Abstract
Nutrition has profound effects on ageing and lifespan. Caloric restriction is the major nutritional intervention that historically has been shown to influence lifespan and/or healthspan in many animal models. Studies have suggested that a reduction in protein intake can also increase lifespan, albeit not as dramatically as caloric restriction. More recent research based on nutritional geometry has attempted to define the effects of nutrition on ageing over a broad landscape of dietary macronutrients and energy content. Such studies in insects and mice indicate that animals with ad libitum access to low-protein, high-carbohydrate diets have longest lifespans. Remarkably, the optimum content and ratio of dietary protein to carbohydrates for ageing in experimental animals are almost identical to those in the traditional diets of the long-lived people on the island of Okinawa.
Keywords: older people; caloric restriction; protein restriction; low-protein, high-carbohydrate diets; Okinawa; ageing biology
An established dietary mantra is that older people need to eat more protein, even if they are not malnourished. The primary focus of such advice is sarcopenia, although other health benefits have been suggested including preventing obesity and osteoporosis, and improving wound healing and recovery from illness. This is based on the observation that dietary protein can drive anabolic responses in the muscle, and that these responses are impaired in older people [1–4].
These recommendations appear to be at odds with basic research in animals and observational studies in humans showing that low-protein or low-protein, high-carbohydrate (LPHC) diets delay ageing and increase lifespan [5–7]. The lifespan-extending effects of these types of diets are consistent with current understanding of cellular mechanisms that link nutrition with ageing biology.
The longest living people are the residents of the Japanese island of Okinawa, who have as many as five times more centenarians than other developed nations [8]. There are many factors that contribute to their exceptional longevity including mild caloric restriction, food quality, genes and physical activity. The energy from their diets was derived from 9% protein and 85% carbohydrates [9] (Figure 1). These Okinawan values for dietary protein and the protein to carbohydrate ratio (1:10) are very low and remarkably similar to those that have been found to optimise lifespan in recent animal studies of ageing [7].
Figure 1.
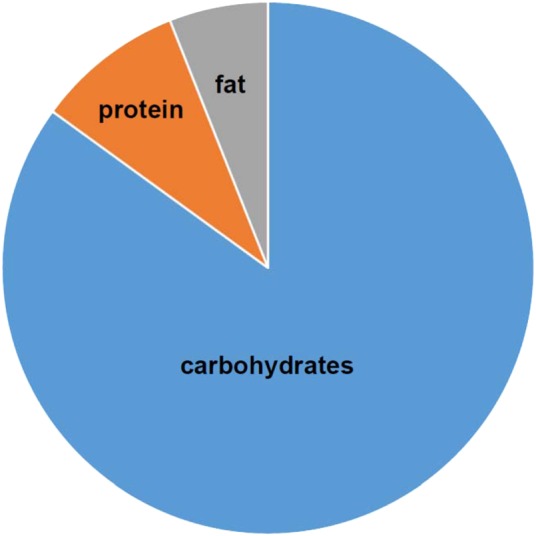
The proportions of macronutrients in the diets of the people of Okinawa in 1949 [9].
The first experimental study of nutrition and ageing is often attributed to McCay et al. [10] who in 1935 showed that reduced access to food led to markedly increased lifespan. This dietary intervention is called ‘caloric restriction’ and is considered to be a robust and reproducible intervention for delaying ageing and increasing lifespan [5, 11]. The McCay publication set the scene for many subsequent animal studies of ageing biology where the focus has been on reducing food, macronutrients or other nutrients to delay ageing. This type of approach is the opposite to much medical research which has tended to focus on the effects of supplementing nutrients on health outcomes in older people. In most caloric restriction studies, access to food is reduced by 10–50% of ad libitum intake. The increase in maximum and/or median lifespan with caloric restriction has been reported across taxa from yeast, worms, flies, mice, rats and dogs; with health and/or lifespan benefits reported in primates including humans [5]. Although caloric restriction is not sustainable for most people, there are a dedicated few worldwide who have been practising long-term caloric restriction (some as long as 15 years or longer) and who have subsequently shown remarkably reduced risk for atherosclerotic disease, as well as other improved risk factors for healthy ageing and longevity [12], albeit with adverse effects such as reduced bone mineral density [13]. Moreover, there is ongoing research underway that is studying the effects of caloric restriction on biomarkers of healthy ageing and longevity in humans [14]. Preliminary results indicate that many of the benefits seen in animal models, particularly in terms of reduced cardiometabolic risk factors, are also seen in humans [15].
There have been major advances in the understanding of the nutrient-sensing cellular pathways that link diet and ageing (Figure 2). The key nutrient-sensing pathways include those regulated by sirtuins (SIRT1), mechanistic target of rapamycin (mTOR), 5′ adenosine monophosphate-activated protein kinase (AMPK) and insulin/insulin-like growth factor-1 (IGF-1)/Growth Hormone (GH) [16]. Decreased activity of mTOR and insulin/IGF-1/GH or increased activity of AMPK and sirtuins have beneficial effects on cellular processes involved with ageing such as mitochondrial biogenesis, autophagy, cellular metabolism, oxidative stress, genome maintenance and protein synthesis. These four pathways are thought to have evolved to allow organisms to survive periods of food shortages by transferring resources from reproduction to survival and cellular maintenance [17]. The ageing benefit of caloric restriction is the long-term side effect of an active, programmed response to food shortages, rather than simply a reduction in harms caused by nutrient excess—although such excesses engender health costs in their own right. Genetic, dietary or pharmacological manipulation of nutrient-sensing pathways alters the rate of ageing in animal models and their response to caloric restriction [18].
Figure 2.
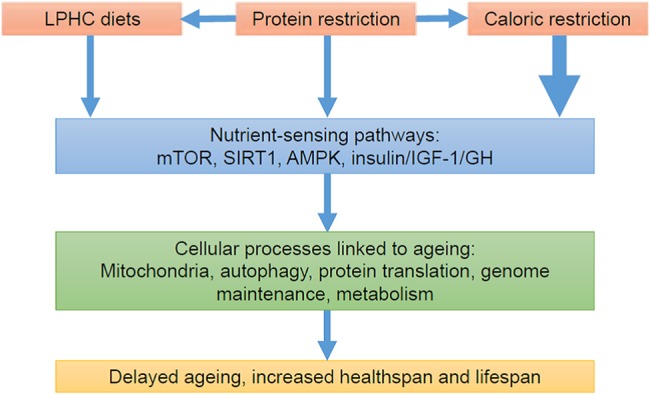
Pathways linking diet and ageing [16].
While modulation of these pathways provides a clear mechanism linking caloric restriction to lifespan extension, other factors may also contribute including periodic hunger, altered gut microbiome, avoidance of captivity-related obesity, altered circadian rhythm and reduced intake of individual macronutrients. Although reduced intake of fat or carbohydrate has not been reported to contribute to the beneficial effects of caloric restriction, there is evidence that some of the benefits of caloric restriction are mediated by reduced protein intake, and that protein restriction as a standalone intervention can extend lifespan [6].
One of most influential early studies compared caloric restriction with 40% protein restriction in ad libitum-fed rats. The caloric restricted rats lived 75% longer than controls, while the protein restricted rats only had an increase in lifespan of 15% [19]. There have been many other animal studies, and overall, protein restriction has been concluded to contribute to some of the benefits of caloric restriction. For example, 16 out of 18 rat and mice studies showed that protein restriction increased maximum lifespan and in these positive studies lifespan was increased by an average of 20%. It was concluded that protein restriction contributes to about half of the life extension of caloric restriction [20]. A recent meta-analysis of 145 animal studies of caloric restriction found that the proportion of protein intake was more important for life extension than any reduction of calories [21].
In humans, a systematic review of 64 interventional and observational studies did not find any conclusive evidence for a link between protein intake and mortality, but did find suggestive evidence that diets that are both high in protein and low in carbohydrates are associated with increased mortality [22]. In a widely reported and recent study of NHANES data, high-protein diets (>20%) were associated with increased mortality and cancer in subjects <65 years, but reduced risks over 65 years [23]. The risk seemed to be conferred by animal-based protein and circulating IGF-1 levels. The evidence in humans is mixed with a suggestion that lower protein diets, at least until old age, are linked with lower mortality.
On first appearances, it would seem straightforward to measure health and ageing outcomes in animals and humans on low-protein diets, which is at odds with the conflicting results of many studies and reviews. However, nutrition is complex and measuring the impact of individual components of the diet does not take into account interactions between nutrients or its impact on appetite and food intake [24]. The health outcomes of a low-protein diet will depend upon whether the protein is replaced by fat or carbohydrates. Furthermore, protein is a major driver of food intake, whereby low-protein diets generally increase food intake, while high-protein diets reduce food intake (‘protein leverage’ [25]). Therefore, any evaluation of the effects of dietary protein on ageing needs to take into account the amounts and interactions between all three macronutrients (protein, carbohydrates, fat) and to carefully measure food intake, especially in ad libitum-fed animals [26]. Recently, a type of nutritional geometry called the Geometric Framework was developed to evaluate the relationship between diet and outcomes such as lifespan across broad landscape of macronutrient and energy intakes [24, 27]. In these experiments, animals are ad libitum-fed one of numerous (typically 20–30) diets varying in protein, carbohydrate (and fat in mammals) and energy content over a lifetime. The methods and analyses are more complicated than traditional one-nutrient-at-a-time manipulations, but necessary to disentangle the effects of each macronutrient, energy intake and their interactions on phenotypic outcomes including ageing.
Initially, this method was applied to study ageing in fruit flies and showed that LPHC diets were associated with the longest lifespan while total calorie intake under ad libitum feeding conditions (i.e. without restricting food availability, but rather manipulating caloric density of the food by dilution) had no impact on lifespan once individual macronutrients were taken into account [28] (Figure 3). Following this approach, we studied 858 ad libitum-fed mice on 25 diets differing in macronutrients and energy and found that, as with the fruit fly, a LPHC diet was associated with the longest lifespan and the best late-life health as assessed by cardiometabolic health and immune function [30, 31]. However, low-protein, high-fat (soya bean oil) diets were associated with poorer outcomes, emphasising the importance of reporting all the macronutrients and their interactions—a low-protein diet may have positive or negative outcomes depending upon the macronutrient that replaces it and presumably also the type and quality of the macronutrients' sources.
Figure 3.
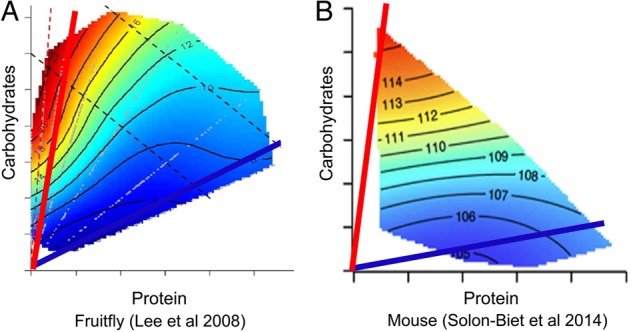
Geometric framework analysis of dietary protein and carbohydrate in fruit flies (A) [28] and mice (B) [29]. The longest lifespans are shown in red on the heat map and the ratio of dietary protein to carbohydrate associated with the longest lifespan is shown by the red line. In both fruit flies and mice, the longest lifespan (red) occurred with low-protein, high-carbohydrate diets, while the shortest lifespan (blue) was associated with high-protein, low-carbohydrate diets.
In a recent review, we reported nine different studies, mostly in insects, using nutritional geometry to study lifespan. These studies confirmed that LPHC diets in ad libitum-fed animals are associated with the longest lifespans. This conclusion was supported by many other indirect studies in animals and some studies in humans [7]. The optimum protein to carbohydrate ratio for lifespan across the different species is consistently ∼1:10 or less, with ∼10% or a little less of total calories coming from protein, i.e. remarkably similar to the Okinawan ratio. Values of 5% of total energy as protein are associated with loss of lean muscle mass and failure to thrive in mice when accompanied with low-energy density, suggesting that this is below the viable dietary limit to maintain health. In humans, it is notable that the Okinawans have among the lowest reported values for dietary per cent protein in human populations with an adequate food supply—at the other extreme, values in the habitual diet of human populations do not exceed 25%, and typically lie close to 15%, with the proportions of non-protein energy coming from fats and carbohydrates varying substantially between populations [32, 33]. Animal data are paralleled by the few human studies that have reported the protein to carbohydrate ratio. In their meta-analysis, Pedersen et al. [22] reviewed the effects of high-protein, low-carbohydrate diets (HPLC), which would be expected to be deleterious on the basis of the animal studies. From five observational studies, they found a suggestive relationship between increased all-cause mortality and long-term HPLC diets. Two studies showed an increase in type 2 diabetes mellitus and one an increase in cardiovascular disease with HPLC diets. Where reported, animal-based protein and diets had the worst effects, while plant-based proteins and diets nullified or reversed the trends.
These animal and human studies seem to indicate that reducing the amount and proportion of dietary protein can delay ageing, and that dietary protein needs to be replaced by healthy carbohydrates but not fat. The mechanisms linking low-protein and LPHC diets with ageing are beginning to be unravelled, and current results indicate that low-protein or LPHC diets can act via some of the same pathways as caloric restriction. In their study of NHANES data, Levine et al. [23] found that IGF-1 mediated the relationship between diet, mortality and cancer, and this was able to be replicated in a mouse model. In mice, we found that mTOR activation was decreased in LPHC diets in association with reduced circulating levels of branched chain amino acids [29]. Branched chain amino acids are potent activators of mTOR, and this result provided a simple mechanism linking low-protein intake, low-circulating branched chain amino acids and inactivation of mTOR. Intriguingly, the regulation of mTOR was not only influenced by branched chain amino acids, but also by the interaction between glucose and branched chain amino acids, providing a clue as to why the ratio of dietary carbohydrates and protein influences ageing. It is of note that elevated branched chain amino acids are a marker of diabetes mellitus in humans [34], but on the other hand, supplementation with branched chain amino acids has been reported to increase lifespan in mice [35] and nematode worms [36], presumably despite activation of mTOR.
Improved nutrition is a major cause of increased human lifespan in the last two centuries [37, 38], while poor diet is the main risk factor for death and disability in present times in developed nations [39]. There are converging results in many studies that indicate that the reduction of access to and/or intake of calories and/or protein delays ageing. This is presumably because such deficiencies activate nutrient-sensing pathways that have evolved to increase resilience in times of famine and food shortage. Ad libitum access to food that is low in protein and high in carbohydrates also increases lifespan, and the optimum ratio of dietary protein to carbohydrate—in a range of animals—matches the ratio that we see in the traditional Okinawan diet. We have limited our review to the effects of the total macronutrient and calorie content of diet and have not focussed upon the type of macronutrients nor other important nutritional factors. For example, the health effects of low-quality versus high-quality carbohydrates or animal- versus plant-based proteins are well recognised: we are certainly not proposing that a high-carbohydrate diet comes in the form of added simple sugars or highly processed foods, which are major contributors to excess energy intakes in modern dietary patterns. It is noteworthy that the main source of carbohydrate in the traditional Okinawan diet was the anti-oxidant-rich, nutrient-dense sweet potato that was low in both calories and glycaemic load [40], while other dietary factors may also be contributing to their longevity [41]. Our conclusions that reducing the amount and/or proportion of protein in the diet delays ageing has broader societal significance given concerns about the deleterious effects of production of animal protein for food on climate change [42, 43] and the potential influence of the commercial food industry on nutrition and health research, publications and policy [44].
Key points.
Caloric restriction and protein restriction prolong life in many animal models.
Low-protein, high-carbohydrate diets maximise lifespan in ad libitum-fed animals.
The optimum ratio of protein to carbohydrates for ageing in animals is nearly identical to the traditional Okinawan diet.
Conflicts of interest
None declared.
Funding
We acknowledge funding from the Australian National Health and Medical Research Council (Grants 571328, 1084267, 1101913) and the Ageing and Alzheimers Institute. B.J.W. and D.C.W. acknowledge funding from Kuakini Medical Center and the US National Institute on Aging (Grants 5R01AG027060, 5R01AG038707 and R21AG042908). These had no role in the design, execution, analysis, interpretation or writing of this review.
References
- 1.Wolfe RR. Update on protein intake: importance of milk proteins for health status of the elderly. Nutr Rev 2015; 73 (Suppl. 1): 41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr 2008; 27: 675–84. [DOI] [PubMed] [Google Scholar]
- 3.Volpi E, Campbell WW, Dwyer JT et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci 2013; 68: 677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009; 12: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell 2015; 161: 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirzaei H, Suarez JA, Longo VD. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol Metab 2014; 25: 558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Couteur DG, Solon-Biet S, Cogger VC et al. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci 2016; 73: 1237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willcox DC, Willcox BJ, Hsueh WC, Suzuki M. Genetic determinants of exceptional human longevity: insights from the Okinawa Centenarian Study. Age (Dordr) 2006; 28: 313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willcox BJ, Willcox DC, Todoriki H et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci 2007; 1114: 434–55. [DOI] [PubMed] [Google Scholar]
- 10.McCay C, Crowell M, Maynard L. The effect of retarded growth upon the length of life and upon ultimate size. J Nutr 1935; 10: 63–79. [PubMed] [Google Scholar]
- 11.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 2012; 11: 390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 2004; 101: 6659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villareal DT, Fontana L, Das SK et al. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trial. J Bone Miner Res 2016; 31: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickman AD, Williamson DA, Martin CK et al. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemp Clin Trials 2011; 32: 874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravussin E, Redman LM, Rochon J et al. A 2-Year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 2015; 70: 1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solon-Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Le Couteur DG, Simpson SJ. Macronutrients and caloric intake in health and longevity. J Endocrinol 2015; 226: R17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkwood TB, Shanley DP. Food restriction, evolution and ageing. Mech Ageing Dev 2005; 126: 1011–6. [DOI] [PubMed] [Google Scholar]
- 18.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins proper targets for improving healthspan and lifespan? Nat Rev Drug Discov 2012; 11: 443–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 1985; 40: 657–70. [DOI] [PubMed] [Google Scholar]
- 20.Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta 2006; 1757: 496–508. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell 2012; 11: 401–9. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen AN, Kondrup J, Borsheim E. Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res 2013; 57; doi:10.3402/fnr.v57i0.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine ME, Suarez JA, Brandhorst S et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014; 19: 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell 2015; 161: 18–23. [DOI] [PubMed] [Google Scholar]
- 25.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev 2005; 6: 133–42. [DOI] [PubMed] [Google Scholar]
- 26.Le Couteur DG, Wilder SM, de Cabo R, Simpson SJ. The evolution of research on ageing and nutrition. J Gerontol A Biol Sci Med Sci 2014; 69: 1–2. [DOI] [PubMed] [Google Scholar]
- 27.Simpson SJ, Raubenheimer D. Perspective: tricks of the trade. Nature 2014; 508: S66. [DOI] [PubMed] [Google Scholar]
- 28.Lee KP, Simpson SJ, Clissold FJ et al. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA 2008; 105: 2498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solon-Biet SM, McMahon AC, Ballard JW et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014; 19: 418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solon-Biet SM, Mitchell SJ, Coogan SC et al. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep 2015; 11: 1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Couteur DG, Tay SS, Solon-Biet S et al. The influence of macronutrients on splanchnic and hepatic lymphocytes in aging mice. J Gerontol A Biol Sci Med Sci 2014; 70: 1499–507. [DOI] [PubMed] [Google Scholar]
- 32.Simpson SJ, Raubenheimer D. The Nature of Nutrition. A Unifying Framework Form Animal Adaption to Human Obesity. Princeton: Princeton University Press, 2012. [Google Scholar]
- 33.Westerterp-Plantenga MS, Wijckmans-Duysens NA, ten Hoor F. Food intake in the daily environment after energy-reduced lunch, related to habitual meal frequency. Appetite 1994; 22: 173–82. [DOI] [PubMed] [Google Scholar]
- 34.Giesbertz P, Daniel H. Branched-chain amino acids as biomarkers in diabetes. Curr Opin Clin Nutr Metab Care 2016; 19: 48–54. [DOI] [PubMed] [Google Scholar]
- 35.D'Antona G, Ragni M, Cardile A et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 2010; 12: 362–72. [DOI] [PubMed] [Google Scholar]
- 36.Mansfeld J, Urban N, Priebe S et al. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat Commun 2015; 6: 10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKeown T. The Role of Medicine: Dream, Mirage or Nemesis. Oxford: Blackwell, 1979. [Google Scholar]
- 38.Bunker JP. The role of medical care in contributing to health improvements within societies. Int J Epidemiol 2001; 30: 1260–3. [DOI] [PubMed] [Google Scholar]
- 39.Murray CJ, Atkinson C, Bhalla K et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 2013; 310: 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willcox DC, Willcox BJ, Todoriki H, Suzuki M. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr 2009; 28 Suppl: 500S–16S. [DOI] [PubMed] [Google Scholar]
- 41.Willcox BJ, Willcox DC. Caloric restriction, caloric restriction mimetics, and healthy aging in Okinawa: controversies and clinical implications. Curr Opin Clin Nutr Metab Care 2014; 17: 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMichael AJ, Powles JW, Butler CD, Uauy R. Food, livestock production, energy, climate change, and health. Lancet 2007; 370: 1253–63. [DOI] [PubMed] [Google Scholar]
- 43.Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature 2014; 515: 518–22. [DOI] [PubMed] [Google Scholar]
- 44.Nestle M. Food Politics: How the Food Industry Influences Nutrition and Health. Berkeley: University of California Press, 2013. [Google Scholar]


