Abstract
Myopia is the largest cause of uncorrected visual impairments globally and its recent dramatic increase in the population has made it a major public health problem. In observational studies, educational attainment has been consistently reported to be correlated to myopia. Nonetheless, correlation does not imply causation. Observational studies do not tell us if education causes myopia or if instead there are confounding factors underlying the association. In this work, we use a two-step least squares instrumental-variable (IV) approach to estimate the causal effect of education on refractive error, specifically myopia. We used the results from the educational attainment GWAS from the Social Science Genetic Association Consortium to define a polygenic risk score (PGRS) in three cohorts of late middle age and elderly Caucasian individuals (N=5,649). In a meta-analysis of the three cohorts, using the PGRS as an IV, we estimated that each z-score increase in education (approximately 2 years of education) results in a reduction of 0.92 ± 0.29 diopters (P=1.04×10−3). Our estimate of the effect of education on myopia was higher (P=0.01) than the observed estimate (0.25 ± 0.03 diopters reduction per education z-score [~2 years] increase). This suggests that observational studies may actually underestimate the true effect. Our Mendelian Randomization (MR) analysis provides new evidence for a causal role of educational attainment on refractive error.
Keywords: Mendelian randomization, Myopia, Refractive error, Education, Polygenic Risk Scores, Instrumental variable
Introduction
The global prevalence of individuals with visual impairment in 2010 was estimated to be 285 million, of which 15% suffer blindness and 85% low vision [Pascolini and Mariotti 2012]. Uncorrected refractive errors accounts for 43% of the 285 million visually impaired [Pascolini and Mariotti 2012]. Myopia is the most common refractive error and occurs when the eye cannot clearly focus distant objects. More severe myopia has been associated with an increased risk of sight-threatening conditions including retinal detachment, subretinal neovascularization, macular haemorrhage, dense cataract, and glaucoma [Foster and Jiang 2014].
Myopia can often be corrected with optical aids such as spectacles, contact lenses, and, more recently, surgical intervention such as refractive surgery [Foster and Jiang 2014; Javitt and Chiang 1994; Jung, et al. 2012; Young 2009]. However, the high prevalence of myopia and cost of refractive care make this condition a significant public health concern worldwide [Fricke, et al. 2012]. The global economic cost in productivity loss from visual impairment due to uncorrected refractive errors is calculated to be USD$91.3 billion [Smith, et al. 2009]. Also, it has been estimated that the money needed to educate personnel, establish and maintain refractive care facilities is around USD$20 billion globally [Fricke, et al. 2012].
Despite extensive international efforts, the causes of myopia are not yet well understood [Sivak 2012; Wojciechowski 2011]. Environmental factors related to socioeconomic status, time spent outdoors, near work activities and education have been consistently reported as being associated with myopia [Dirani, et al. 2008; Foster and Jiang 2014; Pan, et al. 2012; Sivak 2012]. Also, a growing body of evidence on the biological mechanisms underlying myopia suggests it results from complex interactions between the genetic makeup of an individual and the environmental exposures [Goldschmidt and Jacobsen 2014; Mackey and Hewitt 2014; Verhoeven, et al. 2013; Young 2009].
Educational attainment is the most consistent environmental risk factor for myopia [Dirani, et al. 2008; Verhoeven, et al. 2013]. Onset of myopia usually occurs during childhood, particularly during school years [Morgan and Rose 2005]. People with university-level education are 4× more likely to develop myopia than people with just primary education [Morgan and Rose 2005]. From the perspective of myopia epidemiology, level of education has been widely considered as a proxy measure for near work activity during the first three decades of life [Sivak 2012]. Near work activities, such as spending long hours in front of a computer, reading and writing, are considered important environmental risk factors for the development of myopia [Czepita and Zejmo 2011]. Performing near work activities requires the eye to generate extra optical power to focus the image on the retina, causing retinal defocus and degradation, which could then promote eye elongation as a compensatory mechanism [Czepita and Zejmo 2011; Drexler, et al. 1998]. An alternate hypothesis suggests that individuals with higher education spend less time outdoors and this is the reason for an elevated risk of myopia [Foster and Jiang 2014; French, et al. 2013; Ngo, et al. 2014]. Additional studies have found conflicting evidence regarding the near work hypothesis depending on the unit used to measure near work [Ip, et al. 2008; Jones-Jordan, et al. 2011; Yi and Li 2011].
Recent studies have reported a gene-environment interaction between myopia genes and education [Fan, et al. 2014; Verhoeven, et al. 2013; Wojciechowski, et al. 2013]. It also has been proposed that a part of the association between education and myopia is due to pleiotropic effects (genes affecting both education and myopia, possibly as a result of education affecting subsequent myopia) [Cohn, et al. 1988]. A bivariate twin study has shown some evidence for a proportion of genetic factors influencing educational attainment and refractive error [Dirani, et al. 2008]. A large genome-wide association study (GWAS) meta-analysis estimated that genetic factors contribute to 40% of the variance in educational attainment [Rietveld, et al. 2013]. The heritability of refractive error has been estimated to be as high as 90% [Sanfilippo, et al. 2010].
In this report we investigate the effects of the genetic predisposition of education on refractive error (where a more negative refractive error indicates more myopia) in three independent cohorts of European descent. We hypothesize that the genetic correlation between refractive error and level of education is due to a causal association. We apply a Mendelian randomization (MR) approach using polygenic risk scores (PGRS) of educational attainment as an instrumental variable to establish the causal effect of education on refractive error. MR is considered to be equivalent to a randomized trial in which randomization is achieved with respect to predisposing genotypes. As genotypes are passed-on randomly from parental to offspring generations, they are immune to the confounding factors frequently present in observational studies [Davey Smith and Hemani 2014].
Methods
Data
We analysed data from three different cohorts. Samples descriptors are summarized in Table I.
Table I.
Characteristics of the cohorts. AREDS and BMES educational attainment is coded as the higher level awarded. KORA education level is showed as education years completed. Mean and standard deviation is shown for Age and spherical equivalent (SPHEQ).
| AREDS | BMES | KORA | |
|---|---|---|---|
| N | 1459 | 2344 | 1846 |
| Age (s.d.) | 68.15 (4.80) | 66.73 (8.96) | 55.58 (11.77) |
| Male / Females | 588 / 871 | 1327 / 1017 | 934 / 312 |
| SPHEQ (s.d) | 0.51 (2.13) | 0.57 (2.02) | −0.28 (2.25) |
| Height (s.d) | − | M=1.72 (0.06) F=1.59 (0.07) | − |
| BMI (s.d) | − | M=27 (4), F=28 (5) | − |
| Smoking* | − | Yes=217; No=2040 | − |
| Years of education: | |||
|
Educational* attainment |
1. Grade 11: 83; | 1. Certificate-other: 134; | 8: 168; |
| 2. High school: 344; | 2. Certificate-trade: 219; | 10: 789; | |
| 3. College: 472; | 3. Diploma: 596; | 11: 246; | |
| 4. Bachelors :248; | 4. Bachelors: 191; | 12: 146; | |
| 5. Postgraduate: 312; | 5. Graduate diploma: 31; | 13: 223; | |
| 6. Higher degree: 38; | 15: 12; | ||
| 17: 262; |
Numbers may not add-up due to missing data.
KORA
KORA ("Kooperative Gesundheitsforschung in der Region Augsburg" which translates as “Cooperative Health Research in the Region of Augsburg”) was accessed through dbGaP (dbGaP Study Accession: phs000303.v1.p1). The phenotyping and genotyping information are described in more detail elsewhere [Holle, et al. 2005; Oexle, et al. 2011; Steffens, et al. 2006; Wichmann, et al. 2005]. In brief, between 1984 and 2001, adults from 430,000 inhabitants living in Augsburg and 16 surrounding counties in Germany were randomly selected and separated in 4 different groups (S1–S4). One of the groups (S3/F3) was utilized for this study as was the only group with refractive error measured as spherical equivalent (SPHEQ). This study includes 1,981 subjects without medical conditions predisposing myopia, with education and genotype data along with refractive error measurements. For each subject, eyeglass prescriptions were measured in addition to an evaluation by the Nikon Retinomax. Educational attainment was recorded as number of years of education (range 8 to 17, table I). Genotyping was done using the Illumina 2.5M chip or the Illumina Omni Express chip. Samples and SNPs were excluded if they had a low a genotype rate (<0.98). In addition, SNPs were removed if they had low minor allele frequency (<0.01) or Hardy-Weinberg P-value < 10−6. The study was approved by the local ethics committee. Written informed consent was obtained from all participants before enrolment in accordance with the Declaration of Helsinki.
AREDS
The Age-Related Eye Disease Study (AREDS) was accessed through dbGaP (dbGaP Study Accession: phs000429.v1.p1). Detailed description of genotyping and phenotyping can be found elsewhere [Age-Related Eye Disease Study Research 2001a; Age-Related Eye Disease Study Research 2001b]. In brief, AREDS participants were 55 to 80 years of age at enrolment and had to be free of any illness or condition that would make long-term follow-up or compliance with study medications unlikely or difficult. Based on ophthalmologic evaluations, 4,757 participants were enrolled in one of several categories, including a control group (AREDS 1c). Individuals included in this GWAS study are all Caucasians, who do not have age-related macular degeneration (AMD) and were further screened to also exclude individuals with cataracts, retinitis pigmentosa or other retinal degenerations, colour blindness, other congenital eye problems, LASIK, artificial lenses, and other eye surgery. For this work, we included 1842 participants from the control group (AREDS 1c), with refractive error measurements, education survey and genotype data. Refractive error was measured as SPHEQ, plus baseline measures of axis, sphere and cylinder are available for each eye. Educational attainment was recorded on a five point scale (table I). Genotyping was performed using the Illumina 2.5M chip. We applied the same quality control for samples and SNPs as for the KORA cohort. Written informed consent was obtained from all participants before enrollment in accordance with the Declaration of Helsinki.
BMES
The Blue Mountains Eye Study (BMES) is a population-based eye disease survey in individuals living in the Blue Mountains region, west of Sydney, Australia. Genotyping and phenotyping information is found elsewhere [Foran, et al. 2003; Schache, et al. 2013]. In brief, 3,654 permanent residents aged 49 years or older participated (participation rate of 82.4%). During 1997–99 (BMES II A), 2,335 participants (75.1% of survivors) returned for examinations after 5 years. During 1999–2000, 1,174 (85.2%) new participants took part in an Extension Study of the BMES (BMES IIB). BMES cross-section II thus includes BMES IIA (66.5%) and BMES IIB (33.5%) participants (n=3,509). Participants underwent an eye examination including best-corrected visual acuity, objective and subjective refraction, slit-lamp examination. A Humphrey autorefractor was used to obtain an objective refraction. SPHEQ was calculated using the standard formula: SPHEQ = sphere + (cylinder/2). Educational attainment was recorded on a six point scale (table I). From the BMES cross section II who had blood samples collected, DNA was extracted for 3,189 (90.1 %) participants. Genotyping was performed on the Illumina Infinium platform using the Human660W-Quad, a WTCCC2 designed custom chip containing Human 550 probes with 60,000 additional probes to capture common CNVs from the Structural Variation Consortium[Conrad, et al. 2010]. We applied the same QC for the SNPs as for the other two cohorts. Samples with call rate less than 95% were excluded from analysis. After initial QC, 2412 individuals had genotype and SPHEQ data; however, from these, just 1209 had education recorded. All BMES examinations were approved by the Human Ethics Committees of the Western Sydney Area Health Service and University of Sydney.
Genotype data from the remaining samples in the 3 cohorts were merged to perform relatedness filtering so that no pair of individuals had a probability of sharing an identity by descent allele (IBD) of more than 20% (~ first cousins). Further, principal component analysis was performed together with genotype data from the 1000 Genomes project. We removed all individuals that lay beyond >6 standard deviations from the 1000 Genomes northern European ancestry PC1 and PC2 centroid. The plot for the first two principal components after individuals removed is displayed in Supplementary Figure 1. Table 1 summarizes the sample sizes of each cohort after QC. Finally, we performed identity by state (IBS) clustering using PLINK –cluster which produced a single cluster, suggesting a homogeneous sample. We forced PLINK to generate 6 clusters but these were correlated to PC1 and adding the clusters as covariates did not alter our conclusions.
Statistical analysis
Given that educational attainment was coded differently in the three cohorts, it was transformed to z scores. Spearman correlations between educational attainment and refractive error were performed adjusting by sex and age.
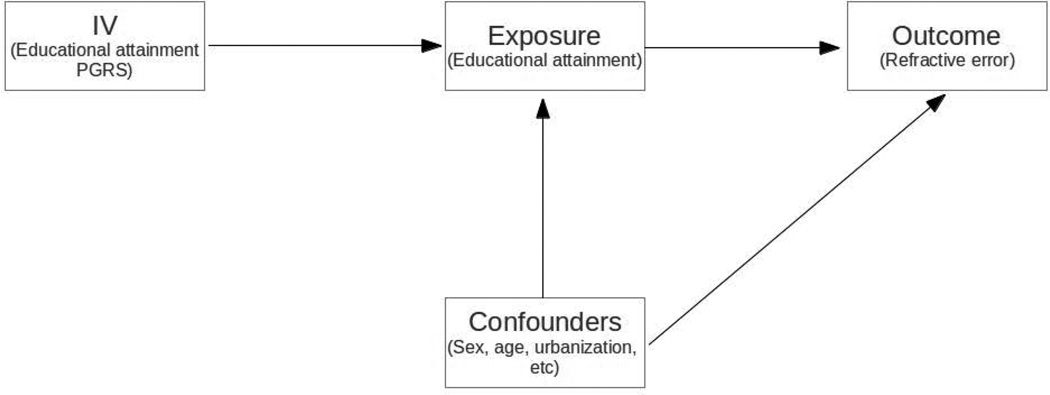
MR is a method that permits the testing of a causal effect from observational data in the presence of confounding factors by using genetic information with a known effect on the exposure as an instrumental variable (IV) [Lawlor, et al. 2008]. There are three fundamental assumptions to ensure the validity of the IV estimate in MR studies [Greenland 2000; Lawlor, et al. 2008]: 1) the IV must be strongly associated with the exposure variable (generally an F statistic > 10 is sufficient to ensure the validity of the IV); 2) the IV is not associated with potential confounders; 3) the IV is only associated with refractive error (outcome variable) via educational attainment (exposure variable) Figure 1.
Figure 1.
Mendelian Randomization assumptions. 1) Educational attainment polygenic risk score (instrumental variable) is robustly associated with educational attainment (exposure variable); 2) IV is only associated with refractive error (outcome variable) via educational attainment (exposure variable); 3) IV is not associated to the confounders.
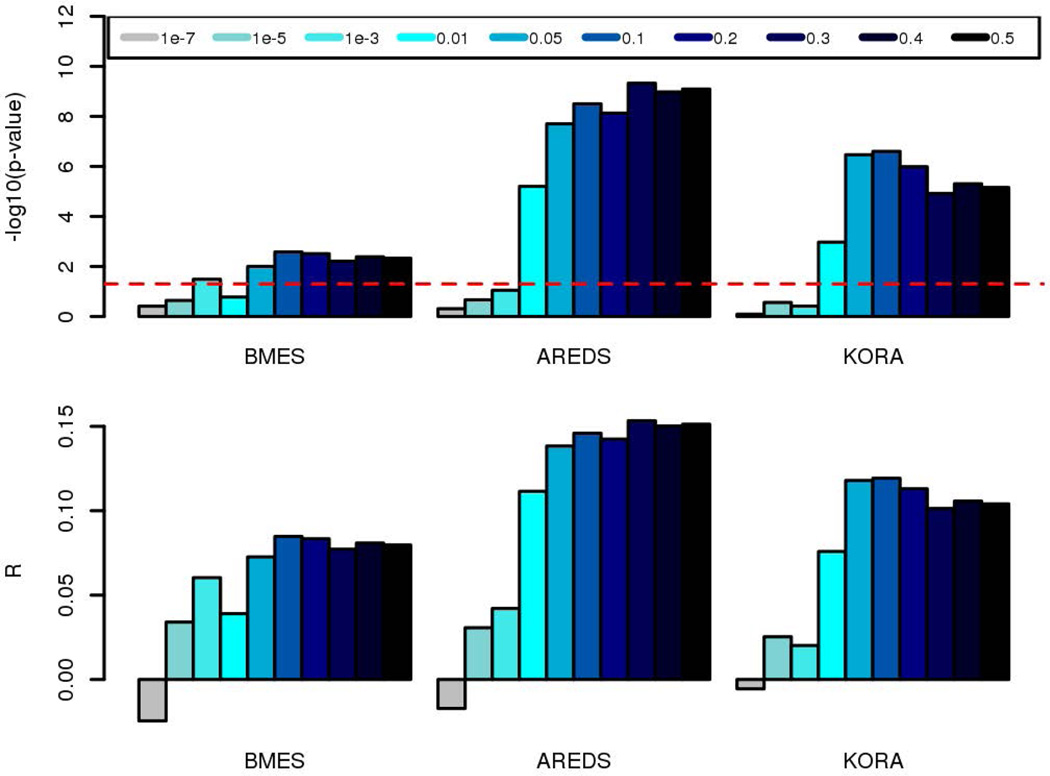
Regression coefficients summarizing the results from Genome-wide association studies (GWAS) are an important source of data for MR studies. Multiple variants from these GWAS can be combined to create a powerful IV [Burgess, et al. 2013]. Here, we computed polygenic risk scores (PGRS) of education per individual based on the educational attainment GWAS summary results from the Social Science Genetic Association Consortium (SSGAC) [Rietveld, et al. 2013]. These GWAS summary results were recomputed from the original SSGAC results [Rietveld, et al. 2013] to exclude the KORA sample which was also involved in that study. The PGRS [International Schizophrenia, et al. 2009; Wray, et al. 2014] were estimated by summing each allele’s estimated effect size multiplied by the number of risk alleles carried by each participant. We used SNPs across 12 different P-value thresholds (i.e. <1e-7, <1e-5, <1e-3, <1e-2, <5e-2, <1e-1, <2e-1, <3e-1, <4e-1, <5e-1), using the –score option in PLINK 1.9 [Purcell, et al. 2007]. Also, the PGRS were computed using the remaining SNPs after clumping for high linkage disequilibrium (clumping threshold: LD r2=0.2 at a distance of <1Mb from the index SNP). In order to choose the PGRS with the best fit to the recorded educational attainment, we performed Spearman correlation after adjusting education by sex, age and the first 3 principal components (derived from the genome-wide genotypes) through linear regression (Figure 2).
Figure 2.
Polygenic risk scores (PGRS) of education predict educational attainment. Each bar represents the p-value threshold used to compute the PGRS of education. The upper panel shows the significance level of the association between PGRS of education and educational attainment; red dotted line represents −log10 (0.05). Lower panel indicates the Spearman correlation estimate.
We carried out the MR using a two-stage least squares (TSLS) approach with the ivreg function of the AER R package. In the first-stage, we predict education from the PGRS. In the second stage, we use the predicted values of education in a linear model with SPHEQ (refractive error). The ivreg function adjusts the second stage with the estimated residuals from the first stage to correctly account for the uncertainty of the predicted values of educational attainment. Age and sex were used as covariates. We used the Wu-Hausman test to test whether the TSLS estimates differed from the estimates obtained from a conventional linear regression between education and SPHEQ. A rejection of the null hypothesis (estimates do not differ) may indicate some inconsistency between conventional linear regression (i.e. the conventional observational study) and the TSLS which could be due to confounding or measurement errors. All the analyses were performed adjusting by sex, age and 3 principal components. Meta-analyses were performed using a weighted fixed-effect meta-analysis using the RMETA R package.
A study investigating genetic correlations showed a significant negative genetic correlation between attending college, obesity and smoking behavior, and a suggestive positive correlation with height [Brendan Bulik-Sullivan 2015]. Also, epidemiological studies have shown association between refractive error and anthropometric traits and smoking [Choi, et al. 2014; Roy, et al. 2015]. In order to investigate potential pleiotropic effects, we performed a series of regressions between the educational attainment PGRS and BMI, height and smoking in the BMES cohort.
Results
Descriptions of the cohorts are displayed in Table I. Phenotypic correlation between educational attainment and refractive error (measured as the mean spherical equivalent, SPHEQ) for the AREDS, BMES and KORA cohorts after correcting by sex and age are summarized in Table II. Consistent with epidemiological studies, a strong negative correlation was observed in the three cohorts (ρ=−0.15 in AREDS; ρ=−0.06 in BMES; ρ=−0.10 in KORA) demonstrated by increased education resulting in more myopia.
Table II.
Phenotypic association (i.e. observational study estimates) of education with spherical equivalent after adjusting by sex and age. B+K+A represents the estimate of a weighted fixed-effect meta-analysis between the three cohorts.
| N | Education level ρ (s.e) | P-value | Education level β (s.e) | |
|---|---|---|---|---|
| AREDS | 1459 | −0.15 (0.013) | 1.9×10−9 | −0.29 (0.06) |
| BMES | 1209 | −0.06 (0.028) | 1.9×10−2 | −0.10 (0.06) |
| KORA | 1846 | −0.10 (0.023) | 2.1×10−6 | −0.32 (0.05) |
| B+K+A | −0.11 (0.012) | <2.2×10−16 | −0.25 (0.03) |
Abreviations: K+B+A: KORA + BMES + AREDS meta-analysis.
We used data from the educational attainment GWAS from SSGAC to compute multiple PGRS of educational attainment based on different p-value thresholds of the genetic association between candidate SNPs and education. Correlation estimates between the PGRS and educational attainment are displayed in Figure 2. The PGRS computed from the top 10% SNPs (17,749 SNPs) of the educational attainment GWAS showed the most consistent and best fit to education in the three cohorts (F=35.5 in AREDS, F=9.1 in BMES and F=26.8 in KORA) and hence was used as IV for the MR analysis (formally, the 10% of SNPs PGRS was a strong instrument, clearly satisfying the first MR assumption). Further, we inspected the association between the PGRS and SPHEQ. The PGRS was significantly associated to SPHEQ in the AREDS (R=−0.09; P=1.4×10−3) and BMES (R=−0.05; P=2.5×10−2) cohorts, but not in KORA, where we observed a smaller effect size (R=−0.03; P=0.16) (Table 3).
Table III.
Association of PGRS of education with spherical equivalent after adjusting by sex and age. B+K+A represents the estimate of a weighted fixed-effect meta-analysis between the three cohorts.
| N | PGRS R (s.e). | P-value | |
|---|---|---|---|
| AREDS | 1459 | −0.09 (0.015) | 4.1×10−4 |
| BMES(all) | 2344* | −0.05 (0.020) | 2.5×10−2 |
| KORA | 1846 | −0.03 (0.022) | 1.6×10−1 |
| B+K+A | −0.05 (0.013) | 1.4×10−4 |
Data available with genotype and spherical equivalent.
Abreviations: K+B+A: KORA + BMES + AREDS meta-analysis.
We proceeded to perform a two-stage least squares IV analysis for the MR estimate. We found that each standard deviation from the mean of educational attainment (equals a 1 unit increase since we are working on a standardized scale, and corresponds to approximately 2 years of education) decreases SPHEQ by 0.64 – 1.33 diopters (Table IV). The IV estimates were statistically significant for the AREDS, but not for KORA and BMES cohorts. This is probably due to the smaller effect sizes seen for these cohorts and the fact that in BMES just 1209 out of 2344 participants had education measures. Further, in order to derive the most precise estimate, we meta-analysed the estimates of the three cohorts to yield the more precise estimate of 0.92 ±0.29 diopters reduction for approximately 2 years of education.
Table IV.
Effect estimates from the two-stage least squares analyses using PGRS as an instrumental variable for education and spherical equivalent as outcome. P-value is for the test of whether beta is significantly different from zero. P-valuediff corresponds to the significance of the endogeneity test (Wu-Hausman test), a rejection of the null hypothesis means that the beta effect estimates here are different from the observed (phenotypic) estimates in table II. B+K+A represents the estimate of a weighted fixed-effect meta-analysis between the three cohorts.
| N | β (s.e) | P-value | P-valuediff | |
|---|---|---|---|---|
| AREDS | 1459 | −1.33 (0.42) | 1.41×10−3 | 7.3×10−3 |
| BMES | 1209* | −0.87 (0.71) | 2.21×10−1 | 2.1×10−1 |
| KORA | 1846 | −0.64 (0.45) | 1.58×10−1 | 2.2×10−1 |
| B+K+A | −0.92 (0.29) | 1.04×10−3 | 1.0×10−2 |
Data available with genotype, spherical equivalent and observed education.
Abreviations: K+B+A: KORA + BMES + AREDS meta-analysis.
We observed that the causal effect estimate for AREDS was significantly higher than that estimated through standard observational methods (Pdiff=7.3×10−3). Provided the assumptions of the MR are satisfied, the MR estimate should reflect the true (i.e., unconfounded) effect of education on myopia. The fact that the observation study estimates are lower may be attributed to confounding (e.g. education in observational studies may be correlated with many other traits which modify myopia risk). Alternatively education SNPs (or SNPs in LD) may be associated with other traits underlying the association with refractive error. To test the latter, we investigated potential confounding effects using the BMES cohort where we had available data on smoking, height and BMI. We found no significant association between the PGRS or SPHEQ with height or BMI (P>0.05) (Supplementary Table 1). Smoking was nominally associated to the PGRS (P=0.039) but not to SPHEQ (P=0.129), thus it is unlikely that smoking mediates the association between the PGRS and SPHEQ. Further, the fact that educational attainment is difficult to assess accurately across studies may also have impacted results.
Discussion
In this Mendelian randomization study, we have estimated the causal effect of education on refractive error (measured as spherical equivalent) by using the genetic predisposition to education as an instrumental variable. As reported in epidemiological (observational) studies, we found a strong negative correlation between educational attainment and refractive error in three different cohorts. We also found that a genetic predictor of higher education was associated with refractive error. We assumed that any effect of education-associated genetic variants on other traits (e.g. obesity) was only via their effect on education (IV assumption 3). We also assumed that the genetic risk score for education was not associated with other traits that may confound the association between education and myopia (MR assumption 2). Our MR analysis showed a significant estimate of the causal effect of education on SPHEQ. Nevertheless, the observed MR estimate was significantly higher than the ones in the phenotypic (observational) association, suggesting some bias in either the instrumental variable or observational analysis (or both). We used principal components derived from genome-wide genotypes to control for potential population bias in all the analyses. Also, IBS clustering did not show evidence of population stratification. We believe that the observed bias may be result of an inaccurate or noisy measure of educational attainment or confounding in the observational studies. It is also possible that education SNPs (or SNPs in LD) may be associated with other traits (pleiotropic effects) underlying the association with refractive error. A recent paper from Bulik Sullivan et al [Brendan Bulik-Sullivan 2015], showed a polygenic risk score for attending college (yes/no) was correlated with the genetic risk score for a range of other traits: Alzheimer’s disease, bipolar disorder, obesity, smoking and serum triglyceride levels. In the case of, say Alzheimer’s, it is unlikely that Alzheimer’s acts as a relevant mediator in the relationship between education-associated genes and refractive errors. For smoking, there was a nominal association between the PGRS and smoking although since we found no association between smoking and refractive error, it is unlikely that our results here are confounded via effects on smoking. Two other variables that are possible mediators [Roy, et al. 2015], obesity and height, were not associated to the education PGRS Also, we note that genetic correlations between education and the other traits described in the Bulik Sullivan paper are weak [Brendan Bulik-Sullivan 2015], hence are unlikely to cause a meaningful violation of assumption 2 (although this is difficult to test). Bulik Sullivan use college yes/no, which is similar to the years of education variable we use here.
A possible source of bias in our estimates is the potential existence of an actual (unknown and/or unmeasured) pleiotropic effect of education-associated markers which cause myopia via pathways other than education. For this to violate MR assumption 3, any pleiotropic effects must not simply exist due to genetic effects influencing refractive error via their effect on education. One scenario would be a gene (or genes) with effects on both brain size and axial length, with bigger brain size being associated with a greater intelligence [Goh, et al. 2011; Kaup, et al. 2011] and higher education, leading to myopia. However, this scenario is unlikely given the propagation rate of genetic variants and the recent dramatic increase in myopia prevalence around the globe. Another scenario could be that education-associated genes could be inversely associated with e.g., athletic prowess, which, in turn, would be associated with increased outdoor exposure and a reduced risk of myopia. Future research should account for outdoor exposure.
A strength of our study is that it includes cohorts from Europe, Australia and the United States. However, due to modest individual sample size our results are strongest when combining the estimates. Our samples were all of European ancestry, as were the data used by the Social Science Genetic Association Consortium to derive the estimated SNP effects for educational attainment. However, as the current myopia epidemic is most marked in East Asian populations, it would be interesting in the future to perform this study in samples of Asian ancestry. Since our results indicate that observational studies may underestimate the true effect of education on myopia, for future studies of myopia where a correction for education is desired, it may be feasible to correct for a genetically derived education variable (particularly in scenarios where the education variable is missing or poorly measured). A practical limitation of this would that currently the education PGRS only explains 2% of the variance in the trait.
In conclusion we have shown that the genetic predisposition of higher education is negatively associated with refractive error. The results of our MR analysis are amongst the strongest to date in support of the notion that educational attainment is causally related to refractive error. Moreover, in the European ancestry samples studied here, the true causal effect of education on refractive error may be larger than predicted from the observational studies conducted to date.
Supplementary Material
Acknowledgments
GCP thanks the University of Queensland and QIMR Berghofer Medical Research Institute for scholarship support. PFK thanks QIMR Berghofer Medical Research Institute for scholarship support. SM is supported by an Australian Research Council Future Fellowship. RW is supported by grant K08EY022943/EY/NEI NIH HHS/United States. DS is supported by grant R01 EY020483/EY/NEI NIH HHS/United States. JEBW is supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
We thank Niels Rietveld (Social Science Genetic Association Consortium) for modifying and making available the summary GWAS results with the KORA samples excluded.
Footnotes
The dataset(s) used for the analyses described in this manuscript were obtained from the Age-Related Eye Disease Study (AREDS) Database found at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000001.v3.p1. Funding support for AREDS was provided by the National Eye Institute (N01-EY-0-2127). We would like to thank the AREDS participants and the AREDS Research Group for their valuable contribution to this research.
The dataset(s) used for the analyses described in this manuscript were obtained from the NEI Refractive Error Collaboration (NEIREC) Database found at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000303.v1.p1. Funding support for NEIREC was provided by the National Eye Institute. We would like to thank NEIREC participants and the NEIREC Research Group for their valuable contribution to this research.
CONFLICTS OF INTERESTS
The authors declare no conflicts of interest exist.
References
- Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001a;119(10):1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001b;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendan Bulik-Sullivan HKF, Anttila Verneri, Gusev Alexander, Day Felix R, ReproGen Consortium, Psychiatric Genomics Consortium. Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Consortium 3. Perry John RB, Patterson Nick, Robinson Elise, Daly Mark J, Price Alkes L, Neale Benjamin M. An Atlas of Genetic Correlations across Human Diseases and Traits. biorxiv. 2015 doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JA, Han K, Park YM, La TY. Low serum 25-hydroxyvitamin D is associated with myopia in Korean adolescents. Invest Ophthalmol Vis Sci. 2014;55(4):2041–2047. doi: 10.1167/IOVS.13-12853. [DOI] [PubMed] [Google Scholar]
- Cohn SJ, Cohn CM, Jensen AR. Myopia and intelligence: a pleiotropic relationship? Hum Genet. 1988;80(1):53–58. doi: 10.1007/BF00451456. [DOI] [PubMed] [Google Scholar]
- Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, Aerts J, Andrews TD, Barnes C, Campbell P, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464(7289):704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepita DA, Zejmo M. Environmental factors and myopia. Ann Acad Med Stetin. 2011;57(3):88–92. discussion 92. [PubMed] [Google Scholar]
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani M, Shekar SN, Baird PN. The role of educational attainment in refraction: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2008;49(2):534–538. doi: 10.1167/iovs.07-1123. [DOI] [PubMed] [Google Scholar]
- Drexler W, Findl O, Schmetterer L, Hitzenberger CK, Fercher AF. Eye elongation during accommodation in humans: differences between emmetropes and myopes. Invest Ophthalmol Vis Sci. 1998;39(11):2140–2147. [PubMed] [Google Scholar]
- Fan Q, Wojciechowski R, Kamran Ikram M, Cheng CY, Chen P, Zhou X, Pan CW, Khor CC, Tai ES, Aung T, et al. Education influences the association between genetic variants and refractive error: a meta-analysis of five Singapore studies. Hum Mol Genet. 2014;23(2):546–554. doi: 10.1093/hmg/ddt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran S, Wang JJ, Mitchell P. Causes of visual impairment in two older population cross-sections: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10(4):215–225. doi: 10.1076/opep.10.4.215.15906. [DOI] [PubMed] [Google Scholar]
- Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond) 2014;28(2):202–208. doi: 10.1038/eye.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Fricke TR, Holden BA, Wilson DA, Schlenther G, Naidoo KS, Resnikoff S, Frick KD. Global cost of correcting vision impairment from uncorrected refractive error. Bull World Health Organ. 2012;90(10):728–738. doi: 10.2471/BLT.12.104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Bansal R, Xu D, Hao X, Liu J, Peterson BS. Neuroanatomical correlates of intellectual ability across the life span. Dev Cogn Neurosci. 2011;1(3):305–312. doi: 10.1016/j.dcn.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E, Jacobsen N. Genetic and environmental effects on myopia development and progression. Eye (Lond) 2014;28(2):126–133. doi: 10.1038/eye.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. An introduction To instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(6):1102. doi: 10.1093/oxfordjournals.ije.a019909. [DOI] [PubMed] [Google Scholar]
- Holle R, Happich M, Lowel H, Wichmann HE, Group MKS. KORA--a research platform for population based health research. Gesundheitswesen. 2005;67(Suppl 1):S19–S25. doi: 10.1055/s-2005-858235. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia C, Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JM, Saw SM, Rose KA, Morgan IG, Kifley A, Wang JJ, Mitchell P. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49(7):2903–2910. doi: 10.1167/iovs.07-0804. [DOI] [PubMed] [Google Scholar]
- Javitt JC, Chiang YP. The socioeconomic aspects of laser refractive surgery. Arch Ophthalmol. 1994;112(12):1526–1530. doi: 10.1001/archopht.1994.01090240032022. [DOI] [PubMed] [Google Scholar]
- Jones-Jordan LA, Mitchell GL, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, Twelker JD, Sims JR, Zadnik K, Group CS. Visual activity before and after the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011;52(3):1841–1850. doi: 10.1167/iovs.09-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul, South Korea. Invest Ophthalmol Vis Sci. 2012;53(9):5579–5583. doi: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci. 2011;23(1):6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- Mackey DA, Hewitt AW. Genome-wide association study success in ophthalmology. Curr Opin Ophthalmol. 2014;25(5):386–393. doi: 10.1097/ICU.0000000000000090. [DOI] [PubMed] [Google Scholar]
- Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24(1):1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ngo CS, Pan CW, Finkelstein EA, Lee CF, Wong IB, Ong J, Ang M, Wong TY, Saw SM. A cluster randomised controlled trial evaluating an incentive-based outdoor physical activity programme to increase outdoor time and prevent myopia in children. Ophthalmic Physiol Opt. 2014;34(3):362–368. doi: 10.1111/opo.12112. [DOI] [PubMed] [Google Scholar]
- Oexle K, Ried JS, Hicks AA, Tanaka T, Hayward C, Bruegel M, Gogele M, Lichtner P, Muller-Myhsok B, Doring A, et al. Novel association to the proprotein convertase PCSK7 gene locus revealed by analysing soluble transferrin receptor (sTfR) levels. Hum Mol Genet. 2011;20(5):1042–1047. doi: 10.1093/hmg/ddq538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld CA, Medland SPHEQ, Derringer J, Yang J, Esko T, Martin NW, Westra HJ, Shakhbazov K, Abdellaoui A, Agrawal A, et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340(6139):1467–1471. doi: 10.1126/science.1235488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kar M, Mandal D, Ray RS, Kar C. Variation of Axial Ocular Dimensions with Age, Sex, Height, BMI-and Their Relation to Refractive Status. J Clin Diagn Res. 2015;9(1):AC01–AC04. doi: 10.7860/JCDR/2015/10555.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo PG, Hewitt AW, Hammond CJ, Mackey DA. The heritability of ocular traits. Surv Ophthalmol. 2010;55(6):561–583. doi: 10.1016/j.survophthal.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Schache M, Richardson AJ, Mitchell P, Wang JJ, Rochtchina E, Viswanathan AC, Wong TY, Saw SM, Topouzis F, Xie J, et al. Genetic association of refractive error and axial length with 15q14 but not 15q25 in the Blue Mountains Eye Study cohort. Ophthalmology. 2013;120(2):292–297. doi: 10.1016/j.ophtha.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Sivak J. The cause(s) of myopia and the efforts that have been made to prevent it. Clin Exp Optom. 2012;95(6):572–582. doi: 10.1111/j.1444-0938.2012.00781.x. [DOI] [PubMed] [Google Scholar]
- Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ. 2009;87(6):431–437. doi: 10.2471/BLT.08.055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M, Lamina C, Illig T, Bettecken T, Vogler R, Entz P, Suk EK, Toliat MR, Klopp N, Caliebe A, et al. SNP-based analysis of genetic substructure in the German population. Hum Hered. 2006;62(1):20–29. doi: 10.1159/000095850. [DOI] [PubMed] [Google Scholar]
- Verhoeven VJ, Buitendijk GH, Consortium for Refractive E. Myopia, Rivadeneira F, Uitterlinden AG, Vingerling JR, Hofman A, Klaver CC. Education influences the role of genetics in myopia. Eur J Epidemiol. 2013;28(12):973–980. doi: 10.1007/s10654-013-9856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann HE, Gieger C, Illig T, Group MKS. KORA-gen--resource for population genetics, controls and a broad spectrum of disease phenotypes. Gesundheitswesen. 2005;67(Suppl 1):S26–S30. doi: 10.1055/s-2005-858226. [DOI] [PubMed] [Google Scholar]
- Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79(4):301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski R, Yee SS, Simpson CL, Bailey-Wilson JE, Stambolian D. Matrix metalloproteinases and educational attainment in refractive error: evidence of gene-environment interactions in the Age-Related Eye Disease Study. Ophthalmology. 2013;120(2):298–305. doi: 10.1016/j.ophtha.2012.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, Middeldorp CM. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55(10):1068–1087. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- Yi JH, Li RR. Influence of near-work and outdoor activities on myopia progression in school children. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13(1):32–35. [PubMed] [Google Scholar]
- Young TL. Molecular genetics of human myopia: an update. Optom Vis Sci. 2009;86(1):E8–E22. doi: 10.1097/OPX.0b013e3181940655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.