Abstract
Skin fluorescence (SF) noninvasively measures advanced glycation end products (AGEs) in the skin and is a risk indicator for diabetes complications. N-acetyltransferase 2 (NAT2) is the only known locus influencing SF. We aimed to identify additional genetic loci influencing SF in type 1 diabetes (T1D) through a meta-analysis of genome-wide association studies (N = 1,359) including Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) and Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR). A locus on chromosome 1, rs7533564 (P = 1.9 × 10−9), was associated with skin intrinsic fluorescence measured by SCOUT DS (excitation 375 nm, emission 435–655 nm), which remained significant after adjustment for time-weighted HbA1c (P = 1.7 × 10−8). rs7533564 was associated with mean HbA1c in meta-analysis (P = 0.0225), mean glycated albumin (P = 0.0029), and glyoxal hydroimidazolones (P = 0.049), an AGE measured in skin biopsy collagen, in DCCT. rs7533564 was not associated with diabetes complications in DCCT/EDIC or with SF in subjects without diabetes (nondiabetic [ND]) (N = 8,721). In conclusion, we identified a new locus associated with SF in T1D subjects that did not show similar effect in ND subjects, suggesting a diabetes-specific effect. This association needs to be investigated in type 2 diabetes.
Introduction
Hyperglycemia and duration of diabetes are the two major risk factors for both microvascular (i.e., retinopathy, nephropathy, and neuropathy) and macrovascular (i.e., cardiac) complications of diabetes (1). Hyperglycemia accelerates glycation, the result of nonenzymatic covalent bonding between the amino groups of proteins, lipids, or nucleic acids and reducing sugars (e.g., glucose or fructose), known as Maillard reactions. These early glycation products undergo further complex reactions to become irreversible advanced glycation end products (AGEs). Glycation and accumulation of AGEs in tissues deteriorate their structural integrity and physiological function (2).
Skin collagens undergo glycation, and due to their long half-life (15 years), they capture decades-long glycemia (3,4). Skin AGEs measured in skin biopsies have been associated with macro- and microvascular complications of diabetes even after adjustment for mean HbA1c levels over time (5–9). However, skin biopsy is an invasive method and is not practical for large studies.
As some AGEs fluoresce, accumulated levels of AGEs in the skin can be measured noninvasively by optical spectroscopy. Two very similar devices, SCOUT DS (Miraculins, Winnipeg, Manitoba, Canada) and AGE Reader (DiagnOptics Technologies BV, Groningen, the Netherlands), both reliably measure skin fluorescence (SF) with high reproducibility (4,10). SF has been associated with both micro- and macrovascular complications of diabetes (11–20). The associations remained significant even after adjustment for decades-long HbA1c, suggesting that SF can capture both glycemic and nonglycemic aspects of tissue damage (14).
Barat et al. (21) compared SF in children with type 1 diabetes (T1D) and their siblings without diabetes (nondiabetic [ND]) and observed a significant correlation of SF levels among siblings even after adjustment for HbA1c and age. Another study found familial correlation of SF in 50 mothers and their children with T1D after adjustment for skin pigmentation and race (22). Both studies suggest that shared genetic and/or environmental factors between family members influence SF. Although there have been no twin studies of SF, a twin study of lens autofluorescence, a related phenotype (23), showed that heritability contributed ∼28% to interindividual variation after adjustment for age, glucose homeostasis, and smoking habits (24). Recently, two genome-wide association studies (GWAS) of SF were performed in parallel: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC), including T1D subjects, and LifeLines, including mainly ND subjects. N-acetyltransferase 2 (NAT2) was the only locus that exceeded the genome-wide significance threshold in both GWAS. These results were replicated in two independent studies of T1D subjects, the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) (25) and Pittsburgh Epidemiology of Diabetes Complications (EDC) (26), and in an independent study of mainly ND subjects, LonGenity (25). The NAT2 effect appeared to be stronger in subjects with T1D with higher levels of HbA1c over time and in subjects with type 2 diabetes (T2D) compared with ND subjects, suggesting larger genetic effects in those with chronic hyperglycemia.
We believe that there is a continuum of loci with different effect sizes on SF. Therefore, in the current study, we aimed to identify additional genetic loci influencing SF in T1D subjects through a meta-GWAS and to determine whether the identified loci show similar associations in ND and T2D subjects.
Research Design and Methods
Design
We performed a meta-GWAS of skin intrinsic fluorescence (SIF) on directly genotyped and imputed single nucleotide polymorphisms (SNPs) from individuals with T1D of European ancestry from two studies, DCCT/EDIC and WESDR. Then, association of the identified locus with SF was investigated in two other studies, LonGenity and LifeLines, including mainly ND subjects with a subset of subjects with T2D (Table 1, Supplementary Table 1, and Supplementary Fig. 1).
Table 1.
Characteristics of the cohorts included in the study
| Study | N | Spectrometer | SF measure | Genotyping platform | Covariates | |
|---|---|---|---|---|---|---|
| T1D | ||||||
| DCCT/EDIC | 1,082 | SCOUT DS | SIF | Illumina 1M | Age, sex, skin tone, clinic latitude, eGFR, smoking status, rs1495741 genotype* | |
| WESDR | 281 | SCOUT DS | SIF | Illumina Omni-quad | Age, sex, skin tone, eGFR, smoking status, rs1495741 genotype† | |
| ND plus T2D | ||||||
| LonGenity | SCOUT DS | SIF | Illumina HumanOmniExpress | Age, sex, skin tone, eGFR, smoking status‡ | ||
| ND (OPEL) | 192 | |||||
| ND (OPUS) | 261 | |||||
| T2D | 58 | |||||
| LifeLines | AGE Reader | SAF | Illumina CytoSNP 12v2 | Age, sex, BMI, eGFR, smoking status, 10 first principle components§ | ||
| ND | 8,678 | |||||
| T2D | 318 | |||||
*Clinic latitude: categorized as a binary variable, with clinic located above 37° N latitude designated as northern clinics. eGFR: mean eGFR trough DCCT/EDIC calculated using annually measured serum creatinine and the Chronic Kidney Disease Epidemiology Collaboration equation (35). Smoking status during EDIC years 1–5, 6–10, and 11–16 as a continuous variable calculated by summing smoking status.
†eGFR: mean eGFR calculated using serum creatinine, measured almost every 5 years, and the Chronic Kidney Disease Epidemiology Collaboration equation (35). Smoking status: mean of smoking status, asked every 5 years through the study.
‡eGFR: eGFR calculated using serum creatinine and the Chronic Kidney Disease Epidemiology Collaboration equation (35). Smoking status: smoker vs. nonsmoker.
§eGFR: Cockcroft-Gault eGFR. Smoking status: smoker vs. nonsmoker.
GWAS
In all four studies, ungenotyped autosomal SNPs were imputed using 1000 Genomes data (worldwide reference panel of all 1,092 samples from the phase I integrated variant set) (v3, released March 2012) (27) using IMPUTE2, version 2.3.0 (https://mathgen.stats.ox.ac.uk/impute/impute_v2.html) (28).
In DCCT/EDIC and WESDR, both genotyped and imputed autosomal SNPs were tested for association primarily with SIF1 (excited at 375 nm, emission range 435–655 nm, kx 0.6, km 0.2, natural log transformed), with the SIF having strongest association with diabetes complications (14), by SNPTEST, version 2.5 (https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html#introduction), using dosage data. Factors influencing SIF including age, sex, skin tone, clinic latitude, smoking status, and estimated glomerular filtration rate (eGFR) (Supplementary Table 2), as well as NAT2 rs1495741 genotype, coded additively, were included in the model as covariates with the aim of increasing statistical power (Table 1).
Association of only genotyped SNPs on chromosome X was tested with SIF1 (natural log transformed) in DCCT/EDIC and WESDR using linear regression under additive genetic model adjusting for the factors mentioned above, except for the NAT2 SNP, using PLINK, version 1.07 (http://pngu.mgh.harvard.edu/∼purcell/plink/).
Meta-GWAS
SNPs with a minor allele frequency (MAF) of >0.01 and high imputation quality (INFO >0.80) were included in meta-analysis performed using METAL, version 1.5 (http://www.sph.umich.edu/csg/abecasis/Metal/index.html), with the STDERR method. P values ≤5E-8 were considered genome-wide significant.
The GWAS in DCCT/EDIC and WESDR were repeated including the newly identified SNP genotype (coded additively) as a covariate in the model, and subsequently, the results were combined through meta-analysis using METAL, version 1.5, with the STDERR method.
Further Investigation of the Identified Locus
Association of identified SNPs was tested with the other SIFs (SIF2–15) similar to SIF1 in both DCCT/EDIC and WESDR considering that NAT2 SNP showed strongest association with SIF12 (Supplementary Fig. 1).
In all four studies, the genotypes of identified SNPs were extracted from dosage data according to the best guess (threshold for calling genotypes = 0.80) using GTOOL, version 0.7.5 (http://www.well.ox.ac.uk/∼cfreeman/software/gwas/gtool.html). Subsequently, extracted genotypes were used for further analyses performed in SAS, version 9.3 (SAS, Cary, NC).
Association of the identified locus with SIF1 in LonGenity, and skin autofluorescence (SAF) in LifeLines, was tested among ND and T2D subjects separately using linear regression under an additive genetic model including factors associated with SF (e.g., age, sex, smoking status, and eGFR) (Supplementary Table 2, Table 1, and Supplementary Fig. 1).
The identified SNP was tested for association with each of the covariates in the model as well as a number of related phenotypes including HbA1c, glycated albumin (GA), 7-point capillary blood glucose profiles, hypoglycemia, AGEs measured by skin biopsy, nephropathy, retinopathy, neuropathy, and coronary artery calcification (Supplementary Table 3 and Supplementary Fig. 1).
For testing of whether association of the identified SNP on SIF1 differs by status of any of the covariates in the model or HbA1c levels, a SNP-covariate/HbA1c interaction term was added in to the model including all covariates (except for NAT2 genotype) in both DCCT/EDIC and WESDR. Similarly, for investigation of whether association of the identified SNP on SIF1 differs by cohort, former treatment group, or caffeine levels (another factor influencing SF) (26) in DCCT/EDIC, a SNP-cohort/group/caffeine interaction term was included in the model.
The association of identified SNP with SIF1 was also tested separately in subjects with time-weighted HbA1c levels above and below the median using linear regression, along with all the other covariates (except for NAT2 genotype). For testing of whether association of the identified locus with SIF1 is statistically different in subjects having time-weighted HbA1c above and below median, HbA1c was dichotomized, and its interaction with SNP was added to the model.
Interaction of the Identified Locus With Diabetes Status
For investigation of whether association of the identified locus with SIF differs by diabetes status, data from all studies that measured SIF (DCCT/EDIC, WESDR, and LonGenity) were combined. Then, linear regression was used to test whether the association of the SNP with SIF was different between T1D subjects (N = 1,363 from DCCT/EDIC and WESDR) and those without diabetes (N = 453 from two subgroups in LonGenity [designated in that study as “OPEL” and “OPUS”]) by including a SNP-T1D interaction term in the model. Linear regression was also used to test whether the association of SNP with SIF differs between subjects with diabetes (N = 1,421 T1D from both DCCT/EDIC and WESDR and T2D from LonGenity) and those without diabetes (N = 453 from OPEL and OPUS) by adding a SNP-diabetes interaction term to the model. Age, sex, smoking status (ever smoker vs. nonsmoker), skin tone, and eGFR at SIF measurement were also included in the model as covariates.
Sample Size Calculation
Br2 (http://www.utstat.toronto.edu/sun/Software/BR2/), which implements a bootstrap-based bias-reduction method to correct for the effect of the winner’s curse in GWAS (29), was used to calculate bias-reduced effect sizes for the SNP identified to be associated with SIF1 in DCCT/EDIC (threshold for significance was 1E-5 for suggestive association). Subsequently, the corrected β for the identified SNP was used to estimate the required sample size to detect the effect of the identified locus among T2D and ND subjects assuming that the SNP effect is similar to its effect in T1D subjects, using QUANTO, version 1.2.4 (http://biostats.usc.edu/Quanto.html).
Results
Meta-GWAS: T1D Subjects
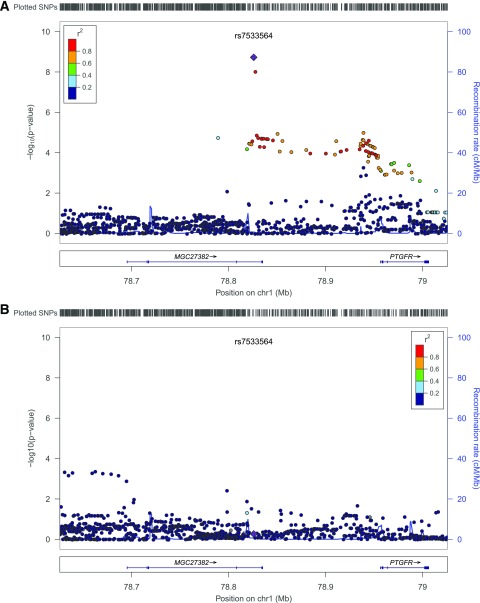
A total of 1,359 subjects (1,081 subjects from DCCT/EDIC plus 278 subjects from WESDR) were included in the meta-analysis (Table 1). Four subjects were excluded from the analysis: one from DCCT/EDIC owing to missing data regarding NAT2 genotype and three from WESDR owing to having end-stage renal disease at baseline (no eGFR data). Subject characteristics and distribution of SIFs are summarized in Tables 2 and 3. In total, 7,735,748 autosomal SNPs having MAF >0.01 and INFO >0.8 in both DCCT/EDIC and WESDR were included in the meta-analysis. Genomic control was 1.02 (Supplementary Fig. 2). We identified two SNPs, rs7533564 (chromosome 1: 78825912 [GRCh37/hg19]; T>C; β = 0.138; P = 1.88E-9) and rs7533823 (chromosome 1: 78828438; T>G; β = 0.134; P = 9.31E-9) (Table 4), 2,526 base pairs apart in complete linkage disequilibrium (r2 = 1, D′ = 1) associated with SIF1 exceeding the genome-wide significance threshold (Supplementary Fig. 3). The SNPs are located within intron 4 of a noncoding RNA, MGC27382, having 6 exons. The nearest gene is prostaglandin F receptor (PTGFR) (Fig. 1).
Table 2.
Characteristics of the study participants
| Variable | DCCT/EDIC: T1D (N = 1,082) |
WESDR: T1D
(N = 281) |
LonGenity |
LifeLines |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPEL, ND
(N = 192) |
OPUS, ND
(N = 261) |
T2D (N = 58) |
ND (N = 8,721) |
T2D (N = 318) |
||||||||||
| M | Mean ± SD or N (%) | M | Mean ± SD or N (%) | M | Mean ± SD or N (%) | M | Mean ± SD or N (%) | M | Mean ± SD or N (%) | M | Mean ± SD or N (%) | M | Mean ± SD or N (%) | |
| Sex (Male) | 0 | 579 (53.51) | 0 | 143 (50.89) | 0 | 79 (41.15) | 0 | 131 (50.19) | 0 | 36 (62.07) | 0 | 3,590 (41.17) | 0 | 168 (52.83) |
| Age (years) | 0 | 51.51 ± 6.98 | 0 | 55.92 ± 8.68 | 0 | 74.1 ± 5.91 | 0 | 75.79 ± 6.67 | 0 | 75.36 ± 7.23 | 0 | 49.1 ± 11.18 | 0 | 58.94 ± 10.81 |
| Smoking status | 0 | 0.87 ± 1.73* | 0 | 0.15 ± 0.27† | 0 | 105 (54.69)‡ | 2 | 129 (49.43)‡ | 2 | 28 (48.28)‡ | 0 | 1,922 (22.04)‡ | 0 | 55 (17.3)‡ |
| 0 | 0.73 ± 1.58§ | |||||||||||||
| 0 | 0.75 ± 1.76|| | |||||||||||||
| Skin tone | 0 | 261.73 ± 41.26 | 0 | 258.5 ± 45.97 | 0 | 241.58 ± 36.83 | 0 | 240.87 ± 37.15 | 0 | 246.62 ± 45.24 | — | — | ||
| Latitude (north) | 0 | 800 (73.94) | — | — | — | — | — | — | ||||||
| eGFR (mL/min/1.73 m2)¶ | 0 | 108.67 ± 10.11 | 3 | 95.44 ± 19.14 | 0 | 68.89 ± 16.16 | 2 | 67.1 ± 17.32 | 1 | 64.15 ± 17 | 19 | 113.07 ± 30.89 | 0 | 118.34 ± 45.45 |
| BMI (kg/m2) | — | — | — | — | — | 3 | 26.41 ± 4.17 | 0 | 30.5 ± 5.39 | |||||
| Diabetes duration (years) | 0 | 29.81 ± 4.88 | 0 | 42.02 ± 6.66 | — | — | 2 | 4.93 ± 1.08 | — | 20 | 5.63 ± 7.96 | |||
| HbA1c (%)# | 0 | 8.17 ± 0.94 | 0 | 8.68 ± 0.92 | — | — | — | 32 | 5.55 ± 0.31 | 0 | 6.84 ± 1.15 | |||
| HbA1c (mmol/mol) | 0 | 66 ± 7.59 | 0 | 71 ± 7.53 | — | — | — | 32 | 37 ± 2.07 | 0 | 51 ± 8.57 | |||
M, number of subjects with missing data.
*Smoking status during EDIC years 1–5 as a continuous variable (minimum = 0, maximum = 5) calculated by summing smoking status (smoker = 1, nonsmoker = 0) in EDIC years 1–5.
†Mean of smoking status (smoker = 1, nonsmoker = 0), asked every 5 years through the study.
‡Current smoker.
§Smoking status during EDIC years 6–10 as a continuous variable (minimum = 0, maximum = 5) calculated by summing smoking status (smoker = 1, nonsmoker = 0) in EDIC years 6–10.
||Smoking status during EDIC years 11–16 as a continuous variable (minimum = 0, maximum = 6), calculated by summing smoking status (smoker = 1, nonsmoker = 0) in EDIC years 11–16.
¶DCCT/EDIC, WESDR, LonGenity used Chronic Kidney Disease Epidemiology Collaboration equation (35) to estimate glomerular filtration rate from serum creatinines, measured annually, every 5 years, and once, respectively; Cockcroft-Gault equation was used in LifeLines to estimate glomerular filtration rate from serum creatinine.
#Time weighted in DCCT/EDIC, calculated by summing (DCCT eligibility HbA1c × duration of diabetes at DCCT baseline), (DCCT mean HbA1c × years of follow-up in DCCT), and (EDIC mean HbA1c × years of follow-up in EDIC) and dividing by total duration of diabetes, and WESDR, calculated by summing (eligibility HbA1c × duration of diabetes at baseline) and (mean HbA1c during the study × years of follow-up) and dividing by total duration of diabetes; single measurement in LifeLine.
Table 3.
SF characteristics in the cohorts included in the study
| SF | LED level (emission range) | kx, km | DCCT/EDIC: T1D | WESDR: T1D | LonGenity |
LifeLines |
|||
|---|---|---|---|---|---|---|---|---|---|
| OPEL: ND | OPUS: ND | T2D | ND | T2D | |||||
| SIF1* | 375 nm (435–655) | 0.6, 0.2 | 3.10 (0.20) | 3.17 (0.22) | 3.05 (0.21) | 3.09 (0.20) | 3.16 (0.18) | ||
| SIF2* | 0.8, 0.2 | 3.20 (0.25) | 3.27 (0.27) | 3.17 (0.27) | 3.21 (0.25) | 3.29 (0.25) | |||
| SIF3* | 0.4, 0.7 | 2.62 (0.19) | 2.70 (0.20) | 2.61 (0.19) | 2.64 (0.19) | 2.70 (0.17) | |||
| SIF4* | 405 nm (440–655) | 0.6, 0.2 | 2.11 (0.23) | 2.19 (0.24) | 2.04 (0.23) | 2.06 (0.22) | 2.14 (0.19) | ||
| SIF5* | 0.8, 0.2 | 2.11 (0.23) | 2.19 (0.24) | 2.06 (0.23) | 2.09 (0.23) | 2.17 (0.20) | |||
| SIF6* | 0.9, 0.0 | 2.27 (0.24) | 2.35 (0.25) | 2.22 (0.24) | 2.24 (0.24) | 2.33 (0.21) | |||
| SIF7* | 416 nm (451–655) | 0.8, 0.2 | 1.84 (0.24) | 1.92 (0.24) | 1.77 (0.24) | 1.80 (0.23) | 1.88 (0.19) | ||
| SIF8* | 0.9, 0.0 | 2.00 (0.24) | 2.07 (0.25) | 1.92 (0.24) | 1.96 (0.24) | 2.04 (0.20) | |||
| SIF9* | 0.4, 0.9 | 1.28 (0.23) | 1.38 (0.23) | 1.23 (0.22) | 1.26 (0.21) | 1.33 (0.17) | |||
| SIF10* | 435 nm (470–655) | 0.9, 0.0 | 1.59 (0.25) | 1.67 (0.26) | 1.52 (0.24) | 1.54 (0.24) | 1.62 (0.19) | ||
| SIF11* | 0.4, 0.8 | 1.02 (0.25) | 1.12 (0.24) | 0.94 (0.23) | 0.97 (0.22) | 1.05 (0.17) | |||
| SIF12* | 0.4, 0.9 | 0.94 (0.24) | 1.04 (0.24) | 0.87 (0.23) | 0.90 (0.22) | 0.97 (0.17) | |||
| SIF13* | 456 nm (491–655) | 0.9, 0.0 | 0.68 (0.24) | 0.78 (0.25) | 0.64 (0.24) | 0.67 (0.24) | 0.75 (0.19) | ||
| SIF14* | 0.4, 0.8 | 0.36 (0.23) | 0.47 (0.24) | 0.30 (0.23) | 0.35 (0.22) | 0.43 (0.17) | |||
| SIF15* | 0.4, 0.9 | 0.28 (0.23) | 0.39 (0.23) | 0.23 (0.23) | 0.27 (0.22) | 0.35 (0.17) | |||
| SAF | 370 nm (420–600) | 2.04 (0.44) | 2.45 (0.59) | ||||||
Data are means (SD) unless otherwise indicated. LED, light-emitting diode.
*Natural log transformed.
Table 4.
Association of rs7533564 (T>C) with SIFs in DCCT/EDIC and WESDR
| SIF* | DCCT/EDIC (N = 1,081) |
WESDR (N = 278) |
Meta-analysis (N = 1,359) |
|||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | Het I2† | Het P‡ | |
| SIF1 | 0.138 (0.026) | 1.65E-07 | 0.140 (0.049) | 0.0042 | 0.138 (0.023) | 1.88E-09 | 0 | 0.964 |
| SIF2 | 0.150 (0.031) | 9.62E-07 | 0.180 (0.059) | 0.0026 | 0.157 (0.027) | 7.85E-09 | 0 | 0.655 |
| SIF3 | 0.126 (0.025) | 7.16E-07 | 0.108 (0.045) | 0.0178 | 0.122 (0.022) | 3.45E-08 | 0 | 0.728 |
| SIF4 | 0.134 (0.029) | 5.00E-06 | 0.161 (0.054) | 0.0034 | 0.140 (0.026) | 5.38E-08 | 0 | 0.663 |
| SIF5 | 0.137 (0.032) | 1.68E-05 | 0.186 (0.057) | 0.0013 | 0.149 (0.028) | 8.35E-08 | 0 | 0.454 |
| SIF6 | 0.139 (0.031) | 6.55E-06 | 0.197 (0.059) | 0.0010 | 0.152 (0.027) | 2.72E-08 | 0 | 0.391 |
| SIF7 | 0.135 (0.031) | 1.63E-05 | 0.193 (0.059) | 0.0012 | 0.148 (0.028) | 8.07E-08 | 0 | 0.383 |
| SIF8 | 0.137 (0.032) | 1.68E-05 | 0.202 (0.060) | 0.0009 | 0.151 (0.028) | 7.04E-08 | 0 | 0.339 |
| SIF9 | 0.129 (0.031) | 3.31E-05 | 0.159 (0.057) | 0.0057 | 0.136 (0.027) | 5.93E-07 | 0 | 0.637 |
| SIF10 | 0.132 (0.033) | 6.76E-05 | 0.202 (0.063) | 0.0014 | 0.147 (0.029) | 4.68E-07 | 0 | 0.321 |
| SIF11 | 0.125 (0.033) | 1.35E-04 | 0.168 (0.061) | 0.0062 | 0.134 (0.029) | 2.89E-06 | 0 | 0.529 |
| SIF12 | 0.124 (0.033) | 1.39E-04 | 0.168 (0.061) | 0.0061 | 0.134 (0.029) | 2.95E-06 | 0 | 0.525 |
| SIF13 | 0.114 (0.031) | 3.14E-04 | 0.194 (0.063) | 0.0022 | 0.130 (0.028) | 3.87E-06 | 23.6 | 0.252 |
| SIF14 | 0.105 (0.031) | 6.06E-04 | 0.163 (0.060) | 0.0074 | 0.117 (0.027) | 1.81E-05 | 0 | 0.396 |
| SIF15 | 0.105 (0.031) | 6.43E-04 | 0.162 (0.061) | 0.0077 | 0.117 (0.027) | 1.99E-05 | 0 | 0.397 |
*Natural log transformed.
†Heterogeneity index (0–100).
‡P value for Cochran Q statistic.
Figure 1.
Regional plot of 200 kb surrounding rs7533564 with SNP P values from DCCT/EDIC and WESDR meta-GWAS (A) and LifeLines (B), with ND subjects on the left y-axis, their genomic position (GRCh37/hg19) on the x-axis, and estimated recombination rates on the right y-axis. The plot was made using LocusZoom (http://locuszoom.sph.umich.edu/locuszoom/) (36). The linkage disequilibrium measures and recombination rates are based on the HapMap CEU population (release 22).
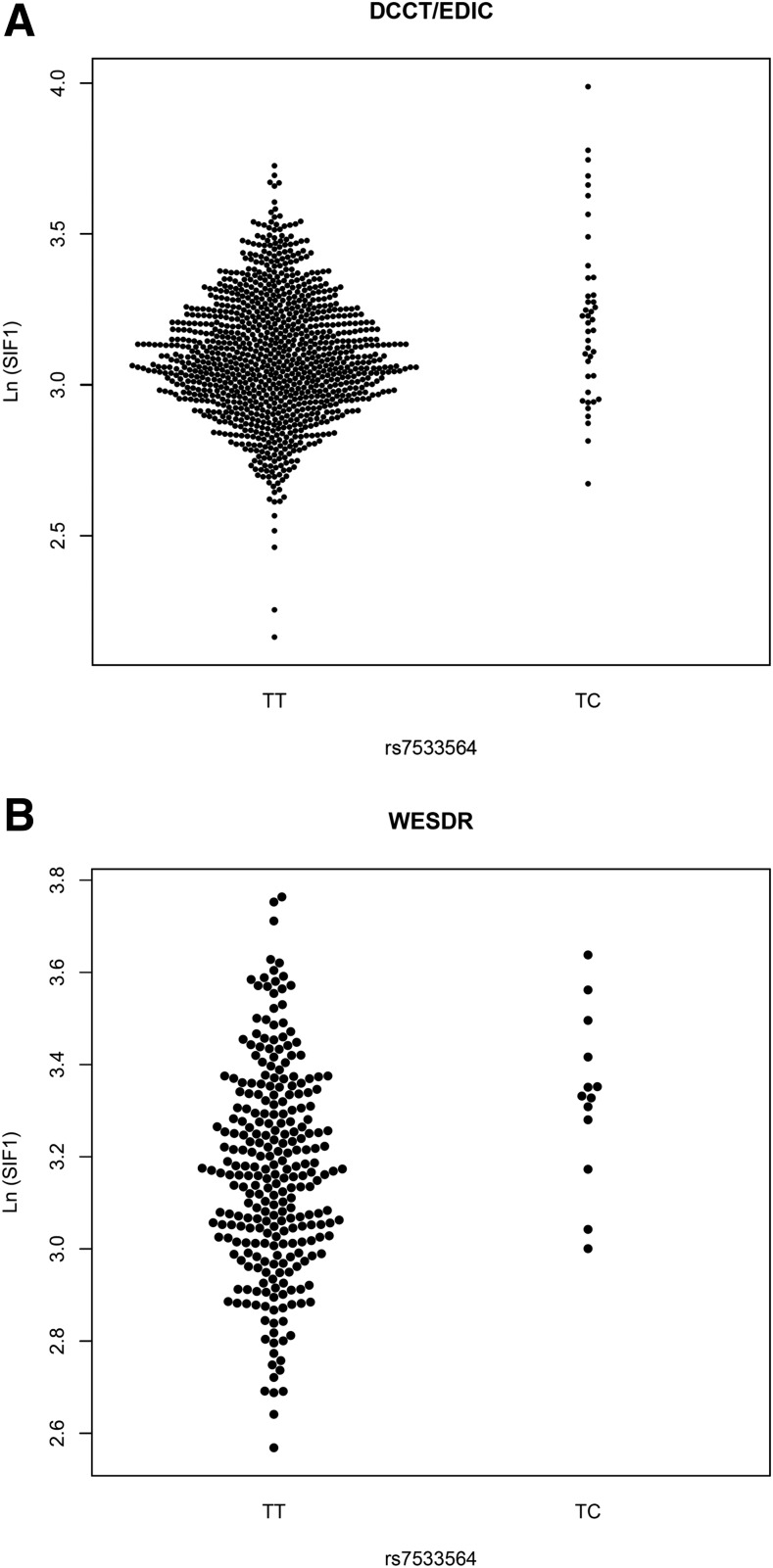
rs7533564 was imputed with high quality in DCCT/EDIC (INFO = 0.958) and genotyped in WESDR (Supplementary Fig. 4). In DCCT/EDIC, we genotyped rs7533564 in a subset (53 common homozygotes and 34 heterozygotes) using TaqMan assays, and genotypes for all 87 subjects perfectly matched with the best guess from imputation. There was no deviation from Hardy-Weinberg equilibrium in either study (P = 1). Considering the low MAF (0.020 and 0.022 in DCCT/EDIC and WESDR, respectively), none of the subjects in DCCT/EDIC or WESDR were homozygous for minor allele of rs7533564 (CC). Heterozygous subjects (TC) (N = 43 and 12 in DCCT/EDIC and WESDR, respectively) had higher levels of SIF1 compared with TT homozygotes (N = 1,038 and 266 in DCCT/EDIC and WESDR, respectively) in both DCCT/EDIC (LnSIF1 mean [SD] 3.22 [0.29] vs. 3.09 [0.20]; Δ = 0.13) and WESDR (LnSIF1 mean [SD] 3.33 [0.18] vs. 3.17 [0.22)]; Δ = 0.16) (Fig. 2), and the effect sizes were similar in both studies, with no evidence for heterogeneity (Table 4).
Figure 2.
Bee swarm plots showing level of SIF1 for each participant in the DCCT/EDIC and WESDR according to rs7533564 genotype. SIF1 is natural log transformed.
rs7533564 showed strong association with SIF2–15, which were highly correlated to SIF1 and to each other (Supplementary Table 4), with slightly less significant P values (Table 4).
After inclusion of rs7533564 in the model, no other SNP reached the genome-wide threshold in DCCT/EDIC or WESDR or in the meta-analysis (Supplementary Figs. 2 and 3).
None of the SNPs on chromosome X reached the genome-significant threshold in DCCT/EDIC (N = 25,095), WESDR (N = 18,156), or the meta-analysis (N = 11,702).
Association of rs7533564 With SF in ND/T2D Studies
rs7533564 was not significantly associated with SIF1 in 447 ND and 55 T2D subjects from LonGenity or with SAF in 8,702 ND and 318 T2D subjects from the LifeLines cohort study (Table 5).
Table 5.
Association of rs7533564 (T>C) with SIF1/SAF in LonGenity and LifeLines
| SF | N | INFO | MAF | HWE P | β (SE) | P | |
|---|---|---|---|---|---|---|---|
| LonGenity | |||||||
| OPEL (ND) | SIF1* | 190 | 1 | 0.023 | 1 | −0.024 (0.062) | 0.703 |
| OPUS (ND) | SIF1* | 257 | 1 | 0.038 | 0.315 | −0.005 (0.036) | 0.892 |
| OPEL plus OPUS (T2D) | SIF1* | 55 | 1 | 0.026 | 1 | −0.040 (0.101) | 0.691 |
| LifeLines | |||||||
| ND | SAF | 8,702 | 0.760 | 0.018 | 0.304 | −0.015 (0.024) | 0.539 |
| T2D | SAF | 318 | 0.811 | 0.017 | 1 | −0.176 (0.169) | 0.299 |
HWE, Hardy-Weinberg equilibrium.
*Natural log transformed.
Interaction of Identified Locus With Diabetes Status
Using data from the three studies that measured SIF, there was significant heterogeneity of an rs7533564 effect on SIF1 dependent on T1D status (β [SE] 0.097 [0.040], P = 0.0157). Similarly, there was also heterogeneity of SNP effect by diabetes status (T1D or T2D vs. ND) on SIF1 (β [SE] 0.090 [0.040], P = 0.0263).
Association of rs7533564 With Covariates and the Other Phenotypes
Covariates
rs7533564 was not significantly associated with any of the covariates in the model in DCCT/EDIC or WESDR (Supplementary Table 5). However, in DCCT/EDIC, the SNP had a positive interaction with smoking status during EDIC years 11–16 (P = 0.003) and a negative interaction with skin tone (P = 0.016) affecting SIF1. In addition, there was a significant interaction with DCCT former treatment group (P = 0.020); the SNP effect was larger in the former conventional group than in the intensive group (β [SE] 0.191 [0.037] vs. 0.072 [0.038]). This interaction remained significant after adjustment for time-weighted HbA1c (P = 0.005). There was also a positive interaction between rs7533564 and mean caffeine consumption during both DCCT (P = 2.66E-4) and EDIC (P = 0.0142) (Supplementary Tables 6 and 7).
HbA1c
Time-weighted HbA1c was significantly associated with SIF1–15, explaining 5.2–8.2% and 2.9–8.3% of their variation in DCCT/EDIC and WESDR, respectively (Supplementary Table 8).
The association of rs7533564 with SIF1 was independent of HbA1c levels and remained strongly significant after further adjustment for time-weighted HbA1c in both DCCT/EDIC (β [SE] 0.127 [0.025], P = 5.49E-7) and WESDR (β [SE] 0.121 [0.048], P = 0.011) as well as in the meta-analysis (β [SE] 0.126 [0.022], P = 1.65E-8).
Although association of rs7533564 with time-weighted HbA1c was not statistically significant in DCCT/EDIC (P = 0.099) or WESDR (P = 0.090) separately (Supplementary Table 5), it was statistically significant in meta-analysis (β [SE] 0.292 [0.128], P = 0.023). Association of rs7533564 with time-weighted HbA1c in the meta-analysis did not remain significant after adjustment for SIF1 (β [SE] 0.138 [0.126], P = 0.27).
In addition, rs7533564 was positively associated with HbA1c at WESDR follow-up years 25 (P = 0.0088) and 30 (P = 0.0379) (Supplementary Table 9), and there was a positive interaction between the SNP and mean HbA1c during DCCT (P = 0.009) affecting SIF1 (Supplementary Table 6).
The estimate of rs7533564 effect on SIF1 was greater for subjects having time-weighted HbA1c levels above median (β [SE] 0.147 [0.036], P < 0.0001) in DCCT/EDIC compared with those having time-weighted HbA1c level below median (β [SE] 0.098 [0.038], P = 0.0096). However, the difference in the SNP effect on SIF1 between the two groups was not statistically significant (P = 0.428). In contrast, in WESDR, the effect estimate was smaller in subjects having time-weighted HbA1c levels above median (β [SE] 0.075 [0.056], P = 0.182) compared with below median (β [SE] 0.228 [0.100], P = 0.0241). However, similar to DCCT/EDIC, the difference between the two groups was not statistically significant (P = 0.165).
GA
The mean GA in a subset of participants measured during DCCT (27.37 mg/mL [SD 6.58]) was associated with SIF1 in all 10 imputed data sets (average β [SE] 0.007 [0.002], P = 0.0024) (Supplementary Table 10).
rs7533564 was associated with mean GA during DCCT in all 10 imputed data sets (N = 455; average β [SE] 0.119 [0.040], P = 0.0029). This association remained significant after further adjustment for indicators of the three case-control statuses (i.e., cardiovascular disease, retinopathy, and nephropathy) (average β [SE] 0.110 [0.038], P = 0.0041). rs7533564 was still associated with mean GA during DCCT after further adjustment for SIF1 (average β [SE] 0.098 [0.043], P = 0.022) (Supplementary Table 11). The SNP did not show any significant interaction with any of the complications affecting mean GA and was not associated with any of them in the GA subset.
Seven-Point Capillary Blood Glucose Profiles
Mean blood glucose during DCCT (190.59 mg/dL [SD 53.26]) was associated with SIF1 (β [SD] = 0.0005 [0.0002], P = 0.0019 [adjusted for former treatment group]). rs7533564 was not associated with mean blood glucose during DCCT (β [SE] 0.041 [0.029], P = 0.164) and was not associated with repeated measures of pre- or postprandial blood glucose either.
Long-term Diabetes Complications
rs7533564 was not associated with nephropathy (Supplementary Tables 12 and 13), retinopathy (Supplementary Table 14), neuropathy, coronary artery calcification, or hypoglycemia (Supplementary Table 12).
AGEs Measured by Skin Biopsy
Characteristics of AGEs measured by skin biopsy are summarized in Supplementary Table 15. Furosine, fructose-lysine, glucosepane, and total glucose-bound skin biopsy AGEs were positively associated with SIF1 in the multivariate analysis (adjusted for age, duration of diabetes, and treatment group). Additional AGEs were also associated with SIF1 in the univariate analysis (Supplementary Table 15). rs7533564 was associated with higher levels of glyoxal hydroimidazolones (G-H1) in the multivariate analysis (β [SE] 1.374 [0.694], P = 0.0494) (Table 6).
Table 6.
Association of rs7533564 with skin biopsy AGEs
| Skin AGE | Univariable |
Multivariable* |
|||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | β (SE) | P | β (SE) | P | ||
| Pepsin soluble collagen (%)* | 170 | 1.78 (0.47) | −0.243 (0.169) | 0.150 | −0.157 (0.152) | 0.303 | |
| Acid soluble collagen (%)* | 170 | −0.72 (0.51) | −0.020 (0.185) | 0.913 | −0.013 (0.181) | 0.942 | |
| Fluorescence (AU)* | 170 | 5.21 (0.26) | 0.148 (0.095) | 0.124 | 0.109 (0.083) | 0.189 | |
| Furosine (pmol/mg collagen)* | 167 | 6.60 (0.29) | 0.214 (0.112) | 0.057 | 0.084 (0.089) | 0.349 | |
| Pentosidine (pmol/mg collagen)* | 166 | 3.21 (0.37) | 0.187 (0.133) | 0.160 | 0.132 (0.085) | 0.126 | |
| Nε-carboxymethyl-lysine (pmol/mg collagen)† | 169 | 13.38 (5.21) | 0.365 (1.892) | 0.847 | 0.124 (1.757) | 0.944 | |
| Carboxyethyl-lysine (pmol/mg)* | 169 | 4.79 (0.65) | −0.106 (0.237) | 0.654 | −0.118 (0.241) | 0.625 | |
| Methylglyoxal hydroimidazolones (nmol/mg)* | 170 | −0.37 (0.55) | 0.156 (0.198) | 0.432 | 0.160 (0.184) | 0.385 | |
| Glyoxal hydroimidazolones (pmol/mg)† | 169 | 7.84 (1.97) | 1.379 (0.707) | 0.053 | 1.374 (0.694) | 0.049 | |
| Fructose-lysine (nmol/mg)* | 170 | 1.63 (0.37) | 0.250 (0.133) | 0.062 | 0.177 (0.118) | 0.135 | |
| Glucosepane (nmol/mg)* | 170 | 0.90 (0.31) | 0.176 (0.112) | 0.117 | 0.110 (0.088) | 0.210 | |
| Total glucose bound (fructose-lysine plus glucosepane)* | 170 | 2.04 (0.30) | 0.226 (0.109) | 0.039 | 0.154 (0.098) | 0.117 | |
| LW1† | 170 | 19.36 (5.01) | 2.004 (1.814) | 0.271 | 1.210 (1.621) | 0.456 | |
Association of the identified SNP with skin biopsy AGEs was tested using linear regression. The results were further adjusted for age, duration of diabetes, and treatment group in the multivariable analysis. AU, arbitrary units.
*Natural log transformed.
†Square root transformed.
Discussion
We used meta-GWAS of two T1D studies, DCCT/EDIC and WESDR, to identify a new locus on chromosome 1 associated with SIF1 (excitation 375 nm, emission 435–655 nm) at genome-wide significance level. The C allele of rs7533564 (T>C) was associated with higher levels of SIF1 in both studies, explaining 1.5 and 2.5% of the variation in SIF1 in DCCT/EDIC and WESDR, respectively. rs7533564 showed similar associations in terms of effect direction and size with SIF2–15 (excitation 375–456 nm, emission 435–655 nm) but at slightly less significance, whereas NAT2, the previously identified locus, had the strongest association with SIF12 (excitation 435 nm, emission 470–655 nm) with much larger effect size compared with SIF1 (25).
rs7533564 did not show similar association with SF in a large population of ND subjects (LifeLines) or in a small population of T2D subjects, suggesting that the effect of SNP on SF is restricted to T1D subjects. The bias-reduced effect estimate for rs7533564 on SIF1 was 0.071 in DCCT/EDIC. With use of this estimate, at least 1,585 subjects would be required to detect the effect with good power (1-β = 80%) for SIF1 (mean [SE] 3.10 [0.20]) with type I error rate (two sided) 0.05 and allele frequency 0.02. Therefore, our study did not have sufficient power to detect an association in T2D subjects (N = 376 including 318 and 58 T2D subjects from LifeLines and LonGenity, respectively) if we assume that the SNP effect is as large as in T1D subjects. However, diabetes duration was much longer in T1D subjects compared with T2D subjects, and diabetes duration is associated with higher levels of AGEs in the skin (7,8) as well as SF (19). In addition, former intensive therapy in DCCT, which results in lower levels of HbA1c, attenuated the effect of the SNP on SIF1 in DCCT/EDIC, indicating that the SNP effect is larger in those who have experienced higher levels of hyperglycemia, and T1D subjects had higher levels of HbA1c over time than T2D subjects (Table 2). Therefore, the SNP effect may be larger in those who have been exposed to higher levels of hyperglycemia for a longer period of time and consequently accumulated more AGEs in skin. Similarly, in ND subjects the effect size could be smaller than in those with diabetes, as they were not exposed to hyperglycemia, and larger studies would be needed to detect this. The interaction analysis also suggested that the SNP effect on SIF differs by diabetes status: having T1D or diabetes in general (i.e., T1D or T2D) compared with ND. The other point to consider is that in T1D subjects, SIF was measured by SCOUT DS, whereas in the majority of our T2D and ND subjects, SAF was measured using AGE Reader. Although the two devices use similar technology, they use different protocols to correct and calculate the final value, which is reported by both in arbitrary units, and to our knowledge the two devices have never been directly compared. We included nongenetic factors influencing SF in the model, and it is unlikely that the results were confounded by any of these factors. Besides, the identified SNP was not associated with any except for HbA1c.
Association of rs7533564 with SIF was independent of mean HbA1c over time. However, the SNP was associated with higher levels of both time-weighted HbA1c and mean GA, indicating that at least part of this association is due to hyperglycemia over decades prior to SIF measurement. The association with mean GA appeared to be partly independent of its association with SIF, and the SNP was not associated with blood glucose levels, but both measurements were obtained during the DCCT, ∼16–26 years before SIF measurement, and did not reflect glycemia when SIF was measured.
We could not confirm whether the SNP effect differed by mean HbA1c over time, as the results from DCCT/EDIC and WESDR were discrepant (e.g., rs7533564 effect on SIF1 appeared to be greater in subjects having higher levels of HbA1c over time in DCCT/EDIC, whereas the opposite was observed in WESDR) and statistically not significant, most probably due to lack of statistical power, especially in WESDR. In addition, there were some differences between DCCT/EDIC and WESDR in terms of age, mean eGFR, and mean HbA1c over time, which makes interpreting the results difficult (Table 2). Besides, HbA1c was measured annually in DCCT/EDIC, whereas it was measured approximately every 5 years in WESDR.
rs7533564 had positive interaction with smoking status during the 6 years prior to SIF measurement (EDIC years 11–16) and with mean caffeine during both DCCT and EDIC, suggesting that smoking and caffeine consumption could augment its effect on SIF1. In contrast, the negative interaction between the SNP and skin tone indicated that the SNP effect could be attenuated in subjects with darker skin.
We could not detect any association between the SNP and incidence of severe hypoglycemia or risk of developing any of the long-term diabetes complications including retinopathy and nephropathy. However, our study may not have sufficient power to detect such associations.
rs7533564 was associated with higher levels of G-H1, an AGE measured by skin biopsy. G-H1 does not fluoresce, but, interestingly, is the same AGE associated with NAT2 (25). The significant association of rs7533564 with G-H1 implies that a process involving free serum glyoxal or glycoaldehyde is implicated in G-H1 formation and influenced by the SNP (30).
PTGFR is the nearest gene to rs7533564; the SNP is located 130 kb 5′ upstream of the gene. PTGFR produces the receptor for prostaglandin F2-α, a potent luteolytic agent, and is mainly expressed in the uterus (http://biogps.org). PTGFR has recently been identified as a fibrosis hormone and is involved in the accumulation of collagen I and III in myocardium in insulin resistance state leading to diabetic cardiomyopathy. Prostaglandin F2-α receptor silencing improves insulin resistance in T2D and has a protective effect on diabetes-induced vascular remodeling (31–33). rs7533564 is not a known expression quantitative trait loci for the nearby genes including PTGFR (http://www.gtexportal.org, eqtl.uchicago.edu, and http://www.muther.ac.uk [as of February 2016]). However, due to low allele frequency of rs7533564, expression studies could be underpowered to detect the effect. The identified locus has not been associated with T1D or related phenotypes in GWAS. The T allele of rs7533564 was nominally associated with higher levels of LDL in meta-GWAS (the opposite of its effect on SIF), but it has not been associated with the other lipids, HbA1c in ND, risk of T2D, or risk of coronary artery disease and myocardial infarction (Supplementary Table 16). rs7533564 was not associated with insulin sensitivity (GENESIS [GENEticS of Insulin Sensitivity] consortium) (J. Knowles, personal communication), whereas a nonsynonymous SNP in NAT2 (rs1208 [803A>G, K268R]) was strongly associated with decreased insulin sensitivity in GWAS (34). However, since the allele frequency of rs7533564 is 2%, insufficient power to detect its association could lead to false negative results.
In conclusion, we identified a locus on chromosome 1 associated with SIF in T1D subjects that needs confirmation. Functional studies are required to identify the exact causal variant and affected gene in the region, which could lead to identification of new pathways involved in development of diabetes complications. The SNP is not significantly associated with SF in ND subjects or has a much smaller effect. Our study was underpowered to detect association with SF in T2D subjects; studies with larger numbers are required. Studies with larger sample sizes are also required to investigate association of the identified locus with long-term complications of diabetes.
Article Information
Acknowledgments. The authors acknowledge the patients and researchers of DCCT/EDIC, WESDR, LifeLines, and LonGenity.
Funding. Industry contributors have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ). The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993 and 2012–2017) and contracts (1982–2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) (current grant nos. U01-DK-094176 and U01-DK-094157) and by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006–present), Bethesda, MD. The skin biopsy ancillary substudy in DCCT/EDIC was funded by the NIDDK (DK-79432 to D.R.S.) and the JDRF International (grant no. 17-2010-318 to V.M.M.). The WESDR was supported by grant R01-EY016379 from the National Eye Institute, National Institutes of Health (NIH), and an unrestricted grant from Research to Prevent Blindness, New York, NY. The LifeLines Cohort Study is supported by the Netherlands Organization for Scientific Research (grant 175.010.2007.006); Economic Structure Enhancing Fund of the Dutch government; Ministry of Economic Affairs, Ministry of Education, Culture and Science; Ministry for Health, Welfare and Sports; Northern Netherlands Provinces Alliance; Province of Groningen; University Medical Center Groningen; Dutch Kidney Foundation; and Dutch Diabetes Research Foundation. Participation in this work was in part also supported by the National Consortium for Healthy Ageing and the BioSHaRE-EU consortium (KP7, project reference 261433). LifeLines (BRIF4568) is engaged in a Bioresource research impact factor policy pilot study, details of which can be found at www.bioshare.eu/content/bioresource-impact-factor. LonGenity is supported by the NIH (P01-AG027734 to N.B.), Glenn Foundation for the Biology of Aging, Nathan Shock Center (P30AG038072), Einstein Institute for Clinical and Translational Research (Clinical and Translational Science Awards grant 8UL1 TR000086 from the National Center for Advancing Translational Sciences), Diabetes Research Center (NIH-5P60-DK-20541), and NIH (RO1-1R01AG042188 to G.A.).
Industry contributors have had no role in the DCCT/EDIC study. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.R. designed the study, researched data, and wrote the manuscript. R.K., B.E.K.K., B.H.R.W., G.A., S.B.B., J.M., and V.M.M. researched data and reviewed and edited the manuscript. M.M.v.d.K., J.V.v.V.-O., D.B.-A., J.P.C., N.B., A.J.C., S.M.H., L.T.H., D.R.S., P.A.C., and B.H.B. researched data. A.D.P. designed the study, researched data, contributed to the discussion, and reviewed and edited the manuscript. R.K., B.E.K.K., B.H.R.W., N.B., and A.D.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1484/-/DC1.
A complete list of participants in the DCCT/EDIC Research Group can be found in N Engl J Med 2011;365:2366–2376.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: a novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol 2015;185:263–268 [DOI] [PubMed] [Google Scholar]
- 3.Verzijl N, DeGroot J, Thorpe SR, et al. . Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000;275:39027–39031 [DOI] [PubMed] [Google Scholar]
- 4.Cleary PA, Braffett BH, Orchard T, et al.; DCCT/EDIC Research Group . Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Technol Ther 2013;15:466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genuth S, Sun W, Cleary P, et al.; DCCT/EDIC Research Group . Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 2015;64:266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genuth S, Sun W, Cleary P, et al.; DCCT Skin Collagen Ancillary Study Group . Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnier VM, Bautista O, Kenny D, et al. . Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monnier VM, Sell DR, Strauch C, et al.; DCCT Research Group . The association between skin collagen glucosepane and past progression of microvascular and neuropathic complications in type 1 diabetes. J Diabetes Complications 2013;27:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monnier VM, Sun W, Gao X, et al.; DCCT/EDIC Research Group . Skin collagen advanced glycation endproducts (AGEs) and the long-term progression of sub-clinical cardiovascular disease in type 1 diabetes. Cardiovasc Diabetol 2015;14:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meerwaldt R, Graaff R, Oomen PH, et al. . Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004;47:1324–1330 [DOI] [PubMed] [Google Scholar]
- 11.Conway B, Edmundowicz D, Matter N, Maynard J, Orchard T. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther 2010;12:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway BN, Aroda VR, Maynard JD, et al. . Skin intrinsic fluorescence is associated with coronary artery disease in individuals with long duration of type 1 diabetes. Diabetes Care 2012;35:2331–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway BN, Aroda VR, Maynard JD, et al. . Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes Care 2011;34:1000–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orchard TJ, Lyons TJ, Cleary PA, et al.; DCCT/EDIC Research Group . The association of skin intrinsic fluorescence with type 1 diabetes complications in the DCCT/EDIC study. Diabetes Care 2013;36:3146–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araszkiewicz A, Naskret D, Niedzwiecki P, Samborski P, Wierusz-Wysocka B, Zozulinska-Ziolkiewicz D. Increased accumulation of skin advanced glycation end products is associated with microvascular complications in type 1 diabetes. Diabetes Technol Ther 2011;13:837–842 [DOI] [PubMed] [Google Scholar]
- 16.Genevieve M, Vivot A, Gonzalez C, et al. . Skin autofluorescence is associated with past glycaemic control and complications in type 1 diabetes mellitus. Diabetes Metab 2013;39:349–354 [DOI] [PubMed] [Google Scholar]
- 17.Noordzij MJ, Mulder DJ, Oomen PH, et al. . Skin autofluorescence and risk of micro- and macrovascular complications in patients with type 2 diabetes mellitus-a multi-centre study. Diabet Med 2012;29:1556–1561 [DOI] [PubMed] [Google Scholar]
- 18.Gerrits EG, Lutgers HL, Kleefstra N, et al. . Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008;31:517–521 [DOI] [PubMed] [Google Scholar]
- 19.Lutgers HL, Graaff R, Links TP, et al. . Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 2006;29:2654–2659 [DOI] [PubMed] [Google Scholar]
- 20.Lutgers HL, Gerrits EG, Graaff R, et al. . Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia 2009;52:789–797 [DOI] [PubMed] [Google Scholar]
- 21.Barat P, Cammas B, Lacoste A, et al. . Advanced glycation end products in children with type 1 diabetes: family matters? Diabetes Care 2012;35:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Báez EA, Shah S, Felipe D, Maynard J, Chalew S. Correlation of advanced glycation endproducts estimated from skin fluorescence in first-degree relatives: the impact of adjustment for skin pigmentation. J Diabetes Sci Technol 2015;9:278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skrha J Jr, Soupal J, Prazny M. Relationship of the lens and skin autofluorescence in patients with diabetes (Abstract). Diabetes 2015;64(Suppl. 1):A172 [Google Scholar]
- 24.Kessel L, Hougaard JL, Sander B, Kyvik KO, Sørensen TI, Larsen M. Lens ageing as an indicator of tissue damage associated with smoking and non-enzymatic glycation--a twin study. Diabetologia 2002;45:1457–1462 [DOI] [PubMed] [Google Scholar]
- 25.Eny KM, Lutgers HL, Maynard J, et al.; LifeLines Cohort Study Group; DCCT/EDIC Research Group . GWAS identifies an NAT2 acetylator status tag single nucleotide polymorphism to be a major locus for skin fluorescence. Diabetologia 2014;57:1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eny KM, Orchard TJ, Miller RG, et al.; DCCT/EDIC Research Group . Caffeine consumption contributes to skin intrinsic fluorescence in type 1 diabetes. Diabetes Technol Ther 2015;17:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abecasis GR, Altshuler D, Auton A, et al.; 1000 Genomes Project Consortium . A map of human genome variation from population-scale sequencing. Nature 2010;467:1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009;5:e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Dimitromanolakis A, Faye LL, Paterson AD, Waggott D, Bull SB; DCCT/EDIC Research Group . BR-squared: a practical solution to the winner’s curse in genome-wide scans. Hum Genet 2011;129:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 1999;344:109–116 [PMC free article] [PubMed] [Google Scholar]
- 31.Ding WY, Liu L, Wang ZH, et al. . FP-receptor gene silencing ameliorates myocardial fibrosis and protects from diabetic cardiomyopathy. J Mol Med (Berl) 2014;92:629–640 [DOI] [PubMed] [Google Scholar]
- 32.Ding WY, Ti Y, Wang J, et al. . Prostaglandin F2α facilitates collagen synthesis in cardiac fibroblasts via an F-prostanoid receptor/protein kinase C/Rho kinase pathway independent of transforming growth factor β1. Int J Biochem Cell Biol 2012;44:1031–1039 [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Han L, Ding WY, et al. . Prostaglandin F2α receptor silencing attenuates vascular remodeling in rats with type 2 diabetes. Exp Mol Pathol 2015;99:517–523 [DOI] [PubMed] [Google Scholar]
- 34.Knowles JW, Xie W, Zhang Z, et al.; RISC (Relationship between Insulin Sensitivity and Cardiovascular Disease) Consortium; EUGENE2 (European Network on Functional Genomics of Type 2 Diabetes) Study; GUARDIAN (Genetics UndeRlying DIAbetes in HispaNics) Consortium; SAPPHIRe (Stanford Asian and Pacific Program for Hypertension and Insulin Resistance) Study . Identification and validation of N-acetyltransferase 2 as an insulin sensitivity gene. J Clin Invest 2015;125:1739–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruim RJ, Welch RP, Sanna S, et al. . LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 2010;26:2336–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]