Abstract
Blue light controls the sexual life cycle of Chlamydomonas, mediated by phototropin, a UV-A/blue-light receptor that plays a prominent role in multiple photoresponses. By using fractionation experiments and immunolocalization studies, this blue-light receptor, in addition to its known localization to the cell bodies, also was detected in flagella. Within the flagella, it was completely associated with the axonemes, in striking contrast to the situation in higher plants and the Chlamydomonas cell body where phototropin was observed in the plasma membrane. Its localization was not perturbed in mutants lacking several prominent structural components of the axoneme. This led to the conclusion that phototropin may be associated with the outer doublet microtubules. Analysis of a mutant (fla10) in which intraflagellar transport is compromised suggested that phototropin is a cargo for intraflagellar transport. The blue-light receptor thus seems to be an integral constituent of the flagella of this green alga, extending the list of organisms that harbor sensory molecules within this organelle to unicellular algae.
INTRODUCTION
Blue light induces a variety of responses in higher and lower plants, many of which are mediated by photoreceptors of the phototropin family (Briggs and Huala, 1999; Christie and Briggs, 2001; Briggs and Christie, 2002). These photoreceptors mediate blue-light responses such as phototropism, chloroplast relocation, leaf expansion, rapid inhibition of hypocotyl growth, and stomatal opening.
Phototropins are UV-A/blue-light receptors that are characterized by their common domain structure of two FMN-binding and light-sensing Per Arnt Sim domains, designated as LOV1 and LOV2, where LOV stands for light, oxygen, and voltage. These domains comprise the amino-terminal half of the protein; a serine/threonine protein kinase catalytic domain is localized to the carboxy-terminal half of the molecule (Briggs et al., 2001; Briggs and Christie, 2002).
Excitation of the photoreceptor by blue light induces a cyclic photoreaction that involves the formation of a covalent adduct between the FMN chromophore and an activesite cysteine residue conserved in LOV1 and LOV2 (Salomon et al., 2000; Kottke et al., 2003). A resulting conformational alteration of the protein is believed to activate the protein kinase catalytic domain, resulting in autophosphorylation at multiple serine residues (Salomon et al., 2003). Correlative evidence suggesting an essential role for this activity in the signaling pathway has been collected (reviewed in Short and Briggs, 1994).
A single phototropin gene (Phot) that encodes a protein with a structure typical for that of other members of the phototropin family was identified in Chlamydomonas reinhardtii (Huang et al., 2002). The functional role of the gene product was investigated using the RNA interference method that allowed the generation of strains with drastically reduced levels of phototropin. In these strains, progression of the sexual life cycle at light-requiring steps (the formation of mating-competent gametes and the germination of zygotes) was found to be impaired when low fluence rates were applied (Huang and Beck, 2003). Maintenance of the gametes' mating ability also was affected. This response, measured by the blue-light–dependent reactivation of gametes that have become mating incompetent due to a short incubation in darkness, primarily seems to involve the flagella (Pan et al., 1997). Incubation in darkness caused a disappearance of the flagella-mediated recognition of gametes of opposite mating type due to an inactivation of the sexual agglutinins, highly glycosylated proteins on the flagellar surface that are involved in the initial contact between gametes. Their reactivation by light was shown to be rapid and independent of cytoplasmic protein synthesis (Pan et al., 1997). This process was shown to be mediated by phototropin because strains with reduced levels of this photoreceptor exhibited a delay in the recovery of mating ability at low fluence rates compared with wild-type cells (Huang and Beck, 2003).
Phototropin in Chlamydomonas gametes also mediates the light control of chemotaxis toward ammonium (Ermilova et al., 2003). Whereas vegetative cells move toward the ammonium source in the light and in the dark, chemotaxis is switched off in the late phase of gamete formation; this switching off is mediated by blue light. In phototropin-defective strains, this turning off of chemotaxis was delayed (Ermilova, Zalutskaya, Huang, and Beck, unpublished data).
In higher plants, phototropin is localized to the inner surface of the plasma membrane (Sakamoto and Briggs, 2002). However, because phototropins are highly hydrophilic and have no membrane-spanning domains, they clearly are not integral membrane proteins. Indeed, a fraction of this protein becomes released to the cytoplasm in response to blue light (Sakamoto and Briggs, 2002). The nature of the membrane attachment remains to be resolved.
C. reinhardtii has two apically positioned flagella. Because flagella lack the machinery for protein synthesis, the site of assembly of the axoneme, at its distal tip, is far removed from the site of synthesis of axonemal proteins in the cell body. This logistical problem has been solved by means of intraflagellar transport (IFT). During IFT, nonmembrane-bound particles are moved continuously along the axonemal doublet microtubules just beneath the flagellar membrane from the base to the tip of the organelle. This anterograde movement is thought to carry materials for the assembly and maintenance of the flagellar axoneme (Qin et al., 2004). After reaching the tip of the flagella, the IFT particles move back to the cell body (retrograde movement), where they are recycled (reviewed in Rosenbaum and Witman, 2002).
Although a principal function of eukaryotic flagella and cilia is thought to be force generation for motility and for the transport of fluids over epithelial cells, they also play an important role in sensory perception. Important evidence for a sensory role of flagella came from studies that focused on defects in the single, nonmotile “+ 0” cilium, called primary cilia, that are present on most vertebrate cells. Based on genetic studies it was concluded that primary cilia initiate signal transduction pathways that, among other functions, control kidney cell proliferation, differentiation, and/or apoptosis (reviewed in Pazour and Witman, 2003). Mechanosensors and receptors for somatostatin and serotonin also have been localized to the plasma membrane of the primary cilium (reviewed in Pazour and Rosenbaum, 2002; Pazour and Witman, 2003).
Cilia also play a crucial role in light perception by vertebrates. Thus, modified nonmotile sensory cilia of certain neuronal cells harbor the membrane-associated photoreceptor machinery of the vertebrate eye. The outer segments of retinal rods, harboring the photopigments, are connected to the rod inner segments by a short “9 + 0” connecting cilium that represents the only path of communication between the outer and the inner segments (reviewed in Rosenbaum and Witman, 2002).
Here, we report on the localization of the blue-light photoreceptor phototropin to the flagella of the green alga C. reinhardtii and thus extend the list of flagella with sensory molecules to the green algae.
MATERIALS AND METHODS
Strains and Culture Conditions
C. reinhardtii strains CC-124 mt- (wild type), oda1 (CC-2228 mt+, CC-2229 mt-) were obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). CF14 (mt+), a sibling from a cross between CC-1010 (mt+) and 137C (mt-), served as the mating partner. The fla10 and pf14 strains were provided by J.L. Rosenbaum (Yale University, New Haven, CT). The ida5 strain was obtained from R. Kamiya (Tokyo University, Tokyo, Japan), and the pf20 strain was from E.F. Smith (Dartmouth College, Hanover, NH). All Chlamydomonas strains were grown either in liquid or on solid (supplemented with 1.5% agar) Tris-acetate-phosphate (TAP) media (Harris 1989) at 23°C in the dark or under continuous irradiation with white light (fluence rate 30 μmol · m2 · s-1) provided by fluorescent tubes (L 36W/25; Osram, Munich, Germany). Flasks with liquid cultures were incubated on a rotary shaker. For tests, mutant strains fla10 and pf20 were grown in R & M media (Sager and Granick, 1954), respectively, with a light/dark regime of 13:11 h at 21°C.
Gametogenesis
For standard gametogenesis, liquid cultures of vegetative cells were centrifuged (2000 × g for 5 min) and resuspended in nitrogen-free (TAP-N) medium at a density of 1 × 107 cells/ml. These cells were incubated for 16 h in the light to generate gametes. The mating ability of gametes was assayed by mixing the cells to be tested with a threefold excess of mature gametes of opposite mating type. These were generated by suspending vegetative cells grown on plates in TAP-N medium at a density of 1–2 × 107 cells/ml, followed by incubation with continuous light for 16–24 h. After mixing and an incubation in the dark for 1 h, the percentage of gametes in the test culture was determined by counting the biflagellated and quadriflagellated cells as described previously (Beck and Acker, 1992).
Flagella and Cell Body Isolation
Flagella were isolated from Chlamydomonas by the pH shock method as described by Witman et al. (1972) with minor modification (Pan and Snell, 2000). Two liters of culture was centrifuged at 2000 × g for 5 min at 4°C, the pellet was resuspended in 40 ml of 10 mM Tris-HCl buffer (pH 7.2), and ice-cold 25% sucrose in 10 mM Tris-HCl (pH 7.2) was added to yield a final concentration of 7% sucrose. While stirring this suspension, its pH was rapidly decreased to pH 4.5 by adding 0.5 M acetic acid. After the flagella have become detached (which typically required ∼20–60 s), the pH was raised to 7.2 with 0.5 M KOH. The suspension of cells and flagella was underlaid with 25% sucrose in 10 mM Tris HCl (pH 7.2) and centrifuged for 10 min at 2500 × g. The upper phase, containing the flagella and a few remaining cell bodies, was underlaid with 25% sucrose and centrifuged again as described above. The upper phase containing purified flagella was carefully removed and centrifuged at 9000 × g for 8 min. For the isolation of flagella from dark grown cells, vegetative cells (∼2 × 106/ml) were put in a dark box and incubated overnight with shaking (∼16 h). The flagella were isolated in dim red light. The sedimented flagella were resuspended in HMDEK buffer (10 mM HEPES, pH 7.2, 5 mM MgSO4, 1 mM dithiothreitol, 1 mM EDTA, and 25 mM KCl) containing a 1/100 dilution of the Sigma protease inhibitor mixture designed for plant cells (Sigma-Aldrich, St. Louis, MO) and either extracted with NP-40 or opened by the freeze/thaw method (see below). For assays using total cell body proteins, the cell body pellet was resuspended in 0.1 M dithiothreitol/0.1 M Na2CO3. Then, 0.66 volumes of 5% SDS/30% sucrose was added. In cases where the lysates were very viscous, samples were sonicated. Homogenization of the suspensions was achieved by rapid shaking at room temperature for 20 min. These samples were used for Western blotting. The cell body pellet used for isolation of the microsomal membrane and plasma membrane was treated differently (see below).
Large-scale isolation of flagella by using dibucaine-HCl was performed as described by King (1995). One to 2 liters of Chlamydomonas cells (∼5 × 106/ml) were sedimented as described above, the pellet was resuspended in 20 ml of 10 mM HEPES buffer (pH 7.2), and then 4 ml of 25 mM dibucaine-HCl solution (MP Biomedicals, Irvine, CA) was added. As soon as the cells were deflagellated (checked by phase contrast microscopy), which usually took 1–2 min, 20 ml of stop solution (0.5 mM EGTA, 29 mM HEPES buffer, pH 7.2, 4% sucrose) was added. Subsequent steps were the same as for the pH shock method.
Preparation of Crude Microsomal Membranes and the Plasma Membrane
The protocol described by Norling et al. (1996) was used with small modifications. The cell body pellet (∼5 g) was frozen in liquid nitrogen. While being suspended in liquid nitrogen, the cells were broken using a mortar and pestle. Then, 40 ml of ice-cold buffer consisting of 0.25 M sucrose, 50 mM MOPS-KOH, pH 7.8, 3 mM EDTA, 0.5 mM EGTA, 5 mM MgCl2, 1% (wt/vol) bovine serum albumin, 5 mM mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride was added. Unbroken cells, chloroplasts, cell debris, mitochondria, and thylakoids were removed by centrifugation for 5 min at 13,000 × g. The supernatant containing the low-density membranes, including microsomes, Golgi, vacuolar membranes, and plasma membranes was subsequently centrifuged for 1 h at 125,000 × g. The resulting pellet, homogenized in 5 ml of 0.25 M sucrose, 5 mM potassium phosphate buffer, pH 7.8, was denoted as total microsomal membrane. This fraction was obtained by a method very similar to that used for the isolation of the cell body membrane fraction described previously (Huang et al., 2002). The only differences were in the buffers used and in the centrifugal forces applied.
For isolation of the plasma membrane, ∼5 g of total microsomal membrane suspension was added to 13.75 g of polymer mixture [6.5% (wt/wt) dextran T-500, 6.5% (wt/wt) polyethylene glycol 3350, 0.25 M sucrose, 5 mM potassium phosphate buffer, pH 7.8, 4 mM KCl), yielding a two-phase system. The partitioning was performed by turning the tube gently upside-down 30 times at 4°C. After mixing, phase settling was facilitated by centrifugation for 3 min at 1000 × g. The upper phase was repartitioned with a lower phase obtained from a phase system with the same final concentrations listed above but without the membrane sample added. This step was repeated a third time, yielding a final upper phase. The final upper phase was diluted 7–10 times with 0.33 M sucrose, 50 mM MOPS-KOH, pH 7.0, 4 mM MgSO4, and the plasma membrane was collected by centrifugation for 75 min at 125,000 × g. The plasma membrane fraction was resuspended in the buffer used for dilution.
Flagella Fractionation
An equal volume of 1% or 0.1% NP-40 in HMDEK buffer (sometimes with 10 mM MgATP) was added to a suspension of isolated flagella (final concentration of 0.5 or 0.05% NP-40), and this mixture was incubated at 4°C on a rocker for 30 min. The mixture was centrifuged for 20 min with 60,000 × g at 4°C. The supernatant was denoted as the membrane + matrix fraction (M+M); the pellet, resuspended in HMDEK buffer, yielded the axoneme fraction.
To permeabilize flagella by the freeze/thaw method (Wang and Snell, 2003), flagella in HMDEK buffer containing a 1/100 dilution of the Sigma protease inhibitor mixture (Sigma-Aldrich) were frozen in liquid nitrogen and subsequently thawed at room temperature. After centrifugation for 20 min with 60,000 × g at 4°C, a supernatant fraction, denoted as soluble fraction (S), was obtained. The pellet was resuspended in HMDEK buffer containing 1% NP-40 and incubated on ice for 30 min. This buffer also contained 10 mM MgATP. Recentrifugation of this fraction (60,000 × g for 20 min at 4°C) yielded the membrane fraction (M) in the supernatant and the axoneme fraction (A) in the pellet.
Immunoblot Analyses
The protein concentration was determined by staining with Amido black (Popov et al., 1975), by using bovine serum albumin as a standard. Proteins from the flagella, the membrane + matrix fractions and the axoneme fractions were separated by SDS-PAGE by using the method of Laemmli (1970) as modified by Jarvik and Rosenbaum (1980)). The gels contained a 4–16% acrylamide gradient and a 2–8 M urea gradient. In some cases, gels with 9% acrylamide were used. The proteins were transferred from the gels to poly-vinylidene difluoride membranes (Hybond-P; Amersham Biosciences, Piscataway, NJ). The antibodies used in this work and their sources are listed in Table 1. Peroxidase-conjugated anti-rabbit serum (Sigma-Aldrich) was used to detect the primary antibodies (see below). For signal detection we used the enhanced chemiluminescence system (Amersham Biosciences).
Table 1.
Antibodies used in this study
| Antigen | Antibody | Reference/Source |
|---|---|---|
| Phototropin of C. reinhardtii | α-Phototropin | Huang et al., 2002 |
| Flagellar membrane glycoprotein | α-FMG-1 | Bloodgood et al., 1986 |
| β-Tubulin of C. reinhardtii | α-β-Tubulin | R. Bloodgood |
| Radial spoke protein RSP1 of C. reinhardtii | α-RSP1 | Williams et al., 1986 |
| Kinesin-II motor protein Fla 10 of C. reinhardtii | α-FLA10 | Cole et al., 1998 |
| Intermicrotubule bridge protein PF20 of C. reinhardtii | pf20 | Smith and Lefebvre, 1997 |
| Chloroplast chaperone HSP70B | αHSP70B | Schroda et al., 1999 |
| Chloroplast GrpE homolog | αCGE1 | Schroda et al., 2001 |
For quantification of the signals, the developed films were scanned and analyzed using the Quantity One program (Bio-Rad, Hercules, CA).
Immunolocalization Experiments
Fixation and immunolocalization experiments were carried out as described in Shevell et al. (2000). Primary antisera were used at a dilution of 1:150 (α-phototropin and α-FMG-1) or 1:100 (rabbit preimmune serum). FMG-1 localizations were performed with sections of Chlamydomonas cells embedded in Historesin. Experiments with preimmune serum and anti-phototropin antiserum were performed with Chlamydomonas cells directly attached to polyethyleneimine-coated glass slides.
RESULTS
Phototropin in Flagella
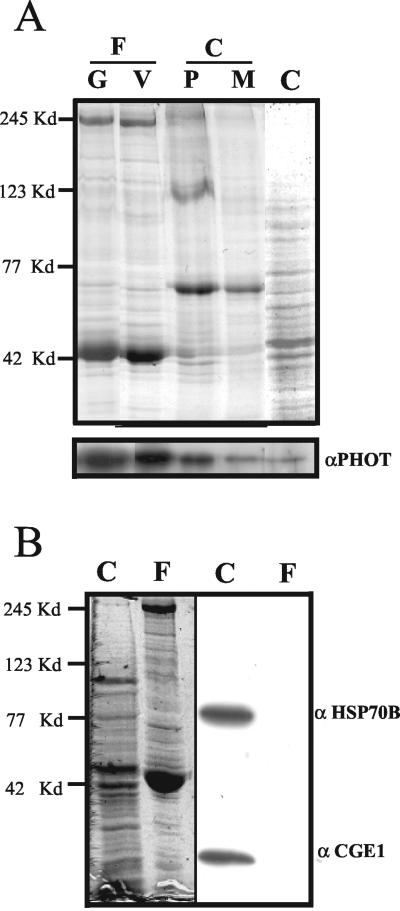
The single polypeptide chain of the blue-light receptor phototropin in C. reinhardtii has a mass of ∼80 kDa. An antibody, directed against its protein kinase catalytic domain (Huang et al., 2002), was used to test for the presence of phototropin in flagella. Flagella released from cell bodies by the pH shock method were isolated. Western blot analysis revealed the presence of phototropin in flagella of both vegetative cells and gametes (Figure 1A). The purity of the flagella fraction was assessed using antibodies directed against two proteins, HSP70B and CGE1, that are expected to be absent from flagella; both of these proteins were previously shown to be localized inside the chloroplast (Schroda et al., 1999, 2001). These proteins were detected in the cell body fraction but not in the flagella fraction (Figure 1B). The localization of phototropin in the flagella-less cell bodies was analyzed as well. In an extension of previous results that showed phototropin in the membrane fraction (Huang et al., 2002), an enrichment of this protein is seen in the plasma membrane fraction of the cell body (Figure 1A). This result is supported by immunolocalization studies (Figure 2A).
Figure 1.
Protein blot to demonstrate the presence of phototropin in flagella. (A) Flagella (F) were released from the cells by the pH shock method. The plasma membrane (P) of the cell bodies (C) was isolated using a two-phase partitioning method, and the microsomal membrane (M) was isolated by differential centrifugation as described in MATERIALS AND METHODS. Flagellar protein (8 μg) from gametes (80% mating competent cells) (G) or vegetative cells (V), and the same amount of protein from cell bodies as well as the plasma membrane fraction (P) and the microsomal membrane fraction (M) of the cell bodies were separated by SDS-PAGE. Top, Coomassie Blue-stained gel. Phototropin was detected by Western blot analysis (bottom) by using an antibody raised against the kinase domain of Chlamydomonas phototropin (Huang et al., 2002). (B) Purity of the flagella fraction was assessed using antibodies directed against proteins HSP70B and CGE1 of the chloroplast (right side). Left side of B shows the gel stained with Coomassie Blue. Protein (40 μg) from cell bodies (C) and flagella (F) were applied per lane. The positions of marker proteins are shown on the left side.
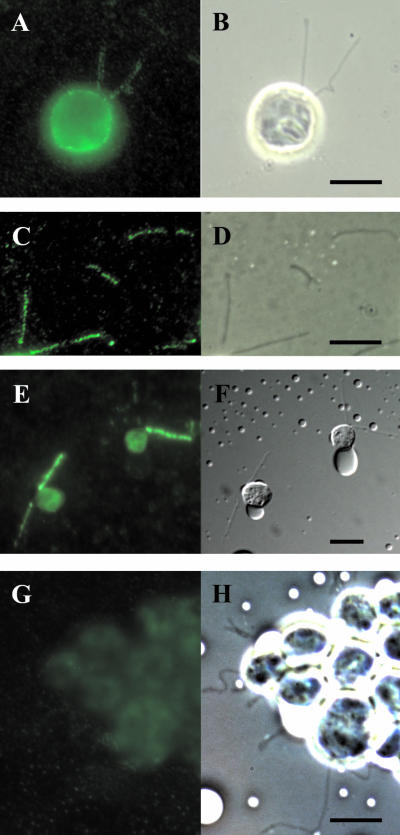
Figure 2.
Localization of phototropin by immunofluorescence. Immunolocalization of phototropin in Chlamydomonas cells (A) and flagella (C). The corresponding bright-field pictures are shown in B and D, respectively. For a control, monoclonal antibody FMG-1, directed against the carbohydrate moieties of the principal high-molecular-weight glycoproteins (Bloodgood et al., 1986) was used (E and F). In G, the rabbit preimmune serum was used. (H) Corresponding bright-field picture. Bars, 10 μm.
The presence of phototropin in flagella was verified using immunofluorescent localization techniques. Treatment of the alga with the C. reinhardtii-specific phototropin antibody and a secondary fluorescent antibody resulted in the emission of a fluorescence signal from both the flagella and the cell body (Figure 2, A–D). Control localizations with phototropin preimmune serum (Figure 2, G and H) resulted in no specific fluorescence signal in either the flagella or in the cell body. As a positive control, we also performed immunolocalizations with an anti-carbohydrate monoclonal antibody (FMG-1) that not only binds preferentially to the principal high-molecular-weight glycoproteins of the flagella but also recognizes surface-exposed epitopes of the cell body (Figure 2, E and F) (Bloodgood et al., 1986).
Within the flagella, the photoreceptor seems to be present over the entire length of this organelle. In the cell bodies, phototropin seems to be localized primarily to the periphery, presumably representing the plasma membrane (Figure 2A). Based on the data presented in Figures 1 and 2, we conclude that the blue-light photoreceptor phototropin is located in both the cell body and the flagella of Chlamydomonas.
Relative Amount of Phototropin in Flagella
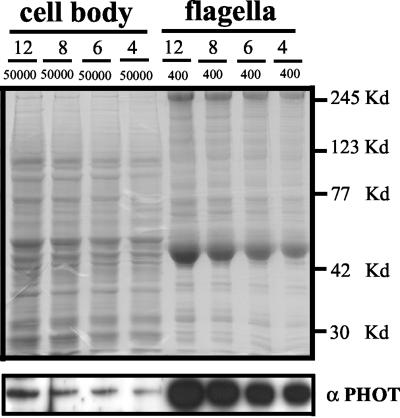
To determine the percentage of phototropin localized in flagella versus cell bodies, the cells were deflagellated by the pH shock method and separated into a cell body and a flagella fraction by centrifugation. The cell bodies were resuspended in 50 ml of buffer, and the flagella in 0.4 ml. Aliquots of protein extracted from the two fractions, after separation by SDS-PAGE, were subjected to Western blot analysis (Figure 3). From a comparison of the intensities of the immunosignals observed, we deduced that ∼3.8% of total phototropin protein is localized within the flagella. We found no significant difference in the concentration of phototropin in gametes and vegetative cells (Figure 1A).
Figure 3.
Determination of the relative amounts of phototropin in flagella and cell bodies. From a Chlamydomonas culture (strain CC-124), the flagella were separated from the cell bodies by the pH shock method. Flagella and cell bodies were resuspended in 0.4 and 50 ml of sample buffer, respectively, and homogenized (see MATERIALS AND METHODS). Different volumes (12, 8, 6, and 4 μl) of flagella and cell body proteins were separated by SDS-PAGE and analyzed by Western blot (bottom). Quantification of the relative amounts of phototropin was achieved using the quantification method described in MATERIALS AND METHODS. The relative amount of phototropin localized in the flagella was calculated according to the formula 400/50000 × 4.8 = 3.84 (the relative amount of phototropin in the flagella samples was determined to be 4.8-fold higher than that in the cell body samples of the same volume). Top, gel stained with Coomassie Blue. The positions of marker proteins are shown on the right side.
Using strain RNAi20 with drastically reduced cellular levels of phototropin (Huang and Beck, 2003), we tested whether the concentration of the photoreceptor in flagella was affected as well. As shown in Figure 4, phototropin in the flagella of this strain was present only at a low level compared with wild-type cells.
Figure 4.
Comparison of phototropin levels in flagella between wild type (CC-124) and a strain with reduced phototropin levels (RNAi20). The flagella from strains CC-124 and RNAi20 (Huang and Beck, 2003) were separated by the pH shock method. Flagellar protein (7 μg) was separated by SDS-PAGE (9% polyacryamide) and immunoblotted using a phototropin-specific antibody. For a loading control, an antibody that reacts with the kinesin-II motor protein FLA10 was used.
Flagellar Phototropin Fractionates with Axonemal Structures
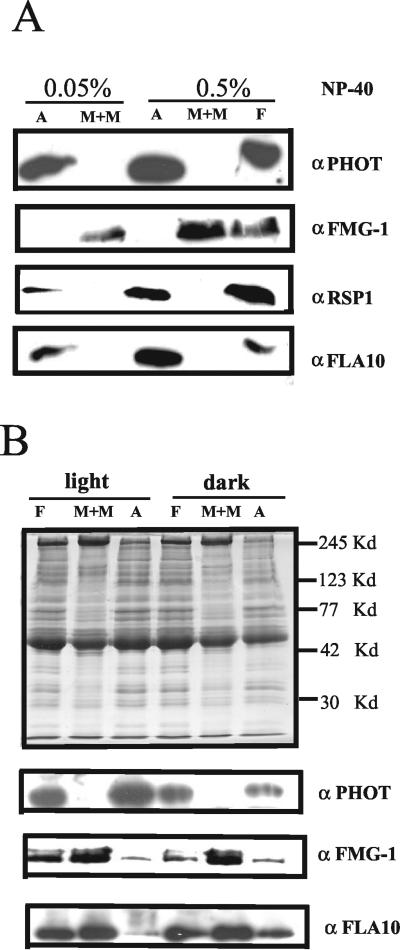
To localize phototropin within the flagella, the nonionic detergent NP-40 was used to fractionate flagella into a membrane/matrix fraction and an axonemal fraction (Witman et al., 1972; Cole et al., 1998). After separation by SDS-PAGE, the fractions were analyzed by Western blot techniques. When flagellar fractionation was performed in the absence of ATP, the majority of phototropin was observed in the axonemal fraction (Figure 5A). The presence of 10 mM ATP during fractionation did not affect this localization (Figure 5B). This axonemal localization was surprising because phototropin in higher plants is found in association with the plasma membrane (Reymond et al., 1992; Sakamoto and Briggs, 2002) as is also the case for C. reinhardtii cell bodies (Figures 1 and 2). Successful fractionation of the flagella was verified using antibodies directed against flagellar proteins known to be associated with either the membrane/matrix or axonemal fractions. As has been previously demonstrated, the major flagellar membrane glycoproteins, recognized by the FMG-1 antibody, fractionated with the membrane/matrix (Figure 5, A and B). In contrast, nearly all of the FLA10 protein, in the absence of ATP in the extraction buffer, is associated with the axoneme (Figure 5A). This protein, which is in the kinesin II family and the motor protein responsible for anterograde IFT, has been shown to be partially released from axonemes by ATP (Walther et al., 1994; Cole et al., 1998). We also observed that ∼50% of FLA10 is released from the axonemes by ATP and becomes localized to the membrane/matrix fraction (Figure 5B). The radial spoke protein 1 (RSP1), located between the outer and central microtubule doublets, was exclusively detected in the axonemal fraction (Figure 5A).
Figure 5.
Association of phototropin with the axoneme fraction of the flagella. (A) Flagella isolated by the pH shock method were separated into an M+M fraction and an axoneme (A) fraction by using 0.05 or 0.5% NP-40 as described in MATERIALS AND METHODS. To 50 μlof flagella suspension (protein concentration 1–2 μg/μl), 50 μl of HMDEK buffer with 2 times detergent was added, and the mixture was centrifuged. The supernatant represents the M+M fraction. The pellet was resuspended in 100 μl of HMDEK buffer with detergent, resulting in the axoneme fraction. One hundred microliters each of the M+M and A fractions as well as 50 μl of total flagella proteins (F) were separated by SDS-PAGE and analyzed by Western blot (note that the amount of protein in these three samples was not the same). In addition to the phototropin-specific antibody, antibodies directed against the major flagellar membrane glycoprotein, FMG-1, RSP1 (one of the radial spoke proteins), and FLA10 (kinesin-II motor protein) were used. (B) Flagella were isolated from cells grown in the light or in the dark. The flagella were fractionated with 0.5% NP-40 in the presence of 10 mM ATP. The same amount of protein (7 μg) from each sample was separated by SDS-PAGE and used for immunoblotting (bottom). Top, gel stained with Coomassie Blue. The positions of marker proteins are shown on the right side.
The analyses described above were performed with flagella isolated from light-grown cells. We next tested whether phototropin, which may be considered as a light-activated protein kinase, was affected in its association with the axonemes when cells were grown and harvested in the dark. We found that, in dark-grown cells, phototropin remained associated with the flagellar axonemes (Figure 5B), suggesting that, at least in flagella, it does not undergo gross changes in its localization upon activation by light.
A possible artifact could arise from a substantial contamination of the axonemal fraction with flagellar membranes. This was investigated by negative staining of flagellar structures extracted with NP-40. Electron microscopic inspection of these structures indicated that the majority of membranes had been removed (our unpublished data), consistent with the absence of the flagellar membrane protein FMG-1 from the axonemal fractions (Figure 5, A and B).
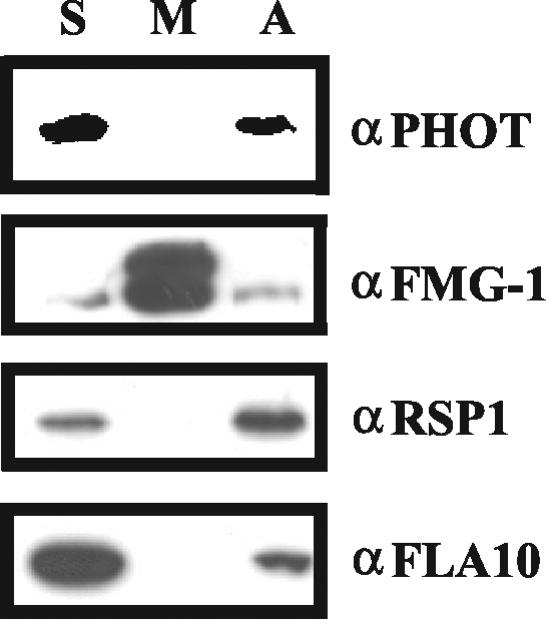
We also used an alternative method to determine the localization of phototropin in the flagella. Freeze/thaw treatment of isolated flagella is thought to put holes into the flagellar membrane and to allow the release of soluble proteins from the flagella. In addition, this treatment may result in a partial denaturation of proteins and thus liberate proteins from larger protein complexes (Wang and Nick, 2001). After freeze/thaw treatment and centrifugation, ∼60% of phototropin was observed in the supernatant (Figure 6). The residual 40% of phototropin was associated with the axonemes after extraction of the freeze-thaw pellet with NP-40. A minor fraction of the radial spoke protein also was released by freeze/thaw treatment (Figure 6). In line with these results, most of the FLA10 protein was found in the supernatant, the rest still being bound to the axonemes. These results indicate that the disruption of flagellar membranes and a partial denaturation of proteins by freeze/thaw treatment may lead to partial release from the axoneme.
Figure 6.
Fractionation of flagella by the freeze/thaw method. One freeze-thaw cycle in HMDEK buffer in the absence of ATP was performed. After centrifugation, a supernatant fraction (soluble fraction, S) was obtained. The pellet was resuspended in HMDEK buffer containing 1% NP-40 and 10 mM ATP. Recentrifugation of this fraction yielded the membrane fraction (M) in the supernatant and the axoneme fraction (A) in the pellet. The same amount of protein (9 μg) was loaded per lane. For a control, antibodies FMG-1 (directed against the major flagellar membrane glycoprotein), RSP1 (one of the radical spoke proteins), and FLA10 (kinesin-II motor protein) were used.
Proteins such as FLA10 that are reversibly bound to axonemes seem to be more susceptible to release than proteins such as RSP1 that are part of the core axonemal structures. The level of phototropin released by freeze/thaw was found to be intermediate between that of FLA10 and RSP1. This finding may indicate that the association of phototropin with the axonemes is tighter than that of FLA10 but weaker than that of RSP1.
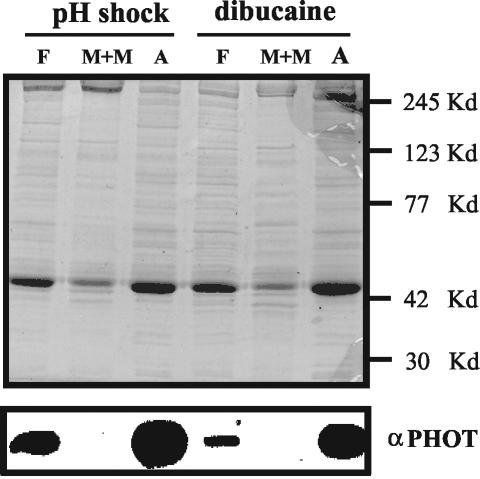
Because the pH shock procedure used to isolate flagella may possibly affect the localization of phototropin, dibucaine treatment was used as an alternative method for removing flagella from the cell bodies (King, 1995). Flagella isolated with dibucaine and extracted with NP-40 have most of the phototropin associated with the axonemes (Figure 7). From these combined results, we conclude that the blue-light receptor phototropin is present in different cytoplasmic compartments in flagella and cell bodies. In cell bodies, it is associated with the plasma membrane, whereas in flagella phototropin is associated with the microtubule cytoskeleton, the flagellar axoneme.
Figure 7.
Comparative fractionation of phototropin from flagella released by the pH shock method or by treatment with dibucaine. The flagella were released from the cell bodies using either the pH shock method or treatment with dibucaine as described in MATERIALS AND METHODS. They were subsequently separated into an M+M fraction and an axonemal fraction using 0.5% NP-40 detergent in the presence of 10 mM ATP in HMDEK buffer. Six micrograms of protein per lane was separated by SDS-PAGE, and after Western blotting, phototropin was detected using the C. reinhardtii-specific antibody (bottom). Top, gel stained with Coomassie Blue. The positions of marker proteins are indicated.
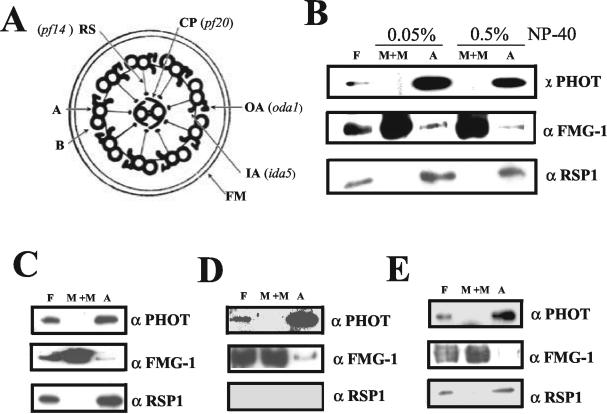
Localization of Phototropin within the Axoneme
To gain further information on the localization of phototropin within the axoneme, we used mutants that lack individual components of the axoneme. These components are indicated in Figure 8A. Fractionation of the flagella after NP-40 extraction of a mutant lacking the outer dynein arms (oda1) (Kamiya 1988; Takada et al., 2002) revealed that the photoreceptor was still associated with the axonemes (Figure 8B). The normal fractionation of RSP1 and the major membrane glycoproteins remained unaffected in this mutant. Mutant pf20 lacks the two central microtubules and associated structures (Smith and Lefebvre, 1997); in this mutant, phototropin continued to fractionate with the axonemes (Figure 8C). Lack of the radial spokes in mutant pf14 (Huang et al., 1977; Diener et al., 1990) also did not affect the association of phototropin with the axonemal fraction (Figure 8D). Absence of the inner dynein arms in mutant ida5 (Kato et al., 1993; Kato-Minoura et al., 1997) again had no effect on the localization of phototropin (Figure 8E).
Figure 8.
Attempts to identify a phototropin interaction partner within the axonemal structure by the use of mutants. (A) Diagram of a cross section of a Chlamydomonas flagellum structure (adapted from Rosenbaum and Witman, 2002). The axonemal structures missing in individual mutants are indicated. Oda1 lacks the outer dynein arms (OA) (Kamiya, 1988; Takada et al., 2002); ida5 lacks most of the inner dynein arms (IA) (Kato et al., 1993; Kato-Minoura et al., 1997); pf14 has no radial spoke structures (RP) (Diener et al., 1990); pf20 lacks the whole central pair complex (CP) (Smith and Lefebvre, 1997). A and B represent the A- and B-tubules of outer doublet microtubules and FM the flagellar membrane. Flagella isolated from mutants oda1 (B), pf20 (C), pf14 (D), and ida5 (E) were separated into M+M and axoneme (A) fractions using 0.05% (oda1 mutant) and 0.5% NP-40 (oda1, ida5, pf14, and pf20 mutants) in the presence of 10 mM ATP in HMDEK buffer. The same amount of protein was loaded in each lane for Western blotting.
These results suggest that the association of phototropin with the axonemes is not mediated via the central microtubules, the radial spokes, or the dynein arms.
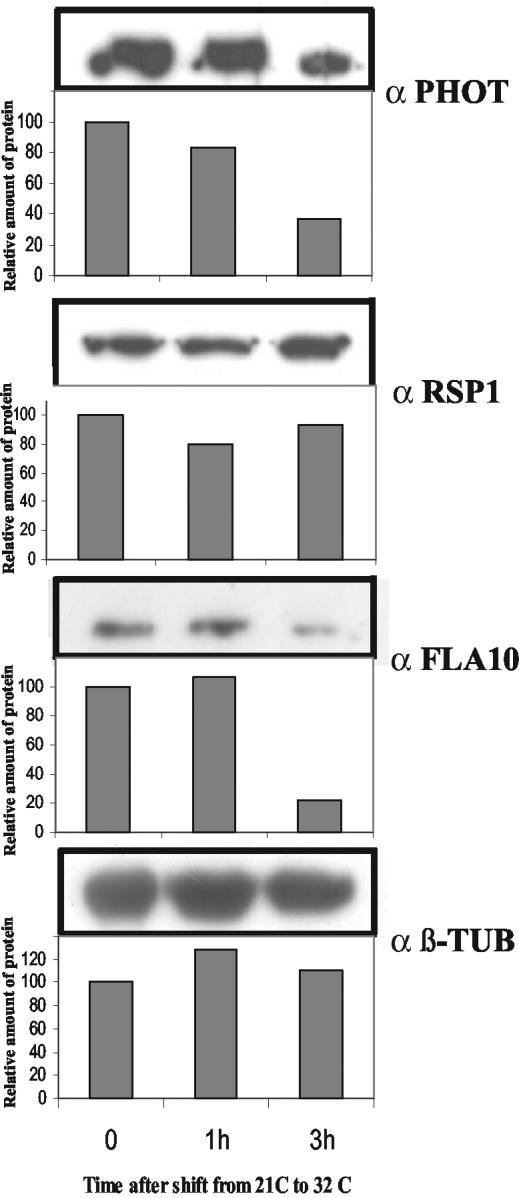
Evidence for a Transport of Phototropin by IFT
Because protein synthesis does not take place within the flagella, the elongated organelle must import all the macromolecules required for its assembly, maintenance, and function from the cytosol. To test whether phototropin is transported by the anterograde transport machinery that is known to deliver macromolecules to the flagellar tips (reviewed in Rosenbaum and Witman, 2002), we used the temperature-sensitive mutant fla10 (Walther et al., 1994) in which, after a shift to the restrictive temperature (32°C), anterograde IFT particle movement ceases and IFT particles are depleted from the flagella (Cole et al., 1998; Pan and Snell, 2002). At the permissive temperature (21°C), IFT is functional in this mutant. After the fla10 strain was shifted to 32°C, samples were taken from 0 to 3 h after the temperature shift, and the isolated flagella processed by SDS-PAGE. Immunoblotting analyses revealed that, after 3 h at the non-permissive temperature, phototropin levels dropped to ∼35% of the initial level (Figure 9). After this same period at 32°C, the FLA10 protein level was found to be reduced to ∼20% of the control level. A reduction in flagellar content of the FLA10 protein at the restrictive temperature is consistent with previous reports (Walther et al., 1994). In contrast, at the nonpermissive temperature, the axonemal components of the flagella, as detected by antibodies against RSP1 and β-tubulin (β-TUB), were not significantly affected in their concentration (Figure 9). This result suggests that the blue-light photoreceptor in the flagella may be transported by IFT.
Figure 9.
Blocking of IFT affects flagellar phototropin levels. The Chlamydomonas mutant strain fla10 at time 0 was shifted from 21°C to the restrictive temperature of 32°C. After 60 and 180 min at 32°C, cells were harvested and the flagella were isolated using the pH shock method. Flagellar proteins (10 μg) from each fraction were analyzed by SDS-PAGE and immunoblotting with antibodies directed against phototropin protein (PHOT), FLA10, RSP1, and β-TUB. For a quantitative evaluation, performed as described in MATERIALS AND METHODS, the amounts of antigen detected at time 0 were used as a reference and set to 100.
DISCUSSION
We showed by cell fractionation studies as well as by immunofluorescence localization analyses that the blue-light receptor phototropin in the green alga C. reinhardtii is located not only at the plasma membrane of the cell body but also in flagella. Even though Chlamydomonas flagella have previously been thought to play sensory roles (Solter and Gibor, 1977), we report here for the first time the presence of a specific known sensory molecule within the flagella of an alga, suggesting a role for this organelle in the perception and transduction of extrinsic signals. A sensory role of flagella or cilia in higher organisms is well established (reviewed in Rosenbaum and Witman, 2002; Pazour and Witman, 2003).
Our studies on the localization of phototropin within the flagella provided a surprising finding. Whereas in higher plants and in the cell body of C. reinhardtii phototropin was always found in association with the plasma membrane fraction (Figures 1 and 2) (Reymond et al., 1992; Sakamoto and Briggs, 2002; Huang et al., 2002), in flagella the photoreceptor seems to be associated with the axoneme (Figure 5). Because the membrane that covers cell body and flagella is a continuum, we also expected phototropin to be associated with the flagellar membrane. The altered location of the photoreceptor in the flagella may indicate a special function in this organelle. The basis for the more typical membrane attachment of phototropin is not yet understood. In the absence of hydrophobic domains (Huang et al., 2002), concepts entertained include a noncovalent binding of phototropin to membrane or membrane-associated proteins (Motchoulski and Liscum, 1999). In this case, the absence of a specific membrane-anchoring protein in the flagellar membrane may be one way to explain the altered localization of the photoreceptor in flagella. The tight association of phototropin with the axonemal fraction observed in all experiments but the freeze/thaw assays (where a partial release not only of phototropin but also of axonemal components such as RSP1 [Figure 6] and β-tubulin [our unpublished data] were observed) may indicate the presence of a specific phototropin binding protein within the axoneme. Some structures that may harbor potential candidates for an axonemal binding protein such as the outer and inner dynein arms, the two central microtubules, and the radial spokes were ruled out because mutants lacking these structures still showed an association of phototropin with the axonemes (Figure 8). This leaves open the possibility of an interaction of phototropin with structural components, possibly directly with the outer doublet microtubules or via microtubule-associated proteins. Following this line of reasoning, we speculate that phototropin in the cell body also may be targeted to the plasma membrane via binding to membrane-associated cytoskeletal components. This possibility seems attractive in view of the reported association of integral membrane proteins with microtubules (Kishi et al., 1997).
The question whether phototropin is transported by the IFT machinery was investigated using a fla10 mutant. In this mutant, at the restrictive temperature, the levels of both the kinesin II motor protein (FLA10) and phototropin were found to be drastically reduced (Figure 9) (Cole et al., 1998; Pan and Snell, 2002). Turnover of flagellar phototropin may account for the decrease observed when, upon blocking of IFT, the delivery of newly synthesized photoreceptor molecules from the cytoplasm to the flagella is prevented. An involvement of kinesein II in the transport of light-sensing molecules also has been observed in mammals (Marszalek et al., 2000).
Approximately 4% of total phototropin protein was observed in flagella. The volume of these organelles comprises ∼0.5% of a whole cell. These considerations imply an enrichment of the photoreceptor in flagella relative to the cell body. This enrichment is even more pronounced when the protein concentration is used as a point of reference: The phototropin concentration in flagella is approximately eight-fold higher than that in cell bodies (our unpublished data). This suggests a preferential targeting of the photoreceptor to the flagella. Because the process of sorting of flagellar proteins in Chlamydomonas is not well understood (Bloodgood, 2000), the basis for this preferential distribution remains open. Possibly, the relative number of phototropin-binding molecules available in flagella exceeds that in the cell body.
Our results suggest that the flagella of C. reinhardtii have a function in light perception in addition to their known roles in cell locomotion and gametic interactions. Flagella are involved in the initial cell–cell recognition during mating of Chlamydomonas gametes. This interaction takes place through sexual agglutinins, which are very large, hydroxyproline-rich glycoproteins located on the gametic flagellar surface. We speculate that one possible function of the photoreceptor in gametic flagella is to maintain the agglutinins in a state of competence for interaction. In darkness, these molecules rapidly loose their ability to interact (Pan et al., 1997). This dark inactivation is reversed by irradiation with blue light and phototropin was shown to be involved in this step because strains with decreased phototropin levels exhibited a reduced reactivation at low fluence rates compared with wild-type strains (Huang and Beck, 2003). Although experimental evidence is still missing, a role of phototropin in the activation of the sexual agglutinins, possibly through molecules that control their activity, as postulated by Pan et al. (1997), may be envisioned. Attempts to reactivate the agglutination activity in isolated flagella thus far have not been successful (our unpublished data).
The observations reported here on the localization of phototropin in flagella support the emerging concept that, in unicellular algae, the flagella are sensory organelles. The flagella of Chlamydomonas, being the best studied organelle of this type, provide a solid basis for a characterization of the blue-light receptor in flagella at the structural and functional level.
Acknowledgments
We thank Drs. D.R. Diener, H. Qin, and J.L. Rosenbaum for antibodies αRSP1, αFLA10, and strains fla10, pf14; Dr. E.F. Smith for antibody αPF20 and strain pf20; and Drs. J. Pan and W.J. Snell for helpful suggestions and strain fla10 (CC-1919). We gratefully acknowledge Dr. J.L. Rosenbaum for stimulating interest and the sharing of unpublished data. The advice of Dr. W. Michalke for isolation of the plasma membrane is greatly appreciated. We especially thank Dr. R. Bloodgood for generous advice, help in improving the manu-script, and for providing antibodies α FMG-1 and α β-TUB. We also thank Dr. M. Schroda for constructive criticism and for critical reading of the manu-script. The work reported in this article was supported by a grant of the Deutsche Forschungsgemeinschaft to C.F.B.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0010. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0010.
Abbreviations used: IFT, intraflagellar transport; M+M, membrane + matrix fraction; RSP, radial spoke protein.
References
- Beck, C.F., and Acker, A. (1992). Gametic differentiation of Chlamydomonas reinhardtii. Plant Physiol. 98, 822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood, R.A. (2000). Protein targeting to flagella of trypanosomatid protozoa. Cell Biol. Int. 24, 857-862. [DOI] [PubMed] [Google Scholar]
- Bloodgood, R.A., Woodward, M.P., and Salomonsky, N.L. (1986). Redistribution and shedding of flagellar membrane glycoproteins visualized using an anti-carbohydrate monoclonal antibody and concanavalin A. J. Cell Biol. 102, 1797-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R., et al. (2001). The phototropin family of photoreceptors. Plant Cell 13, 993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R., and Christie, J.M. (2002). Phototropin 1 and phototropin 2, Two versatile plant blue-light receptors. Trends Plant Sci. 7, 204-210. [DOI] [PubMed] [Google Scholar]
- Briggs, W.R., and Huala, E. (1999). Blue-light photoreceptors in higher plants. Annu. Rev. Cell Dev. Biol. 15, 33-62. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., and Briggs, W.R. (2001). Blue light sensing in higher plants. J. Biol. Chem. 276, 11457-11460. [DOI] [PubMed] [Google Scholar]
- Cole, D.G., Diener, D.R., Himelblau, A.L., Beech, P.L., Fuster, J.C., and Rosenbaum, J.L. (1998). Chlamydomonas kinesin-II–dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory cilia. J. Cell Biol. 141, 993-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener, D.R., Curry, A.M., Johnson, K.A., Williams, B.D., Lefebvre, P.A., Kindle, K.L., and Rosenbaum, J.L. (1990). Rescue of a paralyzed-flagella mutant of Chlamydomonas by transformation. Proc. Natl. Acad. Sci. USA 87, 5739-5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermilova, E.V., Zalutskaya, Zh. M., Lapina, T., and Nikitin, M.M. (2003). Chemotactic behavior of Chlamydomonas reinhardtii is altered during gametogenesis. Curr. Microbiol. 46, 261-264. [DOI] [PubMed] [Google Scholar]
- Harris, E.H. (1989). The Chlamydomonas Sourcebook, San Diego, CA: Academic Press.
- Huang, B., Rifkin, M.R., and Luck, D.J. (1977). Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J. Cell Biol. 72, 67-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, K., and Beck, C.F. (2003). Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 100, 6269-6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, K., Merkle, T., and Beck, C.F. (2002). Isolation and characterization of a Chlamydomonas gene that encodes a putative blue-light photoreceptor of the phototropin family. Physiol. Plant. 115, 613-622. [DOI] [PubMed] [Google Scholar]
- Jarvik, J.W., and Rosenbaum, J.L. (1980). Oversized flagellar membrane protein in paralyzed mutants of Chlamydomonas reinhardtii. J. Cell Biol. 85, 258-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, R. (1988). Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J. Cell Biol. 107, 2253-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, T., Kagami, O., Yagi, T., and Kamiya, R. (1993). Isolation of two species of Chlamydomonas reinhardtii flagellar mutants, ida5 and ida6, that lack a newly identified heavy chain of the inner dynein arm. Cell Struct. Funct. 18, 371-377. [DOI] [PubMed] [Google Scholar]
- Kato-Minoura, T., Hirono, M., and Kamiya, R. (1997). Chlamydomonas innerarm dynein mutant, ida5, has a mutation in an actin-encoding gene. J. Cell Biol. 137, 649-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.M. (1995). Large-scale isolation of Chlamydomonas flagella. Methods Cell Biol. 47, 9-12. [DOI] [PubMed] [Google Scholar]
- Kishi, F., Yoshida, T., and Aiso, S. (1997). Location of NRAMP1 molecule on the plasma membrane and its association with microtubules. Mol. Immun. 33, 1241-1246. [DOI] [PubMed] [Google Scholar]
- Kottke, T., Heberle, J., Hehn, D., Dick, B., and Hegemann, P. (2003). Phot-LOV 1, photocycle of a blue-light receptor domain from the green alga Chlamydomonas reinhardtii. Biophys J. 84, 1192-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Marszalek, J.R., Liu, X., Roberts, E.A., Chui, D., Marth, J.D., Williams, D.S., and Goldstein, L.S.B. (2000). Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell 102, 175-187. [DOI] [PubMed] [Google Scholar]
- Motchoulski, A., and Liscum, E. (1999). Arabidopsis NPH 3, a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286, 961-964. [DOI] [PubMed] [Google Scholar]
- Norling, B., Nurani, G., and Franzen, L.G. (1996). Characterization of the H+-ATPase in plasma membrane isolated from the green alga Chlamydomonas reinhardtii. Physiol. Plant. 97, 445-453. [Google Scholar]
- Pan, J., Haring, M.A., and Beck, C.F. (1997). Characterization of blue light signal transduction chains that control development and maintenance of sexual competence in Chlamydomonas reinhardtii. Plant Physiol. 115, 1241-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J., and Snell, W.J. (2000). Regulated targeting of a protein kinase into an intact flagellum. An aurora/Ipllp-like protein kinase translocates from the cell body into the flagella during gamete activation in Chlamydomonas. J. Biol. Chem. 275, 24106-24114. [DOI] [PubMed] [Google Scholar]
- Pan, J., and Snell, W.J. (2002). Kinesin-II is required for flagellar sensory transduction during fertilization in Chlamydomonas. Mol. Biol. Cell 13, 1417-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G.J., and Rosenbaum, J.L. (2002). Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 12, 551-555. [DOI] [PubMed] [Google Scholar]
- Pazour, G.J., and Witman, G.B. (2003). The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 15, 105-110. [DOI] [PubMed] [Google Scholar]
- Popov, N., Schmitt, S., and Matthices, H. (1975). Eine störungsfreie Micro-methode zur Bestimmung des Proteingehalts in Gewebshomogenaten. Acta Biol. Germ. 31, 1441-1446. [PubMed] [Google Scholar]
- Qin, H., Diener, D.R., Geimer, S., Cole, D.G., and Rosenbaum, J.L. (2004). Intra-flagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond, P., Short, T.W., Briggs, W.R., and Poff, K.L. (1992). Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89, 4718-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, J.L., and Witman, G.B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell. Biol. 3, 813-825. [DOI] [PubMed] [Google Scholar]
- Sager, R., and Granick, S. (1954). Nutritional control of sexuality in Chlamydomonas reinhardtii. J. Gen. Physiol. 37, 729-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto; K., and Briggs, W.R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14, 1723-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., Knieb, E., von Zeppelin, T., and Ruediger, W. (2003). Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42, 4217-4225. [DOI] [PubMed] [Google Scholar]
- Salomon, M., Christie, J.M., Knieb, E., Lempert, U., and Briggs, W.R. (2000). Photochemical and mutational analysis of the FMN binding domains of the plant blue light receptor, phototropin. Biochemistry 39, 9401-9410. [DOI] [PubMed] [Google Scholar]
- Schroda, M., Vallon, O., Whitelegge, J., Beck, C.F., and Wollman, F.A. (2001). Identification and characterization of a chloroplast GrpE homolog. Plant Cell 13, 2823-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda, M., Vallon, O., Wollman, F.A., and Beck, C.F. (1999). A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 11, 1165-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell, D.E., Kunkel, T., and Chua, N.-H. (2000). Cell wall alterations in the Arabidopsis emb30 mutant. Plant Cell 12, 2047-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short, T.W., and Briggs, W.R. (1994). The transduction of blue light signals in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 143-171. [Google Scholar]
- Smith, E.F., and Lefebvre, P.A. (1997). PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol. Biol. Cell 8, 455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter, K.M., and Gibor, A. (1977). Evidence for role of flagella as sensory transducers in mating of Chlamydomonas reinhardtii. Nature 265, 444-445. [DOI] [PubMed] [Google Scholar]
- Takada, S., Wilkerson, C.G., Wakabayashi, K., Kamiya, R., and Witman, G.B. (2002). The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol. Biol. Cell 13, 1015-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, Z., Vashishtha, M., and Hall, J.L. (1994). The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J. Cell Biol. 126, 175-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., and Snell, W.J. (2003). Flagellar adhesion between mating type plus and mating type minus gametes activates a flagellar protein-tyrosine kinase during fertilization in Chlamydomonas. J. Biol. Chem. 278, 32936-32642. [DOI] [PubMed] [Google Scholar]
- Wang, Q.Y., and Nick, P. (2001). Cold acclimation can induce microtubular cold stability in a manner distinct from abscisic acid. Plant Cell Physiol. 42, 999-1005. [DOI] [PubMed] [Google Scholar]
- Williams, B.D., Mitchell, D.R., and Rosenbaum, J.L. (1986). Molecular cloning and expression of flagellar radial spoke and dynein genes of Chlamydomonas. J. Cell Biol. 103, 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman, G.B., Carlson, K., Berliner, J., and Rosenbaum, J.L. (1972). Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J. Cell Biol. 54, 507-539. [DOI] [PMC free article] [PubMed] [Google Scholar]