Abstract
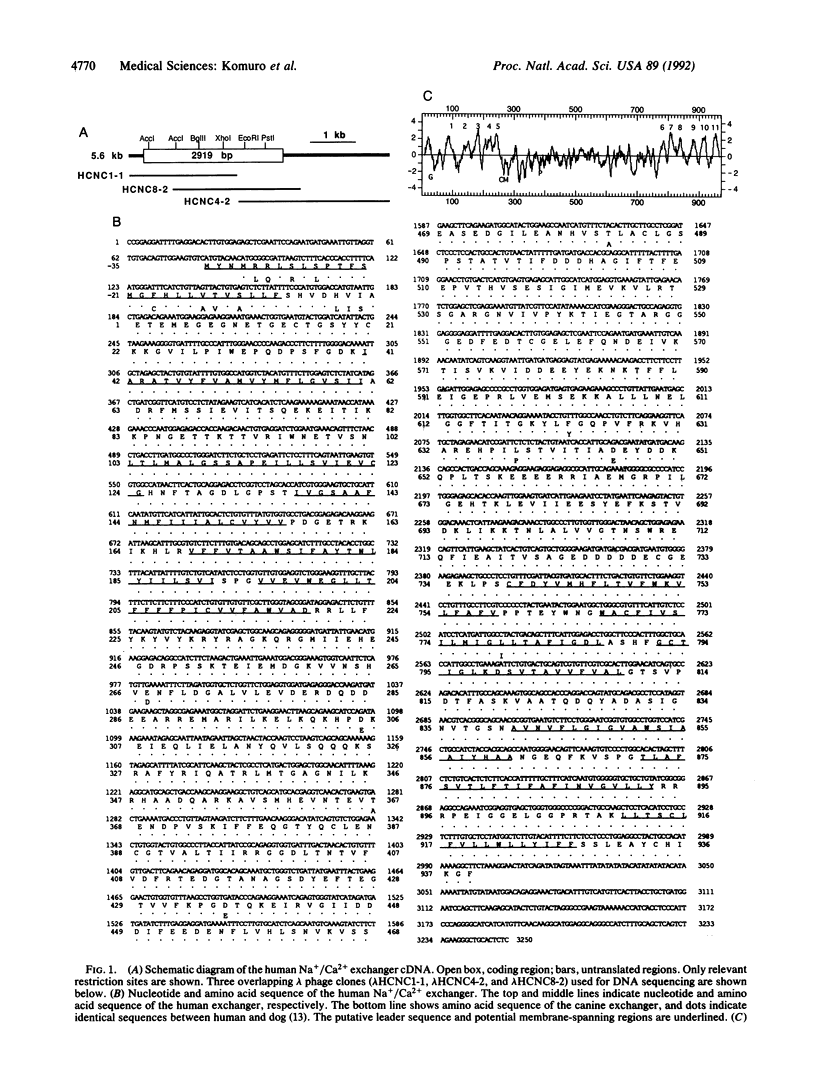
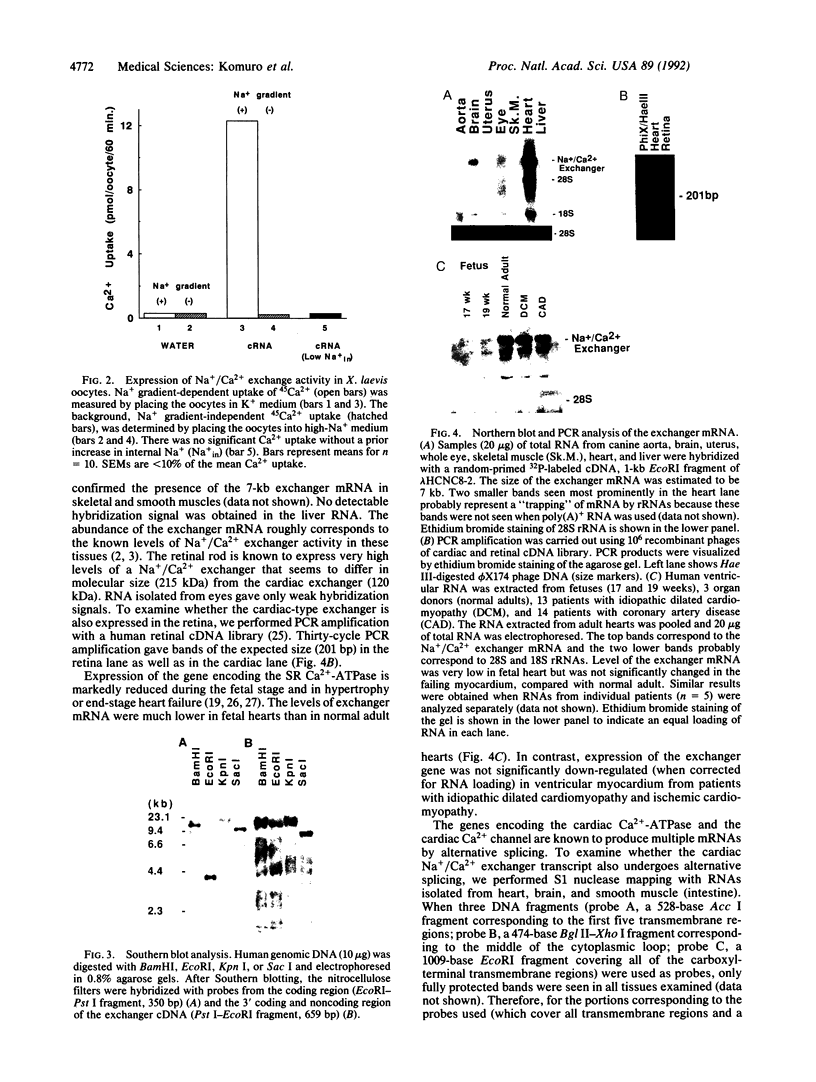
The Na+/Ca2+ exchanger plays important roles in Ca2+ handling in many excitable cells. In particular, the Na+/Ca2+ exchanger is expressed at high levels in the cardiac sarcolemma and is the dominant mechanism of Ca2+ extrusion from the cells. In addition, the exchanger has been suggested to play key roles in digitalis action and in postischemic reperfusion injury of cardiac myocytes. We report here the isolation and characterization of the cDNA encoding the human cardiac Na+/Ca2+ exchanger. Twelve overlapping clones corresponding to 5.6 kilobases of the exchanger cDNA sequence were isolated from 5 x 10(5) phage plaques screened. The sequence predicted a 973-amino acid polypeptide with a putative leader peptide, 11 potential membrane-spanning regions, and one large putative cytoplasmic loop between the fifth and sixth transmembrane helices. When RNA was synthesized in vitro from the cloned cDNA and injected into Xenopus oocytes, it induced expression of Na+/Ca2+ exchange activity at high levels, confirming that this clone encodes the functional Na+/Ca2+ exchanger. Southern blot analysis indicated that the cardiac exchanger gene exists as a single copy in the human genome, although existence of other related genes cannot be ruled out. Northern blot and S1 mapping analyses revealed that the cardiac type exchanger mRNA is expressed most abundantly in the heart and next in the brain. The cardiac-type exchanger mRNA was also expressed in the retina and in skeletal and smooth muscles at very low levels. The levels of mRNA encoding the exchanger were significantly lower in fetal hearts than in adult hearts but were unchanged in the myocardium from patients with end-stage heart failure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achilles A., Friedel U., Haase W., Reiländer H., Cook N. J. Biochemical and molecular characterization of the sodium-calcium exchanger from bovine rod photoreceptors. Ann N Y Acad Sci. 1991;639:234–244. doi: 10.1111/j.1749-6632.1991.tb17310.x. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Durkin J. T., Ahrens D. C., Pan Y. C., Reeves J. P. Purification and amino-terminal sequence of the bovine cardiac sodium-calcium exchanger: evidence for the presence of a signal sequence. Arch Biochem Biophys. 1991 Nov 1;290(2):369–375. doi: 10.1016/0003-9861(91)90553-u. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Na-Ca exchange: stoichiometry and electrogenicity. Am J Physiol. 1985 Mar;248(3 Pt 1):C189–C202. doi: 10.1152/ajpcell.1985.248.3.C189. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Noma A., Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature. 1986 Feb 13;319(6054):596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Komuro I., Kurabayashi M., Shibazaki Y., Takaku F., Yazaki Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989 Apr;83(4):1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I., Kurabayashi M., Takaku F., Yazaki Y. Expression of cellular oncogenes in the myocardium during the developmental stage and pressure-overloaded hypertrophy of the rat heart. Circ Res. 1988 Jun;62(6):1075–1079. doi: 10.1161/01.res.62.6.1075. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Li Z., Nicoll D. A., Collins A., Hilgemann D. W., Filoteo A. G., Penniston J. T., Weiss J. N., Tomich J. M., Philipson K. D. Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. J Biol Chem. 1991 Jan 15;266(2):1014–1020. [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Duc P., Boheler K. R., Fraysse J. B., Wisnewsky C., Allen P. D., Komajda M., Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990 Jan;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T., Jarmakani J. M. Developmental changes in myocardial mechanical function and subcellular organelles. Am J Physiol. 1984 Apr;246(4 Pt 2):H615–H625. doi: 10.1152/ajpheart.1984.246.4.H615. [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983 Oct;34(3):807–814. doi: 10.1016/0092-8674(83)90537-8. [DOI] [PubMed] [Google Scholar]
- Nicoll D. A., Applebury M. L. Purification of the bovine rod outer segment Na+/Ca2+ exchanger. J Biol Chem. 1989 Sep 25;264(27):16207–16213. [PubMed] [Google Scholar]
- Nicoll D. A., Longoni S., Philipson K. D. Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. Science. 1990 Oct 26;250(4980):562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Nicoll D. A., Philipson K. D. Molecular studies of the cardiac sarcolemmal sodium-calcium exchanger. Ann N Y Acad Sci. 1991;639:181–188. doi: 10.1111/j.1749-6632.1991.tb17305.x. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P., Hale C. C. The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem. 1984 Jun 25;259(12):7733–7739. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri R., Haberland M. E., Fong D., Philipson K. D. Identification of the cardiac sarcolemmal Na(+)-Ca2+ exchanger using monoclonal antibodies. J Membr Biol. 1990 Dec;118(3):279–283. doi: 10.1007/BF01868612. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]