Abstract
Recent studies have shown that in addition to their traditionally recognized functions as building blocks, energy stores, or hazardous intermediates, lipids also have the ability to act as signaling molecules with potent effects on systemic metabolism and metabolic diseases. This Perspective highlights this somewhat less apparent biology of lipids, especially focusing on de novo lipogenesis as a process that gives rise to key messenger molecules mediating interorgan communication. Elucidating the mechanisms of lipid-dependent coordination of metabolism promises invaluable insights into the understanding of metabolic diseases and may contribute to the development of a new generation of preventative and therapeutic approaches.
Introduction
Lipids have long been known for their roles in cellular structure and energy storage. Among lipid species, fatty acids incorporated into triglycerides efficiently function as energy-dense storage molecules. Perhaps initially for this purpose, organisms have evolved the ability to synthesize fatty acids endogenously (de novo lipogenesis [DNL]) from alternative carbon sources such as carbohydrates (1). Although storage of excess energy in the form of lipids is a useful defense against starvation, for modern humans, to whom food is often constantly accessible, this adaptive mechanism has become inimical, with energy surplus and excessive lipid exposure generating the basis for a variety of metabolic disorders. However, more recent research has revealed that in addition to energy storage, lipids have wide-ranging actions as signaling molecules that are relevant to systemic metabolism. As described in this review, emerging evidence suggests that the products of DNL in particular have potent effects on systemic metabolism, and hence the tissue-specific regulation of this process is critical for metabolic homeostasis. Here, we describe some of the major lipid metabolism pathways that support DNL and their bioactive products with identified roles in metabolic disease. Further research in these areas may highlight novel endocrine pathways and translational opportunities.
Regulation of DNL
De novo synthesis of fatty acids takes place primarily in the liver (2). Under physiological conditions, excess carbohydrates that are not stored as glycogen in hepatocytes are converted into fatty acids and esterified into triglycerides. Dysregulation of DNL and other aspects of lipid metabolism are common features of obesity and obesity-associated metabolic diseases such as insulin resistance and diabetes. In the liver, obesity causes increased DNL activity and fatty acid esterification, which may be a contributing factor to local and systemic metabolic deterioration, such as hepatic steatosis and increased triglyceride levels in circulation (3). Escalation of the liver lipogenic program observed in obesity is due in particular to an increased level and activity of sterol regulatory element binding protein 1 (SREBP1) (4). In fact, loss- and gain-of-function studies indicate that SREBP1 functions as an important regulator of DNL in the liver (4,5). Interestingly, in spite of the fact that insulin signaling is a key activator of SREBP1 in the liver, enhanced SREBP1 activity and elevated lipogenic capacity persist under even severe insulin resistant states, such as obesity and type 2 diabetes. This suggests that insulin signaling–independent mechanisms may also support the pathological elevation of DNL in the liver. These mechanisms are not completely understood and represent an important area of future research. It is important to note that lipid accumulation in the liver per se is not universally associated with adverse outcomes, and several different mechanistic models have been proposed to underlie these events (6). However, multiple lines of evidence show a high correlation between an increase in liver DNL and metabolic pathologies such as insulin resistance (3).
Unlike liver, which is the main site for endogenous synthesis of fatty acids, the adipose tissue acts as a major storage depot for excess lipids. In adipocytes, these lipids are primarily derived from exogenous sources and only a fraction are endogenously synthesized via DNL (7). Considering the massive quantities of stored lipid in adipocytes, DNL is unlikely to contribute significantly to the lipid mass of adipose tissue. Thus, the fact that adipocytes intrinsically engage in fatty acid synthesis raises the possibility that in comparison with their dietary counterparts, adipocyte-derived fatty acids may have unique functions and roles in important biological processes beyond energy storage.
Under physiological conditions, the regulation of DNL by feeding and fasting is highly synchronized in the liver and adipose tissue. Interestingly, however, this coordination is lost in pathological conditions. For example, contrary to the liver, the de novo lipogenic capacity of adipocytes is substantially reduced in obesity, which was initially revealed by studies tracing labeled glucose in adipocytes isolated from lean and obese rats (8). Since then, multiple groups have confirmed a pronounced suppression of enzymes functioning in the fatty acid synthesis and lipogenesis pathways in the adipose tissues of obese humans and in mouse models of obesity (9–12). These observations raise the possibility that decreased DNL in adipose tissue may in fact contribute to systemic metabolic perturbations observed in obesity. In line with the distinct regulation of DNL in the liver and adipose tissue under pathological conditions, genetic interventions that impinge on DNL in the liver are typically associated with reciprocal regulation of that pathway in adipose tissue. For instance, liver-specific deficiency of SCAP, which results in suppression of liver DNL, drives a concomitant upregulation of lipogenic genes in adipose tissue (13). Likewise, genetic deletion of liver X receptor α and β in leptin-deficient (ob/ob) mice induces the lipogenic program in adipose tissue via activation of carbohydrate response element–binding protein (ChREBP) (14), which is a key transcription factor regulating DNL in adipocytes.
In addition to providing evidence for reciprocal regulation of DNL in the liver and adipose tissue, studies so far have also documented a possible connection between increased DNL in adipose tissue and systemic beneficial outcomes, such as increased insulin sensitivity and improved systemic glucose homeostasis. This possibility is further supported by studies of other mouse models in which adipose tissue DNL is directly targeted. For example, promoting glucose uptake into the adipose tissue via overexpression of Glut4 in adipocytes improves systemic glucose homeostasis (15,16). This effect is largely attributed to ChREBP-dependent transcriptional regulation, since ChREBP loss-of-function abrogates the DNL response as well as the beneficial phenotype seen in this experimental model (17). Reciprocally, adipose tissue–specific overexpression of constitutively active ChREBP is sufficient to provide favorable whole-body metabolic effects, such as improved glucose homeostasis and decreased hepatic triglyceride content (18). Another remarkable connection regarding the systemic effects of adipose tissue DNL is observed in the setting of calorie restriction. Despite being an energetically unfavorable process, calorie restriction results in an increase in adipose tissue DNL (19). This paradoxical consequence raises the possibility that the products of DNL may in fact contribute to the favorable metabolic effects of dietary restriction. Taken together, the accumulating evidence suggests both that the control of the DNL program operates independently and perhaps in an opposite manner in the liver and adipose tissue under pathogenic conditions and that elevated DNL in adipose tissue is highly associated with a favorable overall metabolic phenotype. Hence, a critical question relates to the mechanisms underlying these effects and the identity of the molecules that signal these metabolic activities.
Signaling Actions of Adipose Tissue DNL Products
Work from our group leading to the identification of palmitoleate (C16:1n7) as an adipose tissue–derived lipokine established a direct link between a product of adipose tissue DNL and its beneficial systemic effects (20). This finding originated from the observation that genetic deficiency of lipid chaperone proteins FABP4 and FABP5 (also known as aP2 and mal1, respectively) resulted in elevated adipose tissue expression of DNL genes, such as fatty acid synthase (FAS) and stearoyl-CoA desaturase 1 (SCD1). Unbiased lipid profiling of the adipose tissue in FABP4/5-deficient mice revealed a marked increase in palmitoleate content in multiple lipid classes, such as triglyceride, diacylglycerol, and free fatty acids. The level of palmitoleate was also significantly increased in the plasma of FABP4/5-deficient mice on a high-fat diet. Mechanistically, it was shown in these studies that palmitoleate directly and positively regulated lipid and glucose metabolism by suppressing the expression of lipogenic genes in the liver and increasing insulin sensitivity in the liver and muscle. Taken together, these data suggest that elevated DNL and increased production of palmitoleate in adipose tissue at least in part mediate the whole-body beneficial effects of FABP4/5 deficiency (20). Interestingly, the levels of oleate, another product of SCD1 desaturase activity, were not altered in parallel with palmitoleate. This is perhaps due to the fact that oleate is an abundant fatty acid in mouse chow, and thus relative to palmitoleate, the contribution of adipose tissue DNL to oleate levels is likely to be negligible. In other words, the products of DNL that are rare in the diet, such as palmitoleate, may act as key bioactive signals or lipokines, mediating organ-to-organ communication and regulating systemic metabolic homeostasis through their unique biological activity (Fig. 1).
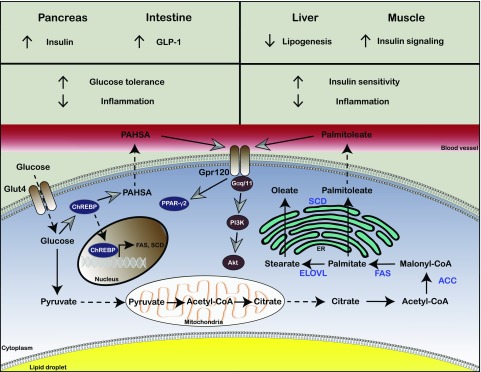
Figure 1.
Products of DNL in adipose tissue regulate systemic metabolism. The de novo lipogenic capacity of adipose tissue is significantly lower than that of the liver. However, interventions that increase DNL in adipose tissue are associated with improved systemic metabolic homeostasis. For example, increased expression of FAS and SCD1 in the adipose tissue of FABP4/5-deficient mice significantly increases de novo production of the monounsaturated fatty acid palmitoleate. In turn, palmitoleate increases insulin sensitivity in muscle and suppresses lipogenesis and steatosis in the liver. Similarly, increased glucose uptake specifically in the adipose tissue of Glut4-overexpressing mice induces DNL, leading to elevated synthesis of many complex lipids including PAHSA via a ChREBP-dependent mechanism. PAHSA positively regulates fatty acid transporter Gpr120 and Glut4-dependent glucose uptake in adipocytes in an autocrine manner and enhances glucagon-like peptide 1 (GLP-1) and insulin secretion from the intestine and pancreas, thereby improving glucose tolerance.
The beneficial role of palmitoleate has also been demonstrated in various other experimental settings. Similar to our findings in an FABP4/5-deficient model, independent groups have reported insulin-sensitizing effects of palmitoleate on muscle (21,22), and palmitoleate administration improves glucose metabolism in the setting of high-fat diet–induced insulin resistance and in the KK-Ay diabetic mouse model (23,24). Palmitoleate has also been shown to antagonize high-fat diet and palmitate-induced proinflammatory activation of macrophages (25,26). Finally, increased palmitoleate production in FABP4-deficient macrophages guards cells against palmitate-induced lipotoxicity and cytotoxicity, which is protective in a mouse model of atherosclerosis (27). These results point to favorable metabolic and anti-inflammatory roles of palmitoleate and underscore its therapeutic potential.
There are several lines of evidence emerging from human studies that are consistent with the beneficial effects of adipose tissue palmitoleate production observed in experimental models (28,29). Interestingly, the relative contribution of adipose tissue–derived palmitoleate to the circulation varies depending on where it is produced in humans. Adipose tissue located in the lower body is a more significant source of circulating palmitoleate than abdominal subcutaneous adipose tissue (30). This may in part explain the relative benefit of “pear-shaped” adipose tissue accumulation compared with abdominal adiposity. However, the numerous studies assessing serum palmitoleate in humans have revealed both positive and negative associations with metabolic disease conditions (28,29,31–34). It is critical to consider the complexity of these serum measurements, as the contrasting findings may be due to the differences between the lipid fractions in which palmitoleate was measured in each investigation. For instance, studies measuring palmitoleate in the circulating nonesterified fatty acid fraction show that palmitoleate level is positively correlated with insulin sensitivity (29). In contrast, an increase in palmitoleate in other lipid fractions, such as cholesterol esters, triglyceride, and diacylglyceride, is correlated with insulin resistance, nonalcoholic fatty liver disease, and diabetes (31–33). These seemingly discrepant results could indicate that the free fatty acid form of palmitoleate acts as a lipokine with systemic beneficial effects, whereas esterified palmitoleate loses this distinct function or may reflect hepatic output. Indeed, esterified fatty acid fractions in the circulation highly resemble liver, but not adipose tissue, fatty acid composition (30,35), and therefore the increased esterified palmitoleate observed in pathological settings may simply be reflective of elevated hepatic DNL. Alternatively, the coupling of DNL and palmitoleate production in the liver may in fact be an adaptive mechanism to offset the potential hazards of increased lipogenesis that would occur in obesity. Altogether, these human studies illustrate the importance of both the form and the source of palmitoleate as determinant factors defining the correlation between palmitoleate levels and disease conditions and emphasize that additional well-controlled human trials will be needed to better understand the action of this lipokine and evaluate its therapeutic utility.
Notably, palmitoleate is not the only mediator of the systemic beneficial effects of increased adipose tissue DNL. Recently, studies from the laboratory of Barbara Kahn described a novel class of adipose tissue–derived lipids with potent metabolic effects under conditions of elevated adipose tissue DNL in the Glut4 transgenic model (36). In these studies, unbiased lipidomic analysis pinpointed a new class of lipids, fatty acid–hydroxy–fatty acids (FAHFAs), which are synthesized in vivo and significantly elevated in the adipose tissue of Glut4-overexpressing mice. An isomer of FAHFA, consisting of palmitic acid and stearic acid (PAHSA), was highly regulated by fasting/feeding cycles and in mice fed a high-fat diet. In addition to its levels in adipose tissue, PAHSA concentration in circulation also fluctuated and was correlated with improved insulin sensitivity. Remarkably, exogenous PAHSA treatment improved glucose tolerance and overall glucose metabolism in mice, mediated in part by enhanced glucagon-like peptide 1 and insulin secretion. Importantly, and similar to palmitoleate, PAHSA administration exerted an anti-inflammatory effect on adipose tissue–resident immune cells (Fig. 1). PAHSA levels were also reduced in the serum and subcutaneous adipose tissue of insulin resistant human subjects (36). These results may indicate that lipid-based communication between metabolically active organs could be a common mechanism of endocrine regulation and that adipose tissue DNL is an important process in the generation of these signaling molecules.
Products of Liver DNL
Lipid-mediated interorgan communication does not only result from adipose tissue–derived lipids. Recent studies focusing on lipid metabolism in the liver have also identified products of DNL that regulate lipid metabolism in peripheral organs, such as adipose tissue and muscle. By modulating the levels of the nuclear receptor peroxisome proliferator–activated receptor (PPAR)δ in mouse liver, the laboratory of Chih-Hao Lee (37) demonstrated that alterations in the hepatic DNL program modulate fatty acid utilization in muscle via a lipid mediator. The Lee lab found that increased hepatic PPARδ activity resulted in elevated fatty acid uptake in muscle, whereas PPARδ loss of function in the liver decreased this process (37). Moreover, unbiased lipid profiling analysis showed that hepatic PPARδ activity regulated the level of a specific phospholipid species, phosphatidylcholine 18:0/18:1, in serum. The liver-derived phosphatidylcholine 18:0/18:1 directly mediated muscle fatty acid uptake, and its systemic administration improved whole-body glucose and lipid homeostasis in leptin receptor–deficient (db/db) mice (37). These findings support the hypothesis that hepatic DNL products can carry out adaptive responses to manage the lipid load by promoting lipid disposal and oxidation in muscle tissue.
Additional evidence for the distant effects of DNL in the liver has emerged from studies focusing on SCD isoforms and the products of their desaturase activities (38). Gain-of-function experiments with two different isoforms of SCD enzyme, mouse SCD3 and human SCD5, which have different preferential saturated fatty acid substrates, demonstrated differential effects of endogenously synthesized palmitoleate and oleate on metabolic homeostasis. Overexpression of SCD5 in the liver of SCD1-deficient mice significantly increased oleate production, which was associated with enhanced triglyceride accumulation in the liver, increased adiposity, and suppression of DNL in adipose tissue. However, SCD3 gain of function in the liver of SCD1-deficient mice, which resulted in increased palmitoleate levels, did not have detrimental effects on peripheral tissues or metabolic homeostasis (38). Taken together, these data suggest that the characteristics of fatty acids, including the site of their endogenous production, are critical factors for determining their identity and physiological or pathophysiological roles.
The studies described above underscore the differing de novo lipogenic capacity of liver and adipose tissue and suggest that the lipid products from these organs may play different or even opposing roles in modulating systemic metabolism. Therefore, approaches to target DNL as metabolic disease therapy would require a high degree of organ specificity, ideally allowing for suppression of hepatic DNL and promotion of DNL in adipose tissue. Achieving this will be a very challenging task because the major lipogenic enzymes lack tissue specificity and are active in both the liver and adipose tissue. However, it may be possible that targeting transcriptional regulators of DNL, such as ChREBP, which has a relatively higher degree of tissue specificity in driving the expression of DNL machinery (4,17), could be a more practical and efficient therapeutic approach.
Other Lipid Classes With Critical Metabolic Effects
The finding that DNL in the liver and adipose tissue are linked to lipid metabolism in distant metabolically active organs and the identification of key mediators of this network have provided significant information about bioactive fatty acid species functioning as mediators of systemic metabolism. In addition to DNL products, many other lipid species also act as mediators of metabolism with both beneficial and detrimental effects. Sphingolipids and the related molecules ceramides are some of the best studied lipids that exert detrimental effects on metabolism (39). Synthesis of sphingolipids and ceramides is induced by cellular stresses including inflammation, and in turn ceramide exerts potent effects on cellular use of glucose and uptake of other lipids (40). Many groups have demonstrated that incubating cells with exogenous cell-permeable ceramide or ceramide analogs blocks insulin signaling (41–46). Further compounding this effect, palmitate-induced ceramide synthesis in macrophages augments the release of inflammatory cytokines (47), and exposure to ceramide has been shown to induce expression of TNF in adipocytes (48). Given these findings, lipids in the ceramide family have emerged as likely mediators of metabolic inflammation and lipid-induced metabolic disease, and indeed inhibition of ceramide synthesis is protective in multiple models of insulin resistance (49–51) and atherosclerosis (52). In line with this, it has also been shown that the adipose tissue hormone adiponectin stimulates the activity of ceramide-degrading enzymes, which may underlie the metabolic benefit of the FGF21/adiponectin signaling axis (53–55). However, it is worth noting that there are some exceptions where such a link may be less critical for metabolic outcomes (56,57), and the accumulation of diacylglycerol, and subsequent activation of protein kinase C, has also been put forward as a mechanism underlying lipid-mediated insulin resistance (58). We will not delve into the details of this aspect of lipid biology, which was recently covered in excellent reviews (40,59,60). It is most likely that engaging the inflammatory networks and consequently impairing insulin action can be triggered by more than a single lipid entity or other signaling molecule. Further research is necessary to clarify the role of these molecules in the pathogenesis of metabolic disease and to better understand how the findings in animal models translate to humans.
At the other end of the spectrum, polyunsaturated fatty acids (PUFAs), particularly those derived from dietary sources, have long been studied for their potential metabolic benefits. In 1979, Dyerberg and Bang linked the high consumption of n-3 PUFAs to decreased platelet aggregation and a low rate of cardiovascular disease in indigenous Greenlanders and speculated that dietary supplementation with the n-3 PUFA eicosapentaenoic acid could reduce cardiovascular risk (61). These findings launched a wave of both clinical research and mechanistic work to unravel this potential, as has been well reviewed previously (62–66). In macrophages and neutrophils, n-3 fatty acids are converted into lipoxins by acetylated cyclooxygenase-2, and these “specialized pro-resolving mediators” have potent anti-inflammatory activities (64,67). Failure of this response has been implicated in the unresolved chronic inflammation that occurs in the setting of obesity and contributes to insulin resistance and atherosclerosis (68). In addition, it is now known that n-3 fatty acids exert their anti-inflammatory effects partly through the membrane receptor GPR120 (69), and polymorphisms in the human GPR120 locus are strongly associated with obesity and insulin resistance (70). Interestingly, this study also demonstrated that GPR120 deficiency was associated with reduced levels of palmitoleate, and palmitoleate infusion acutely repressed expression of lipogenic genes in the livers of GPR120-deficient mice (70), suggesting a complex interaction between dietary and endogenous lipids in maintaining homeostasis. Furthermore, many other G protein–coupled receptors have also been demonstrated to sense and respond to fatty acids, contributing to the regulation of insulin secretion, immune cell activation, and other aspects of immunometabolism (71).
Endocannabinoids are another class of bioactive lipids with multifaceted effects on immunometabolism. These endogenously produced ligands of the cannabinoid receptors (CB1 and CB2) are synthesized from cell membrane phospholipid precursors and have been implicated in controlling gut barrier function as well as communicating the state of the gut microbiota with adipose tissue (72). Endocannabinoids are well characterized for their role in promoting food intake (73), and in mice with diet-induced obesity, the levels of endocannabinoids in the hippocampus are elevated. Indeed, deletion of CB1 in GABAergic neurons confers partial protection from weight gain (74). Interestingly, recent research suggests that the endocannabinoid effect on food intake is nuanced, as activation of CB1 receptors in a specific class of striatal neurons can result in hypophagia (75). However, although endocannabinoid signaling through CB1 may play a net positive role in weight gain, signaling through CB2 receptors has anti-inflammatory effects including blocking Th1 cell differentiation and suppressing macrophage cytokine production (76), which could have important implications for immunometabolic disease. For example, a low-dose administration of the cannabinoid tetrahydrocannabinol to atherogenic apoE−/− mice resulted in reduced development of aortic plaques and lowered the plaque macrophage content (77). Similarly, treating high-fat diet–fed mice with a CB2 agonist reduces diet-induced inflammation in the liver and adipose tissue, and CB2−/− mice exhibit enhanced age-related adipose tissue inflammation (78). Taken together, these studies suggest that a carefully calibrated modulation of the endocannabinoid system, perhaps involving target tissue selection, would be needed to effectively treat or prevent obesity and related immunometabolic diseases.
Conclusions
For many years, peptides and proteins have been the focus of research and therapeutic approaches to diseases, including metabolic disorders. Approaches to modulate hormone levels or neutralize circulating proteins have usually been considered the key to providing promising solutions. However, the identification and characterization of lipid species with bioactive roles in metabolic homeostasis have paved the way for recognizing uncharted avenues for potential therapies. Here, there is much to be learned from natural examples in which bioactive lipids including the products of DNL convey potent metabolic benefit. For example, despite an enormous increase in plasma triglyceride and nonesterified fatty acids after a large meal, Burmese pythons demonstrate a healthy cardiac hypertrophy without accumulation of triglycerides or fatty acids in the heart itself. A pool of fatty acids, which includes palmitoleate, has been found to mediate this effect and, indeed, is sufficient to induce healthy cardiac growth in pythons and mice (79). There are also examples from human history that indicate that food sources of bioactive lipids have long been prized for their health benefits. Indeed, the potency of palmitoleate is the stuff of legend; Genghis Khan, whose horses and soldiers conquered the entire continent of Asia from Korea to Bulgaria, is said to have relied on “chatsargana” or Seabuckthorn to enhance their performance, which is a rich source of palmitoleate (80). This same plant was also said to be the favorite food consumed by Pegasus, the flying horse from Greek mythology (81).
While these legends will remain as such in the absence of direct scientific evidence, they provide enjoyable and inspiring stories to stimulate the imagination. Hopefully this will result in wider appreciation of the lesser-recognized components of metabolic homeostasis, such as bioactive lipids and other metabolites, which take on critical roles in metabolically active organs and systemic health. Among those, palmitoleate, PAHSA, and undoubtedly many more to-be-discovered molecules can be explored for their therapeutic potential against metabolic diseases as safe, efficacious, affordable, and practical entities.
Article Information
Funding. Work in the laboratory of G.S.H. is partly supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases [DK-052539]; National Heart, Lung, and Blood Institute [HL-125753]; and National Institute of Allergy and Infectious Diseases [AI-116901]) and the JDRF (2-SRA-2016-147-Q-R).
Duality of Interest. Work in the laboratory of G.S.H. is also supported through sponsored research agreements with Union Chemique Belge (UCB) and Servier. No other potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this study were presented in abstract form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
References
- 1.Schoenheimer R, Rittenberg D. Deuterium as an indicator in the study of intermediary metabolism. Science 1935;82:156–157 [DOI] [PubMed] [Google Scholar]
- 2.Shrago E, Glennon JA, Gordon ES. Comparative aspects of lipogenesis in mammalian tissues. Metabolism 1971;20:54–62 [DOI] [PubMed] [Google Scholar]
- 3.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 2008;118:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimano H, Shimomura I, Hammer RE, et al. . Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest 1997;100:2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knebel B, Haas J, Hartwig S, et al. . Liver-specific expression of transcriptionally active SREBP-1c is associated with fatty liver and increased visceral fat mass. PLoS One 2012;7:e31812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 2016;126:12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrago E, Spennetta T. The carbon pathway for lipogenesis in isolated adipocytes from rat, guinea pig, and human adipose tissue. Am J Clin Nutr 1976;29:540–545 [DOI] [PubMed] [Google Scholar]
- 8.Czech MP. Cellular basis of insulin insensitivity in large rat adipocytes. J Clin Invest 1976;57:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortega FJ, Mayas D, Moreno-Navarrete JM, et al. . The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2010;18:13–20 [DOI] [PubMed] [Google Scholar]
- 10.Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci U S A 2000;97:11371–11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan H, Rabaglia ME, Stoehr JP, et al. . Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 2003;52:688–700 [DOI] [PubMed] [Google Scholar]
- 12.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 2002;282:E46–E51 [DOI] [PubMed] [Google Scholar]
- 13.Kuriyama H, Liang G, Engelking LJ, Horton JD, Goldstein JL, Brown MS. Compensatory increase in fatty acid synthesis in adipose tissue of mice with conditional deficiency of SCAP in liver. Cell Metab 2005;1:41–51 [DOI] [PubMed] [Google Scholar]
- 14.Beaven SW, Matveyenko A, Wroblewski K, et al. . Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab 2013;18:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab 2005;289:E551–E561 [DOI] [PubMed] [Google Scholar]
- 16.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 1993;268:22243–22246 [PubMed] [Google Scholar]
- 17.Herman MA, Peroni OD, Villoria J, et al. . A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012;484:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuotio-Antar AM, Poungvarin N, Li M, et al. . FABP4-Cre mediated expression of constitutively active ChREBP protects against obesity, fatty liver, and insulin resistance. Endocrinology 2015;156:4020–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab 2010;298:E108–E116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008;134:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 2006;399:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzaka T, Shimano H, Yahagi N, et al. . Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med 2007;13:1193–1202 [DOI] [PubMed] [Google Scholar]
- 23.Yang ZH, Miyahara H, Hatanaka A. Chronic administration of palmitoleic acid reduces insulin resistance and hepatic lipid accumulation in KK-Ay mice with genetic type 2 diabetes. Lipids Health Dis 2011;10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Li H, Xu H, et al. . Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One 2012;7:e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan KL, Pillon NJ, Sivaloganathan DM, et al. . Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J Biol Chem 2015;290:16979–16988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot NA, Wheeler-Jones CP, Cleasby ME. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol Cell Endocrinol 2014;393:129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erbay E, Babaev VR, Mayers JR, et al. . Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 2009;15:1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Cao H, King IB, et al. . Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr 2010;92:1350–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefan N, Kantartzis K, Celebi N, et al. . Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care 2010;33:405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinnick KE, Neville MJ, Fielding BA, Frayn KN, Karpe F, Hodson L. Gluteofemoral adipose tissue plays a major role in production of the lipokine palmitoleate in humans. Diabetes 2012;61:1399–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH; ARIC Study Investigators . Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–98 [DOI] [PubMed] [Google Scholar]
- 32.Puri P, Wiest MM, Cheung O, et al. . The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009;50:1827–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vessby B, Tengblad S, Lithell H. Insulin sensitivity is related to the fatty acid composition of serum lipids and skeletal muscle phospholipids in 70-year-old men. Diabetologia 1994;37:1044–1050 [DOI] [PubMed] [Google Scholar]
- 34.Paillard F, Catheline D, Duff FL, et al. . Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis 2008;18:436–440 [DOI] [PubMed] [Google Scholar]
- 35.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–380 [DOI] [PubMed] [Google Scholar]
- 36.Yore MM, Syed I, Moraes-Vieira PM, et al. . Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014;159:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Brown JD, Stanya KJ, et al. . A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature 2013;502:550–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burhans MS, Flowers MT, Harrington KR, et al. . Hepatic oleate regulates adipose tissue lipogenesis and fatty acid oxidation. J Lipid Res 2015;56:304–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 2015;26:538–550 [DOI] [PubMed] [Google Scholar]
- 40.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 2011;121:4222–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 1996;271:13018–13022 [DOI] [PubMed] [Google Scholar]
- 42.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol 1998;18:5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajduch E, Balendran A, Batty IH, et al. . Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 2001;44:173–183 [DOI] [PubMed] [Google Scholar]
- 44.Kanety H, Hemi R, Papa MZ, Karasik A. Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. J Biol Chem 1996;271:9895–9897 [DOI] [PubMed] [Google Scholar]
- 45.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem 1998;273:16568–16575 [DOI] [PubMed] [Google Scholar]
- 46.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 2001;50:2563–2571 [DOI] [PubMed] [Google Scholar]
- 47.Schilling JD, Machkovech HM, He L, et al. . Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J Biol Chem 2013;288:2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 2006;55:2579–2587 [DOI] [PubMed] [Google Scholar]
- 49.Holland WL, Brozinick JT, Wang LP, et al. . Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 2007;5:167–179 [DOI] [PubMed] [Google Scholar]
- 50.Turpin SM, Nicholls HT, Willmes DM, et al. . Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 2014;20:678–686 [DOI] [PubMed] [Google Scholar]
- 51.Ussher JR, Koves TR, Cadete VJ, et al. . Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010;59:2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park TS, Rosebury W, Kindt EK, Kowala MC, Panek RL. Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol Res 2008;58:45–51 [DOI] [PubMed] [Google Scholar]
- 53.Holland WL, Miller RA, Wang ZV, et al. . Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holland WL, Adams AC, Brozinick JT, et al. . An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 2013;17:790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel SA, Hoehn KL, Lawrence RT, et al. . Overexpression of the adiponectin receptor AdipoR1 in rat skeletal muscle amplifies local insulin sensitivity. Endocrinology 2012;153:5231–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camell CD, Nguyen KY, Jurczak MJ, et al. . Macrophage-specific de novo synthesis of ceramide is dispensable for inflammasome-driven inflammation and insulin resistance in obesity. J Biol Chem 2015;290:29402–29413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumashiro N, Erion DM, Zhang D, et al. . Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2011;108:16381–16385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med 2010;16:400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zechner R, Zimmermann R, Eichmann TO, et al. . FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab 2012;15:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014;510:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dyerberg J, Bang HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet 1979;2:433–435 [DOI] [PubMed] [Google Scholar]
- 62.Oh DY, Olefsky JM. Omega 3 fatty acids and GPR120. Cell Metab 2012;15:564–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calder PC. Polyunsaturated fatty acids and inflammation. Biochem Soc Trans 2005;33:423–427 [DOI] [PubMed] [Google Scholar]
- 64.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serhan CN, Hong S, Gronert K, et al. . Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kristensen SD, De Caterina R, Schmidt EB, Endres S. Fish oil and ischaemic heart disease. Br Heart J 1993;70:212–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 2000;192:1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab 2014;19:21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh DY, Talukdar S, Bae EJ, et al. . GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ichimura A, Hirasawa A, Poulain-Godefroy O, et al. . Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012;483:350–354 [DOI] [PubMed] [Google Scholar]
- 71.Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci 2011;32:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cani PD, Plovier H, Van Hul M, et al. . Endocannabinoids - at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 2016;12:133–143 [DOI] [PubMed] [Google Scholar]
- 73.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 2013;17:475–490 [DOI] [PubMed] [Google Scholar]
- 74.Massa F, Mancini G, Schmidt H, et al. . Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci 2010;30:6273–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellocchio L, Lafenêtre P, Cannich A, et al. . Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 2010;13:281–283 [DOI] [PubMed] [Google Scholar]
- 76.Klein TW, Newton C, Larsen K, et al. . The cannabinoid system and immune modulation. J Leukoc Biol 2003;74:486–496 [DOI] [PubMed] [Google Scholar]
- 77.Steffens S, Veillard NR, Arnaud C, et al. . Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005;434:782–786 [DOI] [PubMed] [Google Scholar]
- 78.Schmitz K, Mangels N, Häussler A, Ferreirós N, Fleming I, Tegeder I. Pro-inflammatory obesity in aged cannabinoid-2 receptor-deficient mice. Int J Obes 2016;40:366–379 [DOI] [PubMed] [Google Scholar]
- 79.Riquelme CA, Magida JA, Harrison BC, et al. . Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science 2011;334:528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stobdan T, Korekar G, Srivastava RB. Nutritional attributes and health application of Seabuckthorn (Hippophae rhamnoides L.)–a review. Curr Nutr Food Sci 2013;9:151–165 [Google Scholar]
- 81.Small E, Catling PM, Li TSC. Blossoming treasures of biodiversity: sea buckthorn (Hippophae rhamnoides)--an ancient crop with modern virtues. Biodiversity 1996;3:25–27 [Google Scholar]