Abstract
Objective
Although vaccine coverage in infants in sub-Saharan Africa is high, this is estimated at the age of 6–12 months. There is little information on the timely administration of birth dose vaccines. The objective of this study was to assess the timing of birth dose vaccines (hepatitis B, BCG and oral polio) and reasons for delayed administration in The Gambia.
Methods
We used vaccination data from the Farafenni Health and Demographic Surveillance System (FHDSS) between 2004 and 2014. Coverage was calculated at birth (0–1 day), day 7, day 28, 6 months and 1 year of age. Logistic regression models were used to identify demographic and socio-economic variables associated with vaccination by day 7 in children born between 2011 and 2014.
Results
Most of the 10,851 children had received the first dose of hepatitis B virus (HBV) vaccine by the age of 6 months (93.1%). Nevertheless, only 1.1% of them were vaccinated at birth, 5.4% by day 7, and 58.4% by day 28. Vaccination by day 7 was associated with living in urban areas (West rural: adjusted OR (AOR) = 6.13, 95%CI: 3.20–11.75, east rural: AOR = 6.72, 95%CI: 3.66–12.33) and maternal education (senior-educations: AOR = 2.43, 95%CI: 1.17–5.06); and inversely associated with distance to vaccination delivery points (≧2 km: AOR = 0.41, 95%CI: 0.24–0.70), and Fula ethnicity (AOR = 0.60, 95%CI: 0.40–0.91).
Conclusion
Vaccine coverage in The Gambia is high but infants are usually vaccinated after the neonatal period. Interventions to ensure the implementation of national vaccination policies are urgently needed.
Keywords: Vaccine, Birth dose, Hepatitis B, BCG, Polio, West Africa
1. Introduction
The Expanded Programme on Immunization (EPI) is an essential, cost-effective health intervention able to reduce child morbidity and mortality worldwide [1]. The World Health Organization (WHO) recommends to administer three vaccines soon after birth, namely hepatitis B virus (HBV) vaccine, Bacillus Calmette-Guerin (BCG) and oral polio vaccine (OPV) [2], [3], [4]. Their early administration aims at preventing both mother-to-child and early horizontal HBV transmission [4]; TB meningitis in childhood [5]; and to increase OPV sero-conversion rates with subsequent doses [3]. Among the 49 countries in sub-Saharan Africa (SSA), BCG and OPV are scheduled at birth in 48 and 39 of them, respectively, whilst only eight countries have introduced HBV vaccine at the birth [6]. According to 2014 WHO/UNICEF reports, coverage of third dose of HBV vaccine, BCG and third dose of OPV in infants in the WHO Africa region was 77%, 84% and 77%, respectively [7]. Nevertheless, these figures were estimated at defined time points (i.e. 12–23 months of age) without considering the national policy and timing of vaccines [8]. For example, recent studies in SSA reported BCG vaccine coverage mostly by 4 or 8 weeks of age [9], [10], [11], [12], [13], and few assessed coverage at birth or at 7 days of age [14], [15].
In The Gambia, a west-African country with high rates of neonatal and infant mortality [16] and high chronic HBV prevalence (>8%) according to the WHO classification [17], [18], [19], the EPI schedule includes HBV vaccine, BCG and OPV immunization at birth or as soon as possible after birth. Although the coverage of these vaccines is above 95% [20], two previous small studies described important delays in BCG administration [9], [10].
Here, we present a large study assessing coverage and timing of the birth dose vaccines (HBV vaccine, BCG and OPV) over a 10-year period (2004–2014) in The Gambia using the Farafenni Health and Demographic Surveillance System (FHDSS). We also assessed demographic and epidemiological factors associated with delayed vaccination.
2. Method
2.1. Study sites and data collection
The Farafenni Health and Demographic Surveillance System (FHDSS) in the North Bank Region of The Gambia was established in 1981 [21]. The details of FHDSS have been described in elsewhere [21]. Briefly, the FHDSS covered all residents living in Farafenni town and surrounding villages located in North Bank Region of The Gambia. In 2012, the FHDSS covered a population of 50,455. Trained field workers visit each household every four months to collect demographic data (e.g. births, deaths, in and out migrations). Vaccination status of children under 5 years of age is collected from their infant welfare cards. If this is not available, children are considered unvaccinated. They constitute no more than 2% of children under 5 years of age [22].
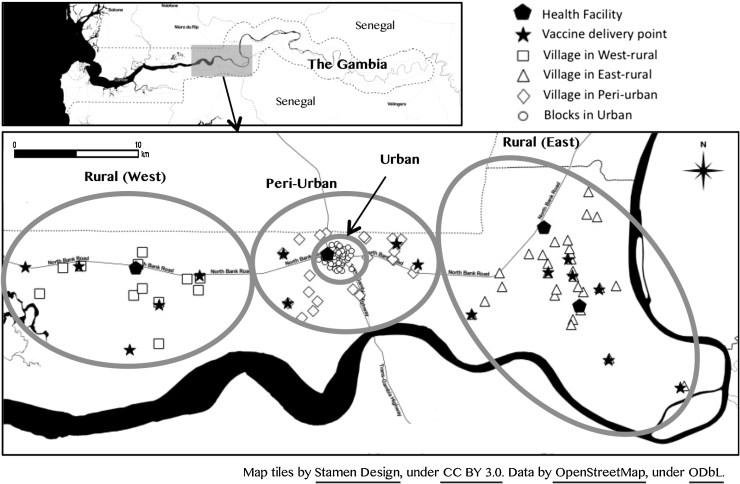
For the purposes of this study, we divided the area covered by the FHDSS into four regions reflecting the catchment areas of the respective health facilities that serve the FHDSS population. These are rural west (11 villages), the catchment area of Illiasa Minor Health Centre; rural east (31 villages) served by Ngaiyen Sanjal and Sarakunda Minor Health Centres in the north and south respectively; and peri-urban (23 villages located between 5 and 10 km from the central point of the urban area) and urban areas (49 blocks) (Fig. 1). The peri-urban and urban regions access health care from a major health centre and a regional hospital located in Farafenni town. The urban area is divided into residential blocks with roughly similar number of inhabitants.
Fig. 1.
Map of Farafenni.
The vaccine data were collected prospectively since mid-2003 as part of routine FHDSS data collection. Socio-economic data were collected in two surveys conducted in 2007 and 2013. Place of birth is collected prospectively since January 2014, as well as other birth information such as birth weight and assistance at delivery.
2.2. Vaccine delivery
Health facilities within the study area are supplied with vaccines by the central medical stores in Banjul, Gambian capital, every month through the office of the Regional Health Team in Farafenni, and stored in fridges with temperature operated by solar panels. Each facility conducts reproductive and child health (RCH) clinics once or twice a week; and undertakes visits to a set schedule of outreach clinics depending on the population of the vicinity within its catchment areas on other days of the week [23]. Vaccines are administered and only available through RCH clinics and these outreach clinics (Fig. 1). Even the hospital in the study area, Farafenni Hospital, does not vaccinate children born in the hospital. Both health facilities and outreach clinics use multiple-dose vials for monovalent HBV vaccine (10 doses/vial), BCG (20 doses/vial) and OPV (20 doses/vial). As recommended by the WHO, an opened vial needs to be used within six hours for BCG and within 28 days for HBV vaccine and OPV [24].
2.3. Statistical analysis
We defined the “Birth dose” of vaccine as vaccination at the day of birth or the day after (day 0 or day 1). Vaccination coverage for the first dose of HBV vaccine, BCG and OPV at the different time points [at birth (day 0–1), day 7, day 28, 6 months and 1 year] were calculated for each year by dividing the number of vaccinated children by the number of live births. The trend of vaccine coverage over the study year was tested by trend test.
Using the data from January 2011 and December 2014, factors associated with the timely administration of the first dose of vaccine [defined as vaccination at birth (day 0–1) and day 7 after birth] were identified by computing odds ratio using logistic regression. p-Value was tested using likelihood ratio tests. Children born between 2004 and 2010 were excluded from this analysis because socio-economic status (SES) was missing. The exposures of interest were area of residence (west-rural, east-rural, peri-urban and urban) and distance to vaccination delivery point (i.e. health centre or outreach clinic). We also assessed the association between the timely administration and other variables [year of birth, sex, ethnicity, season of birth, maternal age, birth spacing, presence of elder sibling(s), maternal education levels and SES]. The factors found to be associated in the univariable analysis (p < 0.2) were included in a multivariable logistic regression model. The impact of the place of birth and category of delivery assistant on vaccine coverage at birth was examined using the 2014 data as information on place of birth only collected from January 2014. All the analyses were performed using STATA 12.0.
SES index were created using asset ownership and household material by principal component analysis and divided into five categories [25]. The locations of villages were mapped using Geographic Information System (GIS). We created the FHDSS map using QGIS and data of OpenSourceMap. The direct distances from villages to vaccination sites were measured using QGIS software.
3. Results
Between 2004 and 2014, 10,851 children were born in the FHDSS and only 14 of these children (0.13%) did not have the infant welfare card. The median age on receipt of the first dose of each of three study vaccines was 24 days [Interquartile range (IQR): 16–35 days for HBV vaccine and BCG, and 16–34 days for OPV]; the overall vaccine coverage by the age of 12 months was 93.6% for HBV vaccine, 95.3% for BCG and 95.9% for OPV (Fig. 2). Because the vast majority [91.8% (9963/10,851)] of infants received these three vaccines on the same day, we only present the data for HBV vaccine in further analysis on risk factors for delayed birth vaccination assuming that those factors are the same factors associated with delayed BCG and OPV vaccination.
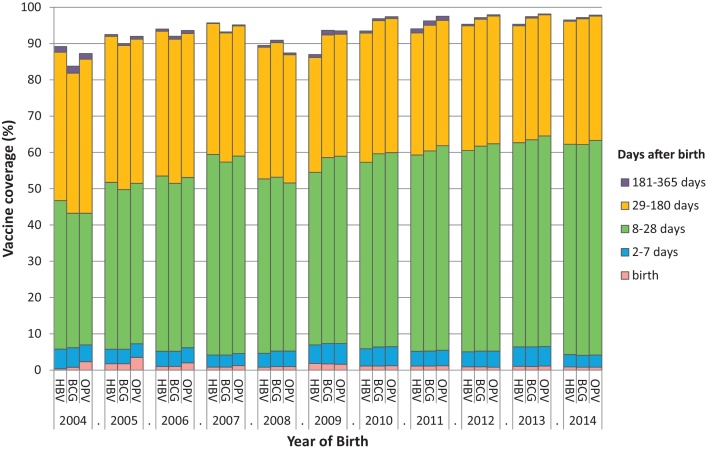
Fig. 2.
BCG, OPV and HBV cumulative vaccine coverage during infancy, from 2005 to 2014, in Farafenni HDSS.
First dose of HBV vaccine coverage at birth (day 0–1), day 7, day 28, 6 months and 12 months over the study period (2004–2014) was 1.1% (117/10,851), 5.4% (586/10,851), 58.4% (6340/10,851), 93.1% (10,100/10,851) and 93.6% (10,162/10,851) respectively. First dose of vaccine coverage did not increase from 6 months to 12 months (Fig. 2). Vaccine coverage at day 1 and 7 were similar throughout the study period (p-value for trend p = 0.516 and p = 0.662 at day 1 and 7, respectively). In contrast, there was an increase in coverage at day 28 and 6 months between 2004 and 2014 [from 46.7% to 62.2% (p-value for trend test <0.001 at day 28; and from 87.6% to 96.2% at 6 months (p-value for trend <0.001)) (Fig. 2).
3.1. Factors associated with vaccination within 7 days of birth
A total of 5994 infants born in the FHDSS between January 2011 and December 2014 were included in the risk factors analysis. Overall, 44.2% of these children lived in rural areas and approximately 50% lived within 1 km from a vaccination delivery point. Ethnicity, maternal age, presence of sibling(s), maternal education and SES index varied according to the study regions (Table 1). Approximately 60% of these children were born in a health facility.
Table 1.
Baseline characteristics of the study participants by region within the area of the Farafenni Health and Demographic Surveillance System from 2011 to 2014 (N = 5994).
| Variable | Category | Total | West-rural | East-rural | Peri-urban | Urban |
|---|---|---|---|---|---|---|
| N = 5994 | N = 767 | N = 1882 | N = 795 | N = 2550 | ||
| Sex | Male | 3114 (52.0) | 401 (52.3) | 1015 (53.9) | 394 (49.6) | 1304 (51.2) |
| Female | 2880 (48.0) | 366 (47.7) | 867 (46.1) | 401 (50.4) | 1246 (48.8) | |
| Ethnicity | Wollof | 2807 (46.8) | 57 (7.4) | 1089 (57.9) | 531 (66.8) | 1130 (44.3) |
| Mandinka | 1710 (28.5) | 561 (73.1) | 300 (15.9) | 66 (8.3) | 783 (30.7) | |
| Fula | 1241 (20.7) | 117 (15.3) | 483 (25.7) | 187 (23.5) | 454 (17.8) | |
| Others | 236 (3.9) | 32 (4.2) | 10 (0.5) | 11 (1.4) | 183 (7.2) | |
| Season of birth | Wet (June–October) | 2933 (48.9) | 393 (51.2) | 932 (49.5) | 367 (46.2) | 1241 (48.6) |
| Dry (November–May) | 3061 (51.1) | 374 (48.8) | 950 (50.5) | 428 (53.8) | 1309 (51.4) | |
| Maternal age (years) | ≦19 | 728 (12.1) | 80 (10.4) | 246 (13.1) | 81 (10.2) | 321 (12.6) |
| 20–29 | 3035 (50.6) | 341 (44.5) | 903 (48.0) | 407 (51.2) | 1384 (54.3) | |
| ≧30– | 2232 (37.2) | 346 (45.1) | 733 (38.9) | 307 (38.6) | 845 (33.2) | |
| Year of birth | 2011 | 1334 (22.3) | 176 (22.9) | 424 (22.5) | 164 (20.6) | 570 (22.4) |
| 2012 | 1649 (27.5) | 225 (29.3) | 519 (27.6) | 221 (27.8) | 684 (26.8) | |
| 2013 | 1519 (25.3) | 183 (23.9) | 475 (25.2) | 188 (23.6) | 673 (26.4) | |
| 2014 | 1491 (24.9) | 183 (23.9) | 464 (24.7) | 222 (27.9) | 622 (24.4) | |
| Birth spacing | Less than 2 years | 644 (10.7) | 85 (11.1) | 215 (11.4) | 82 (10.3) | 263 (10.3) |
| Over 2 years | 5348 (89.2) | 682 (88.9) | 1666 (88.5) | 713 (89.7) | 2287 (89.7) | |
| Siblings (N = 5943) |
No | 1629 (27.4) | 164 (21.6) | 346 (18.4) | 188 (23.7) | 931 (36.9) |
| Yes | 4315 (72.6) | 597 (78.4) | 1521 (81.1) | 604 (76.3) | 1593 (63.1) | |
| Distance to vaccine delivery point | <1 km | 2940 (49.1) | 380 (49.5) | 1232 (65.5) | 256 (32.2) | 1072 (42.1) |
| 1–2 km | 2462 (41.1) | 228 (29.7) | 490 (26.0) | 268 (33.7) | 1476 (57.9) | |
| >2 km | 592 (9.9) | 159 (20.7) | 160 (8.5) | 271 (34.1) | 2 (0.1) | |
| Maternal education (N = 5013) |
No | 790 (15.8) | 53 (8.1) | 86 (5.3) | 122 (19.1) | 529 (25.3) |
| Koranic | 3362 (67.1) | 536 (82.2) | 1390 (85.3) | 443 (69.2) | 993 (47.5) | |
| Basic | 527 (10.5) | 49 (7.5) | 125 (7.7) | 44 (6.9) | 309 (14.8) | |
| Senior | 334 (6.7) | 14 (2.2) | 29 (1.8) | 31 (4.8) | 260 (12.4) | |
| SES index (N = 5463) |
1st poor | 1080 (19.8) | 183 (26.0) | 631 (35.9) | 230 (31.5) | 36 (1.6) |
| 2nd | 966 (17.7) | 284 (40.4) | 465 (26.5) | 174 (23.8) | 43 (1.9) | |
| 3rd | 1058 (19.4) | 131 (18.6) | 482 (27.4) | 215 (29.5) | 230 (10.1) | |
| 4th | 1118 (20.5) | 84 (11.9) | 171 (9.7) | 99 (13.6) | 764 (33.6) | |
| 5th wealthy | 1241 (22.7) | 21 (3.0) | 7 (0.4) | 12 (1.6) | 1201 (52.8) | |
| Place of birth (2014) (N = 1490) |
Own/relative's home | 600 (40.3) | 97 (53.0) | 251 (54.1) | 100 (45.1) | 152 (24.5) |
| Health centers | 268 (18.0) | 68 (37.2) | 191 (41.2) | 3 (1.4) | 6 (1.0) | |
| Hospital | 622 (41.7) | 18 (9.8) | 22 (4.7) | 119 (53.6) | 463 (74.6) | |
| Delivery assistant (2014) (N = 1490) |
Midwife | 444 (32.4) | 58 (31.7) | 140 (30.2) | 61 (27.5) | 223 (35.9) |
| TBA | 444 (29.8) | 89 (48.6) | 204 (44.0) | 80 (36.0) | 71 (11.4) | |
| Doctor | 74 (5.0) | 7 (3.8) | 10 (2.2) | 10 (4.5) | 47 (7.6) | |
| Other | 470 (31.5) | 28 (15.3) | 104 (22.4) | 68 (30.6) | 270 (43.5) | |
| None | 20 (1.3) | 1 (0.6) | 6 (1.3) | 3 (1.4) | 10 (1.6) | |
The multivariable analysis shows that children in rural areas were more likely to be vaccinated by day 7 than those in urban and peri-urban areas [east-rural compared to urban area: Adjusted Odds Ratio (AOR) 6.13, 95%CI: 3.20–11.74; west-rural compared to urban area: AOR 6.72, 95%CI: 3.66–12.34] (Table 2). Increasing distance from a vaccination delivery point decreased the chance of vaccination by day 7 (<1 km vs 1–2 km AOR 0.50, 95%CI: 0.35–0.70, vs ≧2 km AOR 0.41, 95%CI: 0.24–0.70). Ethnicity and education were also associated with vaccine coverage by day 7. Vaccine coverage among Fulas was significantly lower than among Wollof, the most common ethnic group (AOR 0.60, 95%CI: 0.39–0.91). Children born to mothers with high education level had significantly higher vaccine coverage at 7 days than children born from uneducated mothers (AOR 2.43, 95%CI: 1.17–5.07) (Table 2).
Table 2.
Factors associated with vaccination at day 7 after birth in the Farafenni Health and Demographic Surveillance System, 2011–2014 (N = 5994).
| Variables | Total | Day 7 after birth |
||||
|---|---|---|---|---|---|---|
| No. (%) | Crude ORa (95%CIc) |
pd | Adjusted ORb (95%CIc) |
pd | ||
| Area | ||||||
| Urban | 2550 | 43 (1.7) | 1 (reference) | <0.001 | 1 (reference) | <0.001 |
| Peri-urban | 795 | 14 (1.8) | 1.04 (0.57–1.92) | 1.59 (0.69–3.64) | ||
| West-rural | 767 | 66 (8.6) | 5.49 (3.70–8.13) | 6.13 (3.20–11.75) | ||
| East-rural | 1882 | 190 (10.1) | 6.54 (4.67–9.16) | 6.72 (3.66–12.33) | ||
| Distance to vaccine delivery point | ||||||
| <1 km | 2940 | 218 (7.4) | 1 (reference) | <0.001 | 1 (reference) | <0.001 |
| 1–2 km | 2462 | 72 (2.9) | 0.38 (0.29–0.49) | 0.50 (0.35–0.70) | ||
| >2 km | 592 | 23 (3.9) | 0.50 (0.33–0.78) | 0.41 (0.24–0.70) | ||
| Sex | ||||||
| Boys | 3114 | 162 (5.2) | 1 (reference) | 0.94 | ||
| Girls | 2880 | 151 (5.2) | 1.01 (0.80–1.27) | |||
| Ethnicity | ||||||
| Wollof | 2807 | 140 (5.0) | 1 (reference) | <0.001 | 1 (reference) | 0.014 |
| Mandinka | 1710 | 117 (6.8) | 1.40 (1.09–1.80) | 1.18 (0.83–1.70) | ||
| Fula | 1241 | 52 (4.2) | 0.83 (0.60–1.15) | 0.60 (0.40–0.91) | ||
| Others | 236 | 4 (1.7) | 0.33 (0.12–0.9) | 0.67 (0.23–1.96) | ||
| Year of birth | ||||||
| 2011 | 1334 | 69 (5.2) | 1 (reference) | 0.08 | 1 (reference) | 0.06 |
| 2012 | 1649 | 83 (5.0) | 0.97 (0.70–1.35) | 1.19 (0.81–1.73) | ||
| 2013 | 1520 | 97 (6.4) | 1.25 (0.91–1.72) | 1.36 (0.93–1.99) | ||
| 2014 | 1491 | 64 (4.3) | 0.82 (0.58–1.17) | 0.81 (0.53–1.23) | ||
| Season of birth | ||||||
| Wet | 2933 | 160 (5.5) | 1 (reference) | 0.45 | ||
| Dry | 3061 | 153 (5.0) | 0.91 (0.73–1.14) | |||
| Mother age | ||||||
| ≦19 | 728 | 32 (4.4) | 1 (reference) | 0.22 | ||
| 20–29 | 3034 | 151 (5.0) | 1.14 (0.77–1.68) | |||
| ≧30 | 2231 | 130 (5.8) | 1.35 (0.91–2.00) | |||
| Birth spacing | ||||||
| Less than 2 years | 645 | 45 (7.0) | 1 (reference) | 0.041 | 1 (reference) | 0.056 |
| Over 2 years | 5348 | 268 (5.0) | 0.7 (0.51–0.97) | 0.67 (0.45–1.00) | ||
| Sibling | ||||||
| No | 1629 | 75 (4.6) | 1 (reference) | 0.165 | 1 (reference) | 0.75 |
| Yes | 4315 | 237 (5.5) | 1.2 (0.92–1.57) | 0.93 (0.64–1.35) | ||
| Maternal education level | ||||||
| No | 790 | 23 (2.9) | 1 (reference) | 0.005 | 1 (reference) | 0.027 |
| Koranic | 3362 | 198 (5.9) | 2.09 (1.35–3.24) | 0.97 (0.57–1.64) | ||
| Basic | 527 | 28 (5.3) | 1.87 (1.07–3.29) | 1.46 (0.77–2.77) | ||
| Senior | 334 | 16 (4.8) | 1.68 (0.87–3.22) | 2.43 (1.17–5.06) | ||
| SES index | ||||||
| 1st Poor | 1080 | 71 (6.6) | 1 (reference) | <0.001 | 1 (reference) | 0.50 |
| 2nd | 966 | 88 (9.1) | 1.42 (1.03–1.97) | 1.24 (0.85–1.80) | ||
| 3rd | 1058 | 61 (5.8) | 0.87 (0.61–1.24) | 0.95 (0.64–1.43) | ||
| 4th | 1118 | 39 (3.5) | 0.51 (0.34–0.77) | 1.19 (0.73–1.93) | ||
| 5th Wealthy | 1241 | 20 (1.6) | 0.23 (0.14–0.38) | 0.83 (0.39–1.75) | ||
Odd Ratio.
Odds Ratio adjusted for area, distance from vaccine location, ethnicity, year of birth, birth spacing, sibling, maternal education and SES index.
95% confidence interval.
p-Value tested by likelihood ratio test.
Vaccination by day 1 was significantly higher in rural than urban areas (AOR 4.61, 95%CI: 2.27–9.36) (Supplementary Table 1). In 2014, more than half (59.7%) of the 1490 children were born in a health facility (hospital or health center); among these, only 0.6% were vaccinated by day 1 and 3.8% by day 7 after birth (Table 3), not higher percentage than children born in other locations.
Table 3.
Vaccine coverage at birth (day 1) and day 7 after birth by location of birth and by the nature of assistance at delivery in 2014 (N = 1490).
| Number (%) | Day 0–1 (at birth) No. (%) |
AORa (95%CIc) | pd | Day 7 after birth No. (%) |
AORb (95%CIc) | pd | |
|---|---|---|---|---|---|---|---|
| N = 1490 | 13 (0.87) | ||||||
| Place of birth | |||||||
| Home | 600 (40.3) | 8 (1.3) | 1 (reference) | 0.29 | 32 (5.2) | 1 (reference) | 0.34 |
| Health center | 268 (18.0) | 2 (0.8) | 0.32 (0.06–1.59) | 24 (8.5) | 1.49 (0.72–3.07) | ||
| Hospital | 622 (41.7) | 3 (0.5) | 1.19 (0.21–6.76) | 10 (1.6) | 0.63 (0.20–2.03) | ||
| Delivery assistant | |||||||
| Midwife | 482 (32.3) | 4 (1.6) | 1 (reference) | 0.45 | 29 (6.4) | 1 (reference) | 0.90 |
| TBA | 444 (29.8) | 7 (0.8) | 1.58 (0.43–5.68) | 23 (4.6) | 0.93 (0.44–2.01) | ||
| Doctor | 74 (5.0) | 0 (0) | – | 2 (2.7) | 0.50 (0.06–4.22) | ||
| Other | 470 (31.5) | 2 (0.4) | 0.59 (0.11–3.27) | 12 (2.5) | 0.83 (0.34–2.00) | ||
| None | 20 (1.3) | 0 (0) | – | 0 (0) | – | ||
Odds ratio adjusted for living areas, distance to vaccine delivery point and ethnicity.
Odds ratio adjusted for area, distance from vaccine delivery point, ethnicity, birth spacing, sibling, maternal education and SES index.
95% confidence interval.
p-Value tested by likelihood ratio test.
Supplementary Table 1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.05.017.
Factors associated with timely vaccination at birth (day 0–1) in Farafenni Health and Demographic Surveillance System, 2011–2014 (N = 5994).
4. Discussion
Despite the high vaccination coverage by 6 months of age, the proportion of Gambian infants having received the three recommended vaccines within one week of age was particularly low. Few factors were found to be associated with delayed vaccination, namely living in urban and peri-urban settings, long distance from vaccination delivery points, Fula ethnicity, and low maternal education. Interestingly, being born in any health facility did not have any effect on early vaccination, indicating the need to improving awareness on the timely administration of vaccines among health workers. In addition, there is the need for integrating these vaccines in the maternal and neonatal services as is already the case with the tetanus toxoid vaccine. The use of multi-dose vials and the limited time for their use is probably a barrier for this proposed option, specially for BCG where the 20 doses of the multi-vial need to be finalized within hours.
Previous studies, both in The Gambia and in other SSA countries, assessed vaccine coverage at different time points after birth, mostly at 12–23 months [22], [26], [27], and sometimes at 4 and 8 weeks after birth [9], [10], [11], [12], [13], [28], [29], [30]. The few studies that assessed BCG and the first OPV dose at day 7 reported low coverage, in Ghana 38% for BCG and 41% for OPV [14] and in Guinea-Bissau 11% for BCG [15]. In The Gambia, the seemingly better coverage, timely administration of BCG in 90.7% [9] and 94.2% [10] of children, is probably due to the definition of timely vaccination, which was considered so within the first 8 weeks of life.
The current WHO recommendation to provide HBV vaccine within 24 h of delivery aims at preventing hepatitis B mother-to-child transmission [4]. This is extremely important as perinatal maternal transmission not only increases the risk of chronic HBV infection [31], but also that of severe liver disease (i.e. liver cancer) amongst chronic carriers [32], [33]. In The Gambia, approximately 2 out of 3 chronic carriers who require antiviral therapy are attributable to the mother-to-child transmission [33]. Although all SSA countries have introduced HBV vaccine into their national immunization programmes, most countries provide its first dose at 6–8 weeks of age. This happens due to the logistical challenges to provide the vaccine within 24 h of birth in areas where home delivery is common. In addition, the Global Alliance for Vaccines and Immunizations (GAVI) provides only the pentavalent vaccine (DTP-HepB-Hib) which is not licensed to be administered at birth. Consequently, only eight SSA countries (Botswana, Cape Verde, Djibouti, The Gambia, Namibia, Nigeria, Mauritania, and Sao Tome and Principe) have a policy to administer HBV vaccination at birth. Coverage and timeliness have been determined only as part of a vaccine trial [34], [35]; or in Nigeria from a health facility-based cross-sectional study [36], [37]. Ours is the first population-based study in SSA estimating HBV vaccine birth dose coverage in real-life conditions.
In 2014, the WHO's Strategic Advisory Group of Experts on immunization (SAGE) recognized a possible beneficial non-specific effect of BCG on all-cause mortality in addition to the potential direct effect on TB [38], [39]. In addition, a recent randomized controlled trial showed a reduction in all-cause mortality when OPV was given within two days of birth [40]. Because more than half of infant mortality occurs during the first week of life, if non-specific effect of BCG and OPV in fact occur these vaccines should be given immediately after birth. Further research is needed to better understand the benefits of such a strategy.
Several factors were associated with low vaccine coverage by the first week of life. Distance to a vaccination delivery point was one of these and is consistent with previous studies in Africa [27], [41]. Contrary to previous reports of better coverage in urban areas [14], [42], [43], in Farafenni, rural areas had high vaccine coverage by day 7 after birth. The difference between rural and urban areas could be linked to the primary health care (PHC) system, introduced in rural Gambia in 1978, which consists in village-based traditional birth attendants and village health workers operating under the supervision of community health nurses [21]. As part of this system, village health workers have an active role in informing people of the date of outreach clinics, possibly helping in the earlier vaccination uptakes observed in rural areas.
Greater delay in vaccine birth doses among Fulas was consistent with a previous report [9]. Also in agreement with a previous report, we found an association between maternal education and delayed infant's immunization [13].
A recent WHO systematic review on factors associated with higher uptake of the HBV vaccine birth dose identified: integration of the birth dose vaccine with maternal/newborn services, home visits by village health workers, vaccine storage outside the cold-chain and use of a single-dose injection pre-filled with HBV vaccine [44]. The evidence was based mainly on studies done in Asia while no such information has been collected in SSA [44]. Further research is therefore needed in SSA to establish the most suitable intervention in the continent.
The strength of this study is the use of population-based data collected over a period of more than 10 years. Vaccination dates were collected prospectively from infant welfare cards, which were mostly available, minimizing selection bias, and did not rely on mother's recall. A limitation of this study is vaccine coverage may have been overestimated, as children dying during the early neonatal period, less likely to have been vaccinated, may not have been registered in the FHDSS. However, neonatal mortality is a relatively rare event, epidemiologically speaking. The direct distance from the residence to the vaccination delivery point is only a surrogate marker for distance. Although vaccine coverage may differ in different areas within the country, previous data from a smaller study in Sibanor show a similar low coverage of birth vaccines during the early neonatal period. In addition, because timely vaccination coverage was low (approximately 1% at days 0–1 and 5% at day 7), statistical power was insufficient to evaluate factors associated with vaccination at this time point.
5. Conclusion
In conclusion, vaccine coverage at birth and the subsequent neonatal period was extremely low in The Gambia over the last 10 years. Integration of the birth dose vaccines within the maternal and neonatal services in all health facilities could increase early vaccination coverage, at least among children born in health facilities that represent the majority of the population. There is the need to investigate this and other potential approaches to improve birth dose vaccine coverage in SSA.
Author's contributions
All authors contributed significantly to the work of this manuscript. AR and RM conceived the study. RM led the statistical analysis and wrote the first version of the manuscript along with AR. MJ is the manager of the FHDSS and participated in the assessment of the data quality and interpretation of the results. GP manages field works in Farafenni and is responsible for data collection. YS gave direct inputs and suggestions to the analysis and the manuscript. KK, SC, BG and UDA participated significantly in the final version of the manuscript. All authors read and approved the final manuscript.
Funding source
The MRC Unit The Gambia core funding that supports the Farafenni HDSS comes from the MRC UK.
Ethical approval
This study was approved by Gambia Government/Medical Research Council Joint Ethics Committee.
Acknowledgments
We thank the FHDSS field team for coordinating the FHDSS and supporting to access the FHDSS data. Also we are grateful to all participants who consented to join in the FHDSS.
Conflict of interest: All authors declared no conflicts of interest.
References
- 1.Andre F.E., Booy R., Bock H.L., Clemens J., Datta S.K., John T.J. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:140–146. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization BCG vaccine. Wkly Epidemiol Rec. 2004;79(4):25–40. [PubMed] [Google Scholar]
- 3.World Health Organization Polio vaccines: WHO position paper. Wkly Epidemiol Rec. 2014;9(89):73–92. [PubMed] [Google Scholar]
- 4.World Health Organization Hepatitis B vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;28(3):589–590. [Google Scholar]
- 5.Colditz G.A., Berkey C.S., Mosteller F., Brewer T.F., Wilson M.E., Burdick E. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96(July):29–35. [PubMed] [Google Scholar]
- 6.World Health Organization . 2015. WHO vaccine-preventable diseases: monitoring system. Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules. [Google Scholar]
- 7.WHO-UNICEF . 2015, July. Estimates of national immunization coverage. Available from: http://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html [cited 15.11.15] [Google Scholar]
- 8.Burton A., Monasch R., Lautenbach B., Gacic-Dobo M., Neill M., Karimov R. WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull World Health Organ. 2009;87:535–541. doi: 10.2471/BLT.08.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott S., Odutola A., Mackenzie G., Fulford T., Afolabi M.O., Jallow Y.L. Coverage and timing of children's vaccination: an evaluation of the expanded programme on immunisation in The Gambia. PLOS ONE. 2014;9:e107280. doi: 10.1371/journal.pone.0107280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odutola A., Afolabi M.O., Ogundare E.O., Lowe-Jallow Y.N., Worwui A., Okebe J. Risk factors for delay in age-appropriate vaccinations among Gambian children. BMC Health Serv Res. 2015;15(1):346. doi: 10.1186/s12913-015-1015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babirye J.N., Engebretsen I.M.S., Makumbi F., Fadnes L.T., Wamani H., Tylleskär T. Timeliness of childhood vaccinations in Kampala Uganda: a community-based cross-sectional study. PLoS ONE. 2012;7(April (4)):e35432. doi: 10.1371/journal.pone.0035432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadnes L.T., Nankabirwa V., Sommerfelt H., Tylleskär T., Tumwine J.K., Engebretsen I.M.S. Is vaccination coverage a good indicator of age-appropriate vaccination? A prospective study from Uganda. Vaccine. 2011;29(April (19)):3564–3570. doi: 10.1016/j.vaccine.2011.02.093. [DOI] [PubMed] [Google Scholar]
- 13.Schoeps A., Ouédraogo N., Kagone M., Sié A., Müller O., Becher H. Socio-demographic determinants of timely adherence to BCG, Penta3, measles, and complete vaccination schedule in Burkina Faso. Vaccine. 2013;32(December (1)):96–102. doi: 10.1016/j.vaccine.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Gram L., Soremekun S., Asbroek ten A., Manu A., O’Leary M., Hill Z. Socio-economic determinants and inequities in coverage and timeliness of early childhood immunisation in rural Ghana. Trop Med Int Health. 2014;19(July (7)):802–811. doi: 10.1111/tmi.12324. [DOI] [PubMed] [Google Scholar]
- 15.Thysen S., Byberg S., Pedersen M., Rodrigues A., Ravn H., Martins C. BCG coverage and barriers to BCG vaccination in Guinea-Bissau: an observational study. BMC Public Health. 2014;14(1):1037. doi: 10.1186/1471-2458-14-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jasseh M., Webb E.L., Jaffar S., Howie S., Townend J., Smith P.G. Reaching millennium development goal 4 – The Gambia. Trop Med Int Health. 2011;16(June (10)):1314–1325. doi: 10.1111/j.1365-3156.2011.02809.x. [DOI] [PubMed] [Google Scholar]
- 17.Peto T.J., Mendy M.E., Lowe Y., Webb E.L., Whittle H.C., Hall A.J. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in The Gambia Hepatitis Intervention Study (1986–90) and in the nationwide immunisation program. BMC Infect Dis. 2014;14(1):7. doi: 10.1186/1471-2334-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimakawa Y., Lemoine M., Njai H.F., Jatta A., Sanneh B., Taal M. 48 community-based screening for hepatitis B virus infection in The Gambia, West Africa: prevalence of infection and factors affecting the screening attendance. J Hepatol. 2013;58(April):S21. [Google Scholar]
- 19.World Health Organization . 2016. Hepatitis B.http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/ [accessed March 12] [Google Scholar]
- 20.WHO vaccine-preventable diseases: monitoring system. 2015 global summary. Available from: http://apps.who.int/immunization_monitoring/globalsummary [cited 15.11.15].
- 21.Jasseh M., Gomez P., Greenwood B.M., Howie S.R.C., Scott S., Snell P.C. Health & Demographic Surveillance System profile: Farafenni Health and Demographic Surveillance System in The Gambia. Int J Epidemiol. 2015;44:837–847. doi: 10.1093/ije/dyv049. [DOI] [PubMed] [Google Scholar]
- 22.Payne S., Townend J., Jasseh M., Lowe Jallow Y., Kampmann B. Achieving comprehensive childhood immunization: an analysis of obstacles and opportunities in The Gambia. Health Policy Plan. 2014;29(March (2)):193–203. doi: 10.1093/heapol/czt004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Africa Health Workforce Observatory . 2009. Human resources for health country profile – The Gambia. [Google Scholar]
- 24.World Health Organization . 2014. WHO policy statement: multi-dose vial policy. [Google Scholar]
- 25.Quattrochi J., Jasseh M., Mackenzie G., Castro M.C. Spatial analysis of under-5 mortality and potential risk factors in the Basse Health and Demographic Surveillance System, The Gambia. Trop Med Int Health. 2015;20(March (7)):941–951. doi: 10.1111/tmi.12490. [DOI] [PubMed] [Google Scholar]
- 26.Odusanya O.O., Alufohai E.F., Meurice F.P., Ahonkhai V.I. Determinants of vaccination coverage in rural Nigeria. BMC Public Health. 2008;8(1):381. doi: 10.1186/1471-2458-8-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Polain de Waroux O., Schellenberg J.R.A., Manzi F., Mrisho M., Shirima K., Mshinda H. Timeliness and completeness of vaccination and risk factors for low and late vaccine uptake in young children living in rural southern Tanzania. Int Health. 2013;5(June (2)):139–147. doi: 10.1093/inthealth/iht006. [DOI] [PubMed] [Google Scholar]
- 28.Clark A., Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(May (9674)):1543–1549. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 29.Akmatov M.K., Mikolajczyk R.T. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(June (7)):e14–e24. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- 30.Fadnes L.T., Jackson D., Engebretsen I.M., Zembe W., Sanders D., Sommerfelt H. Vaccination coverage and timeliness in three South African areas: a prospective study. BMC Public Health. 2011;11(1):404. doi: 10.1186/1471-2458-11-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edmunds W.J., Medley G.F., Nokes D.J., Hall A.J., Whittle H.C. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253(August (1337)):197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 32.Shimakawa Y., Yan H.-J., Tsuchiya N., Bottomley C., Hall A.J. Association of early age at establishment of chronic hepatitis B infection with persistent viral replication, liver cirrhosis and hepatocellular carcinoma: a systematic review. PLOS ONE. 2013;8(7):e69430. doi: 10.1371/journal.pone.0069430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimakawa Y., Lemoine M., Njai H.F., Bottomley C., Ndow G., Goldin R.D. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut. 2015;(July) doi: 10.1136/gutjnl-2015-309892. [DOI] [PubMed] [Google Scholar]
- 34.Shimakawa Y., Bottomley C., Njie R., Mendy M. The association between maternal hepatitis B e antigen status, as a proxy for perinatal transmission, and the risk of hepatitis B e antigenaemia in Gambian children. BMC Public Health. 2014;14(1):532. doi: 10.1186/1471-2458-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inskip H.M., Hall A.J., Chotard J., Loik F., Whittle H. Hepatitis B vaccine in the Gambian Expanded Programme on Immunization: factors influencing antibody response. Int J Epidemiol. 1991;20(September (3)):764–769. doi: 10.1093/ije/20.3.764. [DOI] [PubMed] [Google Scholar]
- 36.Sadoh A.E., Eregie C.O. Age at presentation for infant immunization in Nigeria: implications for hepatitis B immunization. Public Health. 2008;122(December (12)):1318–1320. doi: 10.1016/j.puhe.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Sadoh A.E., Ofili A. Hepatitis B infection among Nigerian children admitted to a children's emergency room. Afr Health Sci. 2014;14(June (2)):377–383. doi: 10.4314/ahs.v14i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins J., Soares-Weiser K., Reingold A. 2014. Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines: Strategic Advisory Group of Experts on non-specific effects of vaccine. [Google Scholar]
- 39.Sankoh O., Welaga P., Debpuur C., Zandoh C., Gyaase S., Poma M.A. The non-specific effects of vaccines and other childhood interventions: the contribution of INDEPTH Health and Demographic Surveillance Systems. Int J Epidemiol. 2014;43(June (3)):645–653. doi: 10.1093/ije/dyu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lund N., Andersen A., Hansen A.S.K., Jepsen F.S., Barbosa A., Biering-Sørensen S. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin Infect Dis. 2015;(July):civ617. doi: 10.1093/cid/civ617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okwaraji Y.B., Mulholland K., Schellenberg J.R.M.A., Andarge G., Admassu M., Edmond K.M. The association between travel time to health facilities and childhood vaccine coverage in rural Ethiopia. A community based cross sectional study. BMC Public Health. 2012;12(1):476. doi: 10.1186/1471-2458-12-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang X., Bi S., Yang W., Wang L., Cui G., Cui F. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis. 2009;200(July (1)):39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 43.Kuruvilla T.A., Bridgitte A. Timing of zero dose of OPV, first dose of hepatitis B and BCG vaccines. Indian Pediatr. 2009;46(November (11)):1013–1015. [PubMed] [Google Scholar]
- 44.World Health Organization . 2016. Practices to improve coverage of the hepatitis B birth dose vaccine. Available from: http://www.who.int/immunization/documents/control/who_ivb_12.11/ [cited March 12] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Factors associated with timely vaccination at birth (day 0–1) in Farafenni Health and Demographic Surveillance System, 2011–2014 (N = 5994).