Abstract
Treatment of polycystic liver disease (PLD) focuses on symptom improvement. Generic questionnaires lack sensitivity to capture PLD-related symptoms, a prerequisite to determine effectiveness of therapy. We developed and validated a disease-specific questionnaire that assesses symptoms in PLD (PLD-Q). We identified 16 PLD-related symptoms (total score 0–100 points) by literature review and interviews with patients and clinicians. The developed PLD-Q was validated in Dutch (n=200) and US (n=203) PLD patients. We assessed the correlation of PLD-Q total score with EORTC symptom scale, global health visual analogue scale (VAS) of EQ-5D and liver volume. To test discriminative validity, we compared PLD-Q total scores of patients with different PLD-severity stages (Gigot classification), and PLD-Q total scores of PLD patients with general controls and polycystic kidney disease patients without PLD. Reproducibility was tested by comparing original test scores with two week retest scores. In total, 167 Dutch and 124 US patients returned the questionnaire. Correlation between PLD-Q total score and EORTC symptom scale (NL r=0.788; US r=0.811) and global health VAS (NL r=−0.517; US r=−0.593) was good. There was no correlation of PLD-Q total score with liver volume (NL r=0.138, P=0.236; US r=0.254, P=0.052). Gigot type III individuals scored numerically higher than type II patients (NL 46 vs. 40, P=0.089; US 48 vs. 36, P=0.055). PLD patients scored higher on the PLD-Q total score than general controls (NL 42 vs. 17; US 40 vs. 13 points) and polycystic kidney disease patients without PLD (22 points). Reproducibility of PLD-Q was excellent (NL r=0.94 and US 0.96).
Conclusion
PLD-Q is a valid, reproducible and sensitive disease-specific questionnaire which can be used to assess PLD-related symptoms in clinical care and future research.
Keywords: hepatomegaly, hepatic cyst, patient-reported outcome measure, quality of life, autosomal dominant polycystic kidney disease
Polycystic liver disease (PLD) is a condition characterized by multiple liver cysts. PLD is associated with two inherited conditions: isolated in autosomal dominant polycystic liver disease (ADPLD) and as an extra renal manifestation in autosomal dominant polycystic kidney disease (ADPKD).(1, 2) In a proportion of patients, progressive disease leads to mass-related symptoms such as early satiety and abdominal fullness, resulting in reduced quality of life.(3, 4) Treatment is indicated in symptomatic patients only.(5)
Progress in development of new treatments for PLD is hampered by the perceived lack of a widely accepted outcome measure for effectiveness. Past studies investigating the effectiveness of current or new treatments have used liver volume as primary outcome.(3, 6–10) Liver volume is thought to be an objective surrogate marker that could reflect disease severity. However, the relationship between liver volume and disease burden is not straightforward, as it does not always empirically correlate with patient well-being.(4, 11) The ultimate goal of PLD treatment is symptom relief and improvement of quality of life.(5)
Several generic patient-reported outcomes are available to assess either quality of life or gastrointestinal symptoms, but they are not validated in PLD.(12–18) These generic patient-reported outcomes do not capture the specific domains related to PLD such as increased abdominal girth or dyspnea.(4, 11, 19, 20) As a result, there is a substantial gap in the ability to detect clinically significant changes in PLD-related patient reported well-being as a measure of treatment outcome. A questionnaire that assesses the wide range of problems experienced by PLD patients is more likely to be responsive to changes in patients’ wellbeing.
The aim of this study was to develop and validate a PLD-specific questionnaire (PLD-Q) that captures patient-reported frequency and discomfort of PLD associated symptoms in a Dutch and US cohort of PLD patients in order to evaluate effectiveness of therapies.
Patients and methods
We conducted a prospective study from May 2013 – April 2015 at the Department of Gastroenterology and Hepatology, Radboud University Medical Center, Nijmegen, the Netherlands (NL) and at the Department of Internal Medicine, Mayo Clinic, Rochester, Minnesota, United States (US) following the Food and Drug Administration (FDA) guidelines on patient-reported outcome measure development.(21) This study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The protocol was approved by the Institutional Review Board of both centers and patients gave informed consent. We developed and validated the PLD-Q in three cycles, and each step led to further improvement of the questionnaire. Figure 1 shows a schematic overview of the development and validation process.
Figure 1.
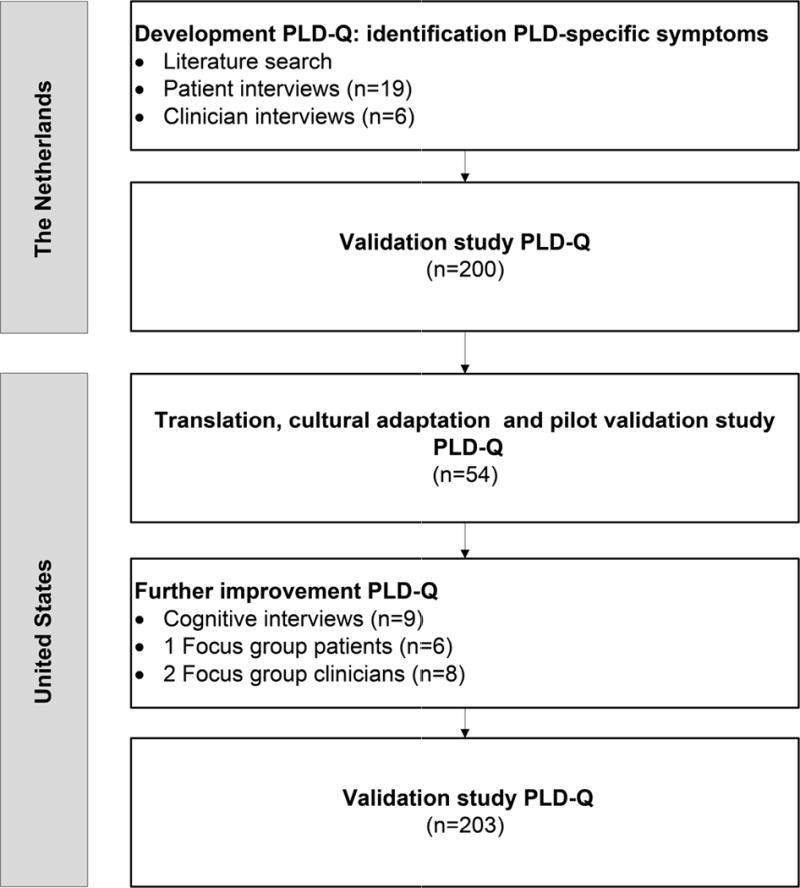
Selection of PLD patients
We selected patients of 18 years and older with PLD defined as >20 liver cysts on ultrasound, magnetic resonance imaging or computed tomography that were evaluated at the medical centers <10 years prior to inclusion.(5) Exclusion criteria included a history of liver or kidney transplantation, dialysis dependency, and liver surgery within six months prior to inclusion. For our validation studies, we additionally excluded patients enrolled in investigational drug studies for PLD or ADPKD (somatostatin analogues or vasopressin V2 receptor antagonists).(22, 23) We identified eligible Dutch patients from the PLD registry of Radboud University Medical Center. The Mayo Clinic Data Discovery and Query Builder was used to identify US PLD-patients with the search terms ‘polycystic liver’ or ‘cystic liver’.
Development of the PLD-Q
We started with building in appropriate questionnaire items relating to key issues for PLD patients (content validity).(24) These PLD-specific symptoms were identified in three resources; extensive literature search and in depth interviews with Dutch PLD patients and clinicians. Interviews were continued until we reached saturation, which was defined as the point where no new items emerged. An established qualitative approach was used to create consensus on the most relevant items for the PLD-Q through survey series (the Delphi method (25), Supplementary File 1 and Figure S1). The questionnaire was pretested in patients to test comprehensibility and changes were made based on their comments. The original Dutch version of the PLD-Q was translated into English using forward and backward translation including three individual translators for each step.(26) We adapted the translated PLD-Q cross-culturally and tested this version in a cohort of US PLD patients for inconsistencies after translation. Finally, we conducted cognitive debriefings, a patient focus group, and two clinician focus groups in the US for further improvement of the PLD-Q. The improved version of the PLD-Q was tested in a large cohort of US patients.
The score of each patient-reported symptom included in the PLD-Q can be calculated by adding a frequency (Likert scale; 1=never, 6=always) and discomfort (Likert scale; 0=not at all, 5= a lot) score of each symptom. Total scores were transformed into a score ranging from 0–100 points, where a higher score represents a higher symptom burden. The total PLD-Q score was calculated if ≤ 1 question score was missing. We chose a recall period of one month due to the chronic nature of PLD.(27)
Validation of the PLD-Q
Data collection
A study package was mailed to eligible patients containing the developed PLD-Q, the symptom scale of the European Organization for Research and Treatment of Cancer (EORTC) and the global health visual analogue scale (VAS) of the EQ5D.(20, 28) The EORTC symptom scale is designed to assess general symptoms in cancer patients participating in clinical trials, while the global health VAS is a general measure for quality of life. A reminder was sent to non-responders four weeks after the initial questionnaire and patients were contacted by phone if they had not returned their questionnaire.
Reliability
The PLD-Q was designed to measure a single domain which can be summed in a total score. We explored unidimensionality of the PLD-Q with exploratory factor analysis (varimax rotation). Unidimensionality was considered suitable when the first factor accounts for more than 20% of the total variability and the variance of the first factor was >4 times the variance of the other factors.(29) Internal consistency of the questionnaire was calculated using Cronbach’s alpha and a value of 0.7 was set as the threshold for good internal consistency.(30)
Score distributions and missing results
To test if the PLD-Q discriminates between patients on the extreme ends of the scale we investigated score distributions and the proportion of patients who got the lowest and highest total score (floor and ceiling effects, respectively). We used a cutoff value of ≥15% to indicate low discrimination.(30) We investigated missing values to assess whether patients understood and accepted the PLD-Q questions. Less than 10% missing values was considered acceptable for the total PLD-Q score and all individual questions.
Correlation PLD-Q scores with quality of life measures
We hypothesized that the PLD-Q total score is positively correlated with the EORTC symptom scale score and negatively correlated with the global health VAS (convergent validity). We calculated Spearman’s correlation coefficients (r) and a correlation coefficient of >0.4, P<0.05 was considered as evidence for convergent validity.
Correlation PLD-Q scores with organ volumes
We also calculated Spearman’s correlation coefficients (r) between questionnaire scores and height-adjusted liver volumes in a subgroup of patients that underwent a CT or MRI within two years prior to study inclusion. Measurements of liver volumes were performed using stereology followed by semi-automated segmentation in the US and manual segmentation in the Netherlands, as published previously.(31, 32) As clinical presentation of symptoms is highly variable across patients with similar liver volumes, we expected a low correlation between the PLD-Q score and height-adjusted liver volume (r=0.2 – 0.4, P<0.05).
Correlation PLD-Q scores with Gigot classification
Polycystic liver disease can be classified according to the Gigot classification.(33) Patients with Gigot type I (<10 large cysts) were excluded in this study since the number of cysts of these patients does not meet the definition of PLD (>20 cysts). Gigot type II classifies cases with diffuse involvement of liver parenchyma by multiple medium-sized cysts with large areas of noncystic liver parenchyma remaining. Gigot type III is defined by large numbers of small and medium-sized liver cysts spread diffusely through the liver parenchyma; only a few areas of normal parenchyma are present. Patients with recent imaging were classified according to the Gigot classification by two independent researchers (NL: T.W. and M.N; US: M.E and T.K.) that were blinded for the PLD-Q scores. We expected that patients classified as Gigot type III score higher on the PLD-Q total score than patients with Gigot type II livers, using the independent t-test.
Reproducibility
To assess the similarity of answers after repeated measurements under consistent conditions, we performed a retest after two weeks in a random subgroup of patients (n=45 in the Netherlands and n=100 in the US). We calculated the intraclass correlation coefficient between scores obtained at the different time points. An intraclass correlation coefficient of >0.7 indicates good reproducibility.(30) To detect systematic error in reproducibility, we plotted the differences between test and retest scores with the Bland-Altman method.(34)
PLD-specificity of PLD-Q items
Finally, we assessed whether the included symptoms of the PLD-Q are liver specific by comparing PLD-Q total scores of patients with two different control groups; a generic control group and an ADPKD control group without PLD (discriminative validity). The ADPKD control group with large polycystic kidneys but without PLD was selected to discriminate between symptoms arising from large kidneys versus symptoms from hepatomegaly, since the majority of patients with PLD have also cystic kidneys. Controls from the generic population were matched on age and gender and were recruited at random public gatherings in the Netherlands. In the US, we mailed a study package to a cohort of generic controls (1:2) that visited the Mayo Clinic within the last year prior to inclusion. US controls were matched on age, gender, race and state using SAS© version 9.3 (SAS Institute Inc., North Carolina, USA). We selected the ADPKD control group from the ADPKD database at the Mayo Clinic. Inclusion criteria were height-adjusted kidney volume of >750 mL and height-adjusted liver volume of <1000 mL or < 20 liver cysts.(11) Exclusion criteria for controls were the similar to the exclusion criteria for patients. Patient and control characteristics were compared using the independent t-test. Scores of patients and both control groups were compared using the Mann-Whitney U test. Significance was defined as P <0.05 in all analyses.
Results
Development of the PLD-Q
Saturation of key issues for PLD patients was reached after interviewing 19 Dutch PLD patients and 6 clinicians (hepatologists n=4, nephrologists n=2). Table S1 shows characteristics of patients included in the interviews. In total, 36 PLD-related items were generated. After the Delphi survey, the 14 items ‘feeling full’, ‘bloating’, ‘stomach tension’, ‘lack of appetite’, ‘early satiety’, ‘pressure or pain rib cage’, ‘pain in side’, ‘stomach pain’, ‘shortness of breath’, ‘limited mobility’, ‘tiredness’, ‘anxiety about the future’, ‘dissatisfaction size abdomen’, and ‘discomfort during intercourse’ were considered relevant to include in the PLD-Q (Table S2). During the pretest of the developed PLD-Q, patients considered the item ‘nausea’ important and this item was therefore retained in the questionnaire. ‘Bloating’ and ‘stomach tension’ were considered redundant to ‘feeling full’ leading to rejection of these items.
After translation, the US PLD-Q pilot study (n=54) showed no large differences compared to the Dutch PLD-Q (Supplementary File 2 and Figure S2). We conducted nine cognitive debriefings, and included six PLD patients in the patient focus group (Table S1). In total, two clinician focus groups were conducted including hepatologists (n=2), nephrologists (n=4), a liver surgeon (n=1), and a transplant surgeon (n=1). Patients reported that the questionnaire was easy to understand and preferred the PLD-Q above general quality of life questionnaires to address their symptoms. Five questions were rephrased for easier understanding. US patients and clinicians felt that three questions were missing; acid reflux, back pain and, concern of liver growth. These 3 questions were added to the 13 items of the Dutch PLD-Q. Table S4 shows changes between the translated PLD-Q used in the pilot study and the improved version of the PLD-Q after cognitive interviews and focus groups in the US. The final version of the PLD-Q is shown in Supplementary File 3.
Validation of the PLD-Q
Characteristics of included patients and controls
The Dutch PLD registry included 211 eligible patients for the validation study. Eleven patients were excluded after sending the questionnaire (death (n=1), renal replacement therapy or liver transplantation (n=7) and use of investigational drugs for PLD (n=3)). Of 200 remaining patients, 167 patients returned the questionnaire (response rate 84%). In the US cohort, 216 eligible patients were identified. In this cohort, 13 patients were excluded after sending the questionnaires (death (n=2), renal replacement therapy or liver transplantation (n=5), use of investigational drugs for PLD (n=5) and recent liver surgery (n=1)). Of 203 remaining patients, 124 patients returned the questionnaire (response rate 61 %). Characteristics of the included patients are shown in Table 1. Age, gender and disease of responders and non-responders were comparable in both groups.
Table 1.
Characteristics of included patients and controls
| Dutch PLD patients (n=167/200) | Dutch general controls (n=183/183) | US PLD patients (n=124/203) | US general controls (n=170/406) | US ADPKD controls (n=32/60) | |
|---|---|---|---|---|---|
| Female (%) | 84 | 79 | 86 | 88 | 47 |
| Age (years) ± SD | 56 ± 11 | 57 (20–90) | 56 ± 15 | 59 ± 10 | 50 ± 13 |
| ADPLD/ADPKD (%) | 54/46 | N/A | 18/82 | N/A | 0/100 |
| Liver volume, mL (IQR) | 2720 (1625–3947)a | N/A | 1649 (1273–2629)a | N/A | 829 (750–984) |
| Kidney volume, mL (IQR) | 536 (374–1045)b | N/A | 672 (486–1395)b | N/A | 1447 (1093–1830) |
| Total liver kidney volume, mL (IQR) | 3307 (2408–4818) | N/A | 2623 (1924–3718)b | N/A | 2337 (1936–2666) |
Data is presented as mean ± standard deviation for normally distributed variables and median (interquartile range) for non-normally distributed variables. Organ volumes are height corrected.
Liver volumes are measured in a subgroup of 76 Dutch and 60 US PLD patients.
Kidney and total liver kidney volumes are measured in ADPKD patients only (Dutch n=49, US n=52).
Abbreviations: ADPLD, isolated autosomal dominant polycystic liver disease; ADPKD, autosomal dominant polycystic kidney disease, N/A, not applicable.
For the general control group, we included 183 Dutch and 170 US controls with similar characteristics as the included PLD cohort. In the ADPKD control group, we identified 69 ADPKD controls of whom nine patients were excluded after sending the questionnaire (renal replacement therapy (n=2), recent nephrectomy (n=1), investigational drugs (n=5) and no correct postal address (n=1)). Of the remaining 60 patients, 32 patients returned their questionnaire (response rate 53%). Table 1 shows the characteristics of the included control groups. ADPKD controls were younger than PLD patients (P =0.009) and more ADPKD controls were male (P <0.001). However, combined total liver kidney volume was not significantly different (P =0.215).
Reliability
Factor analysis showed that the variability of the PLD-Q was largely explained by the first factor (NL 47% and US 54% of the total variability), while the other factors contributed little to the variance (NL: 11 and 8% and US 7%)(Table S4), supporting a unidimensional structure of the PLD-Q. Internal consistency of the PLD-Q was excellent (Cronbachs alpha 0.796 and 0.840 respectively).
Score distributions
Median total PLD-Q score was 42 (IQR 26 – 52) points in the Netherlands and 40 (IQR: 25 – 57) points in the US. Scores were normally distributed (mean 39 ± 18 and 42 ± 21 respectively) and ranged from 0–88 points and 3–93 respectively. A score of 0 points (floor effect) was found in two Dutch patients (1%) and in none of the US patients. No patients scored the maximum of 100 points (ceiling effect). Missing results of the total scores (NL: 1% and US: 4% respectively) and individual items (0–4%) were low, except for sexual intercourse in US patients (7%). However, none of the questions exceeded the predefined cut-off value of 10% missing results.
Correlation PLD-Q scores with quality of life measures
The PLD-Q total score showed a positive correlation with the symptom scale of the EORTC in both cohorts (NL: 0.788 and US: 0.811, P <0.001), indicating that patients with more PLD-associated symptoms on the PLD-Q had also a higher symptom burden on the EORTC symptom scale. The global health VAS showed a negative correlation with the PLD-Q total score (NL: 0.517 and US: 0.599, P<0.001). This shows that patients with a high symptom burden on the PLD-Q score have a low global health status. Both directions and magnitudes of the correlation coefficients were consistent with our predefined hypotheses.
Correlation PLD-Q scores with organ volumes
We identified 76 Dutch patients and 60 US patients with recent imaging. PLD-Q total scores ranged from 3–74 points, (mean 43 ± 16) and 3–93 points (mean 41 ± 23) respectively, comparable to the total validation group scores. There was no significant correlation between the PLD-Q total score and height-corrected liver volume (NL: r=0.138, P =0.236 and US: r=0.254, P =0.052).
Correlation PLD-Q scores with Gigot classification
From the identified PLD patients with recent imaging, 78 patients (42 Dutch; 36 US) were classified as Gigot type II (median height-corrected liver volumes 1871 mL (IQR 1250 – 2586) and 1387 mL (IQR 1211 – 1791 respectively)), and 58 patients (34 Dutch; 24 US) as Gigot type III (median height-corrected liver volume 4073 (IQR 3009 – 5307) and 2657 ml (IQR 1679 – 3473) respectively). Gigot type III individuals scored numerically higher on the PLD-Q total score compared to Gigot type II patients in both countries (respectively 46 ± 16 vs. 40 ± 17 and 48 ± 25 vs. 36 ± 22), although these differences were not significantly different (P =0.089 and P =0.055).
Reproducibility
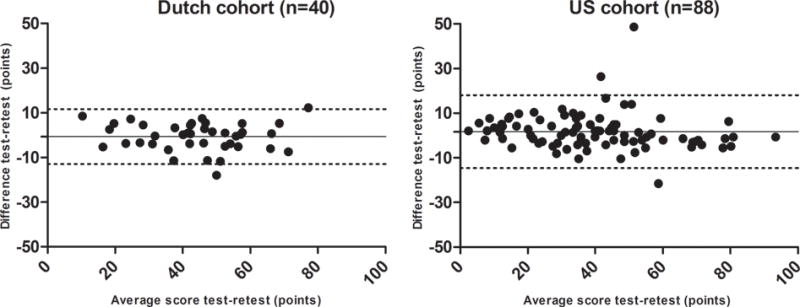
Test-retest was performed in 40 patients that returned the retest in the Netherlands. Mean total score of the test in the Dutch cohort was 45 ± 16 and 44 ± 16 of the retest, showing excellent reproducibility (intraclass correlation coefficient 0.94 (95% CI 0.88 – 0.97)). In the US, 94 patients returned the retest of which 6 could not be used as a result of missing values in test or retest. The mean difference between test and retest in this group was 2 points (95% CI 0 – 3), again resulting in excellent reproducibility (intraclass correlation coefficient 0.96 (95% CI 0.94 – 0.97)).
Bland-Altman plots of the difference between test and retest showed equal spread above and beneath the mean difference indicating the lack of a systematic bias (see Figure 2).
Figure 2.
PLD-specificity of PLD-Q items
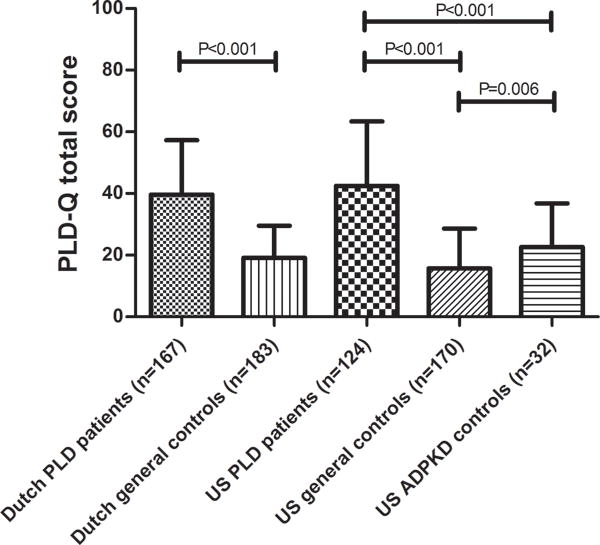
PLD patients scored significantly higher compared to the general controls on the PLD-Q total score (NL: 42 points (IQR 26 – 52) vs. 17 points (IQR: 12 – 26), P <0.001 and US: 40 points (IQR: 26 – 57) vs. 13 points (IQR: 7 – 22),, P <0.001 (see Figure 3)) and all individual items in both cohorts. Compared to the ADPKD controls (without PLD), US PLD patients scored higher on the total score 40 points (IQR: 26 – 57) vs. 22 points (IQR: 12 – 33), P <0.001 and all individual questions except for back pain (P =0.173). ADPKD controls scored higher than US general controls (22 points, (IQR: 12 – 33) vs. 13 points (IQR: 7 – 22); P =0.006).
Figure 3.
Discussion
The PLD-Q is the first prospectively designed disease-specific questionnaire for patients with PLD developed with input from patients, clinicians and experts in quality of life research. This study provides evidence of content validity, reliability, good score distributions, convergent validity, reproducibility and discriminative validity of the PLD-Q in a Dutch and US cohort. We show that the PLD-Q is able to identify PLD-specific symptoms and that these symptoms correlate with quality of life. The PLD-Q was easy to understand which undoubtedly contributed to high completion rates and a small proportion of missing data.
Parallel with development and validation of the PLD-Q, a Belgian group developed a ’polycystic liver disease complaint-specific assessment’ (POLCA) tool, emphasizing the need of a better patient-reported outcome in PLD.(35) Six highly educated panel members designed the POLCA in Dutch by analyzing literature and medical records. POLCA was validated in 61 PLD patients included in a clinical trial. Compared with PLD-Q, POLCA does not include the items dyspnea, pain or pressure in the ribcage, limited mobility, fear or anxiety for the future, and discomfort with intercourse, while our data suggest that these symptoms impact PLD burden. These differences might be explained by the lack of patient involvement in the development of POLCA, which limits content validity.(24, 36) It is unclear whether absence of pretesting in patients has led to reduced comprehensibility of the POLCA, as the proportion of missing data is not provided. Finally, reproducibility of the POLCA was not tested. Therefore clinically important change in POLCA scores cannot be distinguished from measurement error.(30)
The PLD-Q showed no significant correlation with liver volume in the subgroup of patients that had recent imaging. Although we expected that patients with larger liver volumes had more symptoms measured with a disease-specific measure, this finding is consistent with earlier studies. A recent study that used the SF-36, a generic quality of life measure, showed no correlation with liver volume in severe PLD patients.(4) The same applies for the disease-specific POLCA, as no correlation between POLCA scores and liver volume was detected. Only one large study of ADPKD patients with early chronic kidney disease not selected for PLD severity (n=558) demonstrated a weak correlation between physical component scores of the SF-36 and liver volume.(11)
Symptom presentation depends on different personal factors including body habitus, culture and coping strategies,(37, 38)and it might be possible that PLD-Q scores do not correlate with liver volume in the total group, but correlate with individual changes in liver volume over time. Symptoms may also correlate with cyst growth rate rather than total volume. Further validation of the PLD-Q in longitudinal studies will be needed to test these hypotheses.
PLD patients scored higher on all items included in the PLD-Q compared to controls, with the exception of the item back pain. ADPKD controls reported back pain scores similar to our PLD cohort. Indeed, back pain is a frequent symptom reported in a previous study that included ADPKD patients with smaller liver volumes, suggesting that the item is not liver specific.(39) However, we show here that PLD does contribute to back pain, a reason to retain back pain as an item in the PLD-Q.
A strength of this study is the rigorous development and validation of the PLD-Q conform proposed quality criteria.(30) We included patients in all aspects of the development process, as patient-derived measurements are shown to be more valuable than tools derived from non-patient populations such as experts only.(40) We included patients with mild to severe disease and their scores ranged to almost all possible scores of the scale, indicating that this scale provides relevant information for patients in different severity stages. We also confirmed the results from the Dutch cohort in a native English speaking cohort after thorough translation and cultural adaptation. Testing the PLD-Q in a geographically and demographically diverse population improves the generalizability of the results. In a rare disease such as PLD, international collaborations will help accelerate progress towards treatment. Finally, inclusion of two different control groups has led to a better interpretation of PLD-Q scores since patient scores can be compared with reference values.
A limitation of this study is that we have not shown responsiveness, the degree to which a measure can detect clinically important change over time, of the PLD-Q.(30) Obvious changes in PLD symptoms are expected after liver resection or liver transplantation, as the effect of somatostatin analogues on liver volume is relatively small.(41) We are currently testing the responsiveness of the PLD-Q in a prospective study of patients subjected to liver resection. The first four patients that have completed the PLD-Q prior to and six month after the procedure showed large improvements in scores, suggesting responsiveness of the PLD-Q, but additional data are needed to bolster these results. The fact that the PLD-Q can differentiate between patients with different Gigot stages of PLD and control groups provides additional preliminary evidence for responsiveness of the PLD-Q, as virtually all patient-reported outcomes that can differentiate among clinically distinct groups are found to be responsive to change.(42)
The one month recall time frame of the PLD-Q may be less sensitive to symptoms of cystic complications such as cyst hemorrhages, ruptures and infections that may influence quality of life. However, interviews and cognitive debriefings confirmed a 4-week recall period as suitable period in our patient population. In addition, a large case series of 137 PLD cases showed an incidence of 20 cyst hemorrhages, 12 cyst infections and 6 cyst ruptures during a mean follow up period of more than 8 years, indicating that episodes of acute pain are rare.(43)
In the vast majority of PLD patients, the indication of treatment is based on symptoms.(44) The PLD-Q provides a new tool that is likely to be more sensitive to small but important differences in the health status of patients after treatment interventions. The PLD-Q can be considered as patient-reported outcome in clinical trials to support future claims to approve medical treatment.(19) Apart from research purposes, the PLD-Q might be useful in clinical evaluation of PLD. Periodic monitoring of symptoms in PLD patients can guide, start or stop decisions in medical treatment or may determine timing of surgery or liver transplantation. It would be interesting to evaluate the ability of the PLD-Q to distinguish between fast and slow progressors in a population with progressive disease. This requires a rigorous prospective design with long-term follow up and this type of data is not available yet.
We advise to administer the PLD-Q in combination with a generic quality of life questionnaire to provide a complete health status assessment in PLD patients. When adding a generic questionnaire, it is preferably to choose a tool that has been validated previously in the target population. Furthermore, short questionnaires are better to curtail patient burden.
To conclude, we have developed and validated a robust disease-specific questionnaire for polycystic liver disease (PLD-Q) that reflects symptom burden and its impact on patient well-being in a Dutch and US patient cohort. We recommend the PLD-Q as a patient-reported outcome to assess PLD-related symptoms in clinical care and future research.
Potential users of the PLD-Q
The authors reserve copyright for the PLD-Q questionnaire and encourage potential users to contact the authors. No charges or restrictions are placed on using the PLD-Q for non-commercial purposes.
Supplementary Material
Acknowledgments
We would like to thank all patients and experts that participated in this study. Special thanks to Jason Egginton who conducted the cognitive interviews and focus groups.
Financial Support
This study received funding from the Mayo Translational Polycystic Kidney Disease Center (NIDDK P30 DK090728). Myrte K. Neijenhuis received a Kolff PhD Fellowship Abroad Grant from the Dutch Kidney Foundation and Tom J.G. Gevers received an Andrew K. Burroughs Short-Term Training Fellowship Grant from the European Association for the Study of the Liver (EASL).
List of abbreviations
- PLD
polycystic liver disease
- ADPKD
autosomal dominant polycystic kidney disease
- PLD-Q
polycystic liver disease questionnaire
- VAS
visual analogue scale
- EORTC
European Organization for Research and Treatment of Cancer
- NL
the Netherlands
- US
Unites States
Contributor Information
Myrte K. Neijenhuis, Email: Myrte.Neijenhuis@radboudumc.nl.
Tom J.G. Gevers, Email: Tom.Gevers@radboudumc.nl.
Marie C. Hogan, Email: Hogan.Marie@mayo.edu.
Patrick S. Kamath, Email: Kamath.Patrick@mayo.edu.
Titus F.M. Wijnands, Email: Titus.Wijnands@radboudumc.nl.
Ralf C.P.M. van den Ouweland, Email: Rvanden.Ouweland@student.ru.nl.
Marie E. Edwards, Email: Edwards.Marie@mayo.edu.
Jeff A. Sloan, Email: Sloan.Jeff@mayo.edu.
Wietske Kievit, Email: Wietske.Kievit@radboudumc.nl.
Joost P.H. Drenth, Email: Joostphdrenth@cs.com.
References
- 1.Reynolds DM, Falk CT, Li A, King BF, Kamath PS, Huston J, 3rd, Shub C, et al. Identification of a locus for autosomal dominant polycystic liver disease, on chromosome 19p13.2–13.1. Am J Hum Genet. 2000;67:1598–1604. doi: 10.1086/316904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 3.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. Journal of the American Society of Nephrology. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijnands TF, Neijenhuis MK, Kievit W, Nevens F, Hogan MC, Torres VE, Gevers TJ, et al. Evaluating health-related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver Int. 2014;34:1578–1583. doi: 10.1111/liv.12430. [DOI] [PubMed] [Google Scholar]
- 5.Drenth JP, Chrispijn M, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52:2223–2230. doi: 10.1002/hep.24036. [DOI] [PubMed] [Google Scholar]
- 6.Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, van Oijen MG, Coudyzer W, Hoffmann AL, et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35:266–274. doi: 10.1111/j.1365-2036.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 7.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. e1661–1662. doi: 10.1053/j.gastro.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 8.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, Irazabal MV, et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27:3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Wetzels JF, Drenth JP. Effect of lanreotide on polycystic liver and kidneys in autosomal dominant polycystic kidney disease: an observational trial. Liver Int. 2015;35:1607–1614. doi: 10.1111/liv.12726. [DOI] [PubMed] [Google Scholar]
- 10.Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: Results from a randomized controlled trial. J Hepatol. 2013;59:153–159. doi: 10.1016/j.jhep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Hogan MC, Abebe K, Torres VE, Chapman AB, Bae KT, Tao C, Sun H, et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol. 2015;13:155–164. e156. doi: 10.1016/j.cgh.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 13.EuroQol -a new facility for the measurement of health-related quality of life, 1990
- 14.Bovenschen HJ, Janssen MJ, van Oijen MG, Laheij RJ, van Rossum LG, Jansen JB. Evaluation of a gastrointestinal symptoms questionnaire. Dig Dis Sci. 2006;51:1509–1515. doi: 10.1007/s10620-006-9120-6. [DOI] [PubMed] [Google Scholar]
- 15.Agreus L, Svardsudd K, Nyren O, Tibblin G. Reproducibility and validity of a postal questionnaire. The abdominal symptom study. Scand J Prim Health Care. 1993;11:252–262. doi: 10.3109/02813439308994840. [DOI] [PubMed] [Google Scholar]
- 16.Rey E, Locke GR, 3rd, Jung HK, Malhotra A, Choung RS, Beebe TJ, Schleck CD, et al. Measurement of abdominal symptoms by validated questionnaire: a 3-month recall timeframe as recommended by Rome III is not superior to a 1-year recall timeframe. Aliment Pharmacol Ther. 2010;31:1237–1247. doi: 10.1111/j.1365-2036.2010.04288.x. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 18.Guyonnet D, Naliboff B, Rondeau P, Mayer E, Chassany O. Gastrointestinal well-being in subjects reporting mild gastrointestinal discomfort: characteristics and properties of a global assessment measure. Br J Nutr. 2013:1–9. doi: 10.1017/S0007114513000275. [DOI] [PubMed] [Google Scholar]
- 19.Perrone RD, Coons SJ, Cavanaugh K, Finkelstein F, Meyer KB. Patient-Reported Outcomes in Clinical Trials of CKD-Related Therapies: Report of a Symposium Sponsored by the National Kidney Foundation and the US Food and Drug Administration. American Journal of Kidney Diseases. 2013;62:1046–1057. doi: 10.1053/j.ajkd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services Food and Drug Administration. Guidance for the industry. Patient reported outcome measure: tools in medical product development to support labeling claims. www.fda.gov/downloads/drugs/guidance/ucm193282.pdf.
- 22.Gevers TJ, Drenth JP. Somatostatin analogues for treatment of polycystic liver disease. Curr Opin Gastroenterol. 2011;27:294–300. doi: 10.1097/MOG.0b013e328343433f. [DOI] [PubMed] [Google Scholar]
- 23.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, Snyder C, et al. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2012;21:739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 25.Fitch K, Bernstein SJ, Aguilar MS, Burnand B, LaCalle JR, Lazaro P, van het Loo M, McDonnell J, Vader J, Kahan JP. The RAND/UCLA appropriateness method user’s manual. 2001 http://www.rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf.
- 26.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 27.Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:101–108. doi: 10.1038/nrgastro.2012.254. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 29.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45:S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 30.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Warner JD, Irazabal MV, Krishnamurthi G, King BF, Torres VE, Erickson BJ. Supervised segmentation of polycystic kidneys: a new application for stereology data. J Digit Imaging. 2014;27:514–519. doi: 10.1007/s10278-014-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Keimpema L, Ruurda JP, Ernst MF, van Geffen HJ, Drenth JP. Laparoscopic fenestration of liver cysts in polycystic liver disease results in a median volume reduction of 12.5% J Gastrointest Surg. 2008;12:477–482. doi: 10.1007/s11605-007-0376-8. [DOI] [PubMed] [Google Scholar]
- 33.Gigot JF, Jadoul P, Que F, Van Beers BE, Etienne J, Horsmans Y, Collard A, et al. Adult polycystic liver disease: is fenestration the most adequate operation for long-term management? Ann Surg. 1997;225:286–294. doi: 10.1097/00000658-199703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 35.Temmerman F, Dobbels F, Ho TA, Pirson Y, Vanslembrouck R, Coudyzer W, Bammens B, et al. Development and validation of a polycystic liver disease complaint-specific assessment (POLCA) J Hepatol. 2014;61:1143–1150. doi: 10.1016/j.jhep.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Trevisol DJ, da Silva A, de Souza F, Zapelini CM. Development and validation of a polycystic liver disease complaint-specific assessment (POLCA) - use of the Delphi technique for content validation. J Hepatol. 2015;62:988. doi: 10.1016/j.jhep.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 37.Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251–258. doi: 10.7326/0003-4819-88-2-251. [DOI] [PubMed] [Google Scholar]
- 38.Felton BJ, Revenson TA. Coping with chronic illness: a study of illness controllability and the influence of coping strategies on psychological adjustment. J Consult Clin Psychol. 1984;52:343–353. doi: 10.1037//0022-006x.52.3.343. [DOI] [PubMed] [Google Scholar]
- 39.Miskulin DC, Abebe KZ, Chapman AB, Perrone RD, Steinman TI, Torres VE, Bae KT, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1–4: A cross-sectional study. American Journal of Kidney Diseases. 2014;63:214–226. doi: 10.1053/j.ajkd.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orr JG, Homer T, Ternent L, Newton J, McNeil CJ, Hudson M, Jones DE. Health related quality of life in people with advanced chronic liver disease. J Hepatol. 2014;61:1158–1165. doi: 10.1016/j.jhep.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 41.Gevers TJ, Inthout J, Caroli A, Ruggenenti P, Hogan MC, Torres VE, Nevens F, et al. Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology. 2013;145:357–365. e352. doi: 10.1053/j.gastro.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Van Keimpema L, De Koning DB, Van Hoek B, Van Den Berg AP, Van Oijen MG, De Man RA, Nevens F, et al. Patients with isolated polycystic liver disease referred to liver centres: clinical characterization of 137 cases. Liver Int. 2011;31:92–98. doi: 10.1111/j.1478-3231.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- 44.Schnelldorfer T, Torres VE, Zakaria S, Rosen CB, Nagorney DM. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann Surg. 2009;250:112–118. doi: 10.1097/SLA.0b013e3181ad83dc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


