Abstract
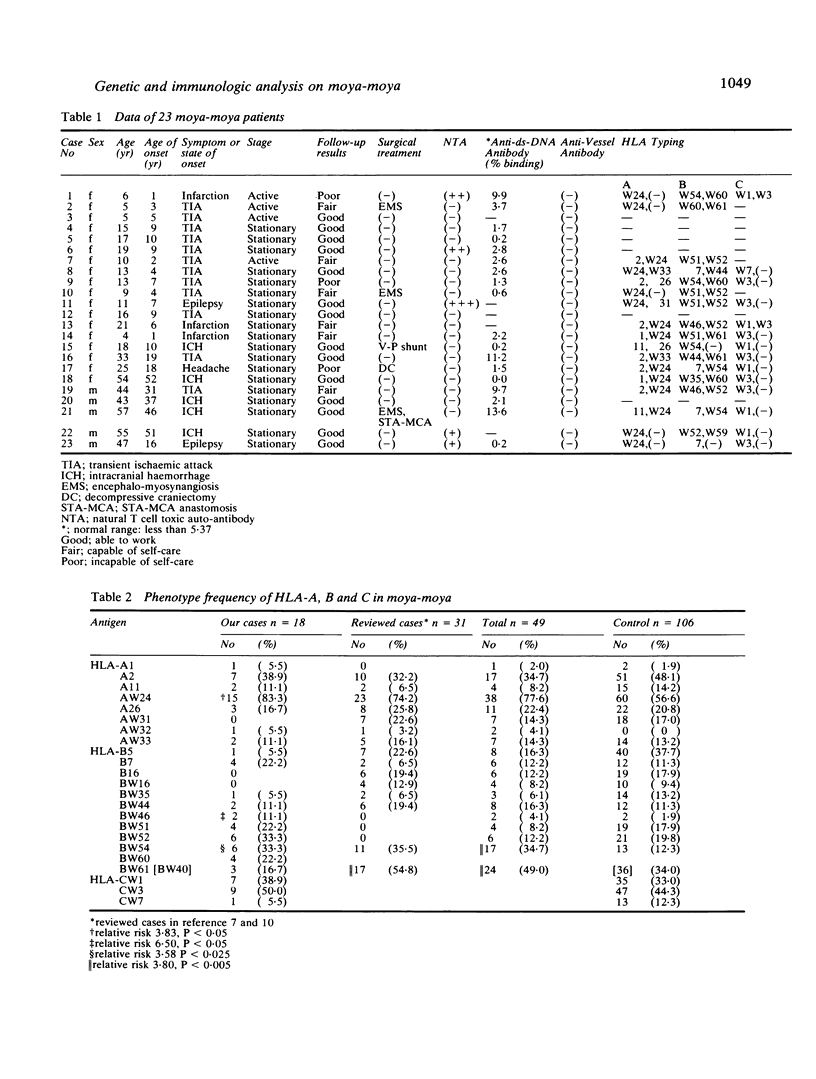
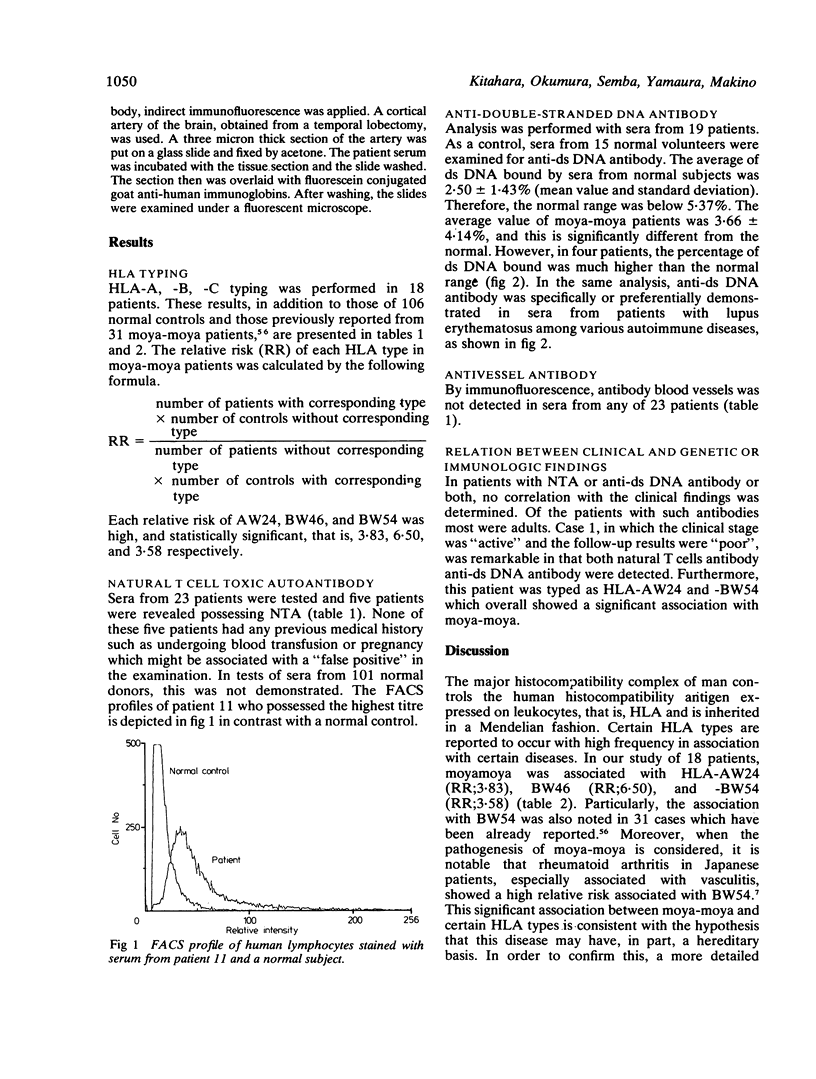
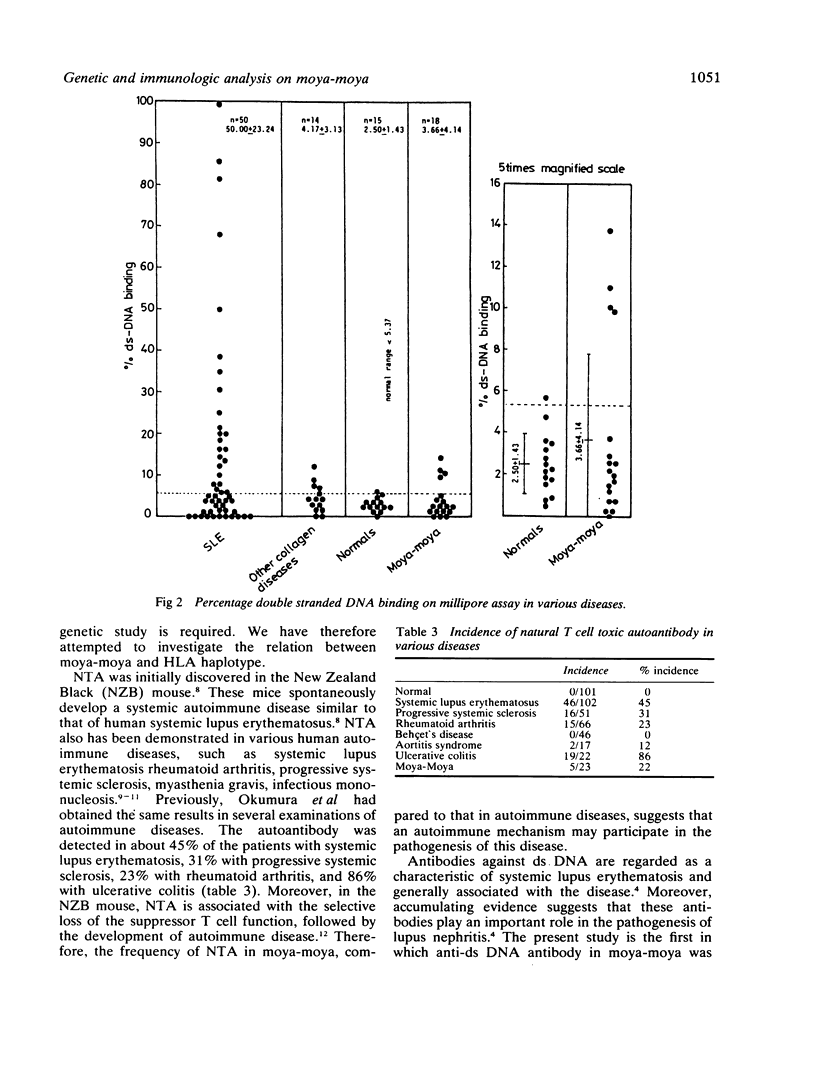
The genetic and immunologic abnormalities associated with the pathogenesis of moya-moya were assessed in 23, 13 children and 10 adults with angiographically diagnosed moya-moya. In HLA-A, -B, -C stereotyping, an association was found of AW24, BW46, and BW54 with relative risks of 3.83, 6.50, and 3.58 respectively. Natural T cell toxic autoantibody was detected by FACS analysis in sera from five out of 23 patients. Millipore filter assay for autoantibody against double-stranded DNA revealed higher than normal binding in sera from four out of 18 patients. Anti-vessel antibody which might be responsible for vascular change associated with moya-moya was not detected in any of the 23 patients studied. Significant association of the disease with certain HLA types, in addition to the presence of natural T cell toxic autoantibody and anti-double-stranded DNA antibody in patients' sera, supports the theory that genetic and immunologic disturbances may underly the pathogenesis of moya-moya.
Full text
PDF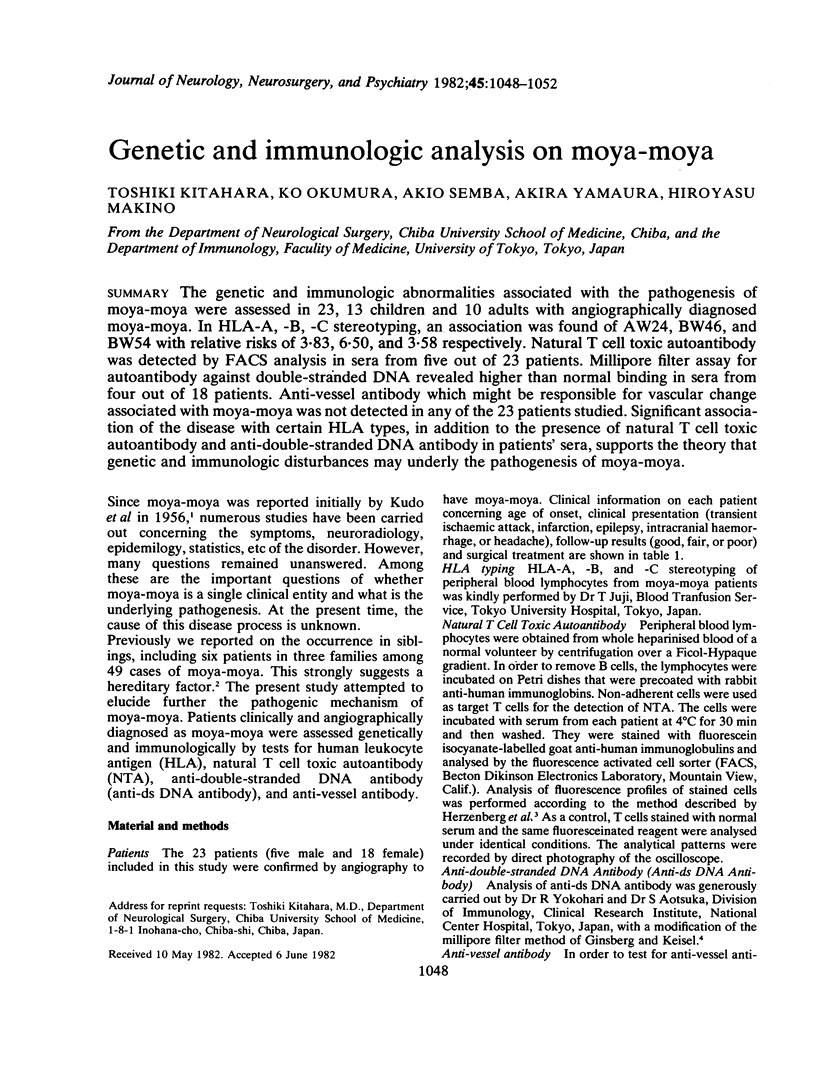

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aotsuka S., Okawa M., Ikebe K., Yokohari R. Measurement of anti-double-stranded DNA antibodies in major immunoglobulin classes. J Immunol Methods. 1979;28(1-2):149–162. doi: 10.1016/0022-1759(79)90337-5. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Sweet R. G., Herzenberg L. A. Fluorescence-activated cell sorting. Sci Am. 1976 Mar;234(3):108–117. doi: 10.1038/scientificamerican0376-108. [DOI] [PubMed] [Google Scholar]
- Kitahara T., Ariga N., Yamaura A., Makino H., Maki Y. Familial occurrence of moya-moya disease: report of three Japanese families. J Neurol Neurosurg Psychiatry. 1979 Mar;42(3):208–214. doi: 10.1136/jnnp.42.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp W., Pateisky K. Lymphocytotoxins in myasthenia gravis. Z Immunitatsforsch Exp Klin Immunol. 1972 Dec;144(4):329–334. [PubMed] [Google Scholar]
- Ooi B. S., Orlina A. R., Pesce A. J., Mendoza N., Masaitis L., Pollak V. E. Lymphocytotoxic antibodies in patients with systemic lupus erythematosus. Clin Exp Immunol. 1974 Jun;17(2):237–243. [PMC free article] [PubMed] [Google Scholar]
- Shirai T., Hayakawa K., Okumura K., Tada T. Differential cytotoxic effect of natural thymocytotoxic autoantibody of NZB mice on functional subsets of T cells. J Immunol. 1978 Jun;120(6):1924–1929. [PubMed] [Google Scholar]
- Shirai T., Mellors R. C. Natural thymocytotoxic autoantibody and reactive antigen in New Zealand black and other mice. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1412–1415. doi: 10.1073/pnas.68.7.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. B. Antibodies to membrane antigen(s) common to thymocytes and a subpopulation of lymphocytes in infectious-mononucleosis sera. Lancet. 1972 Feb 19;1(7747):399–403. doi: 10.1016/s0140-6736(72)90854-9. [DOI] [PubMed] [Google Scholar]


