Abstract
Purpose
Cancer cachexia is characterized by decreased body weight (mainly lean body mass [LBM]) and negatively impacts quality of life (QOL) and prognosis. Anamorelin (ONO-7643) is a novel selective ghrelin receptor agonist under development for treating cancer cachexia.
Methods
In this double-blind, exploratory phase 2 trial, we examined the efficacy and safety of anamorelin in Japanese patients (n = 181) with non-small cell lung cancer (NSCLC) and cancer cachexia (≥5 % weight loss within the previous 6 months). The participants were randomized into three groups and were administered 50 or 100 mg anamorelin, or placebo, orally every day for 12 weeks. The co-primary endpoints were the changes from baseline over 12 weeks in LBM and handgrip strength (HGS). Secondary endpoints included body weight, QOL, Karnofsky Performance Scale (KPS), and serum biomarkers.
Results
The change in LBM over 12 weeks was 0.55 and 1.15 kg in the placebo and 100-mg anamorelin groups, respectively, but the efficacy of anamorelin in HGS was not detected. The changes in body weight were −0.93, 0.54, and 1.77 kg in the placebo, 50-mg anamorelin, and 100-mg anamorelin groups, respectively. Anamorelin (100 mg) significantly improved KPS and QOL-ACD compared with placebo. Administration of anamorelin for 12 weeks was well tolerated.
Conclusions
This phase 2 study showed that 100 mg anamorelin has promising results in improving lean body mass, performance status, and especially, QOL in patients with cancer cachexia.
Electronic supplementary material
The online version of this article (doi:10.1007/s00520-016-3144-z) contains supplementary material, which is available to authorized users.
Keywords: Anamorelin (ONO-7643), Cachexia, Lean body mass, Non-small cell lung cancer, Randomized controlled trial
Introduction
Cachexia is frequently associated with a variety of clinically relevant features, including anorexia, inflammation, insulin resistance, and muscle protein breakdown, and these features are driven by muscle wasting. However, pre-cachexia may occur in the absence of weight loss, and obesity or prior weight loss may mask its symptoms in some patients [1]. Cachexia is a frequent disorder in cancer patients and is cited as the cause of death in about 20 % of cancer patients [2–6]. Furthermore, cancer cachexia may also be accompanied by increased chemotherapy-related toxicity [1], as well as poor prognosis and poor quality of life (QOL) [7].
There are several treatment options available for the management of patients with cachexia. For example, megestrol acetate is available for first-line therapy in AIDS-related cachexia in some countries (but not Japan). In a clinical trial of cancer cachexia, megestrol acetate significantly improved appetite and weight gain compared with placebo [8]. However, megestrol acetate tends to increase fat mass rather than muscle mass [9], and in a clinical trial [8], it did not improve QOL and was associated with a higher incidence of adverse events compared with placebo.
Ghrelin, a neuropeptide secreted by ghrelinergic cells in the gastrointestinal tract, is a regulator of hunger signals that also prepares the body for food intake. In addition, ghrelin acts as a growth hormone (GH) secretagogue [10, 11]. In humans, ghrelin has antagonistic effects on leptin signaling and significantly increases food intake [12–14]. Therefore, ghrelin mimetics have been postulated as possible treatments for cachexia by increasing GH secretion and promoting food intake and weight gain.
Structure–function relationship studies have revealed that the C-terminal part of ghrelin plays an important role in its receptor binding and biological activity [15]. Accordingly, the structure of this region was used as the basis for the development of anamorelin (ONO-7643), an orally administered low-molecular-weight ghrelin-like agonist with the chemical structure 3-{(2R)-3-{(3R)-3-benzyl-3-[(trimethylhydrazino) carbonyl] piperidin-1-yl}-2-[(2-methylalanyl) amino]-3-oxopropyl}-1H-indole [16, 17]. Preclinical studies have demonstrated that anamorelin is a potent ghrelin receptor agonist that significantly increases food intake and body weight in rats [18], and does not promote tumor growth, which is a potential concern for molecules that increase GH and insulin-like growth factor-1 (IGF-1) levels [19]. Subsequent phase 1 and phase 2 studies of anamorelin have been completed in healthy volunteers and patients with cancer cachexia in the USA [20–22]. These studies confirmed that anamorelin significantly increased body weight and food intake compared with placebo. More recently, two completed phase 3 studies of anamorelin showed that anamorelin for 12 weeks was well tolerated and significantly improved lean body mass (LBM), body weight, and anorexia-cachexia symptoms/concerns in advanced non-small cell lung cancer (NSCLC) patients with cachexia; these multinational trials were conducted in North America, Europe, and Australia [23]. However, no studies have examined the effects of anamorelin in Japanese patients with cancer cachexia. Therefore, the aim of this study was to examine the efficacy and safety of anamorelin in Japanese patients with cancer cachexia as part of the clinical development of anamorelin for the treatment of cachexia.
This trial included patients with NSCLC because these patients typically show weight loss and are less likely to drop out owing to disease progression than are patients with other cancer types. Additionally, these patients were expected to tolerate food intake and administration of anamorelin. Furthermore, to minimize any potential confounding effects of the cancer itself, only NSCLC patients were chosen to participate.
Methods
Study design
This multicenter, randomized, double-blind, placebo-controlled study comprised a 2-week observation/run-in period, a 12-week treatment period, and a 4-week follow-up period, and it was conducted at 32 sites in Japan. Visits during the treatment period were scheduled at weeks 0 (baseline/randomization), 4, 8, and 12. The study was conducted in compliance with the Declaration of Helsinki, the study protocol, Paragraph 3 of Article 14 and Article 80-(2) of the Pharmaceutical Affairs Law of Japan, and Good Clinical Practice Guidelines (Ministry of Health and Welfare Ordinance No. 28). The study was approved by ethics committees at all participating institutions. Clinical trial registration: JapicCTI-111415 (Japan Pharmaceutical Information Center Clinical Trials Information).
Patients
Males or females with inoperable stage III or IV NSCLC, or relapsed NSCLC indicated for chemotherapy, were eligible if they satisfied the following inclusion criteria: aged ≥20 years at the time of informed consent; had involuntary weight loss of ≥5 % observed over the preceding 6 months; at least three of anorexia, fatigue, malaise, decreased general muscle strength, arm muscle circumference (AMC) (in cm) <10th percentile and at least one of C-reactive protein (CRP) >5.0 mg/L, hemoglobin <12 g/dL, or albumin <3.2 g/dL; an Eastern Cooperative Group Performance Status of 1 or 2; and an estimated life expectancy of ≥4 months. Anorexia, fatigue, malaise, and decreased muscle strength were to be of grade ≥ 1 according to the Japanese Version of the NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. AMC was calculated using the formula: AMC (cm) = arm circumference (AC) (cm) − 3.14 × triceps skin fold thickness (TSF) (mm)/10.
Patients with known brain metastases or uncontrolled diabetes were excluded from the study. All patients provided written informed consent, and after registration, eligible patients were randomized by a central allocation center with stratification by study site and weight loss over the preceding 6 months (5–15 % and >15 %). Randomization was performed using a randomization table and the sealed envelope method.
Interventions and concomitant therapy
Patients were randomized to receive 50 or 100 mg of anamorelin or placebo once daily during the treatment period. To maintain blinding, all patients took two tablets (two 50-mg tablets, one 50-mg tablet plus placebo tablet, or two placebo tablets). The active and placebo tablets were identical in appearance and packaging. The doses of anamorelin and the duration of administration were chosen based on previous studies [24].
High-emetic-risk chemotherapy (categorized according to the “antiemetics proper use guidelines Version 1” by Japan Society of Clinical Oncology), radiation therapy (except palliative radiotherapy for bone metastasis or radiotherapy for brain metastases), systemic corticosteroids, GH preparations, medroxyprogesterone, megestrol acetate, Chinese herbal medicines (Anchusan, orento, saireito, juzentaihoto, shosaikoto, seishoekkito, ninjin’yoeito, heiisan, hochuekkito, rikkunshito, junshousan), antiarrhythmic drugs, antitumor anthracyclines, CYP3A4 inhibitors, CYP3A4 inducers, grapefruit-containing products, and other investigational treatments were prohibited during the study.
Efficacy assessments
The primary endpoints were the mean changes in LBM, as measured by dual energy X-ray absorptiometry (DEXA) and grip strength of the non-dominant hand from baseline through to Week 12. Other endpoints included the changes in body composition-related variables, as determined by DEXA and bioimpedance, grip strength, body weight, Japanese Version of the M.D. Anderson Symptom Inventory (MDASI-J), QOL Questionnaire for Cancer Patients Treated with Anticancer Drugs (QOL-ACD), ECOG PS, Karnofsky Performance Scale, and serum biomarkers. All efficacy variables were determined at baseline (start of observation) and weeks 4, 8, and 12. The efficacy variables were also measured following treatment discontinuation.
DEXA was performed using Hologic (Bedford, MA, USA) or GE Lunar (Wauwatosa, WI, USA) systems capable of whole-body scans; scans were performed ≥48 h after any use of contrast medium to avoid interference. DEXA was used to measure LBM, body fat, bone mineral content, and total weight using standard procedures. Bioimpedance was also used to record LBM, body fat, bone mineral content, body cell mass, and body weight using established methods. Bioimpedance was performed using an InBody720 (InBody Japan Inc., Tokyo, Japan) instrument provided by the sponsor. Grip strength was measured three times for each hand using a grip dynamometer (Tracker Freedom® Wireless Grip; JTECH Medical Midvale, UT, USA). The maximum value of the three measurements was recorded for each hand, and the dominant/non-dominant hand was noted.
The MDASI-J is a self-rated scale covering 13 symptom items and 6 interference items, which shows good validity and practicality for assessing cancer-related symptoms within the last 24 h by 11 scales [25]. The Japanese version has been validated and showed good consistency with the English version. The QOL-ACD (Supplementary Materials) is a self-rated scale evaluating patients’ status within the past few days by five scales, comprised of four domains (functional, physical, mental, and psychosocial) and a global face scale was developed as a generic questionnaire for evaluating the QOL of Japanese patients undergoing chemotherapy. All four domains displayed moderate–strong associations with clinically relevant variables, such as performance status and weight loss in Japanese patients with NSCLC [26]. Performance status was examined in terms of the ECOG PS and Karnofsky Performance Scale. Blood samples were collected after a ≥12-h fast to measure the concentrations of IGF-1, IGF binding protein-3 (IGFBP-3), interleukin (IL)-6, tumor necrosis factor (TNF)α, IL-1β, prealbumin, acylghrelin, and des-acylghrelin. Laboratory tests were performed at a central laboratory (SRL Medisearch Ltd.).
Safety
Safety variables included vital signs, 12-lead electrocardiography, tumor status (assessed using the Response Evaluation Criteria in Solid Tumors [RECIST] guidelines), patient outcomes/survival, clinical laboratory tests, and adverse events. Overall survival was determined using the life-table method for each treatment group. Adverse events were assessed using the Japanese version of the National Cancer Institute Common Terminology Criteria for Adverse Events ver. 4.0 and were categorized by system organ class/preferred term.
Adverse events were also assessed in terms of their severity, seriousness, causal relationship to the study drug, outcome, clinical course, and whether the study drug was discontinued.
Statistical analyses
In accordance with the pre-specified study protocol and statistical analysis plan, all efficacy variables were first analyzed in the per-protocol set (PPS) and then confirmed using the full analysis set (FAS). The FAS included all eligible subjects who underwent at least one efficacy evaluation after starting the study drug. The PPS comprised all eligible subjects who did not receive any of the prohibited treatments and whose LBM (DEXA) and grip strength were measured during the observation period and at least once after starting the study drug. Safety analyses were performed using the safety analysis set, which was defined as all subjects who were administered the study drug at least once.
The sample size was calculated based on the result of a phase 2 study in which the mean ± standard deviation (SD) difference in LBM (DEXA) between 50 mg anamorelin and placebo was 2.09 ± 3.06 kg. At a significance level of 5 % and power of 80 %, at least 35 subjects were needed per treatment group. Considering that about 35 % of patients were likely to be excluded from the PPS, we planned to enroll 54 patients per group. All efficacy data are presented for the PPS, as specified in the statistical analysis plan, and the results were confirmed using the FAS. Baseline variables were analyzed descriptively to determine the mean ± SD or n (%) of patients. Efficacy variables were analyzed using analysis of covariance with treatment group, time-point, and preceding weight loss (5–15 % and >15 %) as fixed factors and the baseline value as a covariate. The least squares (LS) mean change from baseline to the indicated time-point was calculated for each group. The LS mean difference in the anamorelin groups relative to the placebo groups was also calculated with 95 % confidence intervals (CIs). An interaction of treatment group × time-point was also included for secondary efficacy endpoints (changes in body composition measured by DEXA or bioimpedance, grip strength, body weight, MDASI-J, QOL-ACD, Karnofsky Performance Scale, and serum biomarkers). Safety variables were analyzed descriptively and are shown as n (%) patients. Overall survival was determined using the Kaplan–Meier method and was compared among the three groups using log-rank tests. As this was an exploratory study, no adjustment was made for multiplicity of statistical testing, and missing data were not imputed.
Protocol modifications
Some modifications were made to the statistical analysis plan, but all changes were implemented before unblinding. In brief, the study duration and patient enrollment were extended because it took longer than expected to enroll the target number of patients. We also reassessed the target sample size, as we originally planned to enroll 44 patients per group after anticipating that 20 % of treated patients would be excluded from the PPS; this was increased to 35 %. Additionally, because the US Food and Drug Administration issued new guidance on CYP3A4 inhibitors after starting this trial, the use of these drugs was added to the exclusion criteria and list of prohibited drugs. These criteria were retrospectively applied to all patients in the PPS and FAS.
Results
Patients
Between March 2011 and September 2012, 181 subjects were enrolled and randomized to placebo, 50-mg anamorelin, or 100-mg anamorelin groups (Supplementary Fig. 1). Two patients did not receive treatment. The PPS comprised 115 patients, with 42, 42, and 31 in the placebo, 50-mg anamorelin, and 100-mg anamorelin groups, respectively (Supplementary Fig. 1). There were 12, 8, and 6 deaths in the placebo, 50-mg anamorelin, and 100-mg anamorelin groups, respectively, while 6, 14, and 13 patients, respectively, discontinued owing to adverse events. All three groups were comparable in terms of their baseline characteristics (Table 1).
Table 1.
Patient characteristics
| Parameter | Placebo (n = 60) | 50 mg anamorelin (n = 65) | 100 mg anamorelin (n = 55) | |
|---|---|---|---|---|
| Sex | Male | 39 (65.0) | 50 (76.9) | 35 (63.6) |
| Female | 21 (35.0) | 15 (23.1) | 20 (36.4) | |
| Age (years) | Mean ± SD | 66.0 ± 9.4 | 64.8 ± 8.7 | 65.7 ± 8.8 |
| Weight (kg) | Mean ± SD | 52.31 ± 10.29 | 51.10 ± 8.53 | 52.30 ± 11.57 |
| BMI (kg/m2) | Mean ± SD | 19.80 ± 2.86 | 19.33 ± 2.57 | 20.23 ± 3.21 |
| Weight loss (%) | 5 to <10 | 36 (62.1) | 38 (58.5) | 38 (69.1) |
| 10 to 15 | 20 (34.5) | 16 (24.6) | 14 (25.5) | |
| > 15 | 2 (3.4) | 11 (16.9) | 3 (5.5) | |
| Body composition (DEXA) (kg) | LBM | 38.65 ± 6.80 | 38.77 ± 6.20 | 38.80 ± 7.58 |
| Body fat | 11.30 ± 5.26 | 10.12 ± 4.44 | 11.70 ± 5.06 | |
| BMC | 1.92 ± 0.48 | 1.90 ± 0.47 | 1.91 ± 0.55 | |
| Grip strength (kg) | Dominant hand | 24.91 ± 9.05 | 27.32 ± 8.29 | 25.32 ± 9.28 |
| Non-dominant hand | 23.73 ± 9.31 | 25.79 ± 7.70 | 23.31 ± 9.24 | |
| Sum of both hands | 48.64 ± 18.09 | 53.12 ± 15.59 | 48.63 ± 18.13 | |
| QOL | MDASI-J | 50.9 ± 33.4 | 49.0 ± 36.9 | 55.6 ± 37.2 |
| QOL-ACD | 73.4 ± 13.9 | 71.3 ± 14.9 | 70.6 ± 13.9 | |
| ECOG PS | 1 | 46 (79.3) | 47 (72.3) | 45 (81.8) |
| 2 | 12 (20.7) | 18 (27.7) | 10 (18.2) | |
| Histological type of NSCLC | Adenocarcinoma | 47 (78.3) | 44 (69.8) | 43 (79.6) |
| Squamous cell | 10 (16.7) | 15 (23.8) | 7 (13.0) | |
| Other | 3 (5.0) | 4 (6.4) | 4 (7.4) | |
| Missing | – | 2 | 1 | |
| Disease stage | IIIA | 5 (8.3) | 8 (12.3) | 8 (14.5) |
| IIIB | 3 (5.0) | 9 (13.8) | 4 (7.3) | |
| IV | 45 (75.0) | 38 (58.5) | 38 (69.1) | |
| Recurrence | 7 (11.7) | 9 (13.8) | 5 (9.1) | |
| Other | – | 1 (1.5) | – | |
| Time from diagnosis to starting the study drug (days) | Mean ± SD | 549.0 ± 422.3 | 627.1 ± 572.6 | 466.6 ± 436.7 |
| History of chemotherapy (number of lines) | 0 | – | 1 (1.5) | – |
| 1 | 9 (15.0) | 15 (23.1) | 17 (30.9) | |
| 2 | 19 (31.7) | 12 (18.5) | 14 (25.5) | |
| ≥3 | 32 (53.3) | 37 (56.9) | 24 (43.6) | |
Values are expressed as the n (%) of patients or mean ± SD
SD standard deviation, BMI body mass index, DEXA dual energy X-ray absorptiometry, LBM lean body mass, BMC bone mineral content, QOL quality of life, MDASI-J M.D. Anderson Symptom Inventory, ACD Questionnaire for Cancer Patients Treated with Anticancer Drugs (Kurihara Group Questionnaire), ECOG PS Eastern Cooperative Group Performance Status, NSCLC non-small cell lung cancer, CRP C-reactive protein, Hb hemoglobin, Alb albumin
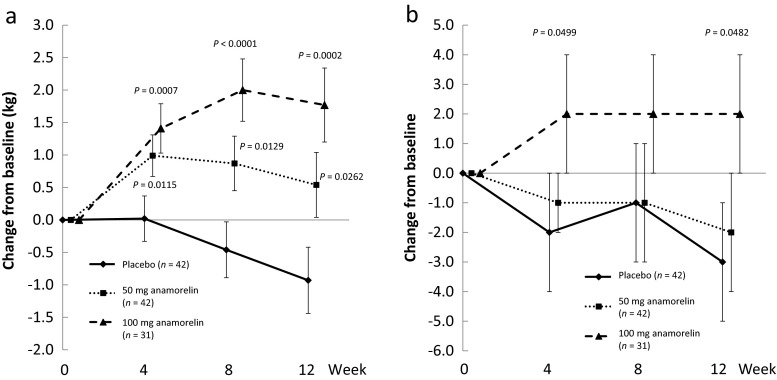
Lean body mass and non-dominant handgrip strength
Compliance with the study drugs was high because ≥96 % of patients took ≥80 % of their allocated drugs. As shown in Fig. 1a, the increase in LBM from baseline was significantly greater in the 100-mg anamorelin group at weeks 8 and 12 than in the placebo group, although the LS mean change from baseline to week 12 was of borderline significance. The results in the FAS were consistent with those in the PPS, with LS mean ± SE changes of 0.19 ± 0.31 kg, and 1.07 ± 0.31 kg in the placebo and 100-mg anamorelin groups, respectively. The difference compared with placebo was 0.89 kg (95 % CI 0.29, 1.48 kg) in the 100-mg anamorelin group (P = 0.0037). Although 50 mg anamorelin also increased LBM, the difference compared with placebo was not significant.
Fig. 1.
Effects of anamorelin on lean body mass (a) and grip strength (non-dominant hand; b). CI confidence interval, LSM least squares mean, SE standard error
There were no changes in non-dominant handgrip strength in any group (Fig. 1b).
QOL-ACD
Significant improvements were identified in the 100-mg anamorelin group compared with the placebo group in the QOL-ACD total score and the scores for items 1–6 “daily activity,” 7–11 “physical condition,” 8 “Did you have a good appetite?,” and 9 “Did you enjoy your meals?” (Fig. 2a–e).
Fig. 2.
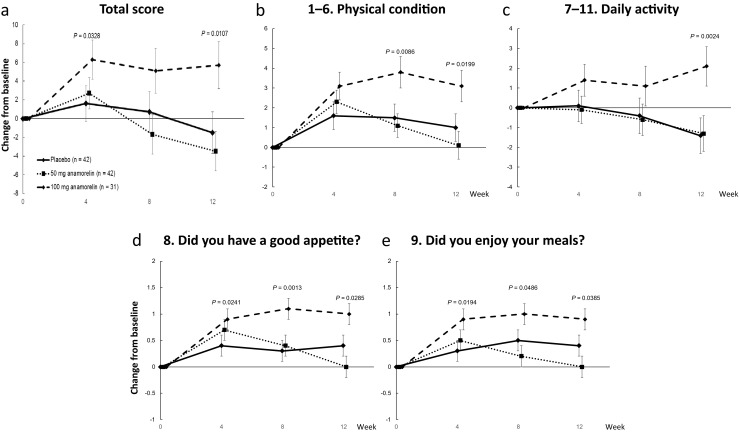
Effects of anamorelin on the QOL-ACD total score (a) and the scores for items 1–6 “daily activity” (b), items 7–11 “physical condition (c), item 8 “Did you have a good appetite?” (d), and item 9 “Did you enjoy your meals?” (e)
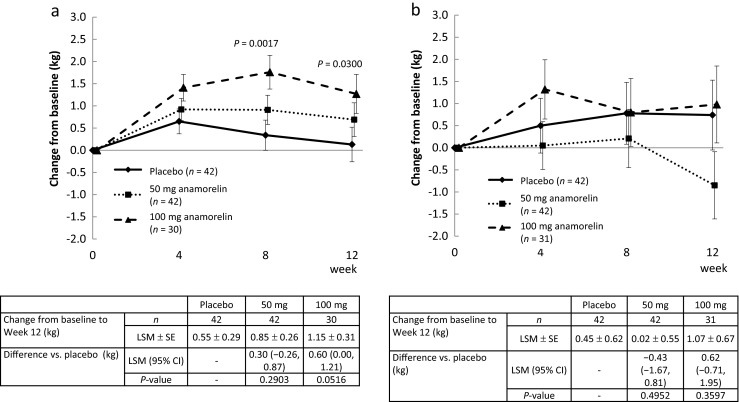
Body weight
Consistent with its mechanism of action and the increase in LBM, Fig. 3a shows that administration of anamorelin 100 and 50 mg resulted in significant body weight gain compared with placebo. These increases in body weight were apparent at week 4 and were sustained for the duration of the study.
Fig. 3.
Effects of anamorelin on body weight (a) and Karnofsky performance scale (b)
Karnofsky performance scale
Administration of 100 mg anamorelin significantly increased patients’ Karnofsky Performance Scale from baseline to weeks 4 and 12 relative to placebo, but no changes were detected in the 50-mg anamorelin group (Fig. 3b).
Other secondary endpoints
The effects of anamorelin on the other secondary endpoints, including serum biomarkers/laboratory variables, measures of body composition (as measured by DEXA and bioimpedance), grip strength, and MDASI-J, are shown in Supplementary Tables 1 and 2. The serum biomarkers IGF-1, IGFBP-3, and prealbumin were significantly increased in patients taking 100 mg anamorelin compared with placebo. Even in patients taking 50 mg anamorelin, these parameters showed increases; however, the magnitude of the changes was smaller than those observed in the 100-mg anamorelin group. (Fig. 4a–c). There were no changes in IL-1β, IL-6, TNFα, acylghrelin, or des-acylghrelin (Supplementary Table 1). In terms of the other secondary endpoints, 100 mg anamorelin was also associated with significant increases in body fat measured by DEXA at each timepoint (Supplementary Table 2).
Fig. 4.
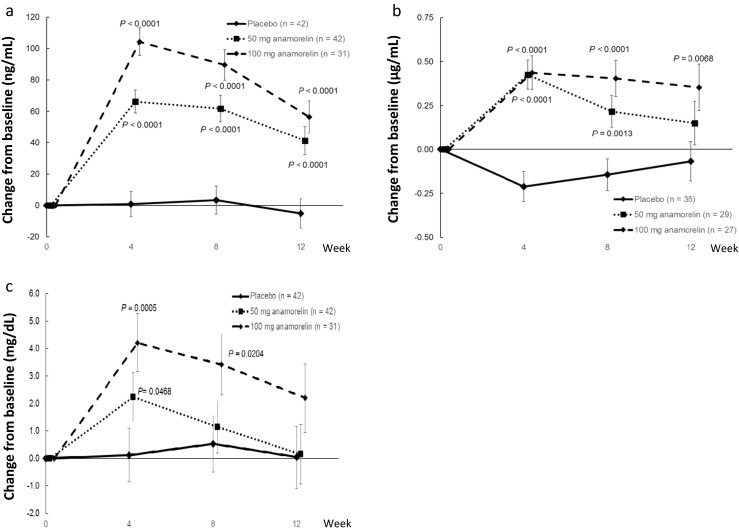
Effects of anamorelin on insulin-like growth factor-1 (a), insulin-like growth factor binding protein-3 (b), and prealbumin (c)
Safety
The tumor responses according to RECIST were classified as complete response, partial response, stable disease, progressive disease, and non-evaluable in 0, 4 (7.0 %), 11 (19.3 %), 24 (42.1 %), and 14 (24.6 %) patients, respectively, in the placebo group; in 0, 3 (4.6 %), 14 (21.5 %), 27 (41.5 %), and 15 (23.1 %) patients, respectively, in the 50-mg anamorelin group; and in 1 (1.8 %), 1 (1.8 %), 20 (36.4 %), 23 (41.8 %), and 9 (16.4 %), patients, respectively, in the 100-mg anamorelin group. The overall survival rates were not significantly different among the three groups (hazard ratio [95 % CI]; placebo vs. 50 mg anamorelin: 0.91 [0.62, 1.33]; placebo vs. 100 mg anamorelin: 0.74 [0.50, 1.10]; Supplementary Fig. 2).
The overall incidences of adverse events, adverse drug reactions, severe adverse drug reactions, and deaths are shown in Table 2. The incidence of adverse events was comparable in all three groups. However, the incidence of adverse drug reactions was significantly greater in both anamorelin groups compared with the placebo group. The incidence of Grade ≥ 3 adverse drug reactions was similar in each group (Table 2). Considering the patient population, most of the deaths were attributed to malignant neoplasm progression, and these events were not deemed related to the study drugs.
Table 2.
Adverse events and adverse drug reactions
| Placebo | 50 mg anamorelin | 100 mg anamorelin | |
|---|---|---|---|
| n | 58 | 65 | 55 |
| Adverse events | 58 (100.0) | 61 (93.8) | 53 (96.4) |
| Difference (%) vs. placebo (95 % CI) | −6.2 (−12.0, −0.3) | −3.6 (−8.6, 1.3) | |
| P value | 0.0548 | 0.1428 | |
| Serious adverse events | 29 (50.0) | 26 (40.0) | 17 (30.9) |
| Discontinuations due to adverse events | 6 (10.3) | 14 (21.5) | 13 (23.6) |
| Adverse drug reactions | 12 (20.7) | 25 (38.5) | 29 (52.7) |
| Difference (%) vs. placebo (95 % CI) | 17.8 (2.0, 33.5) | 32.0 (15.2, 48.9) | |
| P value | 0.0319 | 0.0004 | |
| Serious adverse drug reactions | 4 (6.9) | 3 (4.6) | 0 (0.0) |
| Discontinuations due to adverse drug reactions | 2 (3.4) | 4 (6.2) | 5 (9.1) |
| Deaths | 12 (20.7) | 8 (12.3) | 6 (10.9) |
| Adverse events by grade | |||
| Grade 3 | 17 (29.3) | 23 (35.4) | 15 (27.3) |
| Grade 4 | 13 (22.4) | 9 (13.8) | 11 (20.0) |
| Grade 5 | 13 (22.4) | 11 (16.9) | 7 (12.7) |
| Adverse events in ≥20 % of patients in either anamorelin group | |||
| White blood cell count decreased | 18 (31.0) | 20 (30.8) | 14 (25.5) |
| Neutrophil count decreased | 15 (25.9) | 19 (29.2) | 13 (23.6) |
| CRP increased | 19 (32.8) | 16 (24.6) | 10 (18.2) |
| Hemoglobin decreased | 15 (25.9) | 14 (21.5) | 11 (20.0) |
| Nausea | 10 (17.2) | 9 (13.8) | 17 (30.9) |
| Vomiting | 10 (17.2) | 14 (21.5) | 6 (10.9) |
| Glycosylated hemoglobin increased | 0 (0) | 4 (6.2) | 11 (20.0) |
Values are expressed as the n (%) of patients
CI confidence interval
In either anamorelin-treated group, ≥20 % of patients developed nausea, vomiting, increased C-reactive protein, increased glycosylated hemoglobin, neutrophil count decreased, or decreased hemoglobin and white blood cell count. Of these events, only nausea and increased glycosylated hemoglobin occurred in ≥5 % patients in the anamorelin groups than in the placebo group.
Discussion
This study revealed that administration of 100 mg anamorelin was associated with statistically significant improvements in LBM, body weight, QOL, and statistically significant increases in the serum biomarkers IGF-1, IGFBP-3, and prealbumin compared with placebo. By contrast, 50 mg anamorelin was not associated with marked improvements in most of these variables compared with placebo. These findings indicate that 100 mg anamorelin is the most effective dose in Japanese patients with NSCLC. The efficacy of anamorelin was not confirmed in handgrip strength, IL-1β, IL-6, TNFα, acylghrelin, or des-acylghrelin.
Several clinical trials of anamorelin have been conducted [20, 21, 27]. In a phase 1, multiple-dose study, anamorelin was associated with dose-related increases in body weight over 6 days in healthy volunteers, with the greatest increase observed after once-daily dosing of 50 mg [21]. In a pharmacodynamic study in healthy volunteers, anamorelin (25, 50, and 75 mg) was associated with dose-related increases in body weight and significant increases in GH, IGF-1, and IGFBP-3 concentrations, consistent with its mechanism of action [22]. In a pooled analysis of two phase 2 studies in patients with cancer cachexia, 50 mg anamorelin was associated with a significant increase in LBM (LS mean 1.89 kg, 95 % CI 0.84, 2.95 kg) compared with placebo (−0.20, 95 % CI −1.23, 0.83), corresponding to a between-group difference of 2.09 kg (95 % CI 0.94, 3.25, P = 0.0006) [27]. In two phase 3 studies in patients with NSCLC and cachexia, 100 mg anamorelin was associated with significantly improved LBM, body weight, and anorexia-cachexia symptoms/concerns [23].
In the present study, 50 and 100 mg anamorelin were associated with placebo-subtracted increases in body weight of 0.76 and 0.89 kg, respectively. Consistent with earlier pharmacodynamic studies [22], our study revealed increases in IGF-1 and IGFBP-3 levels in patients treated with 100 mg anamorelin that are suggestive of increased muscle protein synthesis. These changes are consistent with the increases in LBM. We also observed a significant increase in prealbumin, a marker of the nutritional state.
Anamorelin (100 mg) was also associated with significant improvements in QOL-ACD, especially items 8 and 9, which focus on appetite and meal enjoyment, and also increases in daily activity, and improvements in physical condition. These findings indicate that patients treated with 100 mg anamorelin reported greater appetite during treatment and were more likely to enjoy their meals as compared with placebo-treated patients. Additionally, the increase in the Karnofsky Performance Scale suggests there were improvements in the functional status of this cohort of patients.
The overall survival times and tumor responses according to RECIST were generally similar in each treatment group, which indicates that the treatment itself did not modify disease progression. Furthermore, there were no differences in patient characteristics (e.g., disease stage and history of chemotherapy) or existing prognostic factors, among the three groups.
The incidence of adverse drug reactions was significantly greater in both anamorelin groups than in the placebo group, but there were very few serious drug reactions and the distribution of Grade 3–5 events was similar in all three groups. Notably, most deaths and treatment discontinuations were due to disease progression rather than the study drugs themselves. Although nausea and glycosylated hemoglobin occurred in ≥5 % more patients in the anamorelin groups than in the placebo group, neither adverse event was considered a significant risk. This suggests that anamorelin is tolerable in Japanese patients with cancer cachexia.
This study has several limitations. Firstly, the efficacy of anamorelin was not confirmed in handgrip strength. Secondary, the bioimpedance might have not been suitable as a method to determine LBM, body fat, etc. for the patients with cancer cachexia. Thirdly, since this is an exploratory study, multiple comparisons were not performed; it should be performed in a larger-sized study to be conducted in the future.
Conclusions
In conclusion, 100 mg anamorelin once daily had favorable effects on LBM in Japanese NSCLC patients suffering from cachexia, suggesting that this dose should be preferred in this patient population. Treatment with anamorelin was accompanied by increases in IGF-1 and IGFBP-3, which are indicative of an increase in protein synthesis, and an increase in prealbumin, indicative of improved nutritional status. Furthermore, anamorelin was associated with favorable improvements in QOL, appetite, and performance status. Although the incidence of adverse drug reactions and discontinuations because of adverse events were greater in the anamorelin groups, the majority of treatment discontinuations were related to disease progression rather than anamorelin.
Cancer cachexia requires multimodal treatment such as nutrition, exercise, and pharmacotherapies. The profile of anamorelin shown in this study indicates a great potential of the safe and effective drug as an option for the pharmacotherapies for multimodal treatment of cancer cachexia.
Electronic supplementary materials
(DOCX 25 kb)
(DOCX 27 kb)
(DOCX 29 kb)
Patient disposition. *Patients in whom changes from baseline could not be analyzed for both primary efficacy endpoints by DEXA and grip strength. DEXA dual energy X-ray absorptiometry. (PPTX 73 kb)
Overall survival according to treatment group. CI confidence interval, HR hazard ratio, MST median survival time (PPTX 81 kb)
Acknowledgments
The authors thank all of the patients, investigators, and site staff who participated in the study. This study was funded by Ono Pharmaceutical Co., Ltd., Osaka, Japan. The authors thank Nicholas D. Smith, PhD, on behalf of Springer Healthcare Communications, for providing medical writing support, which was funded by Ono Pharmaceutical Co., Ltd.
Author contributions
K. Takayama, H. Saito, Y. Takiguchi, K. Aoe, A. Koyama, N. Komura, and K. Eguchi were responsible for conception and design. N. Katakami, T. Yokoyama, S. Atagi, K. Yoshimori, H. Kagamu, H. Saito, Y. Takiguchi, and K. Aoe were responsible for acquisition of data. K. Takayama, H. Saito, Y. Takiguchi, K. Aoe, A. Koyama, N. Komura, and K. Eguchi were responsible for data analysis and interpretation. All authors were responsible for manuscript writing and final approval of manuscript.
Study sites
Hokkaido University Hospital (First Department of Medicine), Hokkaido University Hospital (Department of Medical Oncology), Okitama Public General Hospital, Funabashi Municipal Medical Center, St.Luke’s International Hospital, National Center for Global Health and Medicine, Kanagawa Cancer Center, Niigata University Medical & Dental Hospital, Aichi Cancer Center Aichi Hospital, Tottori University Hospital, Hiroshima City Hiroshima Citizens Hospital, National Hospital Organization Yamaguchi-Ube Medical Center, Shikoku Cancer Center, Kumamoto University Hospital, Chiba University Hospital, National Hospital Organization Nagoya Medical Center, Kyoto University Hospital, Keio University Hospital, Fukujuji Hospital, Toneyama National Hospital, Institute of Biomedical Research and Innovation, Yokohama Rosai Hospital, National Hospital Organization Kinki-chuo Chest Medical Center, Nagoya University Hospital, Fukuoka University Hospital, Nagaoka Red Cross Hospital, National Hospital Organization Sendai Medical Center, Fukui Prefectural Hospital, Dokkyo Medical University Hospital, Kyorin University Hospital, Itabashi Chuo Medical Center, Japanese Red Cross Society Suwa Hospital.
Compliance with ethical standards
The study was conducted in compliance with the Declaration of Helsinki, the study protocol, Paragraph 3 of Article 14 and Article 80-(2) of the Pharmaceutical Affairs Law of Japan, and Good Clinical Practice Guidelines (Ministry of Health and Welfare Ordinance No. 28). The study was approved by ethics committees at all participating institutions.
Conflict of interest
K. Takayama received remuneration from Ono Pharmaceutical Co., Ltd. H. Saito and K. Eguchi received funding from Ono Pharmaceutical Co., Ltd. Y. Takiguchi received remuneration and funding from Ono Pharmaceutical Co., Ltd. A. Koyama and N. Komura are employees of Ono Pharmaceutical Co., Ltd. All remaining authors have declared no conflicts of interest.
Financial support
Ono Pharmaceutical Co., Ltd., Osaka, Japan.
References
- 1.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E. ABC of palliative care. anorexia, cachexia, and nutrition. BMJ. 1997;315:1219–1222. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69:491–497 [DOI] [PubMed]
- 4.Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148:1586–1591. doi: 10.1001/archinte.1988.00380070082020. [DOI] [PubMed] [Google Scholar]
- 5.von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. J Am Med Dir Assoc. 2014;15:866–872. doi: 10.1016/j.jamda.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachex Sarcopenia Muscle. 2014;5:261–263. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachex Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz Garcia V, Lopez-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;3:Cd004310. doi: 10.1002/14651858.CD004310.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. Body-composition changes in patients who gain weight while receiving megestrol acetate. J Clin Oncol. 1993;11:152–154. doi: 10.1200/JCO.1993.11.1.152. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 11.Smith RG, Cheng K, Schoen WR, Pong SS, Hickey G, Jacks T, Butler B, Chan WW, Chaung LY, Judith F, et al. A nonpeptidyl growth hormone secretagogue. Science. 1993;260:1640–1643. doi: 10.1126/science.8503009. [DOI] [PubMed] [Google Scholar]
- 12.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 13.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 14.Akamizu T, Takaya K, Irako T, Hosoda H, Teramukai S, Matsuyama A, Tada H, Miura K, Shimizu A, Fukushima M, Yokode M, Tanaka K, Kangawa K. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol. 2004;150:447–455. doi: 10.1530/eje.0.1500447. [DOI] [PubMed] [Google Scholar]
- 15.Morozumi N, Hanada T, Habara H, Yamaki A, Furuya M, Nakatsuka T, Inomata N, Minamitake Y, Ohsuye K, Kangawa K. The role of C-terminal part of ghrelin in pharmacokinetic profile and biological activity in rats. Peptides. 2011;32:1001–1007. doi: 10.1016/j.peptides.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Currow DC, Abernethy AP. Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome. Future Oncol. 2014;10:789–802. doi: 10.2217/fon.14.14. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Garcia JM. Anamorelin hydrochloride for the treatment of cancer-anorexia-cachexia in NSCLC. Expert Opin Pharmacother. 2015;16:1245–1253. doi: 10.1517/14656566.2015.1041500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pietra C, Takeda Y, Tazawa-Ogata N, Minami M, Yuanfeng X, Duus EM, Northrup R. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. J Cachex Sarcopenia Muscle. 2014;5:329–337. doi: 10.1007/s13539-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northrup R, Kuroda K, Duus EM, Barnes SR, Cheatham L, Wiley T, Pietra C. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer. 2013;21:2409–2415. doi: 10.1007/s00520-013-1800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21:129–137. doi: 10.1007/s00520-012-1500-1. [DOI] [PubMed] [Google Scholar]
- 21.Garcia JM, Polvino WJ. Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist. 2007;12:594–600. doi: 10.1634/theoncologist.12-5-594. [DOI] [PubMed] [Google Scholar]
- 22.Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Hormon IGF Res. 2009;19:267–273. doi: 10.1016/j.ghir.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Temel SA, Currow DC, Fearon K, Yan Y, Friend J, Abernethy AP. Phase III trials of anamorelin in patients with advanced non-small cell lung cancer (NSCLC) and cachexia (ROMANA 1 and 2) J Clin Oncol. 2015;33:9500. [Google Scholar]
- 24.Moyle GJ, Daar ES, Gertner JM, Kotler DP, Melchior JC, O’Brien F, Svanberg E. Growth hormone improves lean body mass, physical performance, and quality of life in subjects with HIV-associated weight loss or wasting on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2004;35:367–375. doi: 10.1097/00126334-200404010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Okuyama T, Wang XS, Akechi T, Mendoza TR, Hosaka T, Cleeland CS, Uchitomi Y. Japanese version of the MD Anderson symptom inventory: a validation study. J Pain Symptom Manag. 2003;26:1093–1104. doi: 10.1016/j.jpainsymman.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Ohashi Y, Morita S, Kobayashi K, Shibuya M, Yamaji Y, Eguchi K, Fukuoka M, Nagao K, Nishiwaki Y, Niitani H. The quality of life questionnaire for cancer patients treated with anticancer drugs (QOL-ACD): validity and reliability in Japanese patients with advanced non-small-cell lung cancer. Qual Life Res. 2002;11:483–493. doi: 10.1023/A:1015614505929. [DOI] [PubMed] [Google Scholar]
- 27.Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16:108–116. doi: 10.1016/S1470-2045(14)71154-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 25 kb)
(DOCX 27 kb)
(DOCX 29 kb)
Patient disposition. *Patients in whom changes from baseline could not be analyzed for both primary efficacy endpoints by DEXA and grip strength. DEXA dual energy X-ray absorptiometry. (PPTX 73 kb)
Overall survival according to treatment group. CI confidence interval, HR hazard ratio, MST median survival time (PPTX 81 kb)