) samples, i.e. a pool of all included samples, were analyzed at start, end and after every 10th analyzed sample.
2.4 Metabolite identification
In total, 248 plasma samples were metabolically characterized using GC-TOF/MS, from which four were excluded due to low analytical quality. Thus, 244 samples were processed using hierarchical multivariate curve resolution (HMCR) with target processing using in-house spectral library. In total, 108 identified metabolites were considered robust (RSD < 40) and thus included in further multivariate modeling together with relevant clinical, anthropometric and PA variables.
All resolved mass spectral profiles were subjected to spectral database search in NIST MS-Search v. 2.0.38. Match values ranking the spectra were calculated using the dot product of the two spectra (i.e. the resolved spectrum and the database spectrum), with higher m/z peaks having more weight than lower m/z peaks, since higher m/z values are considered to be more compound-specific. The match values range from 0 to 999, where 999 indicates an identical match. Positive identification was confirmed by combining match values with the retention time index, calculated from the analytically characterized alkane series (C10–C40). Only metabolites identified with high certainty and with relative standards deviation, based on QC samples, (RSD) < 40 were included in further multivariate modeling.
2.5 Data treatment
Hierarchical multivariate curve resolution (HMCR) (Jonsson et al. 2005) was used to resolve overlapping metabolites/compounds that where not separated in the chromatographic step. All samples were processed simultaneously except the analytical replicates, which were predicted into the existing HMCR model (Jonsson et al. 2006). To remove instrumental drift and concentration variation, the resolved data were normalized by means of 12 added internal standards eluting over the whole chromatographic range consisted of: [13C5]-proline, [2H4]-succinic acid, [13C5, 15N]-glutamic acid, [1,2,3-13C3]-myristic acid, [2H7]-cholesterol and [13C4]-disodium-ke-toglutarate were purchased from Cambridge Isotope Laboratories (Andover, MA); [13C12]-sucrose, [13C4]-palmitic acid and [2H4]-butanediamine 2HCl were obtained from Campro (Veenendaal, The Netherlands); [13C6]-glucose was purchased from Sigma Aldrich (Steinheim, Germany); [2H6]-salicylic acid was purchased from Icon (Summit, NJ); and NMethyl-N-trimethylsilyltrifluoroacetamide (MSTFA), 1 % trimethylchlorosilane (TMCS) and pyridine (silylation grade) were purchased from Pierce Chemical Co. Stock solutions of the reference compounds and IS were prepared either in Milli-Q water or methanol at the same concentration, 0.5 μg μL−1. First the area under the chromatographic peak for each internal standard was calculated using non-noisy and unique mass-channels. Secondly, to include the weight from all internal standards, a principal component (PC) was calculated based on the chromatographic peak areas from all internal-standards (all variables were scaled to unit-variance, non-centered). Finally, the calculated PC score value, which in this case is comparable to the sample mean, was used to normalize the resolved data by dividing each sample with the corresponding score value (Redestig et al. 2009).
2.6 Statistical analysis
Data are presented as mean ± standard deviation, or percentage. Differences between sites were compared using ANOVA, with Bonferroni posthoc analysis. For the multivariate analysis, initial inspections of the data sets were carried out by principal components analysis (PCA) to detect groupings, outliers and trends within each site. Further sample comparison modeling were performed by using multivariate regression analysis by means of orthogonal partial least squares (OPLS), to elucidate BMI-related metabolic patterns among circulating metabolites and relevant clinical, anthropometric and PA variables. Notably, the axis values on included OPLS figure are OPLS correlations and cannot be interpreted as for example, Pearsons correlations coefficients. Since they are latent variables, the actual value can only be compared within each model, and not between different models. Therefore, we have combined the highlighted OPLS profile with univariate correlation values in plot and table.
Since we did not have a balanced set of normal-weight and obese individuals in US and Ghana we used a continuous response, i.e. the BMI, instead of comparing normal-weight to obese. Thus, we circumvent the problem of an unbalanced multivariate model. To validate the obtained OPLS models, p-values were calculated for the differences between the predefined classes. These p-values were calculated using ANOVA based on the cross validated OPLS scores (CV-ANOVA), only models with p < 0.05 was considered significant. Variables important to the multivariate model projection (VIP), reflects the relative importance of each metabolite to explain the response. A variable was considered significant if it had either a VIP value >1, i.e. the most relevant variables for explaining the response, and/or significant p value (two tailed, paired Student's t-test). A 95 % significance level was applied to all significance tests, i.e. p-values from univariate t-tests as well as CV-ANOVAs and the jack-knifing based confidence intervals (Efron and Gong 1983) from OPLS models.
3 Results
3.1 Subject characteristics
Table 1 presents the demographic, anthropometric and biochemical characteristics of the entire cohort, by site and BMI category (i.e. normal-weight vs. obese). The mean age of all the women was 33.3 ± 6.3 years. SA women were younger than the other sites, and the obese US women were older than their normal-weight counterparts. US women had the most number of years of education, followed by SA and then Ghanaians. The majority of women from SA and Ghana performed manual labor, compared to only a third of US women. Forty percent of SA women used hormonal contraceptives compared to only 14 % of Ghanaians and 9 % of US.
Table 1. Demographic, anthropometric and cardio-metabolic outcomes, by BMI category and site.
| US | South Africa | Ghana | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| NW (N = 24) | Obese (N = 44) | NW (N = 48) | Obese (N = 47) | NW (N = 52) | Obese (n = 26) | |
| Age (years) | 31.5 ± 6.9 | 35.2 ± 5.7 | 32.1 ± 5.2 | 32.7 ± 6.4 | 33.2 ± 7.1 | 35.5 ± 5.7 |
| Education (years) | 13.7 ± 2.8 | 13.8 ± 2.8 | 10.2 ± 1.5β | 9.9 ± 2.3β | 7.7 ± 4.6β | 7.6 ± 4.5β |
| % Manual labor | 35.5 % | 33.3 % | 87.8 %β | 95.5 % | 88.1 %β | 81.8 %β |
| Body composition | ||||||
| Weight (kg) | 59.6 ± 7.4 | 104.5 ± 16.1 | 55.7 ± 5.7α | 93.8 ± 15.9α | 54.1 ± 5.9β | 86.6 ± 11.6β |
| Height (cm) | 1.66 ± 0.06 | 1.64 ± 0.07 | 1.59 ± 0.06α | 1.60 ± 0.06α | 1.58 ± 0.06β | 1.59 ± 0.06β |
| BMI (kg/m2) | 21.6 ± 2.5 | 38.9 ± 6.0 | 21.9 ± 1.9 | 36.5 ± 5.2 | 21.6 ± 2.1 | 34.3 ± 4.58α |
| Fat-free mass (kg) | 39.4 ± 4.0 | 54.1 ± 6.4 | 36.3 ± 3.4β | 48.7 ± 6.5β | 37.1 ± 3.2α | 49.3 ± 4.6α |
| Fat mass (kg) | 20.4 ± 5.4 | 50.4 ± 10.8 | 19.4 ± 4.2α | 45.1 ± 10.1 | 17.0 ± 4.0βγ | 37.2 ± 7.8βδ |
| %body fat | 33.6 ± 6.3 | 47.9 ± 3.7 | 34.6 ± 5.0 | 47.7 ± 3.1 | 31.1 ± 4.8δ | 42.7 ± 3.5βδ |
| Waist (cm) | 75.5 ± 8.5 | 112.6 ± 14.1 | 77.0 ± 6.9 | 104.8 ± 12.7α | 76.0 ± 8.6 | 103.9 ± 14.2α |
| Hip (cm) | 95.4 ± 6.4 | 127.9 ± 11.5 | 94.8 ± 6.1 | 124.0 ± 9.6 | 92.3 ± 11.2 | 116.7 ± 10.0β |
| WHR | 0.79 ± 0.07 | 0.88 ± 0.08 | 0.81 ± 0.06 | 0.85 ± 0.08 | 0.83 ± 0.08 | 0.89 ± 0.08 |
| Cardio-metabolic outcomes | ||||||
| SBP (mmHg) | 116.0 ± 16.1 | 114.2 ± 15.4 | 119.6 ± 17.4 | 118.6 ± 21.7 | 111.1 ± 15.7 | 108.9 ± 14.2γ |
| DBP (mmHg) | 73.9 ± 14.1 | 79.5 ± 12.1 | 74.9 ± 12.2 | 78.2 ± 11.3 | 64.2 ± 12.3α | 70.0 ± 11.6α γ |
| Glucose (mmol/l) | 5.5 ± 3.2 | 5.4 ± 0.8 | 4.1 ± 0.7α | 4.7 ± 0.9α | 5.6 ± 0.8δ | 5.6 ± 0.6δ |
| Cholesterol (mmol/l) | 4.3 ± 1.1 | 4.8 ± 0.9 | 4.1 ± 0.9 | 4.1 ± 0.8α | 4.0 ± 0.9 | 4.5 ± 0.9 |
| HDL-C (mmol/l) | 1.5 ± 0.5 | 1.3 ± 0.3 | 1.4 ± 0.6 | 1.1 ± 0.3α | 1.2 ± 0.4 | 1.1 ± 0.3α |
| LDL-C (mmol/l) | 2.5 ± 0.8 | 2.9 ± 0.8 | 2.4 ± 0.7 | 2.6 ± 0.7 | 2.4 ± 0.7 | 2.9 ± 0.8 |
| Triglycerides (mmol/l) | 0.74 ± 0.28 | 1.3 ± 0.5 | 0.77 ± 0.33 | 0.90 ± 0.43α | 0.78 ± 0.29 | 1.0 ± 0.50 |
| CRP (nmol/l) | 3.8 ± 7.6 | 8.1 ± 7.5 | 7.5 ± 14.3 | 7.9 ± 7.3 | 3.8 ± 10.8 | 8.4 ± 20.6 |
Values are mean ± SD. NW normal-weight, BMI body mass index, WHR waist/hip ratio, SBP systolic blood pressure, SBP diastolic blood pressure, HDL-C high density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, CRP C-reactive protein
Significantly different to US, within BMI category, p < 0.05
Significantly different to US, within BMI category, p < 0.001
Significantly different to South Africa, within BMI category, p < 0.05
Significantly different to South Africa, within BMI category, p < 0.001
The mean BMI of the normal-weight women was similar across sites, while the mean BMI of the obese women was higher in the US compared to the Ghanaian women. Among normal-weight participants, SA women had the least fat-free mass, and the highest % body fat. Among the obese participants, US women had the greatest fat-free mass, and waist circumference, while Ghanaians had the lowest % body fat.
The fasted capillary glucose concentrations were highest in Ghana and lowest in SA, the only country in which differences between normal-weight and obese women were observed. Total cholesterol, LDL-cholesterol and triglycerides were similar across all sites among the normal-weight participants. Obese SA women had significantly lower triglycerides concentrations, while HDL-cholesterol concentrations were higher in obese US compared to obese SA and Ghanaian women (p < 0.01 for both). Serum hsCRP concentrations did not differ between normal-weight US and Ghanaian women, and increased similarly with obesity in both sites, while in normal-weight SA women, hsCRP was higher than US and Ghanaian women and did not increase with obesity.
PA and dietary data by site and BMI group are presented in Table 2. Overall MVPA was greatest among the Ghanaians and lowest among the US women, driven by extremely low MVPA levels among the obese US women. Conversely, sedentary time was greatest among both the normal-weight and obese SA women.
Table 2. Physical activity and dietary energy intake, by BMI category and site (mean ± SD).
| US | South Africa | Ghana | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| NW (N = 24) | Obese (N = 44) | NW (N = 48) | Obese (N = 47) | NW (N = 52) | Obese (n = 26) | |
| Physical activity | ||||||
| MVPA (1-min) | 20.3 ± 20.4 | 11.6 ± 12.1 | 24.6 ± 16.6 | 19.4 ± 14.2α | 28.8 ± 17.5 | 19.6 ± 13.1α |
| MVPA (10-min) | 9.7 ± 12.8 | 4.6 ± 7.9 | 11.4 ± 12.1 | 7.3 ± 7.6 | 13.0 ± 12.1 | 7.6 ± 8.1 |
| Light PA (1-min) | 193.3 ± 53.2 | 196.8 ± 65.2 | 175.6 ± 55.6 | 202.2 ± 58.7 | 256.6 ± 54.1βδ | 197.1 ± 62.9 |
| Light PA (10-min) | 86.7 ± 46.6 | 105.7 ± 66.9 | 76.0 ± 48.2 | 105.0 ± 47.7 | 158.1 ± 61.5βδ | 106.0 ± 60.2 |
| Sedentary (1-min) | 214.7 ± 40.4 | 200.9 ± 53.5 | 228.4 ± 48.6 | 218.3 ± 46.0 | 194.9 ± 33.2 | 187.2 ± 46.4γ |
| Sedentary (10-min) | 46.9 ± 23.3 | 45.2 ± 40.1 | 69.1 ± 42.1α | 59.6 ± 37.7 | 42.6 ± 23.6δ | 41.8 ± 27.4 |
| Dietary intake | ||||||
| Energy (MJ) | 9.2 ± 2.8 | 8.6 ± 3.2 | 4.3 ± 1.3β | 4.1 ± 1.1β | 7.2 ± 1.8β | 7.6 ± 1.6 |
| Protein (%E) | 16.0 ± 3.6 | 14.9 ± 4.1 | 16.0 ± 4.3 | 16.2 ± 4.7 | 11.6 ± 4.6β | 12.3 ± 3.8 |
| Carbohydrate (%E) | 45.9 ± 8.3 | 47.7 ± 10.0 | 57.4 ± 11.4β | 58.8 ± 11.3β | 64.6 ± 10.8β | 62.9 ± 11.1β |
| Fat (%E) | 38.0 ± 6.7 | 36.4 ± 7.0 | 26.6 ± 9.0β | 25.0 ± 8.2β | 23.7 ± 10.1β | 24.5 ± 9.2β |
| SFA (%E) | 12.8 ± 3.3 | 11.6 ± 3.2 | 7.8 ± 3.2β | 7.9 ± 3.3β | 7.8 ± 4.8β | 7.7 ± 4.0β |
| MUFA (%E) | 14.0 ± 3.4 | 13.4 ± 3.3 | 9.1 ± 3.8β | 8.3 ± 3.4β | 8.8 ± 3.8β | 9.4 ± 3.8β |
| PUFA (%E) | 8.0 ± 2.5 | 8.4 ± 3.1 | 6.8 ± 2.9 | 6.0 ± 2.6β | 5.1 ± 2.7β δ | 5.4 ± 3.0β |
Values are mean ± standard deviation. Physical activity presented as 1 and 10 min bouts, measured using accelerometry. NW normal-weight, MVPA moderate-to-vigorous PA, %E percentage of total energy intake, SFA saturated fatty acid, MUFA monounsaturated fatty acid, PUFA polyunsaturated fatty acid
Significantly different to US, within BMI category, p < 0.05
significantly different to US, within BMI category, p < 0.001
Significantly different to South Africa, within BMI category, p < 0.05,
Significantly different to South Africa, within BMI category, p < 0.001
Normal-weight and obese US women reported the highest energy intake, followed by Ghanaian women and then SA women. When expressed relative to total energy intake, protein intake did not differ between US and SA women, but was significantly lower among Ghanaians. US women consumed less carbohydrate, but more total fat, including saturated, monounsaturated and polyunsaturated fat, than their SA and Ghanaian counterparts. Within sites, macronutrient composition did not differ significantly by BMI group.
3.2 Metabolomics
Based on PCA analysis of each site separately, in combination with manual inspection of each crude and resolved spectrum, no significant outlier or clusters were detected among the plasma metabolite profiles. In addition, we could not detect any significant outliers among the QC samples via PCA, calculated from metabolites included in further analysis, i.e. RSD < 40 (Figure S1).
A complete list of all variables included in the multivariate analyses is listed in Supplementary Table 1, together with related statistics.
We detected significant site-specific OPLS models (p < 0.05) that described BMI-related metabolite profiles (Fig. 1), i.e. using BMI as the response. All BMI-related OPLS model included two component one predictive and one orthogonal. All calculated models had a predictive ability >76 %, i.e. Q2 based on cross-validation, and explained >22 % of all variation among the variables and >84 % of the response variation, i.e. R2X and R2Y respectively.
Fig. 1.
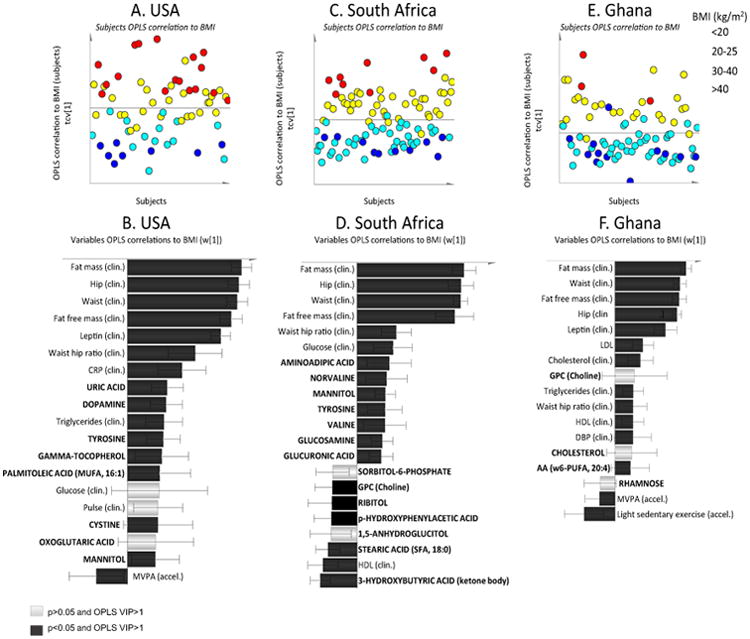
Three separate site-specific OPLS models describing metabolic profiles, i.e. plasma metabolites and clinical anthropometrics, associated with BMI in normal-weight and obese women. Variables/subjects with positive axis value have a positive correlation with BMI and variables/subjects with negative axis value have a negative correlation with BMI. Noteworthy, the axis values are OPLS correlations and cannot be interpreted as for example Pearsons correlations values. They are latent variables, thus the actual value can only be compared within each mode not between different models. Only identified metabolites with variables important to the multivariate model projection (VIP) value >1 are shown and variables are colored according to univariate significance level, i.e. black bars p < 0.05 and white bars p > 0.05. A complete list of all variables included in the OPLS models are listed in Supplementary Table 1. Subjects (plots below) are colored according to their BMI as described in the figure legend. US women revealed a clear BMI metabolite profile (a), which was mainly related to uric acid, amino acids, and lipids (plot b). South African women showed a robust metabolite profile related to BMI (plot c), where a high BMI was mainly related to alterations in amino acids and circulating sugars (plot d). Ghanaian women did not reveal a robust metabolite profile related to BMI (plot e). Among the circulating metabolites, only arachidonic acid was significant (plot e)
In the US, we detected significantly higher levels of circulating uric acid, dopamine and tyrosine, gamma-to-copherol, palmitoleic acid (MUFA 16:1), cysteine and mannitol in women with a high BMI. In the OPLS model, these parameters were highly correlated with high adiposity (i.e. fat mass and waist circumference) and elevated serum lipids (e.g. total-/HDL-cholesterol ratio and triglycerides), and low levels of MVPA (Fig. 1b).
In SA, a high BMI was predominantly related to higher levels of amino acids, such as tyrosine, valine, norvaline, aminoadipic acid, and circulating sugars, such as glucose, glucosamine and glucuronic acid, but lower 3-hydroxybutyric acid, stearic acid (SFA, 18:0), 1,5-anhydroglucitol, p-hydroxyphenylacetic acid, glycerophosphocholine and ribitol. In addition, a high BMI was associated with lower HDL-cholesterol concentrations. Unlike the US, we did not observe any correlation between this BMI-related metabolite profile and MVPA in the SA women (Fig. 1d).
In Ghana, measures of total body fatness, including %body fat and waist circumference, and serum lipids (total- HDL- and LDL-cholesterol and triglycerides) were included in the OPLS model. The only circulating metabolite that correlated with BMI in Ghanaian women was arachidonic acid (n – 6 PUFA) (Fig. 1f).
4 Discussion
The main finding from our study was that we identified a common amino acid profile associated with obesity in black women from the US and SA, which was not detected in Ghanaian women who had a lower HDI, with lower dietary protein and fat intakes, and higher levels of physical activity compared to the US and SA women. Other site-specific metabolites associated with obesity included intermediates in lipid and carbohydrate metabolism, which largely reflected the populations' habitual diet
Previous studies undertaken in developed countries have shown that the component that discriminated most between lean and obese participants included a combination of branched-chain amino acids (BCAA) and aromatic amino acids (AA) (Newgard 2012; Kim et al. 2010; Cheng et al. 2012; Moore et al. 2014). In support of these findings, we showed that in the US and SA women, BMI correlated with tyrosine, an AA, and valine, a BCAA, and its isomer norvaline. In addition, dopamine, which is synthesized from the AAs, phenyalanine and tyrosine, was also associated with obesity in the US women. An elevated BCAA and AA profile in obese individuals has previously been associated with a Westernized diet, typically characterized by high levels of protein and fat intake (Newgard et al. 2009; Bouchard-Mercier et al. 2014). Ghanaian women, consumed significantly less protein and fat than their US and SA counterparts, which could partly account for their lower AA levels. Moreover, there were fewer obese Ghanaian women, and their mean body fat was lower than both their US and SA counterparts (Table 1). While we did not measure insulin sensitivity in this study, it might be suggested that the Ghanaian women were less insulin resistant than the US and SA women. Previous research suggests that elevated BCAA concentrations, in the context of a high fat diet, are closely associated with insulin resistance (Newgard et al. 2009; Cheng et al. 2012). Newgard et al. (2009), after stratifying participants by adiposity, showed that the association between the BCAA-related metabolite component and insulin resistance remained in the obese individuals, but not the normal-weight individuals. Taken together, these findings confirm the association between BCAA and AAs with obesity in populations further along the epidemiologic transition, and typically characterized by a higher prevalence of obesity and greater protein and fat intakes.
Within the SA cohort, we found a strong association between BMI and aminoadipic acid, a metabolite of the essential amino acid lysine. Wang et al. (Wang et al. 2013) recently showed that 2-aminoadipic acid was strongly associated with future diabetes risk in two large independent cohorts as it augments insulin secretion, possibly contributing to the compensatory hyperinsulinemia seen in early insulin resistant states (Wang et al. 2013). Previous research from our lab has shown that black SA women hypersecrete insulin to maintain normoglycemia (Goedecke et al. 2009), but with increasing age, insulin secretion in relation to insulin sensitivity decreases and the prevalence of impaired glucose tolerance and type 2 diabetes increases (Goedecke et al. 2014).
We could also observe alterations in the BMI-related metabolite profile that could be directly linked to the populations' habitual diet. Lipid metabolites, which to a certain extent reflect the dietary fatty acid intake (Ma et al. 1995), were associated with obesity, but the specific lipids differed by country. For example, within the SA cohort, BMI was negatively correlated with stearic acid (18:0), which is in direct contrast to many other studies that have shown a positive association between serum saturated fatty acids and obesity, insulin resistance and the metabolic syndrome (Kim et al. 2010; Vessby 2003). However, research from our lab has shown that black SA women consume more polyunsaturated than saturated fats, and that this is accentuated by obesity (i.e. the polyunsaturated-to-saturated (PS) ratio increases), which was in direct contrast to that reported in white women in whom the PS ratio decreased with obesity (Joffe et al. 2014).
Within the US, BMI was positively associated with palmitoleic acid (16:1) and gamma tocopherol, which is supported by previous studies (Kim et al. 2010; Vessby 2003; Guertin et al. 2014). Palmitic acid (16:0) is desaturated by stearoyl-CoA desaturase (SCD1, Δ9 desaturase) to palmitoleic acid (16:1), and likely is a consequence of a high dietary intake of palmitic acid, in the US women, or alternatively, high activity of SCD1 reported in obese insulin-resistant individuals (Dobrzyn et al. 2010). Gamma tocopherol, a major component of fried foods, has been inversely related to indices of healthy eating in two independent studies (Guertin et al. 2014; Bates et al. 2004).
In Ghanaian women, only arachidonic acid (20:4 n – 6) was positively associated with obesity. Arachidonic acid is metabolized via cyclooxygenase and lipoxygenase pathways into eicosanoids, which promote acute and chronic inflammation seen in numerous diseases including type 2 diabetes and cardiovascular disease (Mathias et al. 2014) and is synthesized from linoleic acid (18:2, n – 6) by the action of desaturase and elongase enzymes. Studies in US have shown that African Americans are more likely to have the variant within the fatty acid desaturase (FADS) gene associated with increased arachidonic acid concentrations (Mathias et al. 2014). Hence, even in the context of a relatively low fat diet, obesity in Ghanaian women was associated with increased arachidonic acid concentrations, likely reflecting increased endogenous production.
Another important finding from our study is the association between uric acid and obesity in the US women. Uric acid inhibits nitric oxide, promotes platelet aggregation and has pro-inflammatory effects, and accordingly has been associated with obesity, diabetes, hypertension, kidney and cardiovascular disease (Soltani et al. 2013). Elevated serum uric acid levels predict future risk of diabetes (Osgood et al. 2013; Kodama et al. 2013), as well as future elevations in blood pressure and cholesterol concentrations (Osgood et al. 2013). Elevated uric acid concentrations have been linked to a diet rich in fructose (Nakagawa et al. 2006). A marked increase in fructose intake in the US has occurred in the last 2 decades, primarily in the form of sucrose (50 % fructose) and high-fructose corn syrup (HFCS, 55 % fructose) (Bray et al. 2004). Unlike glucose, hepatic metabolism of fructose favors de novo lipogenesis, contributing to obesity, hyperlipidaemia and hepatic steatosis (Khitan and Kim 2013). In addition, fructose does not stimulate insulin and leptin secretion or suppress ghrelin, key afferent appetite signals, thereby facilitating a positive energy balance (Havel 2002). Commensurate with increased carbohydrate flux in obesity, we found that obesity was associated with mannitol in the US and SA, and with glucose, glucosamine and glucoronic in SA.
There are a number of limitations to our study. We present cross-sectional data and therefore cannot infer a causal relationship between the biomarkers identified and obesity risk. We also did not have a measure of insulin resistance or type 2 diabetes. Nonetheless, identifying metabolites associated with obesity provides insight into potential predictors of obesity-associated metabolic diseases such as type 2 diabetes in these unique populations. We did not include the dietary intake data in the models, due to difficulties in gathering accurate dietary information. For example, when compared to energy expenditure measured using the doubly labeled water technique, we found significant under-reporting amongst all of our cohorts, most notably among the SA women (Orcholski et al. 2015). In addition to reporting error, this may reflect issues relating to food insecurity, which is not as likely to be captured when dietary intake is assessed using 24-h recalls. Indeed, in the SA cohort, we found that 3-hydroxybutyrate, a ketone body, was associated with a lower BMI. Accordingly, we chose to only include objectively measured data to improve the robustness of the models.
5 Conclusion
We identified site-specific obesity-related metabolites in black women spanning the epidemiologic transition. These findings suggest that the ecological/environmental setting and consequently, lifestyle factors may influence the obesity-related metabolite profile. Future prospective, longitudinal studies are required to confirm these findings and to study if specific metabolite patterns predict obesity-associated metabolic disease, notably diabetes, in developed black populations.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the site-specific clinic staff members as well as the 2500 participants. LRD, EC, JG all conceived the idea, performed the analyses and wrote the manuscript. JPR, EVL, AL, LD, EC all collected the data and wrote the manuscript, EC, GC, LRD and DMS performed the analyses and BTL and RC wrote the manuscript. METS is funded in part by the National Institutes of Health (1R01DK80763). The metabolomics analysis is funded by Thuringsstiftelsen.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11306-016-0960-6) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards: Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Contributor Information
Lara R. Dugas, Email: ldugas@luc.edu.
Elin Chorell, Email: elin.chorell@umu.se.
References
- Bates CJ, Mishra GD, Prentice A. Gamma-tocopherol as a possible marker for nutrition-related risk: results from four National Diet and Nutrition Surveys in Britain. The British journal of nutrition. 2004;92:137–150. doi: 10.1079/BJN20041156. [DOI] [PubMed] [Google Scholar]
- Bouchard-Mercier A, Rudkowska I, Lemieux S, Couture P, Vohl MC. An interaction effect between glucokinase gene variation and carbohydrate intakes modulates the plasma triglyceride response to a fish oil supplementation. Genes & nutrition. 2014;9:395. doi: 10.1007/s12263-014-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American journal of clinical nutrition. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. Journal of Nutrition. 2003;133:601S–608S. doi: 10.1093/jn/133.2.601S. [DOI] [PubMed] [Google Scholar]
- Cheng JS, Niu YH, Lu SH, Yuan YJ. Metabolome analysis reveals ethanolamine as potential marker for improving lipid accumulation of model photosynthetic organisms 2012 [Google Scholar]
- Corona E, Chen R, Sikora M, Morgan AA, Patel CJ, Ramesh A, et al. Analysis of the Genetic Basis of Disease in the Context of Worldwide Human Relationships and Migration. PLoS Genetics. 2013;9:e1003447. doi: 10.1371/journal.pgen.1003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzyn P, Jazurek M, Dobrzyn A. Stearoyl-CoA desaturase and insulin signaling–what is the molecular switch? Biochimica et Biophysica Acta. 2010;1797:1189–1194. doi: 10.1016/j.bbabio.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Dugas LR, Harders R, Merrill S, Ebersole K, Shoham DA, Rush EC, et al. Energy expenditure in adults living in developing compared with industrialized countries: a meta-analysis of doubly labeled water studies. American Journal of Clinical Nutrition. 2011;93:427–441. doi: 10.3945/ajcn.110.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Gong G. A Leisurely Look at the Bootstrap, the Jackknife, and Cross-Validation. The American Statistician. 1983;37:36–48. [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- Goedecke JH, Dave JA, Faulenbach MV, Utzschneider KM, Lambert EV, West S, et al. Insulin response in relation to insulin sensitivity: an appropriate beta-cell response in black South African women. Diabetes Care. 2009;32:860–865. doi: 10.2337/dc08-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedecke J, Peer N, Steyn K, Victor H, Levitt NS. Insulin secretion in relation to insulin sensitivity in black South African men and women with increasing age. Johannesburg, South Africa; JEMDSA: 2014. [DOI] [PubMed] [Google Scholar]
- Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors–an Endocrine Society scientific statement. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1579–E1639. doi: 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. The American journal of clinical nutrition. 2014;100:208–217. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Current Opinion in Lipidology. 2002;13:51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Jiye A, Trygg J, Gullberg J, Johansson AI, Jonsson P, Antti H, Marklund SL, Moritz T. Extraction and GC/MS analysis of the human blood plasma metabolome. Analytical Chemistry. 2005;77:8086–8094. doi: 10.1021/ac051211v. [DOI] [PubMed] [Google Scholar]
- Joffe YT, van der Merwe L, Evans J, Collins M, Lambert EV, September AV, Goedecke JH. Interleukin-6 gene polymorphisms, dietary fat intake, obesity and serum lipid concentrations in black and white South African women. Nutrients. 2014;6:2436–2465. doi: 10.3390/nu6062436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P, Johansson AI, Gullberg J, Trygg J, Jiye A, Grung B, Marklund S, Sjostrom M, Antti H, Moritz T. High-throughput data analysis for detecting and identifying differences between samples in GC/MS-based metabolomic analyses. Analytical Chemistry. 2005;77:5635–5642. doi: 10.1021/ac050601e. [DOI] [PubMed] [Google Scholar]
- Jonsson P, Johansson ES, Wuolikainen A, Lindberg J, Schuppe-Koistinen I, Kusano M, et al. Predictive metabolite profiling applying hierarchical multivariate curve resolution to GC-MS datas - A potential tool for multi-parametric diagnosis. Journal of Proteome Research. 2006;5:1407–1414. doi: 10.1021/pr0600071. [DOI] [PubMed] [Google Scholar]
- Jonsson P, Wuolikainen A, Thysell E, Chorell E, Stattin P, Wikstrom P, Antti H. Constrained randomization and multivariate effect projections improve information extraction and biomarker pattern discovery in metabolomics studies involving dependent samples. Metabolomics. 2015 doi: 10.1007/s11306-015-0818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khitan Z, Kim DH. Fructose: a key factor in the development of metabolic syndrome and hypertension. Journal of nutrition and metabolism. 2013;2013:682673. doi: 10.1155/2013/682673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS) Diabetes Care. 2010;33:1497–1499. doi: 10.2337/dc09-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic Differences in the Relationship Between Insulin Sensitivity and Insulin Response: A systematic review and meta-analysis. Diabetes Care. 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A, Bovet P, Forrester TE, Lambert EV, Plange-Rhule J, Schoeller DA, et al. Protocol for the modeling the epidemiologic transition study: a longitudinal observational study of energy balance and change in body weight, diabetes and cardiovascular disease risk. BMC Public Health. 2012;11:927. doi: 10.1186/1471-2458-11-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A, Bovet P, Plange-Rhule J, Forrester TE, Lambert EV, Schoeller DA, et al. A mixed ecologic-cohort comparison of physical activity & weight among young adults from five populations of African origin. BMC public health. 2014;14:397. doi: 10.1186/1471-2458-14-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke A, Durazo-Arvizu R, Rotimi C, Prewitt TE, Forrester T, Wilks R, et al. Relation between body mass index and body fat in black population samples from Nigeria, Jamaica, and the United States. American Journal of Epidemiology. 1997;145:620–628. doi: 10.1093/oxfordjournals.aje.a009159. [DOI] [PubMed] [Google Scholar]
- Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. The American journal of clinical nutrition. 1995;62:564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- Mathias RA, Sergeant S, Ruczinski I, Torgerson DG, Hugenschmidt CE, Kubala M, et al. The impact of FADS genetic variants on omega6 polyunsaturated fatty acid metabolism in African Americans. BMC Genetics. 2014;12:50. doi: 10.1186/1471-2156-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics: Official journal of the Metabolomic Society. 2014;10:259–269. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. American journal of physiology. Renal physiology. 2006;290:F625–F631. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metabolism. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcholski L, Luke A, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, et al. Under-reporting of dietary energy intake in five populations of the African diaspora. The British journal of nutrition. 2015;113:464–472. doi: 10.1017/S000711451400405X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood K, Krakoff J, Thearle M. Serum uric acid predicts both current and future components of the metabolic syndrome. Metabolic syndrome and related disorders. 2013;11:157–162. doi: 10.1089/met.2012.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redestig H, Fukushima A, Stenlund H, Moritz T, Arita M, Saito K, Kusano M. Compensation for Systematic Cross-Contribution Improves Normalization of Mass Spectrometry Based Metabolomics Data. Analytical Chemistry. 2009;81:7974–7980. doi: 10.1021/ac901143w. [DOI] [PubMed] [Google Scholar]
- Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Current Hypertension Reports. 2013;15:175–181. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn NP, Nel JH, Parker WA, Ayah R, Mbithe D. Dietary, social, and environmental determinants of obesity in Kenyan women. Scand J Public Health. 2011;39:88–97. doi: 10.1177/1403494810384426. [DOI] [PubMed] [Google Scholar]
- Thysell E, Chorell E, Svensson MB, Jonsson P, Antti H. Validated and predictive processing of gas chromatography-mass spectrometry based metabolomics data for large scale screening studies, diagnostics and metabolite pattern verification. Metabolites. 2012;2:796–817. doi: 10.3390/metabo2040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Programme. New York: Human Development Index (HDI) [Online] Available http://hdr.undp.org/en/content/human-development-index-hdi. [Google Scholar]
- Vessby B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Current Opinion in Lipidology. 2003;14:15–19. doi: 10.1097/00041433-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nature Medicine. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. The Journal of Clinical Investigation. 2013;123:4309–4317. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. Journal of Physical Activity and Health. 2011;8:587–591. doi: 10.1123/jpah.8.4.587. [DOI] [PubMed] [Google Scholar]
- Xie B, Waters MJ, Schirra HJ. Investigating potential mechanisms of obesity by metabolomics. Journal of Biomedicine and Biotechnology. 2012:805683. doi: 10.1155/2012/805683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


