Abstract
The ability of regulatory T cells (Treg) to prolong allograft survival and promote transplant tolerance in lymphodepleted rodents is well-established. Very few studies, however, have addressed the therapeutic potential of adoptively-transferred, CD4+CD25+CD127−Foxp3+ (Treg) in clinically-relevant large animal models. We infused ex vivo-expanded, functionally stable, non-selected Treg (up to a maximum cumulative dose of 1.87 billion cells) into anti-thymocyte globulin-lymphodepleted, MHC-mismatched cynomolgus monkey heart graft recipients before homeostatic recovery of effector T cells. The monkeys also received tacrolimus, anti-IL-6R mAb and tapered rapamycin maintenance therapy. Treg administration in single or multiple doses during the early post-surgical period (up to one month post-transplant), when host T cells were profoundly depleted, resulted in inferior graft function compared with controls. This was accompanied by increased incidences of effector memory T cells, enhanced IFNγ production by host CD8+ T cells, elevated levels of proinflammatory cytokines and anti-donor alloAb. The findings caution against infusion of Treg during the early post-transplant period following lymphodepletion. Despite marked but transient increases in Treg relative to endogenous effector T cells and use of reputed “Treg-friendly” agents, the host environment/immune effector mechanisms instigated under these conditions can perturb rather than favor the potential therapeutic efficacy of adoptively-transferred Treg.
Introduction
Regulatory immune cell therapy offers a promising approach to the improvement of long-term outcomes in chronic, immune-mediated inflammatory disorders (1, 2). In particular, the therapeutic potential of regulatory T cells (Treg) has been demonstrated extensively in rodent models, including humanized mice (3). These results provide a strong rationale for testing the efficacy of Treg in suppression of auto- or allo-immunity in humans (4). Indeed, in limited clinical studies, ex-vivo expanded, naturally-occurring (thymic-derived) third-party polyclonal Treg have shown promise in attenuating acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation (5). Furthermore, several phase 1/2 Treg dose-escalation studies are already underway in organ transplantation (4, 6, 7) (https://clinicaltrials.gov/ct2/show/NCT02088931; NCT02091232; NCT02129881; NCT02188719; NCT02244801). Many questions remain however, regarding the optimal conditions for Treg safety and efficacy (8–13). These relate to manufacturing issues, dosage, timing and frequency of cell infusion, Treg specificity, migratory properties and longevity. Of particular concern is Treg functional plasticity (11, 14–16) and their potential reprogramming into pathogenic effectors under inflammatory conditions (17–19), such as exist in transplant recipients (4, 10, 20). Indeed, there is recent evidence that under such conditions, adoptively-transferred regulatory immune cells lose function (21). Additional considerations include the selection of appropriate immunosuppressive agents that do not antagonize and that may promote Treg function. There is also a need to overcome potential barriers to Treg therapy, in particular immunologic memory (22), that is more prevalent in humans than in short-lived, inbred, specific pathogen-free rodent models.
Non-human primates (NHP) offer much closer immunologic proximity to humans than rodents, including high frequencies of alloreactive memory T cells (Tmem) (23). They are regarded as robust, pre-clinical models for testing the most promising innovative approaches to improved long-term graft survival/induction of transplant tolerance (24). In monkeys, endogenous (25) or adoptively-transferred Treg/anergic T cells appear important in suppression of renal allograft rejection (26, 27). Significantly, considerable recent progress has been made in the characterization of ex vivo expanded NHP Treg (28–30). This includes documentation of their ability to suppress alloreactive effector T cell proliferation (29, 31) and their pharmacokinetics/persistence in blood and trafficking to host lymphoid tissue following adoptive transfer (32, 33). Several immunosuppressive agents are regarded as ‘Treg-sparing’, including rabbit anti-thymocyte globulin (ATG) (34, 35) and the serine/threonine kinase mechanistic target of rapamycin inhibitor, rapamycin (31, 36, 37).
We have reported recently (33) that adoptively-transferred, ex vivo expanded cynomolgus monkey polyclonal Treg maintain a signature similar to endogenous Treg, although with progressively diminishing Foxp3 expression, following their transfer to surgically-intact, lymphodepleted hosts given tapered rapamycin maintenance immunosuppression. To investigate the influence of these ex vivo-expanded Treg on heart allograft survival, we administered single or multiple infusions to ATG-treated, MHC-mismatched recipients. The monkeys were also given anti-IL-6R monoclonal antibody (mAb) (to antagonize potential inhibitory/destabilizing effects of IL-6 on Treg function (38–40)) and rapamycin. These agents were administered in an effort to promote/augment Treg survival/function in the early post-surgical period (up to 1 month post-transplant) before homeostatic recovery of effector/memory cells (41, 42), when host T cells were profoundly depleted.
Our data show that, under these conditions, polyclonal Treg infusion(s) results in inferior graft function, associated with increased incidences of circulating T effector memory cells (Temra), enhanced circulating B cell numbers, and augmented proinflammatory cytokine and anti-donor alloAb levels. These data suggest a causative relationship between infusion of large numbers of exogenous Treg and enhanced host immune effector mechanisms under conditions of lymphodepletion. They caution against the infusion of Treg during the early (1 month) post-transplant period following lymphodepletion when, despite marked transient enhancement of the regulatory to effector T cell ratio and use of reputed ‘Treg-friendly’ agents, the host environment/immune effector mechanisms appears to disrupt their potential efficacy.
Materials and Methods
Animals
Healthy cynomolgus monkeys (Macaca fascicularis) of Indonesian origin (3–5 kg; 5–7 years old) were obtained from specific pathogen-free colonies at Alpha Genesis, Inc, or the National Institute of Allergy and Infectious Diseases colony (both Yamassee, SC). All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985), and under a University of Pittsburgh Institutional Animal Care and Use Committee-approved protocol. Environmental enrichment was provided.
MHC typing
Major histocompatibility complex (MHC) typing was performed as described (43) on all 18 monkeys used in the study. The degree of disparity between donors and recipients, that were mismatched in at least 3 major alleles, is shown in Supplemental Table S1.
Heart transplantation and graft monitoring
Nine monkeys received heart transplants from ABO-compatible, MHC-mismatched donors. Anesthesia, excision of the donor organ and heterotopic, intra-abdominal heart transplantation were performed as described (44). Graft function was monitored by regular palpation under chemical restraint (twice a week, by two experienced independent observers). A palpation score (45, 46) was used to assess graft function, including both contraction strength and heart rate, with grade 3 representing strong/excellent, grade 2 moderate, grade 1 weak and grade 0 complete cessation of contraction. Additionally, graft status was monitored by determining cardiac biomarkers in the blood, including cardiac troponin, total creatinine phosphokinase (CPK), CPK-MB isoenzyme and relative CPK-MB relative index (RI) before transplantation and 1 month post-transplant. Samples obtained from healthy monkeys served as controls.
Immunosuppression
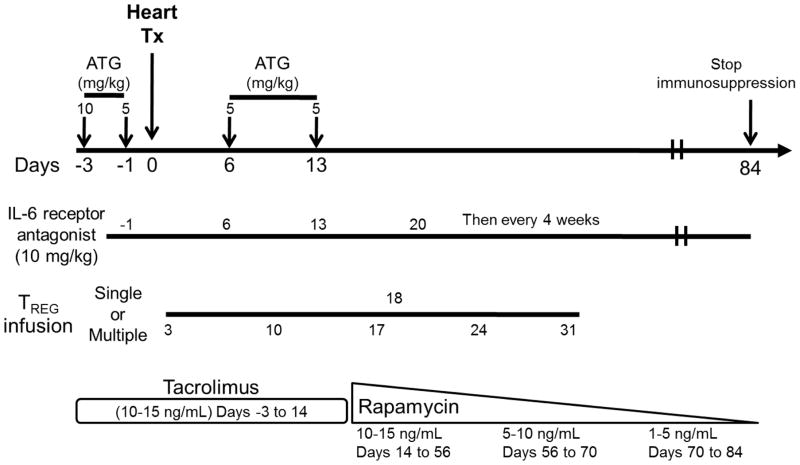
Rabbit ATG (Genzyme; Boston, MA) was given intravenously (i.v.); slowly over 4 hours on days -3 and -1 before transplant and on days 6 and 13 post-transplant at doses of 10, 5, 5 and 5 mg/kg, respectively. Methylprednisolone was given immediately before each ATG infusion at doses of 5, 2.5, 2.5 and 2.5 mg/kg, respectively. Anti-IL-6R mAb (Actemra; Genetech, CA) was administered i.v. over 1 hour at 10 mg/kg on days -1, 6, 13 and 20, and then once every 4 weeks. Tacrolimus was given by intramuscular (i.m.) injection from day -3 to 14 (target whole blood trough levels: 10–15 ng/ml), followed by rapamycin (i.m.; LC Laboratories, Woburn, MA) from days 14 to 54 (target whole blood trough levels: 10–15 ng/ml), after which rapamycin was weaned slowly and discontinued completely on day 84. The immunosuppressive protocol is summarized in Figure 1.
Figure 1. Protocol for infusion of ex vivo-expanded Treg into heart-allografted monkeys treated with ATG, anti-IL-6R mAb, tacrolimus and rapamycin.
Times of immunosuppressive drug administration in relation to the day of heart transplant are shown. The transplant recipients received either no Treg or single or multiple infusions at the time(s) indicated (days) in relation to heart transplantation.
Blood and lymphoid tissue sampling
Peripheral blood mononuclear cells (PBMC) and serum/plasma samples were collected before the start of immunosuppression, at various times pre- and post-transplant, and at the time of euthanasia. Donor spleens and lymph nodes (LN) were collected at the time of transplantation.
Isolation, expansion and infusion of polyclonal Treg
Briefly, cynomolgus CD4+ T cells were negatively-enriched from fresh PBMC using NHP CD4+ T cell isolation kits (Miltenyi Biotech, Auburn, CA). CD4+CD25+CD127− Treg were then flow-sorted (Supplemental Figure S1) using a BD FacsAria (BD Biosciences, San Jose, CA) and expanded over 3 weeks using artificial antigen-presenting cells (aAPCs) (L-32), as described (33, 47). The L-32 cells expressing CD32, CD80 and CD58 were kindly provided by Dr. M. K. Levings, University of British Columbia, Vancouver, Canada (48). Either single or multiple infusions (x4 or 5) of expanded Treg were administered to the heart graft recipients in the doses and at the times shown in Table 1.
Table 1.
Polyclonal Treg infusion(s) administered to heart allograft recipients
| Tx# | Group | Recipient | Treg infusion (s) | ||||
|---|---|---|---|---|---|---|---|
| Number | Treg Source | Total Treg infused (×106) | Treg (×106)/kg | Day(s) post-transplant of infusion | |||
| 1 | Control | M117 | NA | ||||
| 2 | M116 | ||||||
| 3 | M123 | ||||||
| 4 | Treg infusion | M127 | single | 3rd Party (Allogeneic) | 150 | 29.4 | Day 18 |
| 5 | M122 | Autologous | 120 | 26.7 | |||
| 6 | M124 | 3rd Party (Allogeneic) | 300 | 63.8 | |||
| 7 | M125 | 3rd Party (Allogeneic) | 380 | 71.7 | |||
| 8 | M118 | multiple | Autologous (x5) | 596, 588, 154, 422, and 110 | 132, 134, 35, 98 and 26 | Days 3, 10, 17, 24 and 31 | |
| 9 | M120 | Autologous (x4) | 392, 208, 144 and 134 | 73, 40, 28 and 27 | Days 3, 10, 17 and 24 | ||
Treg-specific demethylation region analysis
Expanded Treg were frozen and aliquots submitted to EpigenDX Inc. (Hopkinton, MA) for Treg-specific demethylation region (TSDR) analysis on 10 cytosine-phosphate-guanine (CpG) motifs (49).
In vitro Treg suppression assay
CD4+CD25+CD127− - depleted T cells labeled with 4 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) served as responders. A fixed number (1×105) was stimulated with NHP-specific anti-CD3/CD28 microbeads (Miltenyi), at a cell:bead ratio of 10:1, in the presence of various numbers of ex vivo-expanded Treg for 4 to 5 days. Proliferation was determined by dye dilution and the extent of suppression determined as described previously (29).
Enumeration of host immune cell populations
Distinct PBMC populations were enumerated by flow cytometry, as described (33, 50). Phenotypic analyses were performed using Abs directed against CD3 (clone SP34-2), CD4 (L200), CD8 (RPA-T8), CD14 (M5E2), CD20 (2H2), CD45RA (5H9), CD56 (MY31) and CD127 (HIL-7R-M21) from BD Pharmingen (Franklin Lakes, NJ). Anti-CD159a (NKG2A) (Z199) was from Beckman Coulter and anti-CD28 (CD28.2) from Biolegend (San Diego, CA). Anti-CD25 (BC96) was from eBioscience (San Diego, CA) and anti-CD62L (SK11) from Becton Dickinson (Franklin Lakes, NJ). Data were acquired on a LSR II or LSR Fortessa (BD Bioscience) and analyzed with FlowJo software.
Intracellular cytokine staining and cytokine quantitation
For intracellular cytokine staining, T cells were activated for 3h with PMA and ionomycin in the presence of GolgiStop (BD Bioscience), followed by anti-IFNγ (4S.B3; Biolegend) or anti-IL-17 (eBio64Dec17; ebiosciences). Levels of IFNγ and IL-17 secreted by T cells stimulated for 4 days under Th1-, Th2- or Th17-polarizing conditions were measured using a Cytometric Bead Array (CBA) Flex Set system (BD Bioscience) and analyzed with FCAP Array Software (BD Bioscience). Cytokine levels in serum were determined by the University of Pittsburgh Cancer Institute Luminex Core Laboratory using MILLIPLEX NHP kits from Millipore (Billerica, MA), according to the manufacturer’s instructions.
Measurement of circulating alloAb
Serum samples collected before and 1 month post-transplant and at the time of rejection were stored at −20°C. Donor CD3+ T cells (from either spleen or LN) were used as targets for quantitation of anti-donor MHC class I IgM and IgG alloAbs by flow cytometry, as described (51, 52). Anti-CD3-APC (cat# 557597) was obtained from BD Bioscience, anti-human IgM-PE (cat# 2020-09) from Southern Biotech and goat anti-human IgG-FITC (cat# 62-8411) from Invitrogen.
Histopathological examination of grafts
All transplant and native hearts were examined histologically in a blinded fashion at the time of cessation of graft contraction by an experienced transplant pathologist (MAN). Rejection was graded according to the 2004 and 1990 International Society of Heart and Lung Transplantation (ISHLT) criteria (53, 54). Additionally, the overall pattern of inflammation was recorded and semi-quantitative estimates provided for the extent of confluent necrotic foci, arteritis, and the presence of nodular B cell aggregates (based on CD20 staining).
Statistical analyses
Differences between means were evaluated using Student’s paired ‘t’-test or the non-parametric Mann-Whitney U test, as appropriate. Statistical analyses were conducted using the standard formula in Microsoft Excel software. A ‘p’ value <0.05 was considered significant.
Results
Characterization of ex vivo expanded cynomolgus Treg
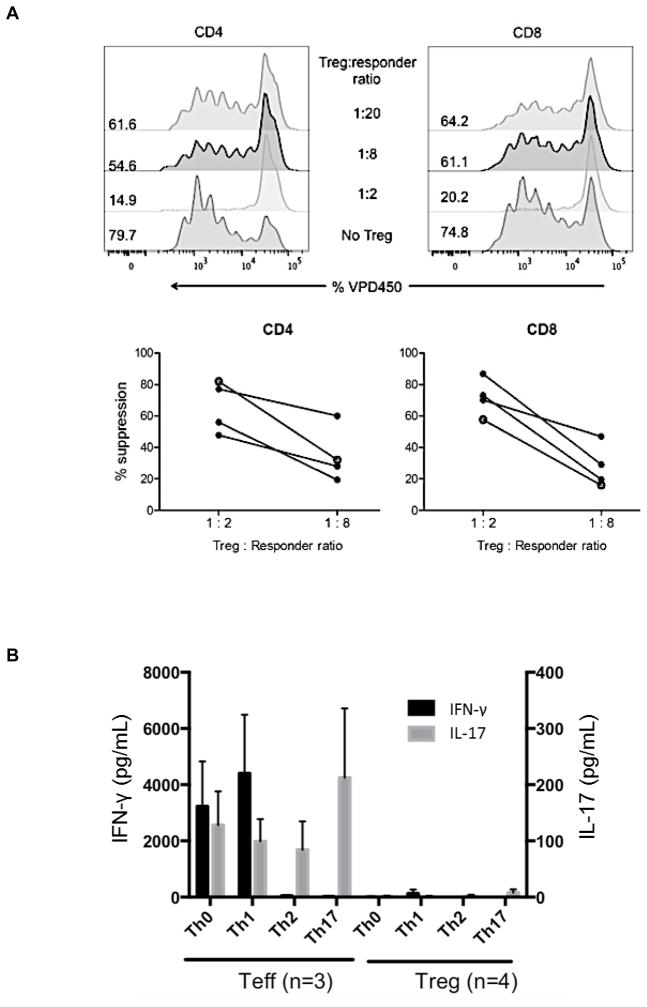
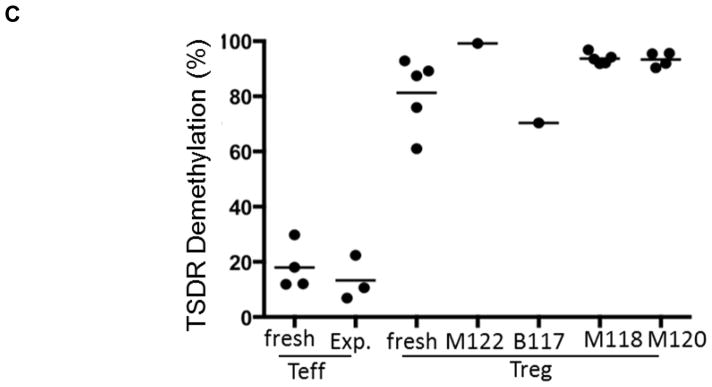
Cynomolgus Treg were flow-sorted and expanded from peripheral blood of healthy monkeys, including prospective heart graft recipients (third party or autologous Treg). The phenotype of the expanded infused Treg is shown in Supplemental Figure 1. Their ability to potently suppress T cell proliferative responses was confirmed (Figure 2A) as described previously in detail (33, 47). To confirm the functional stability of the expanded Treg in vitro, we examined their response to stimulation under culture conditions that promote Th1, Th2 and Th17 cell polarization. As shown in Figure 2B, in contrast to CD4+CD25− T effector cells (Teff), expanded Treg failed to secrete IFNγ or IL-17 and retained high levels of Foxp3+ expression after 4 days of culture under these conditions (data not shown). Each batch of expanded Treg for infusion into transplanted monkeys exhibited high percentages of TSDR demethylation at the Foxp3 locus (Figure 2C) and potently suppressed proliferation of anti-CD3/CD28-activated T cells, obtained from either healthy monkeys or monkeys undergoing heart allograft rejection, as described previously (33) (data not shown).
Figure 2. Suppressive function and stability of ex vivo-expanded cynomolgus Treg infused into heart graft recipients.
(A) Ex-vivo-expanded Treg were tested for their ability to suppress VPD450-labeled autologous or allogeneic anti-CD3/CD28-activated CD4+ and CD8+ T cell proliferation. Percent divided cells was calculated using FlowJo software (top). The suppressive effect of this representative batch of Treg was tested on autologous (open circles) and three different allogeneic (closed circles) activated T cells (bottom). Treg function was expressed as percent suppression of T cell proliferation calculated using the formula: (percent divided T cells without addition of Treg – percent divided T cells with Treg)/percent divided T cells without addition of Treg x 100%. The expanded Treg showed strong suppressive activity when added to bead-stimulated T cells. Data are representative of the 4 batches of expanded Treg used for infusion after transplantation. (B) Primary CD4+CD25− effector T cells (Teff) or Treg expanded ex vivo for 3 weeks were stimulated with NHP-specific anti-CD3/CD28 microbeads at a cell:bead ratio of 10:1 for 4 days under the following culture conditions: Th1 (IL-12 + anti-CD4 mAb), Th2 (IL-4 + anti-IFN-γ mAb), or Th17 (IL-6 + IL-21 + IL-23 + IL-1β + TGF-β + anti-IL-4 mAb + anti-IFN-γ mAb). Supernatants were collected and assayed for IFN-γ and IL-17 using CBA kits. Data (means ± 1SD) are from n=3 or n=4 separate individual Teff or Treg cultures. (C) Demethylation status of Foxp3 TSDR in freshly-isolated Treg and Treg expanded for individual or multiple infusions from 4 different monkeys for 3 weeks before infusion into heart allograft recipients. Data are also shown for freshly-isolated and expanded Teff.
Heart allograft function and survival
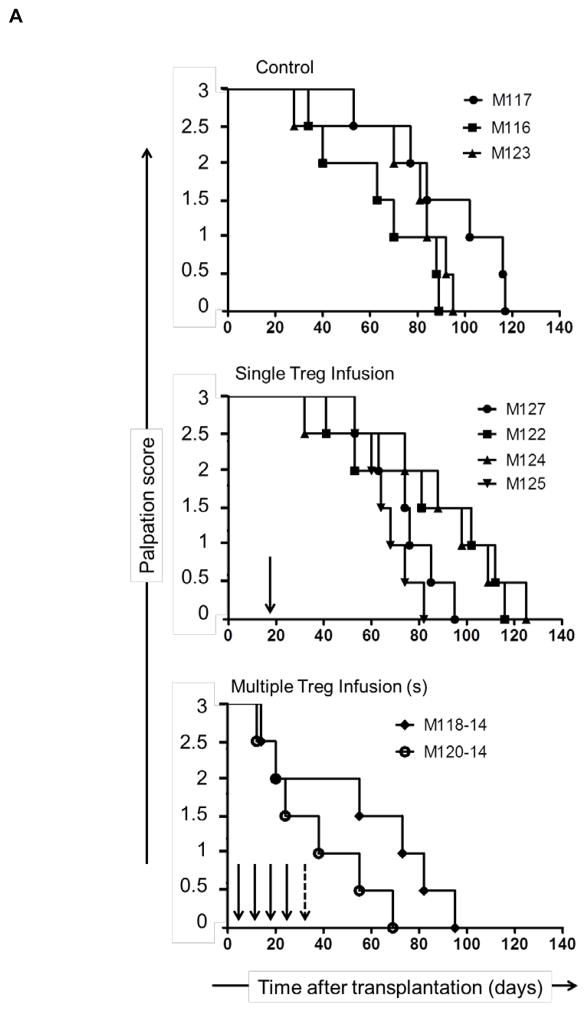
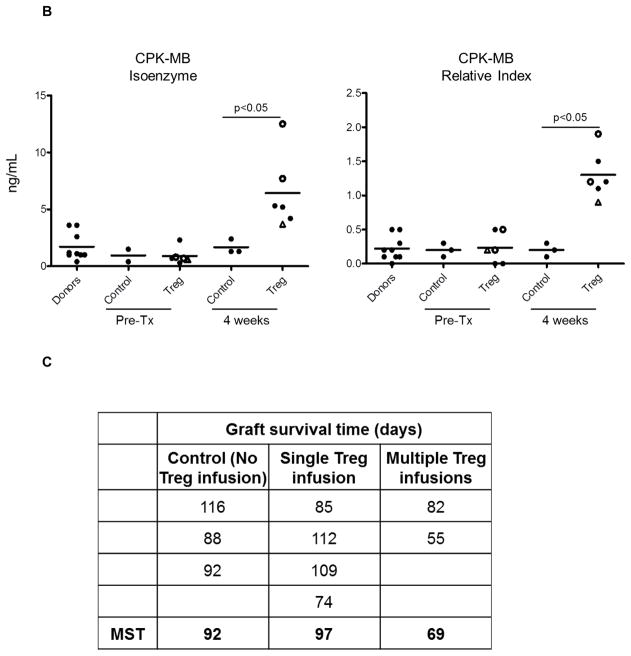
In non-immunosuppressed NHP, heterotopic heart allografts usually reject 6–7 days post-transplant (51). In this study, all monkeys received immunosuppression, as shown in Figure 1, either without (control group; n=3 monkeys) or with Treg infusion (Treg group; n=6 monkeys) at the times and cell doses shown in Table 2. In the control group, graft function (palpation score) began to decline in individual monkeys on days 28, 35 and 53, respectively (Figure 3A). With a single Treg infusion, similar reductions in the palpation score were first observed on days 32, 41, 53 and 53, respectively, whereas with multiple Treg infusions, reductions in the score were first observed earlier, on days 12 and 14, respectively. In all recipients, graft contraction strength declined progressively until total cessation of contraction (graft rejection), as shown in Figure 3A. To evaluate cardiac muscle injury, we monitored cardiac biomarker levels in serum. Significantly higher CPK-MB isoenzyme levels and an increased CPK-MB relative index were found in monkeys given Treg infusions compared to the control group (Figure 3B). No differences in cardiac biomarker expression were observed between the groups at the time of graft rejection (experimental endpoint; data not shown). In control monkeys (no Treg infusion), graft survival times were 88, 92 and 116 days post-transplant (median survival time [MST] = 92 days), whereas, in monkeys given a single Treg infusion, graft survival times were 74, 85, 109 and 112 days (MST = 97 days) and in animals given multiple infusions, 55 and 82 days (MST = 69 days) (Figure 3C). All allografts exhibited ISHLT grade 3B ejection, as described in Supplemental Table S2.
Figure 3. Heart allograft function and survival in control and Treg-infused monkeys.
(A) Heart graft palpation scores at various times post-transplant in monkeys that did not receive Treg (control; n=3) and in those that received single (n=4) or multiple infusions (n=2). Vertical arrows indicate time(s) of Treg infusion. (B) Markers of cardiac muscle injury (CPK-MB isoenzyme) and CPK-MB Relative index in control and Treg-infused graft recipients pre-treatment and one month post-transplant. Open circles denote monkeys that received multiple Treg infusions. Open triangles denote the monkey that received a single autologous Treg infusion. (C) Heart graft survival times in the control and Treg groups. MST= median graft survival time.
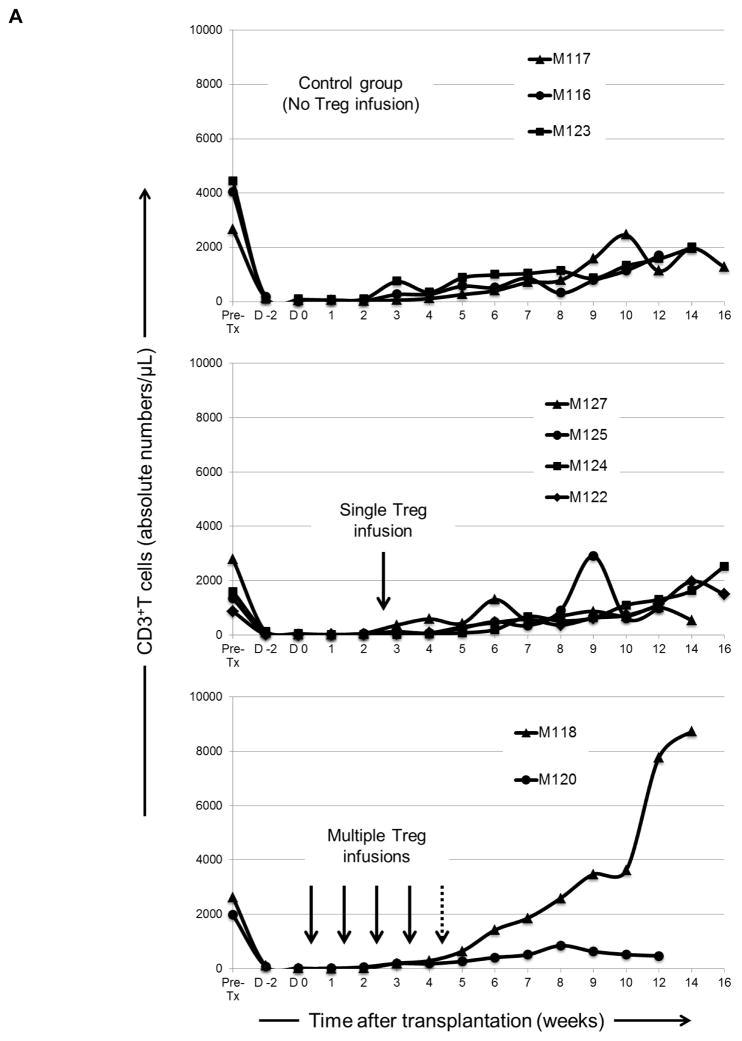
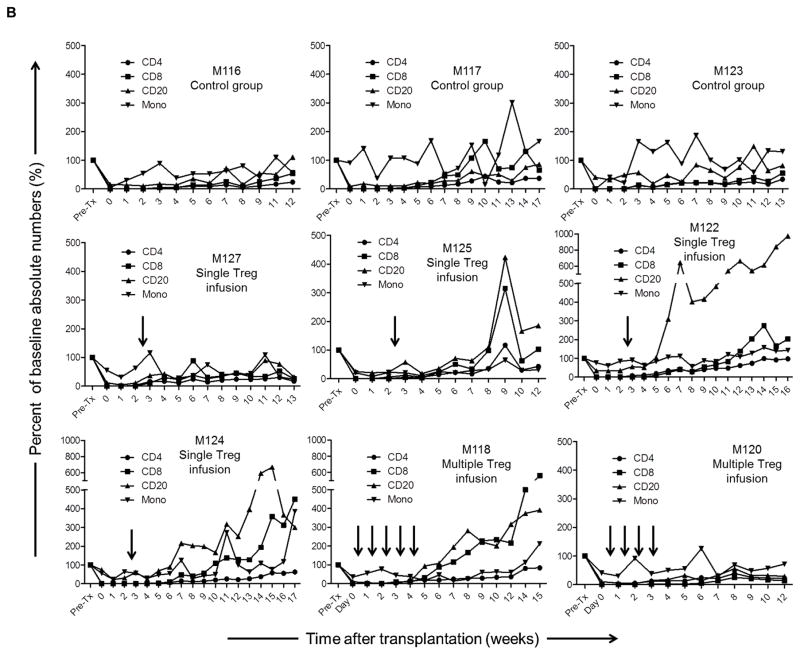
Kinetics of immune cell recovery in transplanted monkeys
Following lymphodepletion, absolute numbers of peripheral blood CD3+T cells remained profoundly below pre-treatment levels (> 95 % depletion) in all graft recipients until 6–8 weeks post-transplant (Figure 4A), after which they began to recover. While CD4+T cells exhibited a similar depletion and recovery pattern to CD3+ T cells in all groups (Figure 4B), CD8+T cells (that also remained below baseline for at least 12 weeks in the control group [no Treg]) recovered earlier and achieved higher than baseline levels by 9–12 weeks post-transplant in four (M125, M122, M124 and M118) of the six graft recipients given Treg. In control monkeys, CD20+B cell numbers remained below baseline until 10–12 weeks post-transplant, when a 200–600% increase above baseline was observed in four (M125, M122, M124 and M118) of the six recipients given Treg infusion. In all graft recipients, circulating monocytes were depressed initially, but after 2–4 weeks they were restored to levels similar to baseline.
Figure 4. Kinetics of peripheral blood T and B cell and monocyte numbers in lymphodepleted control and Treg-infused heart allograft recipients.
(A) Kinetics of CD3+ T cell depletion and recovery in individual monkeys. (B) Absolute numbers of CD4+ and CD8+ T cells, CD20+ B cells, and monocytes in individual monkeys at various times pre-transplant and post-transplant. Vertical arrows denote the time(s) of Treg infusion(s).
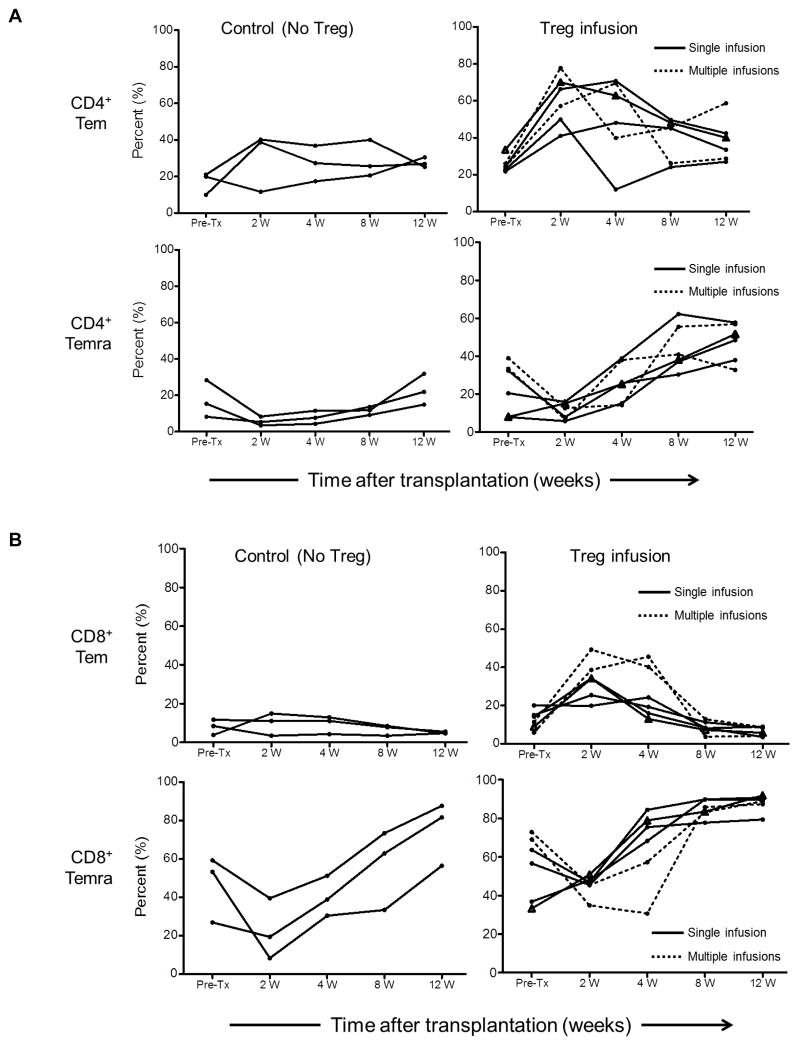
Recovery of Tmem after transplantation
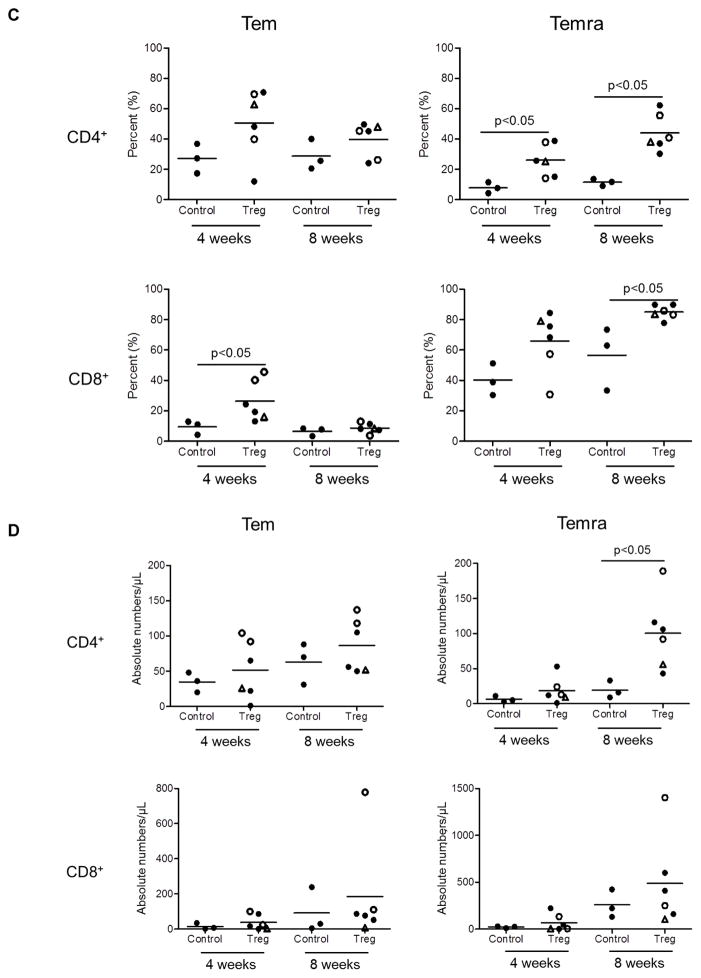
Tmem subsets were identified in peripheral blood based on their differential expression of CD45RA and CD62L. Effector memory (Tem; CD45RA−CD62L+) and terminally-differentiated effector memory (Temra; CD45RA+CD62L−) CD4+ and CD8+ T cells were evaluated sequentially in individual graft recipients 2, 4, 8 and 12 weeks post-transplant (Figure 5). In the control group (no Treg infusion), no to modest increases in the incidences of CD4+Tem at 4 weeks were observed, whereas CD4+Temra were reduced initially, but recovered gradually to baseline levels by 12 weeks post-transplant. In transplant recipients with Treg infusion, increases in the incidences of CD4+Tem compared to baseline levels were observed at 4 weeks, followed by gradual decreases towards baseline levels by 12 weeks. Concomitantly, the incidences of CD4+Temra were reduced initially at 2 weeks, but increased progressively starting 4 weeks post-transplant to significantly above baseline levels by 8 and 12 weeks (Figure 5A). CD8+Tmem showed minimal change over time in the control group and remained similar to baseline levels at 12 weeks. Although they were reduced at 2 weeks, CD8+ Temra levels increased progressively but not above baseline levels until 8 and 12 weeks. With Treg infusion, similar kinetics (compared with CD4+ T cells) of circulating CD8+Tem and CD8+Temra were observed (Figure 5B). CD8+Tem were markedly increased above baseline levels at 4 weeks in the two recipients given multiple infusions. In all recipients with Treg infusion, CD8+Temra levels were similar to baseline levels by 4 weeks and were increased significantly at 8 and 12 weeks compared to baseline levels. Interestingly, an inverse association between CD8+Tem and CD8+Temra levels was observed in the two recipients with multiple Treg infusions. Notably, the incidences of both CD4+ and CD8+ Temra were elevated significantly (p<0.05) in the Treg-infused animals compared to control monkeys at 4 and 8 weeks post-transplant (Figure 5C). Similarly, absolute numbers of Tem and Temra CD4+ and CD8+ T cells were higher at 4 and 8 weeks in the Treg-infused monkeys compared to controls, although the differences were not statistically significant, except for CD4+ Temra 8 weeks after transplantation (Figure 5D).
Figure 5. Levels of CD4+ and CD8+ effector memory (Tem) and terminally-differentiated effector memory (Temra) cells in peripheral blood of lymphodepleted control and Treg-infused heart allograft recipients.
(A) CD4+ and (B) CD8+ Tmem and Temra at various times pre-treatment and post-transplant. (C) and (D) Mean values and significances of differences in relative and absolute numbers between control and Treg-infused monkeys at one month and two months post-transplant. Open circles denote monkeys that received multiple Treg infusions. Triangles denote the one monkey that received a single autologous Treg infusion.
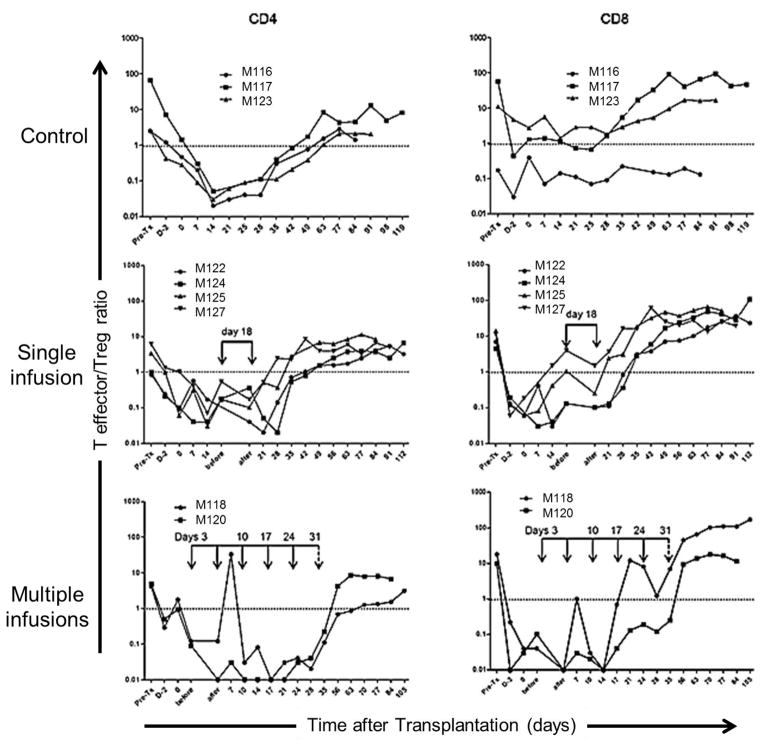
Kinetics of Treg relative to Teff in transplanted monkeys
An important consideration concerning the potential efficacy of adoptively-transferred Treg is their impact on the regulatory: effector cell ratio in vivo. Figure 6 shows the ratio of Teff:Treg in peripheral blood of individual control and Treg-infused monkeys at various times pre- and post-transplant. Although in all graft recipients, CD4+ and CD8+ T cell numbers were profoundly reduced as the result of ATG administration (Figure 4B), the CD4+ Teff:Treg ratio was skewed markedtly in favor of Treg during the first 4 weeks post-transplant. As anticipated, multiple Treg infusions resulted in more profound skewing of the Teff:Treg ratio towards Treg. In each experimental group, however, progressive recovery of the Teff/Treg ratio was observed, with the ratio returning towards baseline levels by the time of graft rejection.
Figure 6. T effector:Treg ratios in control and Treg-infused heart allograft recipients.
Ratios of CD4+ or CD8+ T effector cells (Teff) to CD4+ Treg in peripheral blood are shown pre-treatment, one day after the initial ATG infusion, on the day of transplant and thereafter in control animals and those that received single or multiple Treg infusion(s). Values before and 30 min after the single or the first of multiple infusions are shown in the middle and lower panels, respectively.
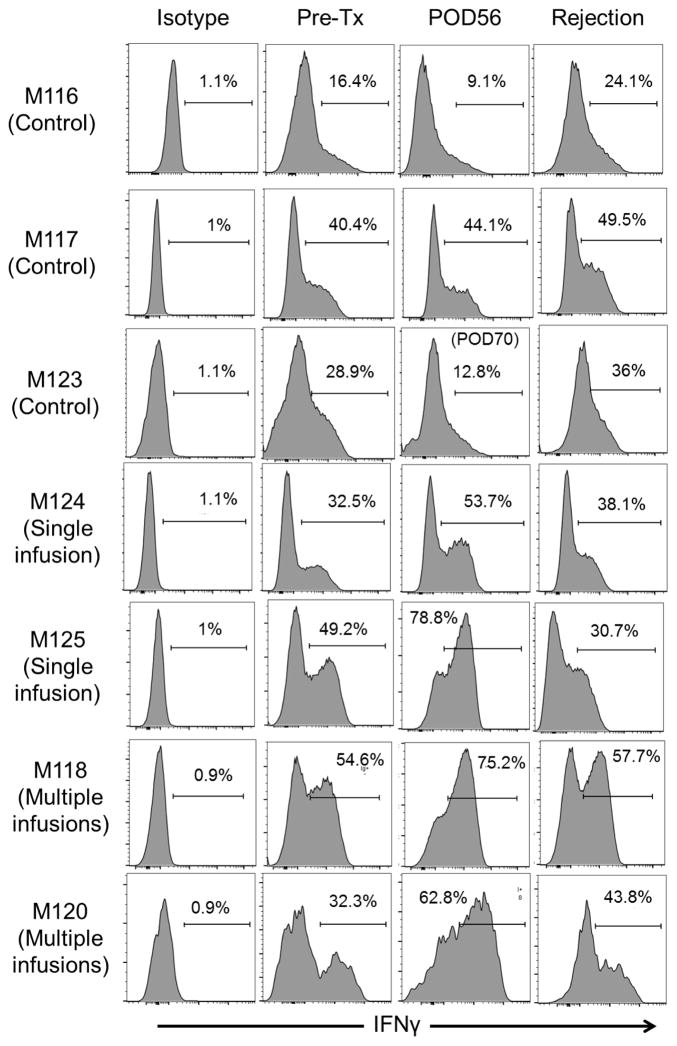
Incidence of CD8+IFNγ+ Teff
Figure 7 shows the incidence of IFNγ+CD8+ T cells in control and Treg-infused graft recipients following ex vivo host CD8+ T cell stimulation at various times pre- and post-transplant. The data show higher levels of IFNγ+CD8+ T cells in the graft recipients given Treg infusion (mean 67.6%; range 53.7–78.8%) compared with controls (mean 22.0%; range 9.1–44.1%) 8 weeks post-transplant.
Figure 7. Incidences of CD8+ IFNγ+ effector T cells in Treg-infused heart allograft recipients.
Incidences of CD8+ IFNγ+ cells in control and Treg-infused monkeys determined by flow cytometry after ex vivo stimulation of circulating CD8+ T cells with PMA and for 3 h, as described in the Materials and Methods. Pre-transplant values and those obtained 8 weeks post-transplant, and at the time of rejection are shown. POD=post-operative day.
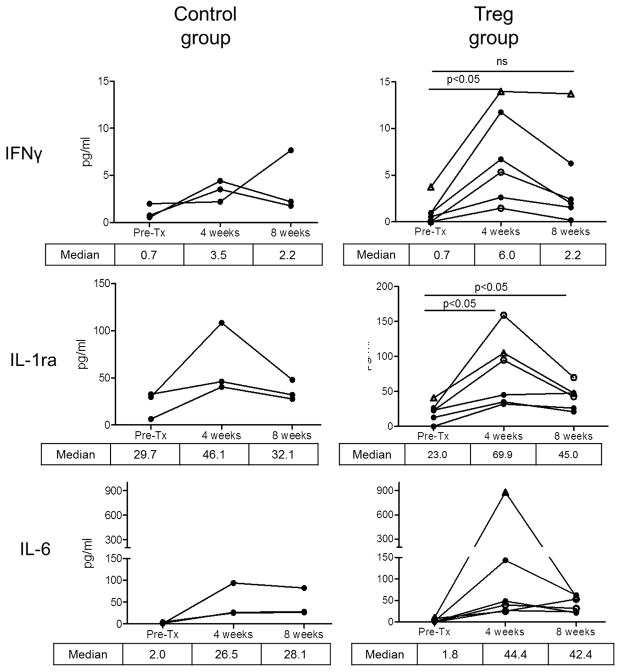
Circulating cytokine levels
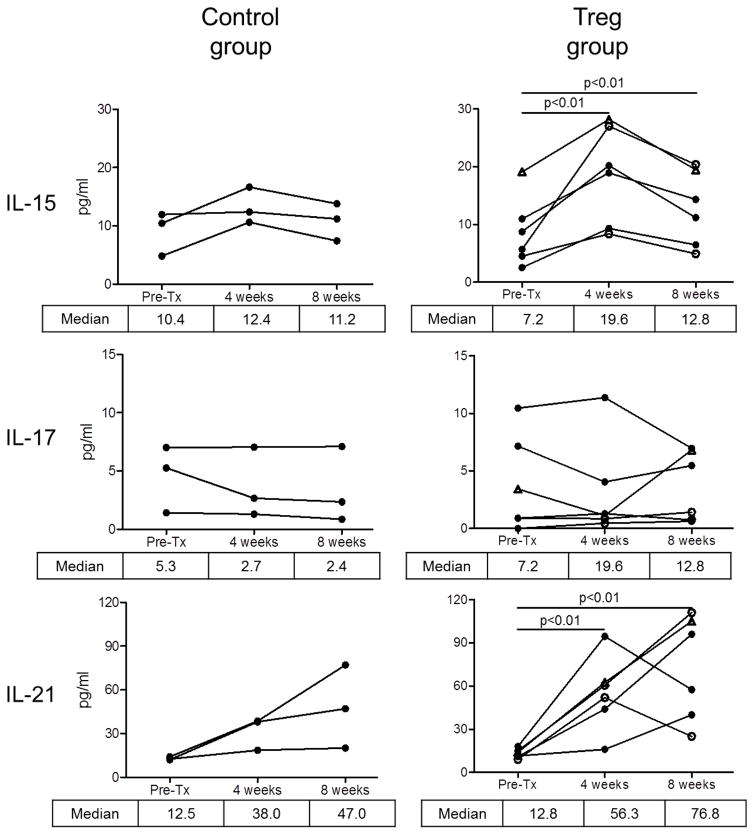
Figure 8 shows systemic levels of specific cytokines in individual graft recipients pre- and post-transplant. Compared to pre-transplant levels, higher median levels of IFNγ, IL-1Ra, IL-6, IL-15, and IL-21 were observed 4 weeks post-transplant in the Treg-infused monkeys. No comparable significant increases were observed in the control group at 4 weeks compared to pre-transplant levels.
Figure 8. Systemic levels of proinflammatory cytokines in Treg-infused heart allograft recipients.
Various cytokines were determined in serum of individual monkeys by Luminex assay (as described in the Materials and Methods) pre-transplant and at one and two months post-transplant in individual control and Treg-infused heart allograft recipients. Open circles denote monkeys that received multiple Treg infusions. Open triangles denote the monkey that received a single autologous Treg infusion.
Circulating alloAb levels
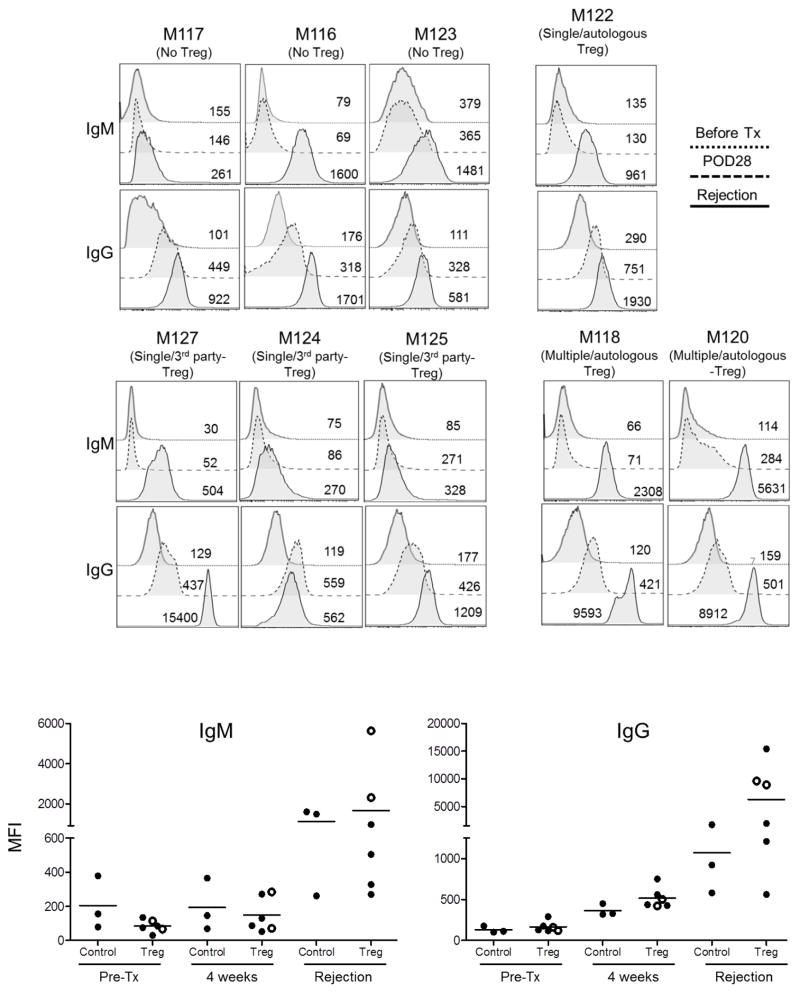
All heart graft recipients developed circulating anti-donor IgM and IgG alloAbs. In the control group, all monkeys developed high IgM titers. Increased IgG alloAb levels were also detected by day 28, with further increases by the time of rejection. Similar increases in levels of both anti-donor IgM and IgG alloAbs were observed in monkeys given Treg infusion (Figure 9). No significant differences in IgM levels were observed between the two groups, either 4 weeks post-transplant or at rejection. Similarly, no significant differences in IgG levels were observed between the groups 4 weeks post-transplant; however, at the time of euthanasia, higher IgG levels were detected in those monkeys given Treg infusion.
Figure 9. Circulating alloAb levels in heart allograft recipients.
Levels of IgM and IgG alloAbs levels were determined by flow cytometry in the serum of control (no Treg) and Treg-infused monkeys before transplant, on day 28 post-transplant and at graft rejection. Numbers on panels denote mean fluorescence intensity (MFI). Horizontal bars in lower panels denote mean values obtained from individual monkeys.
Discussion
The successful expansion of otherwise rare, functional CD4+CD25+Foxp3+ Treg has made Treg therapy a feasible experimental approach to reducing patients’ dependence on immunosuppressive agents and improved long-term allograft survival. In lymphodepleted rodent models, such as T and B cell-deficient (RAG−/−) (55), T cell-depleted (56, 57) or irradiated mice (58), many studies have shown that adoptively-transferred Treg can prolong allograft survival/promote tolerance. Only limited data have demonstrated control of allograft rejection with adoptively-transferred Treg in lymphoreplete animals (59). Our goal was to determine the influence of ex vivo-expanded, non-selected Treg infused following ATG-induced lymphodepletion, on heart allograft survival in a clinically-relevant NHP model. Our rationale was that (1), compared to the steady-state, adoptively-transferred functional Treg would be enhanced numerically relative to severely-depleted endogenous effector T cells in hosts treated with a lymphocyte-depleting immunosuppressive regimen and, therefore, better able to regulate alloimmunity and that (2), incorporation of anti-IL-6R mAb and rapamycin might spare/promote the suppressive function of the infused Treg in an environment that might promote their enrichment during immune reconstitution after ATG administration (60, 61).
The Treg that we expanded and infused either once or multiple times during the early post-transplant period were phenotypically and functionally stable in vitro. They markedly suppressed ex vivo proliferative responses of naïve and memory T cells from normal monkeys or from monkeys acutely rejecting cardiac allografts. We considered this (in vitro) ability to suppress Tmem responses to be of crucial importance since (unlike short-lived, specific pathogen-free mice), NHP normally have high frequencies of alloreactive Tmem (23, 62) that can dominate anti-donor responses following lymphodepletion at the time of transplantation (50, 63, 64). Moreover, CD4+ and CD8+ Tmem are relatively resistant to suppression by CD4+CD25+ Treg (22, 65). The cynomolgus Treg we generated exhibited high CXCR3, but low CD62L and CCR7 expression (33), consistent with potential to accumulate at sites of inflammation (e.g., an organ allograft), where their suppressive effects appear to be important (66, 67). They also retain a Treg signature in monkeys conditioned with ATG and rapamycin following their infusion, although Foxp3 expression diminishes over time (33). Despite these attributes considered favorable to their therapeutic potential, Treg infusion did not prolong heart allograft survival. Indeed, we show that, compared with identically-immunosuppressed recipients that did not receive Treg, allograft function in Treg-infused monkeys was inferior, particularly in those monkeys given multiple cell infusions. Furthermore, Treg infusion was associated with significant increases in the incidences of circulating CD4+ and CD8+ Temra and IFNγ-producing CD8+ T cells, and in levels of anti-donor alloAb.
Our findings clearly differ from previous reports of the ability of adoptively-transferred Treg to promote organ allograft survival in rodents. In these mouse studies, Treg were injected into lymphodepleted hosts either before or after transplantation (56–58). In the present study, failure of adoptively-transferred Treg infused post-transplant to prolong heart graft survival is also at variance with a recent report (27) that ex vivo-expanded Treg could prolong renal allograft survival in ATG-treated cynomolgus monkeys. In this latter investigation however, a much larger number of infusions (total=14) of expanded, donor alloAg-specific, host spleen-derived CD4+CD25+ Treg were infused daily (107 per day). Infusions were started earlier and in closer proximity to ATG administration than in the present study. They were commenced at the time of transplant, i.e., 1 day after a 3-day course of ATG (20mg/kg) and were combined with low-dose rapamycin for 14 days. Thus, renal allograft survival was prolonged from a MST of 22 days to 48.5 days in splenectomized hosts. In the only other NHP study (26) relevant to the present report, autologous anergic T cells generated ex vivo by blocking the CD80/CD86-CD28 costimulatory pathway, suppressed renal allograft rejection after their adoptive transfer (79–155 ×106), once only, 13 days post-transplant to splenectomized rhesus monkeys given cyclophosphamide (30 mg/kg on days 6, 7 and 8) and cyclosporine. Thus, in each of these studies in which regulatory/anergic T cells were reported to prolong NHP organ allograft survival, host splenectomy was performed. In addition, the timing, frequency and doses of Treg and the immunosuppressive regimen employed differed in each case from those in the present study. Also, in one case, the autologous Treg were alloAg-reactive.
The doses of polyclonal Treg that we infused into profoundly lymphodepleted (peripheral blood and secondary lymphoid tissue (68)) 3–5 kg cynomolgus monkeys in this study ranged from 120–380×106 total cells in a single infusion, to cumulative totals of 878×106 –1.87×109 given in multiple infusions. These doses were generally higher than those used in the two aforementioned NHP organ transplant studies. Currently, it is thought (69, 70) that billions of Treg and high Treg:T effector cell ratios (1:1; 1:2) are needed, together with ATG induction at the time of transplant, to dramatically alter the Treg to effector/memory cell balance and to control graft rejection in humans. Technological advances indicate that this is feasible. However, despite infusing numbers of Treg that achieved and markedly exceeded this balance in NHP, albeit transiently, as the result of single or multiple infusions, we did not observe a therapeutic effect on heart graft survival.
A crucial factor for retention of the immunosuppressive potency by Treg, especially under chronic inflammatory conditions (14, 15, 40), including transplantation (16, 71), is the maintenance of Foxp3 expression and their functional stability following adoptive transfer. While we did not monitor Foxp3 in Treg following their infusion in this study, due to concerns that labeling might impair their survival/function (72), we (33) and others (32) have previously observed progressive Foxp3 diminution in infused Treg that persist (in much reduced numbers) in monkeys over time (3 weeks). In the case of our studies (33), this diminution was observed in animals similarly ATG-conditioned and rapamycin-treated (but non-transplanted) to those in the present report. Importantly, others have reported that Treg can lose Foxp3 expression and differentiate into non-regulatory (Foxp3−) pathogenic Th1/Th17 cells, including under lymphopenic conditions (17, 73) and that Foxp3 instability leads to generation of pathogenic memory T cells in vivo (15). In the current graft recipients, environmental cues that can destabilize Treg and enhance Tmem and alloAb responses, in particular IFNγ, IL-6 and IL-15, were elevated, whereas increased B cell-activating factor levels and rapamycin-augmented B cell activation have been reported in human lymphodepleted graft recipients (74). In the current study, rapamycin maintenance therapy was tapered until its complete withdrawal, 84 days post-transplant. Notably, enhanced expansion of CD8+ Tmem, like we observed after Treg infusion, has been correlated with failure of immunosuppression withdrawal after organ transplantation using ATG induction and rapamycin (75). Thus, it is likely that a combination of factors underlie the untoward influence of Treg infusion on graft function and host alloimmunity that we observed in the present study.
In conclusion, multiple infusions of non-selected Treg into ATG-lymphodepleted NHP during the early post-transplant period, resulted in inferior graft function/survival, associated with elevated effector memory and anti-donor alloAb responses. In organ transplantation trials that have been instigated, Treg are being infused into patients at different times post-transplant and under different immunosuppressive drug regimens, including use of ATG-induced lymphodepletion and mTOR inhibition. Further appraisal of the conditions under which Treg are evaluated to promote graft survival/minimize immunosuppression, especially in clinically-relevant NHP models, is justified in order to assure both their safety and therapeutic potential.
Supplementary Material
Supplemental Figure S1. Sort gate for Treg selection and phenotype of the infused Treg.
Table S1. MHC typing of heart allograft donors and recipients
Table S2. Grading of heart allograft rejection
Acknowledgments
The work was supported by National Institutes of Health (NIH) grant U01 AI51698, part of the NIH NHP Transplantation Tolerance Cooperative Study Group and sponsored by the NIAID and NIDDK. HZ was in receipt of a NIAID T32 (AI74490) institutional training grant postdoctoral fellowship. This project used the UPCI Cancer Biomarkers Facility Luminex Core Laboratory that is supported, in part, by NIH award P30CA047904. We thank Ms. Miriam Freeman for excellent administrative support.
Abbreviations
- ATG
anti-thymocyte globulin
- CFSE
carboxyfluorescein succinimidyl ester
- Foxp3
foxhead box P3
- IS
immunosuppression
- MFI
mean fluorescence intensity
- NHP
non-human primate
- Teff
effector T cells
- Tmem
memory T cells
- Treg
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7(8):650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 2.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 3.Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood K. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90(12):1321–1327. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant. 2014;14(4):750–763. doi: 10.1111/ajt.12647. [DOI] [PubMed] [Google Scholar]
- 5.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson JA, Geissler EK. Now or never? The case for cell-based immunosuppression in kidney transplantation. Kidney International. 2015 Mar 4; doi: 10.1038/ki.2015.50. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7(280):280rv282. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald KG, Orban PC, Levings MK. T regulatory cell therapy in transplantation: stability, localization and functional specialization. Curr Opin Organ Transplant. 2012;17(4):343–348. doi: 10.1097/MOT.0b013e328355aaaf. [DOI] [PubMed] [Google Scholar]
- 9.Burrell BE, Nakayama Y, Xu J, Brinkman CC, Bromberg JS. Regulatory T cell induction, migration, and function in transplantation. Journal of Immunology. 2012;189(10):4705–4711. doi: 10.4049/jimmunol.1202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braza F, Durand M, Degauque N, Brouard S. Regulatory T Cells in Kidney Transplantation: New Directions? Am J Transplant. 2015;15(9):2288–2300. doi: 10.1111/ajt.13395. [DOI] [PubMed] [Google Scholar]
- 11.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunological Reviews. 2014;259(1):173–191. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Q. Pharmacokinetics of therapeutic Tregs. Am J Transplant. 2014;14(12):2679–2680. doi: 10.1111/ajt.12933. [DOI] [PubMed] [Google Scholar]
- 13.Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, Ten Brinke A, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015;7(304):304ps318. doi: 10.1126/scitranslmed.aaa7721. [DOI] [PubMed] [Google Scholar]
- 14.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, et al. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39(5):949–962. doi: 10.1016/j.immuni.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degauque N, Mariat C, Kenny J, Zhang D, Gao W, Vu MD, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118(2):735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yurchenko E, Shio MT, Huang TC, Da Silva Martins M, Szyf M, Levings MK, et al. Inflammation-driven reprogramming of CD4+ Foxp3+ regulatory T cells into pathogenic Th1/Th17 T effectors is abrogated by mTOR inhibition in vivo. PLoS One. 2012;7(4):e35572. doi: 10.1371/journal.pone.0035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, et al. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19(12):2522–2534. doi: 10.1097/MIB.0b013e3182a85709. [DOI] [PubMed] [Google Scholar]
- 19.d’Hennezel E, Piccirillo CA. Functional plasticity in human FOXP3(+) regulatory T cells: implications for cell-based immunotherapy. Hum Vaccin Immunother. 2012;8(7):1001–1005. doi: 10.4161/hv.20203. [DOI] [PubMed] [Google Scholar]
- 20.Yeh H, Moore DJ, Markmann JF, Kim JI. Mechanisms of regulatory T cell counter-regulation by innate immunity. Transplant Rev (Orlando) 2013;27(2):61–64. doi: 10.1016/j.trre.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehn BH, Apostolova P, Haverkamp JM, Miller JS, McCullar V, Tolar J, et al. GVHD-associated, inflammasome-mediated loss of function in adoptively transferred myeloid-derived suppressor cells. Blood. 2015;126(13):1621–1628. doi: 10.1182/blood-2015-03-634691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afzali B, Mitchell PJ, Scotta C, Canavan J, Edozie FC, Fazekasova H, et al. Relative resistance of human CD4(+) memory T cells to suppression by CD4(+) CD25(+) regulatory T cells. Am J Transplant. 2011;11(8):1734–1742. doi: 10.1111/j.1600-6143.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3(86):86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, current challenges and unmet needs. Am J Transplant. 2006;6(5 Pt 1):884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 25.Torrealba JR, Katayama M, Fechner JH, Jr, Jankowska-Gan E, Kusaka S, Xu Q, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. Journal of Immunology. 2004;172(9):5753–5764. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 26.Bashuda H, Kimikawa M, Seino K, Kato Y, Ono F, Shimizu A, et al. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. Journal of Clinical Investigation. 2005;115(7):1896–1902. doi: 10.1172/JCI23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma A, Qi S, Song L, Hu Y, Dun H, Massicotte E, et al. Adoptive transfer of CD4+CD25+ regulatory cells combined with low-dose sirolimus and anti-thymocyte globulin delays acute rejection of renal allografts in Cynomolgus monkeys. Int Immunopharmacol. 2011;11(5):618–629. doi: 10.1016/j.intimp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Anderson A, Martens CL, Hendrix R, Stempora LL, Miller WP, Hamby K, et al. Expanded nonhuman primate Tregs exhibit a unique gene expression signature and potently downregulate alloimmune responses. Am J Transplant. 2008;8(11):2252–2264. doi: 10.1111/j.1600-6143.2008.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dons EM, Raimondi G, Zhang H, Zahorchak AF, Bhama JK, Lu L, et al. Ex vivo-expanded cynomolgus macaque regulatory T cells are resistant to alemtuzumab-mediated cytotoxicity. Am J Transplant. 2013;13(8):2169–2178. doi: 10.1111/ajt.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dons EM, Raimondi G, Cooper DK, Thomson AW. Non-human primate regulatory T cells: current biology and implications for transplantation. Transplantation. 2010;90(8):811–816. doi: 10.1097/TP.0b013e3181ebf782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh K, Kozyr N, Stempora L, Kirk AD, Larsen CP, Blazar BR, et al. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant. 2012;12(6):1441–1457. doi: 10.1111/j.1600-6143.2011.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh K, Stempora L, Harvey RD, Kirk AD, Larsen CP, Blazar BR, et al. Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. Am J Transplant. 2014;14(12):2691–2703. doi: 10.1111/ajt.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Guo H, Lu L, Zahorchak AF, Wiseman RW, Raimondi G, et al. Sequential monitoring and stability of ex vivo-expanded autologous and nonautologous regulatory T cells following infusion in nonhuman primates. Am J Transplant. 2015;15(5):1253–1266. doi: 10.1111/ajt.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boenisch O, Lopez M, Elyaman W, Magee CN, Ahmad U, Najafian N. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4(+) T cells and requires the presence of monocytes. Am J Transplant. 2012;12(4):856–866. doi: 10.1111/j.1600-6143.2011.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. Journal of Immunology. 2008;180(9):5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. Journal of Immunology. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 38.Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: role of dendritic cells and toll-like receptors. Crit Rev Immunol. 2006;26(4):291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 40.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 41.Neujahr DC, Chen C, Huang X, Markmann JF, Cobbold S, Waldmann H, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176(8):4632–4639. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 42.Zwang NA, Turka LA. Homeostatic expansion as a barrier to lymphocyte depletion strategies. Curr Opin Organ Transplant. 2014;19(4):357–362. doi: 10.1097/MOT.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiseman RW, Karl JA, Bohn PS, Nimityongskul FA, Starrett GJ, O’Connor DH. Haplessly hoping: macaque major histocompatibility complex made easy. ILAR J. 2013;54(2):196–210. doi: 10.1093/ilar/ilt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper DKC, Ye Y, Niekrasz M. Heart transplantation in primates. In: VCD, Podesta LLM, editors. Handbook of Animal Models in Transplantation Research. Boca Raton, FL: CRC Press, Inc; 1994. pp. 173–200. [Google Scholar]
- 45.Stalder M, Tye T, Lam TT, Chan MC, Berry GJ, Borie DC, et al. Improved assessment of graft function by echocardiography in cynomolgus monkey recipients of hDAF-transgenic pig cardiac xenografts. J Heart Lung Transplant. 2005;24(2):215–221. doi: 10.1016/j.healun.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 46.Kwun J, Oh BC, Gibby AC, Ruhil R, Lu VT, Kim DW, et al. Patterns of de novo allo B cells and antibody formation in chronic cardiac allograft rejection after alemtuzumab treatment. Am J Transplant. 2012;12(10):2641–2651. doi: 10.1111/j.1600-6143.2012.04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo H, Zhang H, Lu L, Ezzelarab MB, Thomson AW. Generation, cryopreservation, function and in vivo persistence of ex vivo expanded cynomolgus monkey regulatory T cells. Cell Immunol. 2015;295(1):19–28. doi: 10.1016/j.cellimm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. Journal of Immunology. 2013;190(5):2001–2008. doi: 10.4049/jimmunol.1201379. [DOI] [PubMed] [Google Scholar]
- 49.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 50.Marco MR, Dons EM, van der Windt DJ, Bhama JK, Lu LT, Zahorchak AF, et al. Post-transplant repopulation of naive and memory T cells in blood and lymphoid tissue after alemtuzumab-mediated depletion in heart-transplanted cynomolgus monkeys. Transplant Immunology. 2013;29(1–4):88–98. doi: 10.1016/j.trim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. 2010;120(4):1275–1284. doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azimzadeh AM, Pfeiffer S, Wu G, Schroder C, Zorn GL, 3rd, Kelishadi SS, et al. Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation. 2006;81(2):255–264. doi: 10.1097/01.tp.0000190099.62847.e6. [DOI] [PubMed] [Google Scholar]
- 53.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):587–593. [PubMed] [Google Scholar]
- 54.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 55.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166(6):3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 56.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 57.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant. 2008;8(2):298–306. doi: 10.1111/j.1600-6143.2007.02088.x. [DOI] [PubMed] [Google Scholar]
- 58.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng SG, Meng L, Wang JH, Watanabe M, Barr ML, Cramer DV, et al. Transfer of regulatory T cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int Immunol. 2006;18(2):279–289. doi: 10.1093/intimm/dxh368. [DOI] [PubMed] [Google Scholar]
- 60.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdez-Ortiz R, Bestard O, Llaudo I, Franquesa M, Cerezo G, Torras J, et al. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transpl Int. 2015;28(1):108–119. doi: 10.1111/tri.12448. [DOI] [PubMed] [Google Scholar]
- 62.Nadazdin O, Boskovic S, Murakami T, O’Connor DH, Wiseman RW, Karl JA, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10(6):1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 64.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7(5):1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104(50):19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MKt, Moore DJ, Jarrett BP, Lian MM, Deng S, Huang X, et al. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J Immunol. 2004;172(11):6539–6544. doi: 10.4049/jimmunol.172.11.6539. [DOI] [PubMed] [Google Scholar]
- 67.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71(3):460–468. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 69.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Curr Opin Organ Transplant. 2012;17(4):349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 70.Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant. 2013;13(11):3010–3020. doi: 10.1111/ajt.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Last’ovicka J, Budinsky V, Spisek R, Bartunkova J. Assessment of lymphocyte proliferation: CFSE kills dividing cells and modulates expression of activation markers. Cell Immunol. 2009;256(1–2):79–85. doi: 10.1016/j.cellimm.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39(4):948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 74.Bloom D, Chang Z, Pauly K, Kwun J, Fechner J, Hayes C, et al. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9(8):1835–1845. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donckier V, Craciun L, Miqueu P, Troisi RI, Lucidi V, Rogiers X, et al. Expansion of memory-type CD8+ T cells correlates with the failure of early immunosuppression withdrawal after cadaver liver transplantation using high-dose ATG induction and rapamycin. Transplantation. 2013;96(3):306–315. doi: 10.1097/TP.0b013e3182985414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Sort gate for Treg selection and phenotype of the infused Treg.
Table S1. MHC typing of heart allograft donors and recipients
Table S2. Grading of heart allograft rejection