Abstract
It is well established that the luteinizing hormone surge triggers ovulation, a dynamic process leading to the release of the mature oocyte from the ovarian follicle. But how this process controlled by LH signaling remains largely unknown in non-mammalian species. In this study, we investigated the roles of nuclear progesterone receptor (npr) in LH-induced ovulation. Our results indicate that the nuclear progesterone receptor serves as an important mediator of LH action on ovulation. This conclusion is based on the following results: (1) the expression level of npr peaks at the full-grown stage of the follicles; (2) the expression of npr is stimulated by LH signaling in vitro and in vivo; and (3) the npr null females are infertile due to ovulation defects. Moreover, we further show that LH signaling could induce ptger4b expression in an npr-dependent manner, and blockage of Ptger4b could also block hCG-induced ovulation. Collectively, our results not only demonstrate that npr serves an indispensable role in mediating the action of LH on ovulation in zebrafish, but also provide insights into the molecular mechanisms of the regulation of ovulation in fish.
Luteinizing hormone (LH) signaling has attracted much research attention for a long time because of its critical roles in reproduction1,2,3,4,5. In mammals, LH signaling has been extensively studied using gene knockout mice1,3,6,7. Deletion of LHβ causes infertility in both males and females in mice3. Recently, we have established the lhβ knockout model in zebrafish and found that final oocyte maturation and ovulation were disrupted in the mutants2. Interestingly, we also found that the gonad-specific igf3 serves as an important mediator of the action of LH on oocyte maturation8. However, the downstream factors in mediating the action of LH on ovulation are largely undetermined.
The nuclear progesterone receptor (nPR; official symbol PGR), which is a member of the nuclear receptor transcription factor superfamily, has been proposed as an important factor for LH-dependent ovulation in mammals. Studies in mice and in human have demonstrated the critical role of nPR in ovulation9,10. Female mice lacking nPR exhibit anovulation, impaired sexual behavior, uterine dysfunction, impaired ductal branching morphogenesis and lobuloalveolar differentiation of the mammary gland11,12,13,14. Although the functional roles of nPR in the ovulation of rodents and human are well recognized15,16, its functional roles in non-mammalian species are not well understood.
Ovulation is a precise and complex biological process during which one or more mature fertilizable oocytes are released from the surrounding follicle wall into the ovarian cavity17. The molecular mechanisms for ovulation have been investigated in many studies. Previous studies have shown that control of the ovulation process may involve the cooperative action of a number of regulators including a variety of proteases18,19,20, matrix metalloproteinases (mmps)21,22,23 and tissue inhibitors of metalloproteinases (timps)22,23,24, etc. Furthermore, prostaglandins (PGs) also participate in regulating key aspects of the ovulatory process25,26. However, how npr and these factors act to mediate the stimulatory action of LH on ovulation has yet to be established.
In this study we have investigated whether npr could mediate the action of LH on ovulation in zebrafish. Our data indicated that npr is stimulated by LH signaling in vitro and in vivo. Moreover, the npr null fish are infertile due to ovulation defects. Further studies suggest that ptger4b is a downstream factor of npr in mediating the action of LH on ovulation. These results revealed that npr and its downstream factor ptger4b are indispensable for ovulation in zebrafish.
Results
Differential gene expression of mprα, mprβ and npr during zebrafish folliculogenesis
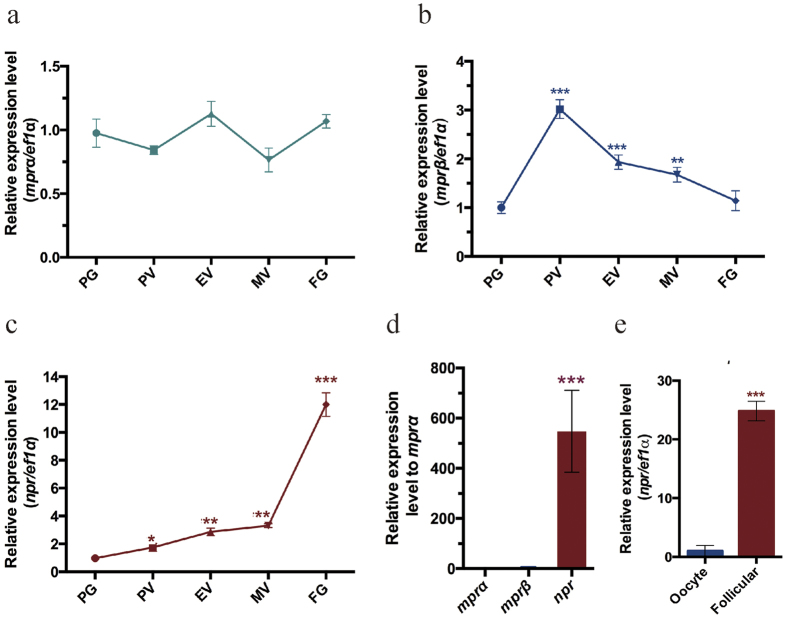
Three progesterone receptors including the nuclear progestin receptor (npr) and membrane progestin receptors (mprα and mprβ) have been identified in zebrafish. We first examined the relative levels of the three progesterone receptors in the ovarian follicles of different developmental stages using real-time PCR. The expression of mprα was not significantly changed during zebrafish folliculogenesis (Fig. 1a). However, the level of mprβ increased from primary growth (PG) stage and reaching the highest level in previtellogenic (PV) stage, and then decreased afterwards (Fig. 1b). The expression of npr gradually increased with a sharp increase from the midvitellogenic (MV) stage to full grown but immature (FG) stage (Fig. 1c). At the FG stage, npr was the most abundantly expressed progesterone receptor (Fig. 1d). The expression of npr was significantly higher in the follicular cells than the oocyte (Fig. 1e). These results suggest that npr may play an important role in final oocyte maturation or in the ovulation of zebrafish.
Figure 1. Expression of mprα, mprβ and npr during folliculogenesis.
(a–c) Real-time PCR detection the expression of mprα (a), mprβ (b) and npr (c) in the follicles of different stages isolated from the ovaries of adult zebrafish. (d) The relative expression of mprα, mprβ and npr in FG stage follicles isolated from the ovaries of adult zebrafish. (e) The expression of npr in the oocyte and follicular cells isolated from FG stage follicles. Each value represents the mean value ± SEM of quadruplicate assays of 4 independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001 vs control).
Regulation of npr expression by LH signaling in vitro and in vivo
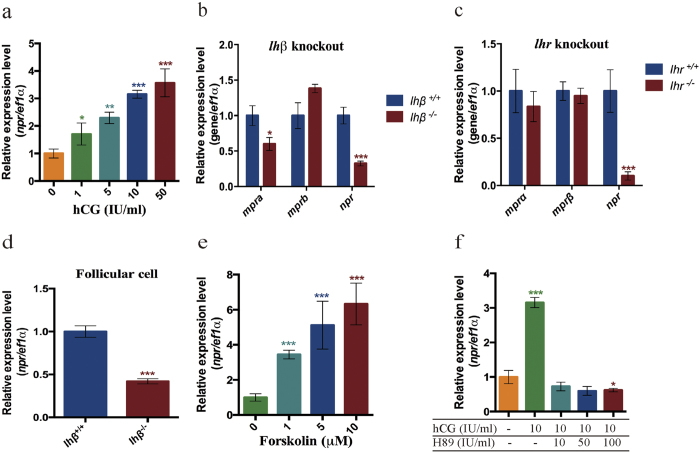
We then examined whether the expression of npr is regulated by LH signaling. FG stage follicles were incubated with different concentrations of human chorionic gonadotropin (hCG). After two hours of incubation, the npr expression was dramatically increased in a dose dependent manner (Fig. 2a). Moreover, the npr expression was significantly decreased in the lhβ or lhr mutants (Fig. 2b–d), indicating that npr expression is also regulated by endogenous LH signaling. However, the mprα expression was only significantly decreased in the lhβ mutant but not the lhr mutant, and mprβ mRNA was not significantly changed in the lhβ or lhr mutant (Fig. 2b,c). These data indicate that the expression of npr is regulated by LH signaling in vitro and in vivo.
Figure 2. Regulation of npr expression by LH signaling.
(a) Dose dependence of hCG treatment for 2 hours on the expression of npr in the intact FG stage follicles, indicating that the expression of npr is regulated by LH signaling in vitro. (b,c) Relative expression of mprα, mprβ and npr in intact FG stage follicles of the lhβ mutant and lhr mutant, indicating that the expression of npr is regulated by LH signaling in vivo. (d) Relative expression of npr in denuded follicular cells isolated from FG stage follicles of the wild-type fish and lhβ mutant, demonstrating that the regulation of npr expression was occurrs at the follicular cells. (e) Dose response of forskolin treatment for 2 hours on the expression of npr in the primary cultured follicular cells. (f) Treatment by hCG on the expression of npr in the presence or absence of H89 in the primary cultured follicular cells, demonstrating that the cAMP-PKA pathway is involved in the regulation of npr expression by LH signaling in vitro. Each value represents the mean value ± SEM of quadruplicate assays of 3 independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001 vs control).
LH signaling mainly acts via the cAMP-PKA pathway in zebrafish27. We therefore investigated whether this pathway is indeed involved in the regulation of npr expression. Two pharmalogical agents, forskolin (an activator of adenylate cyclase to increase the intracellular cAMP level) and H89 (an inhibitor of PKA to decrease the intracellular cAMP level) were used in the experiments. Treatment of cultured zebrafish follicular cells with forskolin for 2 h significantly increased npr expression (Fig. 2e), and H89 effectively suppressed hCG (50 IU/ml) induced npr expression (Fig. 2f). These data demonstrate that the expression of npr is regulated by LH signaling through the cAMP-PKA pathway.
Gene fragment deletion of npr in zebrafish
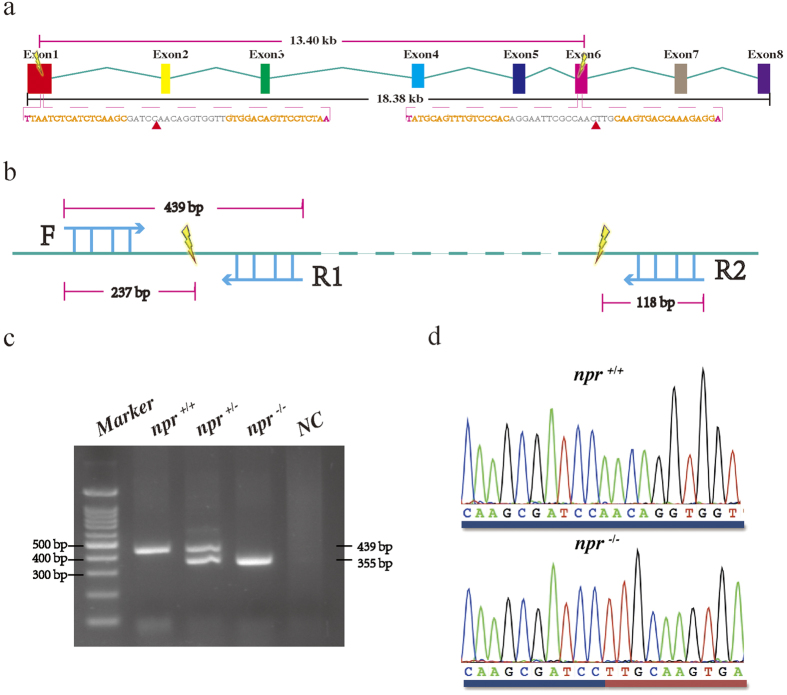
To assess the in vivo function of npr in mediating LH signaling in fish, we have established an npr gene knockout line using TALENs. There are eight exons in the zebrafish npr. Two TALEN target sites were designed in the first and the sixth exon to delete the flanked genomic fragment (13.40 kb) (Fig. 3a). The npr fragment deletion could be ascertained by properly designed primers (Fig. 3b). Successful npr fragment deletion was confirmed by genomic PCR (Fig. 3c) and sequencing (Fig. 3d).
Figure 3. Targeted fragment deletion of the zebrafish npr gene.
(a) Location of the TALEN binding sites on the zebrafish npr gene. Two TALEN target sites in exon 1 and exon 6 were designed to delete the 13.4 kb flanking region. The mutated genotype missing the genomic sequences between the two red triangles was analyzed in this study. (b) The primer design strategy to detect npr fragment deletion in this study. (c) A representative agarose gel analysis of PCR genotyping. Three primers shown in (b) were used in the genomic PCR to distinguish the npr+/+, npr+/− and npr−/− genotypes. (d) DNA sequence analysis of the npr−/− with large genomic deletion.
Ovulation is disrupted in the npr mutant
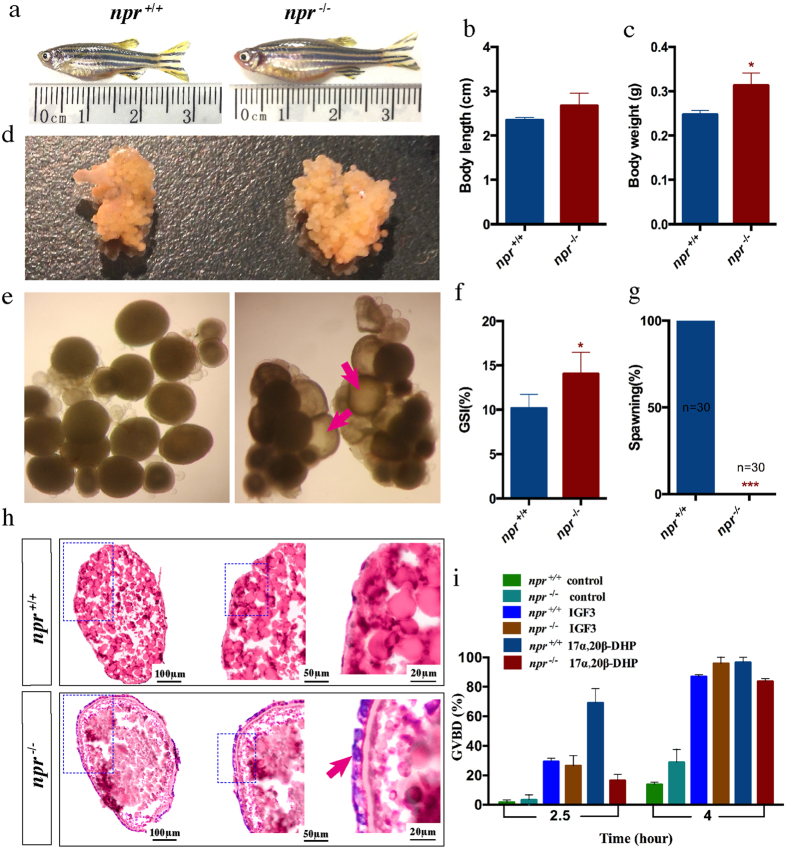
The npr mutants were viable and developed normally. The genotypes were inherited in the expected Mendelian ratio. Interestingly, only female mutants were infertile while males were fertile. The body size and the abdomen of the female npr mutants were bigger compared with the wild-type at adult stage (Fig. 4a). The body length was not affected but the body weight was significant higher in the mutants (Fig. 4b,c). A significant increase in the ovary size and the gonadosomatic index (GSI) was found in the npr mutants (Fig. 4d,f). Further examination of the ovaries showed that mature follicles were present in the mutants but not in the wild-type fish at 15:00 in the afternoon (Fig. 4e). Successful spawning in the morning was observed in the wild-type fish but not in the mutants (Fig. 4g). Ovarian histology analysis showed that follicles of different developmental stages could be found in the ovary of the npr mutants (Fig. 4h). The mature oocytes were trapped within the follicular cells in the mutants, indicating that the npr null fish fail to ovulate (Fig. 4h). Treatment of FG stage follicles from the npr mutant with 17α, 20β-DHP or IGF3 could induce oocyte maturation, suggesting that these FG stage follicles from the mutant fish are of good quality (Fig. 4i). However, injection of hCG could not rescue the ovulatory defect of the npr null mutants, suggesting that npr acts as an downstream factor of LH signaling. These data indicate that disruption of npr mainly causes ovulation defects in zebrafish.
Figure 4. Phenotype analysis of the npr+/+ and npr−/− female zebrafish at 75 dpf.
(a) Gross morphology of the npr+/+ and npr−/− fish. (b,c) Body length (b) and body weight (c) of npr+/+ and npr−/− mutants (n = 6). (d) Ovary morphology of the npr+/+ and npr−/− fish. (e), Microscopic examination of follicles from npr+/+ and npr−/− mutants. Follicles were collected at 15:00 pm and matured oocytes (red arrows) could be observed in the npr−/− mutant. (f) The gonado somatic index (GSI) of npr+/+ and npr−/− fish (n = 6). (g) Spawning assay. No npr−/− fish succeeded in spawning (n = 30). (h) Ovary histology of the npr+/+ and npr−/− fish. The outer layer cells of the matured follicles (indicated by arrow) failed to breakdown in the npr−/− fish. (i) Oocyte maturation assay. FG stage follicles from npr+/+ and npr−/− fish were treated with IGF3 or 17α, 20β-DHP, and the percentages of follicles that underwent germinal vesicle breakdown (GVBD) were recorded. Each value represents the mean value ± SEM (n = 4) of three independent experiments (*P < 0.05; ***P < 0.001 vs npr+/+).
Ptger4b is regulated by LH signaling in an npr-dependent manner
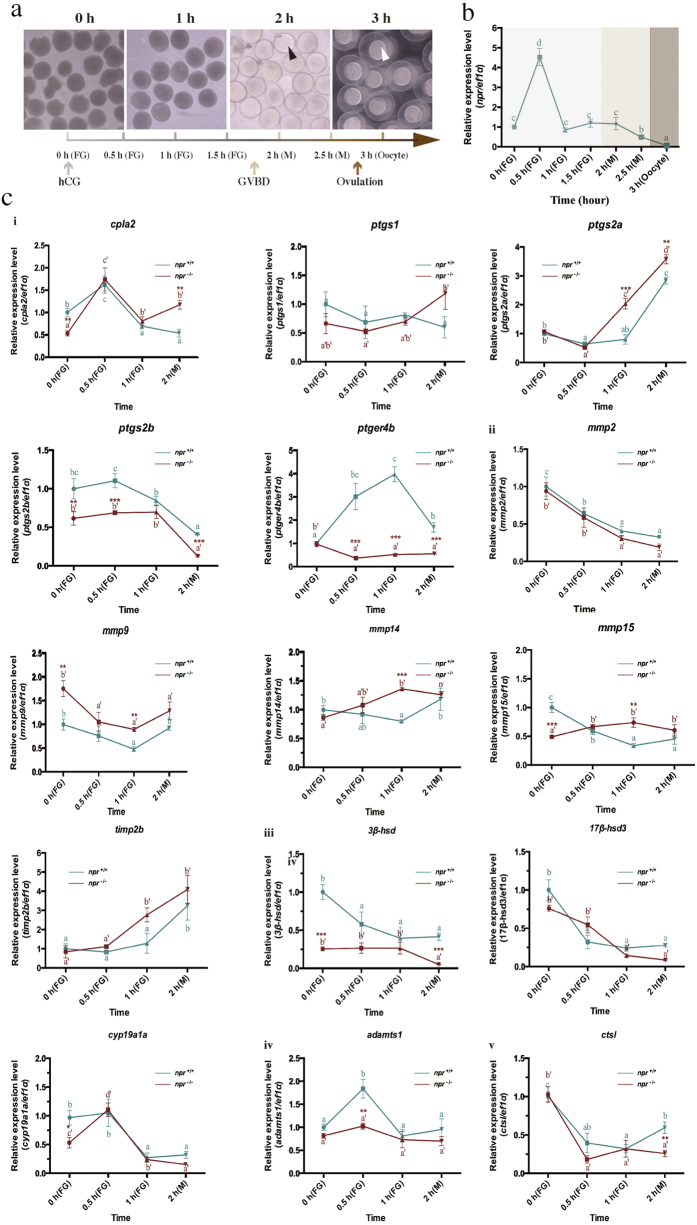
To further analyze the molecular mechanism underlining anovulation observed in the npr mutants, we have established an in vivo ovulation system in zebrafish by intraperitoneal injection of hCG (10 IU/μl) to mimic the endogenous LH surge to induce oocyte maturation and ovulation. The timescale of the hCG-induced oocyte maturation and ovulation in zebrafish is shown in Fig. 5a. Maturation of the follicles and ovulation of the oocytes were observed around 2 hours and 3 hours after hCG injection respectively (Fig. 5a). The temporal expression profiles of npr after hCG injection were analyzed. The expression of npr was drastically up-regulated at 0.5 hour and then decreased afterwards (Fig. 5b), further confirming that npr expression is regulated by LH signaling.
Figure 5. Regulation of ovulation-related genes by LH signaling in npr+/+ and npr−/− fish.
(a) The timescale of hCG-induced oocyte maturation and ovulation in npr+/+ zebrafish. Follicles were isolated at different time points after hCG (10 IU/μl) injection. Matured follicle with transparent appearance is indicated by black arrow and ovulated oocyte with enlarged fertilization membrane is indicated by white arrow. (b) The expression profile of npr after hCG injection. (c) The expression profile of ovulation related genes in npr+/+ and npr−/− fish after hCG injection. (i) Genes involved in prostaglandin biosynthesis; mmps and tissue inhibitors of metalloproteinases (timp2b); (iii) Genes involved in steroidogenesis; (iv) A disintegrin and metalloprotease with thrombospondin motifs1 (adamts1) and (v) cathepsin L (ctsl). The expression levels were normalized to that of ef1α and then to the expression level in npr+/+ fish at 0 h. Different letters in each dataset indicated statistical significance (P < 0.05). Each value represents the mean value ± SEM (n = 4) of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001 vs npr+/+).
The ovulation-related genes could be regulated by LH signaling in an npr-dependent or npr-independent manner. The expression profiles of the ovulation-related genes, including the prostaglandin biosynthesis genes, matrix metalloproteinases (mmps) and tissue inhibitors of mmps, steroidogenesis genes, adamts1 and ctsl were analyzed in the wild-type and npr−/− mutant after hCG injection (Fig. 5c). Ptger4b and adamts1 expression was significantly up-regulated in the npr+/+ but not in the npr−/− fish after hCG injection, indicating that these genes were induced by LH signaling through activation of npr. Other genes including cpla2, ptgs2a and timp2b were up-regulated in both npr+/+ and npr−/− fish after hCG injection, suggesting that these genes may be induced by LH signaling in an npr-independent manner.
Ptger4b is a downstream mediating factor of npr in ovulation
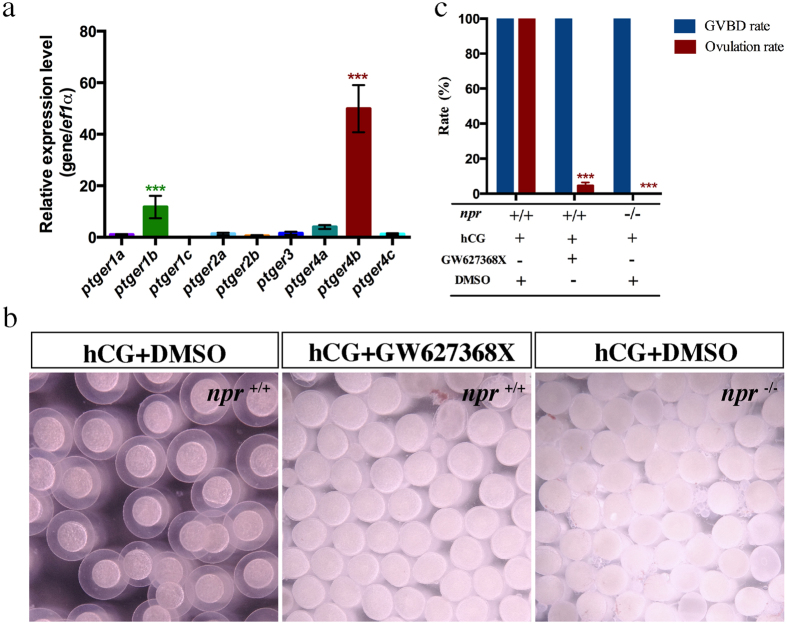
To investigate whether the npr downstream genes are functionally involved in ovulation, we investigated the roles of ptger4b which is the most up-regulated gene by hCG (Fig. 5). A search of PGE2 receptor genes reveals that the zebrafish genome contains nine different genes encoding for the PGE2 receptor. Of the nine ptger genes, the ptger4b was most predominant expressed (Fig. 6a). An antagonist of ptger4b (GW627368X) was employed to block ptger4b action. Interestingly, co-injection of GW627368X with hCG could effectively block the hCG-induced oocyte ovulation but not oocyte maturation (Fig. 6b), demonstrating that activation of ptger4b by LH signaling is required for successful ovulation. This effective blockade of ovulation by the ptger4b antagonist was similar to the ovulation defects observed in the npr null zebrafish after hCG injection (Fig. 6c), suggesting that both npr and ptger4b are downstream factors of LH signaling required for ovulation in zebrafish.
Figure 6. In vivo action of Ptger4 antagonist (GW627368X) on hCG induced oocyte maturation and ovulation.
(a) Relative expression of ptger genes in FG stage follicles. (b) Morphology of follicles from npr+/+ and npr−/− fish treated with hCG(10 IU/μl) or hCG + GW627368X(10 μM) for 4 hours. (c) Oocyte maturation and ovulation rates in npr+/+ and npr−/− fish treated with hCG or hCG + GW627368X for 4 hours. Each value represents the mean value ± SEM of triplicate assays from 3 independent experiments. (***P < 0.001 vs control).
Discussion
It is well established that LH signaling plays an important role in female ovulation in vertebrates including fish2,5. However, the downstream factors mediating LH action remain largely unknown. In this study, we have provided in vivo evidence that npr is an essential mediator of LH signaling on ovulation possibly through ptger4b in zebrafish.
Progesterone has been identified as an essential factor in female reproduction across vertebrates28,29. However, the receptor(s) that mediates the action of progesterone has been a subject of much debate for a long time30,31,32,33,34. Both the nuclear progestin receptor (npr)35 and a pair of membrane progestin receptor (mprα and mprβ)36 have been identified in zebrafish. All of them are expressed in zebrafish ovaries34,35,36,37,38. In order to provide clues to the functional roles of these receptors, we examined the expression levels of these receptors during folliculogenesis. The expression level of mprβ is increased from PG to PV stage follicles, similar to a report using nonquantitative gel electrophoresis37. Interestingly, the npr mRNA level is much higher than that of the mprα and mprβ levels during folliculogenesis, reaching the highest level at FG stage just prior to final oocyte maturation and ovulation. These data strongly argue for a pivotal role of npr on the regulation of final oocyte maturation or ovulation in zebrafish.
The ovarian expression patterns of npr and lhr are similar in zebrafish39, thus prompting us to investigate whether expression of the progesterone receptors is regulated by LH signaling. We found that npr is responsive to hCG, a human gonadotropin that has been widely used in fish to mimic the endogenous gonadotropin(s)40. Moreover, the regulation of npr by LH signaling is further substantiated by zebrafish mutant lines lacking lhβ or lhr2. Among all these receptors expressed in the ovary, only npr expression is dramatically decreased in the lhβ or lhr mutant fish. Using primary cultured zebrafish follicular cells, we have further demonstrated that npr is indeed upregulated by hCG, possibly through the cAMP-PKA pathway. The regulation of npr expression by LH signaling has also been observed in rat, mouse and medaka41,42,43, suggesting that this phenomenon is conserved in many species.
Although the functional roles of npr in mediating the action of LH signaling have been investigate in mammal15,16, studies in non-mammalian vertebrates lag behind. Recent studies suggested that npr may also regulate the ovulatory process in fish43,44, but robast genetic evidence is lacking. Using our optimized TALENs system, we have generated the npr fragment deletion mutant zebrafish line. We found that the npr null male zebrafish are fully fertile while the npr mutant female zebrafish are infertile. Folliculogenesis is normal in the npr mutant but ovulation is disrupted. Histological analysis reveals that mature oocytes are released from the surrounding follicle wall into the ovarian cavity in the ovaries of the wide-type but not the mutant. Similar phenotypes were also observed in a recent study45. These results clearly indicate that npr is indispensible for ovulation in zebrafish. In mice, targeted deletion of the progesterone receptor gene also leads to profound and complete anovulation, with the oocytes retained in the unruptured follicles even hyper-stimulated with gonadotropins12,46. It appears that npr function is well conserved from fish to mammals.
In order to understand the molecular mechanism of the action of LH on ovulation, we have established an in vivo ovulation model in zebrafish by hCG injection. A number of putative ovulation-related genes described in fish and mammals including the prostaglandin biosynthesis genes, various proteases, protease inhibitors and steroid biosynthesis genes were chosen and their expression profiles in npr+/+ and npr−/− fish were analyzed after hCG injection. Down-regulation of cpla2, ptgs2b, mmp15, 3β-hsd and cyp19a1a expression and up-regulation of mmp9 expression were observed in the npr mutant, suggesting that these ovulation-related genes were regulated by npr signaling in zebrafish. Several genes including cpla2, ptgs2a and timp2b were up-regulated in both npr+/+ and npr−/− fish after hCG injection, suggesting that these genes may also be involved in ovulation but in an npr-independent manner. More interestingly, we have identified two genes, namely ptger4b and adamts1, which were induced by LH signaling in an npr-dependent manner. The most responsive one is ptger4b, which is a receptor for PGE2. Prostaglandin (PG) has long been regarded as a critical regulator of ovulation in mammalian species47,48,49. The role of PG in mediating ovulation has also been studied in several fish species26,50,51,52,53,54,55,56. Interestingly, we found that the pgter4 antagonist (GW627368X) could block hCG induced ovulation. These findings suggest that ptger4b is a downstream factor of npr in inducing ovulation upon stimulation by the endogenous LH surge.
In summary, the present study provides robust in vivo evidence indicating that npr mediates the action of LH signaling on ovulation possibly through ptger4b in zebrafish. Information obtained from the present study helps to elucidate the biochemical pathways that link the initial stimulation by LH to the actualization of ovulation.
Methods
Zebrafish husbandry
AB strain zebrafish were reared in the laboratory of the Chinese University of Hong Kong and Sun Yat-Sen University, following the protocols described in Westerfield57. Briefly, fish were maintained in flow-through aquaria under an artificial photoperiod of 14 hours light (9:00–23:00): 10 hours dark (23:00–9:00) at 28 ± 1 °C. The larval and adult zebrafish were fed with brine shrimp (hatched from eggs in 20 mL in 4L salt water) daily. All animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of the Chinese University of Hong Kong and Sun Yat-Sen University.
Isolation of ovarian follicles
The staging system we adopted in this study was based on recent studies8,58. The ovaries were carefully dissected out from 15–20 female zebrafish after anesthetization and decapitation, and placed in a 100-mm culture dish containing 60% Leibovitz L-15 medium. Follicles of different stages were manually isolated and grouped into the following stages: primary growth (PG) (stage I; below 0.1 mm in diameter), previtellogenic (PV) (stage II; ~0.30 mm in diameter), early vitellogenic(EV) (stage III; ~0.40 mm in diameter), midvitellogenic (MV)(stage III; ~0.50 mm in diameter), and full grown but immature (FG) (stage III;~0.65 mm in diameter). The isolation process normally lasted for 4–6 hours at room temperature before incubation and drug treatment at 28 °C for different periods of time.
Primary culture of ovarian follicular cells
Primary culture of zebrafish ovarian follicular cells was performed according to an established protocol59. Briefly, follicles of vitellogenic stage from 15 to 25 females were carefully selected and washed with M199. The follicles were then cultured in a 25 cm2 flask for 6 days in M199 medium plus 10% fetal bovine serum under the condition of 28 °C and 5% CO2. The medium was changed on the third day. After 6 days, the cells were subcultured in 24-well plates at a density of 100 000 cells per well for 24 hours before hormone or drug treatment.
RNA isolation and RT-PCR
Total RNA was extracted from the ovarian follicle samples, cultured follicular cells or ovary of zebrafish using TRIzol reagent (Invitrogen). The cDNA was produced from 1μg total RNA using Rever Tra Ace α-first strand cDNA Synthesis Kit (TOYOBO) and used as template. The specific primers used in this study are listed in Supplemental Table 1. The transcription levels of the target genes were measured using the SYBR Green PCR Master Mix Kit60 carried out on an ABI Real-Time PCR Fast System60. Quantitative RT-PCR conditions were as follows: denaturation at 95 °C for 1 minute, followed by 40 cycles of 95 °C for 15 seconds, 58 °C for 15 seconds, 72 °C for 20 seconds, and then 84 °C for 10 seconds (fluorescent data collection). All mRNA quantification data were normalized to ef1α and expressed as the fold differences of the target gene expression relative to the control.
Establishment of an npr gene fragment disruption zebrafish line by TALEN
The TALENs were assembled using the golden gate method as described previously61,62. The two TALEN somatic expression backbones pCS2-TALEN-ELD and pCS2-TALEN-KKR were developed by our group62. For detailed protocol of the TALEN preparation, see ref. 61. TALEN mRNAs (200–500 pg) were microinjected into one-cell stage zebrafish embryos. Two days after injection, genomic DNA was isolated from 8–10 pooled larvae. The target genomic regions were amplified by PCR. To obtain germline mutations, the TALEN-injected embryos were raised to adulthood and the P0 founders were outcrossed with wild-type fish. The F1 progeny were genotyped by sequencing. To obtain homozygous mutants, heterozygous mutant of the same mutation were obtained and crossed. The primers used in this study are listed in Supplemental Table 1.
PCR genotyping
To assess TALEN induced mutation of the npr target region and to determine the mutation rate, PCR genotyping was performed. Genomic DNA was extracted by the phenol-chloroform method from the tail fin of F2 fish. Genomic PCR was performed two reverse primers specific for the npr+/+(439 bp) or npr−/− (355 bp) and a common forward primer as listed in Supplementary Table 1. The PCR products were electrophoresed on 1.5% agarose gels to resolve and identify the mutations.
Morphological and histological analyses of the zebrafish mutant line
Morphological and histological analyses were performed as described63. Briefly, gross morphology of the adult fish was analyzed at 75 days post fertilization (dpf). Fish were euthanized using MS-222 and images were taken using a digital camera. Body length and body weight were measured. Then the ovary was isolated from the body cavity for histological examination after noting the gonad weight. The gonad-somatic index (GSI) was calculated as (gonad weight/body weight) × 100%. For gonad histology, the ovarian samples were fixed in Bouin’s solution overnight at room temperature. The samples were dehydrated through a graded series of ethanol and embedded in paraffin wax. The samples were serially cut into 7 μm sections on a Leica microtome. After rehydration, the sections were stained with hematoxylin and eosin and mounted with Canada balsam (Sigma-Aldrich) for microscopic examination.
Fertility assessment
Fertility assessment was performed as described63. The fertility of npr−/− female fish was assessed by natural mating with wild-type males in a spawning tray. One hour after light on in the morning, the spawned eggs were collected. Individuals that failed to spawn or produce fertilized embryos after at least 10 trials were considered infertile5.
Maturation assay
Maturation assay was performed as described8. Female zebrafish were sacrificed and ovaries excised as described above. FG stage follicles from the mutant line and wild-type fish were collected and incubated (30–40 follicles/well) in 24-well culture plates at 28 °C. After treatment with 17α, 20β-DHP (Sigma-Aldrich) or recombinant zebrafish IGF38, follicles that underwent GVBD were identified by their ooplasmic clearing (due to proteolytic cleavage of vitellogenin). Each group had 4 replicate wells, and each experiment was repeated 3 times.
Induction of ovulation in vivo
hCG (Sigma-Aldrich) was dissolved in sterile distilled saline solution (NaCl 0.7%) at a concentration of 10 IU/μl. GW627368X (Cayman) was dissolved in DMSO at a concentration of 10 mM and then dissolved in sterile distilled saline solution (NaCl 0.7%) at a concentration of 10 μM. Before the administration of these agents, adult female fish were anesthetized. Fish were injected intraperitoneally with a volume of 5 μl/g body weight. After injection, the fish were placed individually in the tank.
Statistical analyses
All data were expressed as mean values ± SEM. P < 0.05 was considered statistically significant using one-way ANOVA, followed by Fisher’s least significant difference test using the GraphPad Software. Statistical comparisons of the expression levels between wild-type and mutant fish were conducted using an unpaired 2-tailed Student’s t test.
Additional Information
How to cite this article: Tang, H. et al. Gene knockout of nuclear progesterone receptor provides insights into the regulation of ovulation by LH signaling in zebrafish. Sci. Rep. 6, 28545; doi: 10.1038/srep28545 (2016).
Supplementary Material
Acknowledgments
We thank Mi Yao, Zeyao Zhu, Taian Liu and Kathy Sham for expert technical assistance. This work was supported by National Natural Science Foundation of China (NSFC Grant No. 31372512, 31172394) and the Research Grant Council of Hong Kong (14103014) and Guangdong Provincial Natural Science Foundation (2015A030313069).
Footnotes
Author Contributions Conceived and designed the experiments: H.T., Y.L., H.L., X.L. and C.H.K.C. Performed the experiments: H.T., J.L., Y.Y., G.L., Y.C., S.L. and Y.Z. Analyzed the data: H.T., Y.L. and J.L. Wrote the paper: H.T., Y.L., X.L. and C.H.K.C.
References
- Lei Z. M. et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Molecular Endocrinology 15, 184–200 (2001). [DOI] [PubMed] [Google Scholar]
- Chu L., Li J., Liu Y., Hu W. & Cheng C. H. Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Molecular Endocrinology 28, 1785–1795 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Dong Y., Matzuk M. M. & Kumar T. R. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proceedings of the National Academy of Sciences of the United States of America 101, 17294–17299 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. P., Poutanen M., Wilbertz J. & Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Molecular Endocrinology 15, 172–183 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhu B. & Ge W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Molecular Endocrinology 29, 76–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I., Zhang F. P., Kero J., Hamalainen T. & Poutanen M. Transgenic and knockout mouse models for the study of luteinizing hormone and luteinizing hormone receptor function. Molecular and Cellular Endocrinology 187, 49–56 (2002). [DOI] [PubMed] [Google Scholar]
- Kumar T. R. Functional analysis of LHbeta knockout mice. Molecular and Cellular Endocrinology 269, 81–84 (2007). [DOI] [PubMed] [Google Scholar]
- Li J., Chu L., Sun X., Liu Y. & Cheng C. H. IGFs mediate the action of LH on oocyte maturation in zebrafish. Molecular Endocrinology 29, 373–383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robker R. L., Akison L. K. & Russell D. L. Control of oocyte release by progesterone receptor-regulated gene expression. Nuclear Receptor Signaling 7, e012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akison L. K. & Robker R. L. The critical roles of progesterone receptor (PGR) in ovulation, oocyte developmental competence and oviductal transport in mammalian reproduction. Reproduction in Domestic Animals = Zuchthygiene 47 Suppl 4, 288–296 (2012). [DOI] [PubMed] [Google Scholar]
- Mani S. K. et al. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Molecular Endocrinology 10, 1728–1737 (1996). [DOI] [PubMed] [Google Scholar]
- Lydon J. P. et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & Development 9, 2266–2278 (1995). [DOI] [PubMed] [Google Scholar]
- Chappell P. E., Lydon J. P., Conneely O. M., O’Malley B. W. & Levine J. E. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 138, 4147–4152 (1997). [DOI] [PubMed] [Google Scholar]
- Chappell P. E. et al. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology 140, 3653–3658 (1999). [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Mulac-Jericevic B., DeMayo F., Lydon J. P. & O’Malley B. W. Reproductive functions of progesterone receptors. Recent Progress in Hormone Research 57, 339–355 (2002). [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Markstrom E., Andersson M. & Billig H. Progesterone receptor-mediated inhibition of apoptosis in granulosa cells isolated from rats treated with human chorionic gonadotropin. Biology of Reproduction 63, 1457–1464 (2000). [DOI] [PubMed] [Google Scholar]
- Lubzens E., Young G., Bobe J. & Cerda J. Oogenesis in teleosts: how eggs are formed. General and Comparative Endocrinology 165, 367–389 (2010). [DOI] [PubMed] [Google Scholar]
- Beers W. H. Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell 6, 379–386 (1975). [DOI] [PubMed] [Google Scholar]
- Tsafriri A. Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Advances in Experimental Medicine and Biology 377, 121–140 (1995). [DOI] [PubMed] [Google Scholar]
- Murdoch W. J. & McDonnel A. C. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction 123, 743–750 (2002). [DOI] [PubMed] [Google Scholar]
- Ogiwara K., Takano N., Shinohara M., Murakami M. & Takahashi T. Gelatinase A and membrane-type matrix metalloproteinases 1 and 2 are responsible for follicle rupture during ovulation in the medaka. Proceedings of the National Academy of Sciences of the United States of America 102, 8442–8447 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagglund A. C., Ny A., Leonardsson G. & Ny T. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology 140, 4351–4358 (1999). [DOI] [PubMed] [Google Scholar]
- Chaffin C. L. & Stouffer R. L. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: time course and steroid regulation. Biology of Reproduction 61, 14–21 (1999). [DOI] [PubMed] [Google Scholar]
- Liu K., Wahlberg P. & Ny T. Coordinated and cell-specific regulation of membrane type matrix metalloproteinase 1 (MT1-MMP) and its substrate matrix metalloproteinase 2 (MMP-2) by physiological signals during follicular development and ovulation. Endocrinology 139, 4735–4738 (1998). [DOI] [PubMed] [Google Scholar]
- Bradley J. A. & Goetz F. W. The inhibitory effects of indomethacin, nordihydroguaiaretic acid, and pyrrolidinedithiocarbamate on ovulation and prostaglandin synthesis in yellow perch (Perca flavescens) follicle incubates. Prostaglandins 48, 11–20 (1994). [DOI] [PubMed] [Google Scholar]
- Patino R., Yoshizaki G., Bolamba D. & Thomas P. Role of arachidonic acid and protein kinase C during maturation-inducing hormone-dependent meiotic resumption and ovulation in ovarian follicles of Atlantic croaker. Biology of Reproduction 68, 516–523 (2003). [DOI] [PubMed] [Google Scholar]
- Leung P. C. & Steele G. L. Intracellular signaling in the gonads. Endocrine Reviews 13, 476–498 (1992). [DOI] [PubMed] [Google Scholar]
- Gellersen B., Fernandes M. S. & Brosens J. J. Non-genomic progesterone actions in female reproduction. Human Reproduction Update 15, 119–138 (2009). [DOI] [PubMed] [Google Scholar]
- DeQuattro Z. A. et al. Effects of progesterone on reproduction and embryonic development in the fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry/SETAC 31, 851–856 (2012). [DOI] [PubMed] [Google Scholar]
- Stormshak F. & Bishop C. V. Board-invited review: Estrogen and progesterone signaling: genomic and nongenomic actions in domestic ruminants. Journal of Animal Science 86, 299–315 (2008). [DOI] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in Neuroendocrinology 29, 292–312 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs C. & Thomas P. Functional characteristics of membrane progestin receptor alpha (mPRalpha) subtypes: a review with new data showing mPRalpha expression in seatrout sperm and its association with sperm motility. Steroids 73, 935–941 (2008). [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hanna R. N., Schaaf M. J., Spaink H. P. & Thomas P. Candidates for membrane progestin receptors–past approaches and future challenges. Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP 148, 381–389 (2008). [DOI] [PubMed] [Google Scholar]
- Hanna R. N. & Zhu Y. Controls of meiotic signaling by membrane or nuclear progestin receptor in zebrafish follicle-enclosed oocytes. Molecular and Cellular Endocrinology 337, 80–88 (2011). [DOI] [PubMed] [Google Scholar]
- Chen S. X. et al. Molecular cloning and functional characterization of a zebrafish nuclear progesterone receptor. Biology of Reproduction 82, 171–181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna R., Pang Y., Thomas P. & Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. The Journal of Endocrinology 190, 247–260 (2006). [DOI] [PubMed] [Google Scholar]
- Hanna R. N. & Zhu Y. Expression of membrane progestin receptors in zebrafish (Danio rerio) oocytes, testis and pituitary. General and Comparative Endocrinology 161, 153–157 (2009). [DOI] [PubMed] [Google Scholar]
- Hanna R. N. et al. Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain. Biology of Reproduction 82, 112–122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok H. F., So W. K., Wang Y. & Ge W. Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors–evidence for their distinct functions in follicle development. Biology of Reproduction 72, 1370–1381 (2005). [DOI] [PubMed] [Google Scholar]
- Wang Y. & Ge W. Involvement of cyclic adenosine 3′,5′-monophosphate in the differential regulation of activin betaA and betaB expression by gonadotropin in the zebrafish ovarian follicle cells. Endocrinology 144, 491–499 (2003). [DOI] [PubMed] [Google Scholar]
- Clemens J. W., Robker R. L., Kraus W. L., Katzenellenbogen B. S. & Richards J. S. Hormone induction of progesterone receptor (PR) messenger ribonucleic acid and activation of PR promoter regions in ovarian granulosa cells: evidence for a role of cyclic adenosine 3′,5′-monophosphate but not estradiol. Molecular Endocrinology 12, 1201–1214 (1998). [DOI] [PubMed] [Google Scholar]
- Sriraman V., Sharma S. C. & Richards J. S. Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility. Molecular Endocrinology 17, 436–449 (2003). [DOI] [PubMed] [Google Scholar]
- Hagiwara A., Ogiwara K., Katsu Y. & Takahashi T. Luteinizing hormone-induced expression of Ptger4b, a prostaglandin E2 receptor indispensable for ovulation of the medaka Oryzias latipes, is regulated by a genomic mechanism involving nuclear progestin receptor. Biology of Reproduction 90, 126 (2014). [DOI] [PubMed] [Google Scholar]
- Nagahama Y. & Yamashita M. Regulation of oocyte maturation in fish. Development, Growth & Differentiation 50 Suppl 1, S195–219 (2008). [DOI] [PubMed] [Google Scholar]
- Zhu Y. et al. Nuclear progestin receptor (pgr) knockouts in zebrafish demonstrate role for pgr in ovulation but not in rapid non-genomic steroid mediated meiosis resumption. Frontiers in Endocrinology 6, 37 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Conneely O. M. & O’Malley B. W. Reproductive phenotpes of the progesterone receptor null mutant mouse. The Journal of Steroid Biochemistry and Molecular Biology 56, 67–77 (1996). [DOI] [PubMed] [Google Scholar]
- Orczyk G. P. & Behrman H. R. Ovulation blockade by aspirin or indomethacin–in vivo evidence for a role of prostaglandin in gonadotrophin secretion. Prostaglandins 1, 3–20 (1972). [DOI] [PubMed] [Google Scholar]
- Murdoch W. J., Hansen T. R. & McPherson L. A. A review–role of eicosanoids in vertebrate ovulation. Prostaglandins 46, 85–115 (1993). [DOI] [PubMed] [Google Scholar]
- Armstrong D. T. Prostaglandins and follicular functions. Journal of Reproduction and Fertility 62, 283–291 (1981). [DOI] [PubMed] [Google Scholar]
- Crespo D., Goetz F. W. & Planas J. V. Luteinizing hormone induces ovulation via tumor necrosis factor alpha-dependent increases in prostaglandin F2alpha in a nonmammalian vertebrate. Scientific Reports 5, 14210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetta F. & Goetz F. W. Ovarian and plasma prostaglandin E and F levels in brook trout (Salvelinus fontinalis) during pituitary-induced ovulation. Biology of Reproduction 27, 1216–1221 (1982). [DOI] [PubMed] [Google Scholar]
- Lister A. L. & Van Der Kraak G. An investigation into the role of prostaglandins in zebrafish oocyte maturation and ovulation. General and Comparative Endocrinology 159, 46–57 (2008). [DOI] [PubMed] [Google Scholar]
- Jalabert B. & Szollosi D. In vitro ovulation of trout oocytes: effect of prostaglandins on smooth muscle-like cells of the theca. Prostaglandins 9, 765–778 (1975). [DOI] [PubMed] [Google Scholar]
- Stacey N. E. & Pandey S. Effects of indomethacin and prostaglandins on ovulation of goldfish. Prostaglandins 9, 597–607 (1975). [DOI] [PubMed] [Google Scholar]
- Goetz F. W. & Theofan G. In vitro stimulation of germinal vesicle breakdown and ovulation of yellow perch (Perca flavescens) oocytes. Effects of 17 alpha-hydroxy-20 beta-dihydroprogesterone and prostaglandins. General and Comparative Endocrinology 37, 273–285 (1979). [DOI] [PubMed] [Google Scholar]
- Davies D. T. The neuroendocrine control of gonadotrophin release in the Japanese quail. III. The role of the tuberal and anterior hypothalamus in the control of ovarian development and ovulation. Proceedings of the Royal Society of London. Series B, Biological Sciences 206, 421–437 (1980). [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). (M. Westerfield, Eugene, OR; 1993). [Google Scholar]
- Bobe J., Montfort J., Nguyen T. & Fostier A. Identification of new participants in the rainbow trout (Oncorhynchus mykiss) oocyte maturation and ovulation processes using cDNA microarrays. Reproductive Biology and Endocrinology: RB&E 4, 39 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y. & Ge W. Gonadotropin regulation of activin betaA and activin type IIA receptor expression in the ovarian follicle cells of the zebrafish, Danio rerio. Molecular and Cellular Endocrinology 188, 195–205 (2002). [DOI] [PubMed] [Google Scholar]
- Goff D. J. et al. Identification of polymorphic simple sequence repeats in the genome of the zebrafish. Genomics 14, 200–202 (1992). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. A highly effective TALEN-mediated approach for targeted gene disruption in Xenopus tropicalis and zebrafish. Methods 69, 58–66 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Inheritable and precise large genomic deletions of non-coding RNA genes in zebrafish using TALENs. PloS One 8, e76387 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. et al. The kiss/kissr systems are dispensable for zebrafish reproduction: evidence from gene knockout studies. Endocrinology 156, 589–599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.