Abstract
Trillions of beneficial bacteria inhabit the intestinal tract of healthy mammals from birth. Accordingly, mammalian hosts have evolved a series of complementary and redundant pathways to limit pathologic immune responses against these bacteria, while simultaneously protecting against enteric pathogen invasion. These pathways can be generically responsive to the presence of any commensal bacteria and innate in nature, as for IL-22-related pathways. Alternatively, specific bacterial antigens can drive a distinct set of adaptive immune cell responses, including IgA affinity maturation and secretion, and a recently described pathway of intestinal selection whereby MHCII+ ILC3 deletes commensal bacteria-reactive CD4 T cells. These pathways can either promote or inhibit colonization by specific subsets of commensal bacteria, and cooperatively maintain intestinal homeostasis. In this review, we will highlight recent developments in understanding how these diverse pathways complement each other to cooperatively shape the symbiotic relationship between commensal bacteria and mammalian hosts.
Keywords: IL-22, IgA, microbiota, innate lymphoid cells, bacterial infection
Animals have evolved in the presence of a remarkably dense community of commensal prokaryotes that inhabits the gastrointestinal (GI) tract from birth. In humans, these commensal organisms, dominated by bacteria, likely outnumber the host at the cellular level (1). Intestinal commensal bacteria contribute to a wide variety of physiological and disease processes. Accordingly, the relationship between mammalian hosts and commensal bacteria must be tightly regulated and highly sophisticated. On one hand, commensal bacteria provide important developmental cues, contribute to nutrient harvest from the host diet and protect the host against infection (2). In contrast, multiple chronic infectious, inflammatory and metabolic diseases are associated with significant changes in the composition or anatomical localization of commensal bacteria; these changes often contribute to disease progression through metabolic effects or activation of the mammalian immune response (3). While our understanding of host-commensal bacteria relationships is still rapidly evolving, it is abundantly clear that further interrogation of these pathways will provide a greater understanding of human health and guide the development of novel therapeutic approaches in chronic human diseases (4).
Recent technological advances have led to a revolution in our understanding of the relationship between commensal bacteria populations and the human host. In striving to understand the factors that directly underlie healthy versus dysregulated host-commensal bacteria interactions, it has become clear that multiple pathways are acting cooperatively. Commensal bacteria themselves have adapted to exploit resources from the host diet and colonize specific niches in the intestinal tract and associated lymphoid tissues (5, 6). In parallel, mammals have co-evolved a variety of mechanisms to prevent inflammation targeted at innocuous commensal bacteria while simultaneously protecting against tissue invasion by pathogens. Notably, these include both innate pathways (typified by IL-22 responses and barrier maintenance by mucus secretion and epithelial tight junctions), and adaptive pathways that require antigen presentation (including IgA responses, regulatory T cell responses and innate lymphoid cell-mediated selection of commensal bacteria-specific CD4 T cells). Here, we will discuss recent advances in understanding these phenomena, with a particular highlight on the role of host antigen presentation pathways in maintaining intestinal homeostasis.
Distinct antigen-dependent and antigen-independent host pathways regulate symbiosis with commensal bacteria
The mucosal immune system provides a crucial barrier against infections. However, immune responses targeting potential pathogens must exhibit prudent specificity and regulation to prevent chronic inflammation and tissue damage. An estimated 40 trillion commensal bacteria inhabit the colon, all of which have the potential to induce inflammation. Hence, diverse and multi-layered mechanisms have evolved in the host to prevent pathologic, commensal-bacteria-dependent inflammation, while still maintaining mucosal barrier function. Recent work has greatly expanded our understanding in this area.
It is striking that the host has developed processes to both support and inhibit the survival of commensal bacteria. In particular, several host pathways such as IL-22, mucous production and IgA are highly regulated by the presence of commensal bacteria, exhibit a range of bacterial specificities, and function to limit pathogenic tissue invasion by inhibiting bacterial survival and bacterial access to the epithelium. In contrast, complementary host pathways have evolved to simultaneously support bacterial colonization and limit chronic inflammation by restraining immune responses against commensal bacteria-derived antigens. These pathways are typified by commensal bacteria-dependent regulatory T cell (Treg) development (extensively reviewed elsewhere) (7), and by a novel selection pathway involving group 3 innate lymphoid cells (ILC3s) that directly limits T cell reactivity to commensal bacteria-derived antigens through antigen presentation and major histocompatibility complex class II (MHCII). These seemingly contradictory host activities (either supporting or inhibiting commensal bacteria populations by differentially regulating specific immune cell subsets) cooperatively maintain a healthy homeostatic relationship between mammalian hosts and commensal bacteria.
Moreover, the host must adapt to dynamic changes in commensal bacteria populations over a lifetime with an appropriate balance of pro- and anti-bacterial activities. This balance is reflected in host pathways that process and present commensal bacteria-derived antigens. The classic example of such a process is the development of specific IgA antibodies that sequester bacteria from the host epithelium (8). Until recently, a pro-bacterial, antigen-dependent counterpart to the IgA process was not known. The recent discovery of tolerogenic antigen presentation by ILC3s has brought balance to our understanding of host responses to commensal bacterial antigens. Here, we will review the pro- and anti-bacterial host responses, both antigen-independent and antigen-dependent, that collaborate to maintain a healthy relationship between mammalian hosts and commensal bacteria.
Antimicrobial peptides, mucus, and IL-22 are critical innate mediators of homeostatic host-commensal bacteria interactions
IL-22 is an important host-derived factor that is elicited by commensal bacteria and subsequently critically orchestrates host-commensal bacteria symbiosis. An explosion of interest in IL-22 biology in recent years has driven a greater understanding of its function. IL-22 plays a multifaceted role in regulating intestinal homeostasis, through limiting infections by enteric pathogens, regulating the composition of commensal bacteria populations, and influencing anatomical segregation of commensal bacteria from the mammalian immune system (9). In contrast to IgA, however, IL-22 is not known to interact with commensal bacteria directly, and does not respond in an antigen-specific manner. The IL-22 receptor is restricted to non-hematopoietic cells and highly enriched on hepatocytes, keratinocytes, airway epithelium and intestinal epithelial cells (9). Here, we will highlight the substantial recent advances in our understanding of: the impact of IL-22 on enteric bacteria; the mechanisms by which enteric bacteria promote IL-22 responses; and the influence of IL-22 on regulating enteric bacteria in the context of health and disease.
IL-22 is recognized as an essential contributor to epithelial tissue homeostasis and protection against inflammatory disease. In the absence of IL-22, mice are susceptible to intestinal damage and inflammation, including mouse models of dextran sodium sulfate (DSS) exposure, naïve T cell transfer, and graft versus host disease (10–14). In many of these models, it was demonstrated that ILC3-derived IL-22 protects against inflammation by supporting the Lgr5+ intestinal stem cell population (12, 15). These data suggest that IL-22 generally supports epithelial barrier function by supporting the continual regeneration of the epithelium. In contrast, it was recently reported that IL-22 signaling could be pathogenic in other models of intestinal inflammation, such as following anti-CD40 monoclonal antibody (mAb) administration and Toxoplasma gondii infection (16, 17). In the anti-CD40 mAb model administration to immunodeficient mice, ILC3-derived IL-22 is thought to recruit and activate inflammatory monocytes and neutrophils, contributing to tissue damage and inflammation (17). Crucially, the anti-CD40 mAb mouse model of intestinal inflammation lacks all T and B cells, and it’s been suggested that Treg-dependent modulation of inflammation would normally blunt the pathologic inflammation (18). Interestingly, evidence has also arisen to support a role for IL-22 in limiting sensitization to food allergens. In a germ-free mouse model, it was reported that colonization by a handful of Clostridia species, but not a Bacteroides species, could abrogate systemic responses to oral peanut allergens; this protective effect was found to depend on IL-22 induction, which was observed only in the Clostridia-colonized mice (19). Together, these findings argue that IL-22 signaling is crucial for preventing pathologic inflammation, but can become dysregulated and promote inflammation when the adaptive immune system is impaired. Evidence also suggests that the local cytokine milieu may influence the protective versus pathologic functions of IL-22, as co-expression of IL-17 can synergistically promote tissue inflammation (20). Therefore, it will be important to consider IL-22 in the context of immune-deficient patients, which is a frequent presentation of very early onset IBD, or in the context of adult IBD patients where IL-17 is abundantly co-expressed (21–23).
IL-22 may modulate inflammation through several distinct mechanisms including regulation of tissue repair, induction of anti-microbial peptides, and modulation of the composition or anatomical containment of commensal bacteria. For example, IL-22 has a well-documented role in limiting enteric bacterial infection and translocation. Further, depletion of ILCs, a primary source of IL-22, leads to systemic dissemination of otherwise innocuous commensal bacteria, and this phenotype can be rescued by administration of exogenous IL-22 (24). Genetic deletion of IL-22 can lead to a state of dysbiosis, with increased susceptibility to DSS (25). We have recently shown that IL-22 is required for colonization by lymphoid tissue-resident commensal bacteria (LRCs) (6). Given the fact that LRC colonization protects mice from DSS-induced pathology, defective LRC colonization and luminal bacteria dysbiosis may partially contribute to increased DSS susceptibility of Il22−/−mice. IL-22 also plays a crucial and well-documented role in innate immune responses to Citrobacter rodentium, a murine-specific model of attaching and effacing enteric bacterial pathogens such as E. coli O157:H7. IL-22 knockout mice are highly susceptible to infection by C. rodentium (26). This susceptibility pattern was evident even on a Rag2−/− background, suggesting an innate source of functional IL-22 in this infection model. Furthermore, it was shown that IL-22 induces production of anti-microbial peptides (AMPs) in colonic tissues, and C. rodentium susceptibility in IL-22-deficient mice could be partially rescued by administration of the AMP RegIIIγ (26). Subsequently, the innate source of protective IL-22 was found to be the ILC3 cell population in this model (27). Further analysis shows that the LTi-like ILC3 subset, in particular, is the relevant IL-22 source (17, 28).
The critical contribution of ILC3-derived IL-22 in innate immunity against an enteric bacterial pathogen that represents such an important public health burden has motivated careful analyses of both the regulation of IL-22 in infection, and the mechanisms by which IL-22 limits infection. It is clear that IL-23 is an important effector upstream of the IL-22 response in this infection model (26). A recent report extensively characterized the source of IL-23 in C. rodentium infection, and found that CX3CR1+ macrophages are the dominant source in this setting (29). In contrast, a prior study found that CD103+ dendritic cells produce IL-23 that elicits AMP production in an IL-22 dependent manner upon stimulation with bacterial flagellin (30). In combination, these studies argue that multiple enteric phagocytes may produce IL-23 in response to diverse signals with different tropisms in the enteric lamina propria, but IL-22 appears to be a critical integrator of these signals. These findings are in agreement with previous reports that CX3CR1+ macrophages are required for the C. rodentium-elicited IL-22 response (31). Further supporting the paradigm that IL-22 is primarily tissue-protective, the CX3CR1+-dependent ILC3-IL-22 response is found to ameliorate intestinal tissue damage induced by C. rodentium infection (32). Notably, ILC3s themselves also appear to exhibit positive feedback by further stimulating ILC3 production of IL-22 via lymphotoxin receptor signaling (33, 34).
Beyond the prototypical attaching and effacing enteric pathogen, C. rodentium, enteric IL-22 is increasingly being appreciated as a modulator of other infection models as well. Toxoplasma gondii infection at high dose causes acute inflammation of the ileum, or ileitis, in an IL-22-dependent manner (16). Recent work showed that IL-22 in this setting directly elicits IL-18 in the ileum. Although IL-18 was required for effective clearance of C. rodentium, IL-22-dependent IL-18 expression was found to contribute to inflammatory pathology during T. gondii infection (35). Interestingly, IL-18 was also found to elicit further ILC3 production of IL-22 in a positive-feedback loop in the small intestine in T. gondii infection. Surprisingly, aryl hydrocarbon receptor (Ahr)-deficient mice, which exhibit impaired ILC3 development, were recently found to develop increased intestinal pathology relative to wild-type mice during T. gondii infection (36). This increased pathology correlated with T cell hyperactivity. These seemingly contradictory data might be explained by an IL-22-independent role for ILC3 in suppressing T cell responses (37). IL-22 also plays an important role in defense against Clostridium difficile. It was recently shown in mice that C. difficile infection allows the translocation of commensal bacteria and opportunistic pathogens to the liver and the lung, and IL-22 aids in the clearance of these disseminated bacteria through a complement-dependent mechanism (38). Finally, it was recently shown that IL-22 levels correlate with protection from Staphylococcus aureus pneumonia. Remarkably, the presence of SFB was shown to correlate with S. aureus protection, since SFB stimulates IL-22 production (39). Together, these data suggest that enteric IL-22, elicited by the presence of specific commensal bacteria, can have important impact on systemic host immunity.
While experiments with the C. rodentium infection model or bacterial flagellin demonstrated that IL-23 acutely activates IL-22 expression in ILC3s, several other signals may contribute to IL-22 production. Experiments with germ-free mice compared to conventional SPF mice showed that commensal bacteria are required for the development of NKp46+ ILC3 cells, and that even IL-23 stimulation ex vivo is not sufficient to promote ILC3-derived IL-22 in the absence of the microbiota (40). Re-colonization of GF mice with commensal bacteria led to the development of IL-22+ NKp46+ ILC3 cells within 1–2 weeks. Based on comparison of fetal and weanling expression of IL-22 in the gut, it’s also been suggested that microbiota-elicited IL-25 from the epithelium actually suppresses IL-22+ ILC3 cells after birth (41). Beyond IL-23, an additional signal may be required for peak IL-22 expression; one study recently identified the chemokine CXCL16, constitutively expressed by CX3CR1+ macrophages, as a candidate for this second signal, since ILC3s require CXCR6 (the CXCL16 receptor) for proper development of IL-22 responses (42). Stimulation with IL-1β also contributes to IL-22 secretion (43). This result was recently confirmed with an ILC3-like cell line in vitro, where IL-2 and IL-23 were also required for maximal IL-22 production (44). Recent evidence also suggests that IL-1α contributes to IL-22 production elicited by murine rotavirus (45). Interestingly, while IL-22 acts strongly on epithelial cells, evidence of epithelial cell feedback on the ILC3-IL-22 pathway was recently described. In particular, the NF-κB inhibitor IKKβ in intestinal epithelial cells (IECs) is required for induction of ILC3-derived IL-22 upon infection. In its absence, overproduction of TSLP by IECs suppresses ILC3 production of IL-22 and renders mice more susceptible to infection- or chemical-induced intestinal tissue damage (46). Within ILC3s themselves, the developmental factor Id2 was also recently shown to be required for continued ILC3 homeostasis and IL-22 responses to C. rodentium (47). These important advances in understanding IL-22 responses will be crucial for informing the development of therapeutic that modulate the IL-22 pathway for use in infection and inflammatory disease. Much exciting work remains to be done to understand the key points of regulation and the feedback in this pathway.
The role of ILC3-IL-22 pathway in directly stimulating AMP production in the epithelium is well documented, but exciting recent developments have also highlighted distinct mechanisms by which IL-22 may modulate the enteric commensal bacteria population. For example, a recently described subset of commensal bacteria, termed lymphoid-resident commensals (LRCs), is uniquely able to colonize the enteric lymphoid tissues of the healthy humans, primates, and mice (48). IL-22 plays a crucial role in regulating LRC colonization of the host. When IL-22 is acutely depleted from wild-type mice, LRCs can translocate systemically (24). When IL-22 is genetically deleted, however, LRCs cannot colonize mice due to the outgrowth of competing bacteria (6). Furthermore, in Ahr-deficient mice, it was discovered that a lack of ILC3 and IL-22 led to increased SFB colonization, which in turn led to increased inflammatory Th17 responses (49). More recently, it was determined that SFB elicits the serum amyloid A proteins 1 and 2, which may limit bacterial growth in an ILC3 and IL-22 dependent manner (50). This phenomenon is highly restricted to the ileum, and may be a general response to bacterial adherence to IECs (51). These data suggest a complex regulatory connection between epithelial-adherent commensal bacteria, ILC3 and Th17 cell responses, and the subsequent generation of AMPs and other epithelial factors that may shape the commensal bacteria community. The importance of this regulatory network for host health is emphasized by the finding that SFB overgrowth in the absence of ILC3s affects host susceptibility to diet-induced obesity (52). Conversely, it’s also been demonstrated that diet-induced obesity inhibits IL-23-elicited IL-22 production and renders mice susceptible to C. rodentium, highlighting the complexity of the linkage between obesity, IL-22, and mucosal immunity (53). Adding another interesting layer to this regulation, several groups recently described a connection between the ILC3-IL-22 pathway and IEC glycosylation patterns, with important impact for the host-commensal bacteria relationship. IL-22 receptor-deficient mice exhibited severe microbial dysbiosis and were highly susceptible to C. rodentium infection, and this phenotype could be rescued by the administration of fucosylated oligosaccharides (54). It was determined that IEC fucosylation is driven by ILC3-derived IL-22 and is also required for resistance to S. typhimurium (55). Finally, it was demonstrated that Toll-like receptor (TLR) ligands induce dendritic cells to secrete IL-23, which stimulates the ILC3 production of IL-22 and underlies the upregulation of the fucosyltransferase 2 (Fut2) gene in IECs (56). These studies all agree in showing a remarkable impact of IEC-intrinsic Fut2 activity in dictating the population of commensal bacteria that thrive in the lumen, presumably by selecting for commensal bacteria that are capable of metabolizing fucosylated oligosaccharides. Together, these data show that enteric phagocytes produce IL-23 to induce IL-22 production by ILC3s, and this single regulatory event has incredibly wide-ranging impacts on host-commensal bacteria homeostasis by activating IEC production of both negative regulators and positive regulators of bacterial growth. Particular subsets of commensal bacteria can also regulate this IL-22 response, presumably by activating IEC-intrinsic signals upon adhering to the epithelium. This remarkable and intimate feedback between the mammalian host and the enteric commensal bacteria is in keeping with the view that both parties of this relationship have co-evolved regulatory mechanisms to thrive together.
Among all the diverse effector functions of IL-22 at the epithelium, the dominant role of fucosylation suggests that the chemical environment at the barrier between the host epithelium and the commensal bacteria plays a key role in maintaining host-commensal bacteria homeostasis. It is not surprising, then, that the host-derived mucus layer that dominates this environment is a critical player in this homeostasis. The epithelial mucus layer develops and matures in response to the presence of the microbiota over a period of several weeks (57). Continued maintenance of a healthy mucus layer appears to be sensitive to the specific makeup of the commensal bacteria population, as the mucus thins out upon dietary perturbation of a healthy microbiota (58). Interestingly, it was recently shown that MUC2, the dominant mucus protein, actively drives intestinal dendritic cells toward tolerogenic responses (59), arguing that mucus functions extend beyond providing a simple physical barrier. In fact, mucus can also play an active role in modulating host immune responses. Developments in understanding the role of mucus in host-commensal bacteria homeostasis have been extensively reviewed elsewhere (60).
IgA responds to enteric bacteria to sequester them from the immune system
Human adults typically secrete multiple grams of IgA into the gastrointestinal tract each day, an amount that exceeds all other antibodies combined (61). Notably, IgA is present at functional levels in human breast milk, and appears to contribute protective immune functions in neonatal mammals (62). While the potential importance of IgA in human immunity has been recognized for several decades, progress in understanding the biological function of IgA has been complicated by the fact that its regulation is highly complex, as well as the observation that selective IgA deficiency in humans is often asymptomatic due to complementary upregulation of other immunoglobulins (63). Despite these challenges, considerable advances have recently been made in understanding the development and functional consequences of IgA responses.
Several lines of evidence, primarily from studies in mice, argue for a crucial role of IgA in modulating host-commensal bacteria relationships, with important implications for host health. In neonatal mice, for example, a lack of enteric IgA – achieved by genetic deletion of the pIgR gene, which is required for IgA secretion – led to increased levels of the opportunistic bacterium, Ochrobactrum anthropi, translocating to the mesenteric lymph node (mLN), and increased tissue damage upon dextran sodium sulfate (DSS)-elicited inflammation (64). Strikingly, this phenotype correlated with the genotype of the dam rather than the neonate, arguing that maternal IgA in the breast milk is required for protection from opportunistic bacteria and maintenance of tissue repair in suckling mice. In further support of a host-protective role for enteric IgA secretion, a recent study characterized a particular IgA-degrading commensal bacteria community, which was stably transmissible to other mice, and antibiotic-sensitive (65). Remarkably, adoption of this IgA-degrading microbiota led to stable low levels of enteric IgA and a dramatically increased susceptibility to inflammatory tissue damage. This low-IgA, inflammation-sensitive phenotype correlated with increased colonization by bacteria of the Sutterella genus, suggesting complex feedback between IgA levels, host-commensal bacteria homeostasis, and resistance to inflammation (65). Moreover, another recent report investigated the role of IgA-coated commensal bacteria in driving intestinal inflammation. In this study, fecal bacteria samples from IBD patients were sorted based on levels of IgA coating; upon introduction of either highly-IgA-coated or uncoated bacteria into germ-free (GF) mice, it was found that highly-IgA-coated bacteria colonization led to increased host susceptibility to inflammatory tissue damage elicited by DSS (66). Importantly, FISH staining suggested that highly-IgA-coated bacteria were able to colonize the mucus layers, closely apposed to the intestinal epithelium (66). In contrast to the findings outlined above, which suggest that IgA predominantly functions to sequester bacteria from host tissues, it has also recently been suggested that IgA may facilitate the colonization of intestinal lymphoid tissues by specific subsets of bacteria (48). Together, these important findings imply that IgA may function as a diffusible extension of the mucosal barrier: by targeting, with varying degrees of specificity and efficacy, the commensal bacteria that are capable of living in close contact with more inflammatory elements of the mucosal immune system, IgA prevents uncontrolled colonization or dissemination, as well as subsequent tissue inflammation.
Reflecting the immense diversity of bacteria to which enteric IgA must respond over a lifetime, the regulation of IgA production and affinity maturation is multi-faceted. ‘Natural IgA’ is constitutively secreted even in GF mice, but the re-introduction of commensal bacteria in this setting leads to a rapid upregulation of IgA secretion (67). In agreement with this, the full IgA complement from the mature human ileum is dominated by somatically-mutated, antigen-specific IgA+ plasmablasts, displaying reactivity to enteropathogenic and commensal microbes as well as self antigens (68). The microbiota of an individual is expected to drift considerably over time, responding to changes in diet, infections, and antibiotic treatments, among other factors (69). A recent analysis has confirmed that commensal bacteria-specific IgA+ ‘memory’ plasma cells persist well beyond the withdrawal of the antigenic commensal bacterium, and yet new B cell clones can continuously develop against newly presented bacteria, for example in the case of enteric infection (70). Within the GF mouse model, the IgA response depends on the uptake of live commensal bacteria by dendritic cells (DCs), which then migrate to the mLN and induce IgA production by B cells (71). Interestingly, the induction of specific IgA depends on iNOS activity, which is typically considered a pro-inflammatory factor. Evidence points to a role for both DC- and B cell-intrinsic iNOS activation in IgA responses (72, 73).
While it’s clear that T cells are not strictly required for IgA responses, a T cell-dependent pathway does support IgA development (8). A host of recent work has begun to dissect this pathway. Interestingly, Treg cell-intrinsic MyD88 contributes to IgA responses and prevents translocation of commensal bacteria to the liver and the lung (74). Moreover, abrogation of this pathway skews the pattern of reactivity against commensals; whereas IgA typically coats mucosal- and epithelial-associated commensals preferentially, this bias is lost when MyD88 is deleted in Treg cells (75). These mice also showed increased susceptibility to DSS. In a distinct mode of T cell-dependent IgA regulation, Th17 and/or T follicular helper cells also support high-affinity IgA development through the production of IL-21 (76, 77). A new report has also highlighted a requirement for eosinophils in IgA production, which may contribute to IgA development through both T cell-dependent and –independent pathways (78). Interestingly, some commensal bacteria seem more prone to elicit T cell-dependent IgA responses, while others are biased toward T cell-independent IgA development (79). Moreover, T cell presence does not affect the expression of CD11b in IgA+ plasma cells, which was recently reported as a marker of vigorous proliferation and copious IgA secretion. The complexity and the overlapping layers of IgA regulation emphasize the broad role that IgA must play in maintaining host-commensal bacteria homeostasis, while also presenting an ongoing challenge for the field (80).
Aside from simply triggering host-mediated IgA responses, commensal bacteria also appear to play an active role in these responses, with important impacts on host health. Segmented filamentous bacteria (SFB), which are well-known inducers of Th17 cells in the intestine, also appear to induce development of isolated lymphoid follicles in mice lacking Peyer’s patches (PP), which serve as a site of IgA induction in these mice (81). In contrast, E. coli does not show the same capability, implying that some commensal bacteria may actually drive non-canonical modes of IgA induction. Furthermore, a potential bacteria-specific positive feedback loop was recently described (82). In this study, it was shown that a non-invasive Salmonella strain was highly coated by IgA, and subsequently was trafficked preferentially to PPs, where it induced even greater production of fecal IgA upon subsequent enteric antigen challenge (82). It was also demonstrated in this study that different mouse strains show different levels of polyreactive IgA secretion in the GF state, which further argues that stochastic IgA responses, potentially dependent on host genotype, may lead to divergent commensal communities and thus to divergent host phenotypes. In a similar vein, another group recently showed that proteobacteria-directed IgA responses are required for the development of a mature microbiota (dominated by Firmicutes and Bacteroidetes phyla) from the immature, pro-inflammatory, proteobacteria-enriched microbiome (83). In order to minimize the potential host genetic component of IgA responses in a human enteropathy, the Gordon group recently examined a cohort of twins discordant for Kwashiorkor (84). IgA-coated bacteria from the Kwashiorkor donors drove considerable enteropathy when transplanted into GF mice, but this pathology could be rescued by IgA-coated bacteria from the healthy donors (84). Given the recent suggestion of a stochastic IgA positive-feedback loop (65, 82), the data from Kwashiorkor-discordant twins suggests that disease development may also be stochastic, and reinforces the notion that IgA-dependent homeostatic processes can potentially modulate disease processes in humans. Remarkably, it was very recently shown that administration of bacteria-accessible oligosaccharides (derived from bovine milk) could also ameloriate the metabolic defects cause by IgA-coated bacteria from Kwashiorkor donors, without significantly shifting the population of the bacteria (85). This remarkable finding further argues that IgA-coating alone, even within a disease state, may not strictly mark host-detrimental bacteria. The complex feedback between host IgA responses and commensal bacteria suggests that increasingly complex models (beyond GF mice mono-colonized with a single bacterium) will be required to design new therapies for chronic diseases associated with commensal bacteria.
ILC3s in host-commensal bacteria homeostasis: a novel role for antigen presentation
ILC3 cells are an innate immune cell subset with key emerging roles in regulating intestinal health and disease (86). A subset of ILC3s, termed lymphoid tissue inducer (LTi) cells, were initially highlighted for their essential role in the development of secondary lymphoid tissues, including small intestine Peyer’s patches and peripheral lymph nodes (87). ILC3s thus play an indirect role in facilitating IgA responses directed from these tissues. ILC3s elicit these developmental effects by the production of TNF protein family members including Lymphotoxin-α and –β and RANKL, which spawn chemokine gradients contributing to the development of unique T- and B-cell zones in lymphoid tissue (88–90). ILC3s are also required for the formation of isolated lymphoid follicles (ILFs) in the intestine, in a process that depends on the presence of commensal bacteria (91). This observation suggests that the role of ILC3s in lymphoid tissue development continues through adulthood and may play a crucial – though indirect – role in host-commensal bacteria homeostasis. In further support of this concept, it’s recently been shown that ILC3 lymphotoxin responses contribute to both T-cell-dependent and T cell-independent IgA responses, discussed further in the prior section (92). Interestingly, within the context of C. rodentium infection, it was recently shown that IL-22 is regulated downstream of ILC3 lymphotoxin responses to maintain colonic lymphoid tissue organization (33).
Exciting recent evidence argues that ILC3s can regulate homeostatic host-commensal bacteria relationships through modulation of host adaptive immunity, in a pathway that appears to be independent of IL-22 and lymphotoxin. For example, one recently described pathway involved microbiota-dependent crosstalk between macrophages and ILC3s. In this case, it was shown that commensal bacteria stimulate IL-1β production by macrophages, which stimulates the IL-1 receptor on ILC3s to stimulate release of Csf2, which subsequently stimulates Treg maintenance through the development of IL-10- and retinoic acid-producing phagocytes (93). It’s also been shown, using RORγ-deficient mice and bone marrow chimeras, that ILC3s are required for memory CD4 (but not CD8) T cell survival (94). Moreover, it’s recently been shown that ILC3s can regulate CD4 T cells in an IL-1β- and antigen-dependent manner (95). These results together suggest that ILC3 cells can, both directly and indirectly, impact T cell homeostasis. Given the crucial role of regulatory T cells in maintaining intestinal homeostasis, these findings support the notion that ILC3 cells also play a key role in this homeostasis. In support of this, it was shown that MHCII depletion in ILC3 cells led to increased Th17 cell differentiation, independent of SFB (96).
Recent work from our laboratory has more closely investigated the role of ILC3 antigen presentation via MHCII in directly modulating T cell responses, particularly in the context of intestinal homeostasis. In a recent study, we demonstrated that loss of ILC3 (achieved via genetic deletion of RORγ) results in chronic, low-grade inflammation (typified by elevated, commensal bacteria-specific serum IgG responses) driven by the microbiota. However, this could not be recapitulated by targeting classical ILC3-dependent pathways, such as IL-22 or IL-17 (97). Instead, a subset of CCR6+ ILC3 cells were shown to highly express major histocompatibility complex class II (MHCII), and in vitro these cells were capable of processing and presenting model antigens. However, ILC3 did not express classical co-stimulatory molecules and failed to induce CD4 T cell proliferation. In mice, lineage-specific deletion of MHCII resulted in spontaneous chronic intestinal inflammation in a microbiota-dependent manner (97). This microbiota-driven inflammation was associated with increased pro-inflammatory Th17 cells in the colonic tissues and could be transferred into lymphocyte-deficient hosts by T cell adoptive transfers. These data argue that ILC3-intrinsic MHCII directly limits T cell responses to commensal bacteria in order to prevent chronic intestinal inflammation.
More recent work has supported this notion, highlighting a likely mechanism of ILC3-orchestrated ‘intestinal selection’ against commensal bacteria-specific CD4 T cells. This study utilized TCR transgenic mice displaying T cell reactivity against a commensal bacteria flagellin antigen, termed CBir1 (98). Notably, transfer of pre-activated CBir1 T cells into mice expressing either no MHCII, or only ILC3-intrinsic MHCII, showed that CBir1 CD4 T cells were reduced in number in the mLN and intestine of mice expressing ILC3-intrinsic MHCII (37). This reduction was specific to effector, but not regulatory, CBir1 T cells. MHCII+ ILC3 cells could bind large amounts of IL-2 and induce CBir1 CD4 T cell death in vitro. Since T cell death could be rescued by exogenous IL-2 or constitutive STAT5 activation in this setting, it is proposed that ILC3 cells induce T cell death by sequestration of crucial pro-survival cytokines from T cells (37). Finally, examination of Crohn’s disease patients showed an inverse correlation between ILC3 MHCII expression and disease state, Th17 cell frequencies, and commensal bacteria-specific IgG titers (37). These data strongly argue that ILC3-intrinsic MHCII functions to limit pathologic Th17 cell responses to commensal bacteria and prevent chronic inflammation in the intestinal tract of healthy humans. More importantly, these data support that this process may become disrupted in human IBD. This conclusion is supported by a recent unbiased RNA sequencing study of human ILC populations (99). Here, it was shown that one subset of human ILC3 cells expressed multiple HLA-encoding transcripts and associated enzymes to process antigens, but neither of the costimulatory molecules CD80 and CD86 were expressed within this cluster.
Going forward, it will be important to dissect the contribution of the different ILC3 effector functions to human health and disease. A better understanding of these connections will be crucial for the development of therapies for multiple diseases, including cancer, IBD, and enteric infection (86, 100). For example, a recent study in a macaque model of HIV infection showed depleted ILC3 numbers in the intestine, which may be causally connected to the increased levels of bacterial translocation observed in HIV patients (101). Another recent study in a humanized mouse model of HIV showed a similar decrease in ILC3 numbers, which was dependent on plasmacytoid DCs and was reversed by anti-retroviral therapy (102). These recent findings argue that ILC3 effector function may play a host-protective role in the pathology of HIV. However, the relative contributions of ILC3-derived IL-22, ILC3 contributions to lymphoid tissue development, and ILC3 suppression of T cell responses are currently unknown. Interestingly, another recent study examined the impact of MHC polymorphisms on the host-commensal bacteria homeostasis and host susceptibility to infection. Here, it was shown that unique host MHC genotypes underlie unique populations of commensal bacteria, with a range of susceptibility to enteric infections among the different commensal populations (103). Although a role for ILC3s was not examined in this context, the primary role of ILC3 MHCII in modulating intestinal homeostasis would suggests that ILC3s may play a causative role in this context. Finally, it was recently reported that RORγ inhibition can selectively deplete pathogenic inflammatory Th17 cells, but not ILC3s (104). This result emphasizes that careful design and evaluation of immune cell-targeted therapies holds great promise for the treatment of inflammatory disease in the intestinal tract. Future efforts to therapeutically exploit ILC3 cells and their effector pathways will be important to consider moving forward. A continuing challenge remains, however, in carefully elucidating the relevant effector pathways at work in different pathologies, and a further challenge will be the development of therapies that might specifically target one ILC3 effector pathway while leaving others (such as IL-22 signaling, lymphotoxin responses, or intestinal selection) intact.
Conclusions and future directions
In summary, recent efforts focused on host IgA, IL-22, and ILC3 pathways have greatly informed our understanding of the dialogue between mammalian hosts and their intestinal commensal bacteria. IL-22 is driven by generic bacterial signals and thus is not antigen-specific, but it induces both pro-commensal bacteria pathways (such as fucosylated oligosaccharides that may feed a subset of commensal bacteria) as well as anti-commensal bacteria pathways (such as mucus and AMP secretion). Mammals have developed an ILC3 antigen presentation pathway that supports commensal bacteria survival (and limits chronic inflammation) by eliminating inflammatory T cells that recognize commensal bacteria. In contrast, a countervailing host response involves the development of antigen-specific IgA antibodies that serve to limit bacteria from colonizing niches in close proximity to host tissues. Going forward, it will be important to delineate the factors that control the competition (or collaboration) between these two pathways – for example, it is not known whether IgA antigens and ILC3 intestinal selection antigens overlap significantly, and it’s unclear how ILC3s obtain commensal bacterial antigens in the first place. Furthermore, the tendency of a given commensal bacterium to induce IgA responses, ILC3 intestinal selection, or both, is currently unknown. Clearly, modulation of these antigen-dependent pathways could be of great therapeutic benefit. For example, IgA responses contribution to pathogen resistance, while ILC3 responses described above may ameliorate inflammatory bowel disease, infection, and HIV-associated pathology. While many important questions remain concerning the host-commensal bacteria dialogue, we are highly optimistic regarding the long-term therapeutic potential of targeting these antigen-specific pathways.
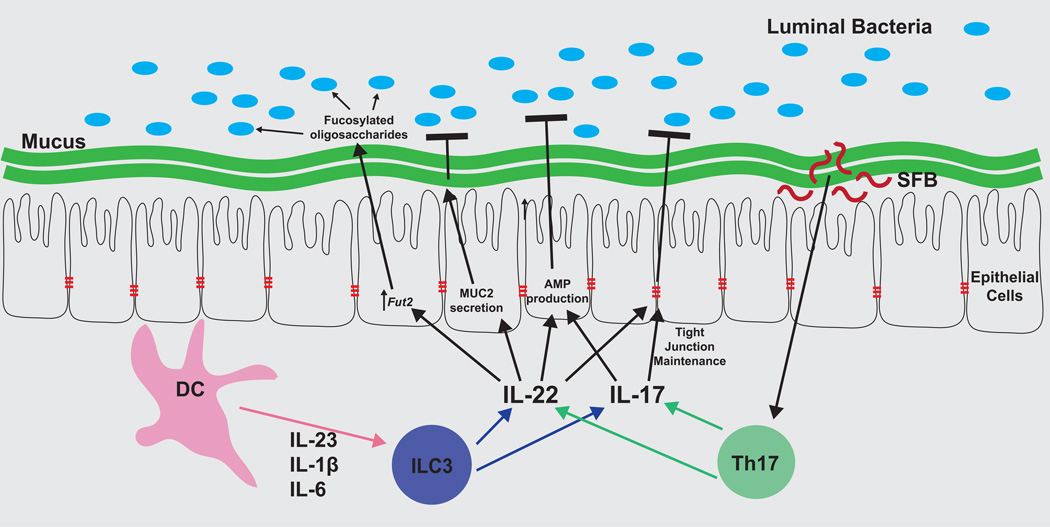
Figure 1. Antigen-independent mammalian pathways regulate host-commensal bacteria symbiosis.
IL-22 is a master regulator of antigen-independent pathways that respond to and modulate the microbiota. IL-22 integrates multiple commensal bacteria-dependent signals, and coordinates diverse responses emanating from the intestinal epithelium. Dendritic cells sense commensal bacteria and release cytokines (IL-23 most notably, as well as IL-1β and IL-6) that stimulate IL-17 and IL-22 production by ILC3 cells. Th17 cells are also stimulated to produce IL-17 and IL-22 by inflammatory signals or, in the steady-state, segmented filamentous bacteria (SFB). IL-17 can induce AMP production and maintenance of epithelial tight junctions. IL-22 is sensed by intestinal epithelial cells, which respond by: secreting mucus to enforce a physical barrier between host and bacteria; maintaining tight junctions to prevent bacterial translocation into tissues; producing anti-microbial peptides (AMPs) that selectively target commensal bacteria; and producing highly fucosylated oligosaccharides, which provide metabolic benefits to specific subsets of commensal bacteria.
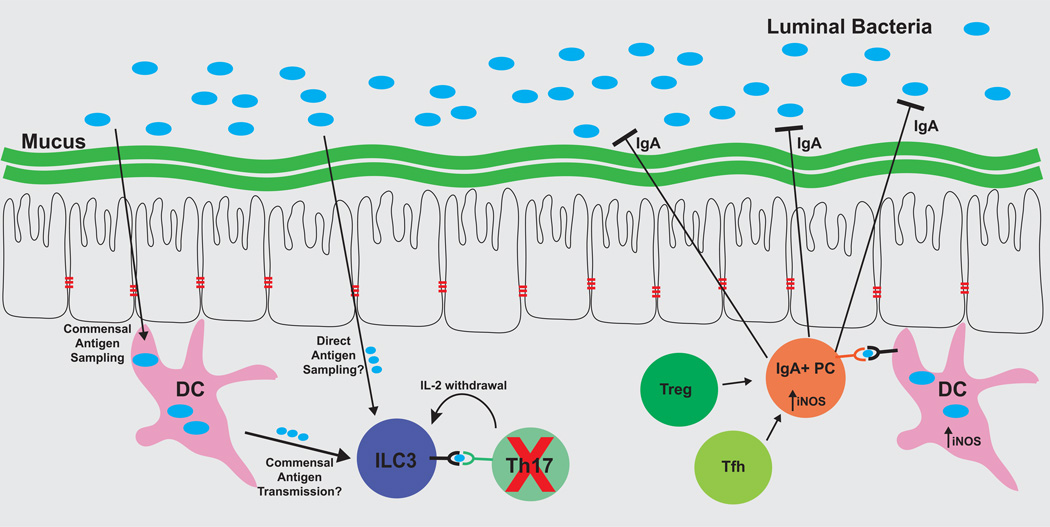
Figure 2. Antigen-dependent host pathways serve both to limit and also to protect commensal bacteria populations.
ILC3 cells and IgA+ plasma cells each respond to commensal bacteria antigens, but ILC3 serves to limit host responses to these antigens, while IgA functions to limit colonization of these bacteria. ILC3 cells acquire antigen through an unknown mechanism, and present commensal bacteria-derived antigens through MHCII, but they do not express co-stimulatory molecules, and instead bind important pro-survival cytokines including IL-2. As a result, ILC3 cells induce apoptosis of T cells reactive to commensal bacteria. In parallel, antigen-specific IgA+ is induced in lymphoid tissues in a DC- and iNOS-dependent manner. T cells can also contribute to antigen-specific IgA responses. Antigen-specific IgA is secreted into the intestinal lumen, where it coats antigenic bacteria and inhibits bacterial colonization of the intestinal epithelium and mucus layers.
Acknowledgments
We thank members of the Sonnenberg laboratory for critical discussions and reading of the manuscript. Research in the Sonnenberg laboratory is supported by the National Institutes of Health (DP5OD012116, R56AI114724 and R01AI123368), the American Asthma Foundation Scholar Award and the Searle Scholars Program.
References
- 1.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. bioRxiv. 2016 doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science (New York, NY) 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40:843–854. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nature reviews Microbiology. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung TC, Bessman NJ, Hepworth MR, Kumar N, Shibata N, Kobuley D, Wang K, Ziegler CGK, Goc J, Shima T, Umesaki Y, Sartor RB, Sullivan KV, Lawley T, Kunisawa J, Kiyono H, Artis D, Sonnenberg GF. Lymphoid tissue-resident commensal bacteria promote IL-10 family cytokines to establish mutualism. Immunity. 2016 doi: 10.1016/j.immuni.2016.02.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends in immunology. 2015;36:3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macpherson AJ, Koller Y, McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends in immunology. 2015;36:460–470. doi: 10.1016/j.it.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 10.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanash AM, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindemans CA, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickert G, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. The Journal of experimental medicine. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugimoto K, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aparicio-Domingo P, et al. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. The Journal of experimental medicine. 2015;212:1783–1791. doi: 10.1084/jem.20150318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz M, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. The Journal of experimental medicine. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song C, et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. The Journal of experimental medicine. 2015;212:1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hue S, et al. Interleukin-23 drives innate and T cell–mediated intestinal inflammation. The Journal of experimental medicine. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefka AT, et al. Commensal bacteria protect against food allergen sensitization. Proceedings of the National Academy of Sciences. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. The Journal of experimental medicine. 2010;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geremia A, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. The Journal of experimental medicine. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelsen JR, Baldassano RN, Artis D, Sonnenberg GF. Maintaining intestinal health: the genetics and immunology of very early onset inflammatory bowel disease. Cellular and molecular gastroenterology and hepatology. 2015;1:462–476. doi: 10.1016/j.jcmgh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 24.Sonnenberg GF, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science (New York, NY) 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zenewicz LA, et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. Journal of immunology (Baltimore, Md: 1950) 2013;190:5306–5312. doi: 10.4049/jimmunol.1300016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature medicine. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 27.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankin LC, et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol. 2016;17:179–186. doi: 10.1038/ni.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aychek T, et al. IL-23-mediated mononuclear phagocyte crosstalk protects mice from Citrobacter rodentium-induced colon immunopathology. Nat Commun. 2015;6 doi: 10.1038/ncomms7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinnebrew Melissa A, et al. Interleukin 23 Production by Intestinal CD103+CD11b+ Dendritic Cells in Response to Bacterial Flagellin Enhances Mucosal Innate Immune Defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manta C, et al. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013;6:177–188. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longman RS, et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. The Journal of experimental medicine. 2014;211:1571–1583. doi: 10.1084/jem.20140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ota N, et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12:941–948. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 34.Tumanov AV, et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munoz M, et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity. 2015;42:321–331. doi: 10.1016/j.immuni.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Wagage S, Harms Pritchard G, Dawson L, Buza EL, Sonnenberg GF, Hunter CA. The Group 3 Innate Lymphoid Cell Defect in Aryl Hydrocarbon Receptor Deficient Mice Is Associated with T Cell Hyperactivation during Intestinal Infection. PloS one. 2015;10:e0128335. doi: 10.1371/journal.pone.0128335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hepworth MR, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science (New York, NY) 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa M, et al. Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage. Immunity. 2014;41:620–632. doi: 10.1016/j.immuni.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauguet S, et al. Intestinal Microbiota of Mice Influences Resistance to Staphylococcus aureus Pneumonia. Infection and immunity. 2015;83:4003–4014. doi: 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanos SL, et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46(+) cells. Nature immunology. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawa S, et al. ROR[gamma]t+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 42.Satoh-Takayama N, et al. The chemokine receptor CXCR6 controls the functional topography of interleukin-22 producing intestinal innate lymphoid cells. Immunity. 2014;41:776–788. doi: 10.1016/j.immuni.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allan DS, et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol. 2015;8:340–351. doi: 10.1038/mi.2014.71. [DOI] [PubMed] [Google Scholar]
- 45.Hernández PP, et al. Interferon-λ and interleukin-22 cooperate for the induction of interferon-stimulated genes and control of rotavirus infection. Nature immunology. 2015;16:698–707. doi: 10.1038/ni.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giacomin PR, et al. Epithelial-intrinsic IKKalpha expression regulates group 3 innate lymphoid cell responses and antibacterial immunity. The Journal of experimental medicine. 2015;212:1513–1528. doi: 10.1084/jem.20141831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Innate Lymphoid Cells Control Early Colonization Resistance against Intestinal Pathogens through ID2-Dependent Regulation of the Microbiota. Immunity. 2015;42:731–743. doi: 10.1016/j.immuni.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Obata T, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7419–7424. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu J, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sano T, et al. An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses. Cell. 2015;163:381–393. doi: 10.1016/j.cell.2015.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atarashi K, et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upadhyay V, et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol. 2012;13:947–953. doi: 10.1038/ni.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 54.Pham TA, et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 2014;16:504–516. doi: 10.1016/j.chom.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goto Y, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science (New York, NY) 2014;345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pickard JM, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johansson ME, et al. Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe. 2015;18:582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earle KA, et al. Quantitative Imaging of Gut Microbiota Spatial Organization. Cell Host Microbe. 2015;18:478–488. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shan M, et al. Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science (New York, NY) 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelaseyed T, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunological reviews. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conley ME, Delacroix DL. Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense? Annals of internal medicine. 1987;106:892–899. doi: 10.7326/0003-4819-106-6-892. [DOI] [PubMed] [Google Scholar]
- 62.Rogier EW, et al. Lessons from mother: Long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut microbes. 2014;5:663–668. doi: 10.4161/19490976.2014.969984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gommerman JL, Rojas OL, Fritz JH. Re-thinking the functions of IgA(+) plasma cells. Gut microbes. 2014;5:652–662. doi: 10.4161/19490976.2014.969977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogier EW, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moon C, Baldridge MT, Wallace MA, Burnham C-AD, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palm NW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science (New York, NY) 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 68.Benckert J, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. The Journal of Clinical Investigation. 121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koenig JE, et al. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindner C, et al. Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat Immunol. 2015;16:880–888. doi: 10.1038/ni.3213. [DOI] [PubMed] [Google Scholar]
- 71.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science (New York, NY) 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 72.Fritz JH, et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481:199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tezuka H, et al. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 74.Wang S, et al. MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity. 43:289–303. doi: 10.1016/j.immuni.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kubinak Jason L, et al. MyD88 Signaling in T Cells Directs IgA-Mediated Control of the Microbiota to Promote Health. Cell Host & Microbe. 17:153–163. doi: 10.1016/j.chom.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao AT, Yao S, Gong B, Nurieva RI, Elson CO, Cong Y. Interleukin (IL)-21 promotes intestinal IgA response to microbiota. Mucosal Immunol. 2015;8:1072–1082. doi: 10.1038/mi.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirota K, et al. Plasticity of TH17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu Van T, et al. Eosinophils Promote Generation and Maintenance of Immunoglobulin-A-Expressing Plasma Cells and Contribute to Gut Immune Homeostasis. Immunity. 40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 79.Bunker Jeffrey J, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kunisawa J, et al. Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun. 2013;4:1772. doi: 10.1038/ncomms2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lécuyer E, et al. Segmented Filamentous Bacterium Uses Secondary and Tertiary Lymphoid Tissues to Induce Gut IgA and Specific T Helper 17 Cell Responses. Immunity. 40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 82.Fransen F, et al. BALB/c and C57BL/6 Mice Differ in Polyreactive IgA Abundance, which Impacts the Generation of Antigen-Specific IgA and Microbiota Diversity. Immunity. 43:527–540. doi: 10.1016/j.immuni.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 83.Mirpuri J, et al. Proteobacteria-specific IgA regulates maturation of the intestinal microbiota. Gut microbes. 2014;5:28–39. doi: 10.4161/gmic.26489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kau AL, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Science translational medicine. 2015;7:276ra224. doi: 10.1126/scitranslmed.aaa4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Charbonneau MR, et al. Sialylated Milk Oligosaccharides Promote Microbiota-Dependent Growth in Models of Infant Undernutrition. Cell. 2016;164:859–871. doi: 10.1016/j.cell.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonnenberg GF, Artis D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nature medicine. 2015;21:698–708. doi: 10.1038/nm.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 88.Kim MY, et al. Function of CD4+CD3− cells in relation to B- and T-zone stroma in spleen. Blood. 2007;109:1602–1610. doi: 10.1182/blood-2006-04-018465. [DOI] [PubMed] [Google Scholar]
- 89.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nature reviews Immunology. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 90.White A, et al. Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood. 2007;110:1950–1959. doi: 10.1182/blood-2007-01-070003. [DOI] [PubMed] [Google Scholar]
- 91.Bouskra D, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 92.Kruglov AA, et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science (New York, NY) 2013;342:1243–1246. doi: 10.1126/science.1243364. [DOI] [PubMed] [Google Scholar]
- 93.Mortha A, et al. Microbiota-Dependent Crosstalk Between Macrophages and ILC3 Promotes Intestinal Homeostasis. Science (New York, NY) 2014;343 doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Withers DR, et al. Cutting Edge: Lymphoid Tissue Inducer Cells Maintain Memory CD4 T Cells within Secondary Lymphoid Tissue. The Journal of Immunology. 2012;189:2094–2098. doi: 10.4049/jimmunol.1201639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.von Burg N, et al. Activated group 3 innate lymphoid cells promote T-cell–mediated immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:12835–12840. doi: 10.1073/pnas.1406908111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goto Y, et al. Segmented Filamentous Bacteria Antigens Presented by Intestinal Dendritic Cells Drive Mucosal Th17 Cell Differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bjorklund AK, et al. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016 doi: 10.1038/ni.3368. advance online publication. [DOI] [PubMed] [Google Scholar]
- 100.Goc J, Hepworth MR, Sonnenberg GF. Group 3 innate lymphoid cells: regulating host-commensal bacteria interactions in inflammation and cancer. International immunology. 2016;28:43–52. doi: 10.1093/intimm/dxv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu H, Wang X, Lackner AA, Veazey RS. Type 3 innate lymphoid cell depletion is mediated by TLRs in lymphoid tissues of simian immunodeficiency virus–infected macaques. The FASEB Journal. 2015;29:5072–5080. doi: 10.1096/fj.15-276477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Z, et al. Plasmacytoid dendritic cells promote HIV-1–induced group 3 innate lymphoid cell depletion. The Journal of Clinical Investigation. 125:3692–3703. doi: 10.1172/JCI82124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kubinak JL, et al. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nat Commun. 2015;6 doi: 10.1038/ncomms9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Withers DR, et al. Transient inhibition of ROR-[gamma]t therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nature medicine. 2016 doi: 10.1038/nm.4046. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]