Abstract
Adolescence and prenatal cocaine exposure can impact risk-taking. In this study, we evaluated risk-taking and gender-related differences in adolescents with prenatal cocaine exposure in terms of electrophysiological correlates of inhibitory control and sustained attention. No differences related to gender were found within measures of risk-taking, or electrophysiological response relating to risk-taking. Greater responses during inhibition versus attention trials support previous studies, with boys showing the largest responses. Gender-related differences were found when comparing the trials before and after frustration was induced, with greater initial attention indices for girls in both trial types and greater sustained attention for both genders during inhibition trials and for boys during attention trials. These data suggest neural correlates of response inhibition show important gender-related differences in this population. Considering these relationships allows us to further understand underlying processes among adolescents who, as a group, tend to be more inclined toward greater risk behaviors.
Keywords: Prenatal Cocaine Exposure, Response Inhibition, Adolescent, Event-Related Potential, Risk-Taking
Introduction
Adolescence is characterized by multiple cognitive, neural, and behavioral developmental changes. The effects of prenatal cocaine exposure (PCE) on these developmental processes remain incompletely understood [1,2]. PCE-related differences may be more subtle among adolescents than in children [3]. Yet, compared to their healthy peers, deficits in adolescents with PCE may be pervasive [2].
Deficits in two neurocognitive facets, inhibitory control [4] and sustained attention [5], have been identified in children with PCE. Among healthy adolescents, inhibitory control [6,7] and sustained attention [8] are associated with risk-taking. So too, risk-taking behaviors have been associated with neural connectivity differences in networks linked to emotion regulation among adolescents [9].
Cortical regions associated with the processes of inhibitory control, sustained attention, emotion regulation, and risk-taking undergo developmental changes during adolescence. Specifically, the prefrontal cortex (PFC) and anterior cingulate cortex (ACC) have been linked to response inhibition and attentional control [10]. Greater risk-taking in healthy adolescents has been related to decreased activity in the PFC and ACC [11]. In both preclinical and human models of PCE, this exposure significantly impacts both PFC and ACC, suggesting broad PCE impacts on frontal cortical function [12,13,14]. Together, these data suggest differences may be seen in adolescents with PCE when evaluating attention and response inhibition.
Risk-taking, which increases during adolescence, may reflect maturational differences, specifically in the neurobiological underpinnings of behavioral and affective circuitry involving the PFC and limbic system [15]. Decisions to take risks may be influenced by affective systems [16]. Higher propensities for risk-taking during adolescence may confer vulnerability to both substance and behavioral addictions [15,17,18]. Additionally, PCE has been related to increased risk-taking during adolescence [19-21].
Risk-taking behavior differs across adolescent boys and girls [22]. Among those with PCE, risk-taking behaviors have been reported to be elevated in boys relative to girls. Risky sexual behavior during adolescence has been reported more frequently in boys with PCE compared to girls with PCE and non-drug-exposed (NDE) boys and girls [21]. Propensity for risk-taking has been reported to be highest among boys with PCE compared to NDE boys and girls, with girls with PCE reporting the lowest propensity amongst these four groups [23]. Gender-related differences in NDE, between PCE and NDE, and among adolescents with PCE suggest this pattern may extend to additional processes linked to risk-taking.
Response inhibition is a critical facet of risk taking, as it represents one’s ability to not engage in a rewarding response/activity. Two neurophysiological correlates relevant to response inhibition are the N2 and P3 event-related potential (ERP) components. Though modalities such as functional magnetic resonance imaging may have comparably better spatial resolution, electrophysiology has comparably better temporal resolution. ERPs are peaks of activity that occur in a designated timeframe after a stimulus. As such N2 is a negative peak that occurs between 200 to 300 ms post-stimulus and P3 is a positive peak that occurs 300 to 500 ms post-stimulus. While electrode recording sites may not correspond directly to specific brain regions, the current components have been suggested to reflect ACC, dorsal lateral and ventral PFC, and medial frontal activity [24,25].
ERP methods are effective, noninvasive markers that are frequently used to delineate neural function within groups or between populations across specific tasks, such as Go/NoGo tasks. Go/NoGo tasks typically have subjects follow rule sets which ask for a response when a rule is met (Go), and another which occurs more infrequently and requires withholding a prepotent response (NoGo), an effective manner of examining inhibitory control [26]. Both ERP components are elicited through Go/NoGo tasks. Robust N2s are present in both successful Go and NoGo trials, while a robust P3 is typically in response to successful NoGo trials. N2 responses during Go/NoGo tasks have been suggested to reflect conflict monitoring [24]. The P3 component has been suggested to represent the end of the inhibition window in Go/NoGo tasks [27]. Additionally, in the context of risk-taking, both N2 and P3 have been related to response inhibition [24] and sensation-seeking [27]. Together, data suggest N2 and P3 responses elicited through Go/NoGo tasks may reflect both overt and covert inhibition [28], and this ERP complex may reflect variation in inhibitory and environmental processing.
Previously, a variant of the Go/NoGo task that increases task difficulty in the middle of three blocks has been used to investigate ERP responses when a task becomes frustratingly difficult [29]. Specifically, amplitudes of both components were larger after frustration induction and also larger for NoGo trials than Go trials in adolescents [29]. Decreased N2 amplitude during this task has also been related to better performance in the Iowa Gambling Task and Stroop task [30]. Together, these data suggest the N2 and P3 components may be influenced by frustration during response inhibition in adolescents with PCE as has been seen in other populations [29].
Current Study
We examined ERPs during Go/NoGo performance within a population of adolescents with PCE. We compared youth characterized as high risk-taking versus low risk-taking groups and boy and girl participants on a validated measure of behavioral risk-taking [31]. Higher risk-taking has been observed in adolescent boys with PCE than in adolescent girls with PCE [32] and for externalizing behavior problems [32]. As such, we sought to determine if responses related to response inhibition would differ according to risk-taking in a gender-specific manner between NoGo and Go conditions. An added component of the current study sought to evaluate the effects a frustration-inducing block would have on response inhibition in later blocks, and analyses were conducted to examine possible effects related to risk-taking and gender.
Materials and Methods
Participants
All participants provided informed consent approved by the Human Investigations Committee at Yale School of Medicine. Participants visited a laboratory within the Yale School of Medicine campus located in northeastern United States (New Haven, CT) for the study. The sample was initially composed of 30 (15 girl) adolescents with PCE. Gender was determined through self-report not biological testing or visual inspection of external genitalia. As such, terminology in line with “gender” from the American Psychological Association’s definitions for sex versus gender are used here [33]. Of these 83 percent reported race/ethnicity as African-American, 3.5 percent Hispanic, 3.5 percent Asian, and 10 percent as Caucasian. These individuals were part of a larger cohort (89 children (44 girls)), all of whom were prenatally exposed to cocaine, alcohol, other drugs, and experienced postnatal environmental adversity such as extreme poverty. Individuals were recruited from an ongoing longitudinal investigation in to the effects of PCE on development; further description of the sample has been published previously [34]. Urine toxicology in the prenatal or postpartum period, meconium toxicology, and maternal report were used to determine PCE [34,35]. Laboratory risk-taking behavior was evaluated in all 89 children for inclusion in the current study. From this, group, boy and girl youth with high or low risk-taking behavior (screening detailed below) were evaluated for an electrophysiological assessment.
The Balloon Analogue Risk Task
The Balloon Analogue Risk Task (BART) was utilized to evaluate the propensity for risk-taking. Through the use of this computer-based task, reward versus loss processing during risk-taking was evaluated through virtual pumping of balloons. Individuals were presented on-screen with a small balloon, a pump, a “Collect $$$” button which would reset the task, a display of the total amount earned, and the last balloon, labeled as such, with the final amount of money earned. During the 30 trials, individuals were prompted to fill the balloon with air through mouse clicks. With each successful pump, money was accrued in a temporary account; if the balloon was pumped past the predetermined bursting point, the balloon would pop with an audible cue, and the money from the temporary account was lost. However, at any time prior to the explosion of the balloon, the individual was able to discontinue pumping and collect the money from the temporary account by clicking the “Collect $$$” button. A more detailed description of the BART has previously been published [31].
The current study used the measure of adjusted mean pumps as described elsewhere in order to classify children as high versus low risk-takers [31]. The adjusted mean number of pumps has been linked previously to real-life risk-taking propensities [31] and was calculated on the trials during which the balloon did not burst. Children in the top 20 percent were categorized as high risk-taking and those in the bottom 20 percent were categorized as low risk-taking. These previously used guidelines [31,34] were used to identify the 30 individuals at the extreme ends of the risk-taking spectrum for inclusion in the current study; all individuals identified as high and low risk-taking participated in the current study.
Go/NoGo Task
Using E-Prime software (Psychological Software, Pittsburgh, PA), a frustration-induction Go/NoGo task was used to evaluate response inhibition under varying task difficulty [29]. In this task, participants were asked to respond with a button click when a new letter was presented, the Go condition. In the NoGo condition, the response needed to be withheld after a letter was presented two consecutive times. Participants were only able to earn points by correctly withholding a response during the NoGo condition but would lose points for an incorrect response during the Go or NoGo condition. When points were earned, a pleasant “chime” sound was presented, and a buzzer noise occurred when points were lost due to an incorrect response (omission or comission). The current task was composed of three blocks (easy, difficult, easy again), which varied in difficulty through changes in the interstimulus intervals. Additionally, letter stimuli varied between blocks; Block A used “o” and “p”, Block B “x” and “y”, and Block C “u “ and “d”. During Block A, points were easily earned in response to the correct NoGo responses. In Block B, the interstimulus interval was significantly shorter compared to Block A, increasing the difficulty of the task and resulting in individuals losing all or most of the money initially earned during Block A. The interstimulus intervals increased during Block C; this allowed for more correct responses, fewer incorrect responses, and increased rewards. Further detailed description of the current task has previously been published [36].
Percentage of correct responses and reaction times were collected for Go trials. However, because a correct response for a NoGo trial would be to withhold a button press when letter stimuli were presented consecutively, only accuracy was collected for NoGo trials.
Procedure
Each individual’s head size was measured to determine the appropriate net size. Geodesic Sensor Nets (Electrical Geodesics, Inc.) with 128 Ag/AgCl electrodes were used and applied according to standard procedure including soaking nets in potassium-chloride solution in order to facilitate brain-wave data recordings. Individuals were seated one meter from a 15-inch Dell computer monitor that was running E-Prime to present the Go/NoGo task.
EEG data were recorded and processed using the Netstation 4.0 software package (Electrical Geodesics, Inc.) and recorded with high impedance amplifiers (Electrical Geodesics, Inc.). Filters were set at 0.1 to 30 Hz, and the sampling rate was set at 250 Hz. During recording, all electrodes were referenced to Cz and during data analysis were re-referenced offline. Single-trial data were re-referenced to an average reference of all electrodes, which has previously been presented as a better representation of true zero [37]. Impedances for all trials were under or at 40 kΩ. Offline, data segmented to epochs which began 100 ms pre-stimulus and continued 600 ms post-stimulus. ERP data that included participant movement or eye artifacts were removed from further analysis (one individual). Electrodes that were identified with poor signal quality for 10 percent or more of the trials were replaced through spherical spline interpolation. Data were only included if less than 12 channels were considered to have generated a poor signal. Baseline correction was applied to the averaged data by the subtraction of the average microvolt value of the 100 ms pre-stimulus segment from the post-stimulus segment.
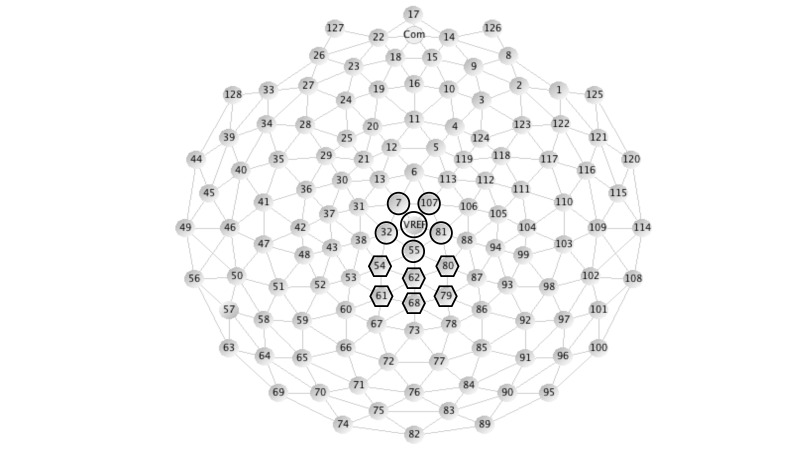
In the current study, N2 and P3 data were localized to Cz and Pz (10-10 system); the Cz and Pz sites are located along the midline and have been previously used in collecting N2 and P3 data [24,27,28-30]. After visual inspection, we relied on an average signal of six electrodes in each of these areas; Cz: electrodes 7, 32, 55, 81, 107, and vertex reference (VREF), and Pz: electrodes 54, 61, 62, 68, 79, and 80 (see Figure 1). For ERP analysis, the N2 amplitude was defined as the mean around the negative peak amplitude between 200 and 300 ms, and the P3 amplitude was defined as the mean amplitude around the positive peak amplitude between 300 and 500 ms within the listed electrode clusters. ERP data were only evaluated for those epochs surrounding correct Go and NoGo responses.
Figure 1.
Diagram of electrode clusters for each composite site, site Cz represented with circles; site Pz represented with hexagons.
ERP Data
Current ERP data were analyzed with repeated measures ANOVA using F tests corrected with Greenhouse-Geisser methods using IBM SPSS Statistics 21 (IBM Corp., Armonk, NY). P-values were used to report differences in outcomes between the groups, with significance set at 0.05. Groups were defined by gender (girl, boy) and risk-taking (high, low). The initial analyses tested a possible interaction between risk-taking and gender in Go versus NoGo electrophysiological responses for N2 and P3. Secondary analyses were used to compare within each of the task blocks (A, B, and C) for each condition (Go, NoGo) between risk-taking and gender. In order to evaluate lasting effects of block B, the frustration block, block A was compared to block C; analyses also included risk-taking and gender as variables.
Results
Participants
Of the 32 individuals who participated in the study, electrophysiological data collected from one boy participant were not useable. The mean age of the sample used was 15.07 (SD = .57) years. All individuals were fluent in English, lacked serious mental illness, and were right-handed as determined by the Edinburgh Handedness Inventory [38]. Descriptive data for BART scores may be found in Table 1.
Table 1. Age and data from the BART characterizing high and low risk-taking groups by gender.
| n | Age | Min Age | Max Age | Mean Score | Min Score | Max Score | |
| Boys | |||||||
| High BART | 7 | 14.90 ± .35 | 14.45 | 15.52 | 44.22 ± 7.52 | 35.19 | 54.94 |
| Low BART | 7 | 15.41 ± .51 | 14.77 | 16.02 | 13.49 ± 3.15 | 7.76 | 16.23 |
| Total Boys | 14 | 15.16 ± .49 | 14.45 | 16.02 | 28.85 ± 16.88 | 7.76 | 54.94 |
| Girls | |||||||
| High BART | 9 | 14.91 ± .60 | 14.07 | 15.47 | 41.79 ± 5.97 | 33.52 | 50.11 |
| Low BART | 6 | 14.86 ± .61 | 14.09 | 15.97 | 7.07 ± 3.89 | 1.8 | 13.00 |
| Total Girls | 15 | 14.88 ± .58 | 14.07 | 15.97 | 20.96 ± 18.20 | 1.8 | 50.11 |
| Total Sample | 29 | 15.01 ± .55 | 14.07 | 16.02 | 24.77 ± 17.72 | 1.8 | 54.94 |
Note: Data presented with means ± SD.
Go versus NoGo
Percentage of correct responses was significantly larger in Go conditions compared to NoGo conditions, F(1.87, 25) = 217.00, p < .001. Data for this comparison may be found in Table 2.
Table 2. Descriptive data for the Go/NoGo task averaged across blocks.
| Go Percent | Go Reaction | NoGo Percent | |
| Correct Total | Time (ms) | Correct Total | |
| Boys | |||
| High BART | .85 ± .06 | 260.28 ± 39.51 | .52 ± .06 |
| Low BART | .89 ± .06 | 288.82 ± 34.51 | .48 ± .06 |
| Total Boys | .87 ± .06 | 274.55 ± 38.46 | .50 ± .06 |
| Girls | |||
| High BART | .85 ± .07 | 273.25 ± 15.87 | .50 ± .05 |
| Low BART | .83 ± .08 | 276.86 ± 15.87 | .48 ± .12 |
| Total Girls | .84 ± .08 | 275.41 ± 35.87 | .49 ± .10 |
| Total Sample | .85 ± .07 | 275.00 ± 36.47 | .49 ± .08 |
Note: Data presented with means ± SD.
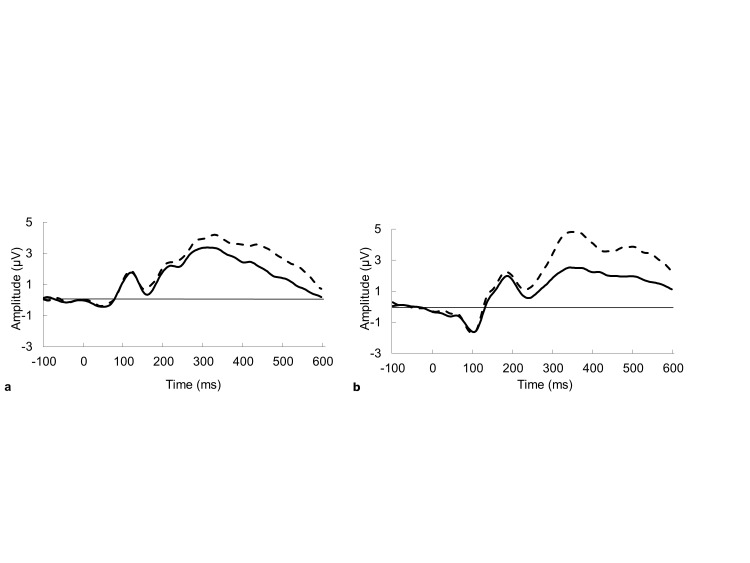
No significant main effects were observed for N2 at site Cz or Pz between Go and NoGo conditions. However, significant main effects for P3 were found at site Cz, F(1, 25) = 8.83, p = .006, and Pz, F(1, 25) = 5.36, p = .03, for condition type. At both sites, the NoGo condition elicited a larger positive amplitude than the Go condition.
A task-condition-by-gender interaction was significant for P3 at Pz, F(1, 25) = 4.83, p = .04. Post-hoc analyses showed the P3 amplitude for boys was significantly larger for Go (p = .04) and NoGo conditions (p = .003) than they were for girls; however, differences between condition types (Go, NoGo) was only significant within boys (p = .004; Figure 2).
Figure 2.
Grand average ERP for Go/NoGo response. Go response is represented by the solid black line and the NoGo response by the dashed black line; a., for site Cz, b., for site Pz.
Block Effects
The percentage of correct responses between all three blocks was significantly different within the Go condition, F(1.62, 40.50) = 27.12, p < .001. The reaction time to correct responses in the Go condition was significant, F(1.98, 49.38) = 46.85, p < .001, with reaction times fastest during block A and slowest during block B. No relationship with gender or risk-taking was found.
The percentage of correct responses between all three blocks was significantly different for the NoGo condition, F(1.79, 44.67) = 31.72, p < .001, with block C having the highest accuracy and block B having the lowest. No relationship with gender or risk-taking was found. All block data may be found in Table 3.
Table 3. Descriptive data for Go/NoGo task for each block; time values are in milliseconds.
| Go Percent Correct | Reaction Time | NoGo Percent Correct | |||||||
| Block A | Block B | Block C | Block A | Block B | Block C | Block A | Block B | Block C | |
| Boys | |||||||||
| High BART | .85 ± .05 | .80 ± .07 | .89 ± .05 | 294.62 ± 54.47 | 215.15 ± 45.04 | 271.05 ± 28.31 | .56 ± .09 | .36 ± .08 | .56 ± .05 |
| Low BART | .91 ± .09 | .84 ± .08 | .90 ± .05 | 322.14 ± 50.16 | 241.04 ± 22.08 | 303.28 ± 51.61 | .48 ± .17 | .39 ± .08 | .53 ± .08 |
| Total Boys | .90 ± .08 | .81 ± .07 | .92 ± .05 | 308.38 ± 52.29 | 228.10 ± 36.63 | 287.17 ± 43.35 | .52 ± .14 | .38 ± .06 | .54 ± .06 |
| Girls | |||||||||
| High BART | .90 ± .08 | .80 ± .12 | .87 ± .07 | 302.59 ± 37.31 | 247.69 ± 34.67 | 269.45 ± 31.73 | .53 ± .08 | .38 ± .07 | .54 ± .06 |
| Low BART | .88 ± .07 | .72 ± .15 | .86 ± .05 | 315.52 ± 54.44 | 221.48 ± 57.42 | 293.58 ± 41.20 | .46 ± .17 | .40 ± .13 | .55 ± .10 |
| Total Girls | .88 ± .07 | .75 ± .14 | .87 ± .06 | 310.35 ± 47.26 | 231.96 ± 49.90 | 283.93 ± 38.46 | .49 ± .14 | .39 ± .11 | .56 ± .08 |
| Total Sample | .88 ± .07 | .78 ± .12 | .89 ± .06 | 309.40 ± 48.86 | 230.60 ± 43.26 | 285.49 ± 40.18 | .50 ± .14 | .38 ± .10 | .54 ± .07 |
For both Go and NoGo conditions, no main effect differences were found between all block types at either site for the N2 component. Additionally, no main effect differences were found between all presentation blocks for either condition at site Cz or site Pz for the P3 component.
Block A versus Block C
The percentage of correct responses between block A and block C was significantly different between boys and girls for the Go condition, F(1, 25) = 7.31, p = .01, with boys performing with greater accuracy. There was a significant main effect of block for reaction time, F(1, 25) = 9.82, p = .004, with faster responses in block C than block A. No significant differences were found between blocks A and C for accuracy during the NoGo conditions.
No significant main effects were observed between blocks A and C at site Cz or Pz for the N2 component within the Go condition. No differences were seen relating to risk-taking or gender.
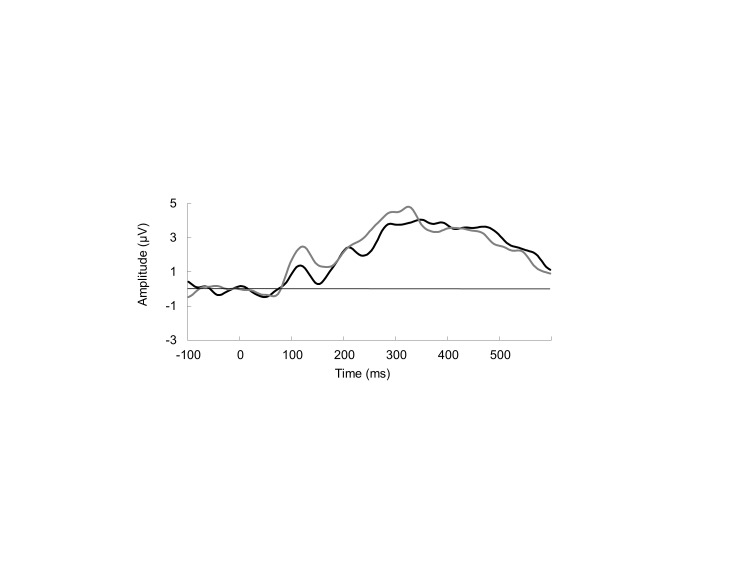
Significant main effects between blocks were found at Cz for N2 in the NoGo condition, F(1, 25) = 5.97, p = .02, with block C having a more negative amplitude than block A. No differences between gender or risk-taking groups were found (Figure 3).
Figure 3.
Grand average ERP for Go/NoGo response comparison between block A, solid black line, and block C, solid gray line.
No significant main effects were observed between blocks A and C at site Pz for the N2 component within the NoGo condition. No differences were seen for risk-taking or gender.
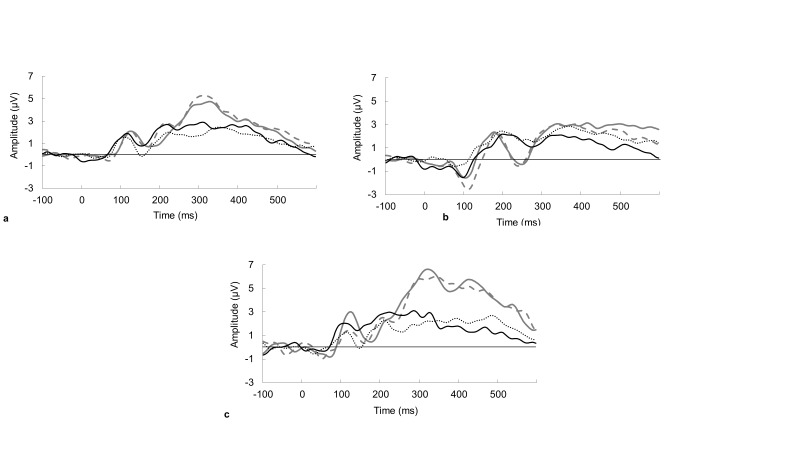
No significant main effects were found for P3 at Cz or Pz within either the Go or NoGo conditions. No significant differences were found for gender or risk-taking within Go or NoGo at site Cz or Pz for the P3 component. Data reflecting gender comparisons may be found in Figure 4.
Figure 4.
Grand average ERP gender comparisons for Go and NoGo responses between block A and block C. Boy block A is represented by the solid black line, block C by dashed black line, and girl block A solid gray lines, and block C by dotted gray line; a., Go response at Cz, b., Go response at Pz, c., NoGo response at C.
Discussion
Our current data from the initial Go versus NoGo comparisons support previous studies suggesting that greater responses in correct NoGo trials reflect better response inhibition. Surprisingly, greater gender-related differences were observed than those relating to risk-taking. For example, Go versus NoGo differences seemed to be driven by responses in boys, similar to previous studies [34]. The current neurophysiological correlates suggest greater neural responses in the NoGo condition between gender groups relating to overt response inhibition.
A unique interest was the effect of a frustration-inducing block on non-frustrating trials, and particularly if there were gender-related differences. Early response differences and greater accuracy among boys during the Go condition suggest girls may have greater lasting effects after frustration induction. While not significant, later responses during pre-frustration trials were larger than post-frustration responses in both the Go condition (boys only) and NoGo condition (both genders), which suggest lasting gender-related effects of frustration for response inhibition. These effects may reflect differences in choice to engage in rewarding behavior even with known risks, and suggest stressful or pressured situations may elicit different decision-making in boys compared to girls with PCE. Here, we suggest girls with PCE may not have found the task as frustrating, may be less susceptible to practice effects, or may mature through greater reactivity difficulties earlier, or these findings may be neurophysiological correlates of gender-related differences like those reported for risk-taking [21,23,39]. Since risk-taking is a multifaceted construct, lack of risk-taking effects may be a result of these thoughts as well. While this pattern may be thought to present among a NDE population, additional study is needed to understand the pattern and relationship between a NDE population and those with PCE. The current data support the perspective of gender being an important factor to consider [40,41] and may hold greater implications related to inhibition, sensation-seeking, and conflict-monitoring with PCE.
These data suggest regulatory-system deficits observed in children with PCE may persist into adolescence, although a control group without PCE is needed to make this statement with greater authority [39,43]. Greater neurophysiological reactivity related to inhibition and sensation-seeking in boys provides support and insight into the more frequently reported risk behavior among boys with PCE [21,23]. The effects of a frustration trial for the correlate of conflict-monitoring and inhibition in girls may be related to risk behaviors, although this possibility warrants additional direct examination. The current data provide neurophysiological evidence for the persisting effect PCE may have on regions impacted by PCE [12,13,14].
While the current findings and perspectives are sound, there exist limitations warranting mention. First, the cohort evaluated did not include a NDE group. As such, comparisons between adolescents with and without PCE as a whole were not evaluated or whether gender-related differences are within the healthy control population or only adolescents with PCE. However, cocaine use during pregnancy remains an important problem; therefore, studies such as this are necessary to understand this at-risk population. The sample size of the current study is also of note. With the multiple comparisons conducted, there is risk of type-1 errors. However, similar sample sizes have been successfully used in comparable studies [34]. The lack of differences between high and low risk-taking groups may reflect the small sample size, though risk-related differences have been noted in this population elsewhere [34]. The sample was also geographically confined to the greater New Haven area and primarily African-American. Thus, the extent to which the findings generalize to other groups warrants investigation. Sustained-attention impairments have been reported in children with PCE [5]; if such impairment persists into adolescence, it may impact performance on the Go/NoGo. Additionally, potential changes in frustration during task blocks were not directly evaluated and could not be examined with respect to gender, BART performance or ERP changes. It is important to note these limitations, as well as suggest these as important avenues to be evaluated in future studies.
Though the current study has limitations, the data suggest important differences among adolescents with PCE, and these differences may be more related to gender than risk-taking propensity. Thus, considering gender-related differences is important in future studies evaluating adolescents with PCE. While Go/NoGo tasks are used to evaluate inhibition, the added component of a frustration block during the task allowed for a comparison between pre- and post-frustration blocks. Further study is required to understand how inhibition and conflict monitoring in adolescents with PCE may vary from NDE adolescents but also may reflect the development of risky behaviors, such as substance abuse, in both populations.
Acknowledgments
The authors would like to thank the participants and their families for their time and participation in the current study. We would also like to thank all research assistants and others involved with the data collection process.
Abbreviations
- PCE
Prenatal cocaine exposure
- NDE
Non-drug exposed
- PFC
Prefrontal cortex
- ACC
Anterior cingulate cortex
- ERP
Event-related potential
- BART
Balloon Analogue Risk Task
Funding
This study supported by a NARSAD Young Investigator Award (MJC), NIAAA grants T32 AA015496 (BCB) and RL1 AA017539 (MNP), NIDA grants T32 DA007238 (BCB), K01 DA034125 (MJC), R01 DA06025 (LCM), RL1 DA017863 (LCM), R01 DA018647 (MNP) and K05 DA020091 (LCM), a grant from the Gustavus and Louise Pfeiffer Research Foundation (LCM), the Connecticut State Department of Mental Health and Addictions Services (MNP), CASAColumbia (MNP), and the Yale Gambling Center of Research Excellence grant from the National Center for Responsible Gaming (MNP). None of the funding agencies had a role in the drafting of the manuscript or the decision to submit the paper for publication.
References
- Lester BM, Lagasse LL. Children of addicted women. J Addict Dis. 2010;29(2):259–276. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA. et al. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131(6):e1917–e1916. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lester BM. Reconceptualizing in a dual-system model the effects of prenatal cocaine exposure on adolescent development: a short review. Int J Dev Neurosci. 2011;29(8):803–809. doi: 10.1016/j.ijdevneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, Mayes LC. Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicol Teratol. 2011;33(1):47–60. doi: 10.1016/j.ntt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125(3):554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. . Dev Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V. et al. Developmental effects of reward on sustained attention networks. Neuroimage. 2011;56(3):1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- DeWitt SJ, Aslan S, Filbey FM. Adolescent risk-taking and resting state functional connectivity. Psychiatry Res. 2014;222(3):157–164. doi: 10.1016/j.pscychresns.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Casey B, Orendi JL, Trainor RJ. et al. A functional MRI study of sex differences in prefrontal activation during performance of a go-no-go task. Neuroimage. 1996;3(3):S174. [Google Scholar]
- Eshel N, Nelson EE, Blair RJ. et al. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG. Prenatal cocaine exposure decreases parvalbumin-immunoreactive neurons and GABA-to-projection neuron ratio in the medial prefrontal cortex. Dev Neurosci. 2012;34(2-3):174–183. doi: 10.1159/000337172. [DOI] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27(8):751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Yip SW, Potenza EB, Balodis IM. et al. Prenatal cocaine exposure and adolescent neural responses to appetitive and stressful stimuli. Neuropsychopharmacology. 2014;39(12):2824–2834. doi: 10.1038/npp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh KN, Mayes LC, Potenza MN. Risk-taking and decision-making in youth: relationships to addiction vulnerability. J Behav Addict. 2013;2(1) doi: 10.1556/JBA.2.2013.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F. Affective and Deliberative Processes in Risky Choice: Age Differences in Risk Taking in the Columbia Card Task. J Exp Psychol Learn Mem Cogn. 2009;35(3):21. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H. et al. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, Mayes LC, Potenza MN. Neurobiology of adolescent substance use disorders: implications for prevention and treatment. Child Adolesc Psychiatr Clin N Am. 2010;19(3):479–492. doi: 10.1016/j.chc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH. et al. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol. 2011;33(1):110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Lin H, Degarmo DS. et al. Neurobehavioral disinhibition predicts initiation of substance use in children with prenatal cocaine exposure. Drug Alcohol Depend. 2012;126(1-2):80–86. doi: 10.1016/j.drugalcdep.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert BL, Bann CM, Bauer CR. et al. Risk-taking behavior among adolescents with prenatal drug exposure and extrauterine environmental adversity. J Dev Behav Pediatr. 2013;34(9):669–679. doi: 10.1097/01.DBP.0000437726.16588.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JP, Miller DC, Schafer WD. Gender differences in risk taking: A meta-analysis. Psychol Bull. 1999;125(3):367–383. [Google Scholar]
- Allen JW, Bennett DS, Carmody DP. et al. Adolescent risk-taking as a function of prenatal cocaine exposure and biological sex. Neurotoxicol Teratol. 2014;41:65–70. doi: 10.1016/j.ntt.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. Eur J Neurosci. 2004;20(9):2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y, v J, Jin Y. et al. The time course of novelty processing in sensation seeking: an ERP study. Int J Psychophysiol. 2010;76(2):57–63. doi: 10.1016/j.ijpsycho.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Oddy BW, Barry RJ. The relationship of N2 and P3 to inhibitory processing of social drinkers in a Go/NoGo task. Int J Psychophysiol. 2009;72(3):323–330. doi: 10.1016/j.ijpsycho.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoorman J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101(2-3):25. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Harper J, Malone SM, Bernat EM. Theta and delta band activity explains N2 and P3 ERP component activity in a go/no-go task. Clin Neurophysiol. 2014;125(1):124–132. doi: 10.1016/j.clinph.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ. et al. Neurophysiological Correlates of Emotion Regulation in Children and Adolescents. J Cogn Neurosci. 2006;18(3):13. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: disentangling the contributions of age and executive function. Neuropsychologia. 2006;44(11):2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW. et al. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent Health Risk Behavior as a Function of Prenatal Cocaine Exposure and Gender. J Dev Behav Pediatr. 2007;28(6):467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Guidelines for psychological practice with lesbian, gay, and bisexual clients. Am Psychol. 2012;67(1):10–42. doi: 10.1037/a0024659. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C. et al. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Dev Neurosci. 2009;31(1-2):137–148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key AP. et al. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27(6):797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Lamm C, Granic I, Zelazo PD. et al. Magnitude and chronometry of neural mechanisms of emotion regulation in subtypes of aggressive children. Brain Cogn. 2011;77(2):159–169. doi: 10.1016/j.bandc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM. et al. he polar reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol. 1999;110(6):7. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9(1):16. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D. et al. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42(4):688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler L, Bennett DB, Carmody DP, In: Gender Differences in Prenatal Substance Exposure. Lewis M, Kestler L, editors. Washington DC: American Psychological Association; 2012. Gender dependent effects of prenatal cocaine exposure. pp. 11–29. [Google Scholar]
- Min MO, Minnes S, Lang A. et al. Effects of prenatal cocaine exposure on early sexual behavior: Gender difference in externalizing behavior as a mediator. Drug Alcohol Depend. 2015;153:59–65. doi: 10.1016/j.drugalcdep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, McAuliffe S, Kachadourian L. et al. Effects of prenatal cocaine exposure on infant reactivity and regulation. Neurotoxicol and Teratol. 2009;31(1):60–68. doi: 10.1016/j.ntt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN. et al. Functional MRI and response inhibition in children exposed to cocaine in utero. Dev Neurosci. 2009;31(1-2):159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]