Abstract
The unfolded protein response (UPR) maintains protein homeostasis by governing the processing capacity of the endoplasmic reticulum (ER) to manage ER client loads; however, key regulators within the UPR remain to be identified. Activation of the UPR sensor PERK (EIFAK3/PEK) results in the phosphorylation of the α subunit of eIF2 (eIF2α-P), which represses translation initiation and reduces influx of newly synthesized proteins into the overloaded ER. As part of this adaptive response, eIF2α-P also induces a feedback mechanism through enhanced transcriptional and translational expression of Gadd34 (Ppp1r15A),which targets type 1 protein phosphatase for dephosphorylation of eIF2α-P to restore protein synthesis. Here we describe a novel mechanism by which Gadd34 expression is regulated through the activity of the zinc finger transcription factor NMP4 (ZNF384, CIZ). NMP4 functions to suppress bone anabolism, and we suggest that this occurs due to decreased protein synthesis of factors involved in bone formation through NMP4-mediated dampening of Gadd34 and c-Myc expression. Loss of Nmp4 resulted in an increase in c-Myc and Gadd34 expression that facilitated enhanced ribosome biogenesis and global protein synthesis. Importantly, protein synthesis was sustained during pharmacological induction of the UPR through a mechanism suggested to involve GADD34-mediated dephosphorylation of eIF2α-P. Sustained protein synthesis sensitized cells to pharmacological induction of the UPR, and the observed decrease in cell viability was restored upon inhibition of GADD34 activity. We conclude that NMP4 is a key regulator of ribosome biogenesis and the UPR, which together play a central role in determining cell viability during endoplasmic reticulum stress.
Keywords: endoplasmic reticulum stress (ER stress), eukaryotic initiation factor 2 (eIF2), protein synthesis, translation control, unfolded protein response (UPR), protein homeostasis
Introduction
Professional secretory cells balance the synthesis, folding, and trafficking of proteins to ensure optimal protein export. Upon differentiation and physiological cues, increased synthesis of polypeptides slated for secretion can lead to accumulation of unfolded proteins in the endoplasmic reticulum (ER)3 that trigger the unfolded protein response (UPR) (1). The UPR features multiple sensory proteins, including PERK (EIF2AK3/PEK), IRE1 (ERN1), and ATF6, which are each situated in the ER and are activated by unfolded proteins in the ER (1). Induction of the UPR leads to a program of translational and transcriptional gene expression that collectively serve to expand the processing capacity of the ER to effectively manage an expanded ER client load (1).
In response to ER stress, PERK phosphorylates the α subunit of eIF2 (eIF2α-P), which represses global translation initiation that reduces influx of newly synthesized proteins into the overloaded ER (2, 3). Coincident with dampening of global protein synthesis, eIF2α-P leads to preferential translation of Atf4, encoding a transcription activator of UPR genes involved in nutrient import, metabolism, and alleviation of oxidative stress (4–6). ATF4 also directly induces the transcriptional expression of Gadd34 (Ppp1r15A), which targets type 1 protein phosphatase for dephosphorylation of eIF2α-P (7–9). Translational expression of Gadd34 is also enhanced by eIF2α-P, and the resulting increased GADD34 serves in feedback control that restores protein synthesis, allowing for translational expression of UPR gene transcripts (7, 10, 11).
UPR-directed transcription is also driven by IRE1, a riboendonuclease that facilitates splicing of Xbp1 mRNA, leading to translation of an activated version of the XBP1 transcription factor. In response to ER stress, ATF6 is transported from the ER to Golgi for proteolytic cleavage that allows for release of the amino-terminal portion of ATF6 to enter the nucleus and direct transcription of targeted UPR genes (1). Oasis is another transcription factor closely related to ATF6 that is also activated via regulated intramembrane proteolysis during ER stress (12). Together the ATF4, XBP1, ATF6, and Oasis transcription factors serve to enhance expression of UPR genes involved in protein folding and assembly, vesicular transport, ER and Golgi expansion, and degradation of unfolded proteins (1, 12). Hence the UPR provides for key transcriptional, translational, and proteolytic processes that are central to professional secretory cells.
Emphasizing the importance of the key UPR regulators in secretory cells, loss of function of Perk, Atf4, Ire1, Xpb1, or Oasis, disrupts the health and secretory functions of osteoblasts and subsequent bone formation (13–15). Treatment of precursor osteoblasts with bone morphogenetic protein BMP2 is suggested to activate each of the UPR branches, directing expression of target genes that contribute to secretion and bone formation (13, 14, 16). Given the central role of the UPR in protein homeostasis and expansion of secretory capacity, there is a growing consensus that the UPR functions in conjunction with additional regulators of bone development. For example, ATF4 is an essential regulator of osteoblast biology, and there are likely to be additional regulatory networks integrating the UPR to bone development (13, 17). We previously reported that the zinc finger transcription factor NMP4 (ZNF384, CIZ) functions to suppress bone anabolism, partially through the repression of genes such as Plaur, Spp1, and Col1a1, which play important roles in osteogenic lineage commitment and mineralization (18–20). Targeted deletion of Nmp4 in mice enhances bone response to PTH and BMP2 and protects these animals from osteopenia. Furthermore, ChIP-Seq analyses of NMP4-binding sites in preosteoblasts, embryonic stem cells, and two blood cell lines suggest that NMP4 binds to the promoters of genes encoding UPR regulators and modulates their gene expression (20).
In this study we addressed the role of NMP4 in the regulation of the UPR and its control of transcription and protein synthesis processes. Our analysis indicates that NMP4 regulates protein synthesis through transcriptional repression of both c-Myc and Gadd34. Loss of Nmp4 results in an increase in c-Myc and Gadd34 expression, enhancing ribosome biogenesis and global protein synthesis. Importantly, protein synthesis is sustained during pharmacological induction of the UPR through a mechanism suggested to involve GADD34-mediated dephosphorylation of eIF2α-P. Sustained protein synthesis sensitizes cells to pharmacological induction of the UPR. Furthermore, the observed decrease in cell viability upon activation of the UPR is restored upon inhibition of GADD34 activity. These results emphasize the importance of appropriate regulation of Gadd34 expression and its role in the maintenance of cellular homeostasis through regulation of eIF2α-P.
Experimental Procedures
Mice
WT and Nmp4−/− mice are as previously described (20). The local Institutional Animal Care and Use Committee approved all husbandry practices and experimental procedures.
Cell Culture
Mesenchymal stem progenitor cells (MSPCs) were isolated from bone marrow using a Ficoll gradient as described (20, 22) and were cultured in Mesencult Media with Mesencult Stimulatory Supplement (StemCell Technologies).
Immunoblot Analysis
MSPCs were treated with 2 μm tunicamycin for up to 9 h, 10 μm salubrinal for 6 h, or left untreated. Spleen, liver, and bone marrow tissues were isolated from Nmp4+/+ and Nmp4−/− mice, and protein lysates were collected and quantified from the tissues and MSPCs followed by immunoblot analyses as previously described (10). Antibodies used for immunoblot analyses are listed in Table 1.
TABLE 1.
Description of antibodies used for immunoblots in this study
| Protein of interest | Supplier and catalog number |
|---|---|
| NMP4 | Sigma #HPA004051 |
| GADD34 | Proteintech #10449-1-AP |
| CReP | Proteintech #14634-1-AP |
| ATF4 | Santa Cruz #sc-22800 |
| eIF2α∼P | Abcam #ab32157 |
| RPS6∼P ribosomal protein | Cell Signaling #2211 |
| RPS6 total ribosomal protein | Cell Signaling #2317 |
| RPL11 ribosomal protein | Cell Signaling #18163 |
| c-MYC | Cell Signaling #5605 |
| β-actin | Sigma #A5441 |
| eIF2α total | Monoclonal antibody kindly provided by Dr. Scott Kimball (Pennsylvania State University College of Medicine, Hershey, PA) |
mRNA Measurement by qPCR
RNA was isolated from MSPCs and tissues using TRIzol reagent (Invitrogen), and single-strand cDNA synthesis was conducted with the TaqMan reverse transcriptase kit (Applied Biosystems) following the manufacturer's instructions. Transcript levels were quantified by qPCR using SYBR Green (Applied Biosystems) on a Realplex2 Master Cycler (Eppendorf). ΔΔCT values were calculated for each transcript in which β-actin levels were used for normalization. Primers used for qPCR analysis are listed in Table 2.
TABLE 2.
Description of primers used for qPCR in this study
| Primer name | Primer sequence |
|---|---|
| Gadd34 forward | 5′-AGGACCCCGAGATTCCTCTA-3′ |
| Gadd34 reverse | 5′-CCTGGAATCAGGGGTAAGGT-3′ |
| Crep forward | 5′-GGCTACAGTGGCCTTCTCTG-3′ |
| Crep reverse | 5′-CATCCATCCCTTGCAAATTC-3′ |
| c-Myc forward | 5′-GAAAACGACAAGAGGCGGAC-3′ |
| c-Myc reverse | 5′-AATGGACAGGATGTAGGCGG-3′ |
| 45S rRNA forward | 5′-TTTTTGGGGAGGTGGAGAGTC-3′ |
| 45S rRNA reverse | 5′-CTGATACGGGCAGACACAGAA-3′ |
| Rpl11 forward | 5′-CCTCAATATCTGCGTCGGGG-3′ |
| Rpl11 reverse | 5′-TTCCGCAACTCATACTCCCG-3′ |
| Rps6 forward | 5′-CAGGACCAAAGCACCCAAGA-3′ |
| Rps6 reverse | 5′-CAGTGAGGACAGCCTACGTC-3′ |
| β-Actin forward | 5′-TGTTACCAACTGGGACGACA-3′ |
| β-Actin reverse | 5′-GGGGTGTTGAAGGTCTCAAA-3′ |
Plasmid Constructs and Luciferase Assays
The DNA segments containing 1 kb of the human Gadd34 and Crep promoters were inserted between KpnI and BglII in a pGL3 basic backbone. The Gadd34 promoter-Luc and CReP promoter-Luc constructs were transiently co-transfected with a Renilla reporter plasmid into WT or Nmp4−/− MSPCs for 24 h followed by a 6-h 2 μm tunicamycin treatment. Lysates were collected, and Firefly and Renilla luciferase activities were measured as described (5). At least three independent biological experiments were conducted for each luciferase measurement, and relative values are represented with S.D. indicated.
Total RNA and DNA Measurement
RNA and DNA was isolated from MSPCs using TRIzol reagent (Invitrogen) following the manufacturer's instructions. Quantification of RNA and DNA was determined by absorbance measurement at 260 and 280 nm by nanodrop.
Polysome Profiling and Sucrose Gradient Ultracentrifugation
MSPCs were treated with 2 μm tunicamycin or 10 μm salubrinal for 6 h or left untreated. Lysates were collected, sheared, and layered on top of 10–50% sucrose gradients as described (10). Whole-cell lysate polysome profiles were measured with a Piston Gradient Fractionator (BioComp) and a 254-nm UV monitor with Data Quest Software.
Cell Viability Assays
MTT and Caspase 3/7 assays were conducted by seeding cells at 5000 cells/well in a 96-well plate. For MTT time course analysis, cells were cultured for 24 h followed by up to 24 h of treatment with 2 μm tunicamycin alone or in combination with either 10 μm salubrinal or 250 nm torin 1 (Tocris) for an additional 24 h, and MTT activity was measured using CellTiter 96 well non-radioactive cell proliferation assay (Promega). For Caspase 3/7 assays, cells were seeded, cultured for 24 h, and treated in the presence or absence of 2 μm tunicamycin for an additional 24 h, and Caspase 3/7 activity was measured using the Caspase-Glo 3/7 Assay System (Promega).
Statistical Analyses
Values indicate the mean ± S.D. and represent at least three independent experiments. Statistical significance was calculated using the two-tailed Student's t test. Differences between multiple groups were analyzed using a two-way analysis of variance followed by a post hoc Tukey HSD test. For the statistical analyses, genotype and treatment were set as fixed factors, and StatPlus software was used to calculate significance. p values <0.05 were considered statistically significant with differences between treatment groups indicated by asterisks (*) and differences between genotypes indicated by a number (#) symbol.
Results
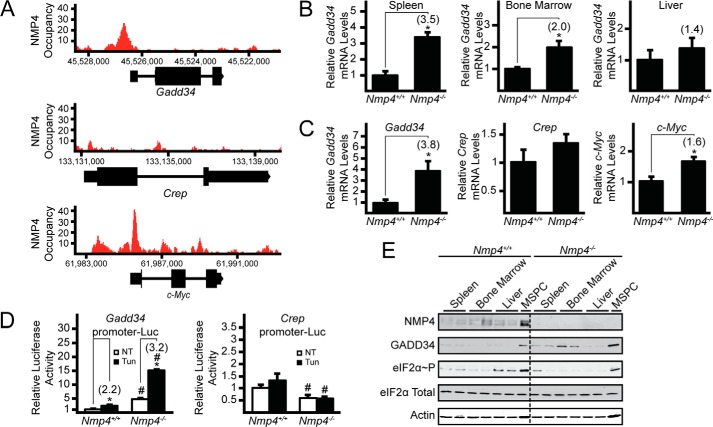
Genome-wide analyses of NMP4-binding sites in multiple cell lines, including preosteoblast MC3T3-E1, embryonic cell line ES-E14, B cell lymphoma Ch12, and murine erythroleukemia cells, suggest that NMP4 binds to specific regions in the Gadd34 promoter at the described NMP4 binding consensus sequence (AAAAAAAAA) (see the UCSC Genome Bioinformatics website and Ref. 20). The reported NMP4-binding site in the Gadd34 promoter in MC3T3-E1 cells is illustrated in Fig. 1A (20), and we posited that NMP4 alters transcriptional expression of Gadd34 mRNA. To address this idea we used qPCR to measure Gadd34 mRNA in tissues involving bone and blood homeostasis, including bone marrow, spleen, and liver, from wild-type (WT) mice and those containing a whole body deletion of Nmp4. In either bone marrow or spleen, loss of Nmp4 led to >2-fold induction in Gadd34 mRNA, whereas there was a trend toward an increase, although not significant, in the Nmp4−/− liver tissues (Fig. 1B). These findings suggest that NMP4 can serve as a repressor of Gadd34 mRNA expression in multiple tissues, although likely to different extents.
FIGURE 1.
Expression of Gadd34 is increased upon deletion of Nmp4. A, NMP4 occupancy on the genomic loci corresponding to sequences encoding Gadd34, Crep, and c-Myc genes that were reported in the genome-wide ChIP-Seq analysis (20). NMP4 occupancy (read count) is indicated on the y axis. Boxes indicate exonic sequences encoding Gadd34, Crep, and c-Myc mRNAs, and horizontal lines indicate intronic regions. B, total RNA was collected from spleen, bone marrow, and liver tissues from Nmp4+/+ and Nmp4−/−, mice and relative levels of Gadd34 mRNA were measured by qRT-PCR. C, levels of Gadd34, Crep, and c-Myc mRNA were also measured in WT and Nmp4−/− MSPCs. D, Gadd34 and Crep transcriptional control was measured in WT or Nmp4−/− MSPCs in the presence or absence of tunicamycin (Tun) via Dual-Luciferase assay. E, the indicated proteins were measured in the indicated Nmp4+/+ and Nmp4−/− tissues and MSPCs by immunoblot. Panel A is representative of three independent biological experiments, and relative values are represented as histograms with the S.D. indicated in panels B, C, and D. Panel E is representative of three independent biological experiments. Differences between treatment groups are indicated by asterisks (*), and differences between genotypes are indicated by number (#) symbols. NT, not treated.
Given the diversity of cell types in bone marrow and spleen, we next prepared MSPCs from bone marrow from the WT and Nmp4-deleted mice and measured Gadd34 mRNA in these cultured primary cells. Consistent with our bone marrow measurements, there was almost a 4-fold increase in Gadd34 mRNA levels in the Nmp4-depleted cells compared with WT (Fig. 1C). By comparison there was minimal difference between the WT and mutant MSPCs for the amount of Crep (Ppp1r15B) mRNA (Fig. 1C), which encodes a constitutively expressed targeting subunit for dephosphorylation of eIF2α-P (23).
To determine whether NMP4 serves to repress transcription of the Gadd34 gene, we transfected luciferase reporter constructs with transcriptional expression directed by the Gadd34 or Crep promoters. There was a >4-fold increase in Gadd34 promoter activity in the MSPCs deleted for Nmp4 compared with WT (Fig. 1D). ATF4 and CHOP are both known to directly increase the transcriptional expression of the Gadd34 gene in response to ER stress, and the Gadd34 promoter luciferase reporter also contains the ATF4 and CHOP-binding sites that are at locations distinct from NMP4 (8, 24). In both WT and Nmp4−/− cells, luciferase activity was sharply increased upon the addition of tunicamycin, an inhibitor of N-linked glycosylation and potent inducer of ER stress. However, Nmp4-deleted cells showed the greatest extent of Gadd34 promoter activity upon ER stress, with a >6-fold increase compared with WT (Fig. 1D). By comparison, luciferase expressed from the Crep promoter showed a 40% decrease in reporter activity compared with WT, and as expected, Crep promoter activity was not significantly changed upon tunicamycin treatment.
Next we measured GADD34 protein in bone marrow, spleen, and liver from WT mice and MSPCs and their Nmp4 knock-out counterparts. Levels of GADD34 protein were increased in Nmp4−/− tissues and MSPCs, with the most significant changes observed in bone marrow and MSPCs (Fig. 1E). Elevated levels of GADD34 protein would be expected to lead to lowered levels of eIF2α-P even in conditions not subject to overt stress, and this finding was confirmed in our immunoblot analyses (Fig. 1E). NMP4 was also present in all tissues analyzed as well as MSPCs, although the levels were varied, with NMP4 being the most abundant in MSPCs. These findings suggest that NMP4 can serve as a major regulator of Gadd34 expression in multiple cell types.
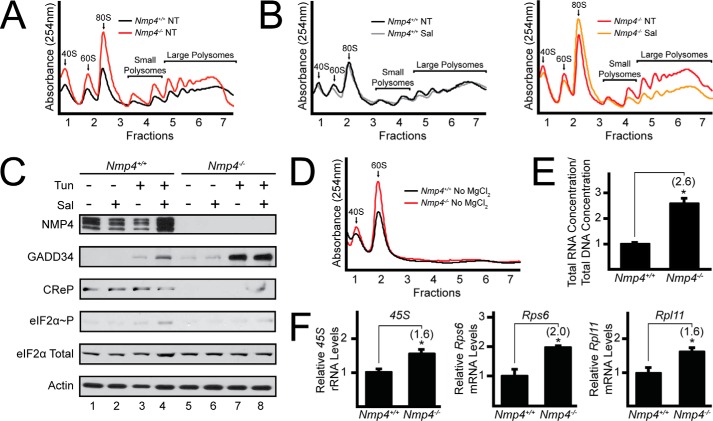
Loss of Nmp4 in MSPCs Increases Protein Synthesis
Phosphorylation of eIF2α represses global translation by lowering the levels of eIF2-GTP available for delivery of aminoacylated initiator tRNA to ribosomes (25). Therefore, elevated levels of GADD34 and the consequential reduction of eIF2α-P would be predicted to enhance global protein synthesis. Lysates prepared from WT and Nmp4−/− MSPCs were analyzed by sucrose gradient ultracentrifugation to visual the amounts of translated mRNAs in polysomes (Fig. 2A). There was a sharp increase in large polysomes upon loss of Nmp4 in the MPSCs, indicative of much higher levels of protein synthesis. Interestingly there was also an increase in free 40S and 60S ribosomal subunits and monosomes in the Nmp4−/− cells, suggesting that the enhanced translation was also accompanied by an increase the amount of ribosomes.
FIGURE 2.
Deletion of Nmp4 in MSPCs increases ribosome biogenesis and protein synthesis. A, lysates were collected from WT and Nmp4−/− MSPCs, and equal amounts of total RNA were layered on top of 10–50% sucrose gradients followed by ultracentrifugation and analysis of whole-lysate polysome profiles at 254 nm. B, polysome profiles were conducted as in panel A with the addition of treatment of WT and Nmp4−/− cells with salubrinal for 6 h or no treatment (NT). C, WT and Nmp4−/− MSPCs were treated individually or in combination with salubrinal and tunicamycin for 6 h as indicated, and the indicated proteins were measured by immunoblot. Quantification of eIF2α∼P was conducted using ImageJ software. Values feature the lane number in the immunoblot, with the first lane on the left designated as lane 1, followed by quantification of eIF2α-P in parentheses: 1(1); 2(1); 3(1.6); 4(3.3); 5(0.6); 6(0.8); 7(1.3); 8(2.4). D, levels of 40S and 60S ribosomal subunits were measured as in panel A with the exception that MgCl2 was omitted in the lysis and sucrose gradients. E, total DNA and total RNA lysates were quantified from WT and Nmp4−/− MSPCs. F, the 45S rRNA and Rps6 and Rpl11 mRNAs were measured by qRT-PCR in WT and Nmp4−/− MSPCs. Panels A, B, C, and D are representative of three independent biological experiments. Relative values of three biological replicates are represented as histograms with the S.D. indicated for panels E and F. Differences between treatment groups are indicated by *.
To test the idea that increased global translation in Nmp4−/− cells is a consequence of elevated GADD34 activity, we treated the MSPCs with salubrinal, a small molecule inhibitor of GADD34, and CReP-targeted dephosphorylation of eIF2α-P (26). Treatment of the Nmp4-deleted cells led to a marked reduction in large polysomes, indicating that the inhibition of GADD34 and CReP lowered global protein synthesis (Fig. 2B). By comparison, salubrinal did not appreciably change the polysome profile in WT MSPCs. In conjunction, we also measured the levels of eIF2α-P in WT and Nmp4−/− MSPCs left untreated, treated individually, or treated in combination with salubrinal or tunicamycin (Fig. 2C). Levels of eIF2α-P in the WT MSPCs remained largely unchanged with salubrinal treatment alone but were increased with tunicamycin treatment that was further elevated with combined drug treatment. Measurement of eIF2α-P was largely decreased in the Nmp4−/− MSPCs due to increased Gadd34 expression; however, there was a modest increase in eIF2α-P with either salubrinal or tunicamycin treatment alone that was further exacerbated with the combined drug treatment (quantified in Fig. 2C). We conclude that elevated Gadd34 expression in the Nmp4−/− MSPCs contributes to a portion of the observed increase in global protein synthesis.
Deletion of Nmp4 in MSPCs Increases Ribosome Biogenesis
Given that more ribosomes are suggested to be present in the Nmp4−/− cells, we carried out sucrose gradient ultracentrifugation using lysates depleted for Mg2+, a condition that leads to release of ribosomes from mRNAs. There were significant increases in both free 40S and 60S ribosomal subunits in the Nmp4−/− cells compared with WT (Fig. 2D). Equal amounts of total RNA, as determined by absorbance at 260 nm, were applied to the sucrose gradients, and we noted that there were consistently more RNA in the MSPCs deleted for Nmp4 compared with equal numbers of WT cells. We confirmed this key finding by purifying and measuring total RNA and DNA from equal numbers of the MSPCs. Although there were similar amounts of DNA between the Nmp4−/− and WT cells, there was 2-fold more total RNA in the Nmp4-deleted cells compared with WT (Fig. 2E). The majority of total RNA in cells consists of rRNA, and these results support the idea that there is increased ribosome biogenesis in the MSPCs upon deletion of Nmp4.
We next sought to understand the underlying basis for increased ribosomes in the MSPCs deleted for Nmp4. mTORC1 and c-MYC are potent inducers of ribosome biogenesis (27, 28), and prior ChIP-Seq analyses indicated that NMP4 can bind to the promoter of the c-Myc gene (Fig. 1A) (20). There were increased levels of c-Myc mRNA in the Nmp4−/− cells as compared with WT (Fig. 1C). Furthermore, there were elevated c-MYC protein levels in the Nmp4−/− cells, whereas levels of phosphorylated S6, a measure of mTORC1 activity, were similar between the Nmp4-depleted cells and WT (Fig. 3B). We note that total S6 protein levels were significantly increased in the Nmp4−/− cells, consistent with increased ribosome biogenesis. These results suggest that increased expression of c-Myc in the MSPCs deleted for Nmp4 is an underlying reason for increased ribosomes. To address this idea, we used qPCR to measure expression levels of c-MYC target genes in WT and Nmp4-deleted MSPCs. Consistent with our measurements of increased 40S and 60S ribosomal subunits, there was a 1.6-fold increase in expression of 45S rRNA and Rpl11 mRNA and a 2-fold increase in Rps6 mRNA (Fig. 2F). These results support that idea that ribosome biogenesis is increased in MSPCs deleted for Nmp4 by a mechanism involving c-MYC.
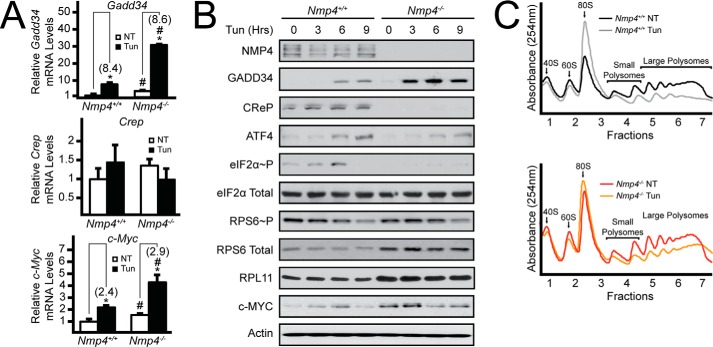
FIGURE 3.
Deletion of Nmp4 facilitates maintenance of global translation during activation of the UPR. A, Gadd34, Crep, and c-Myc mRNAs were measured by qRT-PCR in WT and Nmp4−/− MSPCs that were treated with tunicamycin (Tun) for 6 h or left untreated. NT, not treated. B, WT and Nmp4−/− MSPCs were treated with tunicamycin for 3, 6, or 9 h or left untreated, and the indicated proteins were measured by immunoblot. C, lysates were collected from WT and Nmp4−/− MSPCs treated with tunicamycin for 6 h or left untreated, and equal amounts of total RNA were layered on top of 10–50% sucrose gradients followed by ultracentrifugation and analysis of whole-lysate polysome profiles at 254 nm. Relative values of three biological replicates are represented as histograms with the S.D. indicated for Panel A. Panels B and C are representative of three independent biological experiments. Differences between treatment groups are indicated by asterisks (*), and differences between genotypes are indicated by a number # symbols.
Deletion of Nmp4 Sensitizes MSPCs to Chronic ER Stress
In the UPR, expression of Gadd34 is induced transcriptionally via ATF4 and translationally in response to eIF2α-P (8, 10). Consistent with this idea, we observed a >8-fold increase in the level of Gadd34 transcript upon treatment of WT cells with tunicamycin (Fig. 3A). A similar induction of Gadd34 mRNA was also observed upon tunicamycin treatment of Nmp4−/− cells; however, given that Nmp4-deleted cells have much higher basal levels of Gadd34 transcripts, there was about a 4-fold increase in Nmp4−/− cells exposed to tunicamycin compared with similarly stress WT MSPCs. Analysis of Gadd34 mRNA levels also resulted in a statistically significant two-way analysis of variance for genotype × treatment interactions. These patterns of induction of GADD34 protein were also observed in the WT and Nmp4−/− cells (Fig. 3B). Together these results are consistent with the idea that NMP4 serves to lower Gadd34 transcription expression during both basal and stressed conditions.
Upon ER stress in WT cells there was induced eIF2α-P, with a maximum ∼6 h of tunicamycin treatment. By 9 h of ER stress, increased expression of endogenous Gadd34 led to feedback dephosphorylation of eIF2α-P (Fig. 3B). The greater levels of GADD34 protein in the Nmp4−/− cells culminated in minimal induction of eIF2α-P during ER stress, which led to lowered levels of ATF4 expression and largely sustained protein synthesis during tunicamycin treatment (Fig. 3, B and C). Of interest, Nmp4−/− cells showed a sharp reduction in the amounts of CReP protein. Despite being designated a constitutively expressed targeting subunit for type 1 protein phosphatase dephosphorylation of eIF2α-P, Crep expression was reported to be sharply reduced upon overexpression of GADD34 (10), suggesting that there can be cross-regulation between the Crep and Gadd34 genes.
We next measured mTORC1 activity and expression of c-Myc in the MSPCs subjected to ER stress. Consistent with prior reports (29), mTORC1 was repressed by ER stress in WT cells as illustrated by lowered phosphorylation of RPS6 during the time course of tunicamycin treatment (Fig. 3B). A similar reduction in RPS6 phosphorylation during ER stress also occurred in the Nmp4-deleted cells. We did note that total RPL11 levels as well as RPS6 were increased in the Nmp4−/− cells independent of ER stress, which further supports the idea that there are increased amounts of ribosomes upon deletion of Nmp4. Levels of c-MYC protein were higher in Nmp4−/− cells compared with WT in the absence of stress (Fig. 3B). However, with longer exposure to ER stress the Nmp4-deleted cells showed some lowering of c-MYC protein.
Phosphorylation of eIF2α can provide for protection against acute ER stress (1, 30, 31). Given that Nmp4−/− cells exhibit elevated Gadd34 expression concomitant with lower eIF2α-P, we measured the viability of WT and Nmp4-deleted cells exposed to tunicamycin for up to 24 h. WT cells were largely resistant to ER stress, with only a modest 10% reduction in cell viability as measured by MTT assay (Fig. 4A). By comparison, Nmp4−/− cells showed a striking sensitivity to the ER stress, culminating in a 60% reduction of cells by 24 h of tunicamycin treatment. Furthermore, there was increased caspase 3/7 activity in the Nmp4-deleted cells upon ER stress, which was absent in similarly treated WT cells (Fig. 4B). Finally, we addressed the role of increased Gadd34 expression in the sensitization of Nmp4−/− cells to acute ER stress. The WT and Nmp4-deleted cells were treated with tunicamycin in the presence or absence of salubrinal (Fig. 4C). Salubrinal treatment provided for cell resistance to tunicamycin in the Nmp4−/− cells. Furthermore, combination treatment with torin 1, a potent small molecule inhibitor of mTORC1 (32), did not significantly alter the sensitivity of the Nmp4−/− cells to the ER stress. These findings indicate that increased Gadd34 expression resulting from loss of Nmp4 in MSPCs renders cells more sensitive to acute ER stress.
FIGURE 4.
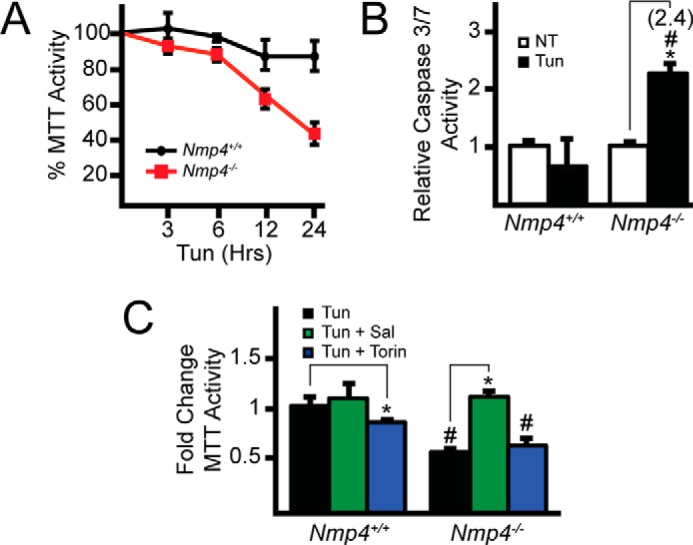
Deletion of Nmp4 sensitizes MSPCs to pharmacological induction of ER stress. A, equal numbers of MSPCs were cultured for 24 h followed by treatment with or without tunicamycin for up to an additional 24 h. MTT activity was measured by the conversion of tetrazolium to formazan. B, caspase 3/7 activity was measured in MSPCs treated with tunicamycin for 24 h or no ER stress. C, MTT activity was measured in the MSPCs treated with tunicamycin or combined treatment with either salubrinal or torin 1. Relative values of three biological replicates are illustrated with the S.D. indicated for panels A, B, and C. Differences between treatment groups are indicated by asterisks (*), and differences between genotypes are indicated by a number (#) symbols.
Discussion
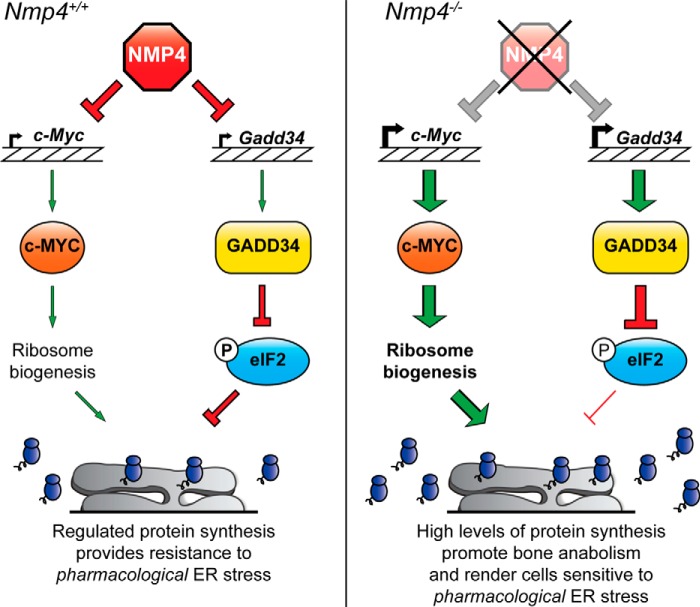
In this study we showed that NMP4 represses Gadd34 and c-Myc expression and that loss of Nmp4 culminates in increased ribosome biogenesis and protein synthesis (Fig. 5). Translational control is central to the maintenance of cellular homeostasis and is critical for the implementation of the UPR, especially in professional secretory cells. In the UPR, eIF2α-P is central for resistance to acute ER stress, and premature resumption of translation can reduce cell viability (24, 33, 34). This idea is illustrated by the finding that pharmacological induction of ER stress resulted in decreased cell viability of Nmp4−/− MSPCs that was ameliorated upon inhibition of GADD34 and CReP activity. Furthermore, we showed that loss of Nmp4 increases ribosome biogenesis by a process suggested to involve c-MYC, contributing to further increases in protein synthesis (Fig. 5). Combined, these results suggest a prominent role for NMP4-mediated dampening of translational control in the UPR, which is critical in the ability of cells to appropriately sense and respond to ER stress. A model for NMP4-mediated regulation of c-Myc and Gadd34 expression and subsequent translation control is presented in Fig. 5. NMP4 serves to repress both c-Myc and Gadd34 expression, helping to maintain appropriate regulation of ribosome biogenesis and translation initiation through eIF2α-P (Fig. 5). Loss of Nmp4 results in heightened c-Myc and Gadd34 expression that contributes to increases in ribosome biogenesis and translation initiation, likely facilitating enhanced bone matrix deposition in mice. However, high levels of protein synthesis incurred through the loss of Nmp4 rendered cells sensitive to acute ER stress induced by pharmacological agents, such as tunicamycin, due to an inability to appropriately regulate translation and activate some of the adaptive features of the UPR (Fig. 5).
FIGURE 5.
Model for NMP4 regulation of ribosome biogenesis and the UPR. NMP4 serves to dampen transcriptional expression of c-Myc and Gadd34, which are important for regulation of ribosome biogenesis and eIF2α-P and the UPR, respectively. ER stress and induction of the UPR in Nmp4+/+ cells results in decreased protein synthesis that promotes stress alleviation, partially through the regulated expression of c-Myc and Gadd34. However, in Nmp4−/− cells there are high levels of c-Myc and Gadd34 expression and subsequent elevation of ribosome biogenesis and translation initiation through GADD34-mediated dephosphorylation of eIF2α-P. As a consequence, heightened levels of synthesized proteins slated to be retained in the cytosol and those directed into the ER for secretion are maintained during pharmacological induction of the UPR, thwarting stress adaptation that renders cells sensitive to the acute ER stress. Loss of Nmp4 has also been shown to increase bone anabolism in mice, which is likely due, at least in part, to increased c-MYC- and GADD34-mediated protein synthesis and secretion.
Previous work suggested that activation of the UPR plays a significant and obligatory role in osteoblast differentiation, proliferation, and function, supporting the idea that the UPR promotes cellular homeostasis in highly secretory cells by regulating changes in gene expression and protecting cells from defects in protein folding (1, 13, 35). Emphasizing the importance of the key UPR regulators in secretory cells, loss of function of Perk, Atf4, Ire1, Xpb1, or Oasis disrupts the health and secretory functions of osteoblasts and subsequent bone formation (13–15). Here we show that NMP4 also plays a role in the appropriate regulation of the UPR through repression of the expression of Gadd34. Loss of Nmp4 resulted in a GADD34-mediated increase in protein synthesis basally that was largely sustained during pharmacological induction of ER stress, which then sensitized cells to the underlying stress. This finding emphasizes the role of NMP4 in maintaining the cell in a homeostatic state in which protection from proteotoxicity is balanced with the secretory requirements of the cell. In contrast to pharmacological stress, more mild, physiological stresses would likely result in maintenance of translation and some acceleration of protein secretion in the Nmp4−/− background without the toxicities associated with sustained pharmacological induction of ER stress. Indeed, targeted deletion of Nmp4 in mice enhances bone response to PTH and BMP2 and protects these animals from osteopenia likely through increased production and secretion of factors that facilitate bone formation (20).
The regulated expression and activity of NMP4 in response to pharmacological and physiological stresses also likely plays a role in NMP4-mediated regulation of ribosome biogenesis and the UPR. Nmp4 mRNA was reported to be expressed in all major organs analyzed, although there were two distinct transcripts that were differentially expressed by a mechanism suggested to involve alternative mRNA splicing (36). Transcription of Nmp4 is also mediated through the activity of two alternative promoters that both respond to PTH treatment and result in the production of Nmp4 mRNAs with different transcription start sites (37). Collectively, these regulatory mechanisms result in the production of multiple NMP4 protein isoforms, some of which contain an in-frame amino-terminal extension and all of which contain Cys2His2 zinc finger binding domains, which can range from five to eight in number (36–38). Although we observed multiple NMP4 protein isoforms, we did not detect an appreciable change in the pattern of NMP4 expression in response to pharmacological induction of the UPR (Fig. 3B). This suggests that protein modifications identified in NMP4 (39, 40) or availability of NMP4 interacting proteins (36) may play a role in regulating the localization and activity of NMP4 in response to cellular cues to modulate protein production and secretion (21).
Of note is the decrease in CReP protein expression that was observed upon loss of Nmp4 and overexpression of Gadd34. Despite being designated a constitutively expressed targeting subunit for type 1 protein phosphatase dephosphorylation of eIF2α-P, Crep expression was previously reported to be sharply reduced upon overexpression of GADD34 (10), suggesting an unexplored cross-regulation between the Crep and Gadd34 genes. The changes in ribosome biogenesis and eIF2α-P described herein emphasize the importance of regulation of NMP4 in Gadd34 and c-Myc expression in the maintenance of cellular homeostasis and provide a better understanding of the processes that maintain appropriate levels of protein synthesis in highly secretory tissues.
Author Contributions
S. K. Y. designed, performed, and analyzed the experiments and wrote the manuscript. Y. S. performed the experiments. R. C. W. and J. P. B. conceived and coordinated the study, designed and analyzed the experiments, and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM049164 (to R. C. W.). This work was also supported by the Ralph W. and Grace M. Showalter Research Trust Fund (to R. C. W.) and Department of Defense Grant PR120563 (to J. P. B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest with the contents of this manuscript.
- ER
- endoplasmic reticulum
- UPR
- unfolded protein response
- eIF2α-P
- phosphorylation of the α subunit of eIF2
- MSPC
- mesenchymal stem progenitor cell
- qPCR
- quantitative PCR
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
References
- 1. Walter P., and Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 2. Shi Y., Vattem K. M., Sood R., An J., Liang J., Stramm L., and Wek R. C. (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harding H. P., Zhang Y., and Ron D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 [DOI] [PubMed] [Google Scholar]
- 4. Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., and Ron D. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 5. Vattem K. M., and Wek R. C. (2004) Reinitiation involving upstream open reading frames regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 101, 11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., and Ron D. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 7. Novoa I., Zeng H., Harding H. P., and Ron D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma Y., and Hendershot L. M. (2003) Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J. Biol. Chem. 278, 34864–34873 [DOI] [PubMed] [Google Scholar]
- 9. Connor J. H., Weiser D. C., Li S., Hallenbeck J. M., and Shenolikar S. (2001) Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell. Biol. 21, 6841–6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Young S. K., Willy J. A., Wu C., Sachs M. S., and Wek R. C. (2015) Ribosome reinitiation directs gene-specific translation and regulates the integrated stress response. J. Biol. Chem. 290, 28257–28271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee Y. Y., Cevallos R. C., and Jan E. (2009) An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2α phosphorylation. J. Biol. Chem. 284, 6661–6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kondo S., Saito A., Asada R., Kanemoto S., and Imaizumi K. (2011) Physiological unfolded protein response regulated by OASIS family members, transmembrane bZIP transcription factors. IUBMB life 63, 233–239 [DOI] [PubMed] [Google Scholar]
- 13. Saito A., Ochiai K., Kondo S., Tsumagari K., Murakami T., Cavener D. R., and Imaizumi K. (2011) Endoplasmic reticulum stress response mediated by the PERK-eIF2α-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J. Biol. Chem. 286, 4809–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tohmonda T., Miyauchi Y., Ghosh R., Yoda M., Uchikawa S., Takito J., Morioka H., Nakamura M., Iwawaki T., Chiba K., Toyama Y., Urano F., and Horiuchi K. (2011) The IRE1α-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep. 12, 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murakami T., Saito A., Hino S., Kondo S., Kanemoto S., Chihara K., Sekiya H., Tsumagari K., Ochiai K., Yoshinaga K., Saitoh M., Nishimura R., Yoneda T., Kou I., Furuichi T., Ikegawa S., Ikawa M., Okabe M., Wanaka A., and Imaizumi K. (2009) Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 11, 1205–1211 [DOI] [PubMed] [Google Scholar]
- 16. Jang W. G., Kim E. J., Kim D. K., Ryoo H. M., Lee K. B., Kim S. H., Choi H. S., and Koh J. T. (2012) BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J. Biol. Chem. 287, 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karsenty G. (2008) Transcriptional control of skeletogenesis. Annu. Rev. Genomics Hum. Genet. 9, 183–196 [DOI] [PubMed] [Google Scholar]
- 18. Childress P., Philip B. K., Robling A. G., Bruzzaniti A., Kacena M. A., Bivi N., Plotkin L. I., Heller A., and Bidwell J. P. (2011) Nmp4/CIZ suppresses the response of bone to anabolic parathyroid hormone by regulating both osteoblasts and osteoclasts. Calcif. Tissue Int. 89, 74–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robling A. G., Childress P., Yu J., Cotte J., Heller A., Philip B. K., and Bidwell J. P. (2009) Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J. Cell. Physiol. 219, 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Childress P., Stayrook K. R., Alvarez M. B., Wang Z., Shao Y., Hernandez-Buquer S., Mack J. K., Grese Z. R., He Y., Horan D., Pavalko F. M., Warden S. J., Robling A. G., Yang F. C., Allen M. R., Krishnan V., Liu Y., and Bidwell J. P. (2015) Genome-wide mapping and interrogation of the nmp4 antianabolic bone axis. Mol. Endocrinol. 29, 1269–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feister H. A., Torrungruang K., Thunyakitpisal P., Parker G. E., Rhodes S. J., and Bidwell J. P. (2000) NP/NMP4 transcription factors have distinct osteoblast nuclear matrix subdomains. J. Cell. Biochem. 79, 506–517 [PubMed] [Google Scholar]
- 22. Wu X., Estwick S. A., Chen S., Yu M., Ming W., Nebesio T. D., Li Y., Yuan J., Kapur R., Ingram D., Yoder M. C., and Yang F. C. (2006) Neurofibromin plays a critical role in modulating osteoblast differentiation of mesenchymal stem/progenitor cells. Hum. Mol. Genet. 15, 2837–2845 [DOI] [PubMed] [Google Scholar]
- 23. Jousse C., Oyadomari S., Novoa I., Lu P., Zhang Y., Harding H. P., and Ron D. (2003) Inhibition of a constitutive translation initiation factor 2a phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han J., Back S. H., Hur J., Lin Y. H., Gildersleeve R., Shan J., Yuan C. L., Krokowski D., Wang S., Hatzoglou M., Kilberg M. S., Sartor M. A., and Kaufman R. J. (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baird T. D., and Wek R. C. (2012) Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 3, 307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., and Yuan J. (2005) A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 27. van Riggelen J., Yetil A., and Felsher D. W. (2010) MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer 10, 301–309 [DOI] [PubMed] [Google Scholar]
- 28. Iadevaia V., Liu R., and Proud C. G. (2014) mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin. Cell Dev. Biol. 36, 113–120 [DOI] [PubMed] [Google Scholar]
- 29. Preston A. M., and Hendershot L. M. (2013) Examination of a second node of translational control in the unfolded protein response. J. Cell Sci. 126, 4253–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., and Kaufman R. J. (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 31. Harding H. P., Zhang Y., Bertolotti A., Zeng H., and Ron D. (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 [DOI] [PubMed] [Google Scholar]
- 32. Thoreen C. C., Kang S. A., Chang J. W., Liu Q., Zhang J., Gao Y., Reichling L. J., Sim T., Sabatini D. M., and Gray N. S. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernales S., Papa F. R., and Walter P. (2006) Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 22, 487–508 [DOI] [PubMed] [Google Scholar]
- 34. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., and Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei J., Sheng X., Feng D., McGrath B., and Cavener D. R. (2008) PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J. Cell. Physiol. 217, 693–707 [DOI] [PubMed] [Google Scholar]
- 36. Nakamoto T., Yamagata T., Sakai R., Ogawa S., Honda H., Ueno H., Hirano N., Yazaki Y., and Hirai H. (2000) CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol. Cell. Biol. 20, 1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alvarez M., Shah R., Rhodes S. J., and Bidwell J. P. (2005) Two promoters control the mouse Nmp4/CIZ transcription factor gene. Gene 347, 43–54 [DOI] [PubMed] [Google Scholar]
- 38. Thunyakitpisal P., Alvarez M., Tokunaga K., Onyia J. E., Hock J., Ohashi N., Feister H., Rhodes S. J., and Bidwell J. P. (2001) Cloning and functional analysis of a family of nuclear matrix transcription factors (NP/NMP4) that regulate type I collagen expression in osteoblasts. J. Bone Miner. Res. 16, 10–23 [DOI] [PubMed] [Google Scholar]
- 39. Sharma K., D'Souza R. C., Tyanova S., Schaab C., Winiewski J. R., Cox J., and Mann M. (2014) Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 8, 1583–1594 [DOI] [PubMed] [Google Scholar]
- 40. Mertins P., Yang F., Liu T., Mani D. R., Petyuk V. A., Gillette M. A., Clauser K. R., Qiao J. W., Gritsenko M. A., Moore R. J., Levine D. A., Townsend R., Erdmann-Gilmore P., Snider J. E., Davies S. R., et al. (2014) Ischemia in tumors induces early and sustained phosphorylation changes in stress kinase pathways but does not affect global protein levels. Mol. Cell. Proteomics 13, 1690–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]