Abstract
Background: Choline is an essential nutrient for cell structure, cell signaling, neurotransmission, lipid transport, and bone formation. Choline can be irreversibly converted to betaine, a major source of methyl groups. Trimethylene N-oxide (TMAO), a proatherogenic molecule, is produced from the metabolism of dietary choline by the gut microbiome. The relation between serum choline and its closely related metabolites with linear growth in children is unknown.
Objective: The aim was to characterize the relation between serum choline and its closely related metabolites, betaine and TMAO, with linear growth and stunting in young children.
Design: We measured serum choline, betaine, and TMAO concentrations by using liquid chromatography isotopic dilution tandem mass spectrometry in a cross-sectional study in 325 Malawian children, aged 12–59 mo, of whom 62% were stunted.
Results: Median (25th, 75th percentile) serum choline, betaine, and TMAO concentrations were 6.4 (4.8, 8.3), 12.4 (9.1, 16.3), and 1.2 (0.7, 1.8) μmol/L, respectively. Spearman correlation coefficients of age with serum choline, betaine, and TMAO were −0.57 (P < 0.0001), −0.26 (P < 0.0001), and −0.10 (P = 0.07), respectively. Correlation coefficients of height-for-age z score with serum choline, betaine-to-choline ratio, and TMAO-to-choline ratio were 0.31 (P < 0.0001), −0.24 (P < 0.0001), and −0.29 (P < 0.0001), respectively. Serum choline concentrations were strongly and significantly associated with stunting. Children with and without stunting had median (25th, 75th percentile) serum choline concentrations of 5.6 (4.4, 7.4) and 7.3 (5.9, 9.1) μmol/L (P < 0.0001).
Conclusions: Linear growth failure in young children is associated with low serum choline and elevated betaine-to-choline and TMAO-to-choline ratios. Further work is needed to understand whether low dietary choline intake explains low circulating choline among stunted children living in low-income countries and whether increasing choline intake may correct choline deficiency and improve growth and development. This trial was registered in the ISRCTN registry (www.isrctn.com) as ISRCTN14597012.
Keywords: betaine, children, choline, linear growth, malnutrition, trimethylene-N-oxide, stunting, undernutrition
INTRODUCTION
Linear growth failure, considered to be the best summary measure of chronic malnutrition in children, is prevalent worldwide. Child stunting, defined as a height-for-age z score (HAZ)8 <−2, affects more than one-quarter of children <5 y of age (1, 2). Stunting is associated with impaired cognitive function, increased mortality, reduced economic productivity, and a greater chance of living in poverty in adulthood (3–7). A global target to reduce stunting by 40% was adopted by the World Health Assembly (8) and is Sustainable Development Goal 2 of the UN Development Project (9). Evidence-based nutritional interventions have only a modest impact on stunting and alone will be insufficient to meet this goal (8, 10, 11). Micronutrient deficiencies and infectious diseases are contributors to stunting (1), although in mathematical models these problems do not account for most of the linear growth failure (10). The pathogenesis of linear growth failure in childhood is still incompletely understood.
Choline is an essential nutrient for normal cellular function, growth, and development (12). Choline serves as the precursor for phosphatidylcholine, the major phospholipid in cell membranes, and for the neurotransmitter, acetylcholine. Choline can be oxidized to betaine, a major source of methyl groups, in the liver and kidney. Trimethylene N-oxide (TMAO) is produced when dietary choline is converted to trimethylamine by the gut microbiome and then oxidized to TMAO in the liver. TMAO has been implicated in the pathogenesis of atherosclerosis (13, 14). The largest proportion of choline is used for the synthesis of phosphatidylcholine (15). Choline that is converted to betaine or TMAO is no longer available for phosphatidylcholine or acetylcholine synthesis, because the conversion is irreversible. Choline was recognized as an essential nutrient by the Institute of Medicine in 1998 (16).
Although it has been suggested that dietary choline intake is likely to be lower in low-income countries (17), little is known about serum concentrations of choline and its closely related metabolites in children from low-income countries and their relation with growth and development. We hypothesized that low serum choline concentrations and higher betaine-to-choline and TMAO-to-choline ratios would be associated with linear growth failure in young children. We tested this hypothesis in young children with a high rate of stunting in rural Malawi.
METHODS
Study subjects and design
The study design was cross-sectional. The study subjects consisted of a community-based sample of 650 children identified because they were twins, aged 12–59 mo, residing in 6 villages (Masika, Makhwira, Mitondo, Mbiza, Chamba, and Mayaka) in rural southern Malawi in 2011. Children were eligible for the study if they were between 12 and 59 mo of age, had no evidence of kwashiorkor or marasmus, had no congenital or chronic disease or caretaker-reported diarrhea, or were not undergoing treatment of acute malnutrition. The community-based sample was a study of gut permeability. A total of 540 children fit the eligibility criteria and underwent anthropometric measurements and dual sugar absorption testing. It was possible to obtain venous blood samples from 483 children. A random subsample of 325 of the 483 samples were chosen for analyses of serum choline, betaine, and TMAO.
All of the children underwent anthropometric measurements conducted by trained, experienced fieldworkers. Weight was measured to the nearest 5 g by using a digital scale (Seca 344). Recumbent length (<24 mo old) or standing height was measured to the nearest 0.1 cm by using a rigid height board (Seca 417). HAZ and weight-for-height were calculated by using WHO growth standards, with stunting defined as an HAZ <−2 (18). Venous blood was drawn by study nurses and doctors following standard guidelines for pediatric blood sampling (19). Serum samples were processed, separated into aliquots, and snap-frozen in liquid nitrogen in cryovials within 4 h of blood drawing. Cryovials were transferred to storage at −80°C. Chichewa-speaking Malawian research nurses obtained written and oral informed consent from each child’s caretaker before enrollment in the study. Community consent for the study was also obtained from the village chief and local health officials. The protocol for this study was approved by the College of Medicine Research Ethics Committee of the University of Malawi, the Human Research Protection Office of Washington University in St. Louis, and the Johns Hopkins School of Medicine Institutional Review Board. This trial was registered in the ISRCTN registry as ISRCTN14597012.
Measurement of serum choline, betaine, and TMAO
To measure serum choline, betaine, and TMAO, we used liquid chromatography isotopic dilution tandem mass spectrometry, an HPLC–tandem mass spectrometry (HPLC-MS/MS) method based on a previous method (20) with modifications to account for different instrumentation and conditions. The transitions used to monitor the analytes, assay validation, and details of assay performance are shown in Supplemental Table 1, Supplemental Figure 1, and Supplemental Tables 2–5, respectively. Choline, betaine, and TMAO were obtained from Sigma-Aldrich. Deuterated internal standards choline-d9 and betaine-d9 were obtained from Isotec/Sigma-Aldrich. TMAO-d9 was obtained from Cambridge Isotope Laboratories. Optima liquid chromatography-mass spectrometry grade water, acetonitrile, and methanol were obtained from Fisher Scientific. A Parker Balston nitrogen generator was used to produce nitrogen gas (ultra-pure, >99.9%). Argon gas (ultra-pure, >99.9%) was obtained from Valley.
Each analyte (choline, betaine, TMAO, and internal standards) was dissolved in methanol and water (50:50, vol:vol) to obtain a 1.0-mg/mL stock solution. Stock solutions were then mixed with water to prepare intermediate stock solutions from which aqueous calibration standards and quality controls were prepared as recently described (21). Specifically, intermediate stock solutions were added into pooled human plasma (Sigma) to create 8 standards at concentrations ranging from 0.01 to 1 μg/mL for each analyte. Three quality-control standards (low, medium, and high) were made by adding a separate 1.0-mg/mL stock solution into the same plasma at concentrations of 0.05, 1.0, and 5.0 μg/mL for each analyte. The quantification of each analyte was accomplished by using area ratios calculated with the use of the standard analyte and the corresponding internal standard. The blank plasma area was subtracted from all of the spiked standards to obtain the calibration curve. All stock solutions, standards, and quality-control samples were stored at −80°C to simulate the storage conditions of the study samples.
Briefly, choline, betaine, and TMAO were extracted from 10 μL serum with 40 μL methanol containing 10 μg choline-d9, betaine-d9, and TMAO-d9/mL as internal standards. After vigorous mixing on a vortex for 30 s followed by centrifugation at 4°C at 12,000 × g for 5 min, a 20-μL aliquot of the supernatant was diluted with 100 μL acetonitrile and methanol (75:25, vol:vol). After vigorous mixing on a vortex for 30 s followed by centrifugation at 4°C at 12,000 × g for 5 min, a 10-μL aliquot was removed and injected into the HPLC-MS/MS system.
Targeted metabolite profiling was developed by using reference standards of each metabolite to determine chromatographic retention times and mass spectrometry multiple reaction monitoring transition, declustering potentials, and collision energies. Choline, betaine, and TMAO unlabeled and deuterated standards were cleaned and separated by using a 5500 QTrap (Sciex) mass spectrometer equipped with an electrospray ionization source, a Shimadzu CBM-20A command module, a Shimadzu LC-20AB pump, a Shimadzu SIL-20AC-HT autosampler, and a Shimadzu CTO-10Ac column oven heater. Chromatographic separations were conducted by using an Atlantis HILIC, 5 μm, 2.1 × 10 mm guard column coupled with an Atlantis Silica HILIC 100Å, 5 μm, 2.1 × 150 mm column from Waters. Mobile phases consisted of phase A (0.2% formic acid in acetonitrile) and phase B (10 mmol ammonium acetate/L and 0.2% formic acid in water). The gradient started with 1 min of isocratic elution with 15% phase B. Phase B was increased to 70% over the next 10 min and held at 70% for another 4 min. The mobile phase composition was returned to 15% phase B and the column was equilibrated for 5 min to give a total runtime of 20 min/sample. The flow rate was 400 μL/min. The electrospray ion spray voltage was 4.5 kV, and the source temperature was 450°C. The autosampler oven temperature was 15°C and dwell time was 150 ms. All of the sample data were collected by using Analyst (version 1.5) software and processed by using MultiQuant software (version 2.1) (both Sciex).
Statistical analyses
The sample size of 325 children was based on >90% power to detect ≥12% difference in serum choline between stunted and nonstunted children, given a 60% prevalence of stunting, σ = 2.3 μmol/L, no matching, α = 0.05, and a 2-sided test. Serum choline concentrations were normally distributed. Serum betaine and TMAO concentrations were skewed to higher values. Spearman correlations were used to examine correlations between variables. Wilcoxon’s rank-sum test was used to compare analytes and ratios between groups. Multivariable linear regression analyses were used to examine the relation between serum choline and height. All of the analyses were performed by using R software (version 3.2.2) with a type I error of 0.05.
RESULTS
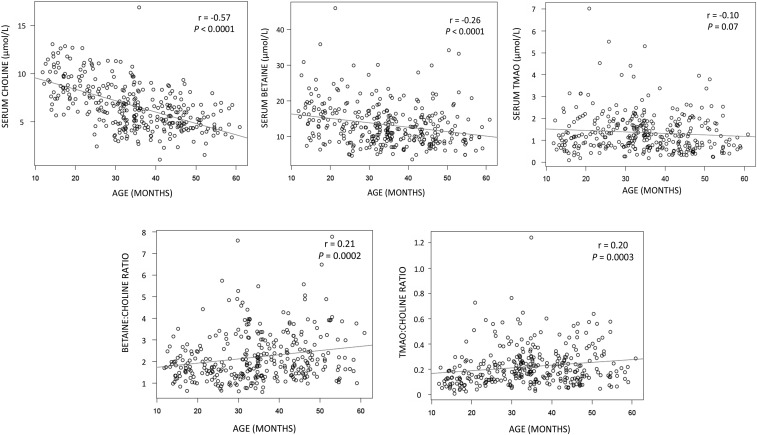
The characteristics of the 325 children in the study are shown in Table 1. There was a nearly equal number of girls and boys. More than 60% of the children were stunted. Median (25th, 75th percentile) serum choline, betaine, and TMAO concentrations were 6.4 (4.8, 8.3), 12.4 (9.1, 16.3), and 1.2 (0.7, 1.8) μmol/L, respectively. Median (25th, 75th percentile) betaine-to-choline and TMAO-to-choline ratios were 1.90 (1.42, 2.76) and 0.18 (0.12, 0.28), respectively. There were no significant differences in serum choline, betaine, TMAO, and betaine-to-choline and TMAO-to-choline ratios between boys and girls (Table 2). Serum choline and betaine concentrations had a significant negative correlation with age (Figure 1). Serum TMAO was not significantly associated with age. Betaine-to-choline and TMAO-to-choline ratios showed significant positive correlations with age (Figure 1).
TABLE 1.
Characteristics of the study population
| Value | |
| Age, mo | 34.4 ± 11.81 |
| Female, % | 49.8 |
| Weight-for-height z score | 0.2 ± 0.9 |
| Height-for-age z score | −2.3 ± 1.3 |
| Stunted,2 % | 62 |
| Caretaker is the mother, % | 96 |
| Father is alive, % | 96 |
| Siblings, n | 3.8 ± 1.7 |
| Individuals that sleep in same room as child, n | 3.3 ± 1.4 |
| Home with a metal roof, % | 19 |
| Family owns bicycle, % | 62 |
| Animals that sleep in house, % | 36 |
| Water from a clean source, % | 67 |
| Child uses pit latrine, % | 80 |
Mean ± SD (all such values).
Height-for-age z score < −2.
TABLE 2.
Serum choline, betaine, and TMAO concentrations and ratios in boys compared with girls1
| Boys (n = 163) | Girls (n = 162) | P2 | |
| Serum choline, μmol/L | 6.2 (4.6, 7.9) | 6.4 (4.8, 8.3) | 0.38 |
| Serum betaine, μmol/L | 12.2 (9.1, 16.2) | 12.6 (9.1, 16.3) | 0.79 |
| Serum TMAO, μmol/L | 1.2 (0.7, 1.8) | 1.1 (0.7, 1.7) | 0.32 |
| Betaine:choline | 1.90 (1.42, 2.80) | 1.90 (1.43, 2.70) | 0.62 |
| TMAO:choline | 0.19 (0.12, 0.29) | 0.18 (0.12, 0.26) | 0.21 |
Values are medians (25th, 75th percentiles). TMAO, trimethylene N-oxide.
Derived by using Wilcoxon’s rank-sum test.
FIGURE 1.
Scatterplots showing serum choline, betaine, and TMAO concentrations and ratios of betaine to choline and TMAO to choline by age, with Spearman correlations, in 325 children. TMAO, trimethylene N-oxide.
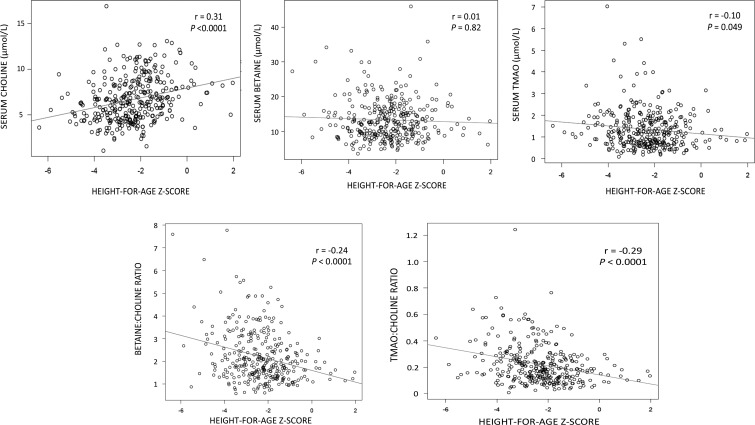
Serum choline concentrations were significantly lower in stunted than in nonstunted children (Table 3). Serum betaine-to-choline and TMAO-to-choline ratios were significantly higher in stunted than in nonstunted children. There were no significant differences in serum betaine concentration or TMAO concentrations between stunted and nonstunted children (Table 3). Serum choline was positively correlated with HAZ (P < 0.0001) (Figure 2). Serum TMAO, betaine-to-choline ratio, and TMAO-to-choline ratio showed significant negative associations with HAZ. There was no significant relation between serum betaine and HAZ (Figure 2). Spearman correlations (r) between HAZ and choline, betaine, TMAO, and betaine-to-choline and TMAO-to-choline ratios, after adjustment for age, were 0.12 (P = 0.002), −0.07 (P = 0.20), −0.14 (P = 0.01), −0.16 (P = 0.004), and −0.23 (P < 0.0001), respectively.
TABLE 3.
Serum choline, betaine, and TMAO concentrations and ratios in stunted compared with nonstunted children1
| Stunted (n = 201) | Not stunted (n = 124) | P2 | |
| Serum choline, μmol/L | 5.6 (4.4, 7.4) | 7.3 (5.9, 9.1) | <0.0001 |
| Serum betaine, μmol/L | 12.5 (9.0, 16.4) | 12.2 (9.2, 16.2) | 0.87 |
| Serum TMAO, μmol/L | 1.2 (0.7, 1.9) | 1.1 (0.7, 1.7) | 0.22 |
| Betaine:choline | 2.09 (1.51, 3.15) | 1.71 (1.28, 2.31) | 0.0004 |
| TMAO:choline | 0.21 (0.13, 0.32) | 0.16 (0.10, 0.24) | 0.0002 |
Values are medians (25th, 75th percentiles). TMAO, trimethylene N-oxide.
Derived by using Wilcoxon’s rank-sum test.
FIGURE 2.
Scatterplots showing serum choline, betaine, and TMAO concentrations and ratios of betaine to choline and TMAO to choline by height-for-age z score, with Spearman correlations, in 325 children. TMAO, trimethylene N-oxide.
In all of the children, a 1-SD (2.3 μmol/L) increase in serum choline was associated with 0.41 cm greater height (P < 0.0001) in a multivariable linear regression model that adjusted for age. By applying similar models among children aged 12–36 mo, a 1-SD increase in serum choline was associated with 0.42 cm greater height (P < 0.0001); among children aged >36–60 mo, a 1-SD increase was associated with 0.33 cm greater height (P < 0.0001). Among girls and boys, in multivariable linear regression models adjusted for age a 1-SD increase in choline was associated with 0.60 and 0.19 cm greater height, respectively (both P < 0.0001).
DISCUSSION
This study shows that young children with low serum choline concentrations are more likely to have linear growth failure. A high serum betaine-to-choline ratio and a high serum TMAO-to-choline ratio were also both associated with linear growth failure. The betaine-to-choline and TMAO-to-choline ratios were used to gain insight into choline that is converted irreversibly to metabolites that are no longer available for phosphatidylcholine and acetylcholine synthesis from choline. The ratios are not to imply that betaine and TMAO have a role in stunting. To our knowledge, this is the first study to examine the relation between serum choline and 2 closely related metabolites with linear growth in young children. These findings support our hypothesis that the availability of choline is a limiting factor for child growth and development. There is considerable evidence for the essential role of choline in the linear growth of bone, cell membrane formation, lipid metabolism, pulmonary function, neurotransmission and central nervous system development, and gut immunity.
Choline is required for the synthesis of phosphatidylcholines through the CDP-choline pathway. Growth and development are highly dependent on phosphatidylcholines, because they are the major component of cell membranes (22), essential for lipoprotein assembly and secretion by the liver (23), the predominant active component of lung surfactant (24), and the major lipid in the protective mucus layer in the gut (25). Chondrogenesis in the growth plate is a main determinant of linear growth in children (26). The growth of limb long bones by endochondral ossification requires the synthesis of phosphatidylcholines by osteoblasts and the endochondral plate (27, 28). Mutant mice that lack choline kinase β, the first enzyme in the CDP-choline pathway for the biosynthesis of phosphatidylcholines, have reduced bone formation, decreased bone mass, and increased osteoblast activity (29). Insufficient choline to meet the requirements for phosphatidylcholine synthesis in bone growth is one potential biological mechanism by which low choline could stunt the linear growth of children.
Sphingomyelin, which is produced from phosphatidylcholine and ceramide in the final step of biosynthesis (30), comprises the main lipid in the myelin sheaths of the central nervous system and is essential in the rapid period of central nervous system myelination that occurs in the first years of life (31, 32). Acetylcholine, which is produced from choline and acetyl-CoA by choline acetyltransferase, serves as a fast-acting neurotransmitter at the neuromuscular junction and in autonomic ganglia and as a major neuromodulator in central nervous system development (33, 34). Acetylcholine also serves as a vagal neurotransmitter and plays a role in the cholinergic anti-inflammatory response in the intestinal epithelium and gut mucosa (35).
In the present study, both the betaine-to-choline and TMAO-to-choline ratios were negatively associated with linear growth in children. As noted previously, choline that is oxidized to betaine or converted to TMAO is no longer available for synthesis of phosphatidylcholines and sphingomyelins or conversion to acetylcholine. Betaine is a major methyl donor in the methionine cycle and serves as an osmolyte in the kidney (36). TMAO is primarily produced in a pathway that involves the conversion of dietary choline, phosphatidylcholine, and carnitine by the gut microbiota (13, 14). TMAO, considered an evolutionary remnant of the osmolyte system (37), may have atherogenic potential in adults (13, 14). The plasma concentrations of TMAO in adults have been described in the range of ∼2–7 μmol/L (13). In the present study, mean serum TMAO concentrations were lower (median: 1.2 μmol/L) than described in adults. Future work is needed to characterize the relation between the gut microbiome in children in low-income settings with serum TMAO.
The rapid and accurate measurement of serum choline and related metabolites is now possible with the use of selected reaction monitoring and mass spectrometry, as developed in our laboratory and by other groups (20, 21, 38–40). There are limited data on serum choline concentrations in humans. Some caution must be taken in comparing serum choline concentrations from different studies, because some earlier studies did not use the more accurate liquid chromatography-MS/MS methodology. Mean serum choline concentrations in normal children aged 12–59 mo in Turkey were ∼13–14 μmol/L (41). In 31 healthy children with a mean age of 10 y, the mean plasma choline concentration was 8.0 μmol/L (42). Mean plasma choline reported in healthy adults was ∼10 μmol/L (16). In pregnant women in Jamaica in the first trimester, mean plasma choline was 8.4 μmol/L (43). The mean plasma choline concentration in 30 women from rural Gambia was 8.2 μmol/L (44). The effect of inadequate dietary intake of choline has been studied in healthy young men. After 3 wk of consumption of a choline-deficient diet, mean plasma choline concentrations decreased from ∼10.5 to ∼7.5 μmol/L (44). The median serum choline concentration of 5.6 μmol/L found in stunted children from rural Malawi in the present study is lower than in previous studies (16, 41–43) and lower than concentrations described in men subjected to experimental choline deficiency (44). There is currently no established definition for choline deficiency or insufficiency on the basis of serum choline concentrations. Genetic factors may affect dietary requirements for choline (45, 46). Further work is needed to characterize serum choline concentrations in health and disease.
In the present study, serum choline concentrations declined with age. Breast milk is a rich source of choline, whereas typical weaning foods in rural Malawi are poor dietary sources of choline. The richest food sources of choline are primarily animal products such as eggs and meats (12), which are infrequently consumed in poor families. Defatted soy flour and wheat germ are high in choline, but it may be difficult for young children to reach the Adequate Intake of choline as established by the Institute of Medicine. The Adequate Intake of choline for children aged 1–3 y is currently set at 200 mg/d (16), which is equivalent to the choline contained in 1 cup of defatted soy flour or 0.5 cup of toasted wheat germ (12). It is probably unlikely that the gut microbiome contributed a great deal to the generation of TMAO, because most dietary choline is absorbed before it reaches the colon. The Institute of Medicine recommendations (16) should be followed with regard to increasing choline intake by food or supplementation. The dietary intake of choline in children and families in low-income countries represents a major gap in the knowledge.
It is important to consider that an inadequate dietary intake of choline is unlikely to occur in isolation from other nutrients in young children with linear growth failure in low-income countries. The synthesis of phosphatidylcholines and sphingomyelins is controlled by transcription factors such as sterol regulatory element-binding protein 1 (SREBP-1). SREBP-1, in turn, is regulated by nutrient-sensing and growth-regulating pathways such as mechanistic target of rapamycin complex 1 (mTORC1) (47). The mTORC1 pathway, widely considered the master regulator of cell and organismal growth, becomes deactivated in the presence of inadequate concentrations of amino acids for protein synthesis. Repression of the mTORC1 pathway inhibits protein synthesis and the production of various classes of lipids, including phosphatidylcholines and saturated and unsaturated fatty acids (48). In other words, if some proteinogenic amino acids are not available for protein synthesis, both protein and lipid synthesis will be inhibited because the cells do not have adequate nutrients for cell growth. A sufficient dietary intake of choline alone may not provide benefit if other proteinogenic amino acids are not sufficient in the diet.
The present study is limited in that a causal relation between low serum choline concentrations and linear growth failure cannot be established by a cross-sectional study. There may not necessarily be a causal link between choline and stunting, because there could be other nutrients found in choline-rich foods that might play a role in linear growth. The findings from this study cannot necessarily be extrapolated to children in other settings, because there may be differences in diet, behavior, environment, and endemic diseases in other populations. Controlled clinical trials will be needed to determine whether increasing dietary choline intake or choline supplementation could improve linear growth and reduce stunting in children in low-income settings.
Acknowledgments
The authors’ responsibilities were as follows—RDS, PZ, RM, IT, KMM, LF, and MJM: designed the research; PZ, IT, KMM, and MJM: conducted the research; RM and LF: provided essential reagents or materials; MG-F and MIO: analyzed the data; RDS, MG-F, IT, LF, and MJM: wrote the manuscript; and RDS: had primary responsibility for the final content. The authors had no conflicts of interest.
Footnotes
Abbreviations used: HAZ, height-for-age z score; HPLC-MS/MS, HPLC- tandem mass spectrometry; mTORC1, mechanistic target of rapamycin complex 1; SREBP-1, sterol regulatory element-binding protein 1; TMAO, trimethylene N-oxide.
REFERENCES
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 2.de Onis M, Blössner M, Borghi E. Prevalence and trends of stunting among pre-school children, 1990-2020. Public Health Nutr 2012;15:142–8. [DOI] [PubMed] [Google Scholar]
- 3.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 2008;371:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, Caulfield LE, Danaei G. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One 2013;8:e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr 2013;98:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudfeld CR, McCoy DC, Danaei G, Fink G, Ezzati M, Andrews KG, Fawzi WW. Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics 2015;135:e1266–75. [DOI] [PubMed] [Google Scholar]
- 8.de Onis M, Dewey KG, Borghi E, Onyango AW, Blössner M, Daelmans B, Piwoz E, Branca F. The World Health Organization’s global target for reducing childhood stunting by 2025: rationale and proposed actions. Matern Child Nutr 2013;9(Suppl 2):6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray CJ. Shifting to Sustainable Development Goals—implications for global health. N Engl J Med 2015;373:1390–3. [DOI] [PubMed] [Google Scholar]
- 10.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr 2008;4(Suppl 1):24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE; Lancet Nutrition Interventions Review Group; Maternal and Child Nutrition Study Group. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382:452–77. [DOI] [PubMed] [Google Scholar]
- 12.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009;67:615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015;290:5647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res 2008;49:1187–94. [DOI] [PubMed] [Google Scholar]
- 16.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: thiamin, riboflavin, niacin, vitamin B-6, vitamin B-12, panthothenic acid, biotin, and choline. Washington (DC): . National Academy of Sciences; 1998. [PubMed] [Google Scholar]
- 17.Mehedint MG, Zeisel SH. Choline’s role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care 2013;16:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Onis M, Onyango A, Borghi E, Siyam A, Blössner M, Lutter C; WHO Multicentre Growth Reference Study Group. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr 2012;15:1603–10. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO guidelines on drawing blood. Geneva (Switzerland): World Health Organization; 2010. [Google Scholar]
- 20.Ocque AJ, Stubbs JR, Nolin TD. Development and validation of a simple UHPLC-MS/MS method for the simultaneous determination of trimethylamine N-oxide, choline, and betaine in human plasma and urine. J Pharm Biomed Anal 2015;109:128–35. [DOI] [PubMed] [Google Scholar]
- 21.Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015;22:228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta 2013;1831:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim Biophys Acta 2012;1821:754–61. [DOI] [PubMed] [Google Scholar]
- 24.Goss V, Hunt AN, Postle AD. Regulation of lung surfactant phospholipid synthesis and metabolism. Biochim Biophys Acta 2013;1831:448–58. [DOI] [PubMed] [Google Scholar]
- 25.Johansson ME, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, Subramani DB, Holmén-Larsson JM, Thomsson KA, Bergström JH, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 2011;68:3635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron J, Sävendahl L, De Luca F, Dauber A, Phillip M, Wit JM, Nilsson O. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol 2015;11:735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Wu G, Sher RB, Khavandgar Z, Hermansson M, Cox GA, Doschak MR, Murshed M, Beier F, Vance DE. Choline kinase beta is required for normal endochondral bone formation. Biochim Biophys Acta 2014;1840:2112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Wu G, van der Veen JN, Hermansson M, Vance DE. Phosphatidylcholine metabolism and choline kinase in human osteoblasts. Biochim Biophys Acta 2014;1841:859–67. [DOI] [PubMed] [Google Scholar]
- 29.Kular J, Tickner JC, Pavlos NJ, Viola HM, Abel T, Lim BS, Yang X, Chen H, Cook R, Hool LC, et al. Choline kinase β mutant mice exhibit reduced phosphocholine, elevated osteoclast activity, and low bone mass. J Biol Chem 2015;290:1729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slotte JP. Biological functions of sphingomyelins. Prog Lipid Res 2013;52:424–37. [DOI] [PubMed] [Google Scholar]
- 31.Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 1987;46:283–301. [DOI] [PubMed] [Google Scholar]
- 32.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 1988;47:217–34. [DOI] [PubMed] [Google Scholar]
- 33.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 2012;76:116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abreu-Villaça Y, Filgueiras CC, Manhães AC. Developmental aspects of the cholinergic system. Behav Brain Res 2011;221:367–78. [DOI] [PubMed] [Google Scholar]
- 35.Dhawan S, Cailotto C, Harthoorn LF, de Jonge WJ. Cholinergic signalling in gut immunity. Life Sci 2012;91:1038–42. [DOI] [PubMed] [Google Scholar]
- 36.Ueland PM, Holm PI, Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med 2005;43:1069–75. [DOI] [PubMed] [Google Scholar]
- 37.Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition 2015;31:1317–23. [DOI] [PubMed] [Google Scholar]
- 38.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 2003;49:286–94. [DOI] [PubMed] [Google Scholar]
- 39.Kirsch SH, Herrmann W, Rabagny Y, Obeid R. Quantification of acetylcholine, choline, betaine, and dimethylglycine in human plasma and urine using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:3338–44. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Zeisel SH, Zhang S. Rapid LC-MRM-MS assay for simultaneous quantification of choline, betaine, trimethylamine, trimethylamine N-oxide, and creatinine in human plasma and urine. Electrophoresis 2015 Jun 17 (Epub ahead of print; DOI: 10.1002/elps.201500055). [DOI] [PubMed] [Google Scholar]
- 41.Ilcol YO, Ozbek R, Hamurtekin E, Ulus IH. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 2005;16:489–99. [DOI] [PubMed] [Google Scholar]
- 42.Misra S, Ahn C, Ament ME, Choi HJ, Jenden DJ, Roch M, Buchman AL. Plasma choline concentrations in children requiring long-term home parenteral nutrition: a case control study. JPEN J Parenter Enteral Nutr 1999;23:305–8. [DOI] [PubMed] [Google Scholar]
- 43.Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S. Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica. West Indian Med J 2005;54:355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominguez-Salas P, Moore SE, Cole D, da Costa KA, Cox SE, Dyer RA, Fulford AJ, Innis SM, Waterland RA, Zeisel SH, et al. DNA methylation potential: dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am J Clin Nutr 2013;97:1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J 1991;5:2093–8. [PubMed] [Google Scholar]
- 46.Silver MJ, Corbin KD, Hellenthal G, da Costa KA, Dominguez-Salas P, Moore SE, Owen J, Prentice AM, Hennig BJ, Zeisel SH. Evidence for negative selection of gene variants that increase dependence on dietary choline in a Gambian cohort. FASEB J 2015;29:3426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 2008;8:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]