Abstract
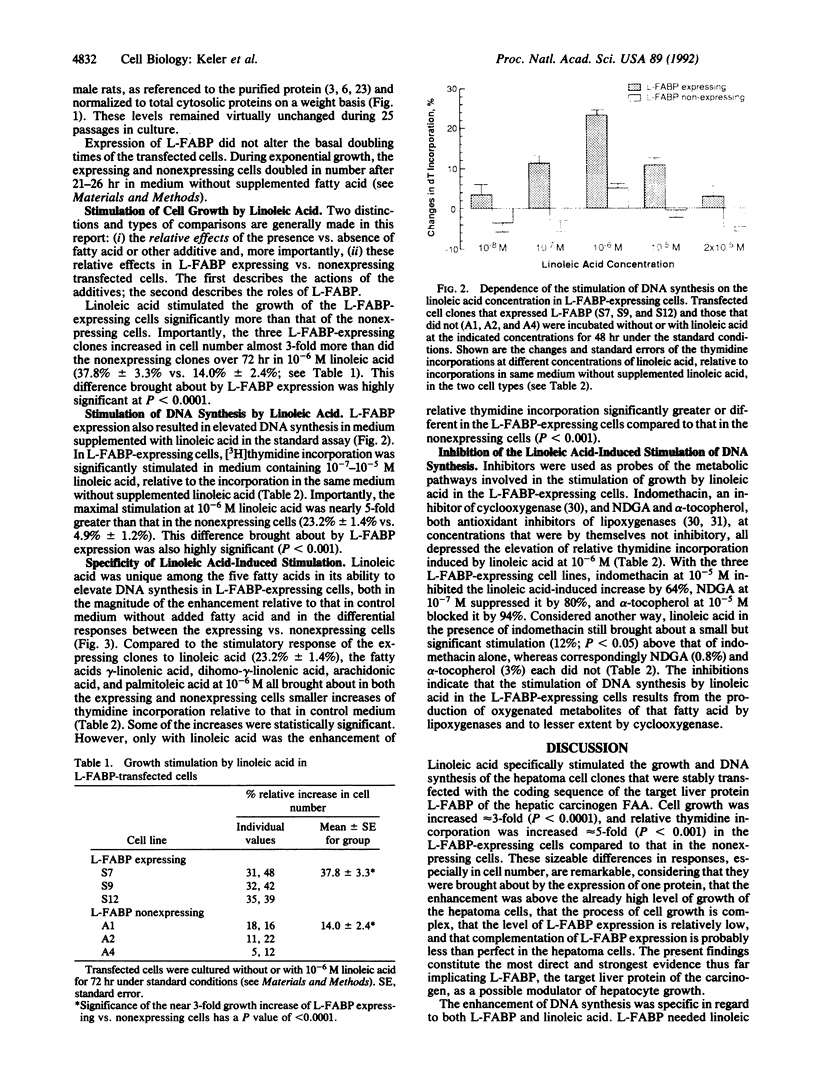
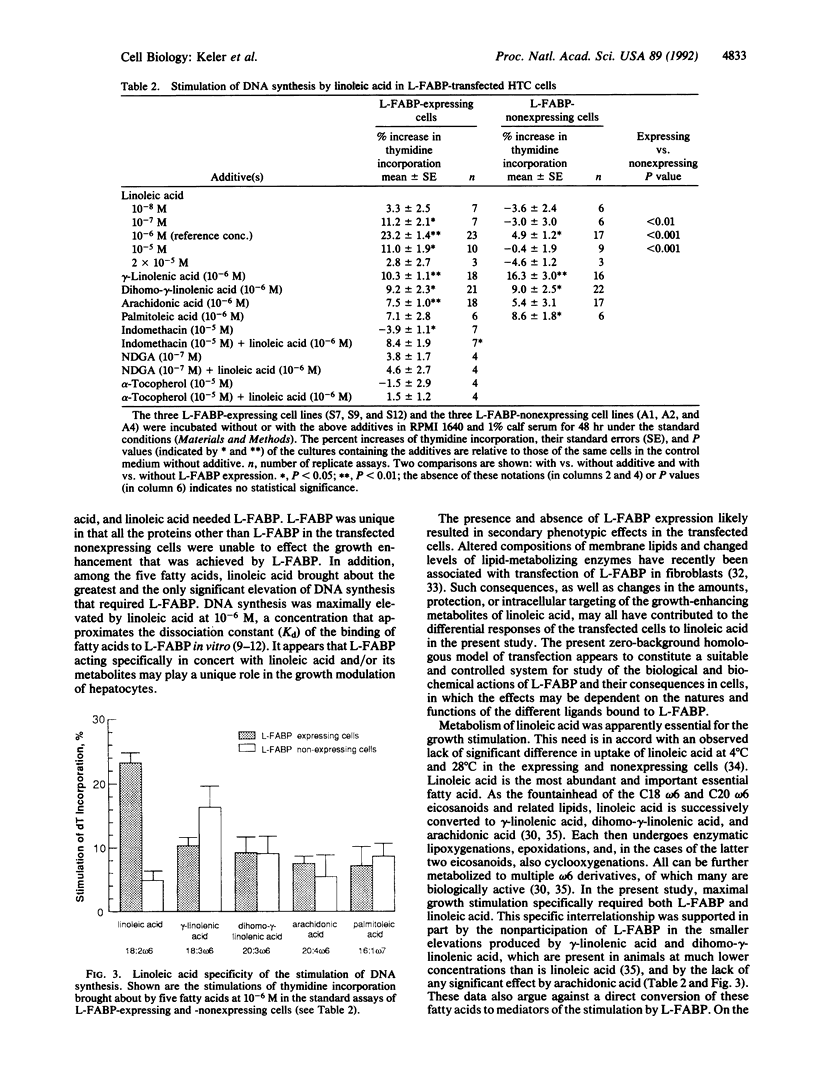
The hepatic carcinogen N-2-fluorenylacetamide (2-acetylaminofluorene) was shown previously to interact specifically with its target protein, liver fatty acid binding protein (L-FABP), early during hepatocarcinogenesis in rats. In search of the significance of the interaction, rat L-FABP cDNA in the sense and antisense orientations was transfected into a subline of the rat hepatoma HTC cell line that did not express L-FABP. After the transfections, the basal doubling times of the cells were not significantly different. However, at 10(-5)-10(-7) M, linoleic acid, which is an essential fatty acid, a ligand of L-FABP, and the precursor of many eicosanoids and related lipids, stimulated the incorporation of [3H]thymidine in three randomly isolated and stably transfected cell clones that expressed L-FABP, but virtually did not stimulate the incorporation of [3H]thymidine in three L-FABP-nonexpressing clones transfected with the antisense DNA. Linoleic acid at 10(-6) M increased cell number almost 3-fold (38% vs. 14%; P less than 0.0001) and thymidine incorporation nearly 5-fold (23.2% vs. 4.9%; P less than 0.001) in the L-FABP-expressing cells compared to that in the transfected nonexpressing cells. L-FABP acted specifically and cooperatively with linoleic acid, inasmuch as all the proteins other than L-FABP in the transfected L-FABP nonexpressing cells and four other fatty acids (gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid, and palmitoleic acid) were unable to effect a significant elevation or difference in the level of DNA synthesis that was attributable to the transfection. Metabolism of the linoleic acid to oxygenated derivatives was apparently necessary, since the cyclooxygenase inhibitor indomethacin partly inhibited and the antioxidant lipoxygenase inhibitors nordihydroguariaretic acid and alpha-tocopherol completely abolished the growth stimulation. The evidence supports the idea that L-FABP, the target protein of the liver carcinogen, acts specifically in concert with oxygenated metabolites of linoleic acid to modulate the growth of hepatocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balakrishnan A., Cramer S., Bandyopadhyay G. K., Imagawa W., Yang J., Elias J., Beattie C. W., Das Gupta T. K., Nandi S. Differential proliferative response to linoleate in cultures of epithelial cells from normal human breast and fibroadenomas. Cancer Res. 1989 Feb 15;49(4):857–862. [PubMed] [Google Scholar]
- Bansal M. P., Cook R. G., Danielson K. G., Medina D. A 14-kilodalton selenium-binding protein in mouse liver is fatty acid-binding protein. J Biol Chem. 1989 Aug 15;264(23):13780–13784. [PubMed] [Google Scholar]
- Bass N. M. The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol. 1988;111:143–184. doi: 10.1016/s0074-7696(08)61733-7. [DOI] [PubMed] [Google Scholar]
- Bassuk J. A., Tsichlis P. N., Sorof S. Liver fatty acid binding protein is the mitosis-associated polypeptide target of a carcinogen in rat hepatocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7547–7551. doi: 10.1073/pnas.84.21.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn G. R., Andrews J. P., Custer R. P., Sorof S. Early events during liver carcinogenesis involving two carcinogen:protein complexes. Cancer Res. 1981 Oct;41(10):4039–4049. [PubMed] [Google Scholar]
- Blackburn G. R., Andrews J. P., Rao K. V., Sorof S. An early event associated with liver carcinogenesis involving loss of a polypeptide that binds carcinogen. Cancer Res. 1980 Dec;40(12):4688–4693. [PubMed] [Google Scholar]
- Blackburn G. R., Schnabel S. J., Danley J. M., Hogue-Angeletti R. A., Sorof S. Principal polypeptide target of carcinogen at the beginning of liver carcinogenesis by three carcinogens. Cancer Res. 1982 Nov;42(11):4664–4672. [PubMed] [Google Scholar]
- Bull A. W., Nigro N. D., Marnett L. J. Structural requirements for stimulation of colonic cell proliferation by oxidized fatty acids. Cancer Res. 1988 Apr 1;48(7):1771–1776. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Custer R. P., Sorof S. Target polypeptide of a carcinogen is associated with normal mitosis and carcinogen-induced hyperplasias in adult hepatocytes. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6738–6742. doi: 10.1073/pnas.81.21.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earles S. M., Bronstein J. C., Winner D. L., Bull A. W. Metabolism of oxidized linoleic acid: characterization of 13-hydroxyoctadecadienoic acid dehydrogenase activity from rat colonic tissue. Biochim Biophys Acta. 1991 Jan 28;1081(2):174–180. doi: 10.1016/0005-2760(91)90023-b. [DOI] [PubMed] [Google Scholar]
- Eriksson L. C., Andersson G. N. Membrane biochemistry and chemical hepatocarcinogenesis. Crit Rev Biochem Mol Biol. 1992;27(1-2):1–55. doi: 10.3109/10409239209082558. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Fukai F., Kase T., Shidotani T., Nagai T., Katayama T. A novel role of fatty acid-binding protein as a vehicle of retinoids. Biochem Biophys Res Commun. 1987 Sep 30;147(3):899–903. doi: 10.1016/s0006-291x(87)80155-9. [DOI] [PubMed] [Google Scholar]
- Funk C. D., Powell W. S. Release of prostaglandins and monohydroxy and trihydroxy metabolites of linoleic and arachidonic acids by adult and fetal aortae and ductus arteriosus. J Biol Chem. 1985 Jun 25;260(12):7481–7488. [PubMed] [Google Scholar]
- Glasgow W. C., Eling T. E. Epidermal growth factor stimulates linoleic acid metabolism in BALB/c 3T3 fibroblasts. Mol Pharmacol. 1990 Oct;38(4):503–510. [PubMed] [Google Scholar]
- Gordon J. I., Alpers D. H., Ockner R. K., Strauss A. W. The nucleotide sequence of rat liver fatty acid binding protein mRNA. J Biol Chem. 1983 Mar 10;258(5):3356–3363. [PubMed] [Google Scholar]
- Jefferson J. R., Powell D. M., Rymaszewski Z., Kukowska-Latallo J., Lowe J. B., Schroeder F. Altered membrane structure in transfected mouse L-cell fibroblasts expressing rat liver fatty acid-binding protein. J Biol Chem. 1990 Jul 5;265(19):11062–11068. [PubMed] [Google Scholar]
- Jefferson J. R., Slotte J. P., Nemecz G., Pastuszyn A., Scallen T. J., Schroeder F. Intracellular sterol distribution in transfected mouse L-cell fibroblasts expressing rat liver fatty acid-binding protein. J Biol Chem. 1991 Mar 25;266(9):5486–5496. [PubMed] [Google Scholar]
- Kaikaus R. M., Bass N. M., Ockner R. K. Functions of fatty acid binding proteins. Experientia. 1990 Jun 15;46(6):617–630. doi: 10.1007/BF01939701. [DOI] [PubMed] [Google Scholar]
- Khan S. H., Sorof S. Preferential binding of growth inhibitory prostaglandins by the target protein of a carcinogen. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9401–9405. doi: 10.1073/pnas.87.23.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z. Effects of phorbol ester on phospholipid metabolism. Prog Lipid Res. 1990;29(3):141–166. doi: 10.1016/0163-7827(90)90001-2. [DOI] [PubMed] [Google Scholar]
- Liem H. H., Grasso J. A., Vincent S. H., Muller-Eberhard U. Protein-mediated efflux of heme from isolated rat liver mitochondria. Biochem Biophys Res Commun. 1990 Mar 16;167(2):528–534. doi: 10.1016/0006-291x(90)92056-6. [DOI] [PubMed] [Google Scholar]
- Raza H., Pongubala J. R., Sorof S. Specific high affinity binding of lipoxygenase metabolites of arachidonic acid by liver fatty acid binding protein. Biochem Biophys Res Commun. 1989 Jun 15;161(2):448–455. doi: 10.1016/0006-291x(89)92619-3. [DOI] [PubMed] [Google Scholar]
- Reddanna P., Rao M. K., Reddy C. C. Inhibition of 5-lipoxygenase by vitamin E. FEBS Lett. 1985 Nov 25;193(1):39–43. doi: 10.1016/0014-5793(85)80075-2. [DOI] [PubMed] [Google Scholar]
- Rose D. P., Connolly J. M. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990 Nov 15;50(22):7139–7144. [PubMed] [Google Scholar]
- San R. H., Shimada T., Maslansky C. J., Kreiser D. M., Laspia M. F., Rice J. M., Williams G. M. Growth characteristics and enzyme activities in a survey of transformation markers in adult rat liver epithelial-like cell cultures. Cancer Res. 1979 Nov;39(11):4441–4448. [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T. Identification of linoleic and arachidonic acids as the factors in hyperlipemic blood that increase [3H]thymidine incorporation in hepatoma 7288CTC perfused in situ. Cancer Res. 1988 Jun 1;48(11):3106–3111. [PubMed] [Google Scholar]
- Sorof S., Custer R. P. Elevated expression and cell cycle deregulation of a mitosis-associated target polypeptide of a carcinogen in hyperplastic and malignant rat hepatocytes. Cancer Res. 1987 Jan 1;47(1):210–220. [PubMed] [Google Scholar]
- Sorof S., Young E. M., Coffey C. B., Morris H. P. On protein binding of fluorenyl carcinogens by minimal deviation hepatomas. Cancer Res. 1966 Jan;26(1):81–88. [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Hoppe P. C., McKeel D. W., Gordon J. I. Mechanisms underlying generation of gradients in gene expression within the intestine: an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988 Oct;2(10):1318–1332. doi: 10.1101/gad.2.10.1318. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Heuckeroth R. O., Gordon J. I. The metabolic significance of mammalian fatty-acid-binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337–359. doi: 10.1146/annurev.nu.07.070187.002005. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Lowe J. B., Gordon J. I. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J Biol Chem. 1986 Apr 25;261(12):5553–5561. [PubMed] [Google Scholar]
- Vancura A., Carroll M. A., Haldar D. A lysophosphatidic acid-binding cytosolic protein stimulates mitochondrial glycerophosphate acyltransferase. Biochem Biophys Res Commun. 1991 Feb 28;175(1):339–343. doi: 10.1016/s0006-291x(05)81240-9. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Peeters R. A., Maatman R. G. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim Biophys Acta. 1991 Jan 4;1081(1):1–24. doi: 10.1016/0005-2760(91)90244-c. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Churey J. J., Haller J. M., Schnabel S. J., Custer R. P., Sorof S. Normal liver chromatin contains a firmly bound and larger protein related to the principal cytosolic target polypeptide of a hepatic carcinogen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2092–2096. doi: 10.1073/pnas.81.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch C. W. Enhancement of mammary tumorigenesis by dietary fat: review of potential mechanisms. Am J Clin Nutr. 1987 Jan;45(1 Suppl):192–202. doi: 10.1093/ajcn/45.1.192. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]