Abstract
Bone marrow-derived human mesenchymal stem cells (BM-hMSCs) have the innate ability to migrate or home towards, and engraft in tumors such as glioblastoma (GBM). Due to this unique property of BM-hMSCs we have explored their use for cell-mediated therapeutic delivery for the advancement of GBM treatment. Extravasation, the process by which blood-borne cells – such as BM-hMSCs – enter the tissue is a highly complex process but is heavily dependent upon glycosylation for glycan-glycan and glycan-protein adhesion between the cell and endothelium. However, in a translationally significant pre-clinical glioma stem cell xenograft (GSCX) model of GBM, BM-hMSCs demonstrate unequal tropism towards these tumors. We hypothesized that there may be differences in the glycan compositions between the GSCXs that elicit homing (“attractors”) and those that do not (“non-attractors”) that facilitate or impede the engraftment of BM-hMSCs to the tumor. In this study, glycotranscriptomic analysis revealed significant heterogeneity within the attractor phenotype and the enrichment of high mannose type N-glycan biosynthesis in the non-attractor phenotype. Orthogonal validation with topical PNGase F deglycosylation on the tumor regions of xenograft tissue, followed by nLC-ESI-MS, confirmed the presence of increased high mannose type N-glycans in the non-attractors. Additional evidence provided by our glycomic study revealed the prevalence of terminal sialic acid-containing N-glycans in non-attractors and terminal galactose and N-acetyl-glucosamine N-glycans in attractors. Our results provide the first evidence for differential glycomic profiles in attractor and non-attractor GSCXs and extend the scope of molecular determinates in BM-hMSC homing to glioma.
Keywords: Glioblastoma, bone marrow-derived human mesenchymal stem cells (BM-hMSCs), transcriptomics, glycomics, N-linked glycosylation, high-mannose, sialic acid
Graphical abstract
Introduction
Glycosylation is the most common protein post-translational modification (PTM). It is estimated that 50% of all proteins within the human proteome are glycosylated though only 10% of proteins have evidence supporting the presence of this PTM1, 2. The glycan moieties on membrane and secreted proteins are important modulators of protein folding, stability, and trafficking2, 3. Glycosylation also mediates biological functions such as cell-cell or cell-matrix adhesion2, 4-6, host-pathogen interactions2, 6, 7, and receptor-ligand interactions6, 8. One important cell-cell adhesion process critically reliant on and predominantly mediated by glycan moieties is extravasation.
Extravasation is the process whereby cells within blood vessels home or migrate to sites of inflammation or damaged tissue5, 9. In the first step, rolling adhesion, carbohydrate-carbohydrate interactions are critical9, 10. Carbohydrate binding proteins (e.g. P- and E-selectin) on the endothelial surface recognize and bind carbohydrates (e.g. Sialyl LewisX) on glycolipids or glycoproteins on the opposing cell surface. The next step, tight binding, predominantly relies on complementary pairs of adhesion molecules on the opposing cell surfaces to strengthen the initial interaction established by rolling adhesion. Many adhesion proteins such as integrins and ICAMs are themselves heavily glycosylated9, 10. The importance of glycosylation in extravasation is highlighted by the consequences of genetic deletion of enzymes related to O-and N-linked glycan processing10. For instance, mice lacking polypeptide N-acetylgalactosamine transferase-1 (Galnt1−/−), which initiates O-linked glycosylation, demonstrate significantly reduced extravasation at every critical step11. Genetic ablation of sialyltransferase ST3Gal-IV in mice reduced CXCR2-mediated firm adhesion12.
Recent observations of intra-arterially delivered bone marrow-derived human mesenchymal stem cells (BM-hMSCs), for cell-based therapeutic delivery of anti-glioma agents13-19, suggest that these cells extravasate from the blood vessel endothelium via diapedesis after intravascular injection to engraft into the tumor mass15. GFP-labeled BM-hMSCs injected into the internal carotid artery of tumor-bearing mice were found in linear arrangements co-localized with endothelial marker CD31 up until two days post-treatment15. By the third day, BM-hMSCs were seen dispersed throughout the tumor parenchyma supporting the hypothesis of extravasation-mediated localization15. However, the fact that some GSCXs attract BM-hMSCs (‘attractors’) and others do not (‘non-attractors’) suggests that there are differences in the tumor expressed glycans14, which may make attractors conducive to BM-hMSC engraftment.
We have previously found alterations in the lipid compositions20 and proteins comprising cell-signaling pathways21 of the attractor and non-attractor GSCXs, which have shed light on the different phenotypes. We have analyzed the glycomic profiles of U373MG xenografts22 and glioma stem cells23, but the differential glycan profile of attractor and non-attractor GSCXs remains unexamined. Thus, we set out to uncover the glycan profile of these tumors to expand our understanding of the variable BM-hMSC tropism. We first used glycogene-targeted transcriptomics to generate an informed data-driven glycomics approach. Data derived from targeted glyco-microarrays prompted an N-linked glycan specific approach using on-tissue digestion of N-glycans from the tumor areas of attractor and non-attractor xenografts followed by nLC-ESI-MS analysis24.
Materials and Methods
Chemicals and reagents
Borane-ammonia complex, sodium hydroxide beads, dimethyl sulfoxide (DMSO), iodomethane, trifluoroacetic acid, chloroform, ammonium bicarbonate, and MS-grade formic acid were obtained from Sigma-Aldrich (St. Louis, MO). Micro-spin columns were supplied by Harvard Apparatus (Holliston, MA). PNGase F (500,000 units/mL) was purchased from New England Biolabs Inc. (Ipswich, MA). Acetic acid and acetonitrile were purchased from Fisher Scientific (Pittsburgh, PA), and HPLC-grade water was acquired from Mallinckrodt Chemicals (Phillipsburg, NJ).
Animals
Male athymic nude mice (nu/nu) were purchased from the Department of Experimental Radiation Oncology, The University of Texas M.D. Anderson Cancer Center (MDACC, Houston, TX) as previously described20, 21. All animal procedures were undertaken within the guidelines prescribed by the MDACC Animal Care and Use Committee, the USDA Animal Welfare Act, and the Guide for the Care and Use of Laboratory Animals (NIH).
Glioma Xenograft Model
GSCs (GSC17, GSC11, GSC229, GSC231, GSC268, and GSC274) were established as previously described25, 26. GSCs (1 × 106) were implanted in mice via the guide-screw method27. Nine attractors (GSCX17, GSCX268, and GSCX274) from three different cell lines each with three biological replicates, and nine non-attractors (GSCX11, GSCX229, and GSCX231)) from three different cell lines each with three biological replicates were used for this study. A total of 18 GSCXs, nine biolgical replicates per phenotype, were used in this study. Attractor and non-attractor phenotypes were determined previously14.
Tissue Dissection, Sectioning and Sampling
Animals were anesthetized by intraperitoneal injection of ketamine/xylazine and sacrificed as previously described20. Brains were removed immediately and flash frozen in liquid nitrogen vapor28 and sliced 1.5 mm thick using a brain matrix. Tissue punches (1.5 mm diameter; Braintree Scientific, Braintree, MA) were taken from the tumor site within each slice and flash frozen in liquid nitrogen as previously described21. Next, brain tissue was sectioned at 20 μm along the coronal plane, and thaw mounted on glass slides for on-tissue deglycosylation24. Slides were stored at -80 °C until further analysis.
Targeted Transcriptomic Analysis
Transcriptomic experiments were conducted on a custom targeted microarray chip containing functional human gene sets related to glioma biology compiled from the NCBI human sequence database as previously described21, 29-31. Briefly, total RNA was extracted from all individual biological replicates of GSCXs (N=9 attractors; N=9 non-attractors), purified, amplified, and then labeled with Cy5. A universal human reference (Stratagene, La Jolla, CA) was labeled with Cy332. Data from chips scanned with a confocal laser (ScanArray 4000XL; Packard Biochip Technologies, Billerica, MA) were processed with BlueFuse (Illumina Fulbourn, Cambridge, UK)21, 29-31 and analyzed by significance of analysis of microarrays algorithm (SAM, v4.0, Stanford University, Palo Alto, CA)33. The significance cutoff was set to an FDR of < 10%21, 29-31. As previously described21, positive fold change values are indicative of an increase in transcript expression in attractors relative to non-attractors and negative fold change values are indicative of a decrease in transcript expression in attractors relative to non-attractors (Supplemental Table 1). This dataset21 was re-analyzed by Gene Set Enrichment Analysis (GSEA) to determine significantly enriched glycomic pathways from a custom-made glycomic pathway database34-36. Significantly enriched data sets are defined at p < 0.05 and a false discovery rate (FDR) q < 0.30. DanteR (version 0.1.1) was used to generate a 3D PCA of all glycogenes from GSCXs.
On-tissue digestion
Coronal sections (20 μm) of all GSCXs were brought to room temperature and spotted with 1 μL of PNGase F (50 Units) on the tumor regions of attractor and non-attractor xenografts. The enzymatic deglycosylation reaction was carried out overnight in a water bath at 37 °C. Released N-glycans were collected, reduced with borane-ammonia followed by solid-phase permethylation (SPP) as previously described24.
nanoLiquid Chromatography-Mass Spectrometry
Samples resuspended in 20% acetonitrile/0.1% FA were subjected to nanoLC-MS analysis as previously described24, 37-40 in a Dionex Ultimate 3000 UHPLC system (Thermo Scientific, Sunnyvale, CA, USA) coupled to an LTQ Orbitrap Velos (Thermo Scientific, San Jose, CA, USA) mass spectrometer. The flow rate of the nanoLC system was set to 350 nL/min. The mobile phase A consisted of 98% water, 2% acetonitrile and 0.1% formic acid while mobile phase B consisted of 100% acetonitrile and 0.1% formic acid. Permethylated glycans were separated on a C18 column (Thermo Scientific, Pittsburgh, PA, USA) using these conditions: 20% mobile phase B for 10 minutes, 20-38% B in one minute, 38- 60% B over 35 min; finally, 90% B was applied and held for 5 minutes. The column oven temperature was set to 55 °C. The nanoLC system was coupled to the mass spectrometer using a nano-electrospray ionization source. The resolution of full MS was set to 15,000, which is adequate to resolve close glycan m/z values. MS/MS was conducted in data-dependent acquisition (DDA) mode; the 4 most intense peaks were subjected to MS/MS analysis using both collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD).
Data Processing and Analysis
MultiGlycan41, 42 was employed to process the raw data files generated by the mass spectrometer. First monoisotopic peaks within 6 ppm difference from theoretical m/z values and with correct charge states were extracted. The theoretical m/z values were generated from a comprehensive N-glycan candidate list consisting of 128 glycans with different charge states and adduct forms (e.g. protonated, sodiated and ammoniated). The peak areas of extracted ion chromatograms were used to represent the abundance of each glycan structure detected in the different samples. Same glycan structures across consecutive MS scans were merged42 and each injection was normalized according to the digestion area on tissue slides. Data were filtered based on percent missing values (%NA) with > 50% NA removed. Grubb's test was used to identify and remove outliers in the dataset. Data presented as mean ± SEM with p ≤ 0.05 considered significant.
Results
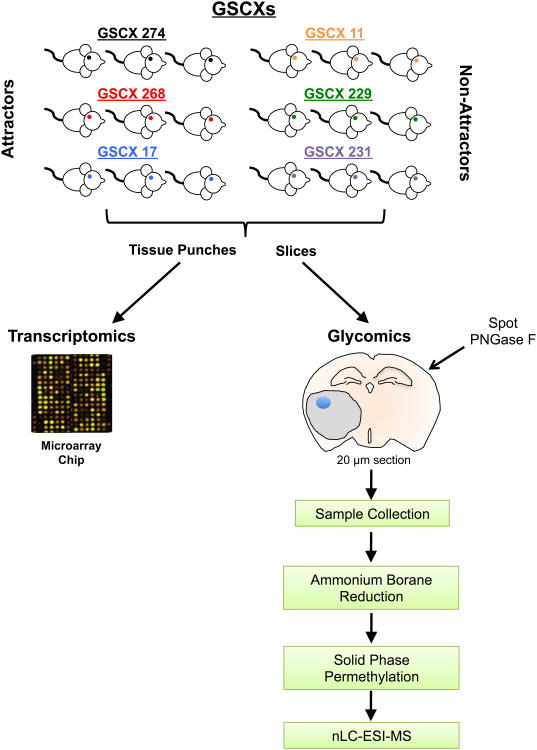
The glycomics of GSCXs exhibiting differential homing for BM-hMSCs – attractors and non-attractors – have not been systematically studied. Because glycans are essential to the extravasation process2, 10, it is critical to gain a better understanding of the differential glycan profiles of the attractor and non-attractor phenotypes. We employed the workflow outlined in Figure 1 to investigate glycotranscripts and N-glycans, differentially expressed in GSCXs exhibiting attractor and non-attractor phenotypes.
Figure 1. Workflow outlining tissue sample preparation for combined transcriptomics and glycomics.
Tissue punches (left) take from GSC xenografts (1.5 mm thick × 1.5 mm diameter) were taken for targeted transcriptomics (as described in Materials and Methods). Serial coronal sections from GSC xenografts at 20 μm (right) were made after the tissue punches were taken and thaw mounted on glass microscope slides. PNGase F (1 μL) was spotted on the tumors of each GSCX for N-glycan release overnight. Release N-glycans were collected, reduced, permethylated, and analyzed by nLC-ESI-MS.
Targeted Transcriptomics Reveals Enrichment of High Mannose Type N-Glycans in Non-Attractor Phenotype
Our previously published data from these same GSCXs using a targeted microarray platform containing 2,577 total transcripts related to glioma biology was re-analyzed, focusing on all the cloned human glycogenes contained on the chip21 (Supplemental Table 1). PCA analysis of all glycotranscripts from attractors and non-attractors (Fig. 2A) demonstrated clear separation between the two phenotypes (PC1). The clustering of biological replicates (individual animals with the same cells line) for attractors (PC3, 8.13%) and non-attractors (PC2, 9%) was similar. Yet, from the overall clustering of both individual cells lines (e.g. GSC17) and biological replicates within a given phenotype, the attractors demonstrated greater glycotranscript heterogeneity compared to non-attractors. GSEA analysis of the transcripts using a custom-made glycogene database revealed high mannose type N-glycan biosynthesis (nominal p-value = 0.0383; FDR q-value = 0.2962) to be significantly enriched in the non-attractor phenotype (Fig. 2B). However, no glycan synthesis or degradation pathway met our threshold requirements in GSEA for attractors (data not shown).
Figure 2. Targeted transcriptomic analysis.
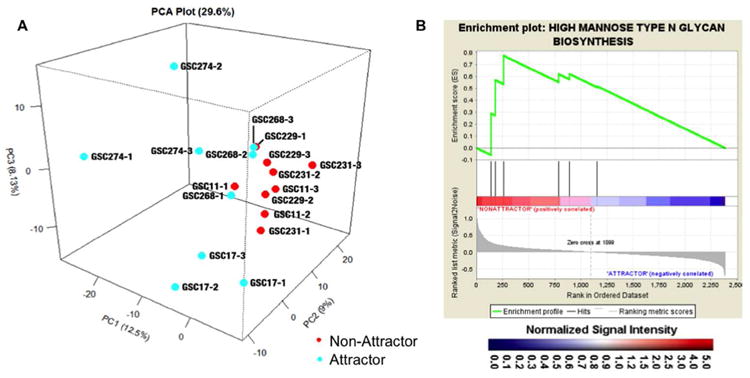
(A) Principal component analysis of all human glycogenes from targeted microarray. Attractors are in blue while non-attractors are shown in red. GSC followed by a number designates the glioma stem cell line used for the xenograft and the number following the hyphen indicates the biological replicate. (B) GSEA enrichment plots for statistically significant genes. The high mannose N-glycan type glycogene set enriched in the non-attractor phenotype is depicted. Black bars illustrate the position of the probe sets in the context of all of the glycoprobes on the array. The running enrichment score plotted as a function of the position of the ranked list of array probes is shown in green. The rank list metric shown in gray illustrates the correlation between the signal-to-noise values of all individually ranked genes according to the class labels (attractor vs non-attractor). The genes overrepresented on the leftmost side of the enrichment plots are those that correlate to differential expression in the non-attractor phenotype. Significantly enriched data sets are defined at a p < 0.05 and a false discovery rate (FDR) < 0.30.
Quantitative Glycomics of Attractor vs Non-Attractor GSCXs
To corroborate the glycotranscriptomic findings and obtain tumor specific N-glycan information we performed nLC-ESI-MS glycomic experiments from on-site PNGase F deglycosylation of 20 μm coronal sections from the GSC xenografts. Of the detectible species passing our filters (Materials and Methods) we identified 18 glycan compositions, 9 of which were significantly differentially expressed between the two phenotypes (Figure 3). Consistent with our previous work21 there were more significant complex N-glycan compositions in the attractors compared to non-attractors. The high mannose glycans were significantly less abundant in the attractor phenotype, in accordance with the GSEA biosynthesis pathway enrichment from the transcriptomic data (Figure 2). Further, the transcript MAN1C1, coding for the protein mannosyl-oligosaccharide 1,2-alpha-mannosidase IC was decreased in attractors relative to non-attractors (Supplemental Table 1). The MAN1C1 enzyme produces Man8GlcNAc2 then Man6GlcNAc2 from Man9GlcNAc2, the nascent N-linked glycan emerging from the Golgi. Notably, Man6GlcNAc2 was one of the significant high mannose N-glycans found to be decreased in attractors compared to non-attractors.
Figure 3. N-glycan Compositions in Attractors and Non-Attractors.
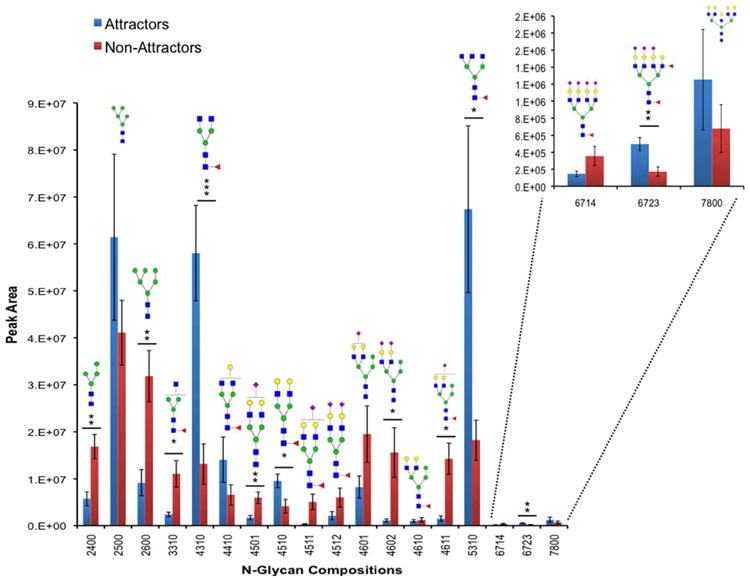
Bar graphs of the permethylated N-glycan peak from attractor (blue) and non-attractor (red) GSCXs through on-tissue (tumor) digestions. The y-axis represents peak area and the x-axis represents glycan compositions (GlcNAc, Man, Gal, Fuc, NeuNAc). Symbols; GlcNAc, blue squares; Man, green circles, Gal, yellow circles, NeuNAc, magenta diamonds, and Fuc red triangles. Values are mean ± SEM; * p < 0.05, ** p < 0.01, and *** p < 0.001 (Student's t-test).
Terminal mono- and disialylation of N-glycans (GlcNAc4Man3Gal2Sia1, GlcNAc4Man4Gal2Sia2, and GlcNAc4Man4Gal2Fuc1Sia1) was significantly decreased in the attractors compared to the non-attractors; the presence of sialic acid on galactose could be either α2-3 or α2-6 linked5. This trend was consistent for those N-glycan compositions (GlcNAc4Man3Gal2Sia1, GlcNAc4Man4Gal2Sia2, and GlcNAc4Man4Gal2Fuc1Sia1) that did not reach significance (Figure 3). The average peak areas of sialic acid-containing N-glycan species (Supplemental Figure 1) support the overall trend of decreased sialic acid species in attractors compared to non-attractors (p = 0.000131735). We note that the exception to this is N-glycan composition GlcNAc6Man3Gal4Fuc2Sia3 (tetrantennary), which was increased in attractors and possess three terminal sialic acids. Interestingly, GlcNAc6Man3Gal4Fuc2Sia3 has a fucose on the antennae in addition to the core fucose, which must be α1-6 linked, as PNGase F cannot cleave N-glycans containing core α1-3 fucose. The fucose present on the N-glycan antenna is linked to the N-acetylglucosamine, which typically occurs as a α1-3 or α1-4 linkage. The transcript FUT5 that codes for the enzyme alpha-(1,3)-fucosyltransferase 5 was increased in attractors relative to non-attractors (Supplemental Table 1). This enzyme is responsible for the placement of a α1-4 linked fucose to N-acetyl-glucosamine (GlcNAc) and thus offers support for the N-glycan composition seen in GlcNAc6Man3Gal4Fuc2Sia3.
In contrast, the N-glycan compositions increased in attractors compared to non-attractors are of the complex type possessing either a terminal galactose or GlcNAc. Composition GlcNAc4Man3Gal2Fuc1 (p = 0.016) terminating in galactose was increased in attractors and compositions GlcNAc4Man3Gal1Fuc1 and GlcNAc7Man3Gal5, while not significant follow the same trend. Complex N-glycans GlcNAc4Man3Fuc1 (p = 0.0005) and GlcNAc5Man3Fuc1 (p = 0.022) are the two most abundant N-glycans in attractors and terminate with two or more GlcNAcs (GlcNAc4Man3Fuc1, fold change = 4.4 & GlcNAc5Man3Fuc1, fold change = 3.7) in a N-acetyllactosamine (LacNAc) formation5. Composition GlcNAc3Man3Fuc1 is an exception to this though, ending with one GlcNAc monosaccharide at the reducing end unlike compositions GlcNAc4Man3Fuc1 and GlcNAc5Man3Fuc1.
Discussion
BM-hMSCs demonstrate significant promise as cell-based delivery vehicles for anti-glioma therapeutics13, 15-17, 19, 43, 44. However, evidence suggests that in GSCXs, the ‘gold standard’ of glioma models, these cells do not home or extravasate equally14. Given the importance of glycosylation in the extravasation process,5, 9, 10 we examined the glycomic profile of attractors and non-attractors. The transcriptomic platform contains all human glycogenes, enabling analysis of all glycosylation pathways including, but not limited to, N-linked and O-linked glycosylation, gangliosides, and glycosaminoglycans. By utilizing this targeted transcriptomic approach, we were able to focus further glycomic studies on protein N-linked glycosylation in a data-driven approach for tumor-specific glycomic profiles. The value of this workflow is that the high-throughput targeted transcriptomic platform yields informative data about genes related to all types of glycosylation, which then serves to inform orthogonal glycomics experiments (TOC and Fig. 1).
The transcriptomic data revealed no significantly enriched glyco-synthetic or degradative pathways in attractors by use of GSEA analysis. This may be attributed to glycan heterogeneity in the attractor phenotype, which can clearly be seen in the PCA analysis (Fig. 2A). However, high mannose biosynthesis was a significantly enriched N-linked glycosylation pathway in the non-attractor phenotype (Fig. 2B) prompting us to examine the N-glycan profile of the GSCXs using the previously developed, highly efficient method for N-glycan profiling of tissue sections24. The utility of this approach is that information relevant to histopathology is obtained from small samples derived from xenografts24.
Orthogonal glycomic experiments confirmed elevated levels of high mannose N-glycans in the non-attractors (Fig. 3), as predicted by GSEA analysis from the glycotranscriptomic data. High mannose N-glycans could be an indicator of an embryonic, undifferentiated phenotype45. Increased expression of high mannose type N-glycans also have been observed in colorectal cancer cell lines of varying malignancy46-48 and in breast cancer cell lines and tissue49-51. The biological significance of this glycomic alteration in cancer is not clear, yet the presence of high (truncated) mannose indicates some level of incomplete N-linked glycosylation prossessing52. To what extent and whether or not the truncated high mannose is protein specific is unknown, as levels of terminal sialic acid (complex and hybrid) N-glycans, which represent uncompromised N-glycan processing were increased in the non-attractors (Fig. 3).
Sialic acids carry a strong negative charge and have dual biological functions5. They can either act as ligands for sialic acid binding proteins or they may serve to “mask” sites like galactose from galactose-binding receptors5, 53. It has been observed that the increase in sialic acid content of tumor cells results in decreased attachment of the cell to the basement membrane via electrostatic repulsion, promoting metastasis5, 53. While GBM is confined within the cranium and does not metastasize, it is possible that via the same phenomenon, the non-attractors repel BM-hMSCs preventing extravasation and dispersion into the tumor parenchyma. Concomitantly, the sialic acid residues may be “masking” cell surface ligands that BM-hMSCs utilize for extravasation and dissemination throughout the tumor paraenchyma. For instance, sialic acid is known to inhibit galectin binding, which binds to either galactose or GlcNAc depending on the galectin isoform54,55. The structures increased in the attractors terminated in either GlcNAc or galactose, in contrast to their sialylated counterparts, which were increased in the non-attractors (Fig. 3 and Supplemental Figure 1). At present, only galectin-1 has been identified on the cell surface of BM-hMSCs56. The functional cell surface expression of other galectin isoforms on BM-hMSCs remains unresolved.
Interestingly, recent evidence suggests that sialic acids may scavenge free radicals, providing an antioxidant effect5, 57-62. Sialic acids on glycosphingolipids have been reported to provide protection against ROS57, 58. Free N-acetylneuraminic acid (Neu5Ac, sialic acid) in solution was able to reduce the concentration of organic peroxides, lipid hydroperoxides and, the arachidonic acid derivative HpETE as well as attenuate cytotoxicity in culture with these agents59, 62. Pharmacologically, the hypersialylated analogue of human erythropoietin (r-HuEPO), was able to attenuated TNF-α-induced ROS and activation of JNK and MSK1, kinases upstream NFkB60. However, upon desialylation, r-HuEPO lost its ability to inhibit JNK and MSK1 and reduce TNF-α-induced ROS60.
We have previously demonstrated that the pentose phosphate pathway (PPP) was down-regulated in attractors relative to non-attractors21. Supporting this was the down-regulation of glutathione S-transferase and superoxide dismutase in attractors relative to non-attractors and compromised fatty acid metabolism – heavily dependent on NADPH generated from the PPP21. These data suggest that reactive oxidative species (ROS), which incite pro-inflammatory reactions, would be more prevalent in attractors than non-attractors. In fact, ROS species have been documented to decrease the sialic acid content of mammalian cell surface oligosaccharides63, 64, which offers a possible explanation for the overall decreased levels of sialic acid containing N-glycans in the attractors (Fig. 3). The role of sialic acid containing N-glycans as free radical scavengers5, 57-61 and its consistent up-regulation in non-attractors in our glycomic study would presumably lead to lower levels of ROS and ROS-mediated inflammation in non-attractors. We note that our previous lipidomic study demonstrated DHA, an inflammatory-resolving lipid, to be increased in the tumor regions of non-attractors20. DHA is a fatty acid dependent upon NADPH generated by the PPP for its biosynthesis65. The PPP and the proteins directly and indirectly involved in DHA metabolism were up-regulated in the non-attractor phenotype relative to the attractor phenotype, supporting decreased inflammation including ROS-generated inflammation21. The increase in terminal sialic acid N-glycans in the non-attractor phenotype generates a new layer of complexity adding new valuable information and supporting our previous work20, 21.
Our study of the N-glycan profile of attractor and non-attractor GSCXs yielded highly informative N-glycan compositions from the tumor regions of glioma xenograft tissue. Because we applied the on-tissue PNGase F deglycosylation protocol24, we are confident that the N-glycan compositions we observed come from the cell surface in contrast to membrane fractionation, which invariably yields membrane contamination from the endoplasmic reticulum and Golgi apparatus45. However, we acknowledge that a limitation to this study is that the N-glycans and subsequent mass spectrometric measurements are derived from a thin slice of tissue, limiting the analytical depth of the tumor microenvironment and any N-glycan micro-heterogeneity associated with differential intratumoral microenvironments. Nonetheless, the results of this study motivate future investigations into the identity of proteins modified by N-glycans and their sites of attachment and linkage, along with mechanistic studies on their biological significance and functional relevance, including N-glycan expression by BM-hMSCs.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financial support of the Cancer Prevention Research Institute of Texas (CPRIT) and The University of Texas Medical Branch to C.L.N. CPRIT (RP130624) and Texas Tech University to Y.M. Grants from the National Cancer Institute CA115729 and 1P50 CA127001, The Dr. Ralph and Marian Falk Medical Research Trust, Chicago IL (to JRM), The Broach Foundation for Brain Cancer Research, The Elias Family Fund, The National Brain Tumor Foundation, The Collaborative Ependymoma Research network (CERN), The Gene Pennebaker Brain Cancer Fund, the Sorenson Foundation, and the Brian McCulloch Fund to F.F.L are gratefully acknowledged.
Footnotes
Supplemental Figure 1 and a complete list of significantly expressed transcripts (Supplemental Table 1) are available free of charge via http://pubs.acs.org/.
Author Contributions: N.C.W. conceived the study, performed experiments, data analysis and interpretation, wrote the manuscript. S.Z. performed experiments, conducted data analysis and interpretation, and assisted in writing in the manuscript. L.G.Z. performed glycan extractions, data acquisition, and data analysis. R.A.K. and J.R.M. contributed transcriptomics data analysis tools and provided helpful discussion. M.S. assisted in transcriptomics experiments and data analysis. P.M. assisted with glycan extractions. J.G. grew stem cell lines and performed all animal work. F.F.L., Y.M. and C.L.N. conceived the project, supervised the work, and critically revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Genomics and proteomics for clinical discovery and development. Springer; New York: 2014. p pages cm. [Google Scholar]
- 3.Dwek RA. Glycobiology: Toward Understanding the Function of Sugars. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A. Essentials of glycobiology. 2nd. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2009. pp. xxix–784. [PubMed] [Google Scholar]
- 6.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Baum LG, Garner OB, Schaefer K, Lee B. Microbe-Host Interactions are Positively and Negatively Regulated by Galectin-Glycan Interactions. Front Immunol. 2014;5:284. doi: 10.3389/fimmu.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferluga S, Hantgan R, Goldgur Y, Himanen JP, Nikolov DB, Debinski W. Biological and structural characterization of glycosylation on ephrin-A1, a preferred ligand for EphA2 receptor tyrosine kinase. J Biol Chem. 2013;288:18448–57. doi: 10.1074/jbc.M113.464008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott DW, Patel RP. Endothelial heterogeneity and adhesion molecules N-glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology. 2013;23:622–33. doi: 10.1093/glycob/cwt014. [DOI] [PubMed] [Google Scholar]
- 10.Wright RD, Cooper D. Glycobiology of leukocyte trafficking in inflammation. Glycobiology. 2014;24:1242–51. doi: 10.1093/glycob/cwu101. [DOI] [PubMed] [Google Scholar]
- 11.Block H, Ley K, Zarbock A. Severe impairment of leukocyte recruitment in ppGalNAcT-1-deficient mice. J Immunol. 2012;188:5674–81. doi: 10.4049/jimmunol.1200392. [DOI] [PubMed] [Google Scholar]
- 12.Frommhold D, Ludwig A, Bixel MG, Zarbock A, Babushkina I, Weissinger M, Cauwenberghs S, Ellies LG, Marth JD, Beck-Sickinger AG, Sixt M, Lange-Sperandio B, Zernecke A, Brandt E, Weber C, Vestweber D, Ley K, Sperandio M. Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation. J Exp Med. 2008;205:1435–46. doi: 10.1084/jem.20070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosztowski T, Zaidi HA, Quiñones-Hinojosa A. Applications of neural and mesenchymal stem cells in the treatment of gliomas. Expert Rev Anticancer Ther. 2009;9:597–612. doi: 10.1586/era.09.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinojima N, Hossain A, Takezaki T, Fueyo J, Gumin J, Gao F, Nwajei F, Marini FC, Andreeff M, Kuratsu J, Lang FF. TGF-β mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells. Cancer Res. 2013;73:2333–44. doi: 10.1158/0008-5472.CAN-12-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, Bogler O, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–40. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 18.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 19.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 20.Wildburger NC, Wood PL, Gumin J, Lichti CF, Emmett MR, Lang FF, Nilsson CL. ESI-MS/MS and MALDI-IMS Localization Reveal Alterations in Phosphatidic Acid, Diacylglycerol, and DHA in Glioma Stem Cell Xenografts. J Proteome Res. 2015;14:2511–9. doi: 10.1021/acs.jproteome.5b00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wildburger NC, Lichti CF, LeDuc RD, Gumin J, Schmidt M, Kroes RA, Moskal JR, Lang FF, Nilsson CL. Quantitative Proteomics Reveals Metabolic Differences in Glioma Stem Cell Xenografts and Stromal Cells. EuPA Open Proteomics. 2015 [Google Scholar]
- 22.Kroes RA, He H, Emmett MR, Nilsson CL, Leach FE, Amster IJ, Marshall AG, Moskal JR. Overexpression of ST6GalNAcV, a ganglioside-specific alpha2,6-sialyltransferase, inhibits glioma growth in vivo. Proc Natl Acad Sci U S A. 2010;107:12646–51. doi: 10.1073/pnas.0909862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He H, Nilsson CL, Emmett MR, Marshall AG, Kroes RA, Moskal JR, Ji Y, Colman H, Priebe W, Lang FF, Conrad CA. Glycomic and transcriptomic response of GSC11 glioblastoma stem cells to STAT3 phosphorylation inhibition and serum-induced differentiation. J Proteome Res. 2010;9:2098–108. doi: 10.1021/pr900793a. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Zhou S, Khalil SI, Renteria CL, Mechref Y. Glycomic profiling of tissue sections by LC-MS. Anal Chem. 2013;85:4074–9. doi: 10.1021/ac400106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 26.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 27.Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–33. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 28.Shavkunov AS, Wildburger NC, Nenov MN, James TF, Buzhdygan TP, Panova-Elektronova NI, Green TA, Veselenak RL, Bourne N, Laezza F. The fibroblast growth factor 14·voltage-gated sodium channel complex is a new target of glycogen synthase kinase 3 (GSK3) J Biol Chem. 2013;288:19370–85. doi: 10.1074/jbc.M112.445924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: social dominance-submission gene expression patterns in rat neocortex. Neuroscience. 2006;137:37–49. doi: 10.1016/j.neuroscience.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 30.Kroes RA, Dawson G, Moskal JR. Focused microarray analysis of glyco-gene expression in human glioblastomas. J Neurochem. 2007;103(1):14–24. doi: 10.1111/j.1471-4159.2007.04780.x. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson CL, Berven F, Selheim F, Liu H, Moskal JR, Kroes RA, Sulman EP, Conrad CA, Lang FF, Andrén PE, Nilsson A, Carlsohn E, Lilja H, Malm J, Fenyö D, Subramaniyam D, Wang X, Gonzales-Gonzales M, Dasilva N, Diez P, Fuentes M, Végvári Á, Sjödin K, Welinder C, Laurell T, Fehniger TE, Lindberg H, Rezeli M, Edula G, Hober S, Marko-Varga G. Chromosome 19 annotations with disease speciation: a first report from the Global Research Consortium. J Proteome Res. 2013;12:135–50. doi: 10.1021/pr3008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Churchill GA. Fundamentals of experimental design for cDNA microarrays. Nat Genet. 2002;(32 Suppl):490–5. doi: 10.1038/ng1031. [DOI] [PubMed] [Google Scholar]
- 33.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 36.Crosson LA, Kroes RA, Moskal JR, Linsenmeier RA. Gene expression patterns in hypoxic and post-hypoxic adult rat retina with special reference to the NMDA receptor and its interactome. Mol Vis. 2009;15:296–311. [PMC free article] [PubMed] [Google Scholar]
- 37.Desantos-Garcia JL, Khalil SI, Hussein A, Hu Y, Mechref Y. Enhanced sensitivity of LC-MS analysis of permethylated N-glycans through online purification. Electrophoresis. 2011;32:3516–25. doi: 10.1002/elps.201100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Mechref Y. Comparing MALDI-MS, RP-LC-MALDI-MS and RP-LC-ESI-MS glycomic profiles of permethylated N-glycans derived from model glycoproteins and human blood serum. Electrophoresis. 2012;33:1768–77. doi: 10.1002/elps.201100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, Desantos-Garcia JL, Mechref Y. Comparative glycomic profiling of isotopically permethylated N-glycans by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2013;27:865–77. doi: 10.1002/rcm.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai TH, Wang M, Di Poto C, Hu Y, Zhou S, Zhao Y, Varghese RS, Luo Y, Tadesse MG, Ziada DH, Desai CS, Shetty K, Mechref Y, Ressom HW. LC-MS profiling of N-Glycans derived from human serum samples for biomarker discovery in hepatocellular carcinoma. J Proteome Res. 2014;13:4859–68. doi: 10.1021/pr500460k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Y, Zhou S, Yu CY, Tang H, Mechref Y. Automated annotation and quantitation of glycans by liquid chromatography/electrospray ionization mass spectrometric analysis using the MultiGlycan-ESI computational tool. Rapid Commun Mass Spectrom. 2015;29:135–42. doi: 10.1002/rcm.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu CY, Mayampurath A, Hu Y, Zhou S, Mechref Y, Tang H. Automated annotation and quantification of glycans using liquid chromatography-mass spectrometry. Bioinformatics. 2013;29:1706–7. doi: 10.1093/bioinformatics/btt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miletic H, Fischer Y, Litwak S, Giroglou T, Waerzeggers Y, Winkeler A, Li H, Himmelreich U, Lange C, Stenzel W, Deckert M, Neumann H, Jacobs AH, von Laer D. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15:1373–81. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 44.Doucette T, Rao G, Yang Y, Gumin J, Shinojima N, Bekele BN, Qiao W, Zhang W, Lang FF. Mesenchymal stem cells display tumor-specific tropism in an RCAS/Ntv-a glioma model. Neoplasia. 2011;13:716–25. doi: 10.1593/neo.101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.An HJ, Gip P, Kim J, Wu S, Park KW, McVaugh CT, Schaffer DV, Bertozzi CR, Lebrilla CB. Extensive determination of glycan heterogeneity reveals an unusual abundance of high mannose glycans in enriched plasma membranes of human embryonic stem cells. Mol Cell Proteomics. 2012;11:M111.010660. doi: 10.1074/mcp.M111.010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethi MK, Thaysen-Andersen M, Smith JT, Baker MS, Packer NH, Hancock WS, Fanayan S. Comparative N-glycan profiling of colorectal cancer cell lines reveals unique bisecting GlcNAc and α-2,3-linked sialic acid determinants are associated with membrane proteins of the more metastatic/aggressive cell lines. J Proteome Res. 2014;13:277–88. doi: 10.1021/pr400861m. [DOI] [PubMed] [Google Scholar]
- 47.Balog CI, Stavenhagen K, Fung WL, Koeleman CA, McDonnell LA, Verhoeven A, Mesker WE, Tollenaar RA, Deelder AM, Wuhrer M. N-glycosylation of colorectal cancer tissues: a liquid chromatography and mass spectrometry-based investigation. Mol Cell Proteomics. 2012;11:571–85. doi: 10.1074/mcp.M111.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holst S, Wuhrer M, Rombouts Y. Glycosylation characteristics of colorectal cancer. Adv Cancer Res. 2015;126:203–56. doi: 10.1016/bs.acr.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Hua S, Saunders M, Dimapasoc LM, Jeong SH, Kim BJ, Kim S, So M, Lee KS, Kim JH, Lam KS, Lebrilla CB, An HJ. Differentiation of cancer cell origin and molecular subtype by plasma membrane N-glycan profiling. J Proteome Res. 2014;13:961–8. doi: 10.1021/pr400987f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Nie H, Zhang Y, Yao Y, Maitikabili A, Qu Y, Shi S, Chen C, Li Y. Cell surface-specific N-glycan profiling in breast cancer. PLoS One. 2013;8:e72704. doi: 10.1371/journal.pone.0072704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Leoz ML, Young LJ, An HJ, Kronewitter SR, Kim J, Miyamoto S, Borowsky AD, Chew HK, Lebrilla CB. High-mannose glycans are elevated during breast cancer progression. Mol Cell Proteomics. 2011;10:M110.002717. doi: 10.1074/mcp.M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hakomori S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspectives. Cancer Res. 1985;45:2405–14. [PubMed] [Google Scholar]
- 53.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–18. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, Yagi F, Kasai K. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–54. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 55.Zhuo Y, Bellis SL. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J Biol Chem. 2011;286:5935–41. doi: 10.1074/jbc.R110.191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. doi: 10.1186/1741-7015-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gavella M, Garaj-Vrhovac V, Lipovac V, Antica M, Gajski G, Car N. Ganglioside GT1b protects human spermatozoa from hydrogen peroxide-induced DNA and membrane damage. Int J Androl. 2010;33:536–44. doi: 10.1111/j.1365-2605.2009.00962.x. [DOI] [PubMed] [Google Scholar]
- 58.Gavella M, Lipovac V. Protective effects of exogenous gangliosides on ROS-induced changes in human spermatozoa. Asian J Androl. 2013;15:375–81. doi: 10.1038/aja.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iijima R, Ichikawa T, Yamazaki M. Sialic acid attenuates the cytotoxicity of the lipid hydroperoxides HpODE and HpETE. Carbohydr Res. 2009;344:933–5. doi: 10.1016/j.carres.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Yang WS, Chang JW, Han NJ, Park SK. Darbepoetin alfa suppresses tumor necrosis factor-α-induced endothelin-1 production through antioxidant action in human aortic endothelial cells: role of sialic acid residues. Free Radic Biol Med. 2011;50:1242–51. doi: 10.1016/j.freeradbiomed.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Ogasawara Y, Namai T, Yoshino F, Lee MC, Ishii K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007;581:2473–7. doi: 10.1016/j.febslet.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 62.Iijima R, Takahashi H, Namme R, Ikegami S, Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004;561:163–6. doi: 10.1016/S0014-5793(04)00164-4. [DOI] [PubMed] [Google Scholar]
- 63.Eguchi H, Ikeda Y, Ookawara T, Koyota S, Fujiwara N, Honke K, Wang PG, Taniguchi N, Suzuki K. Modification of oligosaccharides by reactive oxygen species decreases sialyl lewis x-mediated cell adhesion. Glycobiology. 2005;15:1094–101. doi: 10.1093/glycob/cwj003. [DOI] [PubMed] [Google Scholar]
- 64.Yasuda J, Eguchi H, Fujiwara N, Ookawara T, Kojima S, Yamaguchi Y, Nishimura M, Fujimoto J, Suzuki K. Reactive oxygen species modify oligosaccharides of glycoproteins in vivo: a study of a spontaneous acute hepatitis model rat (LEC rat) Biochem Biophys Res Commun. 2006;342:127–34. doi: 10.1016/j.bbrc.2006.01.118. [DOI] [PubMed] [Google Scholar]
- 65.Kang SW, Lee S, Lee EK. ROS and energy metabolism in cancer cells: alliance for fast growth. Arch Pharm Res. 2015;38:338–45. doi: 10.1007/s12272-015-0550-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.