Abstract
Study Objectives:
While women report sleep interruption secondary to nighttime hot flashes, the sleep disrupting impact of nocturnal hot flashes (HF) is not well characterized. We utilized a model of induced HF to investigate the relationship of nighttime HF to sleep architecture and sleep-stage transitions.
Methods:
Twenty-eight healthy, premenopausal volunteers received the depot gonadotropin-releasing hormone agonist (GnRHa) leuprolide to rapidly induce menopause, manifesting with HF. Sleep disruption was measured on 2 polysomnograms conducted before and after 4–5 weeks on leuprolide, when HF had developed.
Results:
165 HF episodes were recorded objectively during 48 sleep studies (mean 3.4 HF/night). After standardizing to sleep-stage time distribution, the majority of HF were recorded during wake (51.0%) and stage N1 (18.8%). Sixty-six percent of HF occurred within 5 minutes of an awakening, with 80% occurring just before or during the awakening. Objective HF were not associated with sleep disruption as measured by increased transitions to wake or N1, but self-reported nocturnal HF correlated with an increase from pre- to post-leuprolide in the rate of transitions to wake (p = 0.01), and to N1 (p = 0.008).
Conclusions:
By isolating the effect of HF on sleep in women without the confound of age-related sleep changes associated with natural menopause, this experimental model shows that HF arise most commonly during N1 and wake, typically preceding or occurring simultaneously with wake episodes. Perception of HF, but not objective HF, is linked to increased sleep-stage transitions, suggesting that sleep disruption increases awareness of and memory for nighttime HF events.
Clinical Trial Registration:
ClinicalTrials.gov Identifier: NCT01116401.
Citation:
Bianchi MT, Kim S, Galvan T, White DP, Joffe H. Nocturnal hot flashes: relationship to objective awakenings and sleep stage transitions. J Clin Sleep Med 2016;12(7):1003–1009.
Keywords: menopause, hot flashes, vasomotor symptom, polysomnography, sleep, sleep disruption, sleep stages, sleep-wake stages, sleep architecture
INTRODUCTION
During midlife, sleep disturbance and insomnia increase significantly in women, with those who are perimenopausal and experiencing hot flashes most likely to develop such sleep problems. Hot flashes reduce perceived sleep quality by awakening women repeatedly throughout the night. Studies in midlife women have shown that nocturnal hot flashes were most likely to occur during stage N2 sleep and are commonly linked with an awakening.1–3 The link between nocturnal hot flashes and awakenings has raised the question of whether hot flashes directly induce awakenings. Evidence supporting this explanation includes data from peri- and postmenopausal women showing that 54% to 69% of objectively measured nighttime hot flashes are associated with an awakening.1–3 Among those hot flashes occurring proximate to an awakening, the specific temporal sequence between the onset of the awakening and the flash beginning varies, with studies showing that objectively measured nighttime hot flashes precede or begin simultaneously with the awakening 54% to 90% of time.1–3 The observation that a hot flash can begin after the onset of the awakening in some hot flash/wake episode pairs, particularly during the second half of the night,3 raises the possibility that hot flashes do not uniformly induce awakenings,2 even when they are temporally linked.
BRIEF SUMMARY
Current Knowledge/Study Rationale: It is well known that self-reported hot flashes are associated with sleep complaints in menopause. However, the impact of nocturnal hot flashes on objective sleep measures in women remains incompletely understood.
Study Impact: Most objectively measured hot flashes are associated with PSG-defined wake and N1. Objective sleep disruption is linked with the perception of hot flashes.
Using controlled experimental studies, we established that new-onset hot flashes disrupt sleep by increasing the amount of wake time after sleep onset (WASO) and the number of awakenings measured objectively with polysomnography (PSG) proportionate to the number of nighttime hot flashes reported in the morning upon awakening.4 WASO and awakenings similarly increased in association with the number of nighttime hot flashes recorded during the night using skin conductance monitoring that does not rely on subjective perception of or recall for these symptoms. This observed effect of nighttime hot flashes occurred in the absence of any change in sleep architecture measured using standard clinical metrics of stage percentage. However, the possibility remains that nighttime hot flashes might disrupt sleep by increasing the rate of sleep stage transitions to wake and light sleep without altering the overall time (or percentage of time) spent in each sleep stage, as we have previously shown occurs in obstructive sleep apnea using transition-based metrics that are a more sensitive measure of sleep architecture disturbance.5
We investigated the impact of nocturnal hot flashes on sleep-stage transitions in healthy young adult women in whom hot flashes were induced by administration of the gonadotropin-releasing hormone agonist (GnRHa) leuprolide in order to isolate the effect of hot flashes on sleep in the absence of age-related sleep changes. This is similar to the setting in which young women develop hot flashes and sleep disturbance after bilateral oophorectomy or estrogen deprivation treatments in breast cancer patients. We hypothesized that objectively measured hot flashes would most likely occur during or immediately preceding a wake episode and that hot flashes would be linked with an increase in sleep disruption, as measured by the rate of sleep-stage transitions.
METHODS
Premenopausal healthy volunteers without primary sleep disorders were administered open-label depot leuprolide to rapidly suppress endogenous estradiol for the duration of the study,6–11 thereby inducing hot flashes. Two at-home PSG studies were completed prior to and then repeated 4 weeks after leuprolide administration, during which time hot flashes were reported to have developed in a subset of participants. Physiologic evidence of hot flash events was recorded during the night using a hot flash skin conductance monitor that was time synched to the PSG recording system in order to examine the patterns of association of individual nocturnal hot flash events and sleep architecture. All participants provided written informed consent for study procedures, which were approved by our local institutional review board.
Study Participants
Twenty-nine healthy premenopausal volunteers ages 18–45 years were enrolled. All underwent a standard in-laboratory PSG using American Academy of Sleep Medicine (AASM) scoring procedures12 to exclude primary sleep disorders of sleep apnea and periodic limb movement. Eligible participants had regular menstrual cycles, no hot flashes, primary sleep disorders, psychiatric illness, or substance use disorders, normal laboratory studies (prolactin, thyroid, liver, and renal function), and no insomnia on clinical interview. None were using centrally active medications (e.g., antidepressants, benzodiazepines, corticosteroids, hypnotics) or medications known to suppress hot flashes (e.g., birth control preparations, serotonergic agents, gabapentin).
Study Procedures
Detailed study procedures are provided elsewhere.4 Briefly, prior to leuprolide administration, participants completed two ambulatory PSGs. Subjects were hooked up to the unit in our offices and then sent home. They were then given a single open-label intramuscular injection of depot leuprolide 3.75 mg during the mid-luteal phase of the menstrual cycle. Following leuprolide administration, participants were monitored for the onset of hot flashes using daily hot flashes diaries. Serum estradiol levels (liquid chromatography, tandem mass spec-trometry)13,14 confirmed sustained ovarian suppression at 1, 2, and 4 weeks after leuprolide administration. One subject was excluded from the current analysis because her objective hot flash data were not recorded due to a device error. Two post-leuprolide ambulatory PSGs were obtained 4 weeks after leuprolide administration in each subject. One subject completed only one post-leuprolide PSG, leaving 55 recording nights in 28 subjects for analysis. Nighttime hot flashes were measured using a skin conductance monitor during each of the post-leuprolide PSGs.
Study Measures
Polysomnography
The screening PSG recorded standard AASM-recommended channels, including respiration and bilateral anterior tibialis electromyography. Ambulatory PSGs were conducted using the Safiro (Compumedics Limited, Charlotte, NC) ambulatory unit. Standard procedures were used to define sleep staging, including electroencephalography (EEG; C3-A2, C4-A1, O1-A2, O2-A1), bilateral electro-oculography, and submental electromyography. Standard AASM scoring methods were used to define the N1, N2, N3, rapid eye movement (REM), and total sleep time.12 Sleep scoring was completed by Harvard Sleep & EEG Core PSG scorers, who were blinded to hot flash events. Event markers on an actigraphic watch time synched to the PSG were used to establish lights-out and lights-on times.
Total sleep time was a direct summation of the time spent in each sleep stage (REM + N1 + N2 + N3), while total time in bed was calculated as the time between lights off and lights on. Time in bed was calculated as the difference between lights out and lights on clock time based on actigraphic event markers and, where not available (20% of nights), sleep diary reported times.
Sleep was scored manually in standard 30-s epochs. Sleep stages were processed via custom MATLAB scripts to extract the time and duration of bouts for all stages (N1, N2, N3, REM, WASO). Each bout duration represents the amount of consecutive time spent in a given sleep-wake stage, in 30-s epochs. For example, the sequence W-N2-N2-R-R-R-W consists of a 2-epoch duration bout of N2, and a 3-epoch duration bout of REM. In this way, the number of transitions for each stage, as well as the distribution of time spent in each bout, was evaluated. Transitions refer to entry into a stage, such that if a subject had 10 REM transitions, it means that we observed 10 bouts of REM sleep of at least one 30-s epoch duration. Transitions can occur for several reasons during sleep, including normal transitions of sleep stage cycling. Transitions caused by “wake-like activity” may or may not be associated with a change in scoring of the epoch in which it occurs, depending on the duration of the activity within a given epoch and other sleep EEG features. For this reason, we focused on the transitions to wake, or to N1. Increased frequency of transitions to wake or N1 will necessarily reduce the bout duration of other stages, and metrics quantifying disrupted sleep architecture that focus on transitions to wake and N1 are more sensitive markers of objective disturbance than sleep stage percentage.5
Vasomotor Symptoms
Physiologic evidence of hot flashes was recorded using the Bahr skin conductance monitor (Simplex Scientific, Middleton, WI) and identified using the standard Bahr software (Version 1.1.40).4,15 The device detects individual hot flash events in 60-s intervals by measuring transient changes in sternal skin conductance. The monitor was time-synched to the PSG. Daytime and nighttime hot flashes were also recorded subjectively using a daily diary throughout the study, including specifically the night of each PSG.
Sleep Questionnaires
Sleep diaries collected during the week of the post-leuprolide PSG captured self-reported sleep-onset latency, wake time after sleep onset (WASO), number of awakenings, and sleep efficiency. The Insomnia Severity Index (ISI; range 0–28)16 and Pittsburgh Sleep Quality Index (PSQI; range 0–21),17 and Fatigue Severity Scale (FSS; range 9–63)18 were also obtained to characterize the study population.
Analytical Methods
Within-night correlational analyses were conducted using Spearman correlation coefficients. Sleep-stage time distribution analyses were standardized for each subject. Hot flashes were determined to be linked with an awakening if they occurred within 5 minutes before or after an awakening. Consistent with prior studies,3 a hot flash was defined as: (1) preceding an awakening if the hot flash epoch began up to 5 minutes before the start of a wake episode, (2) occurring simultaneous with an awakening if the hot flash epoch began during a wake episode, and (3) occurring after an awakening if the hot flash epoch began up to 5 minutes after the start of a wake episode. For sleep stage transition analyses, mean values between the 2 post-leuprolide PSG nights hot flash frequencies (subjectively reported or objectively measured) and between the 2 pre-leuprolide and 2 post-leuprolide sleep stage transition rates to wake and stage N1 were first calculated for each subject. Correlational analyses were then conducted using Spearman correlation coefficients at the subject level between hot flash frequency and either post-leuprolide or within-subject change from pre- to post-leuprolide in sleep stage transition rates.
RESULTS
Study Participants
Nocturnal hot flashes were recorded during at least one of the 2 post-leuprolide PSGs in 28 study participants. There were a total of 165 nighttime hot flashes recorded on the 48 PSGs during which at least one hot flash was recorded. Women with hot flashes recorded during the PSG were on average 27.3 years old (standard deviation [SD] 7.4), had a mean BMI 25.0 kg/m2 (SD 4.9), and 25% were African American. Serum estradiol was suppressed to postmenopausal levels in all subjects at the time of the PSGs.
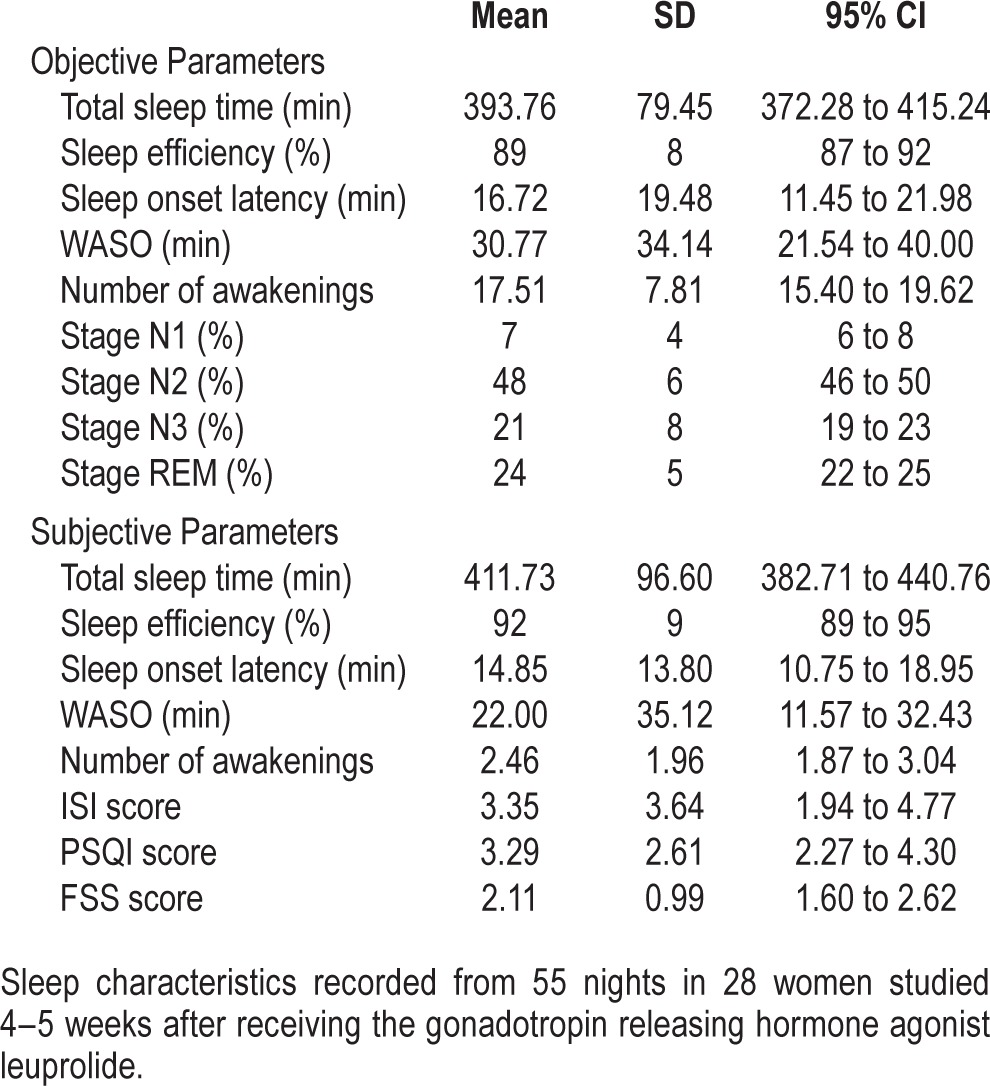
Prior to receiving leuprolide, ambulatory PSGs in the healthy volunteers revealed a mean sleep efficiency of 91.0%, sleep-onset latency of 17.0 min, WASO of 21.0 min, and 16.0 awakenings per night, with normal sleep stage percentages. During the post-leuprolide assessments, there were 3.4 ± 1.8 hot flashes recorded on the monitor per person per PSG night and 2.6 ± 1.6 reported subjectively per night per person in the morning after each PSG. There was no correlation between the number of subjectively reported and objectively measured hot flashes (rs = 0.24, p = 0.08). After developing nocturnal hot flashes, PSG parameters revealed a mean sleep efficiency of 89.0%, sleep-onset latency of 17.3 min, WASO of 31.8 min, and 17.9 awakenings per night (Table 1).
Table 1.
Sleep characteristics.
Total sleep time and time in bed were both positively correlated with the number of nighttime hot flashes measured (rs = 0.29, p = 0.03; rs = 0.37, p = 0.005, respectively). For every additional nighttime hot flash measured, total sleep time was increased by 9.8 minutes (95% confidence interval [CI] 0.4 – 20.0) and time in bed by 15.7 minutes (95% CI 5.4 – 26.0 min).
Distribution of Hot Flashes by Sleep Stage and Temporal Relationship to Awakenings
The distribution of nighttime hot flashes by sleep stage is shown in Figure 1. Before accounting for the amount of time spent in each sleep stage, hot flashes were most common in stage N2 (38.9%), followed by wake (27.4%). However, after adjusting for time spent in each stage, the majority (51.0%) of hot flashes occurred during wake, followed by stage N1 (18.8%). A small fraction of nighttime hot flashes (5.6%) occurred during REM. There was no difference in the hot flash distribution by sleep stage between the first and second half of the night (p > 0.05).
Figure 1. Distribution of objective hot flashes by sleep stage.
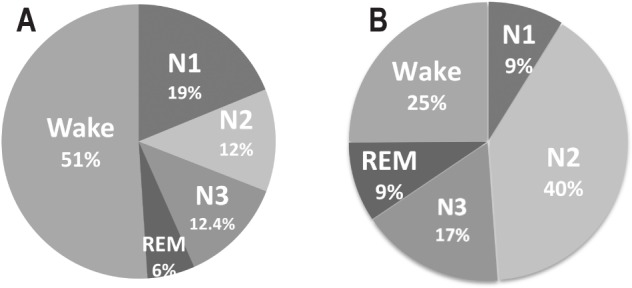
(A) Not adjusted for amount of time spent in each stage and (B) adjusted for amount of time spent in each stage. Proportions of hot flashes calculated from total 165 objective nocturnal hot flashes during 48 post-leuprolide ambulatory PSGs in n = 28 subjects.
Of 165 nighttime hot flashes detected, 109 (66%) occurred during an interval incorporating the wake bout, defined as 5 minutes before through 5 minutes after an awakening. Analysis of the temporal pattern between hot flashes and PSG awakenings indicated that the majority (80%) of nighttime hot flashes occurred before (38.2%) or concurrently (42.2%) with an awakening, with only 20% of nighttime hot flashes occurring after a wake bout. This temporal pattern was consistent across the night and did not differ between the first and second half of the night (Figure 2).
Figure 2. Objective hot flashes by halves of night.
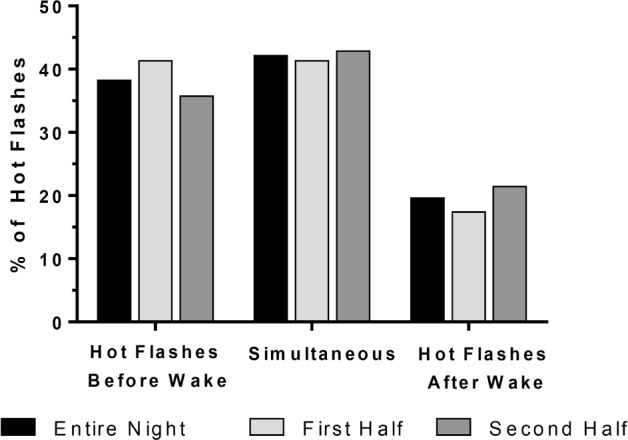
Proportion of objective nocturnal hot flashes measured within 5 minutes of awakenings during post-leuprolide ambulatory PSGs for entire night and by halves of night. Percent of hot flashes were calculated out of a total of 109 objective hot flashes that were linked with an awakening during 48 post-leuprolide ambulatory PSGs in 28 subjects.
Impact of Hot Flashes on Sleep-Wake Stage Dynamics
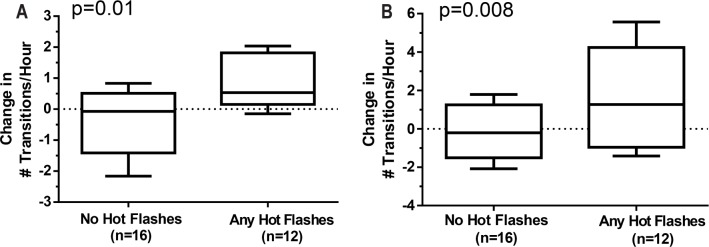
Sleep architecture disturbance can be quantified by an increase in stage transitions per time to wake and N1. The number of hot flashes during the post-leuprolide PSGs did not correlate with the change from pre- to post-leuprolide in the transitions rate to wake or N1, regardless of whether hot flashes were recorded on the monitor or reported subjectively in the morning after the PSG. However, the change in the rate of transition to wake (rs = 0.43, p = 0.01) and to N1 (rs = 0.35, p = 0.008) from pre- to post-leuprolide increased in association with the number of self-reported nocturnal hot flashes reported during the post-leuprolide PSGs (Figure 3).
Figure 3. Change in number of stage transitions on PSG from baseline to post-leuprolide.
(A) # Wake transitions, (B) # N1 transitions, according to the presence or absence of subjectively reported nighttime hot flashes during PSG studies (n = 28).
DISCUSSION
Results of this experimental protocol demonstrate that objectively measured hot flashes arise most commonly during N1 and wake, typically preceding or occurring simultaneously with wake episodes, and that the number of hot flashes reported at night correlates with worsening of sleep disturbance indices. After accounting for the proportion of time spent in each sleep stage, hot flashes occurred most commonly during the sleep stages of wake and N1. Three-quarters of hot flashes were temporally linked to an awakening, the majority of which either preceded or occurred simultaneously with the onset of the awakening. Awakenings preceded the onset of a flash in only 20% of linked hot flash-awakening pairs. While the rate of sleep stage transitions to wake and N1 did not correlate with hot flash frequency as measured with skin conductance methodology, self-reported nocturnal hot flashes correlated strongly with an increase from pre- to post-leuprolide in the rate of transitions to both wake and N1. Taken together, these results provide further evidence that hot flashes are closely linked with sleep interruption and suggest that sleep disturbance increases awareness of and memory for nighttime hot flash events.
The observation that, per unit of time spent in each sleep stage, the majority of hot flashes occur in wake and N1 highlights their non-random distribution by sleep-wake stage. The concentration of hot flashes during times in which the EEG is characterized by high frequency/low amplitude waveforms has several potential explanations. First, hot flashes may cause awakenings and enhance N1 sleep because of the arousing nature of the physical discomfort of sweating at night. We did not observe an increase in time spent in stage N1, but there may not have been enough objective hot flashes per night to detect a change in N1. Alternatively, the transient increase in core body temperature of up to 0.1°C that usually precedes each flash may also induce an awakening,19–21 as has been observed in other clinical settings.22 However, it is also plausible that N1 sleep and brief awakenings are more closely linked with hot flashes because neural factors that drive wakefulness also increase susceptibility to hot flashes, which are similarly triggered by cortical activation.23
While we observed that hot flashes were more likely to be detected physiologically prior to or simultaneous with an awakening rather than following the onset of the awakening, it is important to recognize that this temporal pattern does not imply a specific causal link between the events. The increase in skin conductance measures the heat dissipation response to the central nervous system stimulus for the flash, and is therefore delayed after the brainstem activation that precedes the peripheral measurement of the flash.24 While other studies have concluded that hot flashes do not cause awakenings because more than half were identified after the awakening began,2 this reverse temporal pattern does not negate the possibility that changes in temperature or other perturbations in autonomic nervous system activity that trigger hot flashes may also cause an awakening through shared parallel mechanisms or a cascade phenomenon.
Our analysis did not confirm our hypotheses that there would be more sleep disturbance in association with the number of objectively detected hot flashes. We measured sleep disturbance through transition analysis in order to explore this hypothesis, which can capture a form of disturbance that is not captured by either sleep efficiency or stage percentage. The lack of an effect by these analytics indicates that the sleep-wake stage dynamics are not consistently disrupted in relation to these individual physiologic hot flash events. It is notable, however, that the number of subjectively reported nighttime hot flashes correlated with an increase in sleep disturbance, as indicated by the rate of wake and N1 transitions relative to baseline measurements. Subjective reporting of nighttime events requires that an individual must awaken during the night long enough to consolidate memory for the event or experience sufficiently disrupted sleep to register the event. Given that the number of hot flashes reported to have occurred at night is a limited number of discrete events, it is not likely that these events account for all of the additional sleep-wake stage transitions. We therefore hypothesize that women are more likely to report being aware of hot flashes at night when they experience more sleep stage transitions, which increases awareness of and memory for nighttime events. Individual differences in several concurrent aspects of physiology may be playing roles including susceptibility to objective disruption, susceptibility to objective hot flashes, and the subjective experience of hot flashes. Examples of dissociation between subjective experience and objective measurements are not uncommon in sleep medicine. For example, patients with insomnia may exhibit misperception,25 patients with sleep apnea may not report daytime sleepiness,26 and subjects undergoing sleep deprivation may show performance impairment without corresponding subjective insight.27
REM sleep is associated with decreased thermoregulation relative to non-rapid eye movement (NREM) sleep stages,28 and this is of particular interest with regards to nocturnal hot flashes. Results of our study are consistent with others conducted in midlife women with hot flashes that found a small proportion of hot flashes to occur during REM,1 and inconsistent with other studies which found no flashes during REM sleep.2 Our study also differs from others2 because we observed no difference in the association between hot flashes and awakenings during the first vs. the second half of the night. Other investigators have reported that the distribution of hot flashes in postmenopausal women in REM vs. NREM differed between the first versus second half of the night.3 Our results differ, perhaps due to the different mechanisms of hot flashes (natural versus drug-induced), or the age of the populations studied.
Consistent with other investigations of menopause-related sleep disturbance conducted in ambulatory settings,29,30 we observed that nighttime hot flashes are linked with an increase in total sleep time (TST) and time in bed (TIB). Our study extends these findings by demonstrating this association with objectively measured hot flashes, whereas prior studies observed this association using subjectively reported hot flashes. While increased TST and TIB may be interpreted as an improvement in sleep, alternate explanations are possible. For example, sleep disturbance from chronic nighttime hot flashes could lead to accumulation of homeostatic drive, resulting in increased TST (and indirectly increased TIB). Thus, in the absence of frank sleep deprivation, sleep extension is possible.31 It is also plausible that the association of nocturnal hot flashes with increased TST and TIB may result from increased opportunity to observe hot flashes between lights out and lights on. However, these increases were small relative to the hot flash frequency, and prior studies have made similar observations when nighttime hot flashes were not distinguished from daytime symptoms, eliminating the opportunity bias.29,30
This study advances our understanding of the complex relationship between hot flashes and sleep disturbance by isolating new-onset hot flashes in young women with no other sleep problems, including no age-related sleep changes which may confound cross-sectional studies in midlife women with hot flashes. The experimental design allows for calculations of within-woman change in key sleep parameters. Use of ambulatory PSGs conducted in the subjects' homes introduces additional variability because temperature and other environmental factors cannot be controlled. However, this methodology is also a strength because investigation in the natural home environment eliminates potential confounds introduced by obtaining data in a novel sleeping environment.
In summary, our model of hormone-induced menopause and hot flashes allows for controlled dissection of the relationship of objective and subjective hot flashes to objective sleep architecture dynamics in a natural home setting. Objectively measured hot flashes occur most densely during N1 and wake, when they predominately precede or co-occur with an awakening, but it is subjective recall for nocturnal hot flashes that is linked with an increase in sleep disruption. While hot flashes are linked with individual awakenings, it appears that it is the perception of and memory for these nocturnal events that correlates with global sleep disruption. This differential relationship of sleep disruption with subjective versus objective hot flashes has important implications for clinical practice, in which self-report is the gold standard for both hot flashes and for insomnia.
DISCLOSURE STATEMENT
This was not an industry-supported study. Funding source was 5R01MH082922 (HJ). ClinicalTrials.gov Identifier: NCT01116401. Dr. Joffe receives grant support from the National Institutes of Health, Merck, and serves as a consultant/advisor for Noven, Merck, and Mitsubishi Tanabe. Dr. Bianchi receives funding from the Center for Integration of Medicine and Innovative Technology, the Department of Defense, and the Milton Family Foundation; has a patent pending on a home sleep monitoring device; has received travel funding from Servier; serves on the advisory board of Foramis; is a consultant for GrandRounds; has provided expert testimony in sleep medicine. Dr. White is the Chief Medical Officer for Apnicure Inc and is a consultant for Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Judith Boucher, RPSGT, and Susan Dougherty, RPSGT, for assistance conducting the ambulatory PSG studies, Brandon Lockyer, RPSGT, in the Sleep & EEG Core of the Harvard Medical School Division of Sleep Medicine for assistance with sleep stage scoring, and Aleta Wiley, MPH, for analytic support. The work was performed at Massachusetts General Hospital and the Brigham and Women's Hospital.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- CI
confidence interval
- EEG
electroencephalography
- FSS
Fatigue Severity Scale
- GnRHa
gonadotropin-releasing hormone agonist
- HF
hot flashes
- ISI
Insomnia Severity Index
- NREM
non-rapid eye movement
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- REM
rapid eye movement
- rs
Spearman correlation coefficient
- SD
standard deviation
- TIB
time in bed
- TST
total sleep time
- WASO
wake time after sleep onset
REFERENCES
- 1.de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102:1708–15 e1. doi: 10.1016/j.fertnstert.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82:138–44. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576–83. doi: 10.1097/01.gme.0000227398.53192.bc. [DOI] [PubMed] [Google Scholar]
- 4.Joffe H, Crawford S, Economou N, et al. A gonadotropin-release hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013;36:1977–85. doi: 10.5665/sleep.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi MT, Cash SS, Mietus J, Peng CK, Thomas R. Obstructive sleep apnea alters sleep stage transition dynamics. PloS One. 2010;5:e11356. doi: 10.1371/journal.pone.0011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.San Roman GA, Surrey ES, Judd HL, Kerin JF. A prospective randomized comparison of luteal phase versus concurrent follicular phase initiation of gonadotropin-releasing hormone agonist for in vitro fertilization. Fertil Steril. 1992;58:744–9. doi: 10.1016/s0015-0282(16)55322-9. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum DR, Wisot A, Hamilton F, Gutlay AL, Huynh D, Kempton W. Timing of initiation and dose schedule of leuprolide influence the time course of ovarian suppression. Fertil Steril. 1988;50:400–2. doi: 10.1016/s0015-0282(16)60121-8. [DOI] [PubMed] [Google Scholar]
- 8.Gelety TJ, Pearlstone AC, Surrey ES. Short-term endocrine response to gonadotropin-releasing hormone agonist initiated in the early follicular, midluteal, or late luteal phase in normally cycling women. Fertil Steril. 1995;64:1074–80. doi: 10.1016/s0015-0282(16)57963-1. [DOI] [PubMed] [Google Scholar]
- 9.Blamey RW, Jonat W, Kaufmann M, Bianco AR, Namer M. Goserelin depot in the treatment of premenopausal advanced breast cancer. Eur J Cancer. 1992:810–4. doi: 10.1016/0959-8049(92)90120-q. [DOI] [PubMed] [Google Scholar]
- 10.West CP, Baird DT. Suppression of ovarian activity by Zoladex depot (ICI 118630), a long-acting luteinizing hormone releasing hormone agonist analogue. Clin Endocrinol. 1987;26:213–20. doi: 10.1111/j.1365-2265.1987.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 11.DeFazio J, Meldrum DR, Laufer L, et al. Induction of hot flashes in premenopausal women treated with a long-acting GnRH agonist. J Clin Endocrinol Metab. 1983;56:445–8. doi: 10.1210/jcem-56-3-445. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st ed. [Google Scholar]
- 13.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 14.Siekmann L. Determination of oestradiol-17 beta in human serum by isotope dilution-mass spectrometry. Definitive methods in clinical chemistry, II. J Clin Chem Clin Biochem. 1984;22:551–7. doi: 10.1515/cclm.1984.22.8.551. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter JS, Newton KM, Sternfeld B, et al. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the Menopause Strategies Finding Lasting Answers for Symptoms and Health network. Menopause. 2012;19:664–71. doi: 10.1097/gme.0b013e31823dbbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 19.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–7. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 20.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–8. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause. 2004;11:375–81. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 22.Van Someren EJ. More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17:313–54. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- 23.Freedman RR, Benton MD, Genik RJ, 2nd, Graydon FX. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674–8. doi: 10.1016/j.fertnstert.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Diwadkar VA, Murphy ER, Freedman RR. Temporal sequencing of brain activations during naturally occurring thermoregulatory events. Cereb Cortex. 2014;24:3006–13. doi: 10.1093/cercor/bht155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eiseman NA, Westover MB, Mietus JE, Thomas RJ, Bianchi MT. Classification algorithms for predicting sleepiness and sleep apnea severity. J Sleep Res. 2012;21:101–12. doi: 10.1111/j.1365-2869.2011.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 28.Horne J. REM sleep, energy balance and ‘optimal foraging’. Neurosci Behav Rev. 2009;33:466–74. doi: 10.1016/j.neubiorev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 30.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 31.Horne J. The end of sleep: ‘sleep debt’ versus biological adaptation of human sleep to waking needs. Biol Psychol. 2011;87:1–14. doi: 10.1016/j.biopsycho.2010.10.004. [DOI] [PubMed] [Google Scholar]