Abstract
Neuronal rhythms are ubiquitous features of brain dynamics, and are highly correlated with cognitive processing. However, the relationship between the physiological mechanisms producing these rhythms and the functions associated with the rhythms remains mysterious. This article investigates the contributions of rhythms to basic cognitive computations (such as filtering signals by coherence and/or frequency) and to major cognitive functions (such as attention and multi‐modal coordination). We offer support to the premise that the physiology underlying brain rhythms plays an essential role in how these rhythms facilitate some cognitive operations.
Keywords: attention, beta rhythm, coherence filtering, frequency filtering, gamma rhythm
Introduction
It is well known that the brain produces rhythms that are highly correlated with cognition. However, the underlying mechanisms that bring about their existence and the specificity of the rhythm to the task at hand are still largely debated. The information presented throughout this article offers evidence that addresses these questions and provides strong support for a functional role for rhythms in cognitive functions. We will argue that the physiological processes underlying the multiple mechanisms that generate brain rhythms help to gate the flow of signals within and among brain regions. Furthermore, we offer examples of how the underlying physiology gives clues to the functions of those rhythms.
The brain rhythms that we have in mind are those initially and superficially uncovered in the earliest electroencephalography (EEG) measurements, now studied though a variety of invasive and non‐invasive techniques, including electrophysiology in vitro (Carracedo et al., 2013) and in vivo (Jutras et al., 2013), optogenetics (Cardin et al., 2009), EEG (Tallon‐Baudry et al., 1999), and magnetoencephalography (Siegel et al., 2012). The old classification (e.g. alpha, 9–11 Hz; beta, 12–30 Hz; gamma, 30–90 Hz; delta, 1–4 Hz) based on early human EEG studies has been shown (unsurprisingly) to be too simple. Now, it is understood that any given frequency band may be produced by multiple mechanisms in different areas of the brain (Ainsworth et al., 2011), and that one area may simultaneously produce multiple frequency bands, again via multiple mechanisms (Roopun et al., 2008a,b). Motivated by the working hypothesis made above, we explore ways in which the various rhythms and interactions of rhythms permit, or even compel, specific dynamical processing that is useful for cognitive activity.
We investigate the contributions of rhythms to basic cognitive computations (such as filtering signals by coherence and/or frequency) and to major cognitive functions (such as attention and multi‐modal coordination). The first part of this article focuses on gamma rhythms in the sensory and parietal cortices; however, current experimental and modeling work suggests that this point of view is highly relevant for frontal cortices, as well as for regions of the brain outside the neocortex. The second part looks at instances of interactions among multiple cortical rhythms, mainly gamma and beta. The final part discusses the multiple ways in which beta rhythms may come about mechanistically throughout the neocortex and basal ganglia, and the multiple roles that this rhythm (in all of its various forms) is presumed to play. This section raises many more questions than it answers, but puts these questions in the context of the analyses and simulations discussed in the earlier sections.
Computational properties of gamma rhythms
Physiology of gamma rhythms
Much has already been written about the gamma rhythm (Whittington et al., 2000, 2011; Bartos et al., 2002; Börgers & Kopell, 2005, 2008; Börgers et al., 2005, 2008; Fries et al., 2007; Marinazzo et al., 2007; Kopell et al., 2010a,b; Wang, 2010) . The term ‘gamma rhythm’ is used in multiple ways (Canolty et al., 2006; Colgin et al., 2009). We will use this term to mean rhythms that require the involvement of fast‐spiking (FS) interneurons, with frequencies related to the time scale of the decay of inhibition in the range of 30–90 Hz (Whittington et al., 2000).
Even within this definition, there are multiple mechanisms that produce gamma rhythms. In the interneuron network gamma, a population of tonically excited mutually inhibitory FS interneurons fires in synchronous volleys, each followed by a short (~20‐ms) interval of quiescence, during which the mutual inhibition decays sufficiently to allow another volley. In a family of processes collectively referred to as pyramidal–interneuronal network gamma (PING), synchronous FS volleys are triggered by spikes from excitatory pyramidal cells and separated by intervals of quiescence, during which FS‐to‐pyramid inhibition decays sufficiently to allow more pyramidal spiking. PING is subdivided into rhythms in which individual pyramidal cells spike on most gamma cycles (strong PING), and rhythms in which individual pyramidal cells spike only occasionally (weak PING). We associate strong PING with active coding and cell assemblies, and weak PING with attention and arousal (Börgers et al., 2005, 2008; Börgers & Kopell, 2008). In addition, experimental conditions designed to mimic the background state of excitability in the awake brain (cholinergic neuromodulation alone and/or weak glutamatergic tone) are capable of generating a persistent PING‐like gamma rhythm in which ongoing, patterned noise in principal cell axons provides the excitatory drive (Traub et al., 2000). These general physiological properties of gamma rhythms have been supported by work in vivo (Atallah & Scanziani, 2009; Cardin et al., 2009) and in vitro (Whittington et al., 2000). Note that the cells required by ING and (strong or weak) PING, namely FS interneurons and pyramidal cells, are ubiquitous in the brain.
The presence and power of gamma rhythms depends strongly on the level of tonic drive delivered to the cell populations involved. Gamma rhythms are produced in a simple model of PING only if there is an appropriate balance between excitation and inhibition in the network (Börgers & Kopell, 2003, 2005). In particular, if the FS cells are sufficiently excitable, they lose their phasic relationship with pyramidal cell firing and become incoherent; their activity may lead to the suppression or partial suppression of the pyramidal cells. Thus, gamma rhythms may be modulated by the addition of inhibition to the FS cells [e.g. by another class of interneurons, such as low‐threshold spiking (LTS) cells], which can increase the gamma power by preventing this ‘suppression transition’ (Börgers & Kopell, 2005; Börgers et al., 2008; Börgers & Walker, 2013).
In each of the gamma mechanisms listed above, FS volleys are separated by the decay of inhibition. Therefore, the inhibitory decay time and the size of the population inhibitory postsynaptic potential (IPSP) are crucial to determining the period (when more FS cells fire on a given cycle, the period will be longer). The time scale of this inhibitory decay is the longest important time scale in the gamma rhythm – the rise time of the GABA and AMPA synapses involved is short, currents with longer time constants are generally not as strong during bouts of this rhythm, and, as the inhibition from FS cells decays over the course of a gamma cycle, the effective membrane time constant of inhibited cells (capacitance over the total of all conductances, inward and outward) is small, owing to the large amount of conductance allowed by open inhibitory channels. In particular, the separation between the time scales of inhibitory decay and membrane integration causes pyramidal and FS cells to act as leaky integrators while inhibition persists – if an inward excitatory postsynaptic current to one of these cells does not immediately evoke a spike, it quickly leaks away. This property is important to the processing of inputs, as described below.
Controversy over function of the gamma rhythm
In this article, we argue that the physiology underlying the gamma rhythm is naturally suited to creating cell assemblies and facilitating selective neuronal communication through coherence and frequency selectivity. Some researchers, however, are skeptical about the role of gamma rhythms in the active processing and routing of information. Burns et al. (2011) measured the autocorrelation of gamma phase and frequency over time in the macaque V1, and found that the lengths of gamma cycles were too unpredictable for gamma rhythms to be used as a ‘clock’ for time‐dependent calculations. Ray & Maunsell (2010) showed that the frequency of gamma rhythms in V1 depended strongly on stimulus contrast, and varied across the visual cortex, casting doubt on its capacity to coordinate the timing of V1 output. These observations do seem to limit the capacity of gamma rhythms to perform any function that requires a reliable, precise and uniform gamma frequency. However, they do not rule out functions that rely on phase alignment between directly coupled regions, which could remain phase‐aligned despite variations in frequency over time and space.
Some controversy over gamma rhythms stems from the suspicion that gamma power represents the high‐frequency component of action potentials rather than network‐generated rhythms. Ray et al. (2008) observed that high gamma power (~60–100 Hz) in the local field potential (although not in electrocorticography recordings) was strongly correlated with local firing rates, suggesting that much of this power reflects currents associated with action potentials. However, they found that low gamma power (40–80 Hz) in the local field was not strongly correlated with firing rates, and concluded that their results were ‘not inconsistent’ with the many suggested functional roles of gamma rhythms. Here, we exclude from consideration gamma power associated with firing rates by limiting our scope to the band 30–90 Hz and to rhythms with frequencies related to the decay time of inhibition.
Part of the controversy over the cognitive roles of gamma rhythms and over rhythms in general may stem from the objection that these roles can also be filled by non‐oscillatory mechanisms. In the following, we make no claim that rhythms are absolutely necessary for their proposed cognitive functions, but only that they enhance the performance of those functions.
Gamma rhythms and cell assemblies
We use the term ‘cell assembly’ to mean a group of cells that transiently fire together, whether or not they are synaptically connected. Such assemblies are often hypothesized to encode objects or concepts in the brain. The relationship of gamma rhythms to the formation of cell assemblies has been reviewed several times (Olufsen et al., 2003; Fries et al., 2007; Kopell et al., 2010a). To summarize, strong PING is a perfect set‐up for the formation of assemblies – when one subset of pyramidal cells receives more tonic drive than another, those cells overcome inhibition first and activate a shared FS cell population, which inhibits the other subset and prevents it from spiking (Olufsen et al., 2003; Börgers et al., 2008). This competitive interaction of pyramidal cell populations has been referred to as ‘winner‐take‐all’ dynamics (Lumer, 2000; Fries et al., 2007). Because the time scale of membrane dynamics and the synaptic rise time constant are significantly shorter than the gamma period, the leakiness of both populations erases any spiking history while the inhibition persists during each cycle. Thus, the active assembly represents the most driven subset of cells on each cycle – if the inputs change, so does the assembly. The coding associated with the gamma rhythms is thus a dynamically changing set of assemblies in which the roster of participants is a thresholded representation of the relative strengths of the tonic input drives during each gamma period. However, the response of such a circuit to inputs that vary on time scales shorter than the gamma period can be subtle, as described below.
Gamma rhythms as coherence filters
Although the properties of gamma rhythms have been much investigated (see references cited above), there has been less work on how the physiology of the gamma rhythm affects the processing of the temporally patterned input. We will now refer to FS cells as I‐cells (for inhibitory) and to pyramidal cells as E‐cells (for excitatory).
Gamma‐oscillatory networks of E‐cells and I‐cells receiving periodic input at a gamma frequency have special properties that are not shared by other kinds of forced oscillators [e.g. phase oscillators (Ermentrout & Terman, 2010)]. Because both cell types act as leaky integrators during a gamma cycle, they are driven to spike more readily by a pulse of current concentrated into a time window that is short relative to the time scale of the leak. Figure 1 demonstrates that, in a simple model, an E‐cell population recovering slowly from inhibition responds preferentially to short, sharp pulses over long, shallow ones. This property leads the PING network to act as a coincidence detector – a collection of periodic pulses that arrive at nearly the same time evoke volleys of spikes in the network more effectively than the same pulses in a less coherent distribution. Spike volleys evoked in this way restore inhibition to the network, resetting the phase of the gamma oscillation, so a periodic train of pulses that successfully evokes volleys on every cycle entrains (or ‘phase‐locks’) the gamma rhythm. Coincidence detection by the E‐cell population results in preferential phase‐locking to more coincident volleys of input, i.e. more ‘coherent’ periodic input trains, a behavior that we refer to as ‘coherence filtering’.
Figure 1.
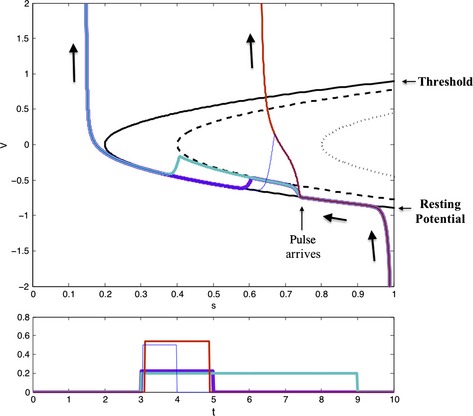
An E–I network acts as a coherence filter. Four different pulses are delivered to the E‐cell after an inhibitory spike. V is the membrane potential of a quadratic integrate and fire neuron (Latham et al., 2000) recovering from inhibition, and s is the strength of the inhibitory current (as a fraction of peak inhibition). V asymptotes to a stable resting voltage, which increases as s decays. The threshold voltage (above which V spikes) decreases with s. When s = 0.2, the stable resting voltage meets the threshold voltage, and the cell spikes. The lower branch of the solid parabola is the stable resting voltage, and the upper branch is the threshold voltage. During a square pulse of height 0.2 (purple, cyan), the resting and threshold voltages shift to the dashed parabola. The membrane potential asymptotes to the new resting voltage and returns after the pulse, so a 6‐ms‐long pulse has the same effect as a 2‐ms‐long pulse. During a square pulse of height 0.4, the resting and threshold voltages shift to the dotted parabola – the leak current is overpowered, and the resting and threshold voltages disappear. A 2‐ms‐long pulse of this height (red) evokes a spike even though it carries less current than the longer, shorter pulse. However, at very short time scales, the amount of current can still be a limiting factor – a 1‐ms‐long pulse of height 0.6 (blue) does not carry enough current to reach a high voltage before the pulse is over.
Coincidence detection is commonplace, but coherence filtering is not – coherence filtering requires coincidence detection within the context of an oscillating system. A simple one‐dimensional model neuron can effectively detect coincidences or oscillate, but not both – an oscillating neuron is generally receiving sufficient tonic drive to overwhelm its leak current, and therefore no longer acts as a leaky integrator capable of coincidence detection. Coherence filtering is explored analytically with quadratic integrate and fire neurons in an upcoming paper. See also Sedley & Cunningham (2013), who review the potential cognitive importance of filtering through gamma rhythms.
Coincidence detection (and hence coherence filtering) may be reinforced by feedforward inhibition. Projections from the thalamus to the neocortex generally send stronger afferents to FS interneurons than pyramidal cells, so a pulse of synaptic input triggers a volley of FS spikes several milliseconds after its arrival (Hull & Scanziani, 2007). If this pulse carries enough current to an E‐cell in its first few milliseconds to push it past threshold, it spikes in response; if not, the rest of the excitatory current arrives under strong inhibition and leaks away. The coincidence‐detecting effect of feedforward inhibition is discussed in Hull & Scanziani (2007). The feedforward inhibition motif may also affect assembly formation, as it creates a hard time window in which active cells must spike in addition to the competitive winner‐take‐all interaction created by feedback inhibition.
One consequence of coherence filtering concerns multiple inputs to an excitatory–inhibitory (E–I) network. Börgers & Kopell (2008) examined periodic input from different streams to an E–I network with a single E‐cell and I‐cell, tuned so that it was quiet in the absence of input. The two streams had (usually somewhat different) frequencies in the gamma range, and one stream was more coherent than the other. They found that the more coherent stream of input entrained the E–I network to its frequency, and that the other input had little effect (Fig. 2). More specifically, a periodic input that is not able to phase‐lock a network at its frequency may be largely ignored in the presence of an input that is able to entrain the target. Hence, a stream of coherent pulses may effectively (but not perfectly) filter out a stream of less coherent pulses; increasing the feedback inhibition makes it harder for signals to entrain the target (shown in Fig. 3), changing which sets of inputs can be effectively separated.
Figure 2.
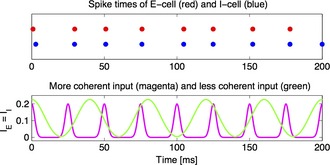
A coherent pulse phase locks a PING circuit and blocks a less coherent signal. The less coherent input is greater not only in temporal average, but also in amplitude. Nonetheless, the target network (an E–I pair) mostly follows the more coherent input, with some perturbations being caused by the less coherent one. Reproduction of fig. 3C of Börgers & Kopell (2008).
Figure 3.
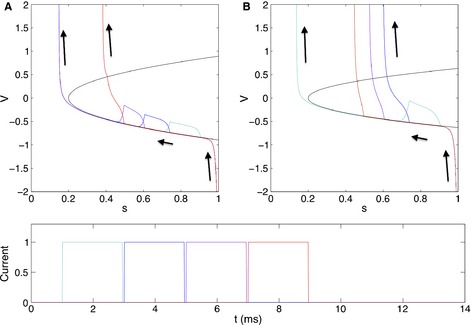
Feedback inhibition determines frequency selectivity. Gamma frequency is more selective for pulse frequency when feedback inhibition is stronger. This is because a pulse can only evoke an excitatory spike and end the gamma cycle if it arrives under sufficiently low inhibition. In this figure, a pulse arrives after an inhibitory spike with four different delays. V is the membrane potential of a quadratic integrate and fire E‐cell, and s is the saturation of the E–I synapse. The lower branch of the parabola is the stable resting voltage, and the upper branch is the threshold voltage. (A) When E–I connections are strong, only the latest pulse arrives under low enough inhibition to evoke an excitatory spike and shorten the period of this gamma cycle. (B) In a system with the same natural frequency but weaker E–I connections, the three later pulses can all evoke excitatory spikes.
It has been shown that input associated with attended stimuli can be more coherent than input from distracting stimuli (Fries et al., 2002; Bichot et al., 2005), and that this is associated with a larger gamma power in the input. The computational and analytical work described above implies that the coherent input associated with attention can act to prevent the distracting stimuli from being heard by the downstream target. Below, we describe other consequences of coherence filtering or closely related phenomena, and discuss reasons why attention is expected to lead to coherence in the gamma band (see section on top‐down beta rhythms and attention).
Gamma rhythms and frequency matching/filtering
In vitro, gamma rhythms have been found over a frequency range of tens of hertz. One important question is whether the frequency in a given cell assembly showing an oscillation in the gamma range can affect whether the input is heard by a target network that has its own ongoing rhythm, and whether the input having another nearby frequency is filtered out. An example of the importance of such frequency matching is given by a striking observation (Middleton et al., 2008) that the rodent entorhinal cortex, in vitro, can produce two different frequencies of gamma rhythm, separated by ~10 Hz, depending on the activity of N‐methyl‐d‐aspartate (NMDA) receptors; furthermore, the slower (low NMDA activity) and faster (normal NMDA activity) frequencies match the in vitro natural frequencies of hippocampal CA3 and CA1, respectively. If the target network listens preferentially to input similar to its own frequency, this suggests that, in the presence of the normal NMDA activity, CA1 would be more responsive to entorhinal cortex input via the temporo‐ammonic pathway, whereas lowered NMDA activity would favor CA3 activation by the input (Kopell et al., 2010b).
The work on coherence by Cannon, Börgers and Kopell described above suggests a modulatable mechanism for frequency matching. We start, for simplicity, with a pulsatile excitatory input to the E‐cell population alone, when there is a background gamma rhythm in the target network. Such input can shorten but not lengthen the period of oscillation; therefore, inputs below its intrinsic frequency cannot phase‐lock the firing of the network, and many arrive while the network is in its heavily inhibited, insensitive phase. This causes the network to filter out frequencies lower than the natural network frequency, while phase‐locking readily to slightly higher frequencies. Thus, any modulation of the natural frequency modulates the range of selected input frequencies. (We note that ‘filter out’ does not preclude some spikes from being evoked; for example, if the input is near half the natural frequency, it may arrive at an appropriate phase at every other gamma cycle).
The amount by which an input pulse can shorten the period of the receiving network depends on how soon the input pulse can overcome the decaying inhibition resulting from a spike volley of the local feedback inhibitory circuit. Larger and/or more coherent pulses can force the network into shorter periods, and weaker E–I connections (e.g. if fewer I‐cells participate) allow a pulse to shorten the network period more (Fig. 3). If a set of pulses arrives with a period shorter than the shortest network frequency that it can evoke, then it too cannot phase‐lock the network; many pulses arrive to find the network in its heavily inhibited, insensitive phase. This causes input at a sufficiently high frequency to be filtered out by the feedback inhibition. (Some subset of the pulses may still cause spikes.) Thus, the upper bound of the range of selected frequencies can be adjusted by modulating the strength of local target network E–I connections and the strength and coherence of the input pulses.
We now consider input to the I‐cells as well. Akam et al. (2012) observed, in CA3, that when excitatory inputs did not quickly evoke a spike, they slightly delayed the gamma period, allowing them to phase‐lock the network at frequencies slightly lower than the natural network frequency. This effect can be accounted for by assuming that a few quiescent interneurons are recruited by every excitatory input, slightly raising the local inhibition and extending the network period. Such inhibitory recruitment may extend the range of selected periods.
Akam & Kullmann (2012) proposed a different mechanism for input frequency selectivity at gamma frequencies. The authors considered asynchronous and oscillatory inputs to both pyramidal and FS interneuronal populations, and showed that an input oscillating at a specific frequency could be decoded from the other inputs by a spiking network filter. (The coherence of the oscillation is also a factor in decodability, so their model may also be considered to be a mechanism for coherence selectivity.) A central feature of their model is feedforward inhibition from interneurons. These interneurons are close to asynchrony, but their firing rates resonate selectively with certain frequencies, owing to mutual connectivity, and allow the population to act as a bandpass filter. By contrast, the work of Cannon, Börgers and Kopell relies centrally on the feedback inhibition that is found in most networks that produce gamma rhythms, and on the near‐synchrony of the oscillating cell populations that is often observed in experiments (Whittington et al., 2011). Feedforward inhibition creates a coarse initial filter – the E‐cells must be very excited to overcome this inhibition. Feedback creates a more temporally precise filter – after the first set of E‐cells spike, the remaining population that might have spiked later are shut down by inhibition.
Figure 4 shows network simulations illustrating frequency matching in a larger E–I network. In the absence of external input, the target network shows weak PING oscillations, driven by stochastic activity in the E‐cells (spike rastergram in Fig. 4A), at a frequency of ~36 Hz. When both E‐cells and I‐cells receive periodic pulses of drive, the response of the network is strong when the input frequency is slightly above the natural frequency (Fig. 4B), but much weaker when the input frequency is below or significantly above the natural frequency (Fig. 4C and D). The reason for the asymmetry is that an input pulse that arrives just before the end of the target network period finds the network in a relatively uninhibited state, whereas a pulse that arrives just after an intrinsic population spike volley of the target network finds the network in a heavily inhibited state. The former is therefore more easily able to elicit a spike volley than the latter. Note that even an input at a favorable frequency (slightly above the intrinsic target frequency) may be ineffective if it arrives at the wrong phase. Figure 4B suggests that such an input may have to shift the phase of the target over several periods before it can become effective. In the simulations of Fig. 4, the inhibitory synapses decay with a time constant of 9 ms. However, much shorter decay time constants, e.g. 4 ms (Bartos et al., 2002), yield very similar results if the inhibitory conductances are raised to prevent a significant change in the intrinsic frequency of the target network.
Figure 4.
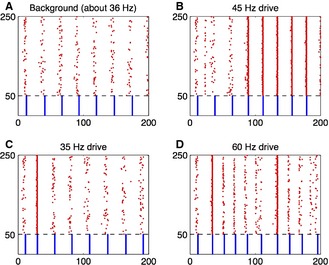
Frequency selectivity occurs in large, heterogeneous E–I networks. Spike rastergram for weak PING. Red dots indicate spike times of E‐cells, and blue dots spike times of I‐cells. The horizontal axis is time in milliseconds. All parameters are as in fig. 1b of Kopell et al. (2010b), with two exceptions: (i) the network is larger here – 200 E‐cells and 50 I‐cells; (ii) synaptic inputs per cell are somewhat stronger – here, using the notation of Kopell et al. (2010b), ĝIE = ĝEI = ĝII = 1.5 (the values used by Kopell et al. were ĝIE = 1.5, ĝEI = ĝII = 0.5.) (A) PING rhythm without forcing. (B–D) Same as (A), but with additional oscillatory input of different frequencies to both E‐cells and I‐cells. The form of the additional input is I(t) = 1 + tanh(10[cos(2πt/T) − 1]), where T is the period, and with a constant of proportionality chosen so that the temporal average of I(t) equals 1 (for E‐cells) and 0.5 (for I‐cells).
Another recent study (Serenevy & Kopell, 2013) showed a mechanism by which a target network can adjust its natural frequency to accommodate a wider range of input frequencies, by changing the number of participating FS cells. In that study, the target network was a set of all‐to‐all coupled inhibitory cells, with each cell receiving sinusoidal excitation at the same frequency but with different oscillator amplitudes and mean drives; the differences in mean drives is equivalent to having heterogeneity in the excitability of the cells. The decay time of inhibition is matched to the period of the driving, as in 40‐Hz‐driven gamma rhythms. In such a network, even without periodic forcing, not all cells participate – when inhibition decays sufficiently, the fast membrane potential dynamics of the FS cells are very sensitive to differential drive, and determine which cells can spike before the resulting inhibition suppresses the rest. If more cells participate at the beginning of a gamma cycle, they produce more inhibition, and the gamma period that follows is longer. When the network is moderately heterogeneous, simulations show that it can entrain to the periodic excitation by allowing just enough cells to participate for the network frequency to match that of the external input. There has been no similar study for a target network showing persistent gamma rhythms, but, as these rhythms are carried mainly by high‐frequency FS cell participation, we conjecture that heterogeneity in the I‐cell population could create a similar response to periodic excitation by allowing differential recruitment. This is consistent with the work of Atallah & Scanziani (2009), who showed that gamma cycles that begin with stronger excitatory postsynaptic potentials (EPSPs) also show stronger IPSPs and have longer periods.
The model presented in Serenevy & Kopell (2013) underscores the importance of heterogeneity in the properties of cells and their connections. This study also investigated the effects of heterogeneous delays from the input sources to different elements of the network. It was shown that, at least under some conditions, inputs whose forcing phases were spread out over a portion of the cycle could entrain the network that could not be entrained when the forcing phases were identical [see White et al. (2000) for the article motivating this study].
Gamma rhythms and communication through coherence (CTC)
The previous results on frequency matching have interesting implications for CTC, a hypothesis popularized by Fries et al. This hypothesis suggests that communication between different regions of the brain can be facilitated by brain rhythms in the two regions, provided that the spikes from the sender appear at the target at the appropriate phase, generally when the level of inhibition at the target is at its lowest. The concept of CTC naturally raises the following question – what mechanism produces this optimal phase relationship between sender and target? It should be emphasized that, when a forced oscillator (receiver) and its forcer (sender), or a pair of mutually coupled oscillators, reach a stable phase relationship, that relationship depends on the details of the two oscillators – there is nothing in the general theory of oscillators that constrains that phase relationship to what might be optimal in the context of CTC.
The forced E–I feedback networks of the kind considered earlier automatically produce such an optimal phase relationship. When a sufficiently strong excitatory pulse is delivered to the network, it generally elicits an I‐cell volley, either directly or by first triggering an E‐cell volley. If the frequency of the input is approximately matched to the target frequency, the feedback inhibition from this volley wears off just when the next signal is due to arrive. Thus, the next signal arrives at an optimal time – the entrainment between the two regions automatically sets up an optimal phase difference for CTC (Fig. 5). This argument holds even in the presence of consistent conduction delays – no matter how long pulses takes to arrive at the entrained network, its IPSP begins immediately after a pulse arrives, and the next pulse arrives when inhibition is low.
Figure 5.
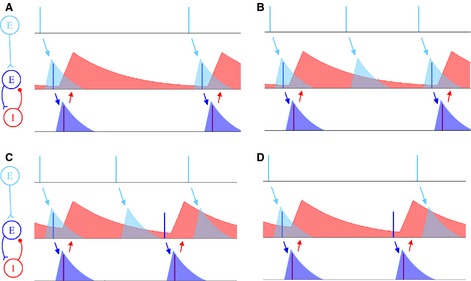
Creation of an optimal phase relationship for CTC. (A) EPSCs from an upstream population successfully induce 1 : 1 phase‐locking by periodically driving the E‐cell above its inhibition and triggering an excitatory volley, which is immediately followed by an inhibitory volley. When the circuit is phase‐locked, input pulses arrive when inhibition is low, an optimal condition for CTC. (B–D) EPSCs fail to phase‐lock the network 1 : 1, owing to frequency mismatch. (B) The forcing period is too short to phase‐lock the PING circuit 1 : 1, but it can phase‐lock the PING circuit 2 : 1 – the second pulse arrives when the E‐cell is under too much inhibition to spike, but the third one evokes an excitatory volley. (C) The forcing period is too short to phase‐lock the circuit. As in B, the second pulse arrives too early to evoke an excitatory spike; unlike in B, the third pulse is too late, and arrives under heavy inhibition. (D) The forcing period is too long to phase‐lock the circuit. The E‐cell recovers from inhibition, spikes, and triggers an inhibitory spike before the second pulse arrives. The second pulse arrives under too much inhibition to evoke another excitatory spike.
If two such E–I networks (e.g. in different cortical regions) are mutually coupled excitation‐to‐excitation, the above mechanisms can turn the interaction into one that is effectively directed – the gamma oscillator with the shorter (but sufficiently close) period will consistently recover from inhibition earlier than the other and spike, triggering the other oscillator to spike immediately afterwards. In this phase alignment, the faster oscillator's excitatory input arrives when the slower oscillator's inhibition is lowest, whereas the slower oscillator's input arrives when the faster oscillator's inhibition is highest. This relationship is optimal for CTC from the faster oscillator to the slower, but prevents the slow oscillator's excitation from having any effect on the faster circuit. The physiology creates not only an optimal phase alignment for CTC, but an enforced hierarchy of oscillators in which the faster one (which, all other parameters being equal, must be the more driven oscillator) can send to but not receive from the slower one. Thus, although it is not always correct that a faster oscillator precedes a slower one when they are mutually coupled (Kopell & Ermentrout, 2002), it is true for E–I networks in which the coupling occurs via mutual excitation.
This mechanism for directed inter‐areal interaction is consistent with recent data of Roberts et al. (2013), which showed that, when stimulus contrast is increased during a visual task (increasing coherence between gamma rhythms in V1 and V2, and the frequency of peak coherence between them), Granger causality becomes more strongly directed from V1 to V2. Such a strengthening of directed causality would be the natural outcome of strong phase‐locking by the mechanism suggested above – increased gamma frequency in V1 would cause it to more reliably phase‐lead V2, such that V2‐to‐V1 signals arrive under high V1 inhibition. It is also noteworthy that fluctuations in gamma frequency were highly correlated between V1 and V2 over a range of 10–15 Hz, which is approximately the degree of frequency matching that was achieved in the simulation of Fig. 4.
Another approach to coordination of sender and target was taken by Akam & Kullmann (2012). The main model in this study relies on a third oscillator that sends a rhythmic ‘external control input’ to synchronize the sender and the target. Their model is very effective at routing signals with the right frequency and phase, and filtering out the rest, but they offer no biophysical analog for their control signal, which must itself be somehow routed to a sender for it to gain an advantage over others. They also describe a bottom‐up method of frequency selectivity that relies on a network of interneurons near asynchrony with intrinsic firing rate resonance to act as a bandpass filter. By contrast, the Cannon–Kopell model assumes that interneurons are firing in nearly synchronous volleys, and take advantage of frequency‐selective phase‐locking rather than frequency‐selective firing rate resonance to filter out distracting inputs. The data suggest that, during gamma rhythms, FS interneurons do fire in discrete volleys (Fisahn et al., 1998), offering support for frequency selection by phase‐locking rather than by resonance.
Coordination between sender and target can also be achieved by two mechanisms that create zero‐phase‐lag synchronization between gamma‐rhythmic sending and receiving circuits. The first, described in Traub et al. (1996) and Ermentrout & Kopell (1998), involves mutual coupling between the two PING circuits, and deals with the effects of small coupling delays by creating two pulses of inhibition on each cycle (‘doublets’) in each circuit, one serving as locally generated feedback inhibition, and one as feedforward inhibition from the other circuit. The second, described in Tort et al. (2007), requires that multiple gamma‐rhythmic circuits be periodically reset by shared pulses of inhibition. In the hippocampus, these pulses are presumably created by oriens lacunosum moleculare interneurons firing together at a theta frequency (4–10 Hz), producing the well‐documented phenomenon of gamma oscillations nested in a theta rhythm. These zero‐phase‐lag mechanisms are useful if both circuits have to send signals to a third circuit and be heard at the same time.
Multiple gamma rhythms and bottom‐up processing
The framework provided by network filtering and CTC (above) shows how rhythms can relate and gate inputs among brain regions. On a spatially smaller scale, rhythms can also gate inputs among layers within a single brain region. One example is the rodent A1, in vitro. It was shown in Ainsworth et al. (2011) that the input layer (L4) and more superficial layers (L2/3) can each produce a different version of gamma rhythm in vitro; the L4 version is mechanistically closest to strong PING (although it requires recurrent excitation mediated by NMDA receptors), whereas the L2/3 version is the persistent variety described above. For changing levels of kainate‐induced excitation to the cortex, the frequencies of the two gammas rhythms behave differently – the L2/3 frequency is almost impervious to increasing excitation, whereas the L4 rhythm is highly sensitive. At different levels of excitatory drive, the two gamma rhythms interact in different ways. At a low level of excitation, there is little firing in L4, whose field potential follows that of L2/3, owing to descending inhibition to excitatory cells. At middle levels of excitation, there is synchrony between the gamma rhythms generated in both layers. At higher levels, the two frequencies break apart spectrally – the L4 gamma rhythms is significantly faster than that of L2/3. L5 follows the output of the layer with the higher expressed frequency, which is L2/3 for low levels of excitation and L4 for the higher levels.
We hypothesize that, in vivo, if there is a large (glutamatergic) input from the thalamus to L4 (e.g. a strong, salient environmental stimulus), this creates a high‐frequency gamma rhythm in L4 that is not coherent with activity in the superficial layers; thus, the spiking of L4 excitatory cells is not well aligned to that in L2/3, and some of the activity is shunted by the feedback inhibition within L2/3. However, the L4 activity is coherent with L5 (the output layer), and can therefore effectively produce spiking in that layer. In contrast, medium sensory inputs to L4 evoke gamma synchrony with L2/3, which may have a direct impact on the output of L5 (Lee et al., 2012). This is consistent with the idea that layers 2 and 3 are needed for higher cognitive functions, perhaps related to contextual processing (Petersen & Crochet, 2013), and may be less important for processing a very salient stimulus, in which gamma rhythms are not coordinated between L2/3 and L4.
The above modeling shows how the different gamma rhythms can gate and route signals within A1. In connection with other modeling work (Lee et al., 2009, 2012), it suggests a role for plasticity in sensory processing – Lee et al. showed that excitatory synapses between gamma‐rhythmic networks experience maximal spike‐time‐dependent facilitation if the presynaptic rhythm is faster than the natural frequency of the postsynaptic rhythm. This plasticity is expected to affect L4‐to‐L2/3 synapses when L4 receives strong drive, causing it to generate fast gamma rhythms while L2/3 continues to generate gamma rhythms at a lower frequency. In particular, such facilitation occurs if the ratio between the two frequencies is 1.6, the ratio between the frequencies of the two gamma rhythms generated in Ainsworth et al. (2011) at the highest dose of kainate. When this facilitation occurs during a salient input, it allows later inputs to L4 to more effectively entrain and transmit information to L2/3 (Fig. 6).
Figure 6.
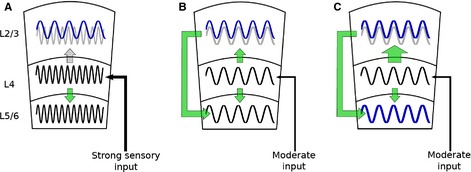
Different inputs to the sensory laminar neocortex may activate different modes of laminar engagement. The laminar schematic shown here illustrates L2/3, L4, and L5/6. Sinusoids depict gamma rhythms in each layer. The light gray sinusoid in L2/3 depicts oscillatory input from L4. Signal flow is depicted by green arrows. The size of the arrow denotes the relative strength of connection. (A) A strong sensory stimulus arrives from the thalamus to L4, producing a gamma rhythm (black trace). The rhythm in L4 is directly coherent with the L5 output layer. We propose that sensory information carried in the gamma frequency goes directly from L4 to L5/6. In contrast, the rhythm in L2/3 is at a lower gamma frequency than that in L4 and L5/6. Although immediate sensory information may not be processed directly by L2/3, the interaction of different gamma rhythms is predicted to create plasticity between L2/3 and L4. (B) In a more difficult task, such as a search task that requires context matching, moderate sensory input engages gamma rhythms in L4 that lock in frequency to L2/3. Sensory information is transmitted from L4 to L2/3. In contrast to A, L2/3 has a direct effect on L5/6 output. (C) Plasticity between E‐cell populations in L4 and L2/3 (denoted by the larger green arrow) is predicted to recruit activity in L2/3 that can increase the output of L5/6.
It is notable that the ratio of the two gamma frequencies is approximately the ‘golden mean’ (Roopun et al., 2008a,b). This number is the ‘most irrational number’, or the one that is least well approximated by a rational number [see Pletzer et al. (2010) on EEG and the golden mean for implications of EEG rhythms]. This fact is equivalent to saying that, for a ratio near this number, there are a large number of cycles of input from one to the other in which the input arrives when the feedback inhibition of the target is large enough to shunt out the input, as described above Thus, the natural frequencies are such that they interfere as little as possible with one another. The ratio between the beta 2 rhythm (described in detail below – 25 Hz) and the persistent gamma rhythm is also ~1.6 (Roopun et al., 2008a,b), which also helps to provide independence between the deep‐layer beta 2 rhythm and the persistent gamma rhythm.
The above results show how the physiological properties of gamma rhythms – and in particular, fast feedback inhibition – endow them with important computational properties. These include the capacity for filtering inputs by coherence and by frequency, and for creating appropriate phase relationships for directed CTC. We also discussed how the differential physiology of the cortex across layers can support multiple gamma rhythms that can interact to dynamically regulate the flow of information within cortical columns.
This section of the article has addressed cognitively relevant computations performed by the gamma rhythm. In the next section, we build upon this framework to discuss interactions of gamma rhythms with other frequency bands, emphasizing the implications for higher‐order cognitive function.
Interaction of gamma and beta rhythms
Top‐down beta rhythms provide gain control
In slices of rodent sensory and parietal cortices, gamma rhythms are found in superficial layers, whereas beta 2 rhythms are found in deep layers (Roopun et al., 2006, 2010). In these in vitro preparations, gamma and beta rhythms interact minimally (Roopun et al., 2008a). However, in the presence of temporally patterned input, the two can interact in ways that have important functional significance. One example of temporally patterned input is top‐down signaling in the beta frequency band, especially in the context of specific attention (Fries et al., 2001, 2008; Saalmann et al., 2007; Bosman et al., 2012).
We can obtain clues about specific attention from the in vitro work; although the experiments have been performed in a different species (rodent vs. monkey) and different structures (S2 and A1 vs. prefrontal cortex and V4), the experiments and modeling help to specify what features the physiology must have to behave in a similar way. The example described below shows how beta frequency input from a higher structure to the deep layers of a target cortex can lead to more activity and more gamma power in the superficial layers; as the superficial layers project to other parts of the cortex, and gamma rhythms are associated with the creation and protection of cell assemblies, it becomes clearer how top‐down signals may help to increase the signal‐to‐noise ratio in primary sensory areas and provide the ‘contextual’ activity required for the complex sensory tasks described above.
The relevance of the beta frequency in the top‐down signal becomes more apparent when we look more closely at the physiology of the rhythms in the target structure. In the case of Roopun et al. (2010), the latter is A1. In A1, in the presence of cholinergic drive, the superficial layers produce a gamma rhythm, as described above, whereas the deep layers produce a beta 2 (25‐Hz) rhythm. The cells most involved in the beta 2 rhythm are intrinsically bursting (IB) excitatory cells and the LTS inhibitory cells, which mediate inhibition that is longer than that of FS interneurons (Otsuka & Kawaguchi, 2009). The latter cells are active when there is cholinergic (nicotinic) drive. The natural beta frequency resonance of this subnetwork allows top‐down signals in the beta frequency to increase power and synchrony in the deep layers, the target of beta frequency input from S2 (Roopun et al., 2010). The activity in the deep layers is conveyed to the superficial ones by ascending fibers that produce slowly decaying inhibition in L4 and activation of LTS cells in L2/3. It is important to note that the enhancement of activity in the deep layers is dependent on beta frequency interactions, but the effect of this enhanced activity on the other layers is essentially tonic inhibition, and is not inherently tied to the beta frequency.
Modeling work (Lee et al., 2013) has shown that such connections can mediate gain control in the superficial layers in the presence of a stimulus by changing the inhibition in those layers – as discussed above under the physiology of gamma rhythms, if the FS population starts in a very active mode, inhibition to those cells from the deep‐layer LTS cells can increase the gamma power in L4, and hence increase the gamma input to L2/3 from L4. The activation of the LTS cells within L2/3 provides a similar function in that layer, preventing local RS cells from firing between gamma cycles. Thus, when the top‐down signals resonate with deep‐layer networks, there is more firing and more gamma power in the superficial layers. Figure 7 shows those connections within and between layers that are relevant to this discussion.
Figure 7.
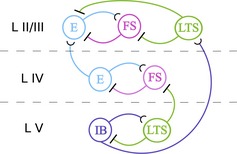
Most relevant cell types and connections for the model of a single cortical column in Lee et al. (2013). E, a population of regular‐spiking pyramidal neurons; FS, a population of FS interneurons; LTS, a population of LTS interneurons; IB, a population of IB pyramidal cells. All interneurons are inhibitory; all pyramidal cells are excitatory.
These ideas are applicable to the work of Fries et al. (2001), in which monkeys were trained to respond to stimuli that were or were not attended to. In the model of Lee et al., there are two columns, one representing the input from an attended stimulus, and one representing the input from the unattended stimulus. Both receive the same bottom‐up input, and the column associated with the attended stimulus receives top‐down beta input as described above. The deep layers of each column send ascending excitatory fibers to inhibitory cells of the other column. In the attention‐related column, the model produces more gamma rhythms and more activity in L2/3 than in the ‘unattended’ column. The modeling simulations reproduce the observation in Fries et al. (2001) that gamma power and synchronization increase with selective visual attention, and explain why the beta frequency signals do not just accompany selective attention, but can help to produce its cognitive effects.
It is well known that there is a connection between cholinergic modulation and attention (Gill et al., 2000; Sarter et al., 2005; Demeter & Sarter, 2013). The key role of the deep layer slow inhibitory (LTS) cells in mediating the gain control suggests one mechanism for this relationship. As mentioned above, these cells are activated via nicotinic receptors, suggesting that the gain control could disappear without this modulation (an effect observed in the models). Indeed, in pathologies that degrade nicotinic regulation, there are also deficits of attention (Martin & Freedman, 2007; Ohmura et al., 2012). Muscarinic modulation also adds to modulation of the LTS cells – muscarinic modulation of deep‐layer FS cells depresses their firing (Xiang et al., 2002), further releasing the slow inhibitory LTS cells from inhibition and thus further enhancing gain control. The gross effects of cholinergic modulation on gain control are evident in vitro – beta activity in S2 produces a beta rhythm or beta coherence in the downstream area A1 only in the presence of cholinergic input (Roopun et al., 2006, 2010).
The focus on physiology also helps to explain how cholinergic modulation, which works via diffuse projections, can appear to act in a selective way – the change in nicotinic and muscarinic tone in the deep layers changes the response of the superficial layers to input from the thalamus in ways that enhance the activity of those columns in response to specific top‐down signals.
Beta 1 rhythms and manipulation of cell assemblies
As with gamma rhythms (see above), rhythms within the classic beta band can have different mechanisms and modal peak frequencies. A particular form of beta rhythm is of special interest – in the rat parietal cortex (S2), but not in sensory cortices, a 15‐Hz rhythm (beta 1) can be seen during decreased excitatory drive following periods of intense excitation. Intense excitation (experimentally induced by kainate) produces a gamma rhythm in the superficial layers via pyramidal–FS cell interaction and a beta 2 (25‐Hz) rhythm in the deep layers via IB cells with slow inhibitory feedback from LTS cells. [Note that the mechanism for beta 2 rhythms in S2 is not the same as that discussed for A1 above (Roopun et al., 2006)]. When this excitation is reduced, both deep and superficial layers switch frequency to 15 Hz in a mechanism that has been shown to involve ‘concatenation’ of rhythms – each beta 1 cycle consists of one superficial gamma cycle (an FS volley, the decay of FS inhibition, and a volley of spikes from superficial pyramidal cells) followed by one deep‐layer beta 2 cycle (an LTS volley, the decay of LTS inhibition, and a burst of IB spikes) (Kramer et al., 2008; Roopun et al., 2008a,b) (Fig. 8). This beta 1 rhythm has the useful property that it can continue without further input (unlike all the forms of gamma and beta 2 rhythms described above). It can do so because each of the two parts of the rhythm is triggered by rebound from inhibition that arises from the previous part. This rhythm requires some baseline of drive, and stops when the voltage decreases enough; it also stops when there is enough new drive to transform the rhythms to superficial gamma and deep beta 2 rhythms.
Figure 8.
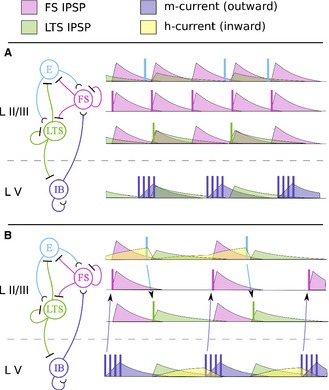
Beta 1 rhythm emerges from concatenation of gamma cycles and beta 2 cycles. Left – circuit diagrams portraying relevant connectivity between superficial FS interneurons, LTS interneurons, regular‐spiking pyramidal neurons (E), and deep‐layer IB neurons. Right – dynamics of each population over time, vertically aligned and color‐coded by cell type. Population spikes are portrayed as vertical lines. The time courses of synaptic and intrinsic currents are color‐coded by current type. (A) Under heavy kainate drive, a column of the cortex generates coexisting gamma and beta 2 rhythms in the superficial layers and deep layers, respectively. Gamma rhythms are paced by the rhythmic production of FS IPSPs, and beta 2 rhythms are paced by the rise and decay of the M‐current in IB neurons. (B) Under less drive and after plasticity, a column of the cortex generates a beta 1 rhythm that is coherent between superficial and deep layers. Owing to low drive, the H‐current builds up in the E and IB populations during IPSPs. When each IPSP wears off, the excitation provided by the H‐current triggers E or IB spikes, which, in turn, trigger LTS or FS spikes, respectively. FS and LTS create IPSPs in alternation, and beta 1 rhythms are paced by the concatenation of the gamma and beta 2 cycles.
Beta 1 rhythms are known to be associated with situations involving coordination of inputs from multiple modalities (von Stein et al., 1999; Senkowski et al., 2008; Dean et al., 2012; Engel et al., 2012). The physiological properties of this parietal beta rhythm can facilitate such coordination – in the absence of another rhythm, cell assemblies that are created in the standard gamma rhythms described above do not allow for integration of inputs over space unless the inputs are well coordinated in both time and strength; otherwise, the shared recurrent inhibition created by excited pyramidal cells can shunt out the excitation cell receiving input via another modality. Thus, the same properties that allow these gamma rhythms to protect assemblies against distractors make it difficult to manipulate them, by adding or subtracting components. By contrast, this beta 1 rhythm can ‘host’ cell assemblies producing a gamma rhythm within the beta rhythm, to which cells can be added (Kopell et al., 2011). The essential mechanistic difference is that, in the simple versions of gamma rhythms described above, the most excitable cells inhibit others via fast feedback inhibition within the superficial layers. In contrast, in the beta 1 rhythm, the FS interneurons and inhibitory cells with slower inhibition are controlled at least partly from the deeper layers (Fig. 7), reducing or eliminating the local competition within the superficial layers. Thus, modulation from the deep layers can change not only the firing rate, but also the degree of competition among cell assemblies, allowing the latter to be modified by incoming activity.
Beta 1 rhythms have also been implicated in the build‐up of evidence for making a decision (Tallon‐Baudry et al., 2004; Donner et al., 2009; Siegel et al., 2011; Spitzer & Blankenburg, 2011), in which the parietal cortex is believed to be involved (Huk & Shadlen, 2005; Kiani & Shadlen, 2009). It is possible that the physiology of this beta 1 rhythm can facilitate such a build‐up, as follows. As described above, the beta 1 rhythm occurs following an input (modeled in vitro by kainate excitation), creating a superficial gamma oscillation and deep‐layer beta 2 oscillation. This switch in frequencies requires plasticity, modeled in Kramer et al. (2008) as progressively enhanced connectivity among deep‐layer IB cells. Now, suppose there is ongoing input to some part of the cortex, as in the random dots paradigm (Mazurek et al., 2003). As relevant cell assemblies in the deep layers become repeatedly activated, it is likely that there will be increased plasticity among such cells, and increased coherence, allowing an increase in the beta 1 power produced in the relevant columns. Indeed, the model in Kramer et al. (2008) shows increased beta 1 power every time there is a burst of gamma rhythms, so it can be considered to be an ‘integral’ of the ongoing gamma power, an analog to an integration of input (Huk & Shadlen, 2005), as in ‘drift‐diffusion’ models of decision‐making (Deco et al., 2013). It is not clear how this build‐up in beta 1 power can be translated into ramping up of rates in single neurons (Wang, 2012). However, recent work of Canolty et al. (2012) has shown that there is a task‐dependent connection between beta power and firing rate in the motor cortex; this beta rhythm is not likely to have the same mechanism, (Yamawaki et al., 2008), but can coordinate with, or even be driven by, parietal beta rhythms. See also Pesaran et al. (2008) for beta rhythms and decisions.
The mystery of multiple beta rhythms
We have shown above that some connections have been drawn between the physiology of the beta rhythm and its roles in cognition. However, the beta rhythm has been ascribed multiple functions, many of which have not yet been connected to mechanisms or physiology. Here, we summarize these functions, and then focus on systems‐level models of beta rhythm in the basal ganglia and thalamus that may serve as a first step towards drawing connections between these functions and physiology.
Functions that have been suggested for the beta rhythm include – coordination among many parts of the neocortex (Siegel et al., 2012); inhibition of movement (Pogosyan et al., 2009); preservation of the status quo (Engel & Fries, 2010); the use of cues for movement (Leventhal et al., 2012); motor planning (Leocani et al., 1997); language processing (Hanslmayr et al., 2009; Weiss & Mueller, 2012); signaling whether there is enough evidence for a decision (Tallon‐Baudry et al., 2004; Donner et al., 2009; Siegel et al., 2011; Spitzer & Blankenburg, 2011); focusing action‐selection network functions (Courtemanche et al., 2003); and rule learning (Buschman et al., 2012). See Engel & Fries (2010) for a review of hypotheses about beta rhythms. Furthermore, it has been noted that beta rhythms tend to mediate signals going from higher‐order structures to lower‐order structures (Fries et al., 2001, 2008; Buschman & Miller, 2007). These beta rhythms come in various overlapping ranges – in addition to the beta 1 and beta 2 rhythms discussed above (in the 12–20‐Hz and 20–30‐Hz range, respectively), a frequency band around 20 Hz is associated with motor planning and control. However, it is unclear why rhythms in those frequency ranges should be especially useful for such functions, and why so many functions are associated with them.
We note that the literature already describes many different mechanisms of beta rhythm generation, including the mechanisms for beta 2 rhythms in A1 and S2 discussed above, the concatenation beta 1 rhythm discussed above, ‘beat‐skipping beta’ in the hippocampus (Traub et al., 1999; Olufsen et al., 2003), and ‘period‐doubling beta’ in the entorhinal cortex (Pervouchine et al., 2006), as well as the many possibilities suggested below. We focus below on hypotheses (not mutually exclusive) about the origins of the beta rhythms most associated with the cortico–basal ganglia–thalamic (CBT) loop, although the other beta rhythms may also interact with them. In light of previous sections, we consider beta oscillations in cortical and subcortical structures in the context of potential functional implications.
Beta rhythms in the basal ganglia and motor cortex are perhaps most well known in the context of motor control (Brittain & Brown, 2013). Many studies have shown that desynchronization in the beta frequency band occurs before and during a voluntary movement in the cortex (Devos et al., 2006), the striatum (Sochurkova & Rektor, 2003), the subthalamic nucleus (STN) (Devos et al., 2006), the internal segment of the globus pallidus (GPi) (Brücke et al., 2008), and the thalamus (Klostermann et al., 2007). Furthermore, beta oscillations have been shown to be coherent between the somatomotor cortex and muscle (van Ede & Maris, 2013). Beta oscillations are elevated in Parkinson's disease, and are correlated with the parkinsonian motor symptoms of bradykinesia and rigidity (Kühn et al., 2006). This has led to the theory that the beta rhythm in the CBT loop is an antikinetic signal (Brown, 2006).
However, recent studies have brought into question the idea of beta rhythms in the CBT loop as being purely movement‐related, and suggest a more cognitive role for this rhythm under normal circumstances. This incorporates CBT beta rhythms into a growing body of evidence for functional roles of subcortical structures such as the basal ganglia in cognition (Ding & Gold, 2013; Mitchell & Chakraborty, 2013; Weintraub & Zaghloul, 2013). In particular Leventhal et al. (2012) have found that the beta rhythm within the CBT loop in rats is not elevated purely in response to movement or to sensory input, but rather is consistently elevated in response to the use of a cue to direct voluntary movement. This suggests that beta rhythms in the CBT loop are perhaps involved in more cognitive functions – perception, attention, decision‐making, and/or working memory. More examples include the following. Beta rhythms in the somatomotor cortex, a part of the cortex that is highly interconnected with the basal ganglia and thalamus, have been tied to perceptual decision‐making, potentially reflecting the accumulation of evidence (Donner et al., 2009; Haegens et al., 2011). Suppression of beta rhythms in the somatomotor cortex has been associated with enhanced tactile perception (Jones et al., 2010). It has been found that even anticipation of a somatosensory demand decreases beta rhythms in the cortex and muscle, and that the decrease in muscular beta power is independent of both motor preparation and movement execution (van Ede & Maris, 2013). Beta rhythms in the CBT loop have also been implicated in working memory – coherence between beta oscillations in the medial dorsal nucleus of the thalamus and the prefrontal cortex, both of which are highly interconnected with basal ganglia, is correlated with working memory (Parnaudeau et al., 2013). See also Brittain & Brown (2013) for more examples.
To understand the functional implications of the beta rhythms for all of the observations above, it is important to identify the physiological bases of the various beta rhythms in the CBT loop. Characterization of the cellular and network components underlying generation of the beta rhythms in the CBT loop should provide much‐needed insights into how this loop transforms inputs into behaviorally relevant signals, as well as the role of the beta rhythm in this transformation. Physiological studies and computational models have suggested multiple sites as potential generators of beta rhythms in the CBT network, with multiple mechanisms. Even within the cortex, there are multiple (non‐exclusive) candidates. Beta oscillations (at ~27 Hz) can be evoked in the primary motor cortex in the presence of carbachol and kainate (Yamawaki et al., 2008), indicating that the primary motor cortex has the intrinsic network connectivity that is necessary to support the generation of beta oscillations. These oscillations were generated in L5/6 of the primary motor cortex, and were dependent on both GABAA receptor transmission and gap junctions (and were independent of AMPA). Deep cortical layers in the rodent A1 and S2 have also been found to produce beta oscillations (at ~25 Hz) (Roopun et al., 2006, 2010). The former requires both AMPA and GABAA receptors, whereas the latter depends on gap junctions and is independent of chemical synapses. Additionally, computational modeling has suggested that beta oscillations may be generated in S1 as a dynamic response to synchronous ~10 Hz input to deep‐layer pyramidal cell dendrites – feedforward input comes from the lemniscal thalamus to the dendrites in the deep layers, and feedback input arrives from the non‐lemniscal thalamus (or other cortical areas) to the dendrites in the superficial layers (Jones et al., 2009). This mechanism suggests that coordination of larger networks involving subcortical structures is necessary to produce a beta rhythm in S1.
Beta oscillations are prevalent in the STN of Parkinson's disease patients (Levy et al., 2002; Steigerwald et al., 2008), raising the question of whether the STN is involved in the generation of basal ganglia beta rhythms. The reciprocal connections between the STN and the external segment of the globus pallidus (GPe) have been investigated as a potential source of beta rhythms in the basal ganglia. The STN and GPe are able to generate slow oscillatory activity (~1 Hz) in organotypic cultures, demonstrating the intrinsic oscillatory capacity of these two interconnected nuclei (Plenz & Kital, 1999). Additionally, computational modeling suggests that, in the presence of parkinsonian perturbations, the STN and GPe can oscillate rhythmically (Terman et al., 2002) and at beta frequency (Holgado et al., 2010).
More recently, it has been suggested that the striatum has the intrinsic cellular and network components to make it a generator of beta rhythms in the CBT network. Robust beta oscillations arise in vivo in the mouse striatum in response to increased cholinergic tone (McCarthy et al., 2011). Computational modeling suggests that the interaction between the synaptic GABAA currents in medium spiny neurons (MSNs) and the intrinsic membrane M‐current within MSNs is critical for the production of beta rhythms in striatal networks (McCarthy et al., 2011). These two currents promote network interactions between MSNs, owing to post‐inhibitory rebound spiking. This mechanism has been investigated extensively both in computational models (McCarthy et al., 2008, 2011) and within a more mathematical framework (McCarthy & Kopell, 2012; Mitry et al., 2013). The M‐current and/or the GABAA current are modulated by various intrinsic [e.g. somatostatin (Moore et al., 1988), dynorphin (Madamba et al., 1999), and neurosteroids (di Michele et al., 2013)] and extrinsic neuromodulators, such as anesthetics and sedatives (Brown et al., 2010), making the production of beta rhythms in this system highly regulated. Additionally, as MSN‐to‐MSN GABAergic inhibition increases with the MSN spiking rate, any regulator of MSN excitability will also modulate the striatal beta oscillation. MSNs are modulated by both dopamine and acetylcholine (Kreitzer, 2009), suggesting that the beta oscillation can be upregulated or downregulated, depending on the levels of these two prominent neuromodulators within the striatum. As beta rhythms are involved in cognitive function, it is particularly noteworthy that cognitive function is altered by many of the neuromodulators that affect, directly and/or indirectly, the MSNs of the striatum, including – dopamine (Jaber et al., 1996), acetylcholine (Zugno et al., 2013), opioids (Iordanova et al., 2006), β‐adrenergic agonists (Beversdorf et al., 2002), and somatostatin (Tuboly & Vécsei, 2013).
An interesting connection between striatally generated beta rhythms and attention to salient sensory stimuli may reside in the projections from the thalamus to the striatum. Shifts in attention in the presence of salient stimuli are thought to involve the intralaminar nuclei of the thalamus (Smith et al., 2009). These intralaminar nuclei project strongly to the striatum (Smith et al., 2004). Recent work by Ding et al. (2010) indicates that these thalamostriatal afferents gate corticostriatal input via their action on striatal cholinergic interneurons – thalamostriatal inputs that mimic a response to salient sensory stimuli induce a burst spiking and pause response in striatal cholinergic interneurons. As described above, computational models suggest that an increased striatal acetylcholine level resulting from the bursting of cholinergic neurons should create a transient increase in beta power in the neurons of the indirect pathway (McCarthy et al. 2011), which are those that primarily respond to acetylcholine with increases in excitability (Benarroch, 2012). This is consistent with the finding of Leventhal et al. (2012) that beta rhythms are elevated after a salient cue (i.e. a cue used for voluntary movement). Thus, the behavioral effect of thalamostriatal input that mimics the response to salient stimuli, i.e. redirecting attention and suppressing ongoing motor activity, may be mediated through the production of beta oscillations in striatal circuits.
Although the combination of physiological and computational studies has provided several plausible ideas regarding the sources of the beta rhythm, there is a huge amount of work to be performed to go from there to a comprehensive mechanistic explanation of how beta rhythms in the CBT loop mediate cognitive and motor functions. (The results described in the previous section suggest functional implications for neocortical beta rhythms.) The explanation may not depend on the origin of the beta rhythm, but rather on its eventual effects on the primary output structure of the basal ganglia – the thalamus. Computational modeling has shown that increased low‐frequency (6 or 10 Hz) synchrony of the output to the thalamus from the GPi decreases the relay reliability of the thalamus (Rubin & Terman, 2004; Agarwal & Sarma, 2012) – increased rhythmicity in the GPi creates phasic inhibition of the thalamus that results in increased bursting of thalamocortical cells and decreased responsiveness to sensorimotor input (Rubin & Terman, 2004). Model simulations also show that deep brain stimulation to the subthalamic nucleus, which sends excitatory projections to the GPi, decreases thalamic relay when the stimulation frequency is 25 Hz (in the beta frequency range) by inducing extended periods of strong GPi inhibition of the thalamus (Rubin & Terman, 2004).
Work by Kane et al. (2009) suggests further complexity – in the thalamic reticular nucleus, it is high frequency (110–170 Hz) rather than low frequency (alpha or beta) that dominates the local field activity in the parkinsonian state. In contrast, when patients are treated with dopaminergic medications, which decrease the power of beta oscillations in STN (Levy et al., 2002), the neurons in the thalamic reticular nucleus show increased coherence in the beta frequency range. This raises the questions of how beta oscillations in the basal ganglia are transformed in the thalamus, and how the alterations in neuromodulation and network‐level dynamics in Parkinson's disease alter normal thalamic computation. Although earlier work has provided us with the important insight that information transfer through the thalamus may be disrupted in the parkinsonian state, the mechanism may not be as straightforward as low‐frequency oscillations from the GPi inducing low‐frequency oscillations in the thalamus.
An additional question of importance concerns the coordination of the many potential beta generators within the CBT loop. In normal rats, episodes of beta rhythm occur almost simultaneously in the cortex, striatum, globus pallidus, and STN (Leventhal et al., 2012). Human cortical and basal ganglia beta oscillations have also been shown to be coherent (Williams et al., 2002). This is consistent with the idea that beta rhythms coordinate long‐range information processing (Kopell et al., 2000; Siegel et al., 2012). A combination of physiological studies and computational modeling will probably suggest mechanisms for coordination of beta rhythms at these multiple sites, and also elucidate the biophysical and network mechanisms that make this coordination functionally important for signaling and information processing.
Summary
Although brain rhythms are associated with all forms of cognitive activity (including sensory and motor processing), and appear in a wide range of neocortical and subcortical structures, their role in facilitating cognitive activity is far from understood. In this article, we support the idea that the physiology underlying brain rhythms contributes to the ways in which the rhythms facilitate function; that is, it is not just the frequencies of the rhythms that matter, but also the mechanisms that produce them. We demonstrated this by discussing how gamma oscillations filter their inputs, and how the associated physiological mechanisms lead to a mechanism for communication through coherence. A more complex interaction of gamma rhythms was discussed in connection with multiple gamma rhythms in A1 that act to route incoming signals, and perhaps prime the network for plasticity that is useful for later processing. These examples illustrate the point that the local dynamic behavior arising from the mechanisms underlying gamma rhythms can be seen as vital for communication in the brain.
We also focused on the interaction between beta and gamma rhythms, adding another layer of complexity. In the first example, we focused on top‐down beta rhythms, and how an incoming signal resonates with circuits in the deep layers that provide ascending inhibition and excitation. The ascending signals, filtered through the slow inhibition provided by LTS cells, change the balance of excitation and inhibition, leading to more gamma and more activity in the superficial layers. This example illustrates some of the subtleties of interacting rhythms – an oscillation‐mediated interaction (in this case, deep‐layer cells resonating with beta frequency input) may have highly significant downstream effects that are not inherently tied to the oscillation (in this case, the creation of tonic inhibition in superficial layers). Furthermore, the effect of the top‐down beta signal on the deep layers depends on nicotinic activation of those LTS cells, so there would be different outcomes in the absence of cholinergic modulation (as in an unattended state). More generally, this story illustrates that the effect of an incoming signal on a target (in this case, the deep layers of the cortex) depends hugely on the dynamic state of that target.
The second example imparts a different lesson – the beta 1 rhythm that appears after intense excitation is reduced shows that rhythms can be history‐dependent. The resulting beta 1 rhythm has special physiological properties (activation of deep and superficial layers sequentially by rebound inhibition) that are useful for coordinating inputs over different sensory modalities and over time. There is much more to understand about how the dynamics of this rhythm can be modulated to gate, coordinate or combine cell assemblies, and this story can be construed as the beginning of a wide‐open study of plasticity and rhythms in cognitive function (Stokes et al., 2013).
Finally, we demonstrate both the difficulty of and the need for further study of rhythms and cognitive function. We have focused on beta oscillations in multiple parts of the brain, especially the cortico–basal ganglia–thalamic loop. We describe multiple functions associated with this frequency band, and the multiple potential physiological mechanisms by which that band can be created. The very large question raised by this is the connection between physiology and function; why are there so many beta rhythms, and are they facilitating different but related computations? For this question, there are only the beginning of answers. For example, we do not have a body of work that has investigated the effects of temporally patterned inputs on the various beta oscillations, parallel to the work described on gamma (which is itself still evolving). The mechanisms of rhythms in the basal ganglia have been investigated far less than those in the cortex or thalamus. The ways in which the beta rhythms are coordinated by still slower rhythms (Dejean et al., 2011) are not well understood [but see Carracedo et al. (2013)]. And, of course, one might ask similar questions regarding all other frequency bands.
Although rhythms are by no means the only mechanisms used by the brain to encode and coordinate signaling (Ainsworth et al., 2012), we believe that they provide important mechanisms. The recent work discussed here is starting to untangle the mystery of why these dynamics are so tightly associated with cognitive activity.
Acknowledgements
This work was partially supported by DMS‐1042134 to N. Kopell, NIH‐5R01NS067199 to N. Kopell and C. Börgers, and DMS‐1225647 to N. Kopell. We thank Mark Kramer and Howard Gritton for helpful conversations.
Abbreviations
- CBT
cortico–basal ganglia–thalamic
- CTC
communication through coherence
- E‐cell
excitatory fast‐spiking cell
- EEG
electroencephalography
- E–I
excitatory–inhibitory
- EPSP
excitatory postsynaptic potential
- FS
fast‐spiking
- GPe
external segment of the globus pallidus
- GPi
internal segment of the globus pallidus
- IB
intrinsically bursting
- I‐cell
inhibitory fast‐spiking cell
- IPSP
inhibitory postsynaptic potential
- LTS
low‐threshold spiking
- MSN
medium spiny neuron
- NMDA
N‐methyl‐d‐aspartate
- PING
pyramidal–interneuronal network gamma
- STN
subthalamic nucleus
References
- Agarwal, R. & Sarma, S.V. (2012) The effects of DBS patterns on basal ganglia activity and thalamic relay. J. Comput. Neurosci., 33, 151–167. [DOI] [PubMed] [Google Scholar]
- Ainsworth, M. , Lee, S. , Cunningham, M.O. , Roopun, A.K. , Traub, R.D. , Kopell, N.J. & Whittington, M.A. (2011) Dual gamma rhythm generators control interlaminar synchrony in auditory cortex. J. Neurosci., 31, 17040–17051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth, M. , Lee, S. , Cunningham, M.O. , Traub, R.D. , Kopell, N.J. & Whittington, M.A. (2012) Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron, 75, 572–583. [DOI] [PubMed] [Google Scholar]
- Akam, T.E. & Kullmann, D.M. (2012) Efficient “communication through coherence” requires oscillations structured to minimize interference between signals. PLoS Comput. Biol., 8, e1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam, T. , Oren, I. , Mantoan, L. , Ferenczi, E. & Kullmann, D.M. (2012) Oscillatory dynamics in the hippocampus support dentate gyrus–CA3 coupling. Nat. Neurosci., 15, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah, B.V. & Scanziani, M. (2009) Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron, 62, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos, M. , Vida, I. , Frotscher, M. , Meyer, A. , Monyer, H. , Geiger, J.R. & Jonas, P. (2002) Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc. Natl. Acad. Sci. USA, 99, 13222–13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch, E.E. (2012) Effects of acetylcholine in the striatum. Recent insights and therapeutic implications. Neurology, 79, 274–281. [DOI] [PubMed] [Google Scholar]
- Beversdorf, D.Q. , White, D.M. , Chever, D.C. , Hughes, J.D. & Bornstein, R.A. (2002) Central β‐adrenergic modulation of cognitive flexibility. NeuroReport, 13, 2505–2507. [DOI] [PubMed] [Google Scholar]
- Bichot, N.P. , Rossi, A.F. & Desimone, R. (2005) Parallel and serial neural mechanisms for visual search in macaque area V4. Science, 308, 529–534. [DOI] [PubMed] [Google Scholar]
- Börgers, C. & Kopell, N. (2003) Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput., 15, 509–538. [DOI] [PubMed] [Google Scholar]
- Börgers, C. & Kopell, N. (2005) Effects of noisy drive on rhythms in networks of excitatory and inhibitory neurons. Neural Comput., 17, 557–608. [DOI] [PubMed] [Google Scholar]
- Börgers, C. & Kopell, N.J. (2008) Gamma oscillations and stimulus selection. Neural Comput., 20, 383–414. [DOI] [PubMed] [Google Scholar]
- Börgers, C. & Walker, B. (2013) Toggling between gamma‐frequency activity and suppression of cell assemblies. Front. Comput. Neurosci., 7, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börgers, C. , Epstein, S. & Kopell, N.J. (2005) Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc. Natl. Acad. Sci. USA, 102, 7002–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börgers, C. , Epstein, S. & Kopell, N.J. (2008) Gamma oscillations mediate stimulus competition and attentional selection in a cortical network model. Proc. Natl. Acad. Sci. USA, 105, 18023–18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman, C.A. , Schoffelen, J.M. , Brunet, N. , Oostenveld, R. , Bastos, A.M. , Womelsdorf, T. , Rubehn, B. , Stieglitz, T. , De Weerd, P. & Fries, P. (2012) Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron, 75, 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittain, J.S. & Brown, P. (2013) Oscillations and the basal ganglia: motor control and beyond. NeuroImage, doi: 10.1016/j.neuroimage.2013.05.084 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, P. (2006) Bad oscillations in Parkinson's disease. J. Neural Transm.‐Supp., 70, 27–30. [DOI] [PubMed] [Google Scholar]
- Brown, E.N. , Lydic, R. & Schiff, N.D. (2010) General anesthesia, sleep, and coma. New Engl. J. Med., 363, 2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brücke, C. , Kempf, F. , Kupsch, A. , Schneider, G.H. , Krauss, J.K. , Aziz, T. , Yarrow, K. , Pogosyan, A. , Brown, P. & Kühn, A.A. (2008) Movement‐related synchornization of gamma activity is lateralized in patients with dystonia. Eur. J. Neurosci., 27, 2322–2329. [DOI] [PubMed] [Google Scholar]
- Burns, S.P. , Xing, D. & Shapley, R.M. (2011) Is gamma‐band activity in the local field potential of V1 cortex a ‘clock’ or filtered noise? J. Neurosci., 31, 9658–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman, T.J. & Miller, E.K. (2007) Top‐down versus bottom‐up control of attention in the prefrontal and posterior parietal cortices. Science, 315, 1860–1862. [DOI] [PubMed] [Google Scholar]
- Buschman, T.J. , Denovellis, E.L. , Diogo, C. , Bullock, D. & Miller, E.K. (2012) Synchronous oscillatory neural ensembles for rules in the prefontal cortex. Neuron, 76, 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty, R.T. , Edwards, E. , Dalal, S.S. , Soltani, M. , Nagarajan, S.S. , Kirsch, H.E. , Berger, M.S. , Barbaro, N.M. & Knight, R.T. (2006) High gamma power is phase‐locked to theta oscillations in human neocortex. Science, 313, 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty, R.T. , Ganguly, K. & Carmena, J.M. (2012) Task‐dependent changes in cross‐level coupling between single neurons and oscillatory activity in multiscale networks. PLoS Comput. Biol., 8, e1002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin, J.A. , Carlén, M. , Meletis, K. , Knoblich, U. , Zhang, F. , Deisseroth, K. , Tsai, L.H. & Moore, C.I. (2009) Driving fast‐spiking cells induces gamma rhythm and controls sensory responses. Nature, 459, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo, L.M. , Kjeldsen, H. , Cunnington, L. , Jenkins, A. , Schofield, I. , Cunningham, M.O. , Davies, C.H. , Traub, R.D. & Whittington, M.A. (2013) A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms. J. Neurosci., 33, 10750–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin, L.L. , Denninger, T. , Fyhn, M. , Hafting, T. , Bonnevie, T. , Jensen, O. , Moser, M.B. & Moser, E.I. (2009) Frequency of gamma oscillations routes flow of information in the hippocampus. Nature, 462, 353–357. [DOI] [PubMed] [Google Scholar]
- Courtemanche, R. , Fujii, N. & Graybiel, A.M. (2003) Synchronous, focally modulated beta‐band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J. Neurosci., 23, 11741–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, H.L. , Hagan, M.A. & Pesaran, B. (2012) Only coherent spiking in posterior parietal cortex coordinates looking and reaching. Neuron, 73, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco, G. , Rolls, E.T. , Albantakis, L. & Romo, R. (2013) Brain mechanisms for perceptual and reward‐related decision‐making. Prog. Neurobiol., 103, 194–213. [DOI] [PubMed] [Google Scholar]
- Dejean, C. , Arbuthnott, G. , Wickens, J.R. , Le Moine, C. , Boraud, T. & Hyland, B.I. (2011) Power fluctuations in beta and gamma frequencies in rat globus pallidus: association with specific phases of slow oscillations and differential modulation by dopamine D1 and D2 receptors. J. Neurosci., 31, 6098–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter, E. & Sarter, M. (2013) Leveraging the cortical cholinergic system to enhance attention. Neuropharmacology, 64, 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos, D. , Szurhaj, W. , Reyns, N. , Labyt, E. , Houdayer, E. , Bourriez, J.L. , Cassim, F. , Krystkowiak, P. , Blond, S. , Destée, A. , Derambure, P. & Defebvre, L. (2006) Predominance of the contralateral movement‐related activity in the subthalamo‐cortical loop. Clin. Neurophysiol., 117, 2315–2327. [DOI] [PubMed] [Google Scholar]
- Ding, L. & Gold, J.I. (2013) The basal ganglia's contributions to perceptual decision making. Neuron, 79, 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, J.B. , Guzman, J.N. , Peterson, J.D. , Goldberg, J.A. & Surmeier, D.J. (2010) Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron, 67, 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipoppa, M. & Gutkin, B.S. (2013) Flexible frequency control of cortical oscillations enables computations required for working memory. Proc. Natl. Acad. Sci. USA, 110, 12828–12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner, T.H. , Siegel, M. , Fries, P. & Engel, A.K. (2009) Buildup of choice‐predictive activity in human motor cortex during perceptual decision making. Curr. Biol., 19, 1581–1585. [DOI] [PubMed] [Google Scholar]
- van Ede, F. & Maris, E. (2013) Somatosensory demands modulate muscular beta oscillations, independent of motor demands. J. Neurosci., 33, 10849–10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, A.K. & Fries, P. (2010) Beta‐band oscillations – signalling the status quo? Curr. Opin. Neurobiol., 20, 156–165. [DOI] [PubMed] [Google Scholar]
- Engel, A.K. , Senkowski, D. & Schneider, T.R. (2012) Multisensory integration through neural coherence In Murray M.M. & Wallace M.T. (Eds), The Neural Bases of Multisensory Processes. CRC Press, Boca Raton. Chapter 7. [PubMed] [Google Scholar]
- Ermentrout, G.B. & Kopell, N. (1998) Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc. Natl. Acad. Sci. USA, 95, 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout, G.B. & Terman, D.H. (2010) Mathematical Foundations of Neuroscience. Springer, New York. [Google Scholar]
- Fisahn, A. , Pike, F.G. , Buhl, E.H. & Paulsen, O. (1998) Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro . Nature, 394, 186–189. [DOI] [PubMed] [Google Scholar]
- Fries, P. , Reynolds, J.H. , Rorie, A.E. & Desimone, R. (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science, 291, 1560–1563. [DOI] [PubMed] [Google Scholar]
- Fries, P. , Schröder, J.H. , Roelfsema, P.R. , Singer, W. & Engel, A.K. (2002) Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J. Neurosci., 22, 3739–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries, P. , Nikolic, D. & Singer, W. (2007) The gamma cycle. Trends Neurosci., 30, 309–316. [DOI] [PubMed] [Google Scholar]
- Fries, P. , Womelsdorf, T. , Oostenveld, R. & Desimone, R. (2008) The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J. Neurosci., 28, 4823–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, T.M. , Sarter, M. & Givens, B. (2000) Sustained visual attention performance‐associated prefrontal neuronal activity: evidence for cholinergic modulation. J. Neurosci., 20, 4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Nácher, V. , Hernández, A. , Luna, R. , Jensen, O. & Romo, R. (2011) Beta oscillations in the monkey sensorimotor network reflect somatosensory decision making. Proc. Natl. Acad. Sci. USA, 108, 10708–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr, S. , Spitzer, B. & Bäuml, K.H. (2009) Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories”. Cereb. Cortex, 19, 1631–1640. [DOI] [PubMed] [Google Scholar]
- Holgado, A.J.N. , Terry, T.R. & Bogacz, R. (2010) Conditions for the generation of beta oscillations in the subthalamic nucleus–globus pallidus network. J. Neurosci., 30, 12340–12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huk, A.C. & Shadlen, M.N. (2005) Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J. Neurosci., 25, 10420–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, C. & Scanziani, M. (2007) It's about time for thalamocortical circuits. Nat. Neurosci., 10, 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova, M.D. , McNally, G.P. & Westbrook, R.F. (2006) Opioid receptors in the nucleus accumbens regulate attentional learning in the blocking paradigm. J. Neurosci., 26, 4036–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber, M. , Robinson, S.W. , Missale, C. & Caron, M.G. (1996) Dopamine receptors and brain function. Neuropharmacology, 35, 1503–1519. [DOI] [PubMed] [Google Scholar]
- Jones, S.R. , Pritchett, D.L. , Sikora, M.A. , Stufflebeam, S.M. , Hämäläinen, M. & Moore, C.I. (2009) Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: rhythmogenesis and modulation of sensory‐evoked responses. J. Neurophysiol., 102, 3554–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S.R. , Kerr, C.E. , Wan, Q. , Pritchett, D.L. , Hamalainen, M. & Moore, C.I. (2010) Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci., 30, 13760–13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras, M.J. , Fries, P. & Buffalo, E.A. (2013) Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc. Natl. Acad. Sci. USA, 110, 13144–13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, A. , Hutchison, W.D. , Godaie, M. , Lozano, A.M. & Dostrovsky, J.O. (2009) Dopamine‐dependent high‐frequency oscillatory activity in thalamus and subthalamic nucleus of patients with Parkinson's disease. NeuroReport, 20, 1549–1553. [DOI] [PubMed] [Google Scholar]
- Kiani, R. & Shadlen, M.N. (2009) Representation of confidence associated with a decision by neurons in the parietal cortex. Science, 324, 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann, F. , Nikulin, V.V. , Kühn, A.A. , Marzinzik, F. , Wahl, M. , Pogosyan, A. , Kupsch, A. , Schneider, G.H. , Brown, P. & Curio, G. (2007) Task‐related differential dynamics of EEG alpha‐ and beta‐band synchronization in cortico‐basal motor structures. Eur. J. Neurosci., 25, 1604–1615. [DOI] [PubMed] [Google Scholar]
- Kopell, N. & Ermentrout, G.B. (2002) Mechanisms of phase‐locking and frequency control in pairs of coupled neural oscillators In Fiedler B. (Ed.), Handbook on Dynamical Systems, Volume 2: Toward Applications. Elsevier, Philadelphia, pp. 3–54. [Google Scholar]
- Kopell, N. , Ermentrout, G.B. , Whittington, M.A. & Traub, R.D. (2000) Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl. Acad. Sci. USA, 97, 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell, N. , Kramer, M.A. , Malerba, P. & Whittington, M.A. (2010a) Are different rhythms good for different functions? Front. Hum. Neurosci., 4, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell, N. , Börgers, C. , Pervouchine, D. , Malerba, P. & Tort, A. (2010b) Gamma and theta rhythms in biophysical models of hippocampal circuits In Cutsuridis V., Graham B.P., Cobb S. & Vida I. (Eds), Hippocampal Microcircuits: A Computational Modeler's Resource Book. Springer, New York, pp. 423–457. [Google Scholar]
- Kopell, N. , Whittington, M.A. & Kramer, M.A. (2011) Neuronal assembly dynamics in the beta1 frequency range permits short‐term memory. Proc. Natl. Acad. Sci. USA, 108, 3779–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M.A. , Roopun, A.K. , Carracedo, L.M. , Traub, R.D. , Whittington, M.A. & Kopell, N.J. (2008) Rhythm generation through period concatenation in rat somatosensory cortex. PLoS Comput. Biol., 4, e1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer, A.C. (2009) Physiology and pharmacology of striatal neurons. Annu. Rev. Neurosci., 32, 127–147. [DOI] [PubMed] [Google Scholar]
- Kühn, A.A. , Kupsch, A. , Schneider, G.H. & Brown, P. (2006) Reduction in subthalamic 8–25 Hz oscillatory activity correlates with clinical improvement in Parkinson's disease. Eur. J. Neurosci., 23, 1956–1960. [DOI] [PubMed] [Google Scholar]
- Latham, P.E. , Richmond, B.J. , Nelson, P.G. & Nirenberg, S. (2000) Intrinsic dynamics in neuronal networks. I. Theory. J. Neurophysiol., 88, 808–827. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Sen, K. & Kopell, N. (2009) Cortical gamma rhythms modulate NMDAR‐mediated spike timing dependent plasticity in a biophysical model. PLoS Comput. Biol., 5, e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Ainsworth, M. , Whittington, M.A. & Kopell, N. (2012) Multiple gamma rhythms and interlaminar synchrony in A1. Poster presented at the annual meeting of the Society for Neuroscience. 366.06. October 2012.
- Lee, J.H. , Whittington, M.A. & Kopell, N.J. (2013) Top‐down beta rhythms support selective attention via interlaminar interaction: a model. PLoS Comput. Biol., 9, e1003164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani, L. , Toro, C. , Manganotti, P. , Zhuang, P. & Hallett, M. (1997) Event‐related coherence and event‐related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self‐paced movements. Electroen. Clin. Neuro., 104, 199–206. [DOI] [PubMed] [Google Scholar]
- Leventhal, D.K. , Gage, G.J. , Schmidt, R. , Pettibone, J.R. , Case, A.C. & Berke, J.D. (2012) Basal ganglia beta oscillations accompany cue utilization. Neuron, 73, 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, R. , Ashby, P. , Hutchison, W.D. , Lang, A.E. , Lozano, A.M. & Dostrovsky, J.O. (2002) Dependence of subthalamic nucleus oscillations on movment and dopamine in Parkinson's disease. Brain, 125, 1196–1209. [DOI] [PubMed] [Google Scholar]
- Lumer, E.D. (2000) Effects of spike‐timing on winner‐take‐all competition in model cortical circuits. Neural Comput., 12, 181–194. [DOI] [PubMed] [Google Scholar]
- Madamba, S.G. , Schweitzer, P. & Siggins, G.R. (1999) Dynorphin selectively augments the M‐current in hippocampal CA1 neurons by an opiate receptor mechanism. J. Neurophysiol., 82, 1768–1775. [DOI] [PubMed] [Google Scholar]
- Marinazzo, D. , Kappen, H.J. & Gielen, S.C. (2007) Input‐driven oscillations in networks with excitatory and inhibitory neurons with dynamic synapses. Neural Comput., 19, 1739–1765. [DOI] [PubMed] [Google Scholar]
- Martin, L.F. & Freedman, R. (2007) Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int. Rev. Neurobiol., 78, 225–246. [DOI] [PubMed] [Google Scholar]
- Mazurek, M.E. , Roitman, J.D. , Ditterich, J. & Shadlen, M.N. (2003) A role for neural integrators in perceptual decision making. Cereb. Cortex, 13, 1257–1269. [DOI] [PubMed] [Google Scholar]
- McCarthy, M.M. & Kopell, N. (2012) The effect of propofol anesthesia on rebound spiking. SIAM J. Appl. Dyn. Syst., 11, 1674–1697. [Google Scholar]
- McCarthy, M.M. , Brown, E.N. & Kopell, N. (2008) Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol‐induced paradoxical excitation. J. Neurosci., 28, 13488–13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, M.M. , Moore‐Kochlacs, C. , Gu, X. , Boyden, E.S. , Han, X. & Kopell, N. (2011) Striatal origin of the pathologic beta oscillations in Parkinson's disease. Proc. Natl. Acad. Sci. USA, 108, 11620–11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Michele, F. , Luchetti, S. , Bernardi, G. , Romeo, E. & Longone, P. (2013) Neurosteroid and neurotransmitter alterations in Parkinson's disease. Front. Neuroendocrin., 34, 132–142. [DOI] [PubMed] [Google Scholar]
- Middleton, S. , Jalics, J. , Kispersky, T. , Lebeau, F.E. , Roopun, A.K. , Kopell, N.J. , Whittington, M.A. & Cunningham, M.O. (2008) NMDA receptor‐dependent switching between different gamma rhythm‐generating microcircuits in entorhinal cortex. Proc. Natl. Acad. Sci. USA, 105, 18572–18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, A.S. & Chakraborty, S. (2013) What does the mediodorsal thalamus do? Front. Syst. Neurosci., 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitry, J. , McCarthy, M. , Kopell, N. & Wechselberger, M. (2013) Excitable neurons, firing threshold manifolds and canards. J. Math. Neurosci., 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S.D. , Madamba, S.G. , Joëls, M. & Siggins, G.R. (1988) Somatostatin augments the M‐current in hippocampal neurons. Science, 239, 278–280. [DOI] [PubMed] [Google Scholar]
- Ohmura, Y. , Tsutsui‐Kimura, I. & Yoshioka, M. (2012) Impulsive behavior and nicotinic acetylcholine receptors. J. Pharmacol. Sci., 11, 413–422. [DOI] [PubMed] [Google Scholar]
- Olufsen, M.S. , Whittington, M.A. , Camperi, M. & Kopell, N. (2003) New roles for the gamma rhythm: population tuning and preprocessing for the beta rhythm. J. Comput. Neurosci., 14, 33–54. [DOI] [PubMed] [Google Scholar]
- Otsuka, T. & Kawaguchi, Y. (2009) Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J. Neurosci., 29, 10533–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau, S. , O'Neill, P.K. , Bolkan, S.S. , Ward, R.D. , Abbas, A.I. , Roth, B.L. , Balsam, P.D. , Gordon, J.A. & Kellendonk, C. (2013) Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron, 77, 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervouchine, D.D. , Netoff, T.I. , Rotstein, H.G. , White, J.A. , Cunningham, M.O. , Whittington, M.A. & Kopell, N.J. (2006) Low‐dimensional maps encoding dynamics in entorhinal cortex and hippocampus. Neural Comput., 18, 2617–2650. [DOI] [PubMed] [Google Scholar]
- Pesaran, B. , Nelson, M.J. & Andersen, R.A. (2008) Free choice activates a decision circuit between frontal and parietal cortex. Nature, 453, 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, C.C. & Crochet, S. (2013) Synaptic computation and sensory processing in neocortical layer 2/3. Neuron, 78, 28–48. [DOI] [PubMed] [Google Scholar]
- Plenz, D. & Kital, S.T. (1999) A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature, 400, 677–682. [DOI] [PubMed] [Google Scholar]
- Pletzer, B. , Kerschbaum, H. & Klimesch, W. (2010) When frequencies never synchronize: the golden mean and the resting EEG. Brain Res., 1335, 91–102. [DOI] [PubMed] [Google Scholar]
- Pogosyan, A. , Gaynor, L.D. , Eusebio, A. & Brown, P. (2009) Boosting cortical activity at beta‐band frequencies slows movement in humans. Curr. Biol., 19, 1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S. & Maunsell, J.H.R. (2010) Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron, 67, 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, S. , Crone, N.E. , Niebur, E.J. , Franaszczuk, P.J. & Hsiao, S.S. (2008) Neural correlates of high‐gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J. Neurosci., 28, 11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M.J. , Lowet, E. , Brunet, N.M. , Ter Wal, M. , Tiesinga, P. , Fries, P. & De Weerd, P. (2013) Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron, 78, 523–536. [DOI] [PubMed] [Google Scholar]
- Roopun, A.K. , Middleton, S.J. , Cunningham, M.O. , Lebeau, F.E. , Bibbig, A. , Whittington, M.A. & Traub, R.D. (2006) A beta2‐frequency (20–30 Hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc. Natl. Acad. Sci. USA, 103, 15646–15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun, A.K. , Kramer, M.A. , Carracedo, L.M. , Kaiser, M. , Davies, C.H. , Traub, R.D. , Kopell, N.J. & Whittington, M.A. (2008a) Period concatenation underlies interactions between gamma and beta rhythms in neocortex. Front. Cell. Neurosci., 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun, A.K. , Kramer, M.A. , Carracedo, L.M. , Kaiser, M. , Davies, C.H. , Traub, R.D. , Kopell, N.J. & Whittington, M.A. (2008b) Temporal interactions between cortical rhythms. Front. Neurosci., 2, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun, A.K. , LeBeau, F.E. , Ramell, J. , Cunningham, M.O. , Traub, R.D. & Whittington, M.A. (2010) Cholinergic neuromodulation controls directed temporal communication in neocortex in vitro . Front. Neural Circuits, 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, J.E. & Terman, D. (2004) High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J. Comput. Neurosci., 16, 211–235. [DOI] [PubMed] [Google Scholar]
- Saalmann, Y.B. , Pigarev, I.N. & Vidyasagar, T.R. (2007) Neural mechanisms of visual attention: how top‐down feedback highlights relevant locations. Science, 316, 1612–1615. [DOI] [PubMed] [Google Scholar]
- Sarter, M. , Hasselmo, M.E. , Bruno, J.P. & Givens, B. (2005) Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal‐driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev., 48, 98–111. [DOI] [PubMed] [Google Scholar]
- Sedley, W. & Cunningham, M.O. (2013) Do cortical gamma oscillations promote or suppress perception? An under‐asked question with an over‐assumed answer. Front. Hum. Neurosci., 7, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski, D. , Schneider, T.R. , Foxe, J.J. & Engel, A.K. (2008) Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci., 31, 401–409. [DOI] [PubMed] [Google Scholar]
- Serenevy, A. & Kopell, N. (2013) Effects of heterogeneous periodic forcing on inhibitory networks. SIAM J. Appl. Dyn. Syst., 12, 1649–1684. [Google Scholar]
- Siegel, M. , Engel, A.K. & Donner, T.H. (2011) Cortical network dynamics of perceptual decision‐making in the human brain. Front. Hum. Neurosci., 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, M. , Donner, T.H. & Engel, A.K. (2012) Spectral fingerprints of large‐scale neuronal interactions. Nat. Rev. Neurosci., 13, 121–134. [DOI] [PubMed] [Google Scholar]
- Smith, Y. , Raju, D.V. , Pare, J.F. & Sidibe, M. (2004) The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci., 27, 520–527. [DOI] [PubMed] [Google Scholar]
- Smith, Y. , Raju, D. , Nanda, B. , Pare, J.F. , Galvan, A. & Wichmann, T. (2009) The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res. Bull., 78, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochurkova, D. & Rektor, I. (2003) Event‐related desynchronization/synchronization in the putamen. An SEEG case study. Exp. Brain Res., 149, 401–404. [DOI] [PubMed] [Google Scholar]
- Spitzer, B. & Blankenburg, F. (2011) Stimulus‐dependent EEG activity reflects internal updating of tactile working memory in humans. Proc. Natl. Acad. Sci. USA, 108, 8444–8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald, F. , Pötter, M. , Herzog, J. , Pinsker, M. , Kopper, F. , Mehdorn, H. , Deuschl, G. & Volkmann, J. (2008) Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J. Neurophysiol., 100, 2515–2524. [DOI] [PubMed] [Google Scholar]
- von Stein, A. , Rappelsberger, P. , Sarnthein, J. & Petsche, H. (1999) Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb. Cortex, 9, 137–150. [DOI] [PubMed] [Google Scholar]
- Stokes, M.G. , Kusunoki, M. , Sigala, N. , Nili, H. , Gaffan, D. & Duncan, J. (2013) Dynamic coding for cognitive control in prefrontal cortex. Neuron, 78, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon‐Baudry, C. , Kreiter, A. & Bertrand, O. (1999) Sustained and transient oscillatory responses in the gamma and beta bands in a visual short‐term memory task in humans. Visual Neurosci., 16, 449–459. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry, C. , Mandon, S. , Freiwald, W.A. & Kreiter, A.K. (2004) Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short‐term memory task. Cereb. Cortex, 14, 713–720. [DOI] [PubMed] [Google Scholar]
- Terman, D. , Rubin, J.E. , Yew, A.C. & Wilson, C.J. (2002) Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J. Neurosci., 22, 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort, A.B. , Rotstein, H.G. , Dugladze, T. , Gloveli, T. & Kopell, N.J. (2007) On the formation of gamma‐coherent cell assemblies by oriens lacunosum‐moleculare interneurons in the hippocampus. Proc. Natl. Acad. Sci. USA, 104, 13490–13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub, R.D. , Whittington, M.A. , Stanford, I.M. & Jefferys, J.G. (1996) A mechanism for generation of long‐range synchronous fast oscillations in the cortex. Nature, 383, 621–624. [DOI] [PubMed] [Google Scholar]
- Traub, R.D. , Whittington, M.A. , Buhl, E.H. , Jefferys, J.G. & Faulkner, H.J. (1999) On the mechanism of the gamma –> beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J. Neurosci., 19, 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub, R.D. , Bibbig, A. , Fisahn, A. , LeBeau, F.E. , Whittington, M.A. & Buhl, E.H. (2000) A model of gamma‐frequency network oscillations induced in the rat CA3 region by carbachol in vitro . Eur. J. Neurosci., 12, 4093–4106. [DOI] [PubMed] [Google Scholar]
- Tuboly, G. & Vécsei, L. (2013) Somatostatin and cognitive function in neurodegenerative disorders. Mini.‐Rev. Med. Chem., 13, 34–46. [DOI] [PubMed] [Google Scholar]
- Wang, X.J. (2010) Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev., 90, 1195–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.J. (2012) Neural dynamics and circuit mechanisms of decision‐making. Curr. Opin. Neurobiol., 22, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub, D.B. & Zaghloul, K.A. (2013) The role of the subthalamic nucleus in cognition. Rev. Neuroscience, 24, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, S. & Mueller, H.M. (2012) “Too many betas do not spoil the broth”: the role of beta brain oscillations in language processing. Front. Psychol., 3, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J.A. , Banks, M.I. , Pearce, R.A. & Kopell, N.J. (2000) Networks of interneurons with fast and slow gamma‐aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma‐theta rhythm. Proc. Natl. Acad. Sci. USA, 97, 8128–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington, M.A. , Traub, R.D. , Kopell, N. , Ermentrout, B. & Buhl, E.H. (2000) Inhibition‐based rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol., 38, 315–336. [DOI] [PubMed] [Google Scholar]
- Whittington, M.A. , Cunningham, M.O. , LeBeau, F.E. , Racca, C. & Traub, R.D. (2011) Multiple origins of the cortical gamma rhythm. Dev. Neurobiol, 71, 92–106. [DOI] [PubMed] [Google Scholar]
- Williams, D. , Tijssen, M. , van Bruggen, G. , Bosch, A. , Insola, A. , Di Lazzaro, V. , Mazzone, P. , Oliviero, A. , Quartarone, A. , Speelman, H. & Brown, P. (2002) Dopamine dependent changes in the functional connectivity between basal ganglia and cerebral cortex in the human. Brain, 125, 1558–1569. [DOI] [PubMed] [Google Scholar]
- Xiang, Z. , Huguenard, J.R. & Prince, D.A. (2002) Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J. Neurophysiol., 88, 740–750. [DOI] [PubMed] [Google Scholar]
- Yamawaki, N. , Stanford, I.M. , Hall, S.D. & Woodhall, G.L. (2008) Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro . Neuroscience, 151, 386–395. [DOI] [PubMed] [Google Scholar]
- Zugno, A.I. , Julião, R.F. , Budni, J. , Volpato, A.M. , Fraga, D.B. , Pacheco, F.D. , Deroza, P.F. , Luca, R.D. , de Oliveira, M.B. , Heylmann, A.S. & Quevedo, J. (2013) Rivastigmine reverses cognitive deficit and acetylcholinesterase activity induced by ketamine in an animal model of schizophrenia. Metab. Brain Dis., 28, 501–508. [DOI] [PubMed] [Google Scholar]


