Abstract
The large-conductance, Ca2+- and voltage-activated K+ (BK) channel is ubiquitously expressed in mammalian tissues and displays diverse biophysical or pharmacological characteristics. This diversity is in part conferred by channel modulation with different regulatory auxiliary subunits. To date, two distinct classes of BK channel auxiliary subunits have been identified: β subunits and γ subunits. Modulation of BK channels by the four auxiliary β (β1–β4) subunits has been well established and intensively investigated over the past two decades. The auxiliary γ subunits, however, were identified only very recently, which adds a new dimension to BK channel regulation and improves our understanding of the physiological functions of BK channels in various tissues and cell types. This chapter will review the current understanding of BK channel modulation by auxiliary β and γ subunits, especially the latest findings.
1. INTRODUCTION
The large-conductance, calcium- and voltage-activated potassium (BK, also known as MaxiK, Slo1, or KCa1.1) channel is a unique member of the potassium channel family. The BK channel has exceptionally large single-channel conductance (200–300 pS) that is 10–20 times larger than most other K+ channels. BK channel activity is dually regulated by two independent physiological signals, membrane voltage, and intracellular free Ca2+ (Ca2+ i), and therefore has a powerful integrative role in regulating cellular excitability and calcium signaling in electrically excitable cells (Ghatta, Nimmagadda, Xu, & O’Rourke, 2006; Salkoff, Butler, Ferreira, Santi, & Wei, 2006). BK channels are ubiquitously expressed in most types of mammalian tissues and cells and are critically involved in various physiological processes. In central nervous system neurons, BK channels mediate the repolarization and fast afterhyperpolarization of action potentials (Shao, Halvorsrud, Borg-Graham, & Storm, 1999; Womack & Khodakhah, 2002), shape dendritic Ca2+ spikes (Golding, Jung, Mickus, & Spruston, 1999), and regulate neurotransmitter release at presynaptic terminals (Hu et al., 2001; Raffaelli, Saviane, Mohajerani, Pedarzani, & Cherubini, 2004; Samengo, Curro, Barrese, Taglialatela, & Martire, 2014; Xu & Slaughter, 2005; see Chapter “BK Channels in Neurons” by Barth and Contet). Neuronal BK channels are involved in motor coordination (Sausbier et al., 2004), learning and memory (Matthews & Disterhoft, 2009; Springer, Burkett, & Schrader, 2014; Typlt et al., 2013; Ye, Jalini, Mylvaganam, & Carlen, 2010), the brain’s intrinsic rhythmicity of the circadian clock (Farajnia, Meijer, & Michel, 2015; Meredith et al., 2006; Montgomery, Whitt, Wright, Lai, & Meredith, 2013; Pitts, Ohta, & McMahon, 2006) and respiration (Onimaru, Ballanyi, & Homma, 2003; Zavala-Tecuapetla, Aguileta, Lopez-Guerrero, Gonzalez-Marin, & Pena, 2008; Zhao, Hulsmann, Winter, Dutschmann, & Richter, 2006), frequency tuning of the cochlear hair cell (Fettiplace & Fuchs, 1999), pain modulation (Cao, Chen, Li, & Pan, 2012; Chen, Cai, & Pan, 2009; Waxman & Zamponi, 2014; Zhang, Mok, Lee, Charbonnet, & Gold, 2012), and neuroprotection in pathological conditions (Mancini et al., 2014; Runden-Pran, Haug, Storm, & Ottersen, 2002; Shen, Kishimoto, Linden, & Sapirstein, 2007; Zhang, Xie, et al., 2009). Defects or dysregulation in human neuronal BK channels can cause epilepsy and paroxysmal dyskinesia (Brenner et al., 2005; Du et al., 2005) and are implicated in mental retardation (Deng et al., 2013; Higgins, Hao, Kosofsky, & Rajadhyaksha, 2008), autism (Laumonnier et al., 2006), and schizophrenia (Zhang, Li, Zhou, & Xing, 2006). BK channels also control contractile tone of almost all types of smooth muscle cells, playing a central role in the regulation of vascular blood pressure (Brenner, Perez, et al., 2000), urinary bladder function (Meredith, Thorneloe, Werner, Nelson, & Aldrich, 2004), and erectile function (Werner, Zvara, Meredith, Aldrich, & Nelson, 2005).
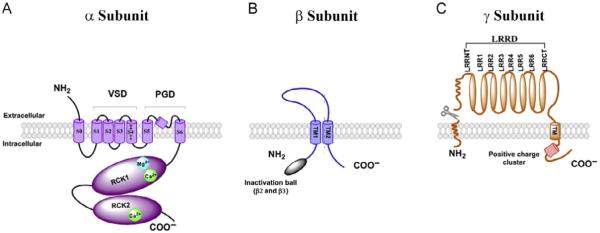
BK channels are homotetramers of the pore-forming, Ca2+-, and voltage-sensing α subunits (BKα). The BKα (rv130 kDa) contains seven-transmembrane (TM) segments (S0–S6), a short extracellular N-terminus, and a large cytosolic C-terminus composed of two RCK (regulating conductance of K+) domains (Fig. 1A). Similar to other voltage-gated K+ channels, the S1–S4 TM segments form the voltage-sensor domain (VSD), and the S5 and S6 TM segments form the pore-gate domain (PGD). Ca2+ and Mg2+ sensitivity is conferred by the two RCK domains in the cytosolic C-terminus. The RCK2 domain contains a well-defined Ca2+-binding site called the Ca2+ bowl, formed by a string of negatively charged residues (Schreiber & Salkoff, 1997; Xia, Zeng, & Lingle, 2002; Yuan, Leonetti, Hsiung, & MacKinnon, 2012; Yusifov, Savalli, Gandhi, Ottolia, & Olcese, 2008). The RCK1 domain is believed to be involved in the formation of another Ca2+-binding site (Xia et al., 2002; Zhang et al., 2010) and a Mg2+-binding site which also involves residues located in the S0–S1 linker (D99) and S2–S3 loop (N172) (Shi et al., 2002; Xia et al., 2002; Yang et al., 2008). The electric (voltage) and chemical (Ca2+ or Mg2+ binding) energies are converted to mechanical forces through their sensory domains to induce conformational change in the PGD, which toggles between the “closed state” and “open state” to control K+ flux (Horrigan & Aldrich, 1999; Horrigan, Cui, & Aldrich, 1999).
Fig. 1.
Schematic structure and membrane topology of BK channel α (A), β (B), and γ (C) subunits and their models of assembly. VSD, voltage-sensor domain; PGD, pore-gate domain; RCK, regulator of K+ conductance; NH2, aminoterminus; COO-, carboxyterminus; LRRD, leucine-rich repeat domain; S or TM, transmembrane segment.
Unlike many other mammalian potassium channels, all BKα subunits are encoded by a single gene (Slo1, KCNMA1). Native BK channels exhibit a wide range of biophysical, pharmacological, and functional properties that differ among various cell types (Ding, Li, & Lingle, 1998; Gessner et al., 2005; Jones, Gray-Keller, Art, & Fettiplace, 1999; Xia, Ding, & Lingle, 1999), at different stages of development (Carvalho-de-Souza, Varanda, Tostes, & Chignalia, 2013), and in different physiological or pathological conditions (Hu et al., 2011; Manzanares et al., 2015; Tao et al., 2015). BK channels with slightly different biophysical properties can be produced by alternative splicing of KCNMA1 precursor mRNA at several different sites (Glauser, Johnson, Aldrich, & Goodman, 2011; Ramanathan, Michael, Jiang, Hiel, & Fuchs, 1999; Saito, Nelson, Salkoff, & Lingle, 1997; Schreiber, Yuan, & Salkoff, 1999; Yu, Upadhyaya, & Atkinson, 2006). BK channel function is differently and potently modulated by auxiliary subunits. To date, four types of beta (β) auxiliary subunits (β1–β4, encoded by the KCNMB1–4 genes) (Behrens et al., 2000; Brenner, Jegla, Wickenden, Liu, & Aldrich, 2000; Knaus, Folander, et al., 1994; Meera, Wallner, & Toro, 2000; Orio, Rojas, Ferreira, & Latorre, 2002; Wallner, Meera, & Toro, 1999; Xia et al., 1999; Xia, Ding, & Lingle, 2003; Xia, Ding, Zeng, Duan, & Lingle, 2000; Zeng, Xia, & Lingle, 2008) and four types of gamma (γ) auxiliary subunits (γ1–γ4, encoded by the LRRC26, LRRC52, LRRC55, and LRRC38 genes) (Yan & Aldrich, 2010, 2012) have been identified (Table 1). These auxiliary subunits affect nearly all aspects of the BK channel’s biophysical and pharmacological properties, including the apparent voltage dependence (Cox & Aldrich, 2000; Wang & Brenner, 2006; Yan & Aldrich, 2010, 2012) and Ca2+ sensitivity (Brenner, Jegla, et al., 2000; McManus et al., 1995; Nimigean & Magleby, 1999; Xia et al., 1999), gating kinetics, inactivation (Brenner, Jegla, et al., 2000; Xia et al., 1999, 2000), ion current rectification (Xia et al., 2000; Zeng, Xia, & Lingle, 2003), and sensitivity to extracellular modulators (Almassy & Begenisich, 2012; Valverde et al., 1999). Regulation by auxiliary subunits is therefore a key mechanism of BK channel functional diversity that adapts the channel for diverse needs in different mammalian tissues and cell types (Table 1). The modulation of BK channels by β subunits has been extensively investigated and comprehensively reviewed elsewhere (Sun, Zaydman, & Cui, 2012; Torres, Granados, & Latorre, 2014; Torres, Morera, Carvacho, & Latorre, 2007). This chapter will review the literature of BK channel modulation by auxiliary β and γ subunits, including a comparison of these two different types of auxiliary subunits, specifically their modulatory effects and underlying mechanisms.
Table 1.
Characteristics of BK Channel Auxiliary Subunits
| Gene Symbol (Human) |
Effect on BK Channels Function |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subunit | Ca2+
Sensitivity |
Voltage Sensitivity |
Inactivation | Deactivation | References | Tissue Expression | Physiologic Relevance | ||
| BKβ1 | KCNMB1 | Increase | Decrease | No inactivation | Slow |
Wallner et al. (1995), Tseng-Crank et al. (1996), Cox and Aldrich (2000), and Orio and Latorre (2005) |
Smooth muscle, kidney, urinary bladder, and brain (Knaus, Garcia-Calvo, Kaczorowski, & Garcia, 1994; Liu et al., 2010; Tseng-Crank et al., 1996) |
Blood pressure, vascular smooth muscle function, bladder function, alcohol dependence, and tolerance (Amberg, Bonev, Rossow, Nelson, & Santana, 2003; Bukiya, Liu, & Dopico, 2009; Kreifeldt, Cates-Gatto, Roberts, & Contet, 2015; Kreifeldt, Le, Treistman, Koob, & Contet, 2013; Werner, Knorn, Meredith, Aldrich, & Nelson, 2007; Werner, Meredith, Aldrich, & Nelson, 2008) |
|
| BKβ2 | KCNMB2 | Increase | No significant decrease |
Fast and complete |
Slow |
Orio and Latorre (2005), Lee, Shi, and Cui (2010), Wallner et al. (1999), Xia et al. (1999, 2000), and Bentrop, Beyermann, Wissmann, and Fakler (2001) |
Pancreas, kidney, spleen, ovary, and brain (Brenner, Jegla, et al., 2000; Uebele et al., 2000; Wallner et al., 1999) |
The adrenal medullary chromaffin cells (CCs) function (Martinez-Espinosa, Yang, Gonzalez-Perez, Xia, & Lingle, 2014) |
|
|
| |||||||||
| BKβ3 | KCNMB3 | β3a | No effect | Increase | Incomplete, comparing to hβ3a, mβ3a: more rapid, more complete |
No effect |
Uebele et al. (2000), Brenner, Jegla, et al. (2000), Orio and Latorre (2005), Zeng, Benzinger, Xia, and Lingle (2007), and Zeng et al. (2008) |
Spleen, placenta, pancreas, heart, and kidney (Brenner, Jegla, et al., 2000; Uebele et al., 2000; Xia et al., 2000) |
Generalized epilepsy (Lorenz, Heils, Kasper, & Sander, 2007) |
|
|
|||||||||
| β3b | No effect | Decrease, while mβ3b: gating shift more to negative potentials at a given Ca2+ concentration |
Inactivation at a given Ca2+ concentration, hβ3b: rapid inactivation; mβ3b: no inactivation |
Slow |
Uebele et al. (2000), Xia et al. (2000), Zeng et al. (2008), and Lingle, Zeng, Ding, and Xia (2001) |
Spleen, pancreas, kidney, heart, testes, brain, placenta, lung, and liver (Brenner, Jegla, et al., 2000; Uebele et al., 2000; Xia et al., 2000) |
|||
|
|
|||||||||
| β3c | No effect | No effect | Incomplete | No effect |
Uebele et al. (2000)
and Brenner, Jegla, et al. (2000) |
Spleen, pancreas, liver, kidney, prostate, placenta, ovary, brain, and lung (Uebele et al., 2000; Xia et al., 2000) |
|||
|
|
|||||||||
| β3d | Not known |
Not known | Not known | Not known |
Uebele et al. (2000)
and Zeng et al. (2008) |
Spleen, pancreas, kidney, testes, Lung, and brain (Brenner, Jegla, et al., 2000; Uebele et al., 2000) |
|||
|
| |||||||||
| BKβ4 | KCNMB4 | Inhibit channel at low Ca2+; activate channel at high Ca2+ |
Decrease | No inactivation |
Slow |
Brenner, Jegla, et al. (2000), Meera et al. (2000), Jin, Weiger, and Levitan (2002), and Wang, Rothberg, and Brenner (2006) |
Brain, neuronal tissue, kidney, and bladder smooth muscle (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Chen & Petkov, 2009) |
Epileptic phenotype, alcohol dependence, and tolerance (Brenner et al., 2005; Kreifeldt et al., 2015, 2013; Martin et al., 2008) |
|
|
| |||||||||
| BKγ1 | LRRC26 | No significant effect |
Largest increase |
No inactivation |
Slow | Yan and Aldrich (2010, 2012) | Prostate, salivary glands, trachea, thyroid gland, thymus, cerebellum, brain (whole), aorta, mucosa, and fetal brain (Yan & Aldrich, 2012) |
Airway hydration, vasodilation, and cancer (Evanson, Bannister, Leo, & Jaggar, 2014; Liu et al., 2012; Manzanares et al., 2014) |
|
|
| |||||||||
| BKγ2 | LRRC52 | No significant decrease |
Increase | No inactivation |
No significant effect |
Yan and Aldrich (2012) | Testis, skeletal muscle, placenta, and sperm cells (Yan & Aldrich, 2012) |
Fertility deficit (Yang, Zeng, Zhou, Xia, & Lingle, 2011; Zeng, Yang, Xia, Liu, & Lingle, 2015) |
|
|
| |||||||||
| BKγ3 | LRRC55 | Decrease | Increase | No inactivation |
Slow | Yan and Aldrich (2012) | Fetal brain, brain (whole), mitral cell layers of olfactory bulb, liver, and spleen (Dolan et al., 2007; Yan & Aldrich, 2012) |
Not known | |
|
| |||||||||
| BKγ4 | LRRC38 | No effect | Increase | No inactivation |
No effect | Yan and Aldrich (2012) | Adrenal gland, skeletal muscle, thymus, cerebellum, brain (whole), testis, and spleen (Yan & Aldrich, 2012) |
Not known | |
2. DISCOVERY
Four different β subunits (β1–β4) have been cloned and identified in mammals. The first β subunit was identified as a binding partner of BKα in the BK channel complexes purified from bovine tracheal smooth muscle by extensive conventional chromatography together with sucrose gradient centrifugation or by immunoprecipitation (Garcia-Calvo et al., 1994; Knaus, Eberhart, Kaczorowski, & Garcia, 1994; Knaus, Folander, et al., 1994; Knaus, Garcia-Calvo, et al., 1994). In these early experiments, charybdotoxin (ChTX), which is a peptide blocker of BK channels, was radiolabeled as a tool for BK channel complex or protein detection, and ChTX was found to be attached to a β subunit upon cross-linking. This smooth muscle-specific auxiliary protein was later named the β1 subunit. With advancements in molecular biology, the other three family members were discovered thereafter by sequence similarity and molecular cloning (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Meera et al., 2000; Uebele et al., 2000; Wallner et al., 1999; Xia et al., 1999).
The first representative of the γ subunit family was identified relatively more recently. An unusual type of K+ current was initially noticed in lymph node carcinoma of prostate (LNCaP) cells, which showed a Kv-like low half-activation voltage (V1/2) of ~30 mV in the absence of intracellular calcium ([Ca2+]i) but had many characteristics of BK channels (Gessner et al., 2005). These BK-like features included large single-channel conductance, activation by Ca2+ and Mg2+, and sensitivity to specific BK channel activator and blockers (Gessner et al., 2005). The range of voltages needed for channel activation for this endogenous BK-like channel in LNCaP cells was shifted to the hyperpolarization direction by more than 120 mV compared with human BKα channels expressed in HEK-293 cells. Therefore, it was concluded that the LNCaP cell contains a special BK channel or a BK-like K+ channel which was designated as BKL (Gessner et al., 2005). Later, it was demonstrated by another research group that the LNCaP cells did express BKα at the protein level, as detected by the BKα antibody, and the BKα existed in a normal zero-splicing form (NCBI accession NP_ 002238), according to reverse transcriptase PCR and sequencing of mRNA (Yan & Aldrich, 2010). A proteomic approach was then used to immunopurify the channel complex, and mass spectrometry was used to identify potential novel interacting partners that may drastically modify the BK channel’s gating property (Yan & Aldrich, 2010). A 35-kDa leucine-rich repeat-containing protein, LRRC26, was specifically identified in the BKα pull-down components. Knockdown of this protein in LNCaP cells resulted in a complete loss of the BK channel’s property of being activated at low voltage in the absence of calcium. Meanwhile, overexpression of LRRC26 in another prostate cancer cell line, PC3, which lacks endogenous LRRC26 expression, converted the endogenous typical BKα channels into the low-voltage-activated LNCaP-type BK channels. In addition, it was shown in a heterologous expression system (HEK-293 cells) that LRRC26 was specifically associated with BKα, as detected by reciprocal coimmunoprecipitation, and shifted the conductance–voltage (G–V) relationship of BK channels to the hyperpolarization direction by ~140 mV, as was seen in the LNCaP cells. LRRC26 is structurally and functionally distinct from the four β subunits and thus was considered a new type of BK channel auxiliary subunit. Later, three other structurally related leucine-rich repeat-containing proteins, LRRC52, LRRC55, and LRRC38, were also reported as able to modulate BK channels when coexpressed heterologously with BKα in HEK-293 cells (Yan & Aldrich, 2012). These proteins have also been shown to produce marked shifts in the ranges of voltages needed for channel activation in the hyperpolarizing direction, although the shifts are smaller than those produced by LRRC26.
3. STRUCTURAL CHARACTERISTICS
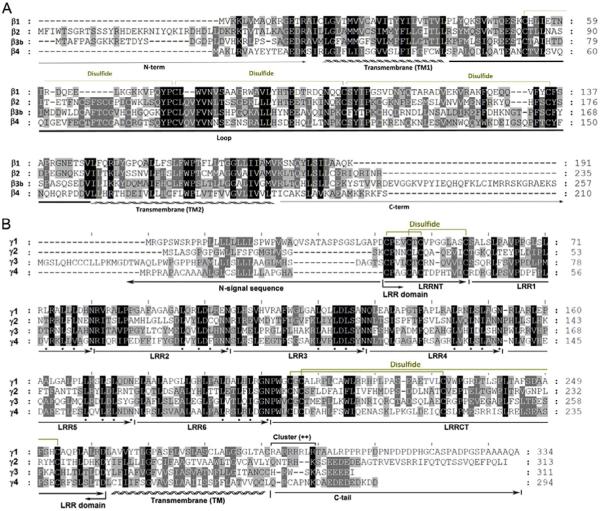
BK channel β subunits are a family of small double-pass membrane proteins (20–30 kDa) (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Uebele et al., 2000). Four types of BKβ genes (KCNMB1-4) have been cloned in mammals (Marty, 1981; Orio et al., 2002; Xia et al., 2003, 2000; Zeng et al., 2008). Among the four β subunits, β1 shares 53% similarity with β2 in amino acid sequence similarity, and 37% with β3, whereas less than 20% with β4 (Behrens et al., 2000; Wallner et al., 1999; Xia et al., 2000) (Fig. 2A). The four β subunits display similar topology containing short N- and C-termini both on the intracellular side, two TM helices, and a large extracellular loop (116–128 amino acid residues) connecting the two TM segments (Orio et al., 2002) (Figs. 1B and 2A). The loop has three or four putative glycosylation sites and multiple pairs of conserved disulfide-forming cysteine residues (Fig. 2A).
Fig. 2.
The protein sequence alignment of human BK channel β (A) and γ (B) subunits. The cysteine pairs for potential disulfide formation are based on previous publications (Brenner, Jegla, et al., 2000; Yan & Aldrich, 2012).
The four γ subunits have similar molecular weights of about 35 kDa. They are type I single-span membrane proteins containing a classic N-terminal cleavable signal peptide for extracellular localization of the N-terminal LRR domain in the mature proteins (Fig. 1C). The signal peptide region was found to be absent in the mature protein. Mutations in this region caused the signal peptide to be retained in the expressed protein and led to a loss of modulatory function in the γ1 subunit, suggesting that proper maturation guided by the signal peptide region is critical for the function of γ subunits (Yan & Aldrich, 2012). The mature proteins of the four γ subunits all contain a single-transmembrane domain, an N-terminal extracellular LRRD (rv240 amino acids), and a short C-terminal tail (Fig. 1C). The four γ subunits share an overall sequence similarity of 35–40%, which is comparable with that of the four β subunits (Fig. 2B).
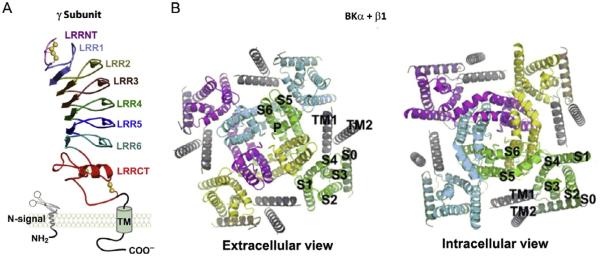
The LRR domain in many proteins is known to provide a structural framework for protein–protein interactions, typically through the concave β-sheet side (Kobe & Kajava, 2001). The LRR domains of γ subunits all contain six LRR units and two cysteine-rich regions, a small one called LRRNT, capped on the N-terminal side, and a large one called LRRCT, capped on the C-terminal side. As in many other LRR-containing proteins, each LRR unit in the γ subunits consists of 24 residues and has a classic consensus sequence of LxxLxLxxN (where x can be any amino acid). Based on structural modeling (Yan & Aldrich, 2012), the LRR domain is a banana-shaped structure with a curved parallel β-sheet lining the inner circumference and small helices or turns flanking the convex circumference, formed by six LRR units stacked together in the middle (Fig. 3A). Each LRR unit forms a β-strand lining the concave face and a short α-helix connected by loops flanking the outer circumference. The hydrophobic core of the LRR domain is tightly packed by the parallel inward-pointing leucine residues, shielded by the LRRCT and LRRNT caps on the N- and C-terminal ends. Both LRRNT and LRRCT contain two pairs of fully conserved cysteine residues that in total potentially form four disulfide linkages in the favorable oxidizing extracellular environment. Consistent with their predicted extracellular location, the LRR domains of the γ subunits all contain single or multiple consensus N-glycosylation sites: Asn-Xaa-Ser/Thr, where Xaa is not a proline. For the γ1 subunit, N147Q mutation and enzymatic removal of the N-linked glycan by PNGase F resulted in the disappearance of an upper glycosylated-mass band on SDS-PAGE (Yan & Aldrich, 2012).
Fig. 3.
Structural models. (A) Predicted leucine-rich repeat domain structure and membrane topology of the γ subunit (Yan & Aldrich, 2012). (B) Docking model of BKβ1 transmembrane segments (TMs) located in BKα subunit (Liu, Zakharov, Yao, Marx, & Karlin, 2015). For panel B, each BKα subunit is shown in a different color (gray shades in the print version). The β1 TM1 and TM2 are in black.
The protein sequences in the LRR domains of γ subunits are closely related but become divergent in the transmembrane and intracellular C-terminal tail regions (Fig. 2B). The single-transmembrane segments of the γ subunits are well predicted from their hydrophobicity and the presence of charged residues on both sides, particularly multiple positively charged residues on the intracellular side following the general “positive-inside rule” for membrane insertion and orientation of membrane proteins. For the C-terminal tail regions, in addition to the cluster of positively charged residues adjacent to the transmembrane domain, it is interesting to note that the rest of the amino acid sequence is enriched in proline residues (11 out of 36 residues) for γ1 and enriched in acidic residues for γ2, γ3, and γ4.
In the absence of direct structural information, a group led by Marx and Karlin have used a biochemical method to engineer disulfide linkage to approximately localize the various structural parts of the β subunits relative to the different BKα TM helices within the BKα/β complex. It was found that the first TM helix (TM1) of β1 was localized near the S1 and S2 helix of α subunits, and the second TM helix (TM2) near the S0 helix. This places the extracellular ends of β1 TM helices within the crevice formed by VSDs of two adjacent α subunits (Liu et al., 2010, 2008). Later, similar interacting patterns between β2, β3, or β4 subunits with BKα subunits were also reported (Wu et al., 2009, 2013). Recently, the cytoplasmic ends of β1 TM1 and TM2 were found to be adjacent and located between the S2– S3 loop of one α subunit and S1 of a neighboring α subunit, but not adjacent to S0, suggesting that the interaction between BKα and the β1 subunit is more complex than previously thought (Fig. 3B) (Liu et al., 2015). As discussed later, it is believed that the extracellular loop of the β subunit is in proximity to the extracellular mouth of the BKα pore gate because of its effect on the ion flow through the channel’s extracellular mouth (Fig. 1B) (Gruslova, Semenov, & Wang, 2012; Zeng et al., 2003). Currently, very little is known about the exact protein regions and residues in BKα that directly interact with β subunits to mediate channel modulation. There is no report yet on the structural location of the γ subunit in the BKα/γ complex. The structural information obtained from the biochemical studies on the location of the β subunits in the BKα/β channel complex might provide an initial biochemical basis from which to decode the molecular mechanisms of BK channel modulation by auxiliary subunits.
The BKα subunit is universally expressed across different species of animals including invertebrates Drosophila melanogaster and Caenorhabditis elegans. The BK channel β subunits are present in both mammal and non-mammal animals such as chicken and turtle. The γ subunits (LRRC26 and its three paralogs) belong to a previously grouped “Elron” subfamily of the “extracellular LRR”-containing proteins that are mainly present in mammals (Dolan et al., 2007), suggesting that in evolution, the acquisition of BK channel proteins occurred in the order of BKα, β, and γ subunits. Although the β and γ subunits belong to distinct protein families with no amino acid sequence similarity, they all share some common structural features. First, they are all single or double membrane-spanning proteins with a large extracellular domain and a relatively much smaller intracellular region. Our previous study showed that the TM domain in γ1, as in the β subunits, is essential for the domain’s modulatory function and physical association with BKα (Yan & Aldrich, 2010). Second, they all contain multiple pairs of disulfide-bond-forming cysteine residues on their extracellular sides. Third, as plasma membrane proteins with an extracellular soluble domain, they are all subject to modification by N-glycosylation. Both glycosylation and extra-cellular disulfide linkages in the β subunits were reported to directly affect the BK channels’ voltage dependence (Hagen & Sanders, 2006) or ion flow through the channel’s extracellular mouth (Zeng et al., 2003), respectively.
4. MODULATION OF THE BK CHANNEL'S BIOPHYSICAL PROPERTIES BY β SUBUNITS
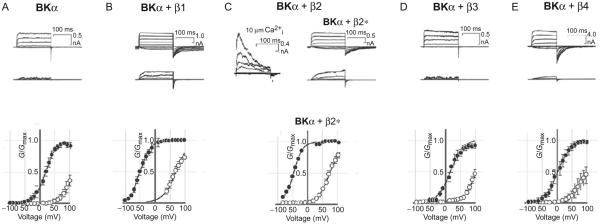
The four β subunits display different and complex effects on apparent calcium and voltage sensitivities, macroscopic current kinetics, and pharmacological sensitivities, which involve multiple distinct mechanisms (Fig. 4). The β1 and β2 subunits overall induce slowing of the macroscopic kinetics and an increase in apparent calcium and voltage sensitivity (Behrens et al., 2000; Brenner, Jegla, et al., 2000; Contreras, Neely, Alvarez, Gonzalez, & Latorre, 2012; Orio & Latorre, 2005; Savalli, Kondratiev, de Quintana, Toro, & Olcese, 2007). The β2 and some splice variants of β3 subunits also cause rapid inactivation through their intracellular N-termini (Uebele et al., 2000; Xia et al., 1999, 2000). In contrast with the classic shaker Kv inactivation peptide, the intrinsically disordered N-terminal peptide of β3 subunit inactivated BK channels in two steps, which involves a stereospecific binding interaction that precedes blockade (Gonzalez-Perez, Zeng, Henzler-Wildman, & Lingle, 2012; Lingle et al., 2001; Xia et al., 2000). The β3 subunits generate rectifying outward currents regulated by their extracellular loops (Xia et al., 2000; Zeng et al., 2003). The brain-specific β4 subunit, in addition to greatly slowing activation and deactivation kinetics, reduces apparent calcium sensitivity in low Ca2+ i conditions but increases apparent sensitivity in high [Ca2+]i conditions (Behrens et al., 2000; Brenner, Jegla, et al., 2000). The β1, β2, and β4 subunits were also found to modulate BK channels by altering BKα expression and trafficking. It was reported that when coexpressed in HEK-293 cells, β1 could reduce the steady-state BKα surface expression and alter the diffused intracellular expression of BKα to a pattern of punctate cytoplasmic localization that overlapped with the β1 expression (Toro et al., 2006). A similar effect on BK channel expression was also observed with the β2 subunit (Lv et al., 2008; Zarei et al., 2007). Expression of β4 subunit was also found to reduce surface expression of BK channels in mouse CA3 neurons which was mediated by a C-terminal ER retention sequence (Shruti et al., 2012). In contrast, the N-terminal domain of β1 was reported to stimulate trafficking of VEDEC (a BKα C-terminal isoform) channels to the plasma membrane (Kim, Zou, Ridgway, & Dryer, 2007). A recent study showed that the palmitoylated β4 subunit regulated surface expression of BK channels through masking a trafficking motif (REVEDEC) at the C-terminus of the BKα subunit (Chen et al., 2013).
Fig. 4.
The voltage- and Ca2+-dependent activities of human BKα alone (A) or together with β1 (B), β2 (C), β3 (D), or β4 (E). The current traces were recorded in an inside-out patch from HEK-293 cells at 10 μM (upper) or1 μM [Ca2+]i (lower). The normalized conductance–voltage relations (G–V) for the activation of BK channels were plotted at 10 μM [Ca2+]i (•) and 1 μM [Ca2+]i (o). *Inactivation was removed by exposing the patch to 1 mg/mL trypsin for 60 s. Modified from Pongs, O., & Schwarz, J. R. (2010). Ancillary subunits associated with voltage-dependent K+ channels. Physiological Reviews, 90, 755–796.
With mathematical modeling and simulation, the mechanisms of action of different β subunits were investigated by multiple laboratories within the framework of the well-established BK channel allosteric HCA (Horrigan et al., 1999) or HA (Horrigan & Aldrich, 2002) models of voltage- and Ca2+-dependent gating. According to the HA model (Fig. 5) (Horrigan & Aldrich, 2002), the activation or open probability (Po) of BK channels is independently affected by voltage and Ca2+, which can be calculated or described by eight gating parameters: L0 and ZL were referred to as equilibrium constant and associated gating charge for the channel pore’s closed $ open transition; J0 and ZJ were referred to as equilibrium constant and associated gating charge for the voltage sensors’ resting $ activated transition; D was considered the allosteric coupling factors between the pore gate and the voltage sensors; C was designated as the allosteric coupling factor between the pore gate and the calcium sensors, and Kd as the elementary Ca2+ dissociation constant when the channel is closed and voltage sensors are not activated; E was referred to as the allosteric coupling factor between the voltage and the calcium sensors, which was considered to be very weak in the case of the BK channel (Horrigan & Aldrich, 2002). The β1 subunit was found to affect multiple gating processes including the voltage-sensor activation (J0), the intrinsic opening of pore gate (L0), and the coupling between voltage sensors and pore gate (D) but with only minor effect on the Ca2+ sensor and its coupling the pore gate (C) (Bao & Cox, 2005; Cox & Aldrich, 2000; Orio & Latorre, 2005; Wang & Brenner, 2006). The apparent increase in sensitivity to Ca2+ is largely caused by modulation on other gating parameters (Ma et al., 2006; Orio & Latorre, 2005; Wang & Brenner, 2006; Yang et al., 2008). The β4 subunit appears to be essentially similar to the β1 subunit in reducing the intrinsic pore gate opening and enhancing voltage-sensor activation, which ultimately results in a shift of the G–V relationship to the depolarizing direction at low Ca2+ but to the hyperpolarizing direction at high Ca2+ (:::rv10 μM) (Wang et al., 2006). By measuring gating currents, the β1, β2, and β4 but not β3 subunits were found to stabilize the BK voltage sensor in the active conformation (Contreras et al., 2012).
Fig. 5.
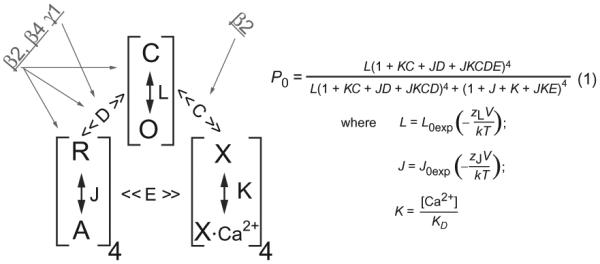
The allosteric HA model of BK channels and the possible effects of the β and γ subunits. The three processes of channel opening (C-O), voltage-sensor activation (R-A), and Ca2+-binding transition (X-XCa2+) are linked, respectively, by the allosteric coupling factors C, D, and E. The possible gating processes or parameters affected by the β and γ subunits are indicated by gray arrows, which are based on previous reports (Contreras et al., 2012; Ma, Lou, & Horrigan, 2006; Orio & Latorre, 2005; Wang & Brenner, 2006; Yan & Aldrich, 2012; Yang et al., 2008). The HA model was taken from Horrigan, F. T., & Aldrich, R. W. (2002). Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. The Journal of General Physiology, 120, 267–305.
Over the past two decades, progress has been made in structurally and functionally identifying the potential protein regions and residues involved in BK channel modulation by β subunits. Studies of chimeric and mutant β subunits indicated that the cytosolic N- and C-termini of the BKβ1 subunit were crucial to altering the channel’s intrinsic opening and voltage-sensor activation (Orio et al., 2006; Wang et al., 2006). It was recently found that substituting two lysine residues (ie, K3 and K4) in the N-terminus of β1 can virtually abolish the effects of β1 on voltage-sensor activation (Castillo et al., 2015). The N-termini of the β2, β3a, and β3c subunits contain an inactivating domain which blocks the entrance to the intracellular pore and inactivates the BK channels (Li et al., 2007; Wallner et al., 1999; Xia et al., 1999, 2003; Zeng et al., 2003; Zhang, Zeng, Xia, & Lingle, 2009; Zhang, Zhou, Ding, Xia, & Lingle, 2006). Different from the simple one-step occlusion mechanism for Kv channel blockade by N-terminal peptides which may mainly involve hydrophobic interactions with the channel pore, the BK channel inactivation by auxiliary β subunits involves two distinguishable kinetic steps (Lingle et al., 2001), a stereospecific binding interaction that precedes blockade (Gonzalez-Perez et al., 2012). Although the sites of stereospecific interactions between the β subunit N-terminus and BK channel pore have not been identified, it was proposed that the relatively larger inner pore of BK channels than Kv channels might require some stereospecific binding to achieve the affinity necessary for BK channel inhibition (Gonzalez-Perez et al., 2012). The extracellular loop and the disulfide bridges within the loop were found to be important in β3 subunit-induced current rectification (Xia et al., 2000; Zeng et al., 2003). The lysine-rich ring (K137, K141, K147, and K150) on the extracellular loop of β2 also was involved in causing the outward rectification (Chen et al., 2008). These results suggest close proximity between the extracellular loop of the β subunits and the extracellular mouth of the channel pore. Several residues (Y74, S104, Y105, and I106) on the extracellular loop of β1, which are also conserved in other β subunits, were also found to be important in the β1 subunit’s modulatory effect on voltage-dependence activation of BK channel (Gruslova et al., 2012). It remains unclear whether the β subunit’s extracellular loop can directly interact and modulate the BK channel or indirectly affect the channel properties by affecting the overall structure of the β subunit. Compared with the other regions, the function of the two TM segments in the β subunits has been relatively less studied. Their likely interposed location between VSDs of adjacent BKα subunits suggests a role in modulating voltage-sensor activation. The two TM segments in β1 subunit were functionally replaceable by those from the β2 subunit (Orio et al., 2006) but not from the β4 subunit even replaced individually (Kuntamallappanavar, Toro, & Dopico, 2014). The latter showed that both TM segments are required to maintain the characteristic modulatory function of the β1 subunit (Kuntamallappanavar et al., 2014).
On the BKα subunit, by using chimeric BKα channels from different species, it was found that the unique S0 TM segment, as well as the extracellular N-terminus of BKα, was important for BK channel modulation by β1 and β2 subunits (Lee et al., 2010; Morrow et al., 2006; Wallner, Meera, & Toro, 1996). The β1 and β2 subunits seem to be different in regulating the various functional domains of the BKα, although both affect the apparent voltage and Ca2+ sensitivity. Mutations on BKα that affected voltage-sensor activation altered the apparent changes in Ca2+ sensitivity induced by the β1 subunit (Yang et al., 2008). In contrast, β2 mainly affected Ca2+ sensitivity, presumably by altering the allosteric coupling between the Ca2+ sensors and the pore gate. Mutations in the BKα voltage sensor were found to have little effect on β2’s modulatory effect on Ca2+ sensitivity (Yang et al., 2008). Instead, mutations on the N-terminus of RCK1 and on the linker region between S6 TM segment and RCK1 had a large impact on β2’s but not β1’s modulation on the BK channel’s Ca2+ sensitivity (Lee et al., 2010). However, experiments with voltage-clamp fluorometry correlated the β2 subunit with changes in voltage sensors (Savalli et al., 2007). The β1 and β2 subunits were also reported to alter the interactions between bound Mg2+ and R213 in the voltage sensor and to disrupt the engineered disulfide-bond formation between the voltage sensor and C-terminal intracellular domain (Sun et al., 2013).
5. MODULATION OF THE BK CHANNEL'S BIOPHYSICAL PROPERTIES BY γ SUBUNITS
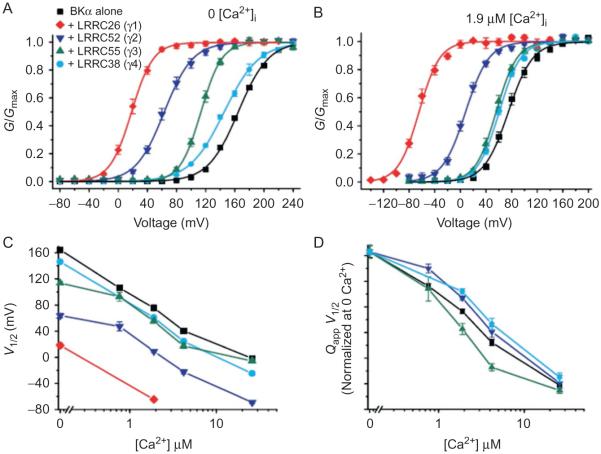
Unlike the complex effects and mechanisms of different β subunits on many aspects of BK channel gating, the actions of the γ subunits appear to be remarkable in mechanistic simplicity and modulatory magnitudes (Yan & Aldrich, 2010; Zhang & Yan, 2014). The four γ subunits have distinct capabilities in shifting the voltage dependence of BK channel activation toward hyperpolarizing voltages by approximately 140 mV (γ1), 100 mV (γ2), 50 mV (γ3), and 20 mV (γ4) in the absence of Ca2+ (Fig. 6) (Yan & Aldrich, 2010, 2012). The gating shift produced by the γ1 subunit is equivalent to the effect of ~10 μM [Ca2+]i on BK channels formed by BKα alone. However, Ca2+ and Mg2+ sensitivities were shown to be largely unaffected by the γ1 subunit (Yan & Aldrich, 2010). In the presence of Ca2+, the γ1–3 subunits all caused slight changes in the slope of the relationship between QappV1/2 (Qapp, apparent gating charge obtained from a Boltzmann fit of the G–V curve for voltage dependence of channel activation) and log [Ca2+]i with some Ca2+-dependent reduction in their V1/2 shifting capabilities, suggesting some small but noticeable effects on the apparent calcium sensitivity of BK channel activation (Yan & Aldrich, 2010). It is unclear whether the observed Ca2+ effects on the function of γ subunits were mediated by the Ca2+ effects on the BKα or on the γ subunits.
Fig. 6.
Modulatory effects of the γ subunits on BK channels heterologously expressed in HEK-293 cells. (A and B) Voltage dependence of the BK channel activation for the BKα alone or together with γ1, γ2, γ3, or γ4 in the virtual absence of [Ca2+]i (A) or in the presence of 1.9 μM [Ca2+]i (B). (C) Plot of V1/2 vs [Ca2+]i (log scale after break). (D) Plot of QappV1/2 vs [Ca2+]i normalized at 0 [Ca2+]i. This figure is taken from Yan, J., & Aldrich, R. W. (2012). BK potassium channel modulation by leucine-rich repeat-containing proteins. Proceedings of the National Academy of Sciences of the United States of America, 109, 7917–7922.
The mechanistic actions of the γ1 subunit were investigated and analyzed within the framework of the HA model in the absence of Ca2+. By measuring the kinetics and open probabilities of the channels at very negative voltages to achieve good estimates of the ZL and L0 parameters, it was found that the pore’s gating parameters L0 and ZL are largely unaffected by the γ1 subunit (Yan & Aldrich, 2010). The lack of effect on L0 also suggests that the γ1 subunit is not a Ca2+-like ligand that directly activates BK channels independently of voltage-sensor activation. By simulation with changes in other gating parameters, the γ1 subunit’s modulatory effect was found to be best explained by an approximate 20-fold increase in the allosteric coupling D factor (Yan & Aldrich, 2010) (Fig. 5). According to this study, the γ1 subunit may mainly affect the coupling between voltage sensors and the pore. The γ subunits may serve as good tools to study BK channel gating mechanisms because little is currently known about the molecular basis underlying allosteric coupling between voltage sensors and the pore in this voltage- and ligand-gated channel. This earlier analysis, which was performed in the absence of Ca2+ and assumed that only one gating parameter was affected in simulation, was not comprehensive enough to allow complete assessment of some other gating parameters, such as some slight changes in the voltage- sensor parameters, J0 and ZJ. Additionally, a later study observed some effects of the intracellular Ca2+ on the modulatory function of the γ subunits (Yan & Aldrich, 2012).
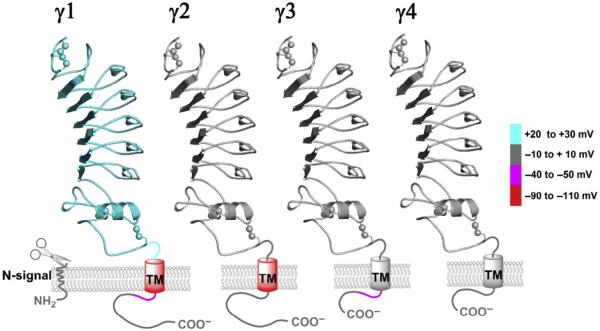
To understand the molecular mechanisms of BK channel regulation by auxiliary γ subunits, it is critical to identify key structural elements underlying their channel modulatory functions. By swapping structural elements among γ subunits and by mutations, we recently found that the differences in the various γ subunit-induced shifts of the BK channel V1/2 are primarily determined by their single TM segments during the approximate -100-mV shift in V1/2, in which the γ1 and γ2 TMs produced low V1/2 BK channels, while the γ3 and γ4 TM domains all resulted in high V1/2 channels (Fig. 7) (Li, Fan, Kwak, & Yan, 2015). We also found that their intracellular C-tails, particularly the juxta-membrane positively charged cluster regions that contain multiple positively charged amino acids, further adjust the modulatory functions of the four γ subunits by conferring to the BK channels an additional approximate -40 to -50 mV shift in V1/2 from the γ1 and γ3 C-tails (Li et al., 2015) (Fig. 7). In a more recent study, we investigated in detail the structure and function in BK channel modulation by the γ1 subunit’s peptide region (rv40 amino acids) encompassing the single TM segment and the adjacent poly-Arg cluster (Li, Guan, Yen, Zhang, & Yan, 2016). We demonstrated that this peptide region, independent of the N-terminal LRR domain and the rest of the C-terminal tail, was sufficient to fully modulate BK channels. We found that Phe273 and its neighboring residues in the middle of the TM segment play a key role in BK channel association and modulation and that a minimum of three Arg residues in the charged cluster are required for the γ1 subunit’s modulatory function. Allosteric coupling between the TM segment and the intracellular positively charged cluster was also observed. We concluded that the TM segment is a key molecular determinant for channel association and modulation, and the intracellular positively charged cluster is involved mainly in channel association likely through its TM anchoring effect (Li et al., 2016). These new findings provide insights into the structure–function relationship of the γ subunits for understanding their potent modulatory effects on BK channels.
Fig. 7.
Schematic of the structure, membrane topology, and generalized relative functional contributions of individual structural elements in the BK channel γ subunits. The different colors (gray shades in the print version) indicate the structural elements’ relative contributions (in millivolts, mV) to the γ subunit-induced shifts of voltage dependence of BK channel activation. This figure is taken from Li, Q., Fan, F., Kwak, H. R., & Yan, J. (2015). Molecular basis for differential modulation of BK channel voltage-dependent gating by auxiliary gamma subunits. The Journal of General Physiology, 145, 543–554.
6. MODULATION OF THE BK CHANNEL'S PHARMACOLOGICAL PROPERTIES BY β AND γ SUBUNITS
As a result of decades of exploration, numerous endogenous and synthetic BK channel modulators have been identified and developed, some of which show BK auxiliary subunit-dependent characteristics. Generally, the β subunits confer alteration in sensitivity of the BK channel to peptide toxins, in which the extracellular loop of β subunits plays a critical role (Chen et al., 2008; Hanner et al., 1998; Meera et al., 2000; Xia et al., 1999). The four residues (Leu90, Tyr91, Thr93, and Glu94) of BKβ1 were found to be crucial for the enhanced affinity of ChTX, and the lysine-rich ring (K137, K141, K147, and K150) of subunit β2 could reduce the ChTX sensitivity to BK channels. In addition, the β2 and β3 subunit were reported to reduce the degrees of blockade by ChTX (Xia et al., 1999, 2000; Zeng et al., 2008), together with a change in the magnitude and kinetics of the blocking reaction (Ding et al., 1998). However, the β4-complexed BK channel is extremely resistant to both iberiotoxin (IbTX) and ChTX (Bergeron & Bingham, 2012; Meera et al., 2000; Schneider, Rogowski, Krueger, & Blaustein, 1989). This kind of resistance is related with the so-called helmet structure constituted by BKα and three residues in extracellular loop (K120, R121, and K125) of the β4 subunit, which can block the entry of ChTX by both electrostatic interaction and the limited space of the pore area (Gan et al., 2008). Moreover, compared with BKα alone, the BKα/β1 channel, but not the BKα/β4 channel, is more sensitive to the blockade by slotoxin (αKTx1.11). But the binding between slotoxin and the BKα/β4 channel seemed to be irreversible in an unknown mechanism (Garcia-Valdes, Zamudio, Toro, & Possani, 2001). In contrast, martentoxin (Shi et al., 2008; Tao, Shi, Liu, & Ji, 2012) and conopeptide Vt3.1 (Li et al., 2014) were identified as the more selective blockers for the BKα/β4 channel, but not BKα/β1 channel, when compared with BKα channel alone. The β subunits were also reported to regulate BK channel modulation by ethanol. In heterologous expression system (HEK-293 cells), the β1 subunit caused blockade of ethanol-induced potentiation of the BK channels (Feinberg-Zadek & Treistman, 2007; Martin et al., 2004). In the supraoptic nucleus neurons, the β4-complexed axonal terminal BK channels were highly potentiated by ethanol, but the β1-complexed somatic and dendritic channels were insensitive to ethanol (Dopico, Widmer, Wang, Lemos, & Treistman, 1999; Wynne, Puig, Martin, & Treistman, 2009). Similarly, the difference in sensitivity of BK channels to ethanol in the dendrites and cell bodies of nucleus accumbens medium spiny neurons can be explained by the differential expression of the β1 subunit in these two compartments (Martin et al., 2004). In contrast to the nervous system, ethanol caused inhibition of BK currents in myocytes that led to cerebrovascular constriction, in which the β1 subunit played a major role (Bukiya, Liu, et al., 2009). This group recently identified K361 on BKα as a putative binding site for ethanol (Bukiya, Kuntamallappanavar, et al., 2014). Further studies will be needed to clarify the different effects of different β subunits on the BK channel’s responses to ethanol. A detailed review of BK channel modulation by ethanol can be found in Chapter “Modulation of BK Channels by Ethanol” by Dopico.
The earliest reported activator, dehydrosoyasaponin-I (DHS-I, the potent compounds extracted from Desmodium adscendens) activates BK channels only when coexpressed with the β1 subunit in Xenopus oocytes (McManus et al., 1993, 1995), and such β1-dependent activation was also observed in smooth muscle membranes (Bukiya, Patil, Li, Miller, & Dopico, 2012). The xenoestrogen, tamoxifen, and the endogenous steroid hormone 17β-estradiol were also found to activate the BK channel in a β1- dependent manner (De Wet et al., 2006; Dick, Rossow, Smirnov, Horowitz, & Sanders, 2001; Duncan, 2005; Valverde et al., 1999). Likewise, the β4-complexed BK channel was more sensitive to corticosterone for channel function potentiation by steroids, whereas the β2-complexed channel was more sensitive to dehydroepiandrosterone (King et al., 2006). The endogenous lithocholate was reported to selectively recognize the steroid- sensing site (T169, L172, and L173) in TM2 of the β1 subunit to activate BK channels (Bukiya, McMillan, Parrill, & Dopico, 2008; Bukiya, Singh, Parrill, & Dopico, 2011; Bukiya, Vaithianathan, Toro, & Dopico, 2009). T169 in TM2 of the β1 subunit was also involved in the activation of BKα/β1 channels by nonsteroidal lithocholic acid (Bukiya et al., 2013). Current research has shown that lipid agents can act through β subunits, although the underlying mechanisms are unclear. Omega-3 docosahexaenoic acid could increase BK channel activity in a β1 and β4 subunit- dependent manner, while not in a β2 or γ1 subunit-dependent manner (Hoshi et al., 2013). In addition, phosphatidylinositol 4,5-biphosphate (PIP2) was reported to inhibit macroscopic currents of BKα and BKα/γ1 channels but increase macroscopic currents of BKα/β1 and BKα/β4 channels without altering those of BKα/β2 channels (Tian et al., 2015). Endogenous leukotrienes (LTB4) can potently activate BK channels when coexpressed with the β1 subunit in X. oocytes, which makes LTB4 a good template for the future development of β1-specific BK channel activators (Bukiya, McMillan, et al., 2014). A recent study reported that bis-(1,3-dibutylbarbituricacid)trimethine oxonol [DiBAC4(3)] and N-arylbenzamide can activate the BK channel in β1 subunit dependence, but DiBAC4(3) partially blocked the BKα/β2 channel’s currents (Kirby, Martelli, Calderone, McKay, & Lawson, 2013; Morimoto et al., 2007). Additionally, HBD2 (human β-defensin 2) could activate BK channels via interactions with Leu41 and Gln43 of the β1 extracellular loop (Liu et al., 2013). A recent report showed that the newly identified BK channel opener GoSlo-SR-5-130, but not its analogue GoSlo-SR-5-6, required the presence of β1 or β4 subunit to achieve its the maximal modulatory effects on BK channels (Large et al., 2015).
Little has been done to investigate the interaction between the newly identified auxiliary γ subunits and the BK channel’s modulators. Nevertheless, the BK channel in LNCaP cells was found to be still sensitive to NS1619, ChTX, IbTX, paxilline, and penitrem A (Gessner et al., 2005), suggesting that most known pharmacological properties of the BK channels are likely retained in the presence of the endogenous γ1 subunit. Interestingly, the γ1 subunit can inhibit the effect of some BK activator (Almassy & Begenisich, 2012). In native salivary gland parotid acinar cells, the endogenous γ1 subunit blocked the activating effect of mallotoxin but not NS1619 (Almassy & Begenisich, 2012). A similar blocking effect of the γ1 subunit on mallotoxin action was also observed in HEK-293 cells when the γ1 subunit was heterologously coexpressed with BKα. It was proposed that mallotoxin may displace the γ1 subunit instead of lacking accessibility to the binding site (Almassy & Begenisich, 2012). Further biophysical studies and biochemical binding assays will be needed to clarify the detailed mechanisms. NS1619 was recently shown to bind to the S6/RCK linker region (Gessner et al., 2012), but little is known about the mallotoxin binding site. Identification of the mallotoxin binding site may complement our understanding of the actions of γ subunits on BK channels.
7. STOICHIOMETRY
When the β1 subunit was first identified from bovine tracheal and aortic smooth muscle with immunoprecipitation, the isolated BK channel complex was found to contain an octameric assembly of BKα and BKβ1 subunits in 1:1 stoichiometry (Knaus, Eberhart, et al., 1994; Knaus, Garcia-Calvo, et al., 1994). In a classic model of tetrameric ion channel complex, auxiliary subunits bind to the pore-forming α subunit with a fourfold symmetry so that the regulatory effect is incremental upon variation in the relative molecular ratio of the auxiliary subunit to core subunit. Consistently, the β subunits regulated the voltage dependence of BK channel activation in the titration-dependent manner in that the G–V curves shifted in a parallel manner as a function of the injected ratio of β to α subunit mRNA in X. oocytes (Wang, Ding, Xia, & Lingle, 2002). Variation in stoichiometry of BKα and β subunits likely contributed to the heterogeneity in BK channel activity in rat chromaffin cells (Ding et al., 1998; Xia et al., 1999) and turtle auditory hair cells (Jones et al., 1999). As the newly identified BK channel auxiliary subunits, no biochemical or stoichiometric study has been reported on the assembly of BKα and γ subunits.
A recent study indicated that the regulatory mechanism of the γ1 subunit may be fundamentally different from that of the β subunit (Gonzalez-Perez, Xia, & Lingle, 2014). In contrast to β subunits (Gonzalez-Perez et al., 2014), the γ1 subunit caused the voltage dependence of channel activation to be either fully shifted or unchanged, independent of the molar ratio of the injected BKα:γ1 RNA to X. oocytes, although the ratio of these two populations of channels varied (Gonzalez-Perez et al., 2014). It is unknown whether one γ1 subunit per channel complex is sufficient to fully modulate BK channels. Alternatively, the γ1 subunit may preferably exist in a tetrameric form when forming a complex with BKα. Additional studies will be required to determine the detailed mechanisms, particularly the stoichiometry and the interaction sites between the BKα and γ subunits in the tetrameric channel complex.
8. PHYSIOLOGICAL AND PATHOLOGICAL RELEVANCE OR ROLES
The BK channel β and γ auxiliary subunits have been reported to be involved in tissue-specific functions in health or disease (Table 1). An early study of epigenetic epidemiology showed that the individuals carrying E65K polymorphism in β1 subunit experienced low prevalence of diastolic hypertension and cardiovascular disease compared to those with normal β1 subunit (Fernandez-Fernandez et al., 2004). The mRNA level of β1 subunit was markedly decreased in the vascular smooth muscle of patients with acquired hypertension (Amberg et al., 2003). Studies with knockout mice showed that the presence of the β1 subunit was critical to normal BK channel function in vascular smooth muscle cells (Werner et al., 2007, 2008). One recent report showed that β1 deficiency exacerbated vascular fibrosis and remodeling (Xu et al., 2015). The knockout mice of β4 subunit showed signs of an epileptic phenotype (Brenner et al., 2005), and the β2 knockout mice had an increased tendency toward spontaneous burst firing in the adrenal medullary chromaffin cells (Martinez-Espinosa et al., 2014). A β3 (delA750) mutant, which led to truncation of the C-terminus, was reported to be associated with a form of generalized epilepsy (Lorenz et al., 2007). The results obtained from the β subunit KO mice so far have used method of global ablation of the β subunit in the whole animal body which could complicate the results and interpretations because of functional communications between different tissues and organs. Studies with tissue- or cell- specific KO mice will be needed to further clarify the physiological functions of different β subunits in different cells or tissues.
The tissue-specific distribution patterns of the four γ subunits at the mRNA level had been investigated with use of TaqMan quantitative PCR in various human tissues (Yan & Aldrich, 2012). The γ1 subunit was highly expressed in the salivary glands, prostate, and trachea, whereas γ2 (LRRC52) was found predominantly in the testes, and γ3 (LRRC55) was found primarily in the nervous system. The γ4 (LRRC38) subunit was observed mainly in skeletal muscle, adrenal glands, and the thymus. These results suggest that like β subunits, γ subunits have different tissue- specific distributions to fit the diverse functional requirements of various tissues and cell types (Yan & Aldrich, 2012). The γ1 subunit’s endogenous functional regulation of BK channels has been confirmed in prostate and salivary gland cells (Almassy & Begenisich, 2012; Yan & Aldrich, 2010). The physiological roles of the γ1 subunit in prostate and salivary glands remain to be determined. Conceivably, constitutive activation of BK channels might be required for K+ flow-mediated fluid secretion in these nonexcitable tissues. A very recent study suggested that the γ1 subunit in airway epithelial cells may participate in BK channel-mediated airway hydration for effective mucociliary clearance (Manzanares et al., 2014). The K+ flow through the apically expressed BK channels in airway epithelial cells provides an electro- chemical driving gradient for Cl− secretion and thus plays a role in airway hydration. It was found that both the mRNA level of the γ1 subunit and the sensitivity of BK channels to mallotoxin were decreased after IFN-γ treatment, suggesting that the γ1 subunit might be involved in IFN-γ-mediated reduction in BK channel activity and the resulting mucociliary dysfunction (Manzanares et al., 2014). The γ1 subunit under a different name (CAPC) was reported to be able to suppress tumor growth and metastasis, which may likely involve ion channel-independent function (Liu et al., 2012). The enhanced K+ channel activity generally promoted cancer cell proliferation (Pardo & Stuhmer, 2014). It will be necessary to determine whether the association of the γ1 subunit with BK channels will affect tumor growth because of change in BK channel activity.
Because of the drastic activating effect caused by the γ1 subunit, expression of this protein even at low levels might exert significant effect on BK channel currents. For example, a low level of mRNA expression of the γ1 subunit has been detected in aorta cells (Yan & Aldrich, 2012). A very recent study reported that knockdown of γ1 subunit expression in rat cerebral artery myocytes led to reductions in the apparent voltage/Ca2+ sensitivity, in current frequency and amplitude of the BK channels, and in the extents of BK channel-specific inhibitor-induced vasoconstriction and activator-induced vasodilation (Evanson et al., 2014). This study suggested that the γ1 subunit may play broad physiological roles that are not limited to nonexcitable cells. In excitable cells, the voltage and Ca2+ sensitivities of the BK channels are more finely tuned to be properly responsive to different levels of voltage and Ca2+ in different cell types; therefore, even a low expression of this potent BK channel modulator might exert a significant physiological effect. It is worth noting that the γ1 subunit is also expressed in fetal brain tissue (Yan & Aldrich, 2012) and that the γ1 subunit might participate in maintaining proper neuronal excitability in the fetal nervous system during early development. The recent finding of independent association and function of the β (β2) and γ (γ1) subunits in the same functional BK channel complex (Gonzalez-Perez, Xia, & Lingle, 2015) suggests that the BK channel function can be extremely diversified across different tissues or cells types when multiple auxiliary subunits (β and γ) are coexpressed.
9. PERSPECTIVES
The four β subunits have been found to confer the BK channel a high diversity in the channel’s biophysical properties. Over the past two decades, BK channel modulation by β subunits has been extensively investigated. Studies in both humans and knockout mice have led to an appreciation of the physiological and pathological significance of the BK channel β subunits. With research efforts using heterologous expression systems in vitro, we have overall achieved a good understanding of the mechanisms underlying the β subunits’ modulatory functions conferring the BK channel inactivation and alterations in ion permeation and toxin sensitivity, which involve more specific regions of interactions between the β and BKα subunits. Significant progress has been made in understanding the mechanisms involved in the β subunits’ effect on BK channel voltage and Ca2+ dependence of channel activation, including the identification of altered gating processes and the important regions and residues involved on the β subunits. However, it remains poorly understood how the β subunit interacts with the BK channel, particularly the interacting amino acid residues on the BKα, to modulate the voltage and/or Ca2+ sensitivity. This is in parallel with our very limited understanding of the detailed molecular mechanisms underlying BK channel activation by voltage and Ca2+, which is partly due to the lack of atomic structure, particularly the structure that includes the channel’s TM domain.
Our understanding of BK channel modulation by γ subunits is still in its very early stage. In particular, very little is known about the physiologic functions and the structural basis underlying the regulatory mechanisms of γ subunits. The few published studies examining the modulatory mechanisms and physiological functions of BK channel γ subunits have mainly focused on the γ1 subunit. The regulation of BK channels by γ2–4 subunits has so far been demonstrated only in the heterologous expression system. It will be important to determine whether γ2–4 subunits also play any functional or physiological role in BK channel modulation in vivo. The mouse γ2 subunit has been found to function as an accessory subunit of the sperm- specific mouse Slo3 channels (Yang et al., 2011), and its ablation in mice was recently found to cause a severe fertility impairment (Zeng et al., 2015). It will be intriguing to determine whether in any stage of germ cells the γ2 subunit also modulates BK channels and whether Slo3 and BK channels can form functional heterotetrameric channels. When the BK and Slo3 channels were coexpressed in a heterologous expression system (X. oocytes), only two distinct BK-like and Slo3-like channels were observed, arguing against the presence of functional heterotetrameric channels (Yang, Zeng, Xia, & Lingle, 2009). BK channels and Slo3 belong to the Slo channel family, which also includes two more distantly related Na+-activated channels, Slo2.1 (slick) and Slo2.2 (slack). It remains an open question whether the γ subunits may broadly function as auxiliary proteins of the Slo channel family.
Effective BK channel openers have been sought or explored to treat a variety of diseases such as stroke, epilepsy, psychoses, bladder overactivity, erectile dysfunction, asthma, arterial hypertension, ischemic heart disease, and gastric hypermotility (Nardi & Olesen, 2008; Chapter “Developing the Molecular Pharmacology of BK Channels for Therapeutic Benefit” by Kaczorowski and Garcia). Although the widely used BK channel opener NS1619 can give an approximate -40-mV shift in V1/2 at a high concentration (30 μM) (Gessner et al., 2012), its specificity was recently questioned because of its direct inhibiting effect on the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) (Wrzosek, 2014) in addition to a previous report on its stimulatory effect on Ca2+ release from caffeine/ryanodine- sensitive intracellular store (Yamamura, Ohi, Muraki, Watanabe, & Imaizumi, 2001). Currently, no BK channel-targeted drug has been approved for clinical use, in spite of extensive academic and pharmaceutical efforts over the past two decades. Drug development specifically targeting auxiliary β or γ subunits of BK channels is likely to be more effective in modulating BK channel function, while minimizing the global adverse effects originated from the ubiquitously expressed BKα subunit. Deciphering the biochemical mechanisms underlying BK channel activation by auxiliary subunits will be useful for the development of new BK channel- targeted drugs.
ACKNOWLEDGMENT
This work is supported by NIH grant NS078152 (J.Y.).
REFERENCES
- Almassy J, Begenisich T. The LRRC26 protein selectively alters the efficacy of BK channel activators. Molecular Pharmacology. 2012;81:21–30. doi: 10.1124/mol.111.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. The Journal of Clinical Investigation. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel’s Ca2+ sensitivity is enhanced by its beta1 subunit. The Journal of General Physiology. 2005;126:393–412. doi: 10.1085/jgp.200509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Letters. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- Bentrop D, Beyermann M, Wissmann R, Fakler B. NMR structure of the “ball-and-chain” domain of KCNMB2, the beta 2-subunit of large conductance Ca2+- and voltage-activated potassium channels. The Journal of Biological Chemistry. 2001;276:42116–42121. doi: 10.1074/jbc.M107118200. [DOI] [PubMed] [Google Scholar]
- Bergeron ZL, Bingham JP. Scorpion toxins specific for potassium (K+) channels: A historical overview of peptide bioengineering. Toxins (Basel) 2012;4:1082–1119. doi: 10.3390/toxins4111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nature Neuroscience. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. The Journal of Biological Chemistry. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, Kuntamallappanavar G, Edwards J, Singh AK, Shivakumar B, Dopico AM. An alcohol-sensing site in the calcium- and voltage-gated, large conductance potassium (BK) channel. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9313–9318. doi: 10.1073/pnas.1317363111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Letters. 2009;583:2779–2784. doi: 10.1016/j.febslet.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, et al. Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel beta1-subunit by a novel nonsteroidal agent. Molecular Pharmacology. 2013;83:1030–1044. doi: 10.1124/mol.112.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, McMillan J, Liu J, Shivakumar B, Parrill AL, Dopico AM. Activation of calcium- and voltage-gated potassium channels of large conductance by leukotriene B4. The Journal of Biological Chemistry. 2014;289:35314–35325. doi: 10.1074/jbc.M114.577825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, McMillan J, Parrill AL, Dopico AM. Structural determinants of monohydroxylated bile acids to activate beta 1 subunit-containing BK channels. Journal of Lipid Research. 2008;49:2441–2451. doi: 10.1194/jlr.M800286-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Patil SA, Li W, Miller DD, Dopico AM. Calcium- and voltage-gated potassium (BK) channel activators in the 5beta-cholanic acid-3alpha-ol analogue series with modifications in the lateral chain. ChemMedChem. 2012;7:1784–1792. doi: 10.1002/cmdc.201200290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Singh AK, Parrill AL, Dopico AM. The steroid interaction site in transmembrane domain 2 of the large conductance, voltage- and calcium-gated potassium (BK) channel accessory beta1 subunit. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20207–20212. doi: 10.1073/pnas.1112901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Vaithianathan T, Toro L, Dopico AM. Channel beta2-4 subunits fail to substitute for beta1 in sensitizing BK channels to lithocholate. Biochemical and Biophysical Research Communications. 2009;390:995–1000. doi: 10.1016/j.bbrc.2009.10.091. [DOI] [PubMed] [Google Scholar]
- Cao XH, Chen SR, Li L, Pan HL. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. Journal of Neurochemistry. 2012;121:944–953. doi: 10.1111/j.1471-4159.2012.07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-de-Souza JL, Varanda WA, Tostes RC, Chignalia AZ. BK channels in cardiovascular diseases and aging. Aging and Disease. 2013;4:38–49. [PMC free article] [PubMed] [Google Scholar]
- Castillo K, Contreras GF, Pupo A, Torres YP, Neely A, Gonzalez C, et al. Molecular mechanism underlying beta1 regulation in voltage- and calcium-activated potassium (BK) channels. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:4809–4814. doi: 10.1073/pnas.1504378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bi D, Tian L, McClafferty H, Steeb F, Ruth P, et al. Palmitoylation of the beta4-subunit regulates surface expression of large conductance calcium-activated potassium channel splice variants. The Journal of Biological Chemistry. 2013;288:13136–13144. doi: 10.1074/jbc.M113.461830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Cai YQ, Pan HL. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. Journal of Neurochemistry. 2009;110:352–362. doi: 10.1111/j.1471-4159.2009.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Gan G, Wu Y, Wang L, Wu Y, Ding J. Lysine-rich extracellular rings formed by hbeta2 subunits confer the outward rectification of BK channels. PLoS One. 2008;3:e2114. doi: 10.1371/journal.pone.0002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. The Journal of Urology. 2009;182:374–381. doi: 10.1016/j.juro.2009.02.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras GF, Neely A, Alvarez O, Gonzalez C, Latorre R. Modulation of BK channel voltage gating by different auxiliary beta subunits. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18991–18996. doi: 10.1073/pnas.1216953109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. The Journal of General Physiology. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wet H, Allen M, Holmes C, Stobbart M, Lippiat JD, Callaghan R. Modulation of the BK channel by estrogens: Examination at single channel level. Molecular Membrane Biology. 2006;23:420–429. doi: 10.1080/09687860600802803. [DOI] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, et al. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. The Journal of Biological Chemistry. 2001;276:34594–34599. doi: 10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- Ding JP, Li ZW, Lingle CJ. Inactivating BK channels in rat chromaffin cells may arise from heteromultimeric assembly of distinct inactivation-competent and non-inactivating subunits. Biophysical Journal. 1998;74:268–289. doi: 10.1016/S0006-3495(98)77785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J, Walshe K, Alsbury S, Hokamp K, O’Keeffe S, Okafuji T, et al. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics. 2007;8:320. doi: 10.1186/1471-2164-8-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN. Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. The Journal of Physiology. 1999;519:101–114. doi: 10.1111/j.1469-7793.1999.0101o.x. Pt. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nature Genetics. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Duncan RK. Tamoxifen alters gating of the BK alpha subunit and mediates enhanced interactions with the avian beta subunit. Biochemical Pharmacology. 2005;70:47–58. doi: 10.1016/j.bcp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Evanson KW, Bannister JP, Leo MD, Jaggar JH. LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circulation Research. 2014;115:423–431. doi: 10.1161/CIRCRESAHA.115.303407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Meijer JH, Michel S. Age-related changes in large-conductance calcium-activated potassium channels in mammalian circadian clock neurons. Neurobiology of Aging. 2015;36:2176–2183. doi: 10.1016/j.neurobiolaging.2014.12.040. [DOI] [PubMed] [Google Scholar]
- Feinberg-Zadek PL, Treistman SN. Beta-subunits are important modulators of the acute response to alcohol in human BK channels. Alcoholism, Clinical and Experimental Research. 2007;31:737–744. doi: 10.1111/j.1530-0277.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, et al. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. The Journal of Clinical Investigation. 2004;113:1032–1039. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annual Review of Physiology. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Gan G, Yi H, Chen M, Sun L, Li W, Wu Y, et al. Structural basis for toxin resistance of beta4-associated calcium-activated potassium (BK) channels. The Journal of Biological Chemistry. 2008;283:24177–24184. doi: 10.1074/jbc.M800179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Calvo M, Knaus HG, McManus OB, Giangiacomo KM, Kaczorowski GJ, Garcia ML. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. The Journal of Biological Chemistry. 1994;269:676–682. [PubMed] [Google Scholar]
- Garcia-Valdes J, Zamudio FZ, Toro L, Possani LD. Slotoxin, alpha-KTx1.11, a new scorpion peptide blocker of MaxiK channels that differentiates between alpha and alpha + beta (beta1 or beta4) complexes. FEBS Letters. 2001;505:369–373. doi: 10.1016/s0014-5793(01)02791-0. [DOI] [PubMed] [Google Scholar]
- Gessner G, Cui YM, Otani Y, Ohwada T, Soom M, Hoshi T, et al. Molecular mechanism of pharmacological activation of BK channels. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3552–3557. doi: 10.1073/pnas.1114321109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner G, Schonherr K, Soom M, Hansel A, Asim M, Baniahmad A, et al. BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. The Journal of Membrane Biology. 2005;208:229–240. doi: 10.1007/s00232-005-0830-z. [DOI] [PubMed] [Google Scholar]
- Ghatta S, Nimmagadda D, Xu X, O’Rourke ST. Large-conductance, calcium-activated potassium channels: Structural and functional implications. Pharmacology & Therapeutics. 2006;110:103–116. doi: 10.1016/j.pharmthera.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Glauser DA, Johnson BE, Aldrich RW, Goodman MB. Intragenic alternative splicing coordination is essential for Caenorhabditis elegans slo-1 gene function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20790–20795. doi: 10.1073/pnas.1116712108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Jung HY, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. The Journal of Neuroscience. 1999;19:8789–8798. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Xia XM, Lingle CJ. Functional regulation of BK potassium channels by gamma1 auxiliary subunits. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4868–4873. doi: 10.1073/pnas.1322123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Xia XM, Lingle CJ. Two classes of regulatory subunits coassemble in the same BK channel and independently regulate gating. Nature Communications. 2015;6:8341. doi: 10.1038/ncomms9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez V, Zeng XH, Henzler-Wildman K, Lingle CJ. Stereospecific binding of a disordered peptide segment mediates BK channel inactivation. Nature. 2012;485:133–136. doi: 10.1038/nature10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruslova A, Semenov I, Wang B. An extracellular domain of the accessory beta1 subunit is required for modulating BK channel voltage sensor and gate. The Journal of General Physiology. 2012;139:57–67. doi: 10.1085/jgp.201110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen BM, Sanders KM. Deglycosylation of the beta1-subunit of the BK channel changes its biophysical properties. American Journal of Physiology Cell Physiology. 2006;291:C750–C756. doi: 10.1152/ajpcell.00116.2006. [DOI] [PubMed] [Google Scholar]
- Hanner M, Vianna-Jorge R, Kamassah A, Schmalhofer WA, Knaus HG, Kaczorowski GJ, et al. The beta subunit of the high conductance calcium-activated potassium channel. Identification of residues involved in charybdotoxin binding. The Journal of Biological Chemistry. 1998;273:16289–16296. doi: 10.1074/jbc.273.26.16289. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Hao J, Kosofsky BE, Rajadhyaksha AM. Dysregulation of large-conductance Ca2+-activated K+ channel expression in nonsyndromal mental retardation due to a cereblon p.R419X mutation. Neurogenetics. 2008;9:219–223. doi: 10.1007/s10048-008-0128-2. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+ The Journal of General Physiology. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. The Journal of General Physiology. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+ The Journal of General Physiology. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, et al. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca(2)(+)-dependent K+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4816–4821. doi: 10.1073/pnas.1221997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. The Journal of Neuroscience. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, et al. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Weiger TM, Levitan IB. Reciprocal modulation between the alpha and beta 4 subunits of hSlo calcium-dependent potassium channels. Journal of Biological Chemistry. 2002;277:43724–43729. doi: 10.1074/jbc.M205795200. [DOI] [PubMed] [Google Scholar]
- Jones EM, Gray-Keller M, Art JJ, Fettiplace R. The functional role of alternative splicing of Ca2+-activated K+ channels in auditory hair cells. Annals of the New York Academy of Sciences. 1999;868:379–385. doi: 10.1111/j.1749-6632.1999.tb11299.x. [DOI] [PubMed] [Google Scholar]
- Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. Journal of Neurophysiology. 2007;97:3508–3516. doi: 10.1152/jn.00009.2007. [DOI] [PubMed] [Google Scholar]
- King JT, Lovell PV, Rishniw M, Kotlikoff MI, Zeeman ML, McCobb DP. Beta2 and beta4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. Journal of Neurophysiology. 2006;95:2878–2888. doi: 10.1152/jn.01352.2005. [DOI] [PubMed] [Google Scholar]
- Kirby RW, Martelli A, Calderone V, McKay NG, Lawson K. Large conductance Ca2+-activated K+ channel (BKCa) activating properties of a series of novel N-arylbenzamides: Channel subunit dependent effects. Bioorganic & Medicinal Chemistry. 2013;21:4186–4191. doi: 10.1016/j.bmc.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Eberhart A, Kaczorowski GJ, Garcia ML. Covalent attachment of charybdotoxin to the beta-subunit of the high conductance Ca2+-activated K+ channel. Identification of the site of incorporation and implications for channel topology. The Journal of Biological Chemistry. 1994;269:23336–23341. [PubMed] [Google Scholar]
- Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, et al. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. The Journal of Biological Chemistry. 1994;269:17274–17278. [PubMed] [Google Scholar]
- Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. The Journal of Biological Chemistry. 1994;269:3921–3924. [PubMed] [Google Scholar]
- Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Current Opinion in Structural Biology. 2001;11:725–732. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Kreifeldt M, Cates-Gatto C, Roberts AJ, Contet C. BK channel beta1 subunit contributes to behavioral adaptations elicited by chronic intermittent ethanol exposure. Alcoholism, Clinical and Experimental Research. 2015;39:2394–2402. doi: 10.1111/acer.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifeldt M, Le D, Treistman SN, Koob GF, Contet C. BK channel beta1 and beta4 auxiliary subunits exert opposite influences on escalated ethanol drinking in dependent mice. Frontiers in Integrative Neuroscience. 2013;7:105. doi: 10.3389/fnint.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntamallappanavar G, Toro L, Dopico AM. Both transmembrane domains of BK beta1 subunits are essential to confer the normal phenotype of beta1-containing BK channels. PLoS One. 2014;9:e109306. doi: 10.1371/journal.pone.0109306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large RJ, Kshatri A, Webb TI, Roy S, Akande A, Bradley E, et al. Effects of the novel BK (KCa 1.1) channel opener GoSlo-SR-5-130 are dependent on the presence of BKbeta subunits. British Journal of Pharmacology. 2015;172:2544–2556. doi: 10.1111/bph.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Roger S, Guerin P, Molinari F, M’Rad R, Cahard D, et al. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. The American Journal of Psychiatry. 2006;163:1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
- Lee US, Shi J, Cui J. Modulation of BK channel gating by the ss2 subunit involves both membrane-spanning and cytoplasmic domains of Slo1. The Journal of Neuroscience. 2010;30:16170–16179. doi: 10.1523/JNEUROSCI.2323-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chang S, Yang L, Shi J, McFarland K, Yang X, et al. Conopeptide Vt3.1 preferentially inhibits BK potassium channels containing beta4 subunits via electrostatic interactions. The Journal of Biological Chemistry. 2014;289:4735–4742. doi: 10.1074/jbc.M113.535898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Fan F, Kwak HR, Yan J. Molecular basis for differential modulation of BK channel voltage-dependent gating by auxiliary gamma subunits. The Journal of General Physiology. 2015;145:543–554. doi: 10.1085/jgp.201511356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Yen K, Zhang J, Yan J. The single transmembrane segment determines the modulatory function of the BK channel auxiliary γ subunit. The Journal of General Physiology. 2016;147:337–351. doi: 10.1085/jgp.201511551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yao J, Tong X, Guo Z, Wu Y, Sun L, et al. Interaction sites between the Slo1 pore and the NH2 terminus of the beta2 subunit, probed with a three-residue sensor. The Journal of Biological Chemistry. 2007;282:17720–17728. doi: 10.1074/jbc.M607063200. [DOI] [PubMed] [Google Scholar]
- Lingle CJ, Zeng XH, Ding JP, Xia XM. Inactivation of BK channels mediated by the NH(2) terminus of the beta3b auxiliary subunit involves a two-step mechanism: Possible separation of binding and blockade. The Journal of General Physiology. 2001;117:583–606. doi: 10.1085/jgp.117.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Niu X, Wu RS, Chudasama N, Yao Y, Jin X, et al. Location of modulatory beta subunits in BK potassium channels. The Journal of General Physiology. 2010;135:449–459. doi: 10.1085/jgp.201010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XF, Xiang L, Zhang Y, Becker KG, Bera TK, Pastan I. CAPC negatively regulates NF-kappaB activation and suppresses tumor growth and metastasis. Oncogene. 2012;31:1673–1682. doi: 10.1038/onc.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zakharov SI, Yang L, Wu RS, Deng SX, Landry DW, et al. Locations of the beta1 transmembrane helices in the BK potassium channel. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10727–10732. doi: 10.1073/pnas.0805212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zakharov SI, Yao Y, Marx SO, Karlin A. Positions of the cytoplasmic end of BK alpha S0 helix relative to S1-S6 and of beta1 TM1 and TM2 relative to S0-S6. The Journal of General Physiology. 2015;145:185–199. doi: 10.1085/jgp.201411337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhang Z, Liu H, Hou P, Lang J, Wang S, et al. Human beta-defensin 2 is a novel opener of Ca2+-activated potassium channels and induces vasodilation and hypotension in monkeys. Hypertension. 2013;62:415–425. doi: 10.1161/HYPERTENSIONAHA.111.01076. [DOI] [PubMed] [Google Scholar]
- Lorenz S, Heils A, Kasper JM, Sander T. Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144B:10–13. doi: 10.1002/ajmg.b.30369. [DOI] [PubMed] [Google Scholar]
- Lv C, Chen M, Gan G, Wang L, Xu T, Ding J. Four-turn alpha-helical segment prevents surface expression of the auxiliary hbeta2 subunit of BK-type channel. The Journal of Biological Chemistry. 2008;283:2709–2715. doi: 10.1074/jbc.M704440200. [DOI] [PubMed] [Google Scholar]
- Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1-S4 voltage sensor of BK channels. The Journal of General Physiology. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Soldovieri MV, Gessner G, Wissuwa B, Barrese V, Boscia F, et al. Critical role of large-conductance calcium- and voltage-activated potassium channels in leptin-induced neuroprotection of N-methyl-D-aspartate-exposed cortical neurons. Pharmacological Research. 2014;87:80–86. doi: 10.1016/j.phrs.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D, Krick S, Baumlin N, Dennis JS, Tyrrell J, Tarran R, et al. Airway surface dehydration by transforming growth factor beta (TGF-beta) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. The Journal of Biological Chemistry. 2015;290:25710–25716. doi: 10.1074/jbc.M115.670885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares D, Srinivasan M, Salathe ST, Ivonnet P, Baumlin N, Dennis JS, et al. IFN-gamma-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2014;306:L453–L462. doi: 10.1152/ajplung.00247.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Hendrickson LM, Penta KL, Friesen RM, Pietrzykowski AZ, Tapper AR, et al. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17543–17548. doi: 10.1073/pnas.0801068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, Treistman S. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. The Journal of Neuroscience. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Espinosa PL, Yang C, Gonzalez-Perez V, Xia XM, Lingle CJ. Knockout of the BK beta2 subunit abolishes inactivation of BK currents in mouse adrenal chromaffin cells and results in slow-wave burst activity. The Journal of General Physiology. 2014;144:275–295. doi: 10.1085/jgp.201411253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981;291:497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Disterhoft JF. Blocking the BK channel impedes acquisition of trace eyeblink conditioning. Learning & Memory. 2009;16:106–109. doi: 10.1101/lm.1289809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus OB, Harris GH, Giangiacomo KM, Feigenbaum P, Reuben JP, Addy ME, et al. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32:6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. The Journal of Biological Chemistry. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nature Neuroscience. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JR, Whitt JP, Wright BN, Lai MH, Meredith AL. Misexpression of the BK K+ channel disrupts suprachiasmatic nucleus circuit rhythmicity and alters clock-controlled behavior. American Journal of Physiology Cell Physiology. 2013;304:C299–C311. doi: 10.1152/ajpcell.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Sakamoto K, Sade H, Ohya S, Muraki K, Imaizumi Y. Voltage-sensitive oxonol dyes are novel large-conductance Ca2+-activated K+ channel activators selective for beta1 and beta4 but not for beta2 subunits. Molecular Pharmacology. 2007;71:1075–1088. doi: 10.1124/mol.106.031146. [DOI] [PubMed] [Google Scholar]
- Morrow JP, Zakharov SI, Liu G, Yang L, Sok AJ, Marx SO. Defining the BK channel domains required for beta1-subunit modulation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5096–5101. doi: 10.1073/pnas.0600907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi A, Olesen SP. BK channel modulators: A comprehensive overview. Current Medicinal Chemistry. 2008;15:1126–1146. doi: 10.2174/092986708784221412. [DOI] [PubMed] [Google Scholar]
- Nimigean CM, Magleby KL. The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. The Journal of General Physiology. 1999;113:425–440. doi: 10.1085/jgp.113.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ballanyi K, Homma I. Contribution of Ca2+-dependent conductances to membrane potential fluctuations of medullary respiratory neurons of newborn rats in vitro. The Journal of Physiology. 2003;552:727–741. doi: 10.1113/jphysiol.2003.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Latorre R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. The Journal of General Physiology. 2005;125:395–411. doi: 10.1085/jgp.200409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News in Physiological Sciences. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- Orio P, Torres Y, Rojas P, Carvacho I, Garcia ML, Toro L, et al. Structural determinants for functional coupling between the beta and alpha subunits in the Ca2+-activated K+ (BK) channel. The Journal of General Physiology. 2006;127:191–204. doi: 10.1085/jgp.200509370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo LA, Stuhmer W. The roles of K+ channels in cancer. Nature Reviews. Cancer. 2014;14:39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Research. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. The Journal of Physiology. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan K, Michael TH, Jiang GJ, Hiel H, Fuchs PA. A molecular mechanism for electrical tuning of cochlear hair cells. Science. 1999;283:215–217. doi: 10.1126/science.283.5399.215. [DOI] [PubMed] [Google Scholar]
- Runden-Pran E, Haug FM, Storm JF, Ottersen OP. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: A study in organotypical hippocampal slice cultures. Neuroscience. 2002;112:277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. The Journal of Biological Chemistry. 1997;272:11710–11717. doi: 10.1074/jbc.272.18.11710. [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nature Reviews. Neuroscience. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Samengo I, Curro D, Barrese V, Taglialatela M, Martire M. Large conductance calcium-activated potassium channels: Their expression and modulation of glutamate release from nerve terminals isolated from rat trigeminal caudal nucleus and cerebral cortex. Neurochemical Research. 2014;39:901–910. doi: 10.1007/s11064-014-1287-1. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli N, Kondratiev A, de Quintana SB, Toro L, Olcese R. Modes of operation of the BKCa channel beta2 subunit. The Journal of General Physiology. 2007;130:117–131. doi: 10.1085/jgp.200709803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MJ, Rogowski RS, Krueger BK, Blaustein MP. Charybdotoxin blocks both Ca-activated K channels and Ca-independent voltage-gated K channels in rat brain synaptosomes. FEBS Letters. 1989;250:433–436. doi: 10.1016/0014-5793(89)80771-9. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophysical Journal. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Yuan A, Salkoff L. Transplantable sites confer calcium sensitivity to BK channels. Nature Neuroscience. 1999;2:416–421. doi: 10.1038/8077. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. The Journal of Physiology. 1999;521:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. Pt. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Kishimoto K, Linden DJ, Sapirstein A. Cytosolic phospholipase A(2) alpha mediates electrophysiologic responses of hippocampal pyramidal neurons to neurotoxic NMDA treatment. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6078–6083. doi: 10.1073/pnas.0605427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, He HQ, Zhao R, Duan YH, Chen J, Chen Y, et al. Inhibition of martentoxin on neuronal BK channel subtype (alpha + beta4): Implications for a novel interaction model. Biophysical Journal. 2008;94:3706–3713. doi: 10.1529/biophysj.107.122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Krishnamoorthy G, Yang Y, Hu L, Chaturvedi N, Harilal D, et al. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 2002;418:876–880. doi: 10.1038/nature00941. [DOI] [PubMed] [Google Scholar]
- Shruti S, Urban-Ciecko J, Fitzpatrick JA, Brenner R, Bruchez MP, Barth AL. The brain-specific beta4 subunit downregulates BK channel cell surface expression. PLoS One. 2012;7:e33429. doi: 10.1371/journal.pone.0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SJ, Burkett BJ, Schrader LA. Modulation of BK channels contributes to activity-dependent increase of excitability through MTORC1 activity in CA1 pyramidal cells of mouse hippocampus. Frontiers in Cellular Neuroscience. 2014;8:451. doi: 10.3389/fncel.2014.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Shi J, Delaloye K, Yang X, Yang H, Zhang G, et al. The interface between membrane-spanning and cytosolic domains in Ca2+-dependent K+ channels is involved in beta subunit modulation of gating. The Journal of Neuroscience. 2013;33:11253–11261. doi: 10.1523/JNEUROSCI.0620-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zaydman MA, Cui J. Regulation of voltage-activated K+ channel gating by transmembrane beta subunits. Frontiers in Pharmacology. 2012;3:63. doi: 10.3389/fphar.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Lin MT, Thorington GU, Wilson SM, Longo LD, Hessinger DA. Long-term hypoxia increases calcium affinity of BK channels in ovine fetal and adult cerebral artery smooth muscle. American Journal of Physiology. Heart and Circulatory Physiology. 2015;308:H707–H722. doi: 10.1152/ajpheart.00564.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Shi J, Liu ZR, Ji YH. Martentoxin: A unique ligand of BK channels. Sheng Li Xue Bao. 2012;64:355–364. [PubMed] [Google Scholar]
- Tian Y, Ullrich F, Xu R, Heinemann SH, Hou S, Hoshi T. Two distinct effects of PIP2 underlie auxiliary subunit-dependent modulation of Slo1 BK channels. The Journal of General Physiology. 2015;145:331–343. doi: 10.1085/jgp.201511363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, Toro L, et al. KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience. 2006;142:661–669. doi: 10.1016/j.neuroscience.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Torres YP, Granados ST, Latorre R. Pharmacological consequences of the coexpression of BK channel alpha and auxiliary beta subunits. Frontiers in Physiology. 2014;5:383. doi: 10.3389/fphys.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: Beta-subunits and voltage-dependent K+ channels. The Journal of Biological Chemistry. 2007;282:24485–24489. doi: 10.1074/jbc.R700022200. [DOI] [PubMed] [Google Scholar]
- Tseng-Crank J, Godinot N, Johansen TE, Ahring PK, Strobaek D, Mertz R, et al. Cloning, expression, and distribution of a Ca2+-activated K+ channel beta-subunit from human brain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typlt M, Mirkowski M, Azzopardi E, Ruettiger L, Ruth P, Schmid S. Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PLoS One. 2013;8:e81270. doi: 10.1371/journal.pone.0081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, et al. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. The Journal of Biological Chemistry. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Ottolia M, Kaczorowski GJ, Latorre R, Garcia ML, et al. Characterization of and modulation by a beta-subunit of a human maxi KCa channel cloned from myometrium. Receptors & Channels. 1995;3:185–199. [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: An additional transmembrane region at the N terminus. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Brenner R. An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating. The Journal of General Physiology. 2006;128:731–744. doi: 10.1085/jgp.200609596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. The Journal of Neuroscience. 2002;22:1550–1561. doi: 10.1523/JNEUROSCI.22-05-01550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Rothberg BS, Brenner R. Mechanism of beta4 subunit modulation of BK channels. The Journal of General Physiology. 2006;127:449–465. doi: 10.1085/jgp.200509436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: Emerging ion channel targets. Nature Neuroscience. 2014;17:153–163. doi: 10.1038/nn.3602. [DOI] [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: Modulation by BK channels. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;292:R616–R624. doi: 10.1152/ajpregu.00036.2006. [DOI] [PubMed] [Google Scholar]
- Werner ME, Meredith AL, Aldrich RW, Nelson MT. Hypercontractility and impaired sildenafil relaxations in the BKCa channel deletion model of erectile dysfunction. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2008;295:R181–R188. doi: 10.1152/ajpregu.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. The Journal of Physiology. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Characterization of large conductance Ca2+- activated K+ channels in cerebellar Purkinje neurons. The European Journal of Neuroscience. 2002;16:1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- Wrzosek A. The potassium channel opener NS1619 modulates calcium homeostasis in muscle cells by inhibiting SERCA. Cell Calcium. 2014;56:14–24. doi: 10.1016/j.ceca.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Wu RS, Chudasama N, Zakharov SI, Doshi D, Motoike H, Liu G, et al. Location of the beta 4 transmembrane helices in the BK potassium channel. The Journal of Neuroscience. 2009;29:8321–8328. doi: 10.1523/JNEUROSCI.6191-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RS, Liu G, Zakharov SI, Chudasama N, Motoike H, Karlin A, et al. Positions of beta2 and beta3 subunits in the large-conductance calcium- and voltage-activated BK potassium channel. The Journal of General Physiology. 2013;141:105–117. doi: 10.1085/jgp.201210891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne PM, Puig SI, Martin GE, Treistman SN. Compartmentalized beta subunit distribution determines characteristics and ethanol sensitivity of somatic, dendritic, and terminal large-conductance calcium-activated potassium channels in the rat central nervous system. The Journal of Pharmacology and Experimental Therapeutics. 2009;329:978–986. doi: 10.1124/jpet.108.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. The Journal of Neuroscience. 1999;19:5255–5264. doi: 10.1523/JNEUROSCI.19-13-05255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Inactivation of BK channels by the NH2 terminus of the beta2 auxiliary subunit: An essential role of a terminal peptide segment of three hydrophobic residues. The Journal of General Physiology. 2003;121:125–148. doi: 10.1085/jgp.20028667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Zeng XH, Duan KL, Lingle CJ. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: Consequences of rapid inactivation by a novel beta subunit. The Journal of Neuroscience. 2000;20:4890–4903. doi: 10.1523/JNEUROSCI.20-13-04890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- Xu H, Garver H, Fernandes R, Phelps JT, Harkema JJ, Galligan JJ, et al. BK channel beta1-subunit deficiency exacerbates vascular fibrosis and remodelling but does not promote hypertension in high-fat fed obesity in mice. Journal of Hypertension. 2015;33:1611–1623. doi: 10.1097/HJH.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JW, Slaughter MM. Large-conductance calcium-activated potassium channels facilitate transmitter release in salamander rod synapse. The Journal of Neuroscience. 2005;25:7660–7668. doi: 10.1523/JNEUROSCI.1572-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura H, Ohi Y, Muraki K, Watanabe M, Imaizumi Y. BK channel activation by NS-1619 is partially mediated by intracellular Ca2+ release in smooth muscle cells of porcine coronary artery. British Journal of Pharmacology. 2001;132:828–834. doi: 10.1038/sj.bjp.0703885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CT, Zeng XH, Xia XM, Lingle CJ. Interactions between beta subunits of the KCNMB family and Slo3: Beta4 selectively modulates Slo3 expression and function. PLoS One. 2009;4:e6135. doi: 10.1371/journal.pone.0006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Zeng XH, Zhou Y, Xia XM, Lingle CJ. LRRC52 (leucine-rich-repeat-containing protein 52), a testis-specific auxiliary subunit of the alkalization-activated Slo3 channel. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19419–19424. doi: 10.1073/pnas.1111104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang G, Shi J, Lee US, Delaloye K, Cui J. Subunit-specific effect of the voltage sensor domain on Ca2+ sensitivity of BK channels. Biophysical Journal. 2008;94:4678–4687. doi: 10.1529/biophysj.107.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Jalini S, Mylvaganam S, Carlen P. Activation of large-conductance Ca2 +-activated K+ channels depresses basal synaptic transmission in the hippocampal CA1 area in APP (swe/ind) TgCRND8 mice. Neurobiology of Aging. 2010;31:591–604. doi: 10.1016/j.neurobiolaging.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Yu JY, Upadhyaya AB, Atkinson NS. Tissue-specific alternative splicing of BK channel transcripts in Drosophila. Genes, Brain, and Behavior. 2006;5:329–339. doi: 10.1111/j.1601-183X.2005.00164.x. [DOI] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Hsiung Y, MacKinnon R. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature. 2012;481:94–97. doi: 10.1038/nature10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusifov T, Savalli N, Gandhi CS, Ottolia M, Olcese R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:376–381. doi: 10.1073/pnas.0705261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Song M, Wilson RJ, Cox N, Colom LV, Knaus HG, et al. Endocytic trafficking signals in KCNMB2 regulate surface expression of a large conductance voltage and Ca2+-activated K+ channel. Neuroscience. 2007;147:80–89. doi: 10.1016/j.neuroscience.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Zavala-Tecuapetla C, Aguileta MA, Lopez-Guerrero JJ, Gonzalez-Marin MC, Pena F. Calcium-activated potassium currents differentially modulate respiratory rhythm generation. The European Journal of Neuroscience. 2008;27:2871–2884. doi: 10.1111/j.1460-9568.2008.06214.x. [DOI] [PubMed] [Google Scholar]
- Zeng XH, Benzinger GR, Xia XM, Lingle CJ. BK channels with beta3a subunits generate use-dependent slow afterhyperpolarizing currents by an inactivation-coupled mechanism. The Journal of Neuroscience. 2007;27:4707–4715. doi: 10.1523/JNEUROSCI.0758-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Xia XM, Lingle CJ. Redox-sensitive extracellular gates formed by auxiliary beta subunits of calcium-activated potassium channels. Nature Structural Biology. 2003;10:448–454. doi: 10.1038/nsb932. [DOI] [PubMed] [Google Scholar]
- Zeng X, Xia XM, Lingle CJ. Species-specific differences among KCNMB3 BK beta3 auxiliary subunits: Some beta3 N-terminal variants may be primate-specific subunits. The Journal of General Physiology. 2008;132:115–129. doi: 10.1085/jgp.200809969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Yang C, Xia XM, Liu M, Lingle CJ. SLO3 auxiliary subunit LRRC52 controls gating of sperm KSPER currents and is critical for normal fertility. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2599–2604. doi: 10.1073/pnas.1423869112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Huang SY, Yang J, Shi J, Yang X, Moller A, et al. Ion sensing in the RCK1 domain of BK channels. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18700–18705. doi: 10.1073/pnas.1010124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li X, Zhou R, Xing G. Possible role of potassium channel, big K in etiology of schizophrenia. Medical Hypotheses. 2006;67:41–43. doi: 10.1016/j.mehy.2005.09.055. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Lee KY, Charbonnet M, Gold MS. Inflammation-induced changes in BK(Ca) currents in cutaneous dorsal root ganglion neurons from the adult rat. Molecular Pain. 2012;8:37. doi: 10.1186/1744-8069-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xie M, Schools GP, Feustel PF, Wang W, Lei T, et al. Tamoxifen mediated estrogen receptor activation protects against early impairment of hippocampal neuron excitability in an oxygen/glucose deprivation brain slice ischemia model. Brain Research. 2009;1247:196–211. doi: 10.1016/j.brainres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yan J. Regulation of BK channels by auxiliary gamma subunits. Frontiers in Physiology. 2014;5:401. doi: 10.3389/fphys.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zeng XH, Xia XM, Lingle CJ. N-terminal inactivation domains of beta subunits are protected from trypsin digestion by binding within the antechamber of BK channels. The Journal of General Physiology. 2009;133:263–282. doi: 10.1085/jgp.200810079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhou Y, Ding JP, Xia XM, Lingle CJ. A limited access compartment between the pore domain and cytosolic domain of the BK channel. The Journal of Neuroscience. 2006;26:11833–11843. doi: 10.1523/JNEUROSCI.3812-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Hulsmann S, Winter SM, Dutschmann M, Richter DW. Calcium-regulated potassium currents secure respiratory rhythm generation after loss of glycinergic inhibition. The European Journal of Neuroscience. 2006;24:145–154. doi: 10.1111/j.1460-9568.2006.04877.x. [DOI] [PubMed] [Google Scholar]