Abstract
Mammalian cells possess multiple DNA glycosylases, including OGG1, NTH1, NEIL1, NEIL2 and NEIL3, for the repair of oxidative DNA damage. Among these, NEIL1 and NEIL2 are able to excise oxidized bases on single stranded or bubble-structured DNA and has been implicated in repair of oxidative damage associated with DNA replication or transcription. We found that Neil1 was highly constitutively expressed in the germinal center (GC) B cells, a rapidly dividing cell population that is undergoing immunoglobulin (Ig) gene hypermutation and isotype switching. While Neil1−/− mice exhibited normal B and T cell development and maturation, these mice contained a significantly lower frequency of GC B cells than did WT mice after immunization with a T-dependent antigen. Consistent with the reduced expansion of GC B cells, Neil1−/− mice had a decreased frequency of Ig gene hypermutation and produced less antibody against a T-dependent antigen during both primary and secondary immune responses. These results suggest that repair of endogenous oxidative DNA damage by NEIL1 is important for the rapid expansion of GC B cells and efficient induction of humoral immune responses.
Keywords: Oxidative damage, Germinal center B cells, DNA glycosylase, Somatic hypermutation, Immune response
1. Introduction
Reactive oxygen species (ROS) are continuously generated as by-products of cellular respiration and are one of the major endogenous causes of DNA damage [1,2]. ROS react with DNA and generate a variety of base lesions, such as 8-oxoguanine (8-oxoG), formamidopyrimidines (Fapys) and thymine glycol (Tg) [1–3]. 8-OxoG pairs with adenine and is thus highly mutagenic whereas Fapys and Tg lesions may block DNA replication and thereby affect cell division and survival. The oxidized lesions are primarily repaired by the base excision repair pathway, which is initiated with excision of the damaged bases by DNA glycosylases. In mammals, five oxidized damage-specific DNA glycosylases, OGG1, NTH1, NEIL1, NEIL2 and NEIL3, have thus far been identified [4,5]. These enzymes have overlapping but distinct substrate specificities presumably to allow for efficient excision of different types of oxidized bases [2]. Among these enzymes, the expression of NEIL1 is elevated during S phase of the cell cycle [6] and is active in excision of oxidized bases on single stranded or bubble-structured DNA [7]. These observations suggest that NEIL1 is involved in repair of oxidative damage associated with DNA replication or transcription. Inhibition of NEIL1 expression by RNAi in ES cells resulted in increased sensitivity to low doses of ionizing irradiation [8]. NEIL1-deficient mice appeared normal up to 4–6 months of age but developed severe obesity, dyslipidemia and fatty liver diseases as they aged although the phenotypes appear to be variable and may also be dependent on the genetic background [9]. Recently, it has been shown that Neil1−/− mice are cancer-prone and that double knockouts of Neil1−/− Nth1−/− had very high frequencies of lung and liver cancers [10]. Additionally, it was shown that downmodulation of NEIL1 by antisense oligonucleotides resulted in elevated oxidative damage in the genome and enhanced spontaneous mutation in the Hprt locus both in human and Chinese hamster cells [11].
During the course of identifying genes that are involved in B cell activation and terminal differentiation, we found that Neil1 was abundantly expressed in the splenic germinal center (GC) B cells and in Peyer’s patches, which are rich in GC B cells. GC B cells represent a unique cell population that arise in the secondary lymphoid organs such as spleen, lymph node, and Peyer’s patch during an immune response against foreign antigens [12]. These rapidly dividing cells undergo dynamic genetic alterations including somatic hypermutation (SHM) of the immunoglobulin (Ig) genes. SHM is initiated by activation-induced cytidine deaminase (AID) [13], which is thought to catalyze the deamination of cytosine (C) to uracil (U) and generate a U:G DNA lesion [14]. Mutations are introduced during replication and repair of the AID-triggered U:G lesion and accumulate as GC B cells undergo cell division [15–17]. SHM results in altered affinity of antibodies and those with increased affinity for antigen are selected to differentiate into antibody-producing plasma cells or memory B cells. Upon re-stimulation by the same antigen, the memory B cells are promptly activated and differentiate to secrete large amounts of antigen-specific antibodies.
The dramatic upregulation of Neil1 expression in GC B cells suggests a role for NEIL1 in B cell activation and terminal differentiation. It is interesting that the preferred substrates of NEIL1 are the oxidized bases on single stranded or bubble-structured DNA. AID, the enzyme responsible for SHM has a similar preference for single stranded regions [14]. In the present study, we have analyzed B cell development, maturation, activation and terminal differentiation in Neil1−/− mice. Our results suggest that repair of endogenous oxidative damage by NEIL1 is important for the rapid expansion of GC B cells and efficient SHM and antibody production.
2. Materials and methods
2.1. Isolation of B-lineage cell subpopulations and RT-PCR analyses
Spleen B cells were isolated by using negative sorting with the IMag B cell purification kit (BD Biosciences, Mountain View, CA). To isolate follicular and marginal zone B cells, spleen cells were stained with APC-B220, FITC-anti-CD21 and PE-anti-CD23. B220+CD23highCD21dull (follicular) and B220+CD23dullCD21high (marginal zone) B cells were then sorted with a FACSVantage™ (BD Biosciences). For GC B cells, mice were injected i.p. with 100 µg of NP-CGG (4-hydroxy-3-nitrophenyl-acetyl coupled to chicken gamma-globulin) precipitated with alum. Two weeks later, spleen cells were stained with PE-B220 and FITCPNA and the B220+PNAhigh GC B cells were sorted using a FACSVantage-turbo cell sorter. RNA was extracted using Trizol reagent (Invitrogen Corp., Carlsbad, CA) and first-strand cDNA was synthesized with Superscript III reverse transcriptase and random primers. The following primers were used in RT-PCR analyses: Neil1/s212, 5′-AGCCACTGTCCCTTGTCTTC-3′; Neil1/as949, 5′-CTGGAAACGGACTTGCTTGA-3′; Neil2/s336, 5′-AGGGAATGTGGCAGAAAGAG-3′; Neil2/as610, 5′-GGAAGCCACCACCACTAAAA-3′; Neil3/s627, 5′-TGCTGTGTGATGTGTTGCTG-3′; Neil3/as1013, 5′-TCCGTAAAGCAATCCTCTCC-3′; Nth1/s225, 5′-TGAGGAAGGCGAAGATGCTG-3′; Nth1/as764, 5′-TGGGGTCTTGGTCATCTTCT-3′; Ogg1/s630, 5′-TCCAAGGTGTGAGACTGCTG-3′; Ogg1/as1083, 5′-CTTAGGATGCCAGCCGTAGT-3′; β-actin/s80,5′-ATGGATGACGATATCGCT-3′; β-actin/as630, 5′-ATGAGGTAGTCTGTCAGGT-3′. RT-PCR was performed using Taq polymerase (TOYOBO, Japan) under the following conditions: Neil1, Neil3, Nth1 and Ogg1, 95°C for 2 min followed by 95 °C for 10 s, 60 °C for 20 s and 72 °C for 1 min for 30 (for Neil1 and Ogg1) or 35 (for Neil3 and Nth1) cycles; Neil2, 95°C for 2 min and then 95 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s for 35 cycles; β-actin, 95°C for 2 min followed by 95 °C for 5 s, 54 °C for 10 s and 72 °C for 1 min for 25 cycles.
2.2. NEIL1-deficient mice and FACS analysis
Neil1−/− mice were generated in a 129/C57BL/6 mixed background [9] and have been backcrossed with C57BL/6 mice for five generations. Mice were kept in specific pathogen-free conditions and all experiments were approved by the Animal Facility Committee of RIKEN Yokohama Institute (permission number 20-025). FACS analysis was performed essentially as described [18,19].
2.3. Somatic hypermutation assays
Two WT and three Neil1−/− mice (10-week old) were immunized with 100 µg of NP-CGG (Biosearch Technologies, Novato, CA) with alum. Two weeks later, B220+PNAhigh GC B cells were sorted from spleens of each mouse and analyzed for SHM as described [20].
2.4. Immune response
Eight pairs of WT and Neil1−/− mice (9-week old) derived from breeding of Neil1+/− mice were injected i.p. with 100 µg of NP-CGG precipitated with alum and boosted 5 weeks later. Mice were bled weekly and serum titers of NP-specific IgG1 were analyzed by ELISA, using NP-specific monoclonal high (clone C6) and low (clone N1G9) affinity antibodies as a standard [21].
2.5. Immunofluorescence
Spleen B cells purified from mice immunized with NP-CGG were first attached to the slide glass by cytospin and then fixed and permeabilized as described previously [18]. The cells were then stained with FITC-GL7 (BD Biosciences) and mouse IgG1 anti-thymine glycol (MTG-100P, Japan Institute for the Control of Aging, Shizuoka, Japan), followed by Texas Red-goat anti-mouse IgG1 (Santa Cruz).
2.6. Sensitivity to hydrogen peroxide (H2O2)
Spleen B cells (5 × 105/ml) were cultured in the presence of 10 µg/ml of lipopolysaccharide (LPS) for 1 day. The cells were then washed once with serum-free medium and resuspended in serum-free medium containing different concentrations of H2O2. After incubation on ice for 10 min, the cells were washed three times with culture medium containing serum and cultured for 1 additional day in the presence of 10 µg/ml of LPS. The cells were then collected and stained with FITC-Annexin V and propidium iodide (PI) to detect apoptotic and necrotic cells, respectively (BioVision, Mountain View, CA). Annexin V−PI− live cells were determined by FACS.
3. Results
3.1. Neil1 is highly expressed in GC B cells
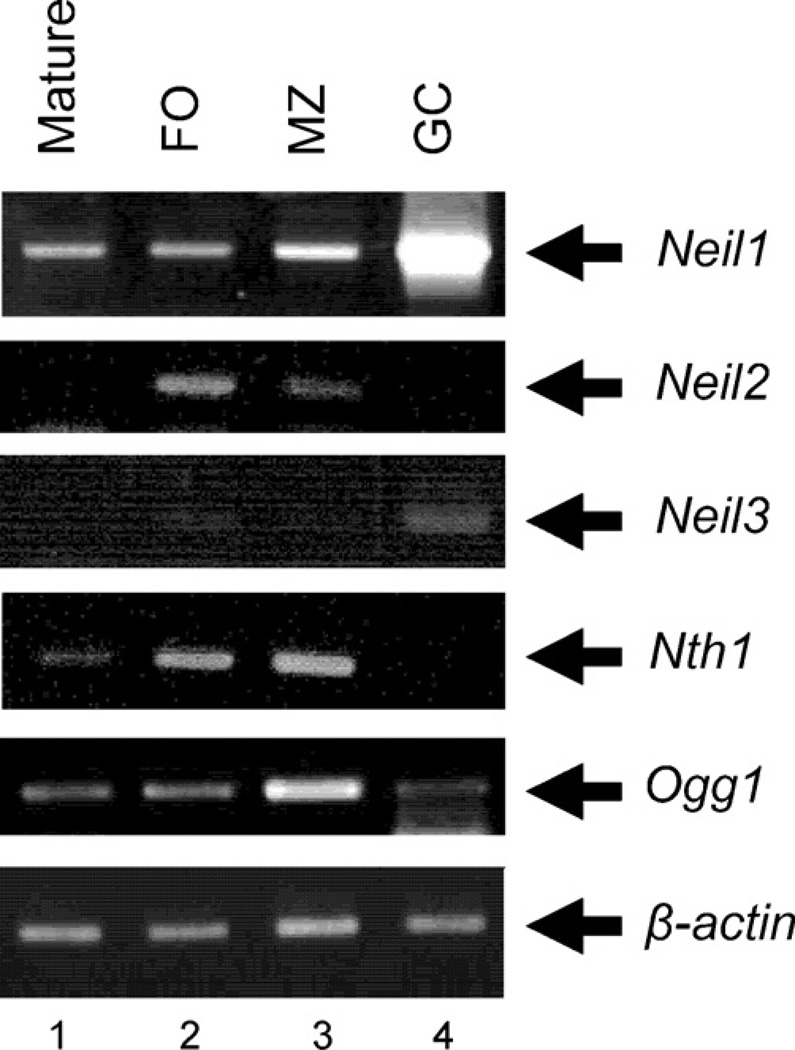
We first examined the expression of DNA glycosylases involved in repair of oxidative damage during B cell differentiation and activation in vivo. Neil1 was expressed at low levels in freshly isolated mature spleen B (Fig. 1, lane 1), follicular B (lane 2) and marginal zone B (lane 3) cell subpopulation, but abundantly expressed in GC B cells (Fig. 1, lane 4) and in Peyer’s patches, which contain a high proportion of GC B cells (not shown). In contrast, Neil2 and Nth1 transcripts were undetectable in GC B cells whereas Neil3 and Ogg1 were only weakly expressed (Fig. 1, lane 4). These results demonstrate that only Neil1 is highly expressed in GC B cells, which are proliferating cells that have been activated by antigen and helper T cells in vivo.
Fig. 1.
Expression of DNA glycosylases involved in repair of oxidative damage during B cell differentiation in vivo. RT-PCR for the indicated genes was performed as described in Section 2. The β-actin gene was used as a cDNA loading control. Similar results were obtained in two independent experiments.
3.2. Reduced GC B cell expansion in Neil1−/− mice
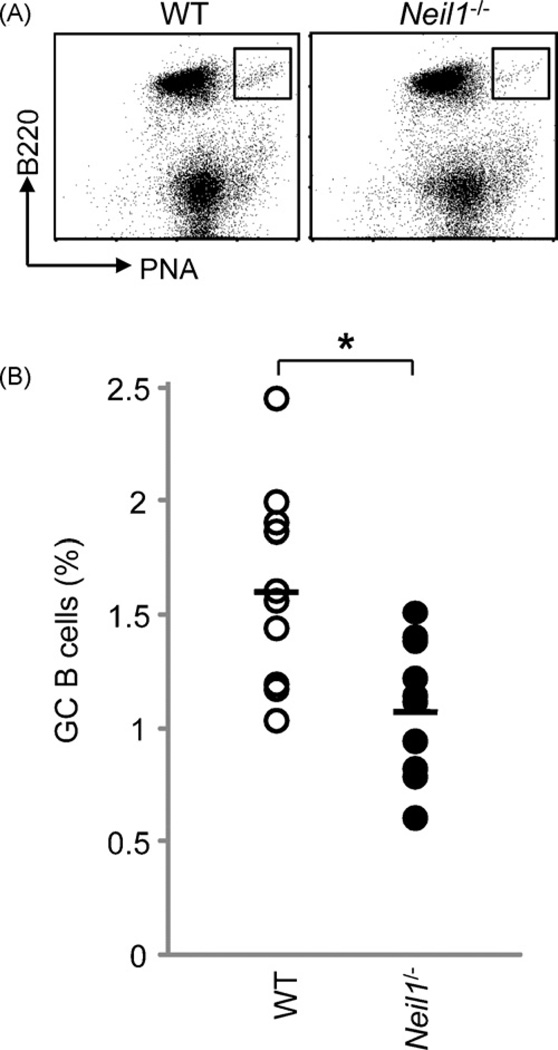
B cell development and maturation, T cell differentiation and B cell Ig class switching appeared normal in Neil1−/− mice (Supplemental Fig. S1). The high levels of Neil1 expression in GC B cells prompted us to examine the role of NEIL1 in the induction of GC B cells. We have analyzed 10 pairs of age-matched WT and Neil1−/− mice and found a moderate but statistically significant reduction in the frequency of the B220+PNAhigh GC B cells in the Neil1−/− mice (Fig. 2). There was no significant difference in the total numbers of splenocytes between WT and Neil1−/− mice. These results demonstrate that NEIL1 deficiency affected the GC B cell expansion in vivo during an immune response.
Fig. 2.
The frequency of B220+PNAhigh GC B cells inWTand Neil1−/− mice. Mice were immunized with NP-CGG in alum and 2 weeks later their spleen cells were stained with PE-B220 and FITC-PNA. (A) Representative FACS profiles. (B) Percentages of B220+PNAhigh GC B cells in 10 pairs of WT and Neil1−/− mice (*p < 0.05, unpaired t-test).
3.3. Accumulation of Tg lesions in WT and Neil1−/− B cells after immunization
Oxidized DNA lesions such as Tg and Fapys are substrates of NEIL1 and are known to block DNA replication. The reduced GC B cell expansion suggests that the absence of NEIL1 might have led to increased levels of oxidative lesions that inhibited DNA replication. We therefore analyzed the levels of Tg lesions in WT and Neil1−/− B cells purified from immunized mice (Supplemental Fig. 2). Examination of the number of Tg spots in 20 randomly selected cells revealed that WT and Neil1−/− B cells contained 6.3 ± 2.9 and 6 ± 3.4 Tg spots per cell, respectively (p > 0.5, unpaired t-test). These results suggest that NEIL1 deficiency did not lead to an obvious increase in the levels of Tg lesions in B cells.
3.4. Reduced frequency of Ig gene hypermutation in Neil1−/− mice
GC B cells undergo Ig gene hypermutation, which accumulates as the cells divide. We next analyzed whether the reduced GC B expansion affected the frequency of Ig gene hypermutation. We analyzed JH4 intronic sequences from 2 WT and 3 Neil1−/− mice and the combined results are shown in Table 1. In agreement with our previous studies, the mutation frequency per mutated sequences was approximately 1% in WT mice and mutations at C:G and A:T were 0.50% and 0.51%, respectively (Table 1). The mutation frequency in Neil1−/− mice dropped to 0.80% and mutations at C:G and A:T were decreased to 0.41% and 0.39% (18 and 24% reduction compared to WT mice, respectively). However, mutation patterns were quite similar between WT and Neil1−/− mice (Supplemental Fig. S3). These results suggest that NEIL1 deficiency affected the frequency but not patterns of Ig gene hypermutation.
Table 1.
Mutation frequency in WT and Neil1−/− mice.
| JH4 intron (509 bp) | WT | Neil1−/− |
|---|---|---|
| Number of sequences | 120 | 327 |
| Mutated sequences (%) | 86 (71.7%) | 252 (77.1%) |
| Total length of mutated sequences | 43,774 | 128,268 |
| Total number of mutations | 443 | 1020 |
| Mutation frequency per total sequences (%) | 0.73 | 0.61 |
| Mutation frequency per mutated sequences (%) | 1.01 | 0.80a |
| Mutation frequency at C:G (%) | 0.50 | 0.41 |
| Mutation frequency at A:T (%) | 0.51 | 0.39 |
| % mutation at C:G: A:T | 49.5:50.5 | 51.4:48.6 |
The values in bold font indicate significant differences from WT mice (p < 0.05, unpaired t-test).
3.5. Reduced antibody production to the T-dependent antigen NP-CGG both during primary and secondary immune responses in Neil1−/− mice
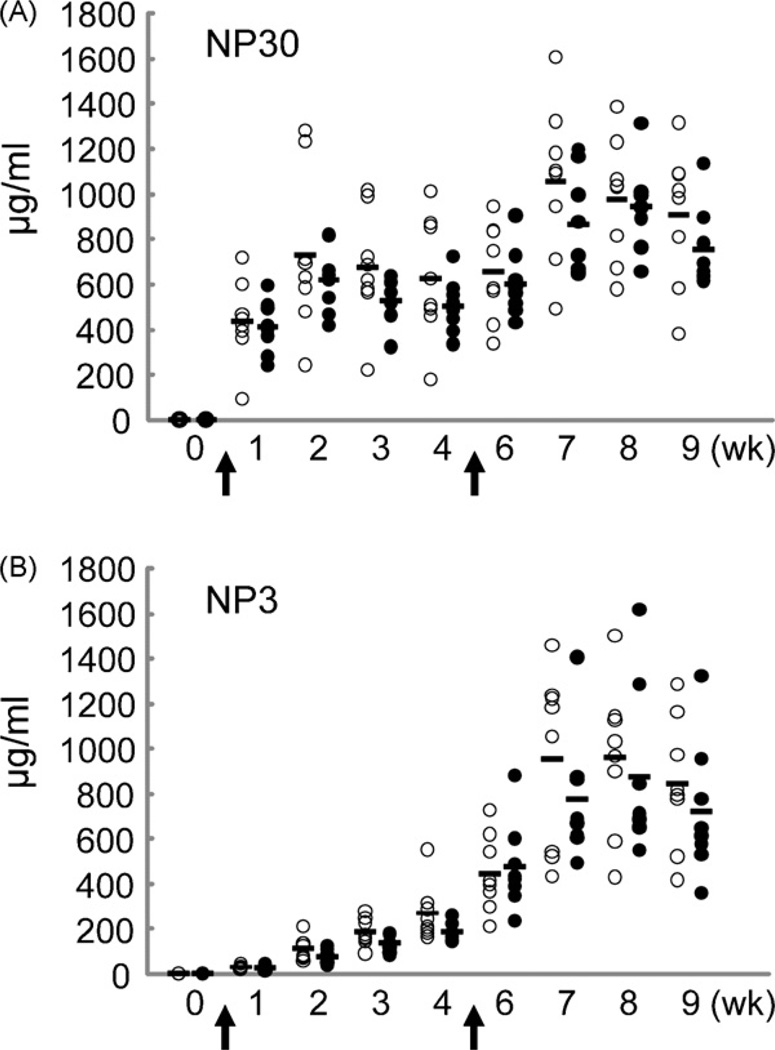
We further compared immune responses against NP-CGG in WT and Neil1−/− mice. Neil1−/− mice had reduced amounts of total (low and high affinity) anti-NP antibodies in both primary and secondary immune responses as measured in an ELISA assay with NP30-BSA (Fig. 3A). Similarly, the production of high affinity antibodies, as measured with NP3-BSA, was also reduced in the Neil1−/− mice (Fig. 3B).
Fig. 3.
Immune responses and affinity maturation in WT and Neil1−/− mice. Mice (8 WT and 8 Neil1−/−) were immunized with NP-CGG and analyzed for the production of NP-specific IgG z1 antibodies as described in Section 2. Open and solid circles represent WT and Neil1−/− mice, respectively. (A) Titers of total (high- and low-affinity) NP-specific antibodies. (B) Titers of high-affinity NP-specific antibodies. The reduced production of total and high-affinity antibodies in Neil1−/− mice relative to WT mice is statistically significant (p < 0.05, two-way ANOVA).
3.6. Normal sensitivity of Neil1−/− B cells to H2O2
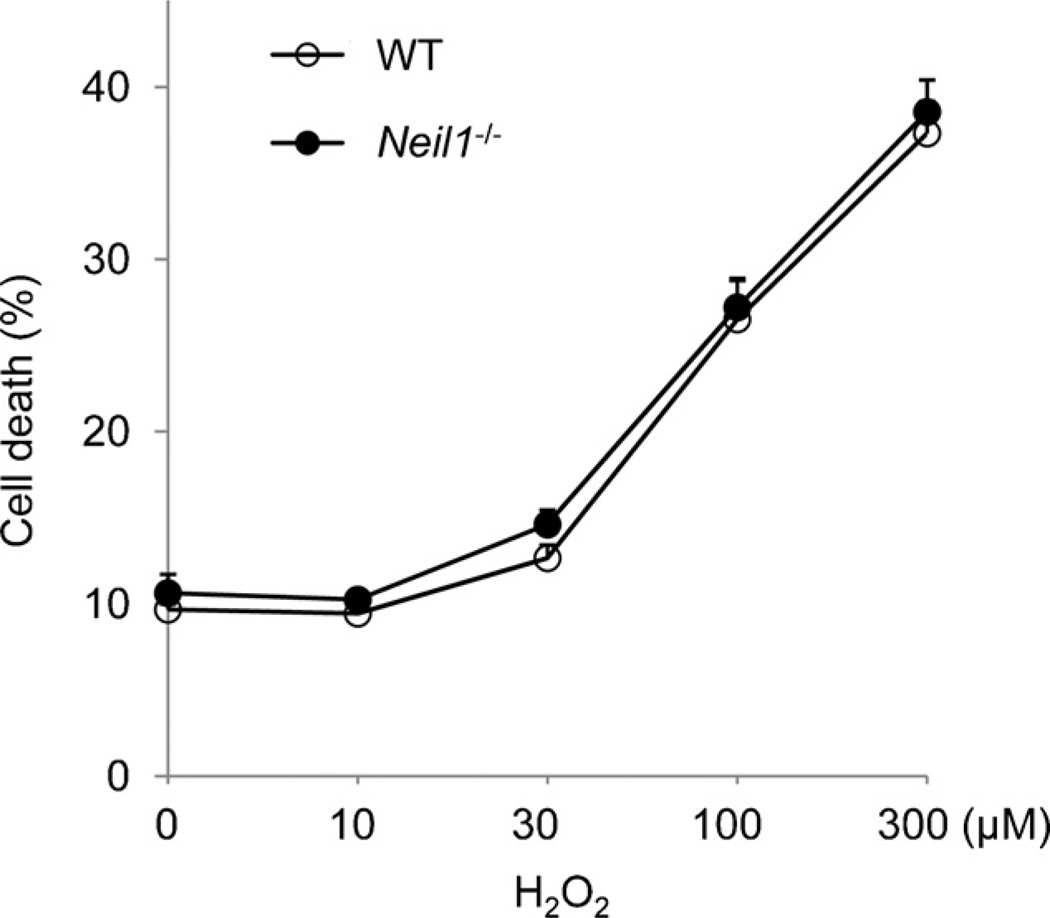
To examine the role of NEIL1 in repair of oxidative damage induced by exogenous sources, we analyzed the sensitivity of spleen B cells to H2O2. The cells were activated and induced to cycle with LPS, treated with H2O2, and then further allowed to proliferate for 1 day before analysis. We found no obvious differences in the survival between WT and Neil1−/− B cells treated with different doses of H2O2 (Fig. 4). Therefore, repair of exogenously induced oxidative damage in B cells did not seem to be affected by the absence of NEIL1.
Fig. 4.
Sensitivity of WT and Neil1−/− B cells to H2O2. Purified WT and Neil1−/− B cells were stimulated with LPS and then treated with different doses of H2O2. Cell survival was analyzed as described in Section 2. Similar results were obtained with two independent experiments.
4. Discussion
Reactive oxygen species are considered to be a major endogenous source that induces DNA damage. ROS interaction with DNA generates a plethora of oxidized base lesions, which can be mutagenic and may block DNA replication and transcription. Among the known DNA glycosylases that initiate the base excision repair of the oxidized bases, we analyzed the function of NEIL1 in the immune system because it is highly expressed in GC B cells. Our results demonstrate that NEIL1-mediated repair of endogenous oxidative damage is indeed important for B cell activation in vivo.
Multiple DNA glycosylases are involved in repair of oxidative damage. These glycosylases have broad substrate specificity but also have unique specificities. Biochemical analysis revealed that NEIL1 is able to excise adenine- and guanine-derived Fapys, Tg, and a number of other lesions. It is unclear what types of oxidized lesions normally repaired by NEIL1 affect the B cell expansion in Neil1−/− mice. We did not observe an obvious increase in the levels of Tg lesions in Neil1−/− B cells purified from immunized mice, suggesting that the repair of Tg lesions is not significantly dependent on the activity of NEIL1 in activated B cells. It is likely that other oxidized lesions such as Fapys, which are another preferred substrate for NEIL1, may have accumulated in the genome and inhibited the rapid expansion of GC B cells. Indeed, both adenine- and guanine-derived Fapys accumulated in all tissues examined (liver, kidney and brain) of Neil1−/− mice, relative to control mice [10]. Further studies are required to determine the type of oxidized lesions that remain unrepaired in GC B cells in the absence of NEIL1.
Although the frequency of Ig gene hypermutation was decreased in Neil1−/− mice, the mutation patterns were similar in WT and Neil1−/− mice. Therefore, Ig gene SHM appeared to be generally repressed in the absence of NEIL1. These observations do not support a direct role for NEIL1 in SHM but rather suggest that the reduction of SHM frequency in Neil1−/− mice is caused indirectly by the reduced GC B expansion. Similarly, the decreased antibody production is likely due to the impaired expansion of GC B cells that would subsequently result in a decreased number of antibody-secreting plasma cells. Notably, antibody production was also reduced during the secondary response, suggesting that the formation of memory B cells during the primary response was defective and/or that the activation and/or differentiation of antigen-specific memory B cells are also affected by the absence of NEIL1.
Although B cells exhibited impaired expansion in Neil1−/− mice, their sensitivity to exogenous oxidative damage induced by H2O2 was not elevated. The existence of multiple oxidized base-specific DNA glycosylases with overlapping enzymatic properties may account for this finding; the function of NEIL1 can probably be compensated for by other glycosylases during repair of H2O2-induced oxidative damage. It is thus curious why the function of NEIL1 in GC B cells cannot be fully compensated for by other related glycosylases. Since only Neil1 is abundantly expressed in GC B cells, one possibility is that the expression levels of other DNA glycosylases are too low to compensate for the activity of NEIL1. Another possibility is that GC B cells undergo rapid cell division and the repair of the replication-associated oxidized lesions is more dependent on the activity of NEIL1. In either case, our results suggest that the elevated Neil1 expression in GC B cells is required for efficient repair of oxidative damage and important for B cell activation and terminal differentiation in vivo.
While Neil1 is abundantly expressed in GC B cells, its transcript levels are low in naïve T and anti-CD3-activated T cells, as well as in many other immune cells (unpublished results). These observations suggest that the reduction of GC B cell expansion in Neil1−/− mice in response to NP-CGG is likely due to an intrinsic effect on B cells. However, since an efficient immune response against T-dependent antigens requires the coordinated function of many different types of immune cells, we cannot exclude the possibility that abnormalities in T and other cells may have also affected the GC B cell expansion and differentiation in vivo. Conditional inactivation of the Neil1 gene in B and other cells should allow the elucidation of the cell type-specific function of NEIL1. Regardless, our results demonstrate for the first time that NEIL1-mediated repair of oxidative damage contributes to the efficient induction of an adaptive immune response.
Supplementary Material
Acknowledgments
The authors wish to thank Akiko Ukai for excellent technical assistance, the RCAI Animal Facility for sbreeding and maintaining the mice and the Immunogenomics group for sequencing. This work was supported by a budget of the Research Center for Allergy and Immunology and NIH R01 DK075974 (RSL).
Abbreviations
- GC
germinal center
- Ig
immunoglobulin
- SHM
somatic hypermutation
- AID
activation-induced cytidine deaminase
- NP-CGG
4-hydroxy-3-nitrophenyl-acetyl coupled to chicken gamma-globulin
- LPS
lipopolysaccharide
- Tg
thymine glycol
- 8-oxoG
8-oxoguanine
- Fapys
formamidopyrimidines
Footnotes
Conflict of interest
The authors declare that no conflicting financial interest exists.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dnarep.2009.08.007.
References
- 1.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazra TK, Das A, Das S, Choudhury S, Kow YW, Roy R. Oxidative DNA damage repair in mammalian cells: a new perspective. DNA Repair. 2007;6:470–480. doi: 10.1016/j.dnarep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dizdaroglu M. Base-excision repair of oxidative DNA damage by DNA glycosylases. Mutat. Res. 2005;591:45–59. doi: 10.1016/j.mrfmmm.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Hazra TK, Izumi T, Kow YW, Mitra S. The discovery of a new family of mammalian enzymes for repair of oxidatively damaged DNA, and its physiological implications. Carcinogenesis. 2003;24:155–157. doi: 10.1093/carcin/24.2.155. [DOI] [PubMed] [Google Scholar]
- 5.Dizdaroglu M. Substrate specificities and excision kinetics of DNA glycosylases involved in base-excision repair of oxidative DNA damage. Mutat. Res. 2003;531:109–126. doi: 10.1016/j.mrfmmm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Hazra TK, Izumi T, Boldogh I, Imhoff B, Kow YW, Jaruga P, Dizdaroglu M, Mitra S. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc. Natl. Acad. Sci. U.S.A. 2002;99:3523–3528. doi: 10.1073/pnas.062053799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazra TK, Mitra S. Purification and characterization of NEIL1 and NEIL2, members of a distinct family of mammalian DNA glycosylases for repair of oxidized bases. Methods Enzymol. 2006;408:33–48. doi: 10.1016/S0076-6879(06)08003-7. [DOI] [PubMed] [Google Scholar]
- 8.Rosenquist TA, Zaika E, Fernandes AS, Zharkov DO, Miller H, Grollman AP. The novel DNA glycosylase, NEIL1, protects mammalian cells from radiation-mediated cell death. DNA Repair. 2003;2:581–591. doi: 10.1016/s1568-7864(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 9.Vartanian V, Lowell B, Minko IG, Wood TG, Ceci JD, George S, Ballinger SW, Corless CL, McCullough AK, Lloyd RS. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1864–1869. doi: 10.1073/pnas.0507444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan MK, Ocampo-Hafalla MT, Vartanian V, Jaruga P, Kirkali G, Koenig KL, Brown S, Lloyd RS, Dizdaroglu M, Teebor GW. Targeted deletion of the genes encoding NTH1 and NEIL1 DNA N-glycosylases reveals the existence of novel carcinogenic oxidative damage to DNA. DNA Repair. 2009;8:786–794. doi: 10.1016/j.dnarep.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiti AK, Boldogh I, Spratt H, Mitra S, Hazra TK. Mutator phenotype of mammalian cells due to deficiency of NEIL1 DNA glycosylase, an oxidized base-specific repair enzyme. DNA Repair. 2008;7:1213–1220. doi: 10.1016/j.dnarep.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 13.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 15.Martomo SA, Gearhart PJ. Somatic hypermutation: subverted DNA repair. Curr. Opin. Immunol. 2006;18:243–248. doi: 10.1016/j.coi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Bransteitter R, Sneeden JL, Allen S, Pham P, Goodman MF. First AID (activation-induced cytidine deaminase) is needed to produce high affinity isotype-switched antibodies. J. Biol. Chem. 2006;281:16833–16836. doi: 10.1074/jbc.R600006200. [DOI] [PubMed] [Google Scholar]
- 17.Longerich S, Basu U, Alt F, Storb U. AID in somatic hypermutation and class switch recombination. Curr. Opin. Immunol. 2006;18:164–174. doi: 10.1016/j.coi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Ouchida R, Yamasaki S, Hikida M, Masuda K, Kawamura K, Wada A, Mochizuki S, Tagawa M, Sakamoto A, Hatano M, Tokuhisa T, Koseki H, Saito T, Kurosaki T, Wang JY. A lysosomal protein negatively regulates surface T cell antigen receptor expression by promoting CD3zeta-chain degradation. Immunity. 2008;29:33–43. doi: 10.1016/j.immuni.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Seimiya M, Bahar R, Wang Y, Kawamura K, Tada Y, Okada S, Hatano M, Tokuhisa T, Saisho H, Watanabe T, Tagawa M, O-Wang J. Clast5/Stra13 is a negative regulator of B lymphocyte activation. Biochem. Biophys. Res. Commun. 2002;292:121–127. doi: 10.1006/bbrc.2002.6605. [DOI] [PubMed] [Google Scholar]
- 20.Masuda K, Ouchida R, Hikida M, Kurosaki T, Yokoi M, Masutani C, Seki M, Wood RD, Hanaoka F, O-Wang J. DNA polymerases eta and theta function in the same genetic pathway to generate mutations at A/T during somatic hypermutation of Ig genes. J. Biol. Chem. 2007;282:17387–17394. doi: 10.1074/jbc.M611849200. [DOI] [PubMed] [Google Scholar]
- 21.Masuda K, Ouchida R, Takeuchi A, Saito T, Koseki H, Kawamura K, Tagawa M, Tokuhisa T, Azuma T, O-Wang J. DNA polymerase theta contributes to the generation of C/G mutations during somatic hypermutation of Ig genes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13986–13991. doi: 10.1073/pnas.0505636102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.