Abstract
Background: Currently, there are few diet quality assessment tools that are predictive of coronary artery disease (CAD) risk that do not require nutrient analysis and substantial time to administer in clinical settings.
Objective: To inform the development of such a tool, we prospectively examined the association between a food-based diet quality score and risk of CAD in 3 separate large US cohort studies.
Design: Between 1984 and 2012, 71,415 women (aged 43–63 y in 1984), 42,945 men (aged 40–75 y in 1986), and 93,131 younger women (aged 27–44 y in 1991) without a history of cardiovascular disease were followed up to 28 y. Diet was assessed ≤7 times by using repeated food-frequency questionnaires. We computed the Food Quality Score (FQS) for each individual based on food groups previously associated with less weight gain. A higher score represented a healthier diet. The FQS and CAD association was modeled with the Cox proportional hazard model, controlling for potential confounders. We also compared the magnitude of association with CAD for the FQS and other diet quality scores.
Results: We ascertained 6817 incident total CAD events, with 4588 cases of nonfatal myocardial infarction and 2131 fatal CAD events. Comparing top to bottom deciles, pooled RRs of the FQS were 0.61 (95% CI: 0.54, 0.69; P-trend < 0.001) for total CAD. These associations were independent of established cardiovascular disease risk factors including body weight, physical activity, and smoking. The magnitude of the RR for 1 SD of the FQS and CAD was generally similar to established diet scores that require detailed nutrient analysis, including the Alternate Healthy Eating Index-2010, the Dietary Approaches to Stop Hypertension score, and the alternate Mediterranean diet score.
Conclusion: A higher food-based diet quality score was associated with lower risk of CAD and was comparable with established diet scores.
Keywords: cardiovascular, diet quality, coronary heart disease, nutrition, diet
INTRODUCTION
Several diet quality indexes have been constructed to measure adherence to dietary recommendations. Many of these scores were associated with lower risk of cardiovascular disease (CVD)10 risk and mortality (1–6). As such, the scientific report of the 2015 Dietary Guidelines Advisory Committee reaffirms the use of dietary patterns as guidance for healthy eating (7). However, most of these diet quality indexes use a combination of both foods and nutrients. Assessment of nutrient intake require an additional step of linking the food intake with a nutrient database; therefore, these scores are not easily adapted for clinic use to assess and track diet change efforts or for incorporation into electronic health records. Food-based scores are easier to adapt for clinical use because they do not require software or a database to perform nutrient analysis. Several food-based dietary quality scores have been associated with CVD risk factors such as inflammatory markers and fasting insulin (8–10). Only 2 food-based scores have been examined for CVD endpoints. The Healthy Nordic Food Index is a food-based score that has been examined for disease prediction (11) but was not associated with CVD mortality. Its purpose was to measure intake of healthy foods common in the Nordic countries. Because it consists of only 6 food groups, it does not fully capture healthy and unhealthy aspects of the diet and may not be fully applicable to the US diet. The Elderly Dietary Index, which consists of 10 food items, has been associated with a lower risk of CVD mortality and incident coronary artery disease (CAD) in a cohort of British elderly men (4). Given the limited data in this area, there is a need to develop methods to easily assess dietary habits in the clinic, assess and track behavior change efforts, and adapt such a score for documentation in Electronic Health Records, which is specified in the Affordable Care Act.
We have previously identified foods that are associated with less long-term weight gain (12). In this study, we examined whether this food-based score predicts long-term risk of CAD in the setting of an epidemiologic study by using data from 3 large cohorts of American adults. We also compared the strength of association between this score and CAD to that of other well-established diet quality scores such as the Alternate Eating Index-2010 (AHEI-2010) (1), the alternate Mediterranean diet Score (aMed) (13), and the Dietary Approaches to Stop Hypertension adherence score (DASH) (14), each of which require a combination of food and nutrient items.
METHODS
Study population
Participants in this analysis were US men and women from the ongoing Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Nurses’ Health Study II (NHS II). The NHS cohort began in 1976 with 121,000 female nurses aged 35–55 y (15). The HPFS cohort began in 1986 with 51,529 male health professionals aged 40–75 y (16). The NHS II began in 1989 with 116,671 female nurses aged 25–42 y (17). Every 2 y, a questionnaire was sent to each participant to assess lifestyle, health outcomes, and medication use. Every 4 y, a validated food-frequency questionnaire (FFQ) was included to assess usual dietary intake (18).
In this analysis, we used 1984 as the start of follow-up (baseline) for the NHS because this was the first administration of a detailed FFQ. Follow-up started in 1986 for the HPFS and in 1991 for the NHS II. We included participants who returned the baseline FFQ (1984 for the NHS, 1986 for HPFS, and 1991 for NHS II) whose energy intake was within a plausible range (500–3500 kcal for women and 800–4000 kcal for men). At baseline, we excluded individuals who reported a history of CAD or stroke and those with missing BMIs. As a result, 71,415 participants from the NHS, 42,495 from the HPFS, and 93,131 from NHS II were included. This study was approved by the Institutional Review Board at Brigham and Women’s Hospital.
Diet assessment and computation of Food Quality Score
An FFQ was administered in 1984 and 1986 and every 4 y thereafter for a total of 8 times in the NHS until 2010, 6 times between 1986 and 2006 in the HPFS, and 5 times between 1991 and 2007 in the NHS II. Each FFQ contained ∼135 items, and each item had 9 frequency choices, ranging from <1 time/mo to ≥6 times/d. A standard portion size was also provided. We computed the Food Quality Score (FQS) for each FFQ year for each individual. The FQS was based on a list of food groups that has previously been found to be associated with weight change in these 3 cohorts (12). In the version used in this analysis, we included these original components: vegetables, fruit, nuts and legumes, whole grains, yogurt, sugar-sweetened beverages, red meats, processed meats, refined grains, desserts and ice cream, potatoes, potato chips, butter, and fried food from outside the home. We also included coffee and nuts because they have been inversely associated with a lower risk of CAD in previous studies (19). We removed milk and cheese from the original version because of their small effect on weight, and trans fatty acids because this is a food component instead of food. Although the food-based score does not necessary contain all foods that have been shown to predict CVD, major foods and food groups that reflect an overall diet pattern were included. For each food group, we ranked intakes of participants into quintiles and assigned the scores of 1–5 to groups previously shown to have favorable health effects (yogurt, nuts, whole grains, fruits, vegetables, and coffee). We assigned reversed quintile rankings (scores of 5 down to 1) for the unhealthy food groups (red meats, processed meats, desserts and ice cream, refined grains, potatoes, potato chips, fried foods prepared away from home, and sugar-sweetened beverages). The rankings of each food group were summed to give an overall score (total of 14 food groups with a possible score range of 14–70). A higher score represents a healthier diet.
In this analysis, we also compared the FQS with other diet quality scores on their ability to predict CAD. These other scores were the AHEI-2010 (1), the aMed (13), and the DASH (14). The components of these scores are listed in Supplemental Table 1.
Assessment of outcome
In the analysis, CAD was nonfatal myocardial infarction (MI) and fatal coronary disease. CAD was first self-reported in each biennial questionnaire during the follow-up period. We then obtained permission from the participants or their next of kin (for deceased participants) to review medical records for confirmation by study physicians who were unaware of the participant’s dietary intake. Nonfatal MI was confirmed by use of WHO criteria that require clinical symptoms and changes on electrocardiogram or cardiac enzymes (20). We also included “probable” cases, for which the questionnaire report was affirmed by interview or letter but for which medical records were not released (or available).
All deaths, including fatal CVD, were identified from searches of state vital records, the National Death Index, or reported by participants’ next of kin or the postal system. Fatal CVD was considered as “definite” if confirmed by review of hospital or autopsy records or when the participant had a history of CAD and death certificate listed CAD as cause of death. For fatal cases confirmed by death certificate only, we classified those as “presumed” cases.
Assessment of lifestyle characteristics
Lifestyle characteristics were assessed every 2 y by self-report in the biennial questionnaires. These characteristics included current smoking status and the number of cigarettes/d. Among women, menopausal status and menopausal hormone use, including the specific type and duration, were collected. BMI for each 2-y period was computed by using reported weight and height, which was reported at baseline. Average duration of leisure-time physical activity over the past year was assessed by using 10 questions that included the most common types of leisure-time activities. We also assessed family history of MI in 1976, 1984, and 1996 for the NHS; in 1986 for the HPFS; and in 1989, 1993, 1997, 2001, and 2005 for the NHS II.
Statistical analysis
For each participant, we calculated person-years from the date of return of the baseline FFQ to the date of diagnosis of CAD, return of the last questionnaire, death, or the end of follow-up (30 June 2012 for the NHS, 31 January 2010 for the HPFS, and 30 June 2011 for the NHS II). To examine the association between the FQS and CAD, we used time-dependent Cox proportional hazard model conditioning on age and follow-up cycle separately for men and women. We then pooled the RRs of the 3 cohorts by use of an inverse variance–weighted meta-analysis by a fixed-effects model, which allowed for between-study heterogeneity (21).
We computed cumulative averages of the FQS to reduce intraperson variation and to better reflect long-term diet quality (22). However, we stopped updating dietary intake when a participant reported a diagnosis of hypertension, hyperlipidemia, angina, or diabetes because individuals might improve their diet but physiologically it might be too late to have an impact. As sensitivity analysis, we repeated our analysis with diet being updated even when an intermediate endpoint was reported. The FQS was classified into deciles to facilitate the ability to detect a potential nonlinear association. We also formally examined the possibly of nonlinear relation between the FQS and CAD nonparametrically with restricted cubic splines (23). Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. Because the food groups in the FQS were previously associated with weight change, we also examined the percentage of excess risk mediated by 4-y weight change (24).
Multivariable models were adjusted for age (continuous), energy intake (quintiles), family history of MI (yes/no), physical activity (5 categories), BMI (9 categories), alcohol intake (5 categories), smoking (5 categories), and menopausal status and postmenopausal hormone use (4 categories). Except for family history, all of the variables were time-dependent variables updated every 2 y. To examine whether association was consistent among different risk strata, we also stratified our analysis by BMI (in kg/m2; <25 or ≥25), physical activity (median level), and family history of MI (yes/no). In these analyses, the FQS was categorized into quintiles to ensure a sufficient number of CAD cases in each stratum in the stratified analysis. Missing values were assigned to a missing indicator variable. The number of individuals with missing values was generally <5% except for physical activity, which the highest for any time period was ∼12%.
To compare the magnitude of the association between various diet quality indexes and CAD, we first assigned a z score to each dietary quality score and modeled 1-SD increase in the score for total CAD. After pooling the RRs from all 3 cohorts, we tested heterogeneity between the FQS and each of the other diet quality scores with the Der Simonian and Laird Q statistics (25).
RESULTS
In ≤26 y of follow-up, we ascertained 6817 incident CAD events, with 4588 cases of nonfatal MI and 2131 fatal CAD events. Of these, 2739 incident CAD cases were in the NHS, 3573 in the HPFS, and 505 in the NHS II. At baseline, participants with a higher FQS score tended to be leaner, had higher levels of physical activity, and consumed more fiber but less energy (Table 1). The diet quality scores were significantly correlated (Supplemental Table 2). Spearman correlation coefficients were the lowest between the FQS and aMed scores (ranging from 0.49 to 0.51 in the 3 cohorts, all P < 0.001) and were up to 0.78 (P < 0.001) between FQS and DASH in NHS II.
TABLE 1.
Age-adjusted baseline characteristics of participants in deciles 1, 5, and 10 of the food-based FQS1
| NHS (n = 71,415) |
HPFS (n = 42,495) |
NHS II (n = 93,131) |
|||||||
| D1 | D5 | D10 | D1 | D5 | D10 | D1 | D5 | D10 | |
| Food-based FQS score | 30 ± 3 | 41 ± 1 | 55 ± 3 | 29 ± 2 | 42 ± 1 | 56 ± 3 | 28 ± 3 | 42 ± 1 | 56 ± 3 |
| BMI, kg/m2 | 25.5 ± 5.3 | 25.1 ± 4.7 | 24.3 ± 4.1 | 25.2 ± 5.1 | 25.1 ± 5.1 | 24.2 ± 4.9 | 25.9 ± 6.6 | 24.7 ± 5.3 | 23.5 ± 4.2 |
| Current smokers, % | 26 | 25 | 17 | 15 | 10 | 4 | 14 | 12 | 9 |
| Physical activity, MET | 10 ± 18 | 13 ± 19 | 22 ± 31 | 15 ± 22 | 20 ± 27 | 32 ± 40 | 13 ± 19 | 19 ± 25 | 34 ± 38 |
| Energy intake, kcal | 2043 ± 512 | 1734 ± 534 | 1580 ± 459 | 2357 ± 605 | 1959 ± 610 | 1846 ± 561 | 2086 ± 526 | 1768 ± 549 | 1666 ± 475 |
| Alcohol intake, g | 5 ± 10 | 7 ± 11 | 7 ± 11 | 11 ± 16 | 12 ± 16 | 10 ± 14 | 2 ± 5 | 3 ± 6 | 4 ± 6 |
| Total fiber, g | 14 ± 3 | 16 ± 4 | 21 ± 6 | 15 ± 4 | 20 ± 5 | 30 ± 9 | 14 ± 3 | 18 ± 4 | 25 ± 7 |
| Vegetables, servings/d | 3.0 ± 1.5 | 3.8 ± 1.9 | 5.5 ± 2.6 | 2.5 ± 1.3 | 3.3 ± 1.8 | 5.0 ± 2.8 | 2.2 ± 1.2 | 3.0 ± 1.8 | 4.7 ± 2.6 |
| Whole fruit, servings/d | 0.9 ± 0.7 | 1.3 ± 1.0 | 2.3 ± 1.3 | 0.8 ± 0.7 | 1.5 ± 1.2 | 2.8 ± 1.8 | 0.6 ± 0.5 | 1.1 ± 0.9 | 2.0 ± 1.2 |
| Sweets and ice cream, servings/d | 1.8 ± 1.4 | 1.2 ± 1.2 | 0.6 ± 0.8 | 2.4 ± 1.7 | 1.5 ± 1.4 | 0.6 ± 0.8 | 1.8 ± 1.3 | 1.2 ± 1.0 | 0.6 ± 0.6 |
| Potatoes, servings/d | 0.6 ± 0.3 | 0.4 ± 0.3 | 0.2 ± 0.2 | 0.7 ± 0.4 | 0.4 ± 0.3 | 0.3 ± 0.3 | 0.6 ± 0.3 | 0.4 ± 0.3 | 0.2 ± 0.2 |
| Red meat, servings/d | 0.9 ± 0.4 | 0.7 ± 0.4 | 0.3 ± 0.3 | 1.3 ± 0.6 | 0.8 ± 0.5 | 0.3 ± 0.3 | 1.2 ± 0.6 | 0.7 ± 0.5 | 0.3 ± 0.3 |
| Processed meat, servings/d | 0.3 ± 0.3 | 0.1 ± 0.2 | 0.0 ± 0.1 | 0.7 ± 0.6 | 0.4 ± 0.4 | 0.1 ± 0.2 | 0.5 ± 0.4 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| Coffee intake, servings/d | 2.0 ± 1.9 | 2.5 ± 1.9 | 2.7 ± 1.9 | 1.6 ± 1.7 | 2.0 ± 1.8 | 2.0 ± 1.8 | 0.8 ± 1.4 | 1.6 ± 1.7 | 2.1 ± 1.7 |
| Sweetened beverages, servings/d | 0.7 ± 0.9 | 0.3 ± 0.5 | 0.1 ± 0.2 | 0.9 ± 0.9 | 0.3 ± 0.5 | 0.1 ± 0.2 | 1.2 ± 1.3 | 0.4 ± 0.7 | 0.1 ± 0.3 |
| Yogurt, servings/d | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.3 | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.0 ± 0.1 | 0.1 ± 0.2 | 0.3 ± 0.4 |
| Nuts, servings/d | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.4 ± 0.6 | 1.6 ± 1.8 | 1.6 ± 1.8 | 1.6 ± 1.8 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.2 |
| Whole grains, servings/d | 0.5 ± 0.7 | 0.9 ± 0.9 | 1.6 ± 0.7 | 0.6 ± 0.7 | 1.3 ± 1.2 | 2.5 ± 1.8 | 0.7 ± 0.7 | 1.3 ± 1.1 | 2.2 ± 1.4 |
| Refined grains, servings/d | 2.2 ± 1.3 | 1.3 ± 1.0 | 0.7 ± 0.6 | 2.1 ± 1.4 | 1.2 ± 1.0 | 0.7 ± 0.7 | 2.2 ± 1.2 | 1.5 ± 0.9 | 1.2 ± 0.7 |
| Snack chips, servings/d | 0.3 ± 0.3 | 0.1 ± 0.2 | 0.0 ± 0.1 | 0.3 ± 0.3 | 0.1 ± 0.2 | 0.0 ± 0.1 | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.0 ± 0.1 |
| Fried foods away from home, times/wk | 0.4 ± 0.3 | 0.2 ± 0.3 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.1 |
Values are means ± SDs unless otherwise indicated. Baseline is 1984 for NHS, 1986 for HPFS, and 1989 for NHS II. D, decile; FQS, Food Quality Score; HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent of task; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
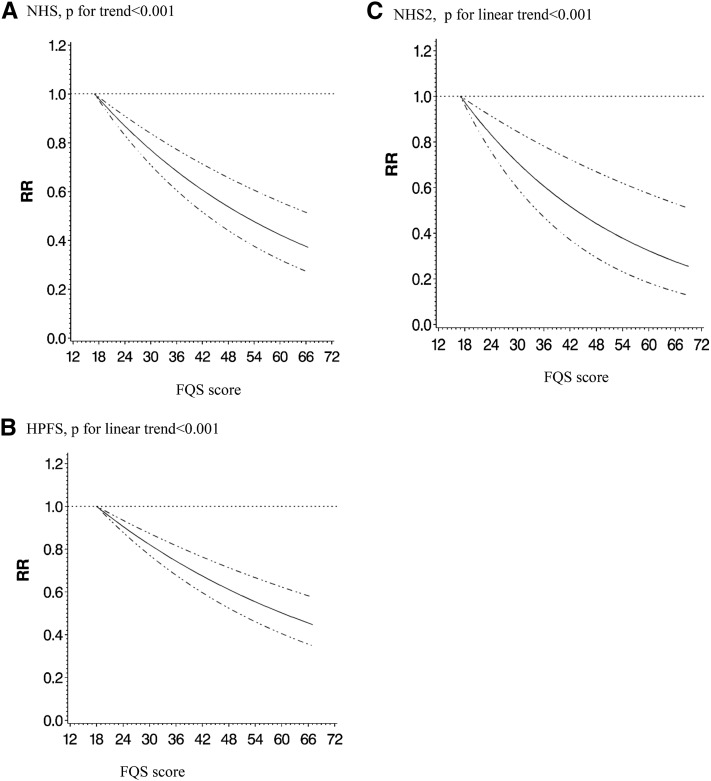
After adjustment for potential confounders, a higher FQS score was associated with lower risk of total CAD in both men and women (Table 2, Supplemental Table 3). The pooled RRs comparing top to bottom deciles of FQS were 0.61 (95% CI: 0.54, 0.69; P-trend < 0.001) for total CAD, 0.56 (95% CI: 0.49, 0.65; P-trend < 0.001) for nonfatal CAD, and 0.71 (95% CI: 0.58, 0.88; P-trend < 0.001) for fatal CAD. Generally, the RRs reached statistical significance in the middle deciles and higher. In a sensitivity analysis that used updated dietary data even when participants reported intermediate endpoints, the results were similar. When we restricted the analysis to “definite” cases only, the results did not change. Spline regression showed significant association of FQS scores with risk of CAD in each cohort with no significant nonlinear association (Figure 1). We did not detect significant mediation of the association between FQS and risk of CAD by 4-y weight change (P values for significant mediation were >0.05 in all 3 cohorts). In addition, we used the FQS assessed at each time point to predict the subsequent 4-y risk of CAD to mimic potential use of diet quality score in a clinical setting when only one diet assessment is available. Results for total CAD were essentially unchanged for the NHS and the NHS II. In the HPFS, results for nonfatal CAD were essentially unchanged but somewhat attenuated for fatal MI (RR comparing extreme deciles: 0.88, 95% CI: 0.69, 1.12; P-trend = 0.07).
TABLE 2.
RRs (95% CIs) of deciles of the food-based Food Quality Score for risk of CAD1
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D10 | P-trend | |
| Total CAD | |||||||||||
| NHS | |||||||||||
| Cases, n | 290 | 258 | 304 | 317 | 299 | 295 | 262 | 258 | 255 | 201 | |
| Age and energy adjusted | 1 | 0.71* | 0.77* | 0.79* | 0.66* | 0.65* | 0.58* | 0.51* | 0.50* | 0.42 (0.35, 0.51)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.76* | 0.86 | 0.91 | 0.78* | 0.80* | 0.74* | 0.65* | 0.67* | 0.58 (0.48, 0.70)* | <0.001 |
| Above + BMI | 1 | 0.76* | 0.87 | 0.92 | 0.79* | 0.80* | 0.75* | 0.66* | 0.68* | 0.60 (0.49, 0.72)* | <0.001 |
| HPFS | |||||||||||
| Cases, n | 361 | 401 | 348 | 414 | 398 | 336 | 357 | 369 | 394 | 295 | |
| Age and energy adjusted | 1 | 0.92 | 0.81* | 0.85* | 0.80* | 0.72* | 0.78* | 0.73* | 0.62* | 0.55 (0.47, 0.65)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.95 | 0.86 | 0.87 | 0.85* | 0.77* | 0.81* | 0.81* | 0.68* | 0.63 (0.53, 0.74)* | <0.001 |
| Above + BMI | 1 | 0.95 | 0.86 | 0.97 | 0.85* | 0.77* | 0.82* | 0.82* | 0.70* | 0.65 (0.55, 0.77)* | <0.001 |
| NHS II | |||||||||||
| Cases, n | 70 | 64 | 61 | 58 | 44 | 58 | 46 | 42 | 35 | 27 | |
| Age and energy adjusted | 1 | 0.78 | 0.72 | 0.65* | 0.51* | 0.62* | 0.50* | 0.42* | 0.36* | 0.27 (0.17, 0.42)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.87 | 0.85 | 0.81 | 0.64* | 0.83 | 0.69 | 0.60* | 0.54* | 0.43 (0.27, 0.68)* | <0.001 |
| Above + BMI | 1 | 0.87 | 0.85 | 0.81 | 0.65* | 0.84 | 0.71 | 0.61* | 0.56* | 0.46 (0.28, 0.73)* | <0.001 |
| Pooled | |||||||||||
| Multivariable + BMI adjusted2 | 1 | 0.87* | 0.86* | 0.90 | 0.82* | 0.80* | 0.80* | 0.73* | 0.69* | 0.61 (0.54, 0.69)* | <0.001 |
| Nonfatal CAD | |||||||||||
| NHS | |||||||||||
| Cases, n | 238 | 197 | 234 | 249 | 224 | 225 | 206 | 298 | 197 | 156 | |
| Age and energy adjusted | 1 | 0.68* | 0.75* | 0.79* | 0.64* | 0.64* | 0.59* | 0.51* | 0.51* | 0.43 (0.35, 0.53)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.72* | 0.82* | 0.88 | 0.73* | 0.76* | 0.72* | 0.63* | 0.65* | 0.57 (0.46, 0.71)* | <0.001 |
| Above + BMI | 1 | 0.72* | 0.83* | 0.89 | 0.73* | 0.76* | 0.73* | 0.63* | 0.66* | 0.58 (0.47, 0.72)* | <0.001 |
| HPFS | |||||||||||
| Cases, n | 215 | 233 | 215 | 230 | 202 | 175 | 199 | 192 | 157 | 145 | |
| Age and energy adjusted | 1 | 0.92 | 0.88 | 0.80* | 0.73* | 0.69* | 0.77* | 0.69* | 0.61* | 0.52 (0.44, 0.68)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.95 | 0.93 | 0.85 | 0.78* | 0.72* | 0.82 | 0.75* | 0.65* | 0.57 (0.46, 0.71)* | 0.002 |
| Above + BMI | 1 | 0.94 | 0.93 | 0.85 | 0.78* | 0.72* | 0.83 | 0.76* | 0.67* | 0.60 (0.48, 0.74)* | 0.005 |
| NHS II | |||||||||||
| Cases, n | 62 | 60 | 55 | 55 | 41 | 55 | 35 | 35 | 34 | 26 | |
| Age and energy adjusted | 1 | 0.82 | 0.74 | 0.69 | 0.53* | 0.67* | 0.43* | 0.39* | 0.40* | 0.29 (0.18, 0.46)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.92 | 0.87 | 0.86 | 0.67 | 0.89 | 0.60* | 0.56* | 0.59* | 0.46 (0.29, 0.75)* | <0.001 |
| Above + BMI | 1 | 0.92 | 0.86 | 0.86 | 0.68 | 0.90 | 0.61* | 0.57* | 0.61* | 0.49 (0.30, 0.80)* | 0.002 |
| Pooled | |||||||||||
| Multivariable + BMI adjusted2 | 1 | 0.84* | 0.88 | 0.88 | 0.75* | 0.76* | 0.78* | 0.68* | 0.67* | 0.56 (0.49, 0.65)* | <0.001 |
| Fatal MI | |||||||||||
| NHS | |||||||||||
| Cases, n | 52 | 61 | 70 | 68 | 75 | 70 | 56 | 60 | 58 | 45 | |
| Age and energy adjusted | 1 | 0.86 | 0.85 | 0.80 | 0.76 | 0.69* | 0.54* | 0.51* | 0.48* | 0.39 (0.26, 0.59)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.98 | 1.03 | 1.02 | 0.99 | 0.97 | 0.83 | 0.79 | 0.79 | 0.66 (0.43, 1.00)* | 0.008 |
| Above + BMI | 1 | 0.98 | 1.05 | 1.06 | 1.02 | 0.99 | 0.85 | 0.81 | 0.82 | 0.69 (0.45, 1.04) | 0.02 |
| HPFS | |||||||||||
| Cases, n | 146 | 168 | 133 | 185 | 196 | 162 | 158 | 177 | 137 | 150 | |
| Age and energy adjusted | 1 | 0.92 | 0.70* | 0.81 | 0.82 | 0.73* | 0.69* | 0.73* | 0.60* | 0.60 (0.48, 0.76)* | <0.001 |
| Multivariable adjusted2 | 1 | 0.94 | 0.76* | 0.89 | 0.92 | 0.83 | 0.78* | 0.87 | 0.71* | 0.70 (0.56, 0.91)* | 0.002 |
| Above + BMI | 1 | 0.95 | 0.77* | 0.89 | 0.92 | 0.84 | 0.79* | 0.89 | 0.72* | 0.72 (0.57, 0.92)* | 0.004 |
| Pooled | |||||||||||
| Multivariable + BMI adjusted2 | 1 | 0.96 | 0.85 | 0.94 | 0.95 | 0.89 | 0.82 | 0.87 | 0.76* | 0.71 (0.58, 0.88)* | <0.001 |
n = 71,415 for the NHS, n = 42,495 for the HPFS, and n = 93,131 for the NHS II. RRs were computed by use of the Cox proportional hazard model. 95% CIs for D2–9 are provided in Supplemental Table 3. *P < 0.05. CAD, coronary artery disease; D, decile; HPFS, Health Professionals Follow-Up Study; MI, myocardial infarction; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
Values were adjusted for age, BMI, smoking, physical activity, family history of diabetes, energy intake, alcohol intake, postmenopausal hormone use (NHS and NHS II), and oral contraceptive use (NHS II).
FIGURE 1.
Multivariable spline regression of FQS and coronary artery disease. Values were adjusted for age, BMI, smoking, physical activity, family history of diabetes, energy intake, alcohol intake, postmenopausal hormone use (NHS and NHS II), and oral contraceptive use (NHS II). n = 71,415 from the NHS (A), 42,495 from the HPFS (B), and 93,131 from the NHS II (C). The solid lines indicate RRs, and the dotted lines indicate 95% CIs. FQS, Food Quality Score; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
When the analysis was stratified by BMI, significant inverse associations were observed both for individuals with a BMI of <25 and ≥25 (Table 3, Supplemental Table 4). The pooled RRs comparing extreme quintiles were 0.61 (95% CI: 0.53, 0.69; P-trend < 0.001) for BMI <25 and 0.78 (95% CI: 0.70, 0.87) for BMI ≥25 (P-heterogeneity by BMI = 0.006). The pooled RRs comparing extreme quintiles of the FQS were 0.76 (95% CI: 0.69, 0.84) among those with physical activity levels less than the median and 0.52 (95% CI: 0.45, 0.61) among those above the median. Pooled results also showed that higher FQS scores were associated with a lower risk of total CAD in both smokers and nonsmokers and in participants with and without a family history of MI. However, there were no significant interactions with physical activity, smoking, or family history.
TABLE 3.
Pooled multivariable RRs (95% CIs) of quintiles of original food-based Food Quality Score for total coronary artery disease, stratified by major risk factors1
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | P-heterogeneity2 | |
| BMI, kg/m2 | |||||||
| <25 (2574 cases) | 1 | 0.90 (0.79, 1.02) | 0.85 (0.75, 0.97) | 0.77 (0.67, 0.87) | 0.61 (0.53, 0.69) | <0.001 | |
| ≥25 (4169 cases) | 1 | 0.97 (0.89, 1.07) | 0.88 (0.80, 0.97) | 0.87 (0.78, 0.96) | 0.78 (0.70, 0.87) | <0.001 | 0.006 |
| Physical activity | |||||||
| METs < median (4960 cases) | 1 | 0.95 (0.87, 1.03) | 0.87 (0.80, 0.95) | 0.83 (0.76, 0.92) | 0.76 (0.69, 0.84) | <0.001 | |
| METs ≥ median (1900 cases) | 1 | 0.91 (0.79, 1.05) | 0.79 (0.68, 0.92) | 0.74 (0.64, 0.86) | 0.52 (0.45, 0.61) | <0.001 | 0.17 |
| Smoking status | |||||||
| Nonsmoker (5790 cases) | 1 | 0.93 (0.85, 1.01) | 0.85 (0.78, 0.93) | 0.82 (0.75, 0.90) | 0.68 (0.62, 0.74) | <0.001 | |
| Current smoker (1070 cases) | 1 | 1.06 (0.89, 1.26) | 0.97 (0.80, 1.17) | 0.86 (0.70, 1.06) | 0.85 (0.67, 1.09) | 0.006 | 0.33 |
| Family history of MI | |||||||
| No (3681 cases) | 1 | 0.96 (0.87, 1.06) | 0.85 (0.77, 0.94) | 0.84 (0.76, 0.94) | 0.71 (0.64, 0.80) | <0.001 | |
| Yes (3179 cases) | 1 | 0.95 (0.85, 1.06) | 0.89 (0.80, 1.00) | 0.83 (0.73, 0.93) | 0.69 (0.61, 0.78) | <0.001 | 0.99 |
Adjusted for age, BMI, smoking, physical activity, family history of MI, energy intake, coffee intake, alcohol intake, and postmenopausal hormone use (Nurses’ Health Study only). RRs were computed with the Cox proportional hazard model. MET, metabolic equivalent of task; MI, myocardial infarction; Q, quintile.
P-heterogeneity was tested by comparing the slopes of the food-based Food Quality Score when the score was modeled as a continuous variable at each stratification category.
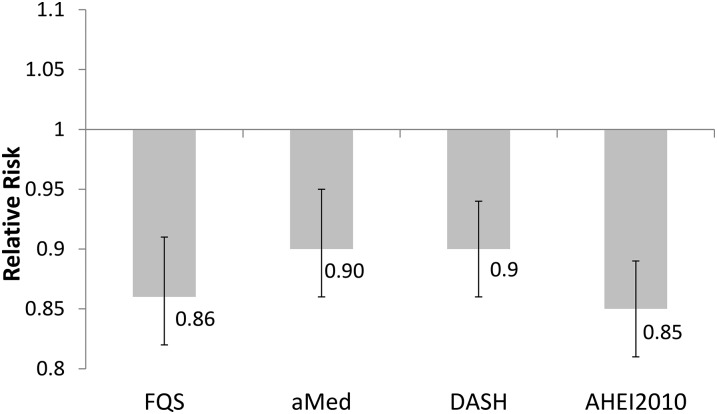
When we compared the magnitude of association between the FQS, AHEI-2010, DASH, aMed, and the risk of total CAD, the risk of CAD for 1 SD increase in the FQS was comparable for all dietary quality scores (Figure 2). There was no significant heterogeneity of the RR for FQS with RRs of the other diet quality score (P values for significant heterogeneity for pairwise comparisons were 0.23 with aMed, 0.35 with DASH, and 0.99 with AHEI-2010).
FIGURE 2.
Multivariable pooled RRs for 1-SD increase in diet quality scores for total coronary artery disease. Values were adjusted for age, BMI, smoking, physical activity, family history of diabetes, energy intake, alcohol intake, postmenopausal hormone use (NHS and NHS II), and oral contraceptive use (NHS II). The test for heterogeneity showed no statistical difference between the RRs of FQS and the RRs of each of the diet quality scores. N = 71,415 from the Nurses’ Health Study, 42,495 from the Health Professionals Follow-Up Study, and 93,131 from the Nurses’ Health Study II. The solid vertical lines indicate 95% CIs. AHEI-2010, Alternate Healthy Eating Index-2010; aMed, alternate Mediterranean diet score; DASH, Dietary Approaches to Stop Hypertension score; FQS, Food Quality Score.
DISCUSSION
In this long-term follow-up of men and women with repeated measurements of diet, we observed that a higher FQS was associated with a lower risk of CAD. The association was seen among subgroups defined by BMI, physical activity, and smoking status. Even though the FQS did not include nutrients, its association with CAD was comparable with diet quality scores containing both food and nutrient components and previously associated with lower CAD risk.
Diet quality scores have been established to measure diet quality in many countries, including the United States (2, 26), the United Kingdom (27), and the Nordic countries (28), as well as various versions of the Mediterranean diet score (3, 13, 29–31). These scores generally include both food and nutrient components. Many of these scores have been associated with disease risk (1, 28, 32–34).
Currently, no diet assessment tool to our knowledge has been demonstrated to predict disease risk and to be quick to use for health care providers and patients in the clinical setting. The traditional methods of 24-h recall, food records, and comprehensive FFQs take considerable time for the patient to complete and require specific software to compute nutrient values. Although these methods can provide detailed information suitable for research or sessions dedicated to nutrition counseling, there is also a need for a quick tool that does not require much time and effort from the patient to input and clinician to analyze. We have previously shown that in a clinical setting, a simple screening questionnaire provides results for key foods and nutrients strongly correlated with those from a full-length semiquantitative FFQ (35). However, this was not studied directly in relation to disease risk. Currently, the USDA SuperTracker (36) and other commercially available applications require substantial burden and responsibility from the individual, and the data are not easily shared with the clinician. Therefore, a tool in the form of a food-based checklist or short questionnaire that is predictive of disease risk, can be completed in a clinic, and does not require a complex scoring algorithm would be a helpful clinical tool. The development of such an assessment tool should include foods and foods groups that have been shown to be associated with CAD risk (37). This list would, at a minimum, need to identify high intakes of fruit, vegetables, whole grains, plant oils, and fish and low intakes of added sugar and saturated and trans fats because these dietary factors would be associated with mediating factors such as serum lipids, inflammation, oxidative stress, endothelial dysfunction, and blood pressure.
Few diet quality indexes have been constructed only with foods groups. The Healthy Nordic Food Index consisted of 6 food groups: whole-wheat bread, oatmeal, root vegetables, apples/pears, cabbage, and fish (28). It has been associated with all-cause and cancer mortality (28) and risk of diabetes (33). On the other hand, it was not associated with breast cancer (38) or cardiovascular mortality (39). Its association with colorectal cancer has been mixed (40, 41). The Elderly Dietary Index consists of 10 food items, and points are awarded for higher intakes of vegetables, fruit, legumes, seafood/fish, and cereals; use of whole grains, low-fat dairy, and olive oil; moderate consumption of dairy; and low consumption of meat (4). Although it was associated with lower risk of CAD incidents in men, it has not been tested in women or middle-aged adults. The Healthy Comprehensive Dietary Pattern consisted of 47 food groups and was associated with lower concentrations of C-reactive protein and fasting insulin in the Multi-ethnic Study of Atherosclerosis cohort (9). The a priori diet quality scores have 34 food groups and contain both healthy and unhealthy food groups (8). In the Iowa Women’s Health Study, this a priori diet quality score is associated with lower total, CVD, and cancer mortality (42). In several diet quality scores, as well as the FQS, significant reductions of CVD risk were observed in the middle and upper categories of intake, suggesting that benefits may be achieved with even a moderately healthy diet (6, 42–44). To our knowledge, the FQS, which has 14 food groups and was built on food groups previously associated with less weight gain (12), is the only food-only comprehensive index shown to be related to a lower risk of incident CAD in both men and women.
Our stratified analysis found greater reduction of risk in lean or more physically active individuals. One previous study showed results similar to our findings (29), although no significant effect modification by BMI was seen in other studies (6, 43). Interestingly, 4-y weight change was not a meaningful mediator in the observed association, in contrast to our hypothesis. It is possible that changes in BMI do not fully capture adiposity-mediated mechanisms such as changes in fat distribution or abdominal obesity. However, the score overall was associated with CAD risk; therefore, the components likely work in a combination of adiposity-mediated (e.g., inflammation and serum lipids) and nonobesity-mediated mechanisms (e.g., cardiac arrthymia and coagulation) (37). In addition, a number of studies on diet quality scores and CVD that presented risk estimates with and without adjusting for BMI generally showed limited confounding or mediation by BMI when other risk factors had already been adjusted (6, 43, 45).
Atherosclerosis progresses slowly, and with <24 y of follow-up, we were able to assess the long-term relation between the FQS and CAD risk. With repeated measurement of diet, we were also able to take into account changes in diet during the long follow-up. Because individuals may change their diet on diagnosis of intermediate endpoints such as hyperlipidemia and hypertension, in the primary analysis we stopped updating diet when intermediate endpoints were reported. Because we had detailed and updated information on lifestyle and health information, we were able to finely adjust for potential confounders. Nonetheless, given the observational nature of the study, residual confounding and incomplete assessment of mediation by BMI cannot be completely ruled out. Because health (including height and weight) and dietary information was self-reported, some degree of misreporting cannot be avoided. However, we have documented reliable reporting from the validated FFQ compared with multiple-week diet records (18). Each food group in the FQS is given the same weight; although straightforward, this does not truly reflect the different magnitude of the association of each food group and CVD risk in the literature. However, the composite food-based score is still useful in capturing an overall dietary pattern.
In this study, we showed that the FQS, a simplified food-based score, performed similarly in CAD prediction compared with previously established diet quality indexes. The FQS could easily be directly adapted, based on a webpage or application, into the clinical setting, with simple software and without the need for any nutrient calculations, nutrient databases, or nutrient updating. Future studies should incorporate and test the FQS in health settings and test potential effects on monitoring current behavior and tracking change. Future research could also evaluate whether simplified questionnaires with fewer items could be developed.
Cardiovascular disease accounted for ∼1 in 4 deaths globally (46), and heart disease is the number one cause of death in the United States (47). Diet is an important contributor to CAD risk directly through multiple mechanisms (48). However, there is almost a total absence of methods for simply assessing and tracking nutrition in the health system. The result of this study is timely because of implementation of electronic health records under the Affordable Care Act. Currently, electronic health records generally do not include nutrition metrics that focus on the dietary intake because of the lack of validated food-based scores.
In conclusion, a higher FQS was associated with a lower risk of CAD. This was consistent in individuals with different BMI status, physical activity level, smoking status, and family health history. The association of the FQS was also similar to food- and nutrient-based diet quality indexes that have previously been associated with lower CAD risk and should be more easily applied in the clinical setting.
Acknowledgments
The authors’ responsibilities were as follows—TTF: designed the study, analyzed the data, wrote the manuscript, and had primary responsibility for the final content; AP, KMR, and WCW: interpreted the data and revised the manuscript; TH: performed the statistical analysis; DM and FBH: designed the study, interpreted the data, and revised the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AHEI-2010, Alternate Healthy Eating Index-2010; aMed, alternate Mediterranean diet score; CAD, coronary artery disease; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension score; FFQ, food-frequency questionnaire; FQS, Food Quality Score; HPFS, Health Professionals Follow-Up Study; MI, myocardial infarction; NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II.
REFERENCES
- 1.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reedy J, Krebs-Smith SM, Miller PE, Liese AD, Kahle LL, Park Y, Subar AF. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 4.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J Nutr 2014;144:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoevenaar-Blom MP, Spijkerman AMW, Boshuizen HC, Boer JMA, Kromhout D, Verschuren WMM. Effect of using repeated measurements of a Mediterranean style diet on the strength of the association with cardiovascular disease during 12 years: the Doetinchem Cohort Study. Eur J Nutr 2014;53:1209–15. [DOI] [PubMed] [Google Scholar]
- 6.Struijk EA, May AM, Wezenbeek NLW, Fransen HP, Soedamah-Muthu SS, Geelen A, Boer JMA, van der Schouw YT, Bueno-de-Mesquita HB, Beulens JWJ. Adherence to dietary guidelines and cardiovascular disease risk in the EPIC-NL cohort. Int J Cardiol 2014;176:354–9. [DOI] [PubMed] [Google Scholar]
- 7.Dietary Guidelines Advisorty Committee. Dietary Patterns, Foods and Nutrients, and Health Outcomes. In: Scientific Report of the 2015 Dietary Guidelines Advisory Committee [Internet]. [cited 2015 May 11]. Available from: http://www.health.gov/dietaryguidelines/2015-scientific-report/07-chapter-2/d2-6.asp.
- 8.Meyer KA, Sijtsma FPC, Nettleton JA, Steffen LM, Van Horn L, Shikany JM, Gross MD, Mursu J, Traber MG, Jacobs DR Jr. Dietary patterns are associated with plasma F2-isoprostanes in an observational cohort study of adults. Free Radic Biol Med 2013;57:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR. A priori–defined dietary patterns and markers of cardiovascular disease risk in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2008;88:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sijtsma FPC, Meyer KA, Steffen LM, Van Horn L, Shikany JM, Odegaard AO, Gross MD, Kromhout D, Jacobs DR Jr. Diet quality and markers of endothelial function: The CARDIA study. Nutr Metab Cardiovasc Dis 2014;24:632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen A, Egeberg R, Halkjær J, Christensen J, Overvad K, Tjønneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr 2011;141:639–44. [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 17.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–73. [DOI] [PubMed] [Google Scholar]
- 18.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Garcia E, van Dam RM, Willett WC, Rimm EB, Manson JE, Stampfer MJ, Rexrode KM, Hu FB. Coffee consumption and coronary heart disease in men and women: a prospective cohort study. Circulation 2006;113:2045–53. [DOI] [PubMed] [Google Scholar]
- 20.Rose GA. Cardiovascular survey methods. 2nd ed. Geneva (Switzerland): World Health Organization; 1982. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Fleming TR, DeGruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–27. [DOI] [PubMed] [Google Scholar]
- 25.Takkouche B, Cadarso-Suárez C, Spiegelman D. Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 1999;150:206–15. [DOI] [PubMed] [Google Scholar]
- 26.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HAB, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julia C, Touvier M, Méjean C, Ducrot P, Péneau S, Hercberg S, Kesse-Guyot E. Development and validation of an individual dietary index based on the British Food Standard Agency Nutrient Profiling System in a French context. J Nutr 2014;144:2009–17. [DOI] [PubMed] [Google Scholar]
- 28.Roswall N, Sandin S, Löf M, Skeie G, Olsen A, Adami H-O, Weiderpass E. Adherence to the Healthy Nordic Food Index and total and cause-specific mortality among Swedish women. Eur J Epidemiol 2015;30:509–17. [DOI] [PubMed] [Google Scholar]
- 29.Mitrou PN, Kipnis V, Thiébaut AM, Reedy J, Subar AF, Wirfält E, Flood A, Mouw T, Hollenbeck AR, Leitzman MF, et al. . Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP diet and health study. Arch Intern Med 2007;167:2461–8. [DOI] [PubMed] [Google Scholar]
- 30.Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 2006;16:559–68. [DOI] [PubMed] [Google Scholar]
- 31.Steffen LM, van Horn L, Daviglus ML, Zhou X, Reis JP, Loria CM, Jacobs DR Jr, Duffey KJ. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br J Nutr 2014;112:1654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George SM, Ballard-Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol 2014;180:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacoppidan SA, Kyrø C, Loft S, Helnæs A, Christensen J, Hansen C, Dahm C, Overvad K, Tjønneland A, Olsen A. Adherence to a Healthy Nordic Food Index is associated with a lower risk of type-2 diabetes: the Danish Diet, Cancer and Health Cohort Study. Nutrients 2015;7:8633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, Sonderman J, Buchowski MS, McLaughlin JK, Shu XO, Steinwandel M, Signorello LB, Zhang X, Hargreaves MK, Blot WJ, et al. . Healthy eating and risks of total and cause-specific death among low-income populations of African-Americans and other adults in the southeastern United States: a prospective cohort study. PLoS Med 2015:12;e1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rifas-Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr 2001;4:249–54. [DOI] [PubMed] [Google Scholar]
- 36.USDA. SuperTracker: food tracker [Internet]. [2015 July 15]. Available from: https://www.supertracker.usda.gov/foodtracker.aspx.
- 37.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–78. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Roswall N, Sandin S, Ström P, Adami H-O, Weiderpass E. Adherence to a Healthy Nordic Food Index and breast cancer risk: results from a Swedish cohort study. Cancer Causes Control 2015;26:893–902. [DOI] [PubMed] [Google Scholar]
- 39.Roswall N, Sandin S, Scragg R, Löf M, Skeie G, Olsen A, Adami HO, Weiderpass E. No association between adherence to the Healthy Nordic Food Index and cardiovascular disease amongst Swedish women: a cohort study. J Intern Med 2015;278:531–41. [DOI] [PubMed] [Google Scholar]
- 40.Kyrø C, Skeie G, Loft S, Overvad K, Christensen J, Tjønneland A, Olsen A. Adherence to a Healthy Nordic Food Index is associated with a lower incidence of colorectal cancer in women: the Diet, Cancer and Health cohort study. Br J Nutr 2013;109:920–7. [DOI] [PubMed] [Google Scholar]
- 41.Roswall N, Li Y, Kyrø C, Sandin S, Löf M, Adami H-O, Weiderpass E. No association between adherence to a Healthy Nordic Food Index and colorectal cancer: results from a Swedish cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:755–7. [DOI] [PubMed] [Google Scholar]
- 42.Mursu J, Steffen LM, Meyer KA, Duprez D, Jacobs DR. Diet quality indexes and mortality in postmenopausal women: the Iowa Women’s Health Study. Am J Clin Nutr 2013;98:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckland G, Agudo A, Travier N, María Huerta J, Cirera L, Tormo M-J, Navarro C, Dolores Chirlaque M, Moreno-Iribas C, Ardanaz E, et al. . Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br J Nutr 2011;106:1581–91. [DOI] [PubMed] [Google Scholar]
- 44.Hoevenaar-Blom MP, Nooyens ACJ, Kromhout D, Spijkerman AMW, Beulens JWJ, van der Schouw YT, Bueno-de-Mesquita B, Verschuren WMM. Mediterranean style diet and 12-year incidence of cardiovascular diseases: the EPIC-NL cohort study. PLoS One 2012;7:e45458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez-González MA, García-López M, Bes-Rastrollo M, Toledo E, Martínez-Lapiscina EH, Delgado-Rodriguez M, Vazquez Z, Benito S, Beunza JJ. Mediterranean diet and the incidence of cardiovascular disease: A Spanish cohort. Nutr Metab Cardiovasc Dis 2011;21:237–44. [DOI] [PubMed] [Google Scholar]
- 46.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J, Murphy SL, Kochanek KD, Bastian BA, Division of Vital Statistics. Deaths: Final Data for 2013 [Internet]. In: National Vital Statistics Reports. 2016 [2015 Dec 22]. Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. [PubMed]
- 48.Medicine I. Promoting cardiovascular health in the developing world: a critical challenge to achieve global health. Washington (DC): Institute of Medicine; 2010. [PubMed] [Google Scholar]