Abstract
The autonomic nervous system influences numerous ocular functions. It does this by way of parasympathetic innervation from postganglionic fibers that originate from neurons in the ciliary and pterygopalatine ganglia, and by way of sympathetic innervation from postganglionic fibers that originate from neurons in the superior cervical ganglion. Ciliary ganglion neurons project to the ciliary body and the sphincter pupillae muscle of the iris to control ocular accommodation and pupil constriction, respectively. Superior cervical ganglion neurons project to the dilator pupillae muscle of the iris to control pupil dilation. Ocular blood flow is controlled both via direct autonomic influences on the vasculature of the optic nerve, choroid, ciliary body, and iris, as well as via indirect influences on retinal blood flow. In mammals, this vasculature is innervated by vasodilatory fibers from the pterygopalatine ganglion, and by vasoconstrictive fibers from the superior cervical ganglion. Intraocular pressure is regulated primarily through the balance of aqueous humor formation and outflow. Autonomic regulation of ciliary body blood vessels and the ciliary epithelium is an important determinant of aqueous humor formation; autonomic regulation of the trabecular meshwork and episcleral blood vessels is an important determinant of aqueous humor outflow. These tissues are all innervated by fibers from the pterygopalatine and superior cervical ganglia. In addition to these classical autonomic pathways, trigeminal sensory fibers exert local, intrinsic influences on many of these regions of the eye, as well as on some neurons within the ciliary and pterygopalatine ganglia.
Introduction
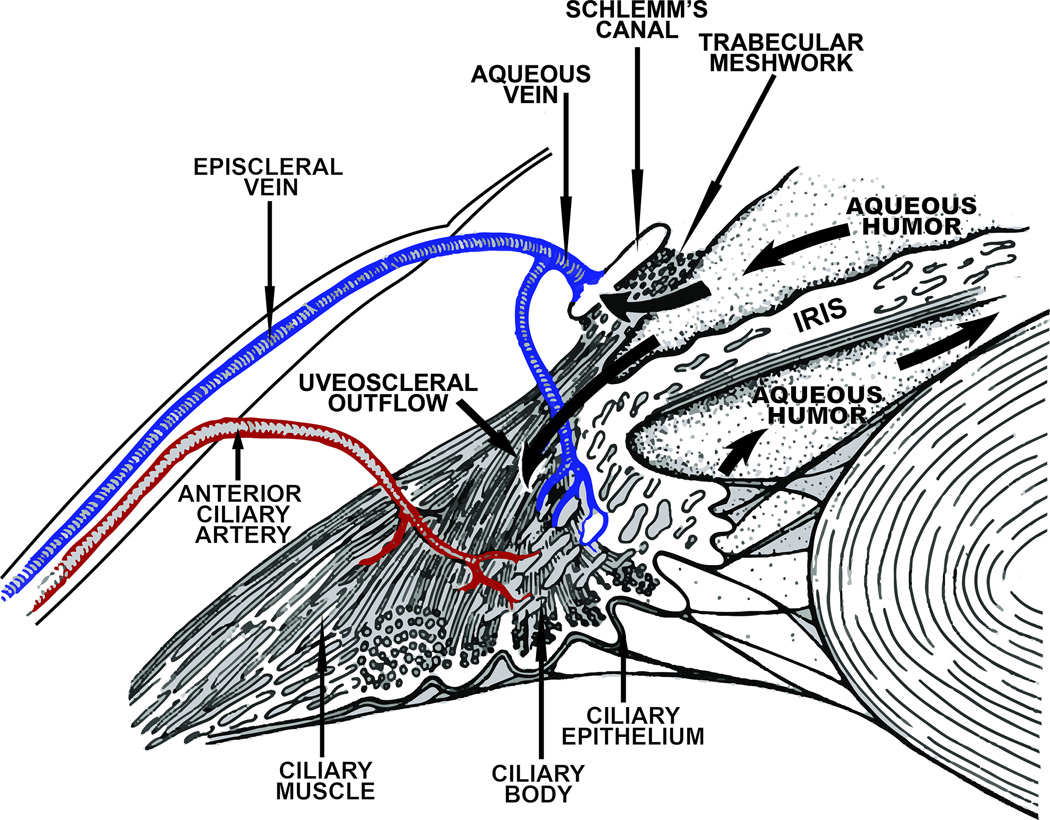
The ocular projections of the autonomic nervous system influence numerous functions of the eye. These include: 1) pupil diameter and ocular accommodation, which are controlled by the intrinsic muscles of the eye located in the iris and ciliary body respectively – these structures are innervated by postganglionic fibers from the ciliary (parasympathetic) and superior cervical (sympathetic) ganglia; 2) Ocular blood flow, which is controlled via innervation of the vasculature within the optic nerve, the retina, choroid, ciliary body, and iris. In mammals, these vascular beds are innervated by postganglionic fibers from the pterygopalatine (parasympathetic) and superior cervical (sympathetic) ganglia. In birds, the ciliary ganglion also contributes to the parasympathetic innervation of the choroid, and there may be a small contribution from this ganglion in mammals; 3) intra-ocular pressure (IOP), which is regulated primarily through changes in aqueous humor formation and outflow. Autonomic regulation of ciliary body blood vessels and the ciliary epithelium are important determinants of aqueous humor formation, while regulation of the trabecular meshwork and episcleral blood vessels are important determinants of aqueous humor outflow – these structures are innervated by postganglionic fibers from the pterygopalatine (parasympathetic) and superior cervical (sympathetic) ganglia (Figure 1). However, as discussed below, these functional subdivisions are somewhat arbitrary since they are often interrelated and do not operate in isolation. For example, both iris and ciliary muscle contraction can influence aqueous humor outflow, while alterations in choroidal blood flow will cause changes in IOP.
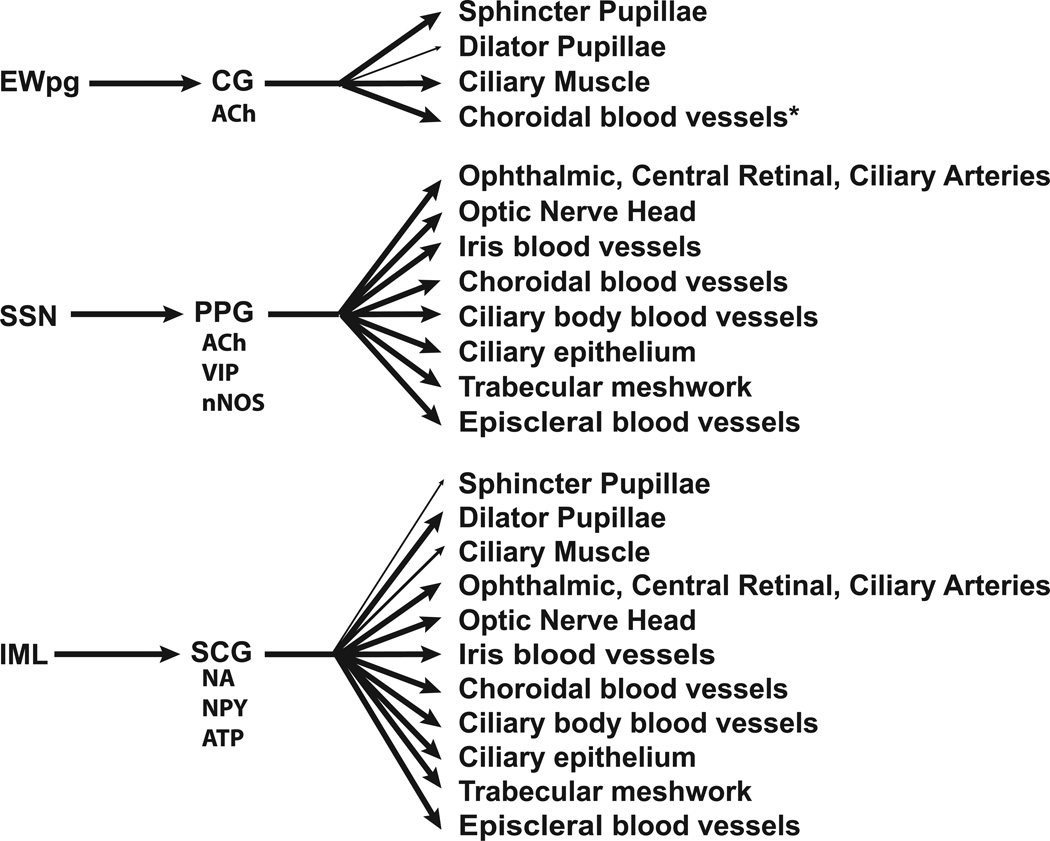
Figure 1.
A schematic diagram showing the parasympathetic and sympathetic innervation of the eye. Neurotransmitters and neuropeptides that are generally present in postganglionic neurons are identified. *Only seen in avian studies to date. Abbreviations: ACh – Acetylcholine; ATP – Adenosine triphosphate; CG – Ciliary ganglion; EWpg – Edinger-Westphal nucleus, preganglionic; IML – Intermediolateral nucleus (cell column); NA – Noradrenaline; nNOS – neuronal nitric oxide synthase; NPY – Neuropeptide Y; PPG – Pterygopalatine ganglion; SCG – Superior cervical ganglion; SSN – Superior salivatory nucleus; VIP – Vasoactive intestinal peptide.
The information presented below focuses not only on the autonomic innervation of the eye, but also the central autonomic pathways controlling pupillary reflexes, accommodation, ocular blood flow, and intraocular pressure. Our discussion of these topics is concentrated on primates, including humans, but includes data from other species where it suggests alternative connections that have yet to be investigated in primates, or where primate data is unavailable. Unless otherwise stated, primary references reflect studies conducted in the primate model. A comprehensive list of abbreviations used in this article can be found in table 1.
Table 1.
List of abbreviations
| ACC | accommodation |
| ACCV | accommodative velocity |
| ATP | Adensoine triphosphate |
| cFN | caudal fastigial nucleus |
| CGRP | calcitonin gene-related peptide |
| CCK | cholecystokinin |
| DAG | diacylglycerol |
| EVP | episcleral venous pressure |
| EW | Edinger-Westphal nucleus |
| EWcp | Edinger-Westphal centrally projecting cells |
| EWm | Medial (choroidal) subdivision of the avian Edinger-Westphal nucleus |
| EWpg | Edinger-Westhal preganglionic cells |
| HRP | horseradish peroxidase |
| IML | intermediolateral cell |
| IOP | intra-ocular pressure |
| IP3 | inositol triphosphate |
| ipRGCs | intrinsically-photosensitive retinal ganglion cells |
| L-NAME | nitro-L-arginine methylester |
| m1, m2, m3 . . . | m1, m2, m3 .. muscarinic receptor subtypes |
| nNOS | neuronal nitric oxide synthase |
| NPY | neuropeptide Y |
| Opn4−/− | melanopsin deficient knockout mice |
| PIN | posterior interposed nucleus |
| PIPR | post-illumination pupil response |
| PLC | phospholipase C |
| PLR | pupillary light reflex |
| PNR | pupillary near response |
| PON | pretectal olivary nucleus |
| rd/rd cl | rodless/coneless knockout mice |
| RPE | retinal pigment epithelium |
| SCN | suprachiasmatic nucleus |
| SOA | supraoculomotor area |
| SP | Substance P |
| SSN | Superior Salivatory Nucleus |
| VIP | vasoactive intestinal peptide |
| WGA | wheat germ agglutinin |
As shown in Figure 1, parasympathetic innervation of the eye arises from two sources: 1) neurons in the Edinger-Westphal preganglionic (EWpg) cell group, the autonomic subdivision of the third cranial nerve nucleus, which lies in the rostral mesencephalon; 2) preganglionic neurons in the superior salivatory nucleus (SSN), the parasympathetic component of the seventh cranial nerve nucleus, which lie in the medulla oblongata. The neurons in EWpg project by way of the oculomotor (III) nerve to postganglionic cells in the ciliary ganglion. Neurons in the SSN project by way of the greater petrosal branch of the facial (VII) nerve to postganglionic cells in the pterygopalatine ganglion (also termed the sphenopalatine ganglion). Sympathetic innervation of the eye arises from preganglionic neurons located in the C8-T2 segments of the spinal cord, a region termed the ciliospinal center of Budge (and Waller). The axons of these preganglionic neurons project to the sympathetic chain ganglia and travel in the sympathetic trunk to the superior cervical ganglion where they contact postganglionic neurons (149, 179). The axons of these postganglionic neurons project from the superior cervical ganglion to the orbit, where they enter the eye via the short and long ciliary nerves, as well as through the optic canal (315).
In addition to these classical, extrinsic autonomic control pathways, it is clear that there are local, intrinsic influences exerted by trigeminal sensory fibers on many regions of the eye, as well as on some neurons within the ciliary and pterygopalatine ganglia (Figure 2). Both sympathetic and parasympathetic fibers are also present in the mammalian cornea. However, their density varies significantly between species (255). In rabbits and cats, sympathetic innervation is quite dense (82, 190, 204, 231, 252, 387). However, in primates, including humans, sympathetic innervation appears to be either absent or very sparse (80, 81, 231, 372, 394). In addition, although some parasympathetic innervation of the cornea has been reported in rat and cat, the existence of such innervation has not been studied in other mammalian species (255). Therefore, the autonomic innervation of the cornea will not be discussed further. A detailed description of the autonomic nervous system in general is provided by Hockman (149) and Robertson (312). Also, the autonomic control of the eye and iris in non-mammalian species has recently been reviewed (261), as has the control of ocular blood flow (183, 306).
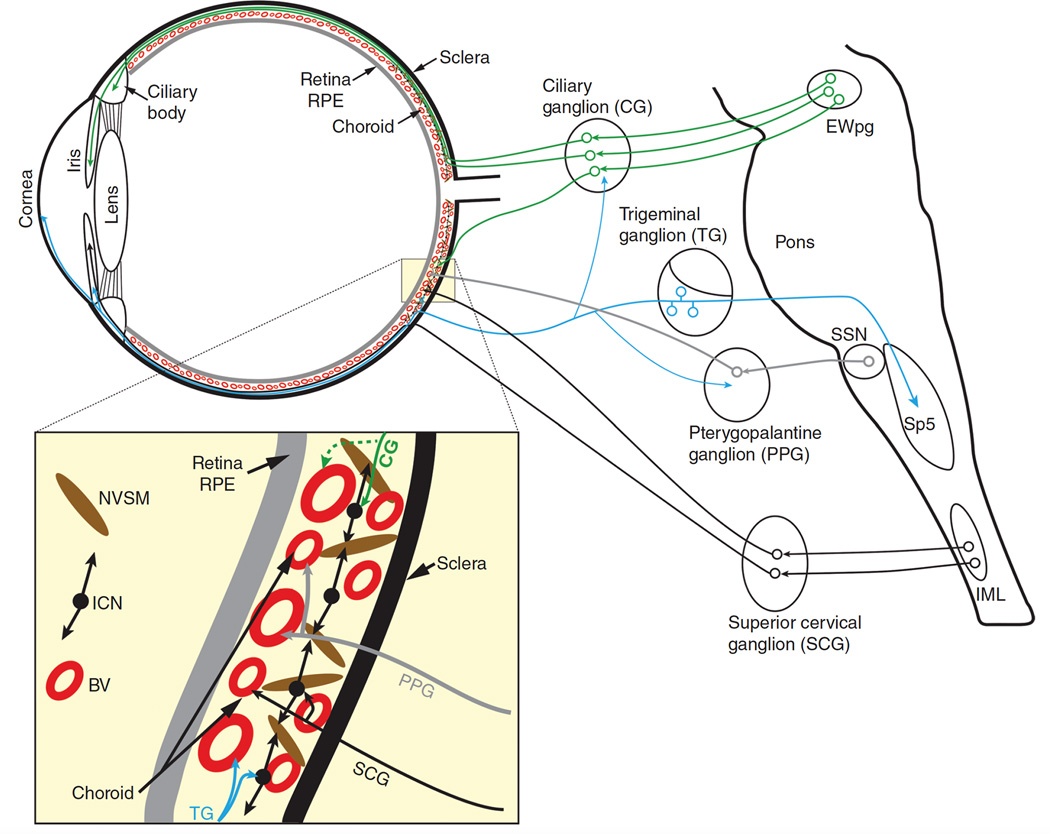
Figure 2.
Autonomic and trigeminal ocular projections. The dotted line shows a ciliary ganglion projection to the choroid which, to date, has been shown only in birds. Line thickness indicates relative strength of projection. Abbreviations: BV - Blood vessels; CNS – Central nervous system; EWpg – Nucleus of Edinger-Westphal, preganglionic division; ICN – Intrinsic choroidal neurons; IML – Intermediolateral nucleus; NVSM – Non-vascular smooth muscle; SSN – Superior salivatory nucleus.
Autonomic Innervation of the Eye
The Third Nerve Pathways Innervating the Eye
The Edinger-Westphal nucleus - preganglionic neurons
The parasympathetic, third nerve pathway originates from preganglionic neurons in the Edinger-Westphal nucleus (EW) and projects via the third cranial nerve to the ciliary ganglion. In most primates, the EW is a distinct nucleus lying immediately dorsal to the somatic subdivisions of the oculomotor complex. It was first described in a developmental study of human neuroanatomical material by Edinger (78) and, a short time afterward, in a neuropathological study by Westphal (419). While both authors recognized this nucleus as being cytoarchitecturally distinct from the oculomotor nucleus, the study by Westphal led to the suggestion that the EW was involved in the innervation of the iris and possibly other intraocular muscles.
The primate EW is composed of relatively large spherical and ovoid cells (approximately 25–40 µm in diameter) and other spindle-shaped neurons (15µm – 30µm in diameter) (e.g. 195, 413). The preganglionic neurons within the EW were initially identified by a retrograde degeneration study (413). Subsequently these neurons were identified by retrograde neuroanatomical tracer studies following injections into the ciliary ganglion of either horseradish peroxidase (HRP) (47, 61), [125I] wheat germ agglutinin (WGA) (4), WGA-HRP (373), or fluorescent tracers (162). All of these studies reported that labeled, preganglionic neurons are generally the larger, more spherical neurons of the EW and are only slightly smaller than the somatic motoneurons of the oculomotor complex.
Based on the number of retrogradely labeled cells and the estimated number of parasympathetic axons in the oculomotor nerve, Burde and Loewy (47) suggested that there were 300–400 preganglionic neurons in EW. However, based on the results of Akert and colleagues (4), Ishikawa and colleagues (162), and upon personal observations, the number of preganglionic neurons in the primate EW is more likely to be in the range of 800–1200.
Additional studies have confirmed in many vertebrate classes that the EW is the preganglionic, parasympathetic component of the oculomotor nuclear complex, and is the central source of parasympathetic innervation of the iris, the ciliary body, and certain additional intraocular muscles and tissues (see 195 for a review). However, in many species including humans, some or all cells of the cytoarchitecturally-defined EW are not preganglionic neurons, but are instead centrally-projecting peptidergic (often urocortin-1) neurons, while the preganglionic neurons are actually located outside of the cytoarchitecturally-defined EW (e.g. 104, 152, 195, 239). For example, in one of the clearest cases of this mismatch, cells in the classically-defined EW of cats project to the spinal cord and cerebellum (48, 217, 218, 314, 370), while the ciliary ganglion receives its central input from a collection of preganglionic cells along the midline of the rostral mesencephalon and in the ventral tegmental area (198, 371, 399). As discussed in a recent review (195), this has led to much confusion in the literature, and it is now generally accepted that the term EW must be supplemented with a terminology based on neuronal connectivity. Specifically, the cholinergic, preganglionic neurons supplying the ciliary ganglion are now termed the Edinger-Westphal preganglionic (EWpg) population, while the centrally projecting, peptidergic neurons are termed the Edinger-Westphal centrally projecting (EWcp) population (195). This new nomenclature and the relative location of the EWpg and EWcp cell populations for various species, including humans, are shown in Figures 3 and 4.
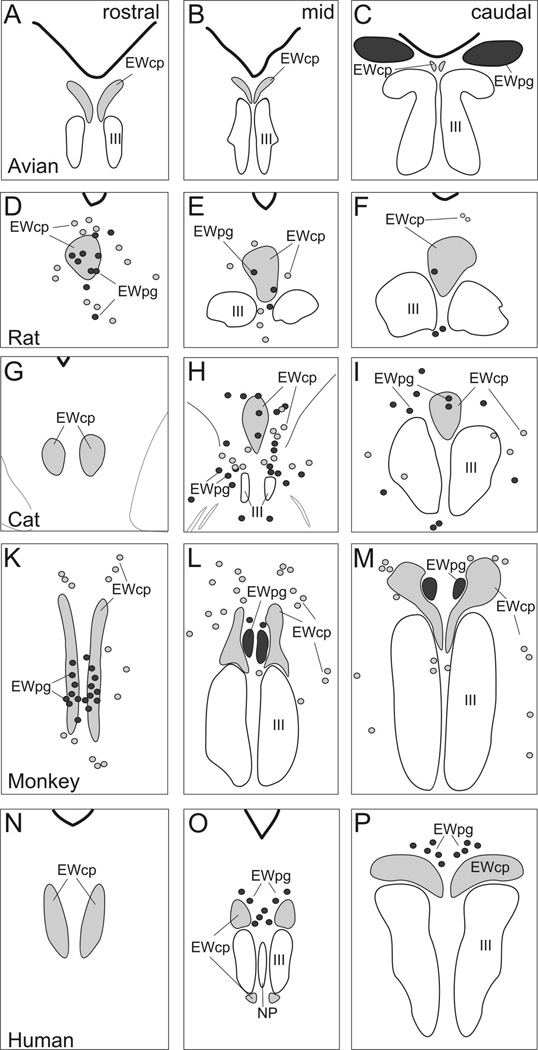
Figure 3.
Line drawings showing the organization of EWpg and EWcp in several selected species: avian, rat, cat, monkey, and human. Representative rostral (left column), middle (middle column) and caudal (right column) sections are shown. The EWcp is indicated by light gray shading and EWpg is indicated by dark gray shading. Scattered cells located outside the nuclear boundaries are indicated by appropriately shaded circles. (From Kozicz et al., 2011) (195).
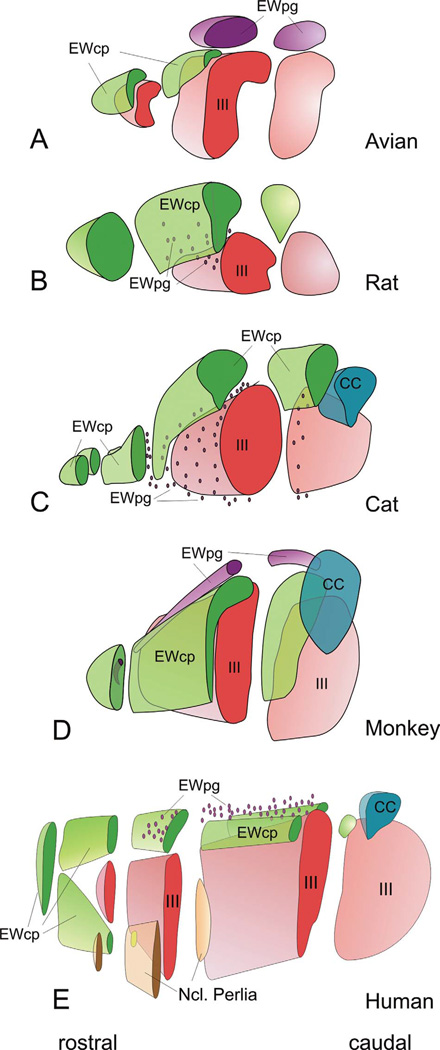
Figure 4.
Three dimensional (3-D) representations of human, macaque, cat, rat and pigeon oculomotor complex, to illustrate the 3-D organization of EWpg and EWcp. The 3-D models are cut at selected points to illustrate how frontal sections through this level would look. In cases where the population is scattered, and so not contained in a discrete nucleus, dots are used. (From Kozicz et al., 2011) (195).
The ciliary ganglion
The ciliary ganglion in humans is approximately 3mm in size, and located 2–3 mm posterior to the globe and lateral to the optic nerve. Within the ciliary ganglion, postganglionic neurons are contacted by the cholinergic, nicotinic synapses of parasympathetic preganglionic axon terminals that in macaque, generally contain neuropeptide Y (NPY) (Grimes et al., 1998). The axons of these postganglionic neurons leave the ciliary ganglion to enter the eye via the short ciliary nerves. Once in the eye, postganglionic axons project in the suprachoroid space to extensively innervate the sphincter pupillae and ciliary muscles and, in birds, the choroidal vasculature (104). They also make a limited number of synapses on sympathetic terminals in the dilator pupillae muscle (174). Although postganglionic neurons in the ciliary ganglion receive input primarily from the preganglionic neurons of EW, there is evidence for additional neuronal inputs that may act to modulate this signal (241). For example, there is good evidence that some (~10–20%) postganglionic neurons receive calcitonin gene-related peptide/Substance P (CGRP/SP)-positive trigeminal inputs (Figure 2) (e.g. 187). Furthermore, ultrastructural analysis of the macaque ciliary ganglion via electron microscopy demonstrated heterogeneity in cellular and synaptic structure, as well as postsynaptic vesicular content (241). Therefore, the ciliary ganglion should not be considered only as a simple relay of preganglionic inputs from EW to the eye, but also as a site of potential neural integration (104). In addition, although many studies have reported that all preganglionic neurons synapse in this ganglion in mammals (e.g. 198, 324), there has been some debate as to the existence of a synapse in this ganglion for both pupil-related and accommodation-related preganglionic neurons. For example, based on physiological studies (418) and on retrograde anatomical studies (164, 285) it has been reported that EW neurons do not synapse in the ciliary ganglion, but instead have a direct projection to the ciliary muscle. Other reports have disagreed with these studies and there is a growing consensus for the existence of a synapse between the pre- and postganglionic neurons in the primate ciliary ganglion (see 316 for a review). It appears likely that some retrograde tracer experiments showed apparent direct connections between the preganglionic neurons of the EW and their eventual peripheral targets because the tracer was taken up by preganglionic fibers that contact intraocular ganglion cells contained within the accessory ciliary ganglia of the primate and other mammals. In some mammals these accessory ganglia are located immediately behind the sclera, while in others they are located in the suprachoroid lamina along the intraocular portions of the ciliary nerves from the scleral canal to the iris and ciliary body (198, 199). Injections in the vicinity of these intraocular ganglion cells would have involved preganglionic fibers from EW neurons and would thus have resulted in retrograde labeling of EW neurons.
Seventh nerve pathways innervating the eye
Superior Salivatory Nucleus – preganglionic neurons
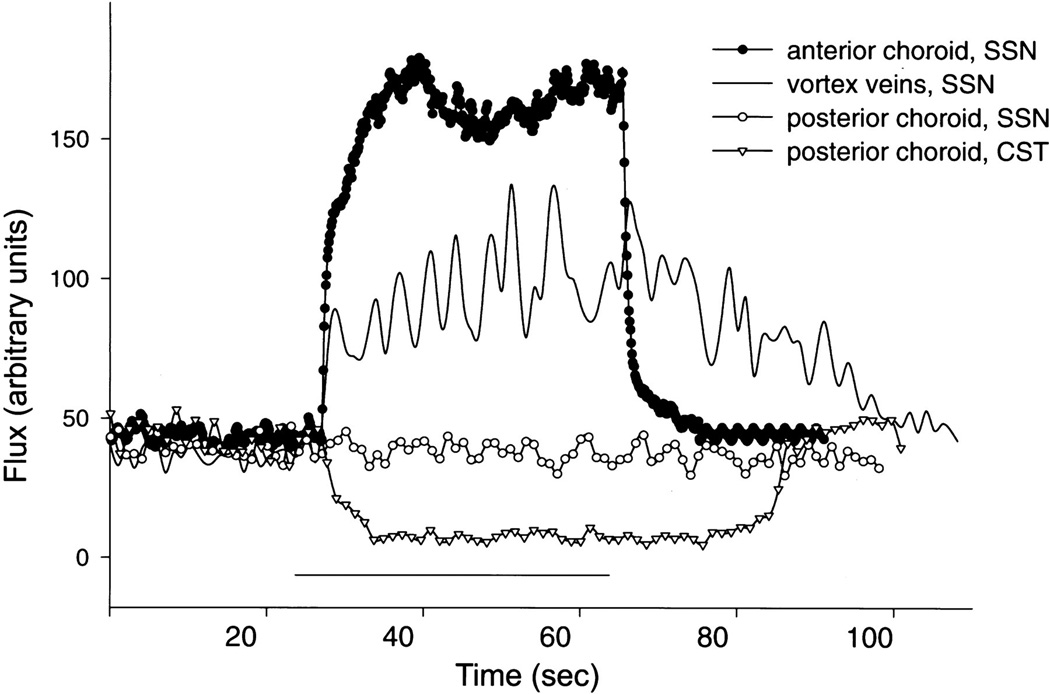
The parasympathetic, seventh nerve pathway innervating the eye originates from preganglionic neurons in the superior salivatory nucleus (SSN), which are located in the ventrolateral medulla, slightly dorsolateral to the facial motor nucleus. In rats, the preganglionic neurons are somewhat intermingled with, and surrounded by neurons of the A5 catecholamine cell group (Figure 5) (e.g. 70, 210). All SSN neurons are cholinergic, and in rabbits and rats many have also been shown to contain neuronal nitric oxide synthase (nNOS) (70, 440, 441). In rats, it has been established that one population of preganglionic SSN neurons projects by way of the greater petrosal branch of the facial nerve to the pterygopalatine ganglion (66, 70, 262, 264, 306, 351, 397), which sends postganglionic fibers to ocular structures which are involved in the regulation of blood flow and IOP (see below). The other population of preganglionic neurons within the SSN are not involved in the autonomic control of the eye. These neurons project by way of the chorda tympani nerve to the submandibular ganglion (66, 167, 262, 264, 306) which sends postganglionic fibers to the submandibular and sublingual glands. A coarse topography exists within the SSN such that neurons projecting to the pterygopalatine ganglion are generally located ventral to those projecting to the submandibular ganglion (66, 70, 167, 262, 264, 397).
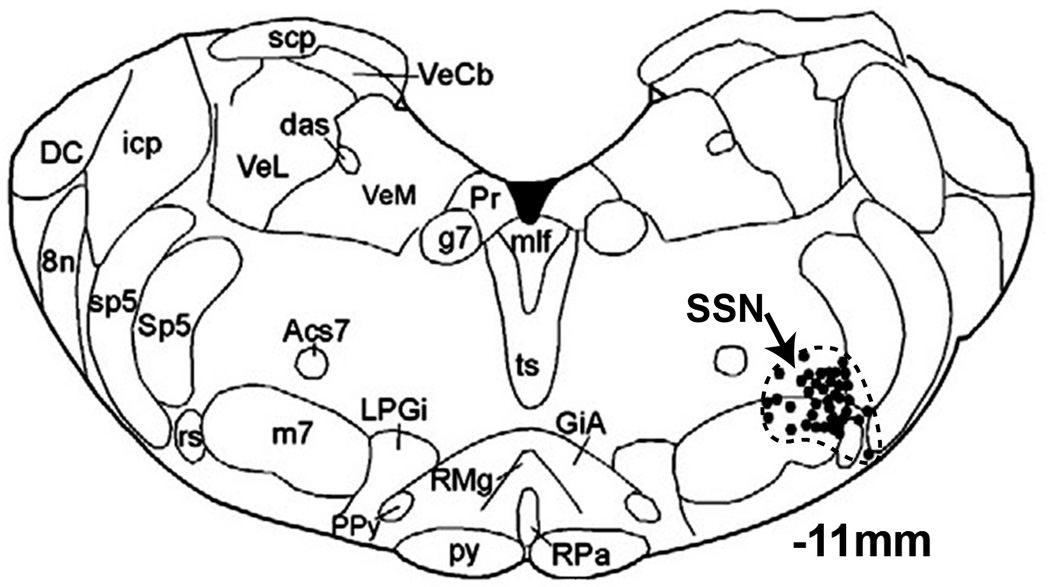
Figure 5.
Diagram showing the location of prechoroidal SSN neurons in rat. Abbreviations: 8n, vestibulocochlear nerve; Acs7, accessory facial nucleus; das, dorsal acoustic stria; DC, dorsal cochlear nucleus; g7, genu of facial nerve; GiA, alpha part of gigantocellular reticular nucleus; icp, inferior cerebellar peduncle; m7, facial nucleus; mlf, medial longitudinal fasciculus; LPGi, lateral paragigantocellular nucleus; PPy, parapyramidal nucleus; Pr, prepositus nucleus; py, pyramid; RMg, raphe magnus; RPa, raphe pallidus nucleus; rs, rubrospinal tract; scp, superior cerebellar peduncle; Sp5, spinal trigeminal nucleus; sp5, spinal trigeminal tract; SSN, superior salivatory nucleus; ts, tectospinal tract; VeCb, vestibulocerebellar nucleus; VeL, lateral vestibular nucleus; VeM, medial vestibular nucleus. (Modified from Li et al. 2010) (210)
Pterygopalatine ganglion
In humans, the pterygopalatine ganglion is located in the pterygopalatine fossa, its shape varies between individuals, being variously described as conical, triangular, or elliptical, and it is reported to be approximately 3mm vertical, 4 mm sagittal, and 2 mm transversely (378). However, more recently it has been recognized that the pterygopalatine ganglion may be bipartite, and a number of associated microganglia may also be present (e.g. 306, 325). Within the pterygopalatine ganglion, the preganglionic neurons form cholinergic, nicotinic synapses with postganglionic neurons. Axons of these postganglionic neurons leave the pterygopalatine ganglion caudally and reach the eye by way of the rami orbitales, the retro-orbital plexus, and the rami oculares (174, 321). These postganglionic fibers target a number of ocular structures including the ciliary epithelium and trabecular meshwork, as well as blood vessels in the optic nerve, iris, ciliary body, choroid, and episclera (Figure 1, 2). In mammals including primates, postganglionic fibers from the pterygopalatine ganglion also project to cerebral blood vessels, the lacrimal gland, blood vessels of the nasal mucosa and palate and the meibomian glands (207, 259, 317, 322, 380, 402, 409).
Postganglionic neurons within the pterygopalatine ganglion are cholinergic, but many also express nNOS and vasoactive intestinal peptide (VIP) (132, 174, 201, 261, 306). Also, similar to the ciliary ganglion, some pterygopalatine ganglion cells in rats are innervated by collaterals of trigeminal afferents (Figure 2) (374).
Sympathetic pathways innervating the eye
The intermediolateral cell column
The preganglionic sympathetic neurons that innervate the eye are located in the intermediolateral cell column (IML) in the C8-T2 segments of the spinal cord, a region termed the ciliospinal center of Budge (and Waller). The axons of these preganglionic neurons project to the sympathetic chain ganglia and travel in the sympathetic trunk to the superior cervical ganglion (149, 179).
Superior Cervical Ganglion
The superior cervical ganglion is located at the C1-C3 vertebral level and, in humans, it lies immediately anterior to the common carotid artery bifurcation (423). Within the superior cervical ganglion, preganglionic axons form nicotinic, cholinergic synapses with postganglionic neurons. The axons of these postganglionic neurons project from the superior cervical ganglion as the internal carotid nerves to the level of the cavernous sinuses where they form the carotid plexus around the carotid arteries. Many of the fibers destined for the eye form the sympathetic root of the ciliary ganglion, which runs along with the ophthalmic artery to enter the orbit. These fibers then traverse the ciliary ganglion to enter the eye via the short ciliary nerves (282, 315). In addition, other sympathetic fibers enter the eye through the long ciliary nerves as well as through the optic canal (315). In addition to containing the neurotransmitter noradrenaline, many postganglionic sympathetic neurons projecting to the eye in mammals also express the vasoconstrictor peptide, neuropeptide Y (NPY) (41, 42, 46, 124, 137, 268, 306, 382), as well as the co-transmitter ATP (295) (Figure 1).
Trigeminal Nervous system - peripheral reflex control
In birds and mammals, the nerve branches arising from the trigeminal nerve provide the sensory innervation of the eye. More specifically, the ophthalmic branch of the trigeminal nerve divides into the lacrimal, frontal and nasociliary nerves. The latter divides into a major branch that travels with the long ciliary nerves to the eye and a minor branch that passes through the ciliary ganglion to enter the eye with the short ciliary nerves (174, 199, 261, 379). Additionally, in monkeys, Ruskell (319) has suggested that there is a small contribution to the sensory innervation of the eye from the maxillary branch of the trigeminal nerve.
Within the eye of mammals and birds, sensory fibers are distributed to the: cornea (170, 230, 363); conjunctiva (84, 222); iris including the sphincter muscle, blood vessels and anterior melanocytes (22, 174); ciliary body including the ciliary processes, blood vessels, and a region of the ciliary muscle near the scleral spur (174, 323, 364); ophthalmic and central retinal arteries (28, 33, 85, 306); choroid including the intrinsic choroidal neurons (337, 345, 364); episcleral vessels (340, 341); trabecular meshwork (205, 339).
It has been recognized that the peripheral nerve endings and collaterals of these sensory fibers contain a number of neuropeptides, and over the past few decades it has been established that these neuropeptides can also serve as neurotransmitters (e.g. 150, 174). Thus, it is now clear that the trigeminal sensory system can act as a local effector system not only within the various ocular structures that it innervates, but also through collaterals that are reported in both the ciliary and pterygopalatine ganglia (174, 261). In general, in mammals including humans, a significant percentage of sensory nerve endings contain substance P (SP) (174, 365, 385, 386) and calcitonin gene-related peptide (CGRP) (174, 366), and both neuropeptides are present in cells in the trigeminal ganglion (123, 174, 208). In addition, other tachykinins such as neurokinin A, neurokinin B and neuropeptide K have been reported, as have cholecystokinin (CCK), galanin, and vasopressin (see 174 for a review). Substance P, and presumably other neuropeptides, are released from peripheral nerve endings in response to antidromic trigeminal nerve stimulation, as well as peripheral stimulation from capsaicin, bradykinin, histamine, nicotine, and prostaglandins. (36, 174, 228, 411, 437).
Autonomic control of the Pupil
The pupil is essentially an optical element that controls the amount of light striking the retina by acting as an aperture stop: the most light-restrictive element of an optical system. Pupillary diameter, or more precisely iris size, is controlled by two muscles, the sphincter pupillae, which is primarily under the control of the parasympathetic nervous system, and the dilator pupillae, which is primarily under the control of the sympathetic nervous system. Contraction of the sphincter, accompanied by relaxation of the dilator, produces pupil constriction (miosis); while contraction of the dilator, accompanied by relaxation of the sphincter, produces pupil dilation (mydriasis). These muscles are innervated by postganglionic neurons originating from the ciliary and superior cervical ganglia, which in turn receive preganglionic inputs from the EWpg and IML respectively (Figure 1, 2). Neurons in the EWpg and IML are, in turn, controlled by central nervous system inputs that are influenced by a variety of factors such as retinal irradiance, viewing distance, alertness, and cognitive load.
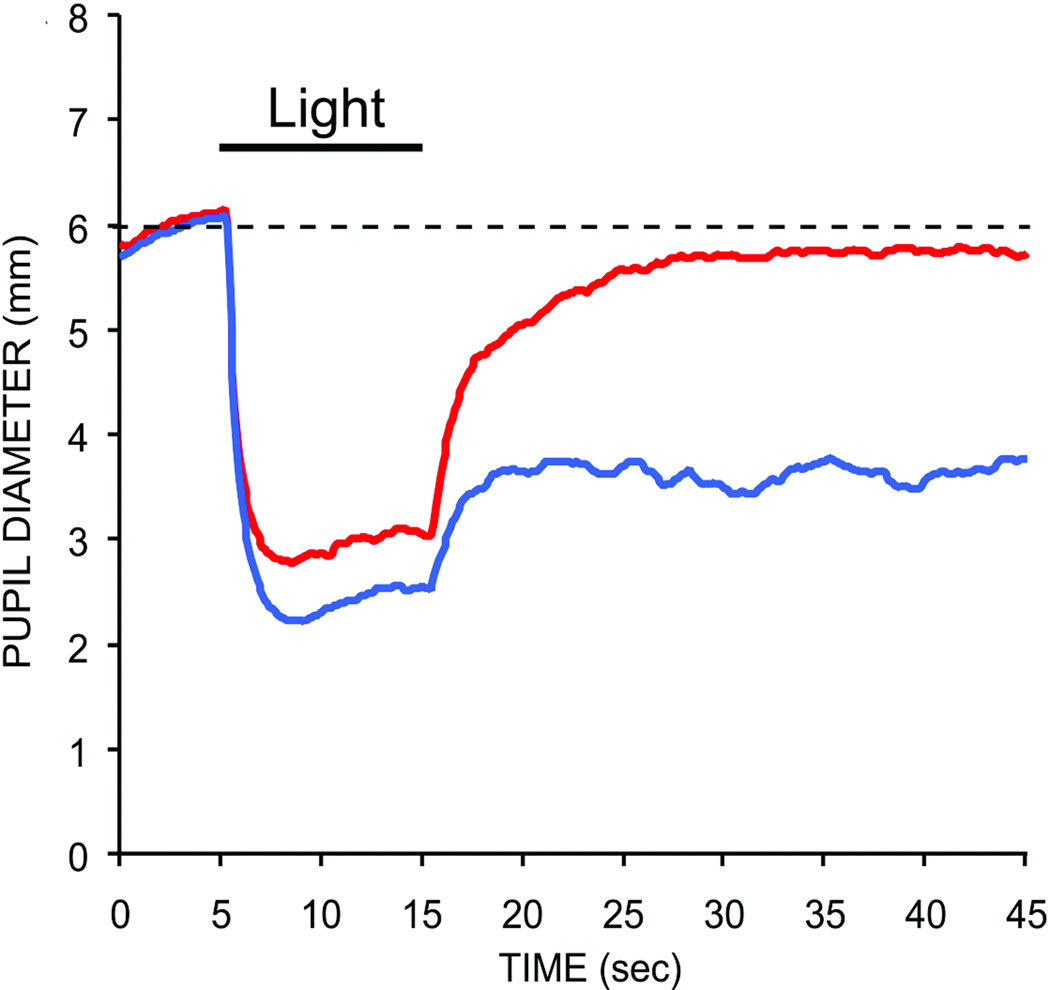
The normal human pupil can change diameter from 8 mm to 1.5 mm, which corresponds to approximately a 28 fold change in area. Thus the movement of the iris can account for almost 1.5 log unit variation in retinal irradiance (282). Although the visual system can operate over a 10 log unit range of lighting levels through the process of adaptation, it can take several minutes for optimum sensitivity to return after an abrupt increase or decrease in retinal illumination. The rapid control of retinal irradiance by the iris allows the visual system to more quickly regain optimal sensitivity by dampening fast changes in ambient lighting levels and requiring less retinal adaptation to a given change in environmental lighting levels (151, 427).
Changes in pupil size modulate not only retinal illumination, but also depth of focus, optical aberrations, and diffraction. As pupil diameter decreases, depth of focus increases (229, 412) and the image degrading effects of optical aberrations decrease (212, 338), but the image degrading effects of diffraction increase (54, 59, 93). However, over the normal range of pupillary diameter, diffraction impacts image quality less than optical aberrations, and the optimal pupil diameter is therefore approximately between 2mm and 4 mm (56, 168, 417). These various factors differentially affect visual performance and, given changing environmental lighting conditions and visual tasks, the nervous system continuously modulates pupil diameter for optimal visual performance.
Clearly, a mobile pupil allows the autonomic nervous system to optimize retinal irradiance, diffraction, ocular aberrations, and depth of focus despite differing conditions and visual tasks. For example, across a range of daylight (photopic) luminance levels, pupil size corresponds to that required for the highest visual acuity (55), and the maximal information capacity of the retinal image (148, 206). On the other hand, under low light (scotopic) conditions in which poorer retinal image quality can be tolerated due to the lower resolution of rod photoreceptors, the pupil dilates sufficiently to maximize retinal illumination. Further evidence for the optimization of pupil diameter for differing visual tasks is evident in the pupillary near response. When viewing distance changes from far to near, the pupils constrict to increase the depth of field and reduce retinal image defocus (see below for more detail).
Overview of the pathways controlling pupil diameter
A summary diagram of our current understanding of the afferent, central, and efferent pathways controlling pupil diameter are shown in figure 6. This figure shows the iris musculature innervated by autonomic efferents from both the parasympathetic and sympathetic components of the autonomic nervous system.
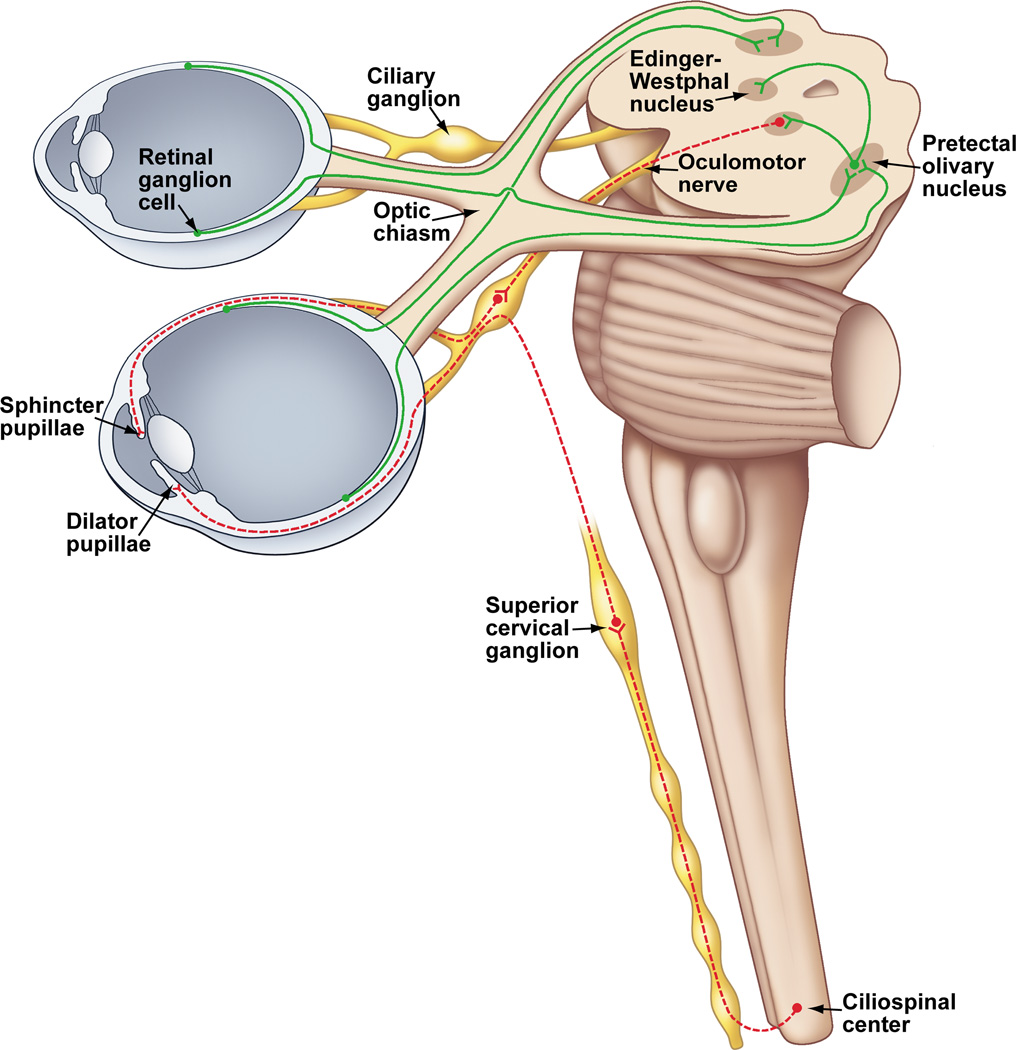
Figure 6.
Anatomical drawing showing the parasympathetic and sympathetic innervation of the iris in primates. The bilateral projection from the retina to the pretectum is also shown. The pretectal olivary nucleus receives input from the temporal retina of the ipsilateral eye and the nasal retina of the contralateral eye. The pretectal olivary nucleus projects bilaterally to the Edinger-Westphal nucleus, which contains parasympathetic, preganglionic, pupilloconstriction neurons. The axons of these preganglionic neurons travel in the third cranial nerve to synapse upon postganglionic pupilloconstriction neurons in the ciliary ganglion. The axons of these postganglionic neurons leave the ciliary ganglion and enter the eye via the short ciliary nerves, and travel through the choroid to innervate the sphincter muscle of the iris. The sympathetic preganglionic pupillodilation neurons are found at the C8-T1 segmental levels of the spinal cord. The axons of these neurons project from the spinal cord via the dorsal roots and enter the sympathetic trunk, and then project rostrally to the superior cervical ganglion where they synapse with the postganglionic neurons. These postganglionic neurons project from the superior cervical ganglion through the neck and carotid plexus, and into the orbit of the eye. These fibers enter the eye either by passing through the ciliary ganglion and entering in the short ciliary nerves, by bypassing the ciliary ganglion and entering via the long ciliary nerves, or through the optic canal (for clarity only one of these alternative pathways is shown). Upon entering the eye, these axons travel through the choroid and innervate the dilator muscle of the iris.
Iris Musculature
In a cross section of the iris, the sphincter pupillae can be seen as an annular band of smooth muscle (100–170 µm thick; 0.7–1.0 mm wide) encircling the pupil (figure 7). The sphincter, which is located in the posterior iris immediately anterior to the pigmented epithelium, interdigitates with the surrounding stroma and connects to dilator muscle fibers (see below). The smooth muscle cells of the sphincter are clustered in small bundles and connected by gap junctions (45). These gap junctions ensure synchronized contraction of the sphincter muscle. The sphincter receives muscarinic, cholinergic innervation from the short ciliary nerves: parasympathetic, postganglionic fibers arising from the ciliary ganglion. The m3 subtype of muscarinic receptor is the predominant receptor subtype expressed by smooth muscle cells of the sphincter pupillae. In the human iris, the m3 receptor subtype comprises 60–75% of the total number of expressed muscarinic receptors, while other muscarinic receptor subtypes (m1, m2, m4, m5), are expressed at lower levels (5–10%) (125). Binding of acetylcholine to m3 receptors initiates a series of events leading to the activation of phospholipase C (PLC) via G-proteins of the Gq family. Activated PLC generates inositol triphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol biphosphate. The increase in IP3 elicits the release of Ca2+ ions from the endoplasmic reticulum and the influx of extracellular Ca2+ ions. The resultant increase in intracellular free Ca2+ concentration produces muscle contraction (see 79 for a review). Muscarinic receptor antagonists such as atropine, scopolamine, or tropicamide produce mydriasis, while agonists such as pilocarpine, bethanechol, metoclopramide, or oxotremorine produce miosis. The reversible cholinesterase inhibitor, physostigmine, also produces a marked miosis.
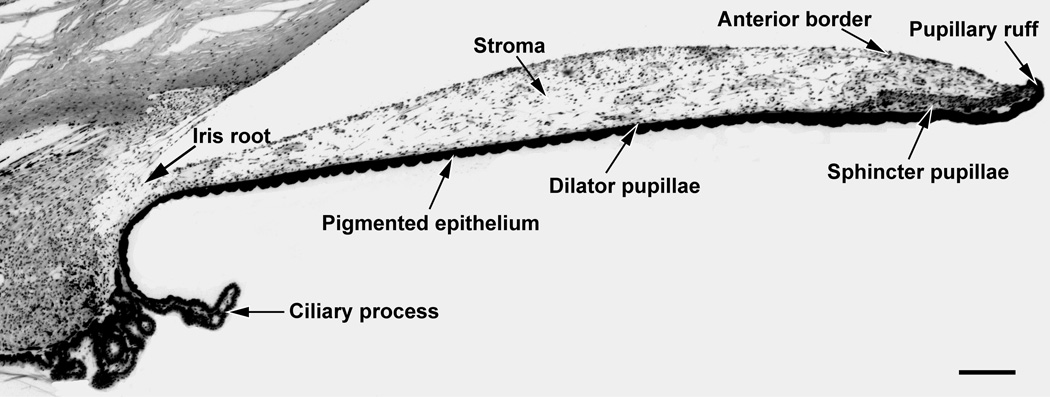
Figure 7.
Low power photomicrograph of a cross section of the macaque iris. The approximate location of the dilator pupillae is shown since it is not clearly evident at this magnification. Scale bar = 200 µm.
The dilator pupillae is composed of radially oriented smooth muscle fibers that are myoepithelial in origin. Individual fibers are approximately 50 µm long and 5–7 µm wide. In the pupillary zone, dilator muscle processes fuse with the sphincter pupillae, while peripherally, their processes attach to the ciliary body. Contraction of the dilator muscle pulls the pupillary margin towards the ciliary body (45). The dilator receives adrenergic innervation from the long ciliary nerves: sympathetic, postganglionic fibers arising from the superior cervical ganglion. The alpha 1a adrenoreceptor appears to be the predominant receptor subtype expressed by the smooth muscle cells of the dilator pupillae (161). Binding of norepinephrine to the alpha 1a adrenoreceptor, a G protein-coupled receptor, produces muscle contraction through the same signaling cascade (PLC/ IP3) as that in the sphincter pupillae muscle.
Alpha-adrenoreceptor antagonists such as dapiprazole or thymoxamine produce miosis (390), as does the more selective alpha 1a adrenoreceptor antagonist, tamsulosin (286). The non-specific adrenoreceptor agonist, phenylephrine, produces mydriasis. Mydriasis is also produced by hydroxyamphetamine and related drugs, which stimulate norepinephrine release from the postganglionic sympathetic nerve endings (390).
Importantly, release of 3H-noradrenaline is inhibited when the muscarinic receptors (M2 subtype) on sympathetic nerve endings in the human iris-ciliary body (175) or whole iris of other mammals (44, 98) are activated by muscarinic agonists (251). Thus, conceivably parasympathetic innervation of the sphincter pupillae can potentially partially antagonize the effect of sympathetic innervation of this tissue.
In addition, trigeminal sensory fibers containing both SP and CGRP innervate the iris in mammals including humans (83, 146, 249, 347, 363–366, 383, 384, 386, 405). SP and CGRP released from trigeminal nerve endings in response to noxious stimuli, temperature increases or decreases, or pressure can produce pupillary constriction, also known as miosis. Indeed, in mammals including humans, stimulation of the trigeminal nerve causes pronounced miosis (14, 228, 360), as does intracameral injection of SP or CGRP (36, 228, 275, 361, 403), and SP eye drops in humans (5).
Pupillary Light Reflex
The pupillary light reflex (PLR) is the constriction of the pupil that is elicited by an increase in illumination of the retina. The direct PLR, present in virtually all vertebrates, is the constriction of the pupil in the same eye as that stimulated with light. The consensual pupillary light reflex is the constriction of the pupil in the eye opposite to the eye stimulated with light. In mammals with laterally placed eyes, such as the rat and rabbit, the direct pupillary light reflex is more pronounced than the consensual PLR. However, in those mammalian species with frontally placed eyes, such as humans and monkeys, the direct and consensual pupillary light reflex are essentially equal (215).
The pupillary light reflex has traditionally been divided into two separate pathways based on the clinical manifestations of the defects in this reflex. The efferent pathway is composed of the preganglionic pupilloconstriction fibers of the EWpg and their postganglionic recipient neurons in the ciliary ganglion, which project to the sphincter muscle of the iris (Figure 6). The afferent pathway is composed of both the retinal cells that project to the pretectum, as well as their recipient neurons, which project to the EWpg (Figure 6).
Efferent Pathway of the Pupillary Light Reflex
By the mid 1900’s, based on experimental lesion studies and clinical studies, it was generally accepted that the final efferent link of the pupillary light reflex consisted of a preganglionic projection from the EW in the midbrain to the ciliary ganglion that, in turn, projected to the sphincter pupillae muscle of the iris (see 215 for a complete review of the literature). Specifically, Bernheimer showed that lesions in the region of the EW resulted in a fixed, dilated pupil ipsilateral to the lesion (30). A later study on primates by Pierson and Carpenter (293) also showed pupillary deficits following discrete lesions in the area of the anterior EW. Similarly, lesions of the EW in birds result in dilated pupils that are unreactive to light and an inability to accommodate (331). Pupillary immobility is also associated with third nerve palsies or selective damage to the axons of the preganglionic neurons coursing to the ciliary ganglion, and with damage to the postganglionic fibers that results in Adie’s syndrome (299, 390).
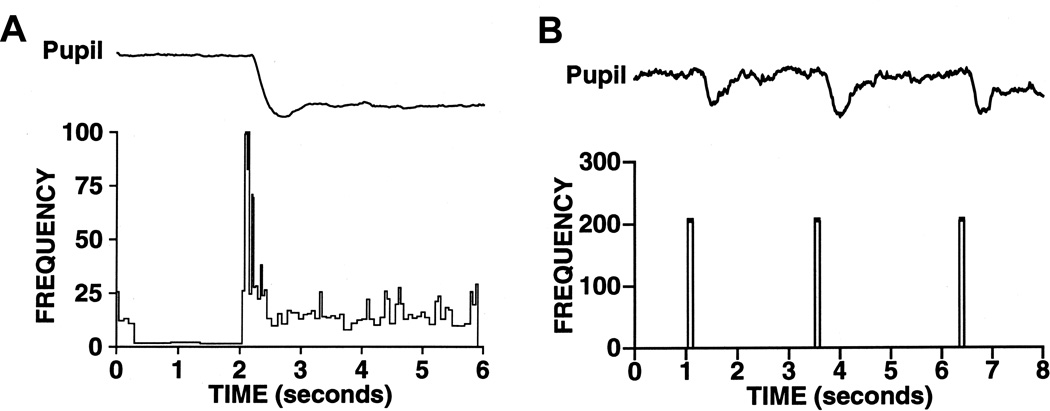
Further support for the course of the efferent parasympathetic pupillary pathway and the importance of the EW in pupilloconstriction in primates came from stereotaxic, electrical stimulation studies in the vicinity of EW that elicited pupilloconstriction, as well as accommodation (24, 165, 418). Other studies in the cat (296, 349), marmoset (61), and chicken (400) showed that electrical stimulation of the EW evokes pupilloconstriction and accommodation in these species. Electrical stimulation of the ciliary ganglion or nerves in cats has also been shown to elicit pupilloconstriction and accommodation (232, 277, 308). In the alert primate, pupilloconstriction can be evoked by electrical microstimulation of the EWpg or the pupillomotor fibers of the intracranial portion of the oculomotor nerve (115). Following stimulation, the pupil constricts with a latency of approximately 100 ms, and peak pupilloconstriction occurs approximately 300–500 msec after stimulation. Pupil diameter then returns to baseline with a time constant of approximately 600 msec. Such pupillary responses were only elicited from the area dorsal to the oculomotor nucleus and from a localized region of the oculomotor nerve.
Responses of EW Neurons During the Pupillary Light Reflex
Sillito and Zbrozyna (348) recorded the activity of preganglionic, pupillomotor neurons in anaesthetized cats. Because of the effects of the chloralose anesthesia, the pupils were relatively constricted, but still showed a small light reflex. To overcome these effects of anesthesia, hypothalamic stimulation was used to elicit a “defense reaction” and hence produce pupil dilation. Using this approach, the authors found that the baseline level of activity of the pupil-related EW neurons was between 6 and 10 spikes/second and was completely inhibited by hypothalamic stimulation. Maximal pupilloconstriction was seen when the pupil-related EW neurons displayed an activity of approximately 8 spikes/second, but these neurons also displayed transient firing rates with light “on” of up to 28 spikes/second. Light “off” was observed to produce a post-excitatory depression lasting as long as 700 msec. In addition to this study, pupil-related parasympathetic activity has been studied postganglionically in the ciliary nerves of rabbits (160, 272), and in the ciliary ganglion of cats (246) and rabbits (169). The reports of these studies are generally consistent with the results of the study by Sillito and Zbrozyna (348).
More recently, in alert primates, a few pupil-related EW neurons have been antidromically identified by electrical stimulation of the intracranial portion of the oculomotor nerve (104). In all cases, antidromic activation was confirmed by collision testing (99). An example of an EW pupillomotor neuron is shown in figure 8. In darkness, the firing rate of the neuron was very low. During sinusoidal modulation of light intensity, the activity of the neuron is modulated sinusoidally and varies from approximately 10 spikes/second at a pupillary diameter of approximately 7 mm to 25 spikes/second for near-maximal pupilloconstriction. This neuron also shows a phase advance with respect to pupilloconstriction. This indicates that, during pupilloconstriction, these neural signals show an additional increase in firing that results in a transient increase in muscle force sufficient to compensate for the sluggish nature of the iris musculature and its associated tissues. Interestingly, the behavior of this pupil-related EW neuron is very similar to the luminance neurons of the pretectal olivary nucleus (see below), which are presumed to provide EW with input related to the pupillary light reflex (111).
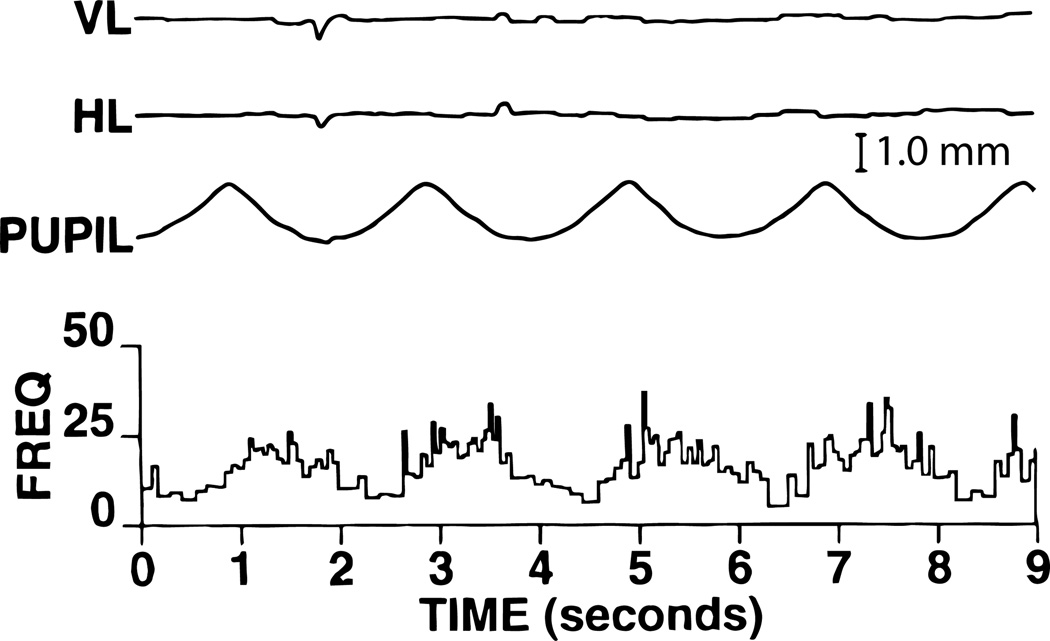
Figure 8.
The response of a pupil-related EW neuron during 0.5 Hz sinusoidal modulations in light intensity and the resultant pupillary responses. The activity of the neuron is modulated sinusoidally and also shows a phase advance with respect to the pupilloconstriction. Note that the animal maintained fixation of the target for the entire period of the trial. Abbreviations: HL - Horizontal position of the left eye; VL - Vertical position of the left eye. (Scale Bar = 1mm).
Pupil-Related Inputs to the Edinger-Westphal nucleus
Once the essential role of the EW in the pupillary light reflex had been established by the early part of last century, studies began to investigate the sources of inputs to this nucleus that mediated the reflex. It was soon shown that the central afferent limb of the reflex began with retinal ganglion cell fibers and includes the brachium of the superior colliculus (180). However, the site of termination of these fibers and their subsequent projections remained unclear until the studies of Magoun and colleagues (225–227, 305). These experimenters used localized stimulation and lesioning techniques to follow the trajectory of the reflex pathway and showed for the first time that the pretectum is essential for the integrity of the pupillary light reflex. Since these pioneering studies, most studies have implicated the pretectum in providing the EW with the pupil-related input that mediates the pupillary light reflex. But there was disagreement as to the precise portion of the mammalian pretectum that projected to the EW (25, 293, 354, 430), and some studies even reported that there was no direct projection from any retinorecipient pretectal nucleus to the EW in the cat (29, 134), tree shrew (414), and rat (263).
Given the distinctiveness, in birds, of the Edinger-Westphal nucleus, the midbrain, and the pretectal nuclei, we conducted studies in pigeons to resolve some of these mammalian issues. To identify the pupil-related inputs to the EW, it was injected with HRP (117). Following these injections, only one retinorecipient region of the pretectum was found to contain HRP labelled cells - a dorsomedially situated region termed the area pretectalis (AP) that receives contralateral retinal input. Approximately 100–250 labelled cells were present in the AP contralateral to the injection site but very few labelled cells were present ipsilaterally. In order to determine the precise portion of the EW to which AP projected, this nucleus was injected with 3H proline/leucine and autoradiographic techniques were used to show that the projection was to the caudal pole of the lateral subdivision of the contralateral EW. The terminal field was confined to a discrete region of the caudolateral EW overlying approximately 100 neurones. These anatomical results suggest that only a small number of EW cells mediate the pupillary light reflex. To establish that this pathway played an essential role in the pupillary light reflex we conducted additional experiments (117). We showed that unilateral lesions that completely destroyed AP resulted in a fixed, dilated pupil in the eye contralateral to the lesions, while the pupillary light reflex of the eye ipsilateral to the lesioned AP appeared normal. Also, microstimulation of AP was found to elicit a pupillary constriction of the contralateral eye only. Thus our data for pigeon clearly indicated that a retinorecipient nucleus in the pretectum (area pretectalis) played a major role in the control of pupilloconstriction. Further, based on topographic and histochemical grounds, we have suggested that the pretectal olivary nucleus of mammals is comparable to the AP of birds (104).
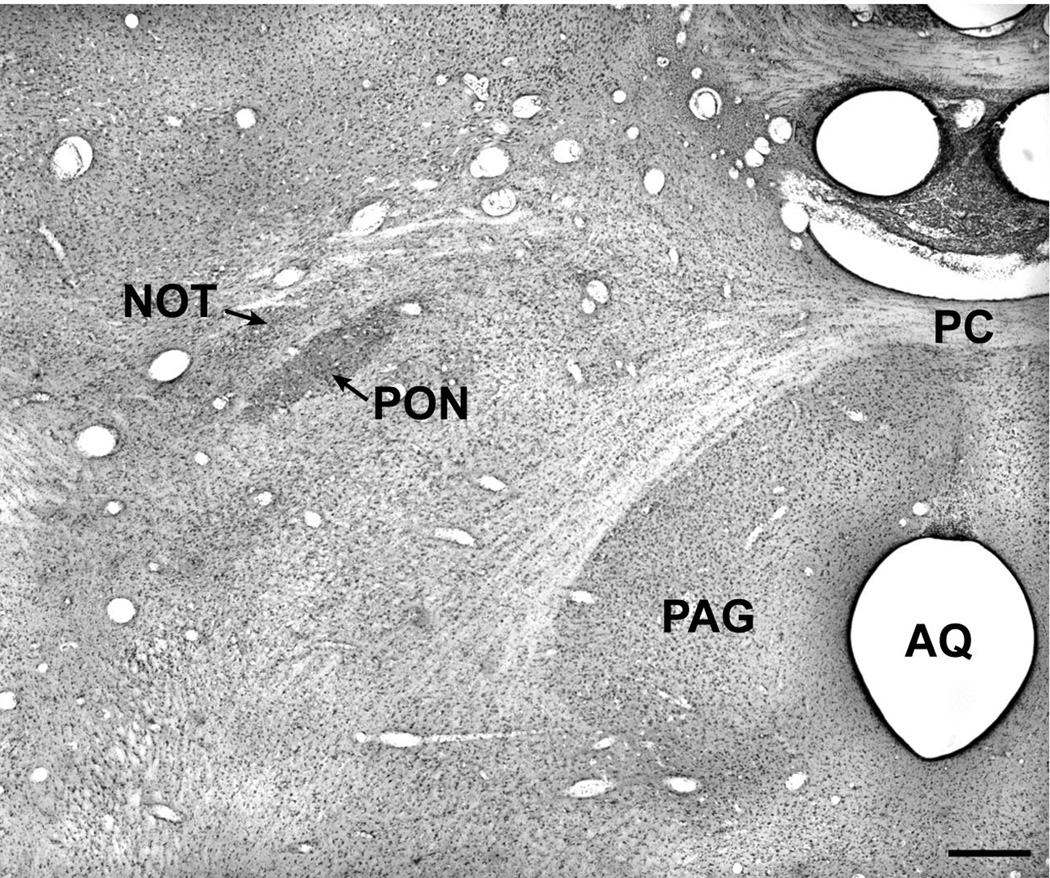
In light of these results in pigeons and the conflicting results in most mammals, we investigated the source of the pretectal input to the EW in the rhesus monkey (106). To identify the afferent pretectal regions, WGA-HRP was injected into the EW under physiological guidance. Intravitreal injections of the same tracer were also made in other animals to define the retinal terminal fields within the pretectum. Following injection of WGA-HRP in the EW and appropriate processing, retrogradely labeled cells were found in only one retinorecipient pretectal nucleus, the pretectal olivary nucleus (PON) (Fig. 9; Fig 10B).
Figure 9.
A Nissl-stained section through the pretectum of the rhesus macaque showing the location of the pretectal olivary nucleus. Abbreviations: AQ – Aqueduct; PAG – Periaqueductal Gray; PC – Posterior commissure; PON – Pretectal olivary nucleus; NOT – Nucleus of the Optic Tract. Scale bar = 500 µm.
Figure 10.
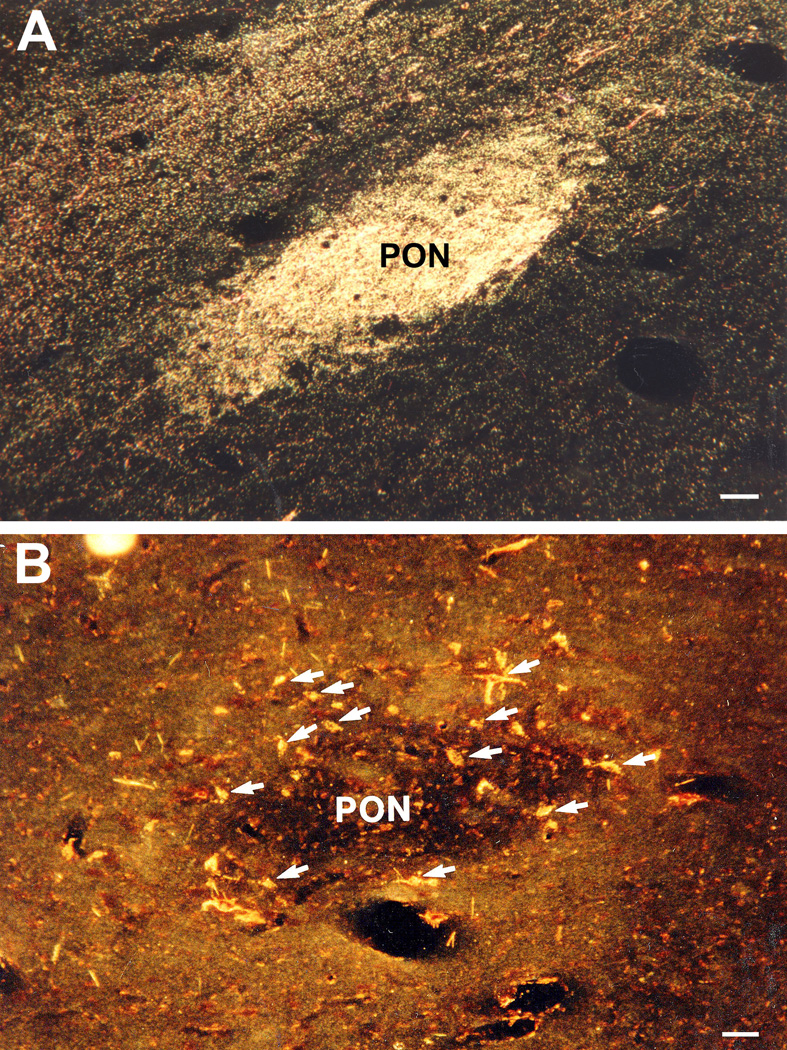
A. WGA-HRP anterograde labeling of retinal afferent terminals in the PON after intraocular injections of the tracer on the contralateral side. B. WGA-HRP retrogradely labeled neurons indicated with arrows in the PON contralateral to an injection site that included the Edinger-Westphal nucleus. Most neurons are encountered in the shell surrounding the central neuropil. Scale bars = 50 µm (Modified from Gamlin and Clarke, 1995) (115).
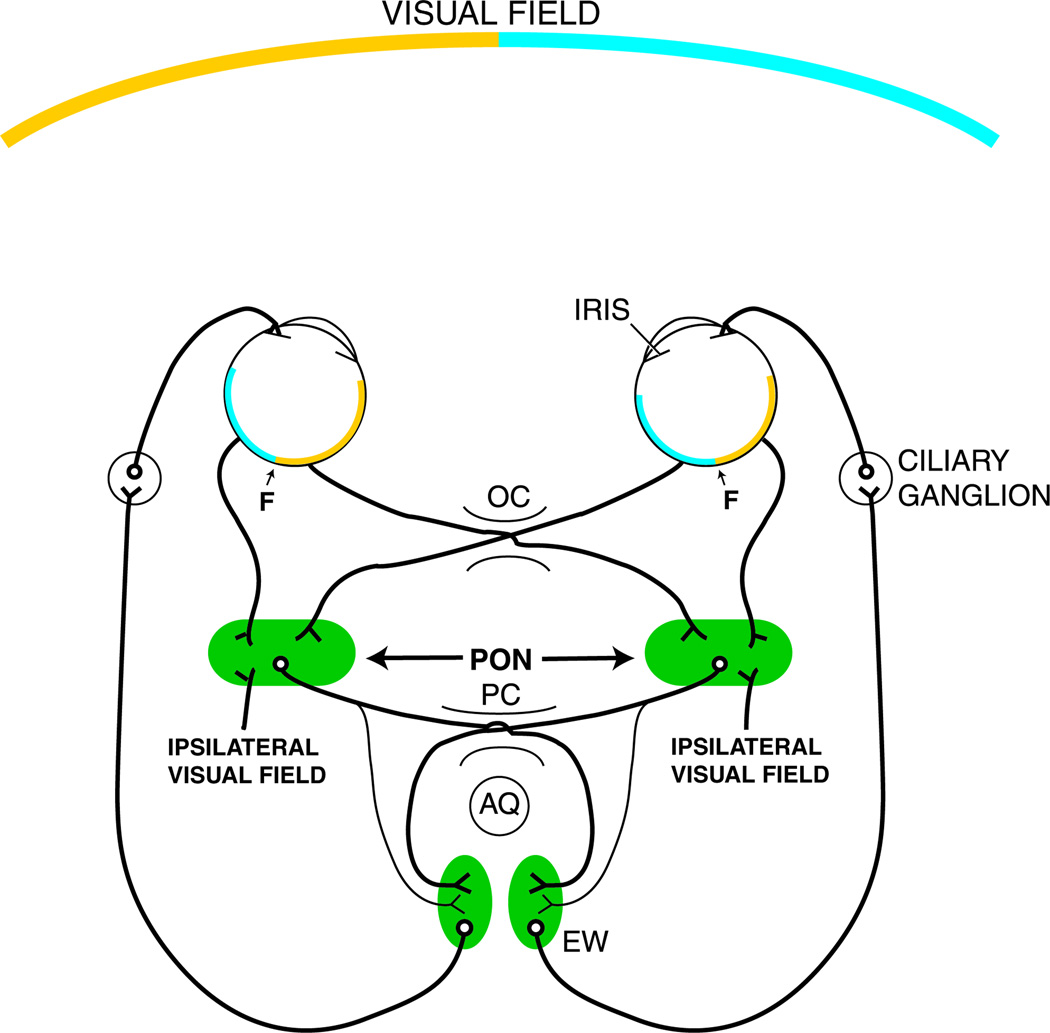
Almost all labeled cells were located contralateral to the injection site. Intravitreal injection of WGA-HRP resulted in anterograde labeling of all the retinorecipient pretectal nuclei including the PON (Fig 10A). The retinal terminal field in the PON coincided with the location of the cells that were retrogradely labeled by the injection of tracer into the EW and its vicinity. These results demonstrate that, in macaques, there is a direct projection from the pretectum to EW, that it arises from only one retinorecipient pretectal nucleus, the PON, and that the PON projects predominantly contralaterally to the EW by way of the posterior commissure (Figure 11).
Figure 11.
Schematic diagram of the direct and consensual pupillary light pathways in primates including humans. It is believed that the ipsilateral visual field is of cortical origin (64). Abbreviations: AQ – Aqueduct; EW - Edinger-Westphal nucleus; F – Fovea; OC – Optic chiasm; PC – Posterior commissure; PON – Pretectal olivary nucleus.
Support for this viewpoint comes from other studies in the monkey that have generally yielded comparable results demonstrating that retrogradely labeled cells in the pretectum are predominantly confined to the contralateral pretectal olivary nucleus after injections of HRP or WGA-HRP into the EW (53, 354). However, the results of two anterograde studies have raised some questions regarding the details of this proposed pathway. One of these studies investigated the pretectal projection to the EW and suggested that the PON projects not to the EW proper, but immediately lateral to it (53). Following intraocular injections of tritiated amino acids, the other study reported transneuronal anterograde labeling over a similar region lateral to the EW proper (194). Specifically, in both studies, the projection was reported to be to the so-called lateral visceral cell column (57) where, except for a report by Burde and Williams (49), preganglionic neurons have not been reported. Another investigation (240) that combined transneuronal retrograde labeling of the pupil-related region of the EWpg with anterograde pretectal injections identified both a monosynaptic connection from the pretectum to EWpg consistent with our previous findings, as well as a projection similar to that reported by Kourouyan and Horton (194), to a region that does not contain parasympathetic, preganglionic neurons. The role of this latter projection in the pupillary light reflex is currently unclear.
In addition to this pretectal, pupil-related input to the EW, the cerebellum has also been reported to project to the EW in cats and monkey (e.g. 238, 389). Electrophysiological and lesion studies in cats (154, 155, 159, 401) have reported that this projection may modulate pupillary function. However, as emphasized by Hultborn and colleagues (154) and as described below, while these papers provide some evidence that the cerebellum modulates the pupillary light reflex, there is even stronger evidence that the cerebellar projection to the EW modulates accommodation.
Afferent Pathway of the Pupillary Light Reflex
The first neurons in the afferent pathway of the pupillary light reflex are retinal ganglion cells. It has recently been recognized that this reflex in rodents and primates is driven predominantly by a unique subset of intrinsically-photosensitive retinal ganglion cells (ipRGCs) which project to the PON [(Figs. 9, 10) (105)]. Early anatomical studies of the pupillary light reflex did not concentrate on the PON specifically, but instead examined all retinal projections to the pretectum, which contains five retinorecipient nuclei (105). Several of these early studies utilized tracers injected into the vitreous of the eye to anterogradely label all retinal ganglion cell projections to the pretectum (see 105 for a review). These studies found that the retinal projections to the pretectum were heaviest to the nucleus of the optic tract and pretectal olivary nucleus and, in primates, were bilateral, with only a slightly heavier contralateral component (e.g. 158). In contrast, the retinal projection to the pretectum of rodents is predominantly contralateral (328–330), and only exhibits a moderate ipsilateral component in cats (e.g. 157, 192). The ratio of crossed to uncrossed projections of the retinopretectal projections appears to correlate with the ratio of the direct versus consensual pupillary light reflex in mammals.
Other early anatomical studies used retrograde tracing to label pretectally-projecting retinal ganglion cells by injecting the tracers into the pretectum. These studies are hard to interpret because fibers projecting to the superior colliculus were also often labeled. Retrograde labeling studies across several different species have found that pretectally projecting cells represented only a small percentage of the total population of ganglion cells (1–6%), and that these cells generally possess small or medium-sized cell bodies and can be classified morphologically as being gamma or W-like (e.g. 17). Very few studies were able to successfully target injections exclusively to the PON and therefore it is unclear to what extent the retinal cells labeled in these studies actually participate in the PLR. However, an injection centered on the PON in macaques gave rise to medium-sized labeled neurons, with a few coarse dendrites and extensive dendritic arbors (291). The morphology of these labeled cells is consistent with the intrinsically photosensitive retinal ganglion cells that have been shown to contribute significantly to the pupillary light reflex (72, 107).
Intrinsically photosensitive retinal ganglion cells (ipRGCs)
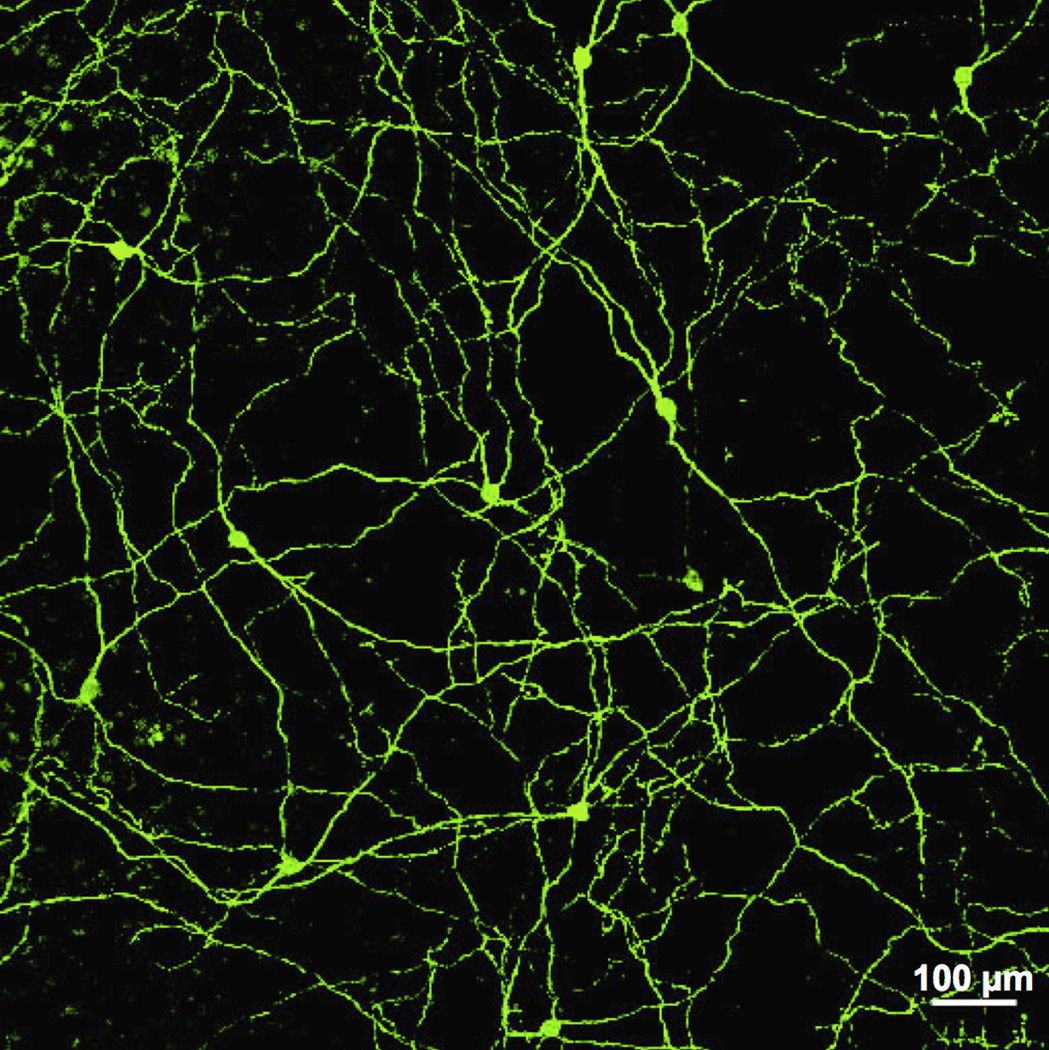
Prior to 2000, it was assumed that the pupillary light reflex was driven by retinal ganglion cells that received light signals exclusively from rod and cone photoreceptors, which up to that time were the only known photoreceptive cells in the retina. Recent findings in rodents and primates suggest that the pupillary light reflex is driven by intrinsically-photosensitive retinal ganglion cells which, unlike any other retinal ganglion cell class, are themselves photosensitive (Figure 12) (32, 72, 107, 129, 142, 221, 245, 256). The intrinsic photoresponse of ipRGCs is mediated by the photopigment melanopsin (130, 301–303), and is well fitted by a single pigment absorbance spectrum centered at 482 nm (72, 96, 143, 303). In addition to their intrinsic signal, it is clear that ipRGCs receive rod and cone inputs (72, 290, 333, 426), as this allows these cells to be sensitive to both photopic and scotopic (following dark adaptation) light stimuli (72). The scotopic sensitivity of ipRGCs appears to be mediated exclusively by rod photoreceptors, while photopic sensitivity is driven by a combination of cone and the intrinsic response (72). In response to a photopic light stimulus, intracellular recordings from this cell type show a characteristic transient burst of firing at stimulus onset, which rapidly decays to a plateau of sustained firing that often extends well past stimulus offset. The initial burst of firing is mediated by a rapidly adapting cone mediated photoresponse and the sustained firing that follows is driven by the intrinsic response of these cells (31, 72, 95, 426).
Figure 12.
Photograph of melanopsin-containing ipRGCs cells in peripheral macaque retina. Scale bar = 100 µm. (Modified from Dacey et al., 2005) (72).
Intrinsically-photosensitive retinal ganglion cells project to the pretectum of rodents (96, 131, 142, 221) and primates (72), and it is clear that the confluence of photoreceptive signals impinging on this cell type has a significant impact on pupillary behavior. Several studies have examined the contribution of the separate photoresponses of ipRGCs to the pupillary light reflex directly. These studies have demonstrated that the intrinsic photoresponse of ipRGCs is necessary, but not sufficient, to produce a normal pupillary light reflex in both rodents and primates. Studies investigating the pupillary light reflex of melanopsin deficient knockout mice (Opn4−/−) have determined that these animals display a pupillary light reflex, but the pupil fails to constrict maximally in bright lights (96, 143, 221, 283). Rodents lacking both rod and cone photoreceptors due to retinal degeneration or transgenic manipulation nevertheless display a pupillary light reflex; however, the reflex has a higher irradiance threshold than in normal animals (96, 143, 221, 283). When rodless/coneless knockout mice (rd/rd cl) are crossed with Opn4−/− mice to eliminate all potential photoreceptive inputs to ipRGCs, the pupillary light reflex is completely absent (96, 143, 221, 283).
Studies in primates have also shown that the primate pupillary light reflex is present in the absence of rod and cone input; however, the reflex has a higher retinal irradiance threshold than normal (107). Taken together, these results show that both the intrinsic photoresponse of ipRGCs and classical photoreceptor inputs provide signals of retinal irradiance that drive the pupillary light reflex. There is evidence that the intrinsic photoresponse compensates for the rapid adaptation of cones (245), and maintains pupilloconstriction during steady state exposure at all photopic (daylight) illuminance levels (129, 245). In contrast, it appears that rod and cone mediated signals predominately drive acute pupillary responses to phasic light stimuli (21, 129, 186). It is still unclear in primates whether the influence of traditional photoreceptors on the pupillary light reflex is mediated exclusively by the rod and cone inputs to ipRGCs or by other classes of retinal ganglion cells that may to project to the PON.
The intrinsic response of ipRGCs also includes an accumulative irradiance history signal, which can affect pupillary behavior in the absence of overt light stimulation. As noted above, recordings from ipRGCs in rodents and macaque monkeys have shown that these cells possess an ability to encode stimulus irradiance through an elevation of firing rate that extends beyond stimulus offset (31, 72, 95, 333, 424, 426). This sustained firing after stimulus offset appears to be a mechanism for encoding stimulus intensity, as the magnitude and duration of this sustained response varies linearly with stimulus intensity (72, 424–426). This cellular response also appears to influence pupillary responses. Studies of the human pupillary light reflex have shown that bright light stimuli can produce a prolonged pupillary constriction that persists for minutes, even when the subject is kept in complete darkness following stimulus presentation (215). An example of a human consensual pupillary light reflex produced by two different wavelengths of light is shown in figure 13. Note the sustained post-illumination pupil response (PIPR) for the shorter wavelength stimulus. This response is mediated almost entirely by the intrinsic photoresponse of ipRGCs in primates including humans (88, 107, 177, 313).
Figure 13.
Pupilloconstriction elicited by a ten second light stimulus of 493 nm wavelength light at 14.0 log quanta/cm2/second irradiance (blue trace), and 613 nm wavelength light at 14.1 log quanta/cm2/second irradiance (red trace). Note that a 473 nm stimulus, which effectively activates the intrinsic photoresponse of ipRGCs, drives a larger pupillary response than the 613 nm stimulus (red trace), which does not effectively activate the intrinsic photoresponse of ipRGCs at this irradiance level. Also note that the pupilloconstriction induced by the 473 nm light is maintained following light cessation - this is termed the post-illumination pupil response (From Kankipati et al, 2010) (177).
Pretectum
The retinorecipient neurons in the afferent pathway of the pupillary light reflex are the luminance neurons that are located within the pretectal olivary nucleus (PON). These neurons are characterized by tonic firing rates that increase with increases in retinal illuminance. In primates, these neurons exhibit a transient burst of activity followed by sustained tonic activity in response to increases in retinal illuminance (Figure 14A). In addition, the tonic firing rate of these cells is proportional to retinal illuminance over at least a three log unit range of stimulus intensities in primates (111) and in rats (62). Although it is clear that these luminance neurons receive inputs from ipRGCs and that the response characteristic of PON luminance neurons to increases in retinal illuminance is reminiscent of that of ipRGCs, it is possible these neurons may receive input from other types of retinal ganglion cells. Furthermore, PON receives significant input from visual areas in cortex, ventral thalamus, and midbrain. Studies in primates have identified well-defined ipsilateral projections from both striate and extrastriate visual areas to the PON (15, 25, 75, 77, 209, 223, 353) as well as inputs from the pregeniculate nucleus and intergeniculate leaflet (105).
Figure 14.
Luminance neurons in the pretectal olivary nucleus (PON) drive the pupillary light reflex. (A) The response of a single neuron is the pretectal olivary nucleus in response to a 100 troland light stimulus. The pupillary response to the same light stimulus is shown in the trace above. (B) Electrical microstimulation at the level of the PON produces pupillary constriction, even in the absence of a light stimulus.
Owing to its importance in the PLR, the best described efferent projection of the PON is to the EWpg. However, the PON has also been shown to have a variety of efferent connections that may influence pupillary behavior, such as the hypothalamus, pons, and medulla (105). Electrical microstimulation of the PON in rats and monkeys (Fig. 14B) elicits pupilloconstriction at short latencies, and lesions of the PON in rats produce deficits in pupillomotor function (105). These results provide strong support for models in which luminance neurons within the PON mediate the pupillary light reflex.
Sympathetic Influences on the PLR
Although it is generally agreed that the parasympathetic pathway discussed above is the primary route of pupillary constriction associated with the pupillary light reflex (178, 215, 390), there is evidence that light also causes a slower reduction in the tone of the dilator muscle of the iris via the sympathetic pathway described in figures 1, 2, and 6, and therefore can enhance the sustained pupillary light reflex. Studies in cats have shown a light induced inhibition of postganglionic pupillodilation fibers at the level of the long ciliary nerves (272), and preganglionic pupillodilation fibers at the level of the cervical sympathetic nerve (289). These studies found that the pupillodilation fibers were inhibited by light in an intensity dependent manner, i.e., a more intense light brought about a greater inhibition in firing rate. These findings have not been replicated in primates, but there is evidence that the sympathetic system plays a tonic role but does not contribute to the dynamics of the PLR (63).
Other studies have been undertaken to determine the route of the inhibitory light signal to the pupillodilation centers of the spinal cord. By determining the effects of selective lesions at various levels of the CNS on the light induced inhibition of pupillodilation in the long ciliary nerves of cats, Okada and colleagues (274) found that this light signal originated at the level of the pretectum, presumably from one of the retinorecipient nuclei contained within the pretectum. Further lesions in more caudal portions of the CNS suggested that the inhibitory light signal descended from the pretectum bilaterally to the pupillodilation centers of the spinal cord. A more recent series of anterograde labeling studies in rat support these findings by demonstrating an anatomical connection between the pretectal olivary nucleus and the pupillodilation center of the spinal cord (189).
It is not known if the light induced inhibitory signal is carried by pretectal neurons responding to an increase in light intensity, similar to the cells driving the parasympathetic pathway, although this would require an intervening sign inverting synapse somewhere in the descending pathway. It is possible that the inhibition is a direct result of cells which decrease their firing in response to light. The existence of so called “darkness detector” cells have been reported in the pretectum of rats. These cells show a reduction in firing rate in response to increases in retinal luminance (62). Taken together, these findings suggest that the pupillary light reflex is potentially augmented by a light induced reduction in tone of the dilator muscle that occurs in conjunction with the increased tone of the sphincter muscle brought about by the parasympathetic pathway.
The Pupillary Near Response
The pupillary near response (PNR) is a pupillary constriction associated with a change in viewing distance from far to near that occurs in primates including humans. When the eyes shift from viewing a distant object to viewing a near one, three oculomotor responses occur. The eyes converge to bring the image of the object onto similar regions of each retina, the refractive power of the crystalline lens is adjusted to bring the image of the object into focus on the retina, and the iris constricts thus reducing the diameter of the pupil. These collective processes, termed convergence, ocular accommodation, and miosis respectively, are classically referred to as the near response or the near triad.
Efferent Pathway of the Pupillary Near Response
The PNR is thought to be driven solely by an increased drive to the sphincter muscle of the iris via the parasympathetic efferent pathway (181). Therefore, the neural control pathway of the pupillary near response shares a common efferent pathway with the pupillary light reflex, although the afferent inputs responsible for the pupillary near response are more complex. These two reflexes appear to converge at the EWpg, since the activity of PON luminance neurons is not correlated with the pupil constriction that occurs during near viewing (434). Further, certain clinical neurological conditions are characterized by an intact pupillary near response with the absence of the pupillary light reflex (light-near dissociation) (220).
It is generally accepted that neurons in EWpg drive the PNR as well as the PLR. However, it has not been determined if separate subpopulations of neurons exist in EWpg devoted exclusively to either the pupillary light reflex or the pupillary near response, or whether the same population of neurons drives pupillary constriction in both reflexes, although the latter seems most likely.
Afferent Influences on the Pupillary Near Response
Modern investigations into the afferent influences driving the PNR began in the late 1940s with the advent of infrared photographic recordings of the pupil during near viewing. This technique allowed researchers to measure the magnitude of the PNR in near darkness, thus eliminating any artifactual change in pupil diameter induced by decreased retinal illuminance originating from the constriction of the pupil during the response. The aim of these original investigations was to determine whether the PNR was driven primarily by ocular convergence or accommodation, the other two components of the near triad. Early studies found that the PNR was more closely associated with accommodation than with convergence (13, 94, 191, 233, 234). Later studies found a greater association with convergence (16, 171), and even showed that the PNR could be totally absent during certain blur driven accommodative responses (292, 352). These conflicting results are likely a product of an incomplete disassociation between the convergence and accommodation systems during these experiments, as these two systems have been shown to be highly interdependent.
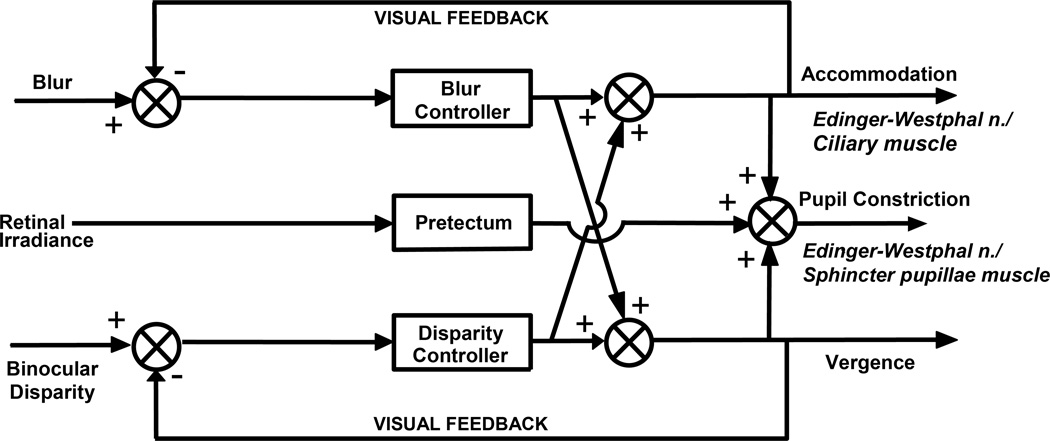
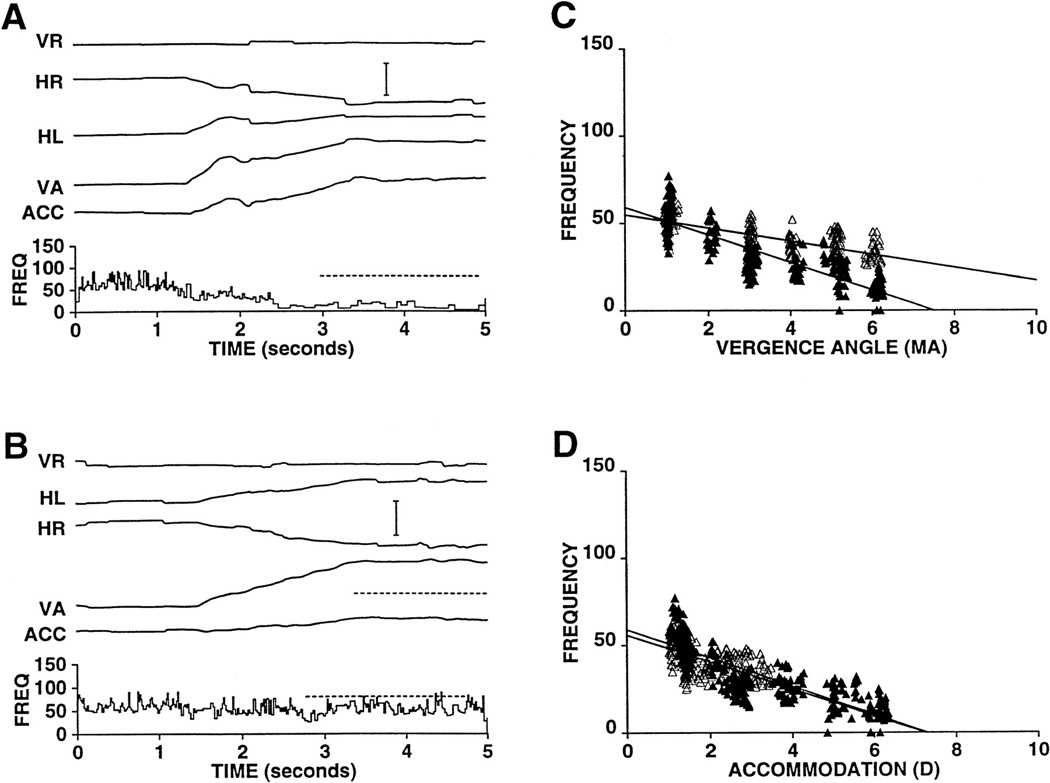
A more modern view of the afferent influences of the PNR has recently emerged, in which the PNR is not seen as resulting from either accommodation or convergence alone, but as a separate output of the neural pathways that drive both accommodation and convergence. This view has resulted from our increased knowledge of the neural circuitry involved in all three oculomotor processes of the near triad. Experiments investigating the interaction between convergence and accommodation lead to the introduction of the dual interaction model of convergence and accommodation [(156, 334, 343); (Figure 15)]. This model proposes that two neural controllers operate in the near triad, one which integrates stimuli for accommodation such as blur, and one which integrates stimuli driving vergence, such as disparity between the images on the retina of each eye (50, 242), and that the PNR is not driven exclusively by either the accommodation controller or the convergence controller, but actually by an interaction of the two controllers (257, 375). These findings also suggest that the three oculomotor components of the near triad share common afferent influences.
Figure 15.
Schematic of the modified dual interaction model that accounts for the pupillary near response component of the near response triad. This model indicates that the combined output of the accommodation controller and the convergence controller drive the pupillary near response.
A number of brain areas play a role in controlling the near triad. These include cortical areas, such as extrastriate cortex, parietal cortex, frontal eye fields, as well as the cerebellum and the midbrain (113). Of particular interest to the pupillary near response, is the supraoculomotor area (SOA) of the midbrain, which lies just dorsal and lateral to the oculomotor nucleus (238). The SOA contains near response cells which are modulated by both vergence and accommodation, and which receive input from both the accommodation and vergence controllers (113). These cells project to medial rectus motoneurons, and thus contribute to vergence eye movements (439). As discussed below, it seems likely that these cells also project to EW and are responsible for carrying the signal from the accommodation and convergence controller to preganglionic, pupilloconstriction neurons, as well as preganglionic, accommodation-related neurons (50, 113, 242).
Cortical Influences on Pupillary Behavior
In addition to cortical afferents mediating the pupillary near response, the pupil is also influenced by both visual and non-visual cortical regions. These afferents manifest themselves in small changes in pupil diameter measured during presentation of visual stimuli such as isoluminant, colored stimuli and gratings, as well as non-visual stimuli such auditory tones, and even during higher order cortical functions such as problem solving. These observations provide clear evidence that cortex exerts an influence on pupillary behavior, which cannot therefore be thought of as entirely reflexive in nature. Recent studies in primates, including humans, have shown that the pupil responds to complex aspects of visual stimuli such as color, motion, and texture. Slight pupillary constrictions have been shown to occur in both human and macaque with the presentation of complex visual stimuli, even when the stimuli do not involve a change in viewing distance or retinal illuminance (20, 112). The three best described stimulus attributes which produce this effect are color, spatial frequency, and apparent motion. It is clear from physiological and functional imaging studies that these stimulus characteristics are encoded in areas of visual cortex. Furthermore, when these cortically driven pupillary responses are assayed in human subjects with well documented lesions to cortical areas involved in processing one or more of these stimulus characteristics, a deficit in the concurrent pupillary response is always observed (20). For example, Heywood and colleagues (145) have demonstrated in macaques that lesions of rostral inferior temporal cortex but not V4 abolish pupillary responses to chromatically modulated gratings. These findings offer conclusive evidence of cortical influences on pupil diameter.
Ascending Neuromodulatory Systems
The ascending neuromodulatory systems of the midbrain and pons can have a variety of effects on pupillary behavior. These nuclei are the origin of neuromodulatory fibers which release dopamine, norepinephrine, histamine, and serotonin at a number of brain areas implicated in pupillary control. These neuromodulatory systems appear to be critical in the regulation of sleep and arousal (327), as well as autonomic regulation (43) and cortical plasticity (138). In addition to these global neuromodulatory effects, all or some of which could have a profound influence on pupillary behavior, there is evidence for a direct inhibition of pupilloconstriction neurons in the EWpg by adrenergic neurons originating from the locus coeruleus in a number of animal models (193). However, studies in humans (202) and rabbits (431) have failed to find such a direct noradrenergic inhibition of pupilloconstriction neurons, and it has been suggested that this effect is mediated by the dopaminergic neurons in these species (431). In ether case, increased activity of adrenergic or dopamine afferents would cause pupil dilation (mydriasis), as does serotonin. Importantly, drugs that agonize or antagonize these neuromodulatory neurotransmitters have been found to differentially affect pupillary behavior in a wide range of animal models and human studies. These differential effects are most likely due to the both interspecies variability in the projections of these neuromodulatory fibers (138), as well as the differential activation of multiple brain areas implicated in pupillary behavior due to the extensive projections of these neuromodulators. Therefore, these experimental findings in animal models do not always generalize well to humans.
Autonomic Control of Ocular Accommodation
The basic mechanism of accommodation was first described by Helmholtz (144). Briefly, changes in focus of the lens of the eye are brought about by changes in force from the ciliary muscle which acts in an antagonistic fashion with the fibers of the zonule. These zonular fibers support the lens capsule and act to maintain the lens in a relatively “flattened” state at rest. Ocular accommodation occurs when ciliary muscle contraction results in a reduction in tension in the zonular fibers which produces a “bulging” (increase in convexity) of the lens and a concomitant increase in its refractive power. Accommodative ranges of up to 20 diopters, i.e. the ability to focus as close as 5 centimeters, are seen in primates (e.g. 60, 67) and birds (e.g. 400). The parasympathetic innervation of the ciliary muscle dominates the dynamics of these accommodative responses. Increases in parasympathetic innervation act rapidly (within 1 second) to produce substantial positive accommodation of up to 20 diopters (e.g. 60, 118, 396), while sympathetic innervation acts with a much slower time course of 10–40 seconds to produce hyperopia (accommodation for far) of at most 1.5 diopters (e.g. 126, 395, 396). A more recent study states that the sympathetic system only slightly affects the dynamics of accommodation and has little effect on the resting level or amplitude of accommodation (278).
Accommodation Evoked by Electrical Stimulation of the EWpg or its Efferent Projections
Electrical stimulation and single-unit recording in the EWpg and its efferent pathways in primates and birds have revealed much about the physiology of accommodation related neural connections. Also, anatomical investigations in primates have revealed much about the sources of afferents to the EWpg that mediate accommodation. A number of studies have shown that electrical microstimulation in or immediately adjacent to the EW evokes ocular accommodation in primates (24, 61, 68, 118, 166, 173, 279, 280, 410, 418);. These results of EW stimulation are also consistent with studies in which electrical stimulation of the ciliary ganglion or nerves produced similar increases in accommodation in cats (232, 308).
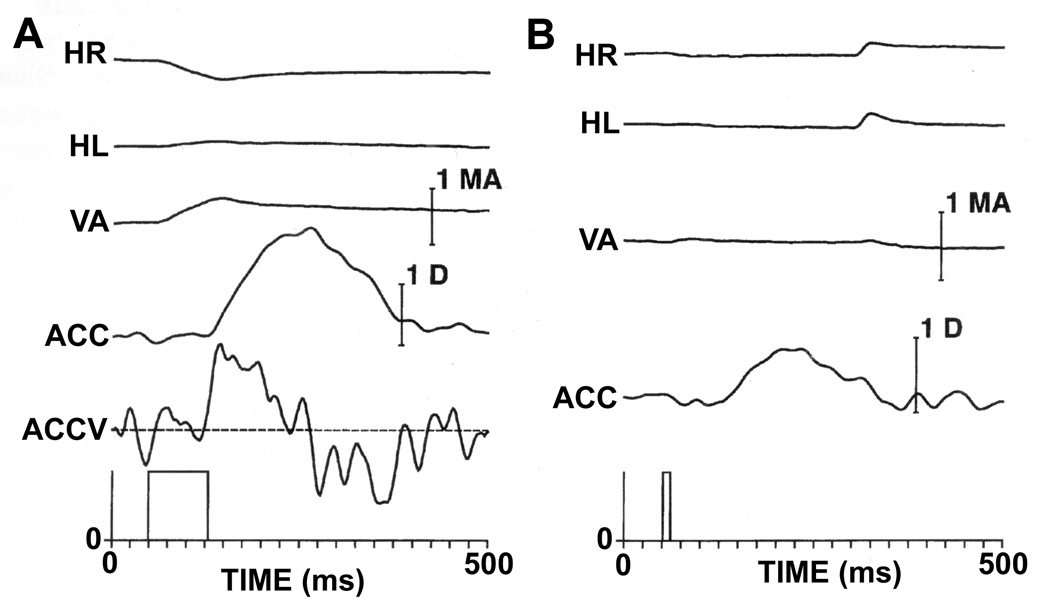
We have examined the effects of electrical microstimulation in the EW of the alert rhesus monkey (figure 16) (118). Accommodation was measured by a continuous recording optometer based on a design of Kruger (197). To facilitate measurement of the latency of the response, both accommodation (ACC) and accommodative velocity (ACCV) are shown in figure 16A. As can be seen in this figure, the ACCV trace crosses the zero velocity line approximately 75 msec after the beginning of the stimulus train. In figure 16A, some convergence and adduction of the right eye are visible. However, figure 16B shows that, with shorter stimulation times, specific changes in accommodation could be elicited that were not associated with significant changes in eye position. Repeated measures of the latency of the stimulation-induced responses were made at a number of sites within the EW, and the results from two animals were essentially identical, with accommodative responses being evoked with latencies of 75 msec.
Figure 16.
Effect of microstimulation of the EW on ocular accommodation. A shows stimulation (80 msec; 500Hz; 40 µA) producing an accommodative response with a latency of 75 msec. Accommodative velocity (ACCV) is also shown to facilitate estimation of the latency of the accommodative response. Note that, in addition to accommodation, there is an adduction of the right eye which presumably results from current spread to the nearby medial rectus motoneurons of the "C" subgroup of the right oculomotor nucleus. The stimulation also produces a small amount of convergence that is either the result of activation of the axon collaterals of near-response neurons that project to medial rectus motoneurons and presumably also to the Edinger-Westphal nucleus or stimulation of nearby medial rectus C-group motoneurons. B confirms the specificity of the microstimulation effect by showing that only accommodation is elicited when a stimulation train of shorter duration (10ms; 500Hz; 40 µA) is used. Abbreviations: HL - horizontal position of the left eye; HR - horizontal position of the right eye; VA - Vergence angle. (Scale Bar = 1 meter angle and 1 diopter). (From Gamlin et al. 1994) (118).
Responses Of EW Neurons During Ocular Accommodation
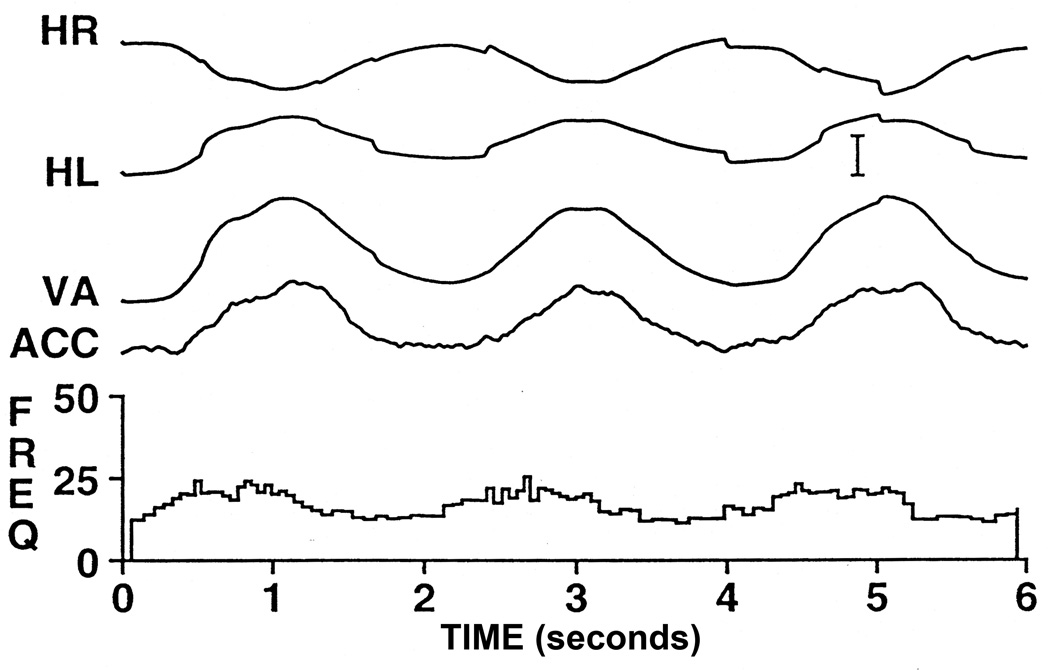
To examine the single-unit responses of EW neurons during accommodation, preganglionic neurons were identified by antidromic activation from a stimulating electrode placed in the intracranial portion of the ipsilateral oculomotor nerve just as was done to identify pupil-related preganglionic EW neurons. Also, as was the case for pupil-related preganglionic neurons, antidromic activation was confirmed by collision testing (99). An example of the behavior of an accommodation-related EW neuron is shown in figure 17 during sinusoidal tracking of a target moving in depth at 0.5 Hz. We found that the behavior of these neurons during normal binocular viewing was qualitatively the same for all cells. In all cases, their firing rates in darkness and at optical infinity were low, but all were active (average activity = 11.6 spikes/sec), and all increased their firing rates with increases in accommodation. On average these neurons showed a sensitivity to accommodation of 3.3 (spikes/s)/diopter. Also as expected, we found that the firing rate of these EW neurons was unaffected by horizontal conjugate eye movements.
Figure 17.
Behavior of a preganglionic EW neuron during sine wave tracking of a target moving in depth. The firing rate modulates between approximately 15 spikes/second and 25 spikes/second for the change in accommodation of 5 diopters. Note that there is a significant phase lead in the firing rate of this cell with respect to accommodation that cannot be accounted for solely by the latency between the activity of the cell and accommodation. This phase lead results from a substantial component of the firing rate being related to the dynamics of the movement. Abbreviations: HL - horizontal position of the left eye; HR - horizontal position of the right eye; VA - Vergence angle. (Scale bar is equal to 2 meter angles and 2 diopters). (From Gamlin et al. 1994) (118).
In addition, as shown in this figure, the neuron shows a phase lead that cannot be accounted for solely based on the 75 msec delay between neural activity and ocular accommodation that would have been expected based on the results from electrical microstimulation. Instead, this phase lead results from a significant sensitivity not just to accommodation but to the speed of accommodation that was seen on this cell and on all the accommodation-related EW neurons that were characterized with sine-wave tracking in depth. Overall, accommodation-related preganglionic neurons were related to the speed of accommodation with a sensitivity of 1.2 (spikes/s)/(diopter/s) at 0.5 Hz. This neural activity is presumably required to provide the additional innervation of the ciliary muscle that is needed to generate sufficient transient force to compensate for the characteristics of this muscle and the peripheral accommodative apparatus, which are presumed to be very sluggish (118).
Accommodation-Related Inputs to the EW
There have been few anatomical studies of the inputs to the primate EW that might control accommodation. However, several electrophysiological studies in alert macaques have characterized the behavior of neurons in the reticular formation immediately dorsal and lateral to the oculomotor nucleus – the supraoculomotor area (SOA) (173, 243, 244, 439). Some of these cells appear to control convergence, while others are more closely linked to the control of accommodation (173, 243, 244, 439). Consistent with this suggestion, some convergence-related midbrain cells project monosynaptically to ipsilateral medial rectus motoneurons in the primate (438). Comparable monosynaptic projections from accommodation-related midbrain neurons to EW neurons are very likely to exist. In addition, we have used anatomical techniques in the rhesus monkey to show that the caudal fastigial nucleus (cFN) of the cerebellum projects to the EW and the SOA while a ventrolateral region of the posterior interposed nucleus (PIN) projects to the SOA alone (238). Electrophysiological studies in the alert rhesus monkey have shown that some neurons in the cFN are related to convergence and accommodation for near (110) while some neurons in PIN are related to divergence and accommodation for far (Fig. 18) (436).
Figure 18.
Response properties of a far accommodation cell in the posterior interposed nucleus during normal binocular viewing and partial dissociation of vergence from accommodation during conflict viewing. Initial VA and ACC of 1 MA and 1 D respectively in A and B. A: during normal binocular viewing, activity of this neuron decreases as a function of near response. B: response of this cell during a convergence movement similar in amplitude to that in A and an accommodative response substantially lower than that in A. This difference is best appreciated by reference to upper of 2 dashed lines that indicates accommodative response in A. For reference, lower of 2 dashed line is located at same relative level as it is in A. Note that decrease in firing rate is much less than in A. C: scatterplot and linear regression of firing rate as a function of vergence angle for normal viewing (▲) and conflict viewing (Δ). D: scatterplot and linear regression of firing rate as a function of accommodation for normal viewing (▲) and conflict viewing condition (Δ). Scale bar = 4 meter angles and 4 diopters. (From Zhang and Gamlin, 1998) (435)
Presumably these neurons modulate the activity of EW and SOA neurons by way of their projections to these regions. Consistent with this hypothesis, muscimol inactivation studies in strabismic monkeys show that exotropia is increased by cFN inactivation and decreased by PIN inactivation (172). In cats, neurons related to accommodation have been reported in both the fastigial and interposed nuclei (18, 153), and accommodation-related preganglionic neurons can be orthodromically activated by electrical microstimulation of the interposed nucleus (19). Thus there is strong evidence from studies in both primates and cats that the cerebellum can significantly modulate accommodation by way of its projection to the EW.
In addition to the near-response cells located immediately adjacent to the oculomotor nucleus, other cells that modulate their activity during the near response have been reported approximately 4–6 millimeters more dorsal in an ill-defined region that includes, or is close to, the nucleus of the posterior commissure (173, 244). In the rhesus monkey, it is possible that these near-response cells in both the midbrain and pretectal region project monosynaptically to the EW. Although this has not been specifically investigated either physiologically or anatomically in this species, there is support for connections of this nature from some anatomical studies in the pigeon (116). The results in pigeons suggest that, if the projections in primates are comparable, there are monosynaptic projections from the near response cells in both the (SOA) and the nucleus of the posterior commissure onto the EWpg. Projections of this nature would presumably mediate or modulate accommodation and possibly the pupillary near response. In mammals, authors have interpreted the projection from the nucleus of the posterior commissure to much of the EW as being involved in the pupillary light reflex (e.g. 25). However, since only a small percentage of the EW cells are involved in the pupillary light reflex in both mammals and birds (104, 117, 240, 413), it is unlikely that this extensive an input would mediate only the pupillary light reflex. It is instead likely that the nucleus of the posterior commissure input to the EW in mammals is related to both accommodation and to the pupilloconstriction associated with the pupillary near response. To resolve the details of these connections, further study of this pathway and of the proposed projection from the SOA to the EW will be needed.
The neural control of ocular accommodation and vergence eye movements has been recently reviewed (50). Cells whose activity is related to lens accommodation and vergence have been recorded in temporal (3, 376) and frontal cortex of the macaque monkey (109), as well as nucleus reticularis tegmenti pontis in cats (147) and macaque monkeys (115). Cells in the frontal eye fields have been shown to be related to ocular accommodation, and electrical microstimulation of this region in alert monkeys produces positive accommodation with appropriate latencies (Fig. 19) (109). However, there is little known about how these signals access the accommodative preganglionic neurons in EW (104, 114, 238).
Figure 19.
Stimulation in the primate frontal eye fields evokes vergence and ocular accommodation. Biphasic electrical microstimulation elicited increases in vergence and ocular accommodation. Stimulation parameters; 500 Hz, 40 ms, 40 µA. AACC, average accommodation; ACC, accommodation; AVA, average vergence angle; AVV, average vergence velocity; VA, vergence angle; STIM, stimulation; VV, vergence velocity. Scale bar, 0.5 degrees, 0.5 diopters, and 5 degrees/s. (From Gamlin and Yoon, 2000) (109)
Autonomic Control of Ocular Blood Flow
The eyes of primates, including humans, have two separate vascular systems, the retinal and the uveal, which comprises the iris, ciliary body, and choroidal vasculature (281). The retinal system supplies the inner retina while the uveal system supplies the outer retina including all the photoreceptors, as well as supplying the retinal pigment epithelium. The uveal component accounts for ~85% of total retinal blood flow (33, 37, 306).
All ocular vessels receive their blood supply from the ophthalmic artery, a branch of the internal carotid artery. The central retinal artery and two or three main ciliary arteries branch off the ophthalmic artery. The central retinal artery then enters and runs within the optic nerve. As it continues anteriorly through the lamina cribrosa to supply the retinal vasculature, it gives off small branches that provide the blood supply to the central core of the optic nerve. The main ciliary arteries give rise to 6–20 short posterior ciliary arteries and two long posterior ciliary arteries. The short posterior ciliary arteries penetrate the sclera around the optic nerve and divide to form the arterioles that constitute the choroid, which is also supplied by anterior ciliary arteries, which originate from arterial branching in the extraocular rectus muscles. The long posterior ciliary arteries penetrate the sclera close to the optic nerve and, along with the anterior ciliary arteries, they provide the blood supply to the iris and ciliary body (281).
Retinal vessels receive minimal, if any, autonomic innervation (183, 203, 306, 429), but demonstrate robust autoregulatory capabilities (e.g. 183). Therefore, the autonomic control of retinal vessels will not be further discussed. Blood flow to the ciliary body includes both the ciliary muscle, as well as the ciliary processes. Separation of these two components in vivo is difficult (183), and blood flow to the ciliary processes has been studied in more depth than that to the ciliary muscle because of its substantial role in aqueous humor secretion (183). Therefore, neural control of the blood flow to the ciliary body will be discussed in “Neural control of Intraocular Pressure”. In general, the role of the parasympathetic innervation appears to be to ensure the continued perfusion of ocular tissues in the face of decreases in mean arterial pressure that result from blood loss or other causes of hypotonia, while the role of the sympathetic innervation appears to be to prevent the over perfusion of ocular tissues in the face of increases in mean arterial pressure that result from exercise or other causes of hypertonia (183, 306). However, in addition to this global role, the autonomic nervous system can independently control the perfusion of individual ocular tissues based on additional factors. For more extensive information of the neural control of ocular blood flow than is provided in this section, the reader is encouraged to consult reviews on this topic (183, 306).
Neural Control of Ophthalmic, Central Retinal, and Ciliary arteries
Blood flow to the eye is under neural control at multiple levels of the vascular supply (Fig. 1) (e.g. 183, 306). The sole artery supplying the eye, the ophthalmic artery, is under parasympathetic, sympathetic, and local trigeminal neural control. Further, except for the retinal blood supply, all ocular blood vessels that derive from the ophthalmic artery are under both parasympathetic and sympathetic neural control with many also under local trigeminal control (306).
In many mammals, the ophthalmic, central retinal artery, and ciliary arteries are innervated by VIP+/nNOS+ fibers arising from cholinergic postganglionic neurons in the pterygopalatine ganglia, NPY+ fibers that arise from noradrenergic postganglionic neurons in the superior cervical ganglion, and SP+/CGRP+ trigeminal sensory nerve fibers (28, 33, 82, 85, 200, 203, 204, 306, 340, 429). However, there is evidence that trigeminal sensory fibers are not present in the central retinal artery of humans (27).
In mammals, parasympathetic input has a vasodilatory effect primarily through nitrergic mechanisms (306, 392, 393), while sympathetic input has a vasoconstrictor effect with norepinephrine acting through alpha-adrenoreceptors, with ATP and possibly NPY also playing a role (Fig. 20) (133, 391). The neuropeptides, SP and CGRP, released from trigeminal nerve endings have a robust vasodilatory effect (176, 306).
Figure 20.
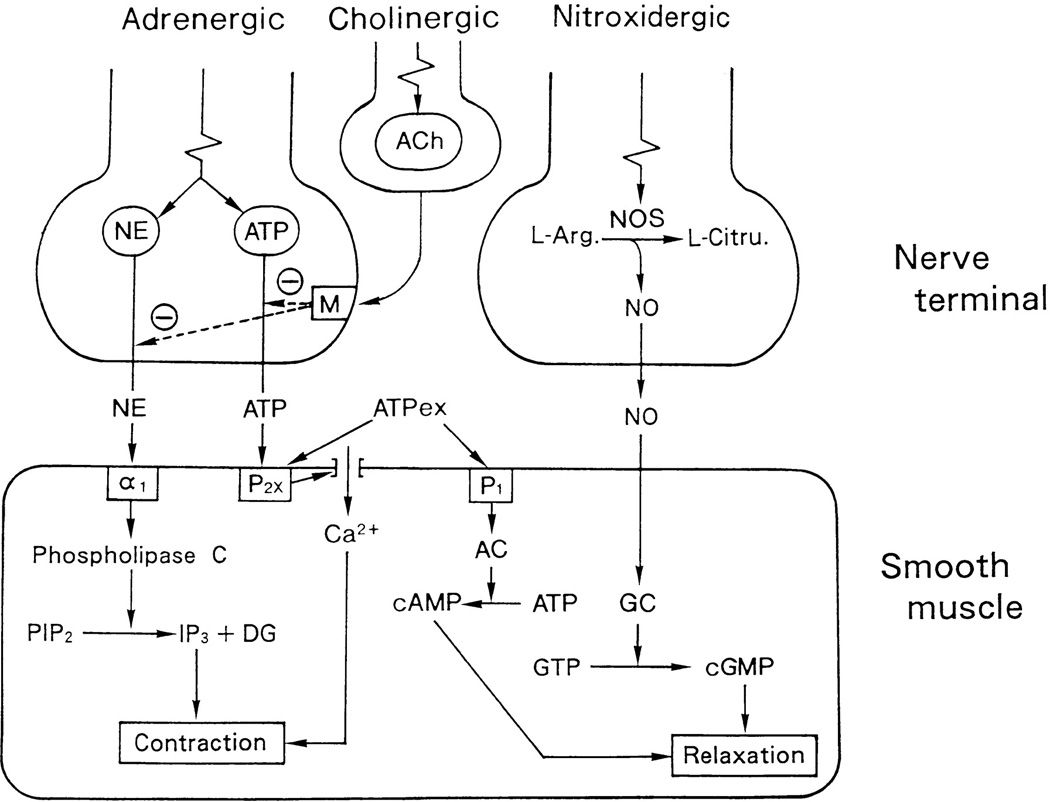
Scheme of adrenergic, cholinergic, and nitrergic innervations and roles of neurotransmitters in the regulation of nerve and smooth muscle functions in ciliary and ophthalmic arteries. Squares in nerve terminal and smooth muscle represent receptors. Abbreviations: NOS, NO synthase; L-Arg., l-arginine; L-Citru., l-citrulline; ATPex, exogenous ATP; PIP2phosphatidyl inositol bisphosphate; IP3inositol trisphosphate; DG, diacylglycerol; AC, adenylate cyclase; GC, soluble guanylate cyclase0. (From Toda et al., 1999) (391)
Neural Control of Optic Nerve Blood Flow
The optic nerve and nerve head can be divided into three regions, the retrolaminar, prelaminar, and laminar, based on their relationship to the lamina cribrosa. The retrolaminar zone is posterior to the lamina cribrosa and is supplied both by pial arteries and small branches from the central retinal artery. The prelaminar zone is anterior to the lamina cribrosa and is supplied by collaterals from the choroid and retina circulations. The laminar zone contains the lamina cribrosa and is supplied by branches from the short posterior ciliary and pial arteries. Since the choroidal blood supply is under autonomic control (see below), blood flow to all regions of the optic nerve is under control of the autonomic nervous system. Facial nerve stimulation and hence activation of the preganglionic input to the pterygopalatine ganglion causes increased blood flow in the optic nerve (271). Further, optic nerve and nerve head blood flow is regulated both in the face of changes in ocular perfusion pressure (9, 121, 253, 309, 350, 415, 416) and with retinal activity due to flicker (87, 309, 310) in many mammals. This regulation of optic nerve blood flow is likely achieved not only through local control by metabolic and myogenic mechanisms (183), but also through central autonomic control.
Neural Control of Iris Blood Flow
The role of the autonomic innervation of the iris vasculature is not entirely clear. As discussed below, iris blood flow can be significantly increased by the parasympathetic nervous system by way of the pterygopalatine ganglion, and decreased by the sympathetic nervous system by way of the superior cervical ganglion. In some animals, this appears to compensate for changes in IOP or mean arterial blood pressure (e.g. 6, 8, 10, 306). However, iridal blood flow in humans shows no evidence of autoregulation when ocular perfusion pressure is reduced (58).
The iris vasculature is innervated by VIP+ fibers arising from cholinergic, postganglionic neurons in the pterygopalatine ganglion in many species, including humans (Fig. 1) (52, 347, 362, 368, 384, 402, 407). Since the majority of pterygopalatine ganglion neurons in mammals, including humans, contain nNOS, it can be assumed that most of these fibers are also NOS+ (12, 132). In monkeys, cats, and rabbits, facial nerve stimulation and hence activation of the preganglionic input to the pterygopalatine ganglion causes increased blood flow in the iris (271). This effect can be blocked, presumably at the level of the pterygopalatine ganglion, by the nicotinic, acetylcholine receptor antagonist, hexamethonium (271) in cats and rabbits. Furthermore, this increased iridal blood flow is most likely mediated by NOS at low frequencies (2Hz) and additionally by VIP at higher frequencies (5Hz) (e.g. 269, 270). Consistent with the proposed role of NOS in this response, NOS inhibition by nitro-L-arginine methylester (L-NAME) in dogs, rabbits and pigs substantially reduces iris blood flow despite increases in mean arterial blood pressure (73, 163, 342).
In addition to the role of the facial nerve pathway in controlling iris blood flow, there have been some reports of decreases in iris blood flow as a consequence of oculomotor nerve stimulation (e.g. 357, 358) in rabbits and monkeys. However, as discussed by Reiner and colleagues (306), this vasoconstriction appears to be likely due to mechanical compression of the iris vessels as a result of the pupil constriction elicited by nerve stimulation, and not a direct effect on the iris vasculature.
The iris vasculature is innervated by NPY+ fibers that arise from noradrenergic postganglionic neurons in the superior cervical ganglion (Fig. 1) (42, 146, 250, 368, 381). Unilateral sympathetic nerve stimulation causes a substantial reduction in iris blood flow (133). However, phenoxybenzamine, an alpha adrenoreceptor antagonist, only partially eliminated the response to such sympathetic stimulation, suggesting that NPY, which is present in postganglionic fibers, is also involved in sympathetically-mediated vasoconstriction (133). This is consistent with the observation that intravenous NPY significantly reduces iridal blood flow (268).
Trigeminal sensory fibers containing both SP and CGRP innervate iris blood vessels in mammals including humans (83, 146, 249, 347, 363–366, 383, 384, 386, 405). SP and CGRP released from trigeminal nerve endings in response to noxious stimuli, temperature increases or decreases, or pressure can have a robust vasodilatory effect (176, 306). Indeed, stimulation of the trigeminal nerve in rabbits causes increased iridal blood flow along with pronounced pupil constriction (360), as does intracameral injection of CGRP (36, 275, 403).
Neural Control of Choroidal Blood Flow
Although the choroid appears to play multiple roles in both birds and mammals (265), its primary role is to provide approximately 85% of the blood supply to the retina including all the photoreceptors and the entire retinal pigment epithelium (RPE) (306). Photoreceptors are metabolically very active, especially in darkness, when approximately 90% of the oxygen supply to the retina comes from the choroidal circulation (40, 213, 214). However, both the choroid and choriocapillaris are physically separated from the retina by the RPE and Bruch’s membrane. Therefore, to ensure a sufficient oxygen supply to the retina, the choroidal blood supply must maintain a very high oxygen tension, which is achieved through a very high blood flow resulting in an arterial/venous oxygen tension difference of only 3% (8, 9). Furthermore, consistent with the high metabolic demands in darkness, in birds and mammals, there is a circadian rhythm of choroidal thickness, with the choroid being thicker at night and thinner in daytime (266, 267, 284, 404). Thus, the choroid is essential for meeting the metabolic, and possibly the thermoregulatory, demands of the outer layers of the retina. In support of this, retinal photoreceptors have been shown to rapidly degenerate when the choroidal blood supply is occluded (e. g. 65, 119, 120), or the innervation to the choroid is compromised (346). Despite its very high resting blood flow, there is also increasing evidence that the choroid shows some local autoregulatory capabilities in response to changes in perfusion pressure, which are in addition to blood flow modulation through autonomic control (183, 298, 306).
Additionally, the choroid of birds and some mammals is characterized by two special anatomical features. First, the choroid possesses a network of neurons that lie in the choroidal layer (so-called intrinsic choroidal neurons; see below) and second, it contains non-vascular smooth muscle cells that form a network of flattened lamellae in the suprachoroid and a layer beneath Bruch's membrane (261, 265). This smooth muscle appears to receive both parasympathetic and sympathetic input (300) and might serve to control the thickness of the choroid independently of blood flow by controlling the size of the choroidal lacunae. This action could also control the focal plane of the retina, yet, this is currently only speculation (265).
Intrinsic choroidal neurons
In 1859, Mueller reported that the human choroid contained numerous neurons (254). These neurons have recently been re-discovered, and are now referred to as either intrinsic choroidal neurons (335) or choroidal ganglion cells (26, 235, 236). These multipolar neurons, 20–40 µm in size, are sparse in most mammals, but are numerous in foveate primates with well-developed ocular accommodation, including humans as well as many species of bird (91, 92, 235, 236, 261, 265). It is thought that these neurons play a role in choroidal blood flow regulation, but their precise role has yet to be determined (261, 265, 306).
Many of the intrinsic choroidal neurons in humans are NADPHd/nNOS/VIP positive, and approximately 50% are also positive for calretinin (261). Also, based on the absence of immunohistochemical markers, they appear to be neither cholinergic nor adrenergic neurons (236). The intrinsic choroidal neurons form an interconnected plexus that makes extensive contacts with both choroidal blood vessels as well as with non-vascular choroidal smooth muscle cells (Fig. 2) (236, 261). In humans, many intrinsic choroidal neurons receive adrenergic, presumably sympathetic, inputs (236) as well as substantial trigeminal inputs (336). In addition, they receive input from cholinergic boutons, many of which are negative for nNOS or VIP, and therefore appear to arise from cells in the ciliary ganglion rather than those in the pterygopalatine ganglion, which are often nNOS/VIP positive (236).
Parasympathetic Control of Choroidal Blood flow
The parasympathetic input to the choroid has a vasodilatory action and serves to increase choroidal blood flow in both mammals (41, 271) and birds (89). In birds, this parasympathetic innervation arises predominantly from the choroidal neurons of the ciliary ganglion (71, 247, 294) which produce choroidal vasodilation through cholinergic stimulation of the M3 receptors on endothelial cells, this in turn results in endothelial nitric oxide production and release (432). In birds, there is a smaller contribution to the choroidal innervation from the facial nerve by way of the pterygopalatine ganglion (69). In contrast, in mammals, based on anatomical studies, the parasympathetic innervation of the choroid arises primarily from the pterygopalatine ganglion (306, 318). However, physiological studies in mammals also suggest a contribution from the oculomotor nerve by way of the ciliary ganglion (122, 260). For example, electrical microstimulation of the Edinger-Westphal nucleus or the ciliary nerves has been shown to evoke increases in choroidal blood flow in the cat (e.g. 122, 260, 358) and the rabbit (e.g. 357).
Studies of the influence of electrical stimulation of the EW on choroidal blood flow have also been conducted in the pigeon using laser Doppler flowmetry (89, 90). Measurements were made transsclerally from the vascular beds beneath the superior rectus or superior oblique muscle. Figure 21 shows an example of the increase in choroidal blood flow that was evoked by electrical microstimulation (100 Hz, 400 µA) of the EW of the pigeon.
Figure 21.
EW and SCN electrical microstimulation effects on choroidal blood flow. Solid bars indicate the timing and duration of electrical stimulation. Choroidal blood flow was measured transsclerally at a site below the superior rectus muscle in an anaesthetized animal. (Scale Bar = 50 Blood Flow Units). (Redrawn from Fitzgerald et al. 1996) (90).
In addition, in pigeons, SP+ neurons in the suprachiasmatic nucleus (SCN) project to the choroidal subdivision of the Edinger-Westphal nucleus, the EWm (108). Electrical microstimulation of the SCN elicits increases in choroidal blood flow (Fig. 21) (90), which can be inhibited with lidocaine blockade of the EW (90). Thus, on the basis of anatomical, electrical stimulation, and lesion studies, this retino-SCN-EWm pathway has been shown to be a significant component of the parasympathetic control of choroidal blood flow in pigeons (306). In pigeons, this centrally-mediated, retino-SCN-EWm, reflex could play a role in preventing the deleterious effects of light on photoreceptors under normal ambient light levels by increasing choroidal blood flow in response to increases in retinal irradiance. Dysfunctions of this reflex may play a role in at least some classes of degenerative diseases of the retina. In monkeys and humans, increases in illumination of a given eye result in increased blood flow within both the stimulated eye and the contralateral eye (97, 287, 288), thus demonstrating the existence of an analogous central pathway in primates controlling choroidal blood flow in response to changes in retinal irradiance.
In mammals, including humans, and to a lesser extent in birds, the choroid is innervated by nNOS/VIP+ fibers arising from cholinergic, postganglionic neurons in the pterygopalatine ganglion (52, 69, 224, 318, 362, 364, 368, 384, 402, 406, 428). Unilateral stimulation of the facial nerve input to the pterygopalatine ganglion elicits increased choroidal blood flow in a number of mammalian species (270, 271, 359). This appears to be due to either a direct release of nitric oxide from postganglionic nerve terminals or from cholinergic/VIP release from postganglionic nerve terminals, or to the resulting stimulation of endothelial cell nitric oxide production (270), or a combination of both. The pterygopalatine ganglion receives input from preganglionic neurons in the SSN, and electrical stimulation of the SSN elicits increases in blood flow in the anterior choroid of the rat (Fig. 22) (355).
Figure 22.
Laser Doppler flux recordings from the anterior choroid, posterior choroid, and the nasal vortex veins during stimulation of either the superior salivatory nucleus (SSN) or the cervical sympathetic trunk (CST). All recordings were obtained from a single rat with the exception of the anterior choroid. Responses obtained from the vortex veins and anterior choroid during CST stimulation were similar to that shown for the posterior choroid. Bar indicates period of SSN stimulation (20Hz, 3V) or CST stimulation (12Hz, 30V). (From Steinle et al., 2000) (355)
The central inputs to the SSN that might modulate preganglionic neuron activity have been studied using a retrograde, transneuronal tracer (70, 210). These studies revealed inputs to the SSN from the paraventricular nucleus and the nucleus of the solitary tract, which was independently shown to project to the SSN using combined retrograde and anterograde techniques (2).. Both of these structures are involved in the autonomic control of blood pressure and are involved in modulating the tone of vascular smooth muscle in the cerebral vasculature (141, 258). Thus, areas involved in sensing and controlling systemic blood pressure project to the SSN, and it has been suggested that the role of this circuit is to maintain choroidal blood flow in the face of decreases in systemic blood pressure (e.g. 306). Furthermore, It has also been suggested that this pathway is involved in the light-mediated control of choroidal blood flow through projections of light-sensitive, hypothalamic neurons to the SSN (306).
Sympathetic Control of Choroidal Blood flow
In both birds and mammals, the sympathetic innervation of the choroid is derived from NPY+ fibers that arise from noradrenergic postganglionic neurons in the superior cervical ganglion (82, 139, 188, 211, 306, 364). In a variety of mammals, including primates, unilateral sympathetic nerve stimulation causes a substantial reduction in choroidal blood flow (Fig. 22) (39, 122, 133, 185, 355). This effect is mediated through alpha-adrenoreceptors (1, 7, 9, 355), likely via alpha1-adrenoreceptors (182). However, phenoxybenzamine, an alpha adrenoreceptor antagonist, only partially eliminated the response to sympathetic stimulation at higher stimulation frequencies in rabbits. This suggests that NPY, which is present in postganglionic fibers, is also involved in sympathetically-mediated vasoconstriction (133). This is consistent with the observation that intravenous NPY significantly reduces choroidal blood flow (268). It has been suggested that the role of this circuit is to maintain choroidal blood flow through vasoconstriction in the face of significant increases in systemic blood pressure, and hence prevent the over perfusion that would otherwise occur (33, 306). Such an over perfusion and consequent vascular leakiness has been reported in sympathectomize rabbits and monkeys (38, 39, 86). In humans, choroidal blood flow is maintained in the face of exercise despite significant increases in mean arterial pressure (219, 311), and it is likely that the sympathetic pathway contributes to this response.
Trigeminal Modulation of Choroidal Blood flow
Trigeminal sensory fibers containing both SP and CGRP innervate choroidal blood vessels in mammals including humans (363, 364, 366, 367, 383, 384), and appear to affect choroidal blood flow. SP and CGRP released from trigeminal nerve endings in response to noxious stimuli, temperature increases or decreases, or pressure can have a robust vasodilatory effect (176, 306). Furthermore, stimulation of the trigeminal nerve causes increased choroidal blood flow in rabbits (360). It has been proposed that this pathway may be involved in the temperature-dependent control of choroidal blood flow (306, 345).
Autonomic Control of Intraocular Pressure
Aqueous humor is continuously produced by the ciliary epithelium to provide nutrients to the avascular lens and cornea, and to maintain the intraocular pressure (IOP) of the eye. The balance of aqueous humor production and aqueous humor outflow determines IOP. In normal individuals, IOP is approximately 15 mmHg, which ensures the optimal mechanical and optical properties of the globe (101).
Aqueous humor is secreted by the ciliary epithelium and enters the posterior chamber where it then flows around the lens and iris into the anterior chamber (Fig. 23). Aqueous humor outflow occurs by way of two independent pathways. With the conventional or trabecular route, outflow occurs, via the iridocorneal angle, through the trabecular meshwork to Schlemm’s canal and eventually to the episcleral venous circulation (Fig 23). With the unconventional or uveoscleral route, outflow occurs across the iris root and through the connective tissue between the ciliary muscle bundles into the supraciliary and suprachoroidal spaces and eventually through the sclera (Fig. 23), although slight outflow may occur from the choroid (Wagner et al., 2004). The rate of aqueous humor production is determined by blood flow to the ciliary body and its rate of active secretion from the ciliary epithelium. Aqueous humor outflow is determined by resistance at the iridocorneal angle, the outflow facility of the trabecular meshwork and Schlemm’s canal for the conventional route, the uveoscleral outflow facility for the unconventional route, and by the resistance of the episcleral venous circulation (101). As discussed below, parasympathetic and sympathetic innervation influence both aqueous humor production and outflow, as well as choroidal blood flow - as previously discussed. In general, in vivo pharmacological, stimulation and lesion studies are able to selectively modulate at least one, but not all of the following: ciliary body blood flow, ciliary epithelium secretion, the outflow facility of the conventional and unconventional routes, or the resistance of the episcleral venous circulation. Therefore, in many cases, the precise pathway(s) and structure(s) underlying any observed changes in IOP are poorly understood.
Figure 23.
Diagram showing the structures involved with aqueous humor formation and outflow through the trabecular meshwork and uveoscleral routes. (Figure adapted from Loewy, 1990) (216).
Neural control of ciliary body blood flow
In mammals, aqueous humor production is independent of ciliary body blood flow unless blood flow falls to approximately 75% of baseline levels, at which point aqueous humor production decreases (35, 307). More specifically, Kiel and colleagues (184) state: “there is a dynamic relationship between ciliary blood flow and aqueous humor production, with production being blood flow independent above a critical level of perfusion, and blood flow dependent below it. The results also show that the plateau portion of the relationship shifts up or down depending on the level of secretory stimulation or inhibition.” Therefore, in the absence of secretory changes, increased blood flow to the ciliary body is unlikely to directly result in significantly increased aqueous humor production, while significant decreases in ciliary body blood flow can be expected to reduce aqueous humor production.
The ciliary body vasculature in many species, including humans, is innervated by VIP+ fibers arising from cholinergic, postganglionic neurons in the pterygopalatine ganglion (Fig. 1) (51, 347, 362, 368, 384, 402, 407). Since the majority of pterygopalatine ganglion neurons in mammals, including humans, contain nNOS, it can be assumed that most of these fibers are also NOS+ (1, 132). Facial nerve stimulation and hence activation of the preganglionic input to the pterygopalatine ganglion causes increased blood flow in the ciliary body (271). This effect can be blocked, presumably at the level of the pterygopalatine ganglion, by the nicotinic, acetylcholine receptor antagonist, hexamethonium (271). Furthermore, this increased blood flow is most likely mediated by NOS at low frequencies (2Hz) and additionally by VIP at higher frequencies (5Hz) (e.g. 269, 270). Consistent with the proposed role of NOS in this response, NOS inhibition by L-NAME in dogs, rabbits and pigs substantially (~50%) reduces ciliary body blood flow (73, 163, 342). These results appear to reflect a high parasympathetic resting tone producing a tonic vasodilation of the ciliary body vasculature through two possible mechanisms: 1) directly through neuronal NOS; 2) indirectly through endothelial NOS activated via muscarinic receptors. Based on this observation, decreased parasympathetic innervation might be expected to reduce blood flow and aqueous humor production, and hence reduce IOP. Consistent with this suggestion, Ruskell (320) observed a long-lasting reduction in IOP following removal of the pterygopalatine ganglion in monkeys. Furthermore, pterygopalatine ganglion stimulation leads to increased IOP in the monkey (271).
The ciliary body vasculature is also innervated by NPY+ fibers that arise from noradrenergic postganglionic neurons in the superior cervical ganglion (Fig. 1) in humans (368) and in rats (381). In rabbits, unilateral sympathetic nerve stimulation causes a substantial reduction in ciliary body blood flow (133). However, phenoxybenzamine, an alpha adrenoreceptor antagonist, only partially eliminated the response to such sympathetic stimulation, suggesting that NPY, which is present in postganglionic fibers, is also involved in sympathetically-mediated vasoconstriction (133). This is consistent with the observation that intravenous NPY significantly reduces ciliary body blood flow (268). Thus, increased sympathetic innervation of the ciliary body vasculature produces a pronounced vasoconstriction, a consequent reduction in aqueous humor production, and a decrease in IOP (23, 135, 408). However, low frequency stimulation of the sympathetic nerves is reported to induce an increase in IOP in rabbits, possibly because NPY is not released at this lower stimulus frequency (103).
Ciliary body blood vessels in mammals, including humans, are innervated by trigeminal sensory fibers containing both SP and CGRP (83, 347, 363–366, 383, 384, 386, 405). SP and CGRP released from trigeminal nerve endings in response to noxious stimuli, temperature increases or decreases, or pressure often have a robust vasodilatory effect (176, 306). Indeed, stimulation of the trigeminal nerve in rabbits causes increased ciliary body blood flow and significantly increased IOP (360), as does intracameral injection of CGRP in cats (36, 275, 276).
Neural Control of the Ciliary Epithelium and Aqueous Humor Secretion
The ciliary epithelium is composed of two layers of epithelial cells, pigmented and non-pigmented, arranged apex-to-apex (174). The basal surface of the pigmented epithelial cells face the ciliary stroma, while the basal surface of the non-pigmented epithelial cells face the posterior chamber. Aqueous humor is formed by diffusion, ultrafiltration, and predominantly (80–90%) through active secretion (101, 128, 248). A plasma ultrafiltrate is formed in the ciliary stroma through diffusion and ultrafiltration from the fenestrated ciliary capillaries. Aqueous humor is formed from this ultrafiltrate by active secretion across the ciliary epithelium, with current models supporting the active transport of Na+ ions from the ciliary stroma to the posterior chamber by the Na+/K+ ATPase of non-pigmented epithelial cells (101, 174). In addition, an active role for aquaporins in aqueous humor formation is also reported (128). More extensive detail on the production of aqueous humor can be found in the following reviews (101, 128, 248).
Adrenergic and muscarinic receptors are found on non-pigmented epithelial cells in the bovine (297) and primate ciliary epithelium (140). Also, carbachol, a muscarinic agonist, and phenylephrine, an α1-adrenoreceptor agonist, have been shown to reduce the coupling between pigmented and non-pigmented epithelial cells in cows and rabbits, in a dose dependent fashion, which would be expected to reduce aqueous humor production (344, 356). In addition, net activation of adenylyl cyclase in ciliary epithelium by VIP or β2-adrenoreceptors stimulates aqueous humor formation while inhibition of adenylyl cyclase by NPY, ACh muscarinic receptors, or α2-adrenoreceptors reduces aqueous humor formation (174). Thus postganglionic, sympathetic fibers can potentially stimulate aqueous humor formation via β2-adrenoreceptors and inhibit formation via α2- adrenoreceptors and NPY (101). Further, since postganglionic, parasympathetic fibers release ACh at lower frequencies and VIP at higher frequencies, parasympathetic innervation could potentially inhibit formation of aqueous humor at low frequencies and stimulate aqueous humor formation at higher frequencies.
Neural control of outflow facility
Iridocorneal angle
Aqueous humor passes via the iridocorneal angle to both the conventional, and to some extent the unconventional, outflow pathways (Figure 23). Resistance to flow at the iridocorneal angle is affected by pupil diameter, especially in those in which the iris volume does not decrease normally with pupil dilation; it is higher when the pupil is dilated and lower when constricted (304). Drugs which constrict the pupil (miotics) such as pilocarpine are used clinically to decrease resistance at the iridocorneal angle as well as to decrease resistance of outflow through the trabecular meshwork due to ciliary muscle contraction (see below) (74).
Trabecular meshwork/Schlemm’s canal
Between 50% and 75% of the aqueous humor leaves the eye through the conventional route. It is well known that contraction of the ciliary muscle through voluntary accommodation or stimulation of the third cranial nerve results in an immediate conformational change of the trabecular meshwork and possibly dilation of Schlemm’s canal leading to a decreased outflow resistance of this route (e.g. 101). In addition, morphological studies have demonstrated cholinergic, adrenergic, and trigeminal innervation of the trabecular meshwork and scleral spur region in the eyes of various mammals, including humans (237, 273, 339, 388). Studies of trabecular meshwork cells in culture and in perfused anterior eye segments have demonstrated that they are contractile, and that their contraction increases aqueous humor outflow facility in cattle and humans (76, 377, 420–422). Noradrenaline appears to increase trabecular meshwork outflow facility, but this is an active area of research, and the role of the autonomic nervous system in directly controlling trabecular meshwork resistivity is currently unclear (101).
Uveoscleral outflow
When the ciliary muscle contracts due to parasympathetic innervation, there is a reduction in uveoscleral drainage, but this reduction is more than offset by the increase in drainage through the conventional route that results from this same action (101). Increase in sympathetic innervation produces an increase in uveoscleral drainage most likely through stimulating β2-adrenoreceptors (11, 34, 398). Prostaglandin F2-alpha very effectively increases uveoscleral flow through relaxation of the ciliary muscle and structural changes in the extracellular matrix, and may interact with the autonomic innervation in ciliary muscle (101, 102).
Episcleral blood vessels
Drainage of aqueous humor by the conventional route is via the episcleral blood vessels. The episcleral vasculature possesses a specialized morphology with no capillaries and numerous arteriovenous anastomoses (340). Further, the veins are characterized by muscle-rich walls, and there is substantial innervation of both arteries and veins by parasympathetic, sympathetic, and trigeminal fibers (340). For fluid to leave the eye via the trabecular outflow pathway, it has to overcome the episcleral venous pressure (EVP) which, in humans under normal conditions, accounts for ~60% of IOP (369). EVP is decreased significantly within minutes of the application of a local anesthetic, proparacaine in rabbits, suggesting that the episcleral circulation is under tonic neural control that is either arterial (vasodilatory) or venous (vasoconstrictor) (433). It appears that either or both the parasympathetic and sympathetic nervous systems provide this tonic control. Consistent with a role for parasympathetic innervation, topical nitric oxide donors raise EVP (100, 433) and topical nitric oxide synthase inhibitors lower EVP (433) in rabbits. Further, in humans, topical treatment with the alpha2-adrenoreceptor agonist, clonidine, decreases EVP (196).
Central control of IOP
There have been few studies on the central control of intraocular pressure to date, and most of these were published over 50 years ago. There were early reports in cats that diencephalic stimulation elicits changes in intraocular pressure (127, 136, 332). However, these studies were confounded by electrical stimulation of fibers of passage in the hypothalamus and by the concurrent changes in mean arterial pressure, and thus their findings were not broadly accepted. More recently, the GABA antagonist, bicuculline, injected into the dorsomedial and perifornical hypothalamus of rats was shown to produce changes in IOP and translaminar pressure (the pressure difference between IOP and intracranial pressure) (Fig. 24) (326). The time course of these changes was dissociated in time from changes in mean arterial pressure, and the use of bicuculline would have selectively excited hypothalamic neurons in the vicinity of the injection site.
Figure 24.
Changes in the translaminar pressure gradient after site-directed microinjection of bicuculline methiodide (•; 30 pmol/75 nL; n = 9) or saline (Δ; 75 nL; n = 10) into the DMH and PeF regions. All injections at T = 0 min. * Denotes significant difference between saline and BMI treatment groups, P < 0.05. (From Samuels et al., 2012) (326).
Thus, there appears to be clear evidence for an involvement of the dorsomedial and perifornical hypothalamus in the control of IOP. However, it is not clear through which autonomic pathway it acts. It may act by way of sympathetic innervation, but the specific mechanisms involved remain unclear. In contrast, there is more empirical support for hypothalamic control via parasympathetic innervation. For example, in addition to modulating choroidal blood flow, the superior salivatory nucleus appears to control episcleral venous pressure, which would modulate IOP. Electrical stimulation of the superior salivary nucleus in rats significantly increases both EVP and IOP; although the rapidity of the latter response is probably a result of increased choroidal blood volume (369). In addition, it has been reported that stimulation of the Edinger-Westphal nucleus elicits increases in intraocular pressure in the cat (e.g. 122). However, the mechanism underlying this increase in intraocular pressure has not been investigated further, and it may be the result of either increasing extraocular muscle tone or intraocular blood flow as a result of stimulating either EWpg or EWcp.
Conclusion
As described in this article, the ocular projections of the parasympathetic and sympathetic nervous systems substantially influence numerous ocular functions. These include the generally considered functions of controlling pupil diameter and ocular accommodation. They also include the lesser considered, but possibly more vital, functions of controlling blood flow to all ocular tissues and of controlling intraocular pressure. In addition to these extrinsic autonomic control pathways, it is clear that there are local, intrinsic influences exerted by trigeminal sensory fibers in many regions of the eye. The central control of the autonomic influences over these various ocular functions is not well understood in mammals, including humans. The cortical pathways influencing pupillary responses are little understood, as are ascending neuromodulatory pathways. To date, the central pathways involved with the motor control of ocular accommodation have been partially described, but the visual input pathways and sensorimotor transformations responsible for ocular accommodation are poorly understood. Central pathways controlling ocular blood flow and intraocular pressure have not been extensively researched. Therefore, in primates, there are clear targets for future research. Research is needed into the cortical pathways influencing the pupillary responses to light, cognitive state, and arousal as well as further research into the ascending neuromodulatory pupil-related pathways to the EWpg. Research is also needed into the central pathways that control ocular accommodation. In particular, we know very little about how blur-related neural signals arise and how they are processed for ocular accommodation. It should also be clear from this review that research into the central control of both ocular blood flow and intra-ocular pressure is sorely needed. Finally, research is needed on the central autonomic and peripheral mechanisms underlying the extensive circadian modulation of all ocular functions.
Acknowledgments
This work was supported by NIH P30 EY03039 CORE grant; EyeSight Foundation of Alabama and EY007558, EY09380, and EY022290 (PDG); GM103528 (DHM)
References
- 1.Abe S, Karita K, Izumi H, Tamai M. Increased and Decreased Choroidal Blood Flow Elicited by Cervical Sympathetic Nerve Stimulation in the Cat. Jpn J Physiol. 1995;45:347–353. doi: 10.2170/jjphysiol.45.347. [DOI] [PubMed] [Google Scholar]
- 2.Agassandian K, Fazan VPS, Adanina V, Talman WT. Direct Projections from the Cardiovascular Nucleus Tractus Solitarii to Pontine Preganglionic Parasympathetic Neurons: A Link to Cerebrovascular Regulation. J Comp Neurol. 2002;452:242–254. doi: 10.1002/cne.10372. [DOI] [PubMed] [Google Scholar]
- 3.Akao T, Mustari MJ, Fukushima J, Kurkin S, Fukushima K. Discharge Characteristics of Pursuit Neurons in Mst During Vergence Eye Movements. Journal of neurophysiology. 2005;93:2415–2434. doi: 10.1152/jn.01028.2004. [DOI] [PubMed] [Google Scholar]
- 4.Akert K, Glicksman MA, Lang W, Grob P, Huber A. Edinger-Westphal Nucleus in the Monkey - Retrograde Tracer Study. Brain Research. 1980;184:491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- 5.Alessandri M, Fusco BM, Maggi CA, Fanciullacci M. In Vivo Pupillary Constrictor Effects of Substance P in Man. Life sciences. 1991;48:2301–2308. doi: 10.1016/0024-3205(91)90266-e. [DOI] [PubMed] [Google Scholar]
- 6.Alm A. The Effect of Stimulation of the Cervical Sympathetic Chain on Regional Cerebral Blood Flow in Monkeys. A Study with Radioactively Labelled Microspheres. Acta physiologica Scandinavica. 1975;93:483–489. doi: 10.1111/j.1748-1716.1975.tb05839.x. [DOI] [PubMed] [Google Scholar]
- 7.Alm A. The Effect of Sympathetic Stimulation on Blood Flow through T,E Uvea, Retina and Optic Nerve in Monkeys (Macacca Irus) Exp Eye Res. 1977;25:19–24. doi: 10.1016/0014-4835(77)90241-x. [DOI] [PubMed] [Google Scholar]
- 8.Alm A. Ocular Circulation. In: Adler FH, Hart WM, editors. Adler's Physiology of the Eye : Clinical Application. 9th. St. Louis: Mosby Year Book; 1992. pp. 198–227. [Google Scholar]
- 9.Alm A, Bill A. The Effect of Stimulation of the Cervical Sympathetic Chain on Retinal Oxygen Tension and on Uveal, Retinal and Cerebral Blood Flow in Cats. Acta Physiol Scand. 1973;88:84–94. doi: 10.1111/j.1748-1716.1973.tb05436.x. [DOI] [PubMed] [Google Scholar]
- 10.Alm A, Bill A. The Oxygen Supply to the Retina. Ii. Effects of High Intraocular Pressure and of Increased Arterial Carbon Dioxide Tension on Uveal and Retinal Blood Flow in Cats. A Study with Radioactively Labelled Microspheres Including Flow Determinations in Brain and Some Other Tissues. Acta Physiol Scand. 1972;84:306–319. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- 11.Alm A, Nilsson SF. Uveoscleral Outflow--a Review. Experimental eye research. 2009;88:760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Alm P, Uvelius B, Ekstrom J, Holmqvist B, Larsson B, Andersson KE. Nitric Oxide Synthase-Containing Neurons in Rat Parasympathetic, Sympathetic and Sensory Ganglia: A Comparative Study. Histochem J. 1995;27:819–831. [PubMed] [Google Scholar]
- 13.Alpern M, Mason GL, Jardinico RE. Vergence and Accommodation. V. Pupil Size Changes Associated with Changes in Accommodative Vergence. Am J Ophthalmol. 1961;52:762–767. [PubMed] [Google Scholar]
- 14.Andersson SE. Responses to Antidromic Trigeminal Nerve Stimulation, Substance P, Nka, Cgrp and Capsaicin in the Rat Eye. Acta physiologica Scandinavica. 1987;131:371–376. doi: 10.1111/j.1748-1716.1987.tb08252.x. [DOI] [PubMed] [Google Scholar]
- 15.Asanuma H, Kosar E, Tsukahara N, Robinson H. Modification of the Projection from the Sensory Cortex to the Motor Cortex Following the Elimination of Thalamic Projections to the Motor Cortex in Cats. Brain research. 1985;345:79–86. doi: 10.1016/0006-8993(85)90838-8. [DOI] [PubMed] [Google Scholar]
- 16.Backer WD, Ogle KN. Pupillary Response to Fusional Eye Movements. Am J Ophthalmol. 1964;58:743–756. [PubMed] [Google Scholar]
- 17.Ballas I, Hoffmann KP, Wagner HJ. Retinal Projection to the Nucleus of the Optic Tract in the Cat as Revealed by Retrograde Transport of Horseradish Peroxidase. Neuroscience letters. 1981;26:197–202. doi: 10.1016/0304-3940(81)90132-4. [DOI] [PubMed] [Google Scholar]
- 18.Bando T, Ishihara A, Tsukahara N. The Mode of Cerebellar Control of Lens Accommodation. In: Ito M, Tsukahara N, Kubota K, Yagi K, editors. Integrative Control Functions of the Brain. New York, New York: Kodansha Ltd; 1978. pp. 149–150. [Google Scholar]
- 19.Bando T, Tsukuda K, Yamamoto N, Maeda J, Tsukahara N. Physiological Identification of Midbrain Neurons Related to Lens Accommodation in Cats. J Neurophysiol. 1984;52:870–878. doi: 10.1152/jn.1984.52.5.870. [DOI] [PubMed] [Google Scholar]
- 20.Barbur JL. A Study of Pupil Response Components in Human Vision. In: Robbins JG, Djamgoz MBA, Taylor A, editors. Basic and Clinical Perspectives in Vision Research : A Celebration of the Career of Hisako Ikeda. New York: Plenum Press; 1995. pp. 3–18. [Google Scholar]
- 21.Barrionuevo PA, Nicandro N, McAnany JJ, Zele AJ, Gamlin P, Cao D. Assessing Rod, Cone, and Melanopsin Contributions to Human Pupil Flicker Responses. Investigative Ophthalmology & Visual Science. 2014;55:719–727. doi: 10.1167/iovs.13-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beckers HJ, Klooster J, Vrensen GF, Lamers WP. Substance P in Rat Corneal and Iridal Nerves: An Ultrastructural Immunohistochemical Study. Ophthalmic research. 1993;25:192–200. doi: 10.1159/000267291. [DOI] [PubMed] [Google Scholar]
- 23.Belmonte C, Bartels SP, Liu JH, Neufeld AH. Effects of Stimulation of the Ocular Sympathetic Nerves on Iop and Aqueous Humor Flow. Investigative ophthalmology & visual science. 1987;28:1649–1654. [PubMed] [Google Scholar]
- 24.Bender MB, Weinstein EA. Functional Representation in the Oculomotor and Trochlear Nuclei. Archives of Neurology & Psychiatry. 1943;49:98–106. [Google Scholar]
- 25.Benevento LA, Rezak M, Santosanderson R. Autoradiographic Study of Projections of Pretectum in Rhesus-Monkey (Macaca-Mulatta) - Evidence for Sensorimotor Links to Thalamus and Oculomotor Nuclei. Brain Research. 1977;127:197–218. doi: 10.1016/0006-8993(77)90536-4. [DOI] [PubMed] [Google Scholar]
- 26.Bergua A, Junemann A, Naumann GO. [Nadph-D Reactive Choroid Ganglion Cells in the Human] Klin Monbl Augenheilkd. 1993;203:77–82. doi: 10.1055/s-2008-1045651. [DOI] [PubMed] [Google Scholar]
- 27.Bergua A, Kapsreiter M, Neuhuber WL, Reitsamer HA, Schrodl F. Innervation Pattern of the Preocular Human Central Retinal Artery. Experimental eye research. 2013;110:142–147. doi: 10.1016/j.exer.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Bergua A, Schrodl F, Neuhuber WL. Vasoactive Intestinal and Calcitonin Gene-Related Peptides, Tyrosine Hydroxylase and Nitrergic Markers in the Innervation of the Rat Central Retinal Artery. Exp Eye Res. 2003;77:367–374. doi: 10.1016/s0014-4835(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 29.Berman N. Connections of Pretectum in Cat. Journal of Comparative Neurology. 1977;174:227–254. doi: 10.1002/cne.901740204. [DOI] [PubMed] [Google Scholar]
- 30.Bernheimer S. Weitere Experimentelle Studien Zur Kenntnis Der Lage Des Sphinkter-Und Levatorkerns. Graefes Arhiv für Ophthalmologie. 1909;70:539–562. [Google Scholar]
- 31.Berson DM. Strange Vision: Ganglion Cells as Circadian Photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 32.Berson DM, Dunn FA, Takao M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 33.Bill A. The Circulation in the Eye. In: Renkin E, Michel C, editors. Handbook of Physiology: The Cardiovascular System Iv: Microcirculation Part 2. Baltimore, Md.: Waverly Press; 1984. pp. 1001–1035. [Google Scholar]
- 34.Bill A. Early Effects of Epinephrine on Aqueous Humor Dynamics in Vervet Monkeys (Cercopithecus Ethiops) Experimental eye research. 1969;8:35–43. doi: 10.1016/s0014-4835(69)80078-3. [DOI] [PubMed] [Google Scholar]
- 35.Bill A. The Effect of Changes in Arterial Blood Pressure on the Rate of Aqueous Humour Formation in a Primate (Cercopithecus Ethiops) Ophthalmic Research. 1970;1:193–200. [Google Scholar]
- 36.Bill A. Effects of Some Neuropeptides on the Uvea. Experimental eye research. 1991;53:3–11. doi: 10.1016/0014-4835(91)90138-5. [DOI] [PubMed] [Google Scholar]
- 37.Bill A. Some Aspects of the Ocular Circulation. Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1985;26:410–424. [PubMed] [Google Scholar]
- 38.Bill A, Linder J. Sympathetic Control of Cerebral Blood Flow in Acute Arterial Hypertension. Acta Physiol Scand. 1976;96:114–121. doi: 10.1111/j.1748-1716.1976.tb10176.x. [DOI] [PubMed] [Google Scholar]
- 39.Bill A, Nilsson SF. Control of Ocular Blood Flow. J Cardiovasc Pharmacol. 1985;7(Suppl 3):96–9102. doi: 10.1097/00005344-198500073-00011. [DOI] [PubMed] [Google Scholar]
- 40.Bill A, Sperber G, Ujiie K. Physiology of the Choroidal Vascular Bed. International ophthalmology. 1983;6:101–107. doi: 10.1007/BF00127638. [DOI] [PubMed] [Google Scholar]
- 41.Bill A, Sperber GO. Control of Retinal and Choroidal Blood Flow. Eye (Lond) 1990;4(Pt 2):319–325. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- 42.Bjorklund H, Hokfelt T, Goldstein M, Terenius L, Olson L. Appearance of the Noradrenergic Markers Tyrosine Hydroxylase and Neuropeptide Y in Cholinergic Nerves of the Iris Following Sympathectomy. J Neurosci. 1985;5:1633–1640. doi: 10.1523/JNEUROSCI.05-06-01633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boehm S, Kubista H. Fine Tuning of Sympathetic Transmitter Release Via Ionotropic and Metabotropic Presynaptic Receptors. Pharmacological Reviews. 2002;54:43–99. doi: 10.1124/pr.54.1.43. [DOI] [PubMed] [Google Scholar]
- 44.Bognar IT, Pallas S, Fuder H, Muscholl E. Muscarinic Inhibition of [3h]-Noradrenaline Release on Rabbit Iris in Vitro: Effects of Stimulation Conditions on Intrinsic Activity of Methacholine and Pilocarpine. British journal of pharmacology. 1988;94:890–900. doi: 10.1111/j.1476-5381.1988.tb11601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bron AJ, Tripathi RC, Tripathi BJ, Wolff E. Wolff's Anatomy of the Eye and Orbit. London; New York: Chapman & Hall Medical; 1997. [Google Scholar]
- 46.Bruun A, Ehinger B, Sundler F, Tornqvist K, Uddman R. Neuropeptide Y Immunoreactive Neurons in the Guinea-Pig Uvea and Retina. Investigative ophthalmology & visual science. 1984;25:1113–1123. [PubMed] [Google Scholar]
- 47.Burde RM, Loewy AD. Central Origin of Oculomotor Parasympathetic Neurons in the Monkey. Brain Research. 1980;198:434–439. doi: 10.1016/0006-8993(80)90757-x. [DOI] [PubMed] [Google Scholar]
- 48.Burde RM, Parelman JJ, Luskin M. Lack of Unity of Edinger-Westphal Nucleus Projections to the Ciliary Ganglion and Spinal Cord: A Double-Labeling Approach. Brain Res. 1982;249:379–382. doi: 10.1016/0006-8993(82)90072-5. [DOI] [PubMed] [Google Scholar]
- 49.Burde RM, Williams F. Parasympathetic Nuclei. Brain Research. 1989;498:371–375. doi: 10.1016/0006-8993(89)91119-0. [DOI] [PubMed] [Google Scholar]
- 50.Busettini C, Davison RC, Gamlin PDR. The near Triad: Vergence, Accommodation, and Pupilloconstriction. In: Squire L, editor. New Encyclopedia of Neuroscience. Oxford: Elsevier; 2007. [Google Scholar]
- 51.Butler JM, Ruskell GL, Cole DF, Unger WG, Zhang SQ, Blank MA, McGregor GP, Bloom SR. Effects of Viith (Facial) Nerve Degeneration on Vasoactive Intestinal Polypeptide and Substance-P Levels in Ocular and Orbital Tissues of the Rabbit. Experimental Eye Research. 1984;39:523–532. doi: 10.1016/0014-4835(84)90052-6. [DOI] [PubMed] [Google Scholar]
- 52.Butler JM, Ruskell GL, Cole DF, Unger WG, Zhang SQ, Blank MA, McGregor GP, Bloom SR. Effects of Viith (Facial) Nerve Degeneration on Vasoactive Intestinal Polypeptide and Substance P Levels in Ocular and Orbital Tissues of the Rabbit. Exp Eye Res. 1984;39:523–532. doi: 10.1016/0014-4835(84)90052-6. [DOI] [PubMed] [Google Scholar]
- 53.Buttner-Ennever JA, Cohen B, Horn AK, Reisine H. Pretectal Projections to the Oculomotor Complex of the Monkey and Their Role in Eye Movements. J Comp Neurol. 1996;366:348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Campbell FW, Green DG. Optical and Retinal Factors Affecting Visual Resolution. J Physiol. 1965;181:576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell FW, Gregory AH. Effect of Size of Pupil on Visual Acuity. Nature. 1960;187:1121–1123. doi: 10.1038/1871121c0. [DOI] [PubMed] [Google Scholar]
- 56.Campbell FW, Gubisch RW. Optical Quality of the Human Eye. J Physiol. 1966;186:558–578. doi: 10.1113/jphysiol.1966.sp008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carpenter MB, Peter P. Accessory Oculomotor Nuclei in the Monkey. J Hirnforsch. 1970;12:405–418. [PubMed] [Google Scholar]
- 58.Chamot SR, Movaffaghy A, Petrig BL, Riva CE. Iris Blood Flow Response to Acute Decreases in Ocular Perfusion Pressure: A Laser Doppler Flowmetry Study in Humans. Exp Eye Res. 2000;70:107–112. doi: 10.1006/exer.1999.0759. [DOI] [PubMed] [Google Scholar]
- 59.Charman WN. Optics of the Eye. In: Bass M, editor. Handbook of Optics. New York: McGraw-Hill; 1995. pp. 24.23–24.54. [Google Scholar]
- 60.Chin NB, Ishikawa S, Lappin H, Davidowitz J, Breinin GM. Accommodation in Monkeys Induced by Midbrain Stimulation. Invest Ophthalmol. 1968;7:386–396. [PubMed] [Google Scholar]
- 61.Clarke RJ, Coimbra CJP, Alessio ML. Distribution of Parasympathetic Motoneurones in the Oculomotor Complex Innervating the Ciliary Ganglion in the Marmoset (Callithrix-Jacchus) Acta Anatomica. 1985;121:53–58. doi: 10.1159/000145942. [DOI] [PubMed] [Google Scholar]
- 62.Clarke RJ, Ikeda H. Luminance and Darkness Detectors in the Olivary and Posterior Pretectal Nuclei and Their Relationship to the Pupillary Light Reflex in the Rat. I. Studies with Steady Luminance Levels. Exp Brain Res. 1985;57:224–232. doi: 10.1007/BF00236527. [DOI] [PubMed] [Google Scholar]
- 63.Clarke RJ, Zhang H, Gamlin PD. Characteristics of the Pupillary Light Reflex in the Alert Rhesus Monkey. J Neurophysiol. 2003;89:3179–3189. doi: 10.1152/jn.01131.2002. [DOI] [PubMed] [Google Scholar]
- 64.Clarke RJ, Zhang H, Gamlin PD. Primate Pupillary Light Reflex: Receptive Field Characteristics of Pretectal Luminance Neurons. Journal of neurophysiology. 2003;89:3168–3178. doi: 10.1152/jn.01130.2002. [DOI] [PubMed] [Google Scholar]
- 65.Collier RH. Experimental Embolic Ischemia of the Choroid. Arch Ophthalmol. 1967;77:683–692. doi: 10.1001/archopht.1967.00980020685025. [DOI] [PubMed] [Google Scholar]
- 66.Contreras RJ, Gomez MM, Norgren R. Central Origins of Cranial Nerve Parasympathetic Neurons in the Rat. J Comp Neurol. 1980;190:373–394. doi: 10.1002/cne.901900211. [DOI] [PubMed] [Google Scholar]
- 67.Crawford K, Terasawa E, Kaufman PL. Reproducible Stimulation of Ciliary Muscle-Contraction in the Cynomolgus Monkey Via a Permanent Indwelling Midbrain Electrode. Brain Research. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- 68.Crawford K, Terasawa E, Kaufman PL. Reproducible Stimulation of Ciliary Muscle Contraction in the Cynomolgus Monkey Via a Permanent Indwelling Midbrain Electrode. Brain research. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- 69.Cuthbertson S, Jackson B, Toledo C, Fitzgerald ME, Shih YF, Zagvazdin Y, Reiner A. Innervation of Orbital and Choroidal Blood Vessels by the Pterygopalatine Ganglion in Pigeons. J Comp Neurol. 1997;386:422–442. [PubMed] [Google Scholar]
- 70.Cuthbertson S, LeDoux MS, Jones S, Jones J, Zhou Q, Gong S, Ryan P, Reiner A. Localization of Preganglionic Neurons That Innervate Choroidal Neurons of Pterygopalatine Ganglion. Invest Ophthalmol Vis Sci. 2003;44:3713–3724. doi: 10.1167/iovs.02-1207. [DOI] [PubMed] [Google Scholar]
- 71.Cuthbertson S, White J, Fitzgerald ME, Shih YF, Reiner A. Distribution within the Choroid of Cholinergic Nerve Fibers from the Ciliary Ganglion in Pigeons. Vision Res. 1996;36:775–786. doi: 10.1016/0042-6989(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 72.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-Expressing Ganglion Cells in Primate Retina Signal Colour and Irradiance and Project to the Lgn. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 73.Deussen A, Sonntag M, Vogel R. L-Arginine-Derived Nitric Oxide: A Major Determinant of Uveal Blood Flow. Exp Eye Res. 1993;57:129–134. doi: 10.1006/exer.1993.1107. [DOI] [PubMed] [Google Scholar]
- 74.Diestelhorst M, Nordmann JP, Toris CB. Combined Therapy of Pilocarpine or Latanoprost with Timolol Versus Latanoprost Monotherapy. Survey of ophthalmology. 2002;47(Suppl 1):S155–S161. doi: 10.1016/s0039-6257(02)00329-6. [DOI] [PubMed] [Google Scholar]
- 75.Dineen JT, Hendrickson A. Overlap of Retinal and Prestriate Cortical Pathways in the Primate Pretectum. Brain research. 1983;278:250–254. doi: 10.1016/0006-8993(83)90247-0. [DOI] [PubMed] [Google Scholar]
- 76.Dismuke WM, Liang J, Overby DR, Stamer WD. Concentration-Related Effects of Nitric Oxide and Endothelin-1 on Human Trabecular Meshwork Cell Contractility. Exp Eye Res. 2013;120C:28–35. doi: 10.1016/j.exer.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Distler C, Hoffmann KP. The Pupillary Light Reflex in Normal and Innate Microstrabismic Cats, Ii: Retinal and Cortical Input to the Nucleus Praetectalis Olivaris. Visual neuroscience. 1989;3:139–153. doi: 10.1017/s0952523800004454. [DOI] [PubMed] [Google Scholar]
- 78.Edinger L. Uber U Den Verlauf Der Centralen Hirnnervenbahnen Mit Demonstrationen Von Praparaten. Arch Psychiat Nervenkr. 1885;16:858–859. [Google Scholar]
- 79.Eglen RM, Hegde SS, Watson N. Muscarinic Receptor Subtypes and Smooth Muscle Function. Pharmacological Reviews. 1996;48:531–565. [PubMed] [Google Scholar]
- 80.Ehinger B. Adrenergic Nerves to the Eye and to Related Structures in Man and in the Cynomolgus Monkey (Macaca Irus) Investigative ophthalmology & visual science. 1966;5:42–52. [Google Scholar]
- 81.Ehinger B. A Comparative Study of the Adrenergic Nerves to the Anterior Eye Segment of Some Primates. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1971;116:157–177. doi: 10.1007/BF00331259. [DOI] [PubMed] [Google Scholar]
- 82.Ehinger B. Distribution of Adrenergic Nerves in Eye and Some Related Structures in Cat. Acta Physiologica Scandinavica. 1966;66 doi: 10.1111/j.1748-1716.1966.tb03176.x. 123-&. [DOI] [PubMed] [Google Scholar]
- 83.Ehinger B, Sundler F, Tervo K, Tervo T, Tornqvist K. Substance P Fibres in the Anterior Segment of the Rabbit Eye. Acta Physiol Scand. 1983;118:215–218. doi: 10.1111/j.1748-1716.1983.tb07265.x. [DOI] [PubMed] [Google Scholar]
- 84.Elsas T, Edvinsson L, Sundler F, Uddman R. Neuronal Pathways to the Rat Conjunctiva Revealed by Retrograde Tracing and Immunocytochemistry. Exp Eye Res. 1994;58:117–126. doi: 10.1006/exer.1994.1201. [DOI] [PubMed] [Google Scholar]
- 85.Elsas T, Uddman R, Sundler F. Pituitary Adenylate Cyclase-Activating Peptide-Immunoreactive Nerve Fibers in the Cat Eye. Graefes Archive for Clinical and Experimental Ophthalmology. 1996;234:573–580. doi: 10.1007/BF00448802. [DOI] [PubMed] [Google Scholar]
- 86.Ernest JT. The Effect of Systolic Hypertension on Rhesus Monkey Eyes after Ocular Sympathectomy. Am J Ophthalmol. 1977;84:341–344. doi: 10.1016/0002-9394(77)90676-6. [DOI] [PubMed] [Google Scholar]
- 87.Falsini B, Riva CE, Logean E. Flicker-Evoked Changes in Human Optic Nerve Blood Flow: Relationship with Retinal Neural Activity. Invest Ophthalmol Vis Sci. 2002;43:2309–2316. [PubMed] [Google Scholar]
- 88.Feigl B, Zele AJ, Fader SM, Howes AN, Hughes CE, Jones KA, Jones R. The Post-Illumination Pupil Response of Melanopsin-Expressing Intrinsically Photosensitive Retinal Ganglion Cells in Diabetes. Acta Ophthalmol (Copenh) 2012;90:e230–e234. doi: 10.1111/j.1755-3768.2011.02226.x. [DOI] [PubMed] [Google Scholar]
- 89.Fitzgerald ME, Vana BA, Reiner A. Control of Choroidal Blood Flow by the Nucleus of Edinger-Westphal in Pigeons: A Laser Doppler Study. Invest Ophthalmol Vis Sci. 1990;31:2483–2492. [PubMed] [Google Scholar]
- 90.Fitzgerald MEC, Gamlin PDR, Zagvazdin Y, Reiner A. Central Neural Circuits for the Light-Mediated Reflexive Control of Choroidal Blood Flow in the Pigeon Eye: A Laser Doppler Study. Visual Neuroscience. 1996;13:655–669. doi: 10.1017/s0952523800008555. [DOI] [PubMed] [Google Scholar]
- 91.Flugel-Koch C, Kaufman P, Lutjen-Drecoll E. Association of a Choroidal Ganglion Cell Plexus with the Fovea Centralis. Invest Ophthalmol Vis Sci. 1994;35:4268–4272. [PubMed] [Google Scholar]
- 92.Flugel C, Tamm ER, Mayer B, Lutjen-Drecoll E. Species Differences in Choroidal Vasodilative Innervation: Evidence for Specific Intrinsic Nitrergic and Vip-Positive Neurons in the Human Eye. Invest Ophthalmol Vis Sci. 1994;35:592–599. [PubMed] [Google Scholar]
- 93.Fry GA. The Optical Performance of the Human Eye. PROG OPTICS. 1970;8:53–131. [Google Scholar]
- 94.Fry GA. The Relation of Pupil Size to Accommodation and Convergence. Am J Optom & Arch Am Acad Optom. 1945;22:451–465. [Google Scholar]
- 95.Fu Y, Liao HW, Do MTH, Yau KW. Non-Image-Forming Ocular Photoreception in Vertebrates. Curr Opin Neurobiol. 2005;15:415–422. doi: 10.1016/j.conb.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Y, Zhong H, Wang MH, Luo DG, Liao HW, Maeda H, Hattar S, Frishman LJ, Yau KW. Intrinsically Photosensitive Retinal Ganglion Cells Detect Light with a Vitamin a-Based Photopigment, Melanopsin. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fuchsjager-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral Light-Dark Transitions Affect Choroidal Blood Flow in Both Eyes. Vision Res. 2001;41:2919–2924. doi: 10.1016/s0042-6989(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 98.Fuder H, Schopf J, Unckell J, Wesner MT, Melchiorre C, Tacke R, Mutschler E, Lambrecht G. Different Muscarine Receptors Mediate the Prejunctional Inhibition of [3h]-Noradrenaline Release in Rat or Guinea-Pig Iris and the Contraction of the Rabbit Iris Sphincter Muscle. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:597–604. doi: 10.1007/BF00717733. [DOI] [PubMed] [Google Scholar]
- 99.Fuller JH, Schlag JD. Determination of Antidromic Excitation by the Collision Test: Problems of Interpretation. Brain Res. 1976;112:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- 100.Funk RH, Gehr J, Rohen JW. Short-Term Hemodynamic Changes in Episcleral Arteriovenous Anastomoses Correlate with Venous Pressure and Iop Changes in the Albino Rabbit. Curr Eye Res. 1996;15:87–93. doi: 10.3109/02713689609017615. [DOI] [PubMed] [Google Scholar]
- 101.Gabelt B, Kaufman PL. Production and Flow of Aqueous Humor. In: Levin LA, Kaufman PL, editors. Adler's Physiology of the Eye : Clinical Application. 11th. New York: Elsevier; 2011. [Google Scholar]
- 102.Gabelt BT, Kaufman PL. Prostaglandin F2 Alpha Increases Uveoscleral Outflow in the Cynomolgus Monkey. Exp Eye Res. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 103.Gallar J, Liu JH. Stimulation of the Cervical Sympathetic Nerves Increases Intraocular Pressure. Investigative ophthalmology & visual science. 1993;34:596–605. [PubMed] [Google Scholar]
- 104.Gamlin PD. Functions of the Edinger-Westphal Nucleus. In: Burnstock G, Sillito AM, editors. Nervous Control of the Eye. Amsterdam: Harwood Academic; 2000. pp. 117–154. [Google Scholar]
- 105.Gamlin PD. The Pretectum: Connections and Oculomotor-Related Roles. Progress in brain research. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- 106.Gamlin PD, Clarke RJ. The Pupillary Light Reflex Pathway of the Primate. Journal of the American Optometric Association. 1995;66:415–418. [PubMed] [Google Scholar]
- 107.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and Macaque Pupil Responses Driven by Melanopsin-Containing Retinal Ganglion Cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gamlin PD, Reiner A, Karten HJ. Substance P-Containing Neurons of the Avian Suprachiasmatic Nucleus Project Directly to the Nucleus of Edinger-Westphal. Proc Natl Acad Sci U S A. 1982;79:3891–3895. doi: 10.1073/pnas.79.12.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gamlin PD, Yoon K. An Area for Vergence Eye Movement in Primate Frontal Cortex. Nature. 2000;407:1003–1007. doi: 10.1038/35039506. [DOI] [PubMed] [Google Scholar]
- 110.Gamlin PD, Yoon K, Zhang H. The Role of Cerebro-Ponto-Cerebellar Pathways in the Control of Vergence Eye Movements. Eye. 1996;10(Pt 2):167–171. doi: 10.1038/eye.1996.42. [DOI] [PubMed] [Google Scholar]
- 111.Gamlin PD, Zhang H, Clarke RJ. Luminance Neurons in the Pretectal Olivary Nucleus Mediate the Pupillary Light Reflex in the Rhesus Monkey. Exp Brain Res. 1995;106:169–176. doi: 10.1007/BF00241367. [DOI] [PubMed] [Google Scholar]
- 112.Gamlin PD, Zhang H, Harlow A, Barbur JL. Pupil Responses to Stimulus Color, Structure and Light Flux Increments in the Rhesus Monkey. Vision Res. 1998;38:3353–3358. doi: 10.1016/s0042-6989(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 113.Gamlin PDR. Neural Mechanisms for the Control of Vergence Eye Movements. ANN NY ACAD SCI. 2002;956:264–272. doi: 10.1111/j.1749-6632.2002.tb02825.x. [DOI] [PubMed] [Google Scholar]
- 114.Gamlin PDR. Subcortical Neural Circuits for Ocular Accommodation and Vergence in Primates. Ophthalmic and Physiological Optics. 1999;19:81–89. doi: 10.1046/j.1475-1313.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 115.Gamlin PDR, Clarke RJ. Single-Unit Activity in the Primate Nucleus-Reticularis Tegmenti Pontis Related to Vergence and Ocular Accommodation. Journal of Neurophysiology. 1995;73:2115–2119. doi: 10.1152/jn.1995.73.5.2115. [DOI] [PubMed] [Google Scholar]
- 116.Gamlin PDR, Reiner A. The Edinger-Westphal Nucleus - Sources of Input Influencing Accommodation, Pupilloconstriction, and Choroidal Blood-Flow. Journal of Comparative Neurology. 1991;306:425–438. doi: 10.1002/cne.903060307. [DOI] [PubMed] [Google Scholar]
- 117.Gamlin PDR, Reiner A, Erichsen JT, Karten HJ, Cohen DH. The Neural Substrate for the Pupillary Light Reflex in the Pigeon (Columba-Livia) Journal of Comparative Neurology. 1984;226:523–543. doi: 10.1002/cne.902260407. [DOI] [PubMed] [Google Scholar]
- 118.Gamlin PDR, Zhang YH, Clendaniel RA, Mays LE. Behavior of Identified Edinger-Westphal Neurons During Ocular Accommodation. Journal of Neurophysiology. 1994;72:2368–2382. doi: 10.1152/jn.1994.72.5.2368. [DOI] [PubMed] [Google Scholar]
- 119.Gaudric A, Coscas G, Bird AC. Choroidal Ischemia. Am J Ophthalmol. 1982;94:489–498. doi: 10.1016/0002-9394(82)90242-2. [DOI] [PubMed] [Google Scholar]
- 120.Gay AJ, Goldor H, Smith M. Chorioretinal Vascular Occlusions with Latex Spheres. Invest Ophthalmol. 1964;3:647–656. [PubMed] [Google Scholar]
- 121.Geijer C, Bill A. Effects of Raised Intraocular Pressure on Retinal, Prelaminar, Laminar, and Retrolaminar Optic Nerve Blood Flow in Monkeys. Invest Ophthalmol Vis Sci. 1979;18:1030–1042. [PubMed] [Google Scholar]
- 122.Gherezghiher T, Hey JA, Koss MC. Parasympathetic Nervous Control of Intraocular Pressure. Exp Eye Res. 1990;50:457–462. doi: 10.1016/0014-4835(90)90032-p. [DOI] [PubMed] [Google Scholar]
- 123.Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-Localization of Calcitonin Gene-Related Peptide-Like Immunoreactivity with Substance P in Cutaneous, Vascular and Visceral Sensory Neurons of Guinea Pigs. Neuroscience letters. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- 124.Gibbins IL, Morris JL. Co-Existence of Neuropeptides in Sympathetic, Cranial Autonomic and Sensory Neurons Innervating the Iris of the Guinea-Pig. Journal of the Autonomic Nervous System. 1987;21:67–82. doi: 10.1016/0165-1838(87)90093-2. [DOI] [PubMed] [Google Scholar]
- 125.Gil DW, Krauss HA, Bogardus AM, WoldeMussie E. Muscarinic Receptor Subtypes in Human Iris-Ciliary Body Measured by Immunoprecipitation. IOVS. 1997;38:1434–1442. [PubMed] [Google Scholar]
- 126.Gilmartin B. A Review of the Role of Sympathetic Innervation of the Ciliary Muscle in Ocular Accommodation. Ophthalmic Physiol Opt. 1986;6:23–37. [PubMed] [Google Scholar]
- 127.Gloster J, Greaves DP. Effect of Diencephalic Stimulation Upon Intra-Ocular Pressure. Br J Ophthalmol. 1957;41:513–532. doi: 10.1136/bjo.41.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous Humor Dynamics: A Review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gooley JJ, Ho Mien I, St Hilaire MA, Yeo SC, Chua EC, van Reen E, Hanley CJ, Hull JT, Czeisler CA, Lockley SW. Melanopsin and Rod-Cone Photoreceptors Play Different Roles in Mediating Pupillary Light Responses During Exposure to Continuous Light in Humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:14242–14253. doi: 10.1523/JNEUROSCI.1321-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in Cells of Origin of the Retinohypothalamic Tract. Nature Neuroscience. 2001;4:1165–1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 131.Gooley JJ, Lu J, Fischer D, Saper CB. Broad Role for Melanopsin in Nonvisual Photoreception. Journal of Neuroscience. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gottanka J, Kirch W, Tamm ER. The Origin of Extrinsic Nitrergic Axons Supplying the Human Eye. J Anat. 2005;206:225–229. doi: 10.1111/j.1469-7580.2005.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Granstam E, Nilsson SF. Non-Adrenergic Sympathetic Vasoconstriction in the Eye and Some Other Facial Tissues in the Rabbit. European journal of pharmacology. 1990;175:175–186. doi: 10.1016/0014-2999(90)90228-x. [DOI] [PubMed] [Google Scholar]
- 134.Graybiel AM, Hartwieg EA. Some Afferent Connections of the Oculomotor Complex in the Cat: An Experimental Study with Tracer Techniques. Brain Res. 1974;81:543–551. doi: 10.1016/0006-8993(74)90850-6. [DOI] [PubMed] [Google Scholar]
- 135.Greaves DP, Perkins ES. Influence of the Sympathetic Nervous System on the Intra-Ocular Pressure and Vascular Circulation of the Eye. The British journal of ophthalmology. 1952;36:258–264. doi: 10.1136/bjo.36.5.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grimes PA, Macri FJ, Von Sallmann L, Wanko T. Some Mechanisms of Centrally Induced Eye Pressure Responses. Am J Ophthalmol. 1956;42:130–147. doi: 10.1016/0002-9394(56)90364-6. [DOI] [PubMed] [Google Scholar]
- 137.Grkovic I, Edwards SL, Murphy SM, Anderson CR. Chemically Distinct Preganglionic Inputs to Iris-Projecting Postganglionic Neurons in the Rat: A Light and Electron Microscopic Study. The Journal of comparative neurology. 1999;412:606–616. [PubMed] [Google Scholar]
- 138.Gu Q. Neuromodulatory Transmitter Systems in the Cortex and Their Role in Cortical Plasticity. NEUROSCI. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 139.Guglielmone R, Cantino D. Autonomic Innervation of the Ocular Choroid Membrane in the Chicken: A Fluorescence-Histochemical and Electron-Microscopic Study. Cell Tissue Res. 1982;222:417–431. doi: 10.1007/BF00213222. [DOI] [PubMed] [Google Scholar]
- 140.Gupta N, McAllister R, Drance SM, Rootman J, Cynader MS. Muscarinic Receptor M1 and M2 Subtypes in the Human Eye: Qnb, Pirenzipine, Oxotremorine, and Afdx-116 in Vitro Autoradiography. British Journal of Ophthalmology. 1994;78:555–559. doi: 10.1136/bjo.78.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guyenet PG. The Sympathetic Control of Blood Pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 142.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-Containing Retinal. Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and Rod-Cone Photoreceptive Systems Account for All Major Accessory Visual Functions in Mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Helmholtz Hv. Handbuch Der Physiologischen Optik. Leipzig: Voss; 1867. [Google Scholar]
- 145.Heywood CA, Nicholas JJ, LeMare C, Cowey A. The Effect of Lesions to Cortical Areas V4 or Ait on Pupillary Responses to Chromatic and Achromatic Stimuli in Monkeys. Exp Brain Res. 1998;122:475–480. doi: 10.1007/s002210050536. [DOI] [PubMed] [Google Scholar]
- 146.Hirai R, Tamamaki N, Hukami K, Nojyo Y. Ultrastructural Analysis of Tyrosine Hydroxylase-, Substance P-, and Calcitonin Gene-Related Peptide-Immunoreactive Nerve Fibers in the Rat Iris. Ophthalmic Res. 1994;26:169–180. doi: 10.1159/000267409. [DOI] [PubMed] [Google Scholar]
- 147.Hiraoka M, Shimamura M. The Midbrain Reticular-Formation as an Integration Center for the near Reflex in the Cat. Neuroscience Research. 1989;7:1–12. doi: 10.1016/0168-0102(89)90032-1. [DOI] [PubMed] [Google Scholar]
- 148.Hirata Y, Yamaji K, Sakai H, Usui S. Function of the Pupil in Vision and Information Capacity of Retinal Image. SYST COMPUT JPN. 2003;34:48–57. [Google Scholar]
- 149.Hockman CH. Essentials of Autonomic Function :The Autonomic Nervous System : Fundamental Concepts from Anatomy, Physiology, Pharmacology, and Neuroscience for Students and Professionals in the Health Sciences. Springfield, Ill., USA: Thomas; 1987. [Google Scholar]
- 150.Holzer P. Local Effector Functions of Capsaicin-Sensitive Sensory Nerve-Endings - Involvement of Tachykinins, Calcitonin Gene-Related Peptide and Other Neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 151.Hood D, Finkelstein M. Sensitivity to Light. In: Boff KR, Kaufman L, Thomas JP, editors. Handbook of Perception and Human Performance. New York: Wiley; 1986. pp. 5/1–5/66. [Google Scholar]
- 152.Horn AK, Eberhorn A, Haertig W, Ardeleanu P, Messoudi A, Buettner-Ennever JA. Perioculomotor Cell Groups in Monkey and Man Defined by Their Histochemical and Functional Properties: Reappraisal of the Edinger-Westphal Nucleus. Journal of Comparative Neurology. 2008;507:1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- 153.Hosoba M, Bando T, Tsukahara N. Cerebellar Control of Accommodation of Eye in Cat. Brain Research. 1978;153:495–505. doi: 10.1016/0006-8993(78)90334-7. [DOI] [PubMed] [Google Scholar]
- 154.Hultborn H, Mori K, Tsukahara N. Cerebellar Influence on Parasympathetic Neurones Innervating Intra-Ocular Muscles. Brain Res. 1978;159:269–278. doi: 10.1016/0006-8993(78)90534-6. [DOI] [PubMed] [Google Scholar]
- 155.Hultborn H, Mori K, Tsukahara N. The Neuronal Pathway Subserving the Pupillary Light Reflex and Its Facilitation from Cerebellar Nuclei. Brain Res. 1973;63:357–361. doi: 10.1016/0006-8993(73)90104-2. [DOI] [PubMed] [Google Scholar]
- 156.Hung GK, Semmlow JL. Static Behavior of Accommodation and Vergence: Computer Simulation of an Interactive Dual-Feedback System. IEEE T BIO-MED ENG. 1980;27:439–447. doi: 10.1109/TBME.1980.326752. [DOI] [PubMed] [Google Scholar]
- 157.Hutchins B. Evidence for a Direct Retinal Projection to the Anterior Pretectal Nucleus in the Cat. Brain research. 1991;561:169–173. doi: 10.1016/0006-8993(91)90764-m. [DOI] [PubMed] [Google Scholar]
- 158.Hutchins B, Weber JT. The Pretectal Complex of the Monkey: A Reinvestigation of the Morphology and Retinal Terminations. The Journal of comparative neurology. 1985;232:425–442. doi: 10.1002/cne.902320402. [DOI] [PubMed] [Google Scholar]
- 159.Ijichi Y, Kiyohara T, Hosoba M, Tsukahara N. The Cerebellar Control of the Pupillary Light Reflex in the Cat. Brain Res. 1977;128:69–79. doi: 10.1016/0006-8993(77)90236-0. [DOI] [PubMed] [Google Scholar]
- 160.Inoue T. The Response of Rabbit Ciliary Nerve to Luminance Intensity. Brain Res. 1980;201:206–209. doi: 10.1016/0006-8993(80)90787-8. [DOI] [PubMed] [Google Scholar]
- 161.Ishikawa H, Miller DD, Patil PN. Comparison of Post-Junctional Alpha-Adrenoceptors in Iris Dilator Muscle of Humans, and Albino and Pigmented Rabbits. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:765–772. doi: 10.1007/BF00166903. [DOI] [PubMed] [Google Scholar]
- 162.Ishikawa S, Sekiya H, Kondo Y. The Center for Controlling the near Reflex in the Midbrain of the Monkey - a Double Labeling Study. Brain Research. 1990;519:217–222. doi: 10.1016/0006-8993(90)90080-u. [DOI] [PubMed] [Google Scholar]
- 163.Jacot JL, O'Neill JT, Scandling DM, West SD, McKenzie JE. Nitric Oxide Modulation of Retinal, Choroidal, and Anterior Uveal Blood Flow in Newborn Piglets. J Ocul Pharmacol Ther. 1998;14:473–489. doi: 10.1089/jop.1998.14.473. [DOI] [PubMed] [Google Scholar]
- 164.Jaeger RJ, Benevento LA. A Horseradish-Peroxidase Study of the Innervation of the Internal Structures of the Eye - Evidence for a Direct Pathway. Investigative Ophthalmology & Visual Science. 1980;19:575–583. [PubMed] [Google Scholar]
- 165.Jampel RS, Mindel J. The Nucleus for Accommodation in the Midbrain of the Macaque. Invest Ophthalmol. 1967;6:40–50. [PubMed] [Google Scholar]
- 166.Jampel RS, Mindel J. The Nucleus for Accommodation in the Midbrain of the Macaque. Investigative ophthalmology. 1967;6:40–50. [PubMed] [Google Scholar]
- 167.Jansen AS, Ter Horst GJ, Mettenleiter TC, Loewy AD. Cns Cell Groups Projecting to the Submandibular Parasympathetic Preganglionic Neurons in the Rat: A Retrograde Transneuronal Viral Cell Body Labeling Study. Brain research. 1992;572:253–260. doi: 10.1016/0006-8993(92)90479-s. [DOI] [PubMed] [Google Scholar]
- 168.Jenkins TCA. Aberrations of the Eye and Their Effects on Vision: Part 2. Br J Physiol Opt. 1963;20:161–201. [PubMed] [Google Scholar]
- 169.Johnson DA, Purves D. Tonic and Reflex Synaptic Activity Recorded in Ciliary Ganglion Cells of Anaesthetized Rabbits. J Physiol. 1983;339:599–613. doi: 10.1113/jphysiol.1983.sp014737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jones MA, Marfurt CF. Peptidergic Innervation of the Rat Cornea. Experimental Eye Research. 1998;66:421–435. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 171.Jones R. The Effect of Accomodation and Convergence on the Pupil. IOVS. 1989;30:45. [Google Scholar]
- 172.Joshi AC, Das VE. Muscimol Inactivation of Caudal Fastigial Nucleus and Posterior Interposed Nucleus in Monkeys with Strabismus. Journal of neurophysiology. 2013;110:1882–1891. doi: 10.1152/jn.00233.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Judge SJ, Cumming BG. Neurons in the Monkey Midbrain with Activity Related to Vergence Eye-Movement and Accommodation. Journal of Neurophysiology. 1986;55:915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- 174.Jumblatt JE. Innervation and Pharmacology of the Iris and Ciliary Body. In: Burnstock G, Sillito AM, editors. Nervous Control of the Eye. Amsterdam: Harwood Academic; 2000. pp. 1–40. [Google Scholar]
- 175.Jumblatt JE, Hackmiller RC. M2-Type Muscarinic Receptors Mediate Prejunctional Inhibition of Norepinephrine Release in the Human Iris-Ciliary Body. Experimental eye research. 1994;58:175–180. doi: 10.1006/exer.1994.1005. [DOI] [PubMed] [Google Scholar]
- 176.Kahl BF, Reid TW. Substance-P and the Eye. Progress in Retinal and Eye Research. 1995;14:473–504. [Google Scholar]
- 177.Kankipati L, Girkin CA, Gamlin PD. Post-Illumination Pupil Response in Subjects without Ocular Disease. Investigative ophthalmology & visual science. 2010;51:2764–2769. doi: 10.1167/iovs.09-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kardon R. Pupillary Light Reflex. Curr Opin Ophthalmol. 1995;6:20–26. doi: 10.1097/00055735-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 179.Kardon RH. Anatomy and Physiology of the Autonomic Nervous System. In: Miller NR, Walsh FB, Hoyt WF, editors. Walsh and Hoyt's Clinical Neuro-Ophthalmology. 6th. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 3 v. [Google Scholar]
- 180.Karplus JP, Kreidl A. Über Die Bahn Des Pupillarreflexes. Pflüger's Arch. 1912;149:115–155. [Google Scholar]
- 181.Kasthurirangan S, Glasser A. Characteristics of Pupil Responses During Far-to-near and near-to-Far Accommodation. OPHTHAL PHYSL OPT. 2005;25:328–339. doi: 10.1111/j.1475-1313.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 182.Kawarai M, Koss MC. Sympathetic Vasoconstriction in the Rat Anterior Choroid Is Mediated by Alpha1-Adrenoceptors. Eur J Pharmacol. 1998;363:35–40. doi: 10.1016/s0014-2999(98)00790-0. [DOI] [PubMed] [Google Scholar]
- 183.Kiel JW. The Ocular Circulation. San Rafael, CA: Morgan & Claypool Life Science; 2010. [PubMed] [Google Scholar]
- 184.Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA. Ciliary Blood Flow and Aqueous Humor Production. Progress in retinal and eye research. 2011;30:1–17. doi: 10.1016/j.preteyeres.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kiel JW, Lovell MO. Adrenergic Modulation of Choroidal Blood Flow in the Rabbit. Invest Ophthalmol Vis Sci. 1996;37:673–679. [PubMed] [Google Scholar]
- 186.Kimura E, Young RSL. Sustained Pupillary Constrictions Mediated by an L- and M-Cone Opponent Process. Vision Research. 2010;50:489–496. doi: 10.1016/j.visres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 187.Kirch W, Neuhuber W, Tamm ER. Immunohistochemical Localization of Neuropeptides in the Human Ciliary Ganglion. Brain research. 1995;681:229–234. doi: 10.1016/0006-8993(95)00299-6. [DOI] [PubMed] [Google Scholar]
- 188.Klooster J, Beckers HJ, Ten Tusscher MP, Vrensen GF, van der Want JJ, Lamers WP. Sympathetic Innervation of the Rat Choroid: An Autoradiographic Tracing and Immunohistochemical Study. Ophthalmic Res. 1996;28:36–43. doi: 10.1159/000267871. [DOI] [PubMed] [Google Scholar]
- 189.Klooster J, Vrensen GFJM. New Indirect Pathways Subserving the Pupillary Light Reflex: Projections of the Accessory Oculomotor Nuclei and the Periaqueductal Gray to the Edinger-Westphal Nucleus and the Thoracic Spinal Cord in Rats. ANAT EMBRYOL. 1998;198:123–132. doi: 10.1007/s004290050170. [DOI] [PubMed] [Google Scholar]
- 190.Klyce SD, Beuerman RW, Crosson CE. Alteration of Corneal Epithelial Ion Transport by Sympathectomy. Investigative ophthalmology & visual science. 1985;26:434–442. [PubMed] [Google Scholar]
- 191.Knoll HA. Pupillary Changes Associated with Accommodation and Convergence. Am J Ophthalmol. 1949;26:346–357. doi: 10.1097/00006324-194908000-00006. [DOI] [PubMed] [Google Scholar]
- 192.Koontz MA, Rodieck RW, Farmer SG. The Retinal Projection to the Cat Pretectum. The Journal of comparative neurology. 1985;236:42–59. doi: 10.1002/cne.902360105. [DOI] [PubMed] [Google Scholar]
- 193.Koss MC. Pupillary Dilation as an Index of Central-Nervous-System Alpha-2-Adrenoceptor Activation. Journal of Pharmacological Methods. 1986;15:1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 194.Kourouyan HD, Horton JC. Transneuronal Retinal Input to the Primate Edinger-Westphal Nucleus. Journal of Comparative Neurology. 1997;381:68–80. doi: 10.1002/(sici)1096-9861(19970428)381:1<68::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 195.Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, Ryabinin AE. The Edinger-Westphal Nucleus: A Historical, Structural, and Functional Perspective on a Dichotomous Terminology. The Journal of comparative neurology. 2011;519:1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Krieglstein GK, Langham ME, Leydhecker W. The Peripheral and Central Neural Actions of Clonidine in Normal and Glaucomatous Eyes. Invest Ophthalmol Vis Sci. 1978;17:149–158. [PubMed] [Google Scholar]
- 197.Kruger PB. Infrared Recording Retinoscope for Monitoring Accomodation. Am J Optom Physiol Opt. 1979;56:116–123. doi: 10.1097/00006324-197902000-00008. [DOI] [PubMed] [Google Scholar]
- 198.Kuchiiwa S, Kuchiiwa T, Nakagawa S. Localization of Preganglionic Neurons of the Accessory Ciliary Ganglion in the Midbrain - Hrp and Wga-Hrp Studies in the Cat. Journal of Comparative Neurology. 1994;340:577–591. doi: 10.1002/cne.903400410. [DOI] [PubMed] [Google Scholar]
- 199.Kuchiiwa S, Kuchiiwa T, Suzuki T. Comparative Anatomy of the Accessory Ciliary Ganglion in Mammals. Anatomy and Embryology. 1989;180:199–205. doi: 10.1007/BF00309772. [DOI] [PubMed] [Google Scholar]
- 200.Kumagai N, Yuda K, Kadota T, Goris RC, Kishida R. Substance P-Like Immunoreactivity in the Central Retinal Artery of the Rabbit. Exp Eye Res. 1988;46:591–596. doi: 10.1016/s0014-4835(88)80015-0. [DOI] [PubMed] [Google Scholar]
- 201.Kuwayama Y, Grimes PA, Ponte B, Stone RA. Autonomic Neurons Supplying the Rat Eye and the Intraorbital Distribution of Vasoactive Intestinal Polypeptide (Vip)-Like Immunoreactivity. Exp Eye Res. 1987;44:907–922. doi: 10.1016/s0014-4835(87)80053-2. [DOI] [PubMed] [Google Scholar]
- 202.Larson MD, Talke PO. Effect of Dexmedetomidine, an Alpha-Adrenoceptor Agonist, on Human Pupillary Reflexes During General Anaesthesia. British Journal of Clinical Pharmacology. 2001;51:27–33. doi: 10.1046/j.1365-2125.2001.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Laties AM. Central Retinal Artery Innervation. Absence of Adrenergic Innervation to the Intraocular Branches. Arch Ophthalmol. 1967;77:405–409. doi: 10.1001/archopht.1967.00980020407021. [DOI] [PubMed] [Google Scholar]
- 204.Laties AM, Jacobowitz D. A Comparative Study of the Autonomic Innervation of the Eye in Monkey, Cat, and Rabbit. The Anatomical record. 1966;156:383–395. doi: 10.1002/ar.1091560403. [DOI] [PubMed] [Google Scholar]
- 205.Laties AM, Stone RA, Brecha NC. Substance P-Like Immunoreactive Nerve Fibers in the Trabecular Meshwork. Invest Ophthalmol Vis Sci. 1981;21:484–486. [PubMed] [Google Scholar]
- 206.Laughlin SB. Retinal Information Capacity and the Function of the Pupil. OPHTHAL PHYSL OPT. 1992;12:161–164. doi: 10.1111/j.1475-1313.1992.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 207.LeDoux MS, Zhou QH, Murphy RB, Greene ML, Ryan P. Parasympathetic Innervation of the Meibomian Glands in Rats. Investigative Ophthalmology & Visual Science. 2001;42:2434–2441. [PubMed] [Google Scholar]
- 208.Lee Y, Kawai Y, Shiosaka S, Takami K, Kiyama H, Hillyard CJ, Girgis S, Macintyre I, Emson PC, Tohyama M. Coexistence of Calcitonin Gene-Related Peptide and Substance-P-Like Peptide in Single Cells of the Trigeminal Ganglion of the Rat - Immunohistochemical Analysis. Brain Research. 1985;330:194–196. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- 209.Leichnetz GR. Preoccipital Cortex Receives a Differential Input from the Frontal Eye Field and Projects to the Pretectal Olivary Nucleus and Other Visuomotor-Related Structures in the Rhesus Monkey. Visual neuroscience. 1990;5:123–133. doi: 10.1017/s095252380000016x. [DOI] [PubMed] [Google Scholar]
- 210.Li C, Fitzgerald ME, Ledoux MS, Gong S, Ryan P, Del Mar N, Reiner A. Projections from the Hypothalamic Paraventricular Nucleus and the Nucleus of the Solitary Tract to Prechoroidal Neurons in the Superior Salivatory Nucleus: Pathways Controlling Rodent Choroidal Blood Flow. Brain research. 2010;1358:123–139. doi: 10.1016/j.brainres.2010.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li H, Grimes P. Adrenergic Innervation of the Choroid and Iris in Diabetic Rats. Curr Eye Res. 1993;12:89–94. doi: 10.3109/02713689308999500. [DOI] [PubMed] [Google Scholar]
- 212.Liang J, Williams DR. Aberrations and Retinal Image Quality of the Normal Human Eye. J Opt Soc Am A. 1997;14:2873–2883. doi: 10.1364/josaa.14.002873. [DOI] [PubMed] [Google Scholar]
- 213.Linsenmeier RA, Braun RD. Oxygen Distribution and Consumption in the Cat Retina During Normoxia and Hypoxemia. The Journal of general physiology. 1992;99:177–197. doi: 10.1085/jgp.99.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Linsenmeier RA, Goldstick TK, Blum RS, Enroth-Cugell C. Estimation of Retinal Oxygen Transients from Measurements Made in the Vitreous Humor. Experimental eye research. 1981;32:369–379. doi: 10.1016/s0014-4835(81)80016-4. [DOI] [PubMed] [Google Scholar]
- 215.Loewenfeld IE, Lowenstein O. The Pupil : Anatomy, Physiology, and Clinical Applications. Iowa State University Press; 1993. [Google Scholar]
- 216.Loewy AD. Autonomic Control of the Eye. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 266–283. [Google Scholar]
- 217.Loewy AD, Saper CB. Edinger-Westphal Nucleus: Projections to the Brain Stem and Spinal Cord in the Cat. Brain Res. 1978;150:1–27. doi: 10.1016/0006-8993(78)90650-9. [DOI] [PubMed] [Google Scholar]
- 218.Loewy AD, Saper CB, Yamodis ND. Re-Evaluation of the Efferent Projections of the Edinger-Westphal Nucleus in the Cat. Brain Res. 1978;141:153–159. doi: 10.1016/0006-8993(78)90624-8. [DOI] [PubMed] [Google Scholar]
- 219.Lovasik JV, Kergoat H, Riva CE, Petrig BL, Geiser M. Choroidal Blood Flow During Exercise-Induced Changes in the Ocular Perfusion Pressure. Invest Ophthalmol Vis Sci. 2003;44:2126–2132. doi: 10.1167/iovs.02-0825. [DOI] [PubMed] [Google Scholar]
- 220.Lowenstein O. The Argyll Robertson Pupillary Syndrome; Mechanism and Localization. American journal of ophthalmology. 1956;42:105–121. doi: 10.1016/0002-9394(56)90362-2. [DOI] [PubMed] [Google Scholar]
- 221.Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished Pupillary Light Reflex at High Irradiances in Melanopsin-Knockout Mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 222.Luhtala J, Uusitalo H. The Distribution and Origin of Substance P Immunoreactive Nerve Fibres in the Rat Conjunctiva. Exp Eye Res. 1991;53:641–646. doi: 10.1016/0014-4835(91)90224-3. [DOI] [PubMed] [Google Scholar]
- 223.Lui F, Gregory KM, Blanks RH, Giolli RA. Projections from Visual Areas of the Cerebral Cortex to Pretectal Nuclear Complex, Terminal Accessory Optic Nuclei, and Superior Colliculus in Macaque Monkey. The Journal of comparative neurology. 1995;363:439–460. doi: 10.1002/cne.903630308. [DOI] [PubMed] [Google Scholar]
- 224.Lutjen-Drecoll E. Choroidal Innervation in Primate Eyes. Experimental eye research. 2006;82:357–361. doi: 10.1016/j.exer.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 225.Magoun HW, Atlas D, Hare WK, Ranson SW. The Afferent Path of the Pupillary Light Reflex in the Monkey. Brain. 1936;59:234–249. [Google Scholar]
- 226.Magoun HW, Ranson SW. The Afferent Path of the Light Reflex - a Review of the Literature. Archives of Ophthalmology. 1935;13:862–874. [Google Scholar]
- 227.Magoun HW, Ranson SW. The Central Path of the Light Reflex - a Study of the Effect of Lesions. Archives of Ophthalmology. 1935;13:791–811. [Google Scholar]
- 228.Mandahl A, Bill A. Effects of the Substance P Antagonist, (D-Arg1, D-Pro2, D-Trp7,9, Leu11)-Sp on the Miotic Response to Substance P, Antidromic Trigeminal Nerve Stimulation, Capsaicin, Prostaglandin E1, Compound 48/80 and Histamine. Acta physiologica Scandinavica. 1984;120:27–35. doi: 10.1111/j.1748-1716.1984.tb07369.x. [DOI] [PubMed] [Google Scholar]
- 229.Marcos S, Moreno E, Navarro R. The Depth-of-Field of the Human Eye from Objective and Subjective Measurements. Vision Res. 1999;39:2039–2049. doi: 10.1016/s0042-6989(98)00317-4. [DOI] [PubMed] [Google Scholar]
- 230.Marfurt C. Nervous Control of the Cornea. In: Burnstock G, Sillito AM, editors. Nervous Control of the Eye. Amsterdam: Harwood Academic; 2000. pp. 41–92. [Google Scholar]
- 231.Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and Sympathetic Innervation of the Mammalian Cornea - a Retrograde Tracing Study. Investigative Ophthalmology & Visual Science. 1989;30:461–472. [PubMed] [Google Scholar]
- 232.Marg E. Accommodative Response of the Eye to Electrical Stimulation of the Ciliary Ganglion in Cats. American journal of optometry and archives of American Academy of Optometry. 1954;31:127–136. doi: 10.1097/00006324-195403000-00003. [DOI] [PubMed] [Google Scholar]
- 233.Marg E, Morgan MW. The Pupillary near Reflex. Am J Optom. 1949;26:183–198. [PubMed] [Google Scholar]
- 234.Marg E, Morgan W. Further Investigation of the Pupillary near Reflex. Am J Optom. 1950;27:217–225. [PubMed] [Google Scholar]
- 235.May CA. Description and Function of the Ciliary Nerves--Some Historical Remarks on Choroidal Innervation. Exp Eye Res. 1997;65:1–5. doi: 10.1006/exer.1997.0312. [DOI] [PubMed] [Google Scholar]
- 236.May CA, Neuhuber W, Lutjen-Drecoll E. Immunohistochemical Classification and Functional Morphology of Human Choroidal Ganglion Cells. Invest Ophthalmol Vis Sci. 2004;45:361–367. doi: 10.1167/iovs.03-0624. [DOI] [PubMed] [Google Scholar]
- 237.May CA, Skorski LM, Lutjen-Drecoll E. Innervation of the Porcine Ciliary Muscle and Outflow Region. J Anat. 2005;206:231–236. doi: 10.1111/j.1469-7580.2005.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.May PJ, Porter JD, Gamlin PD. Interconnections between the Primate Cerebellum and Midbrain near-Response Regions. J Comp Neurol. 1992;315:98–9116. doi: 10.1002/cne.903150108. [DOI] [PubMed] [Google Scholar]
- 239.May PJ, Reiner AJ, Ryabinin AE. Comparison of the Distributions of Urocortin-Containing and Cholinergic Neurons in the Perioculomotor Midbrain of the Cat and Macaque. J Comp Neurol. 2008;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.May PJ, Sun W, Erichsen JT. Defining the Pupillary Component of the Perioculomotor Preganglionic Population within a Unitary Primate Edinger-Westphal Nucleus. In: Kennard C, Leigh RJ, editors. Using Eye Movements as an Experimental Probe of Brain Function - a Symposium in Honor of Jean Buttner-Ennever. 2008. pp. 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.May PJ, Warren S. Ultrastructure of the Macaque Ciliary Ganglion. J Neurocytol. 1993;22:1073–1095. doi: 10.1007/BF01235750. [DOI] [PubMed] [Google Scholar]
- 242.Mays LE, Gamlin PDR. Neuronal Circuitry Controlling the near Response. Current Opinion in Neurobiology. 1995;5:763–768. doi: 10.1016/0959-4388(95)80104-9. [DOI] [PubMed] [Google Scholar]
- 243.Mays LE, Porter JD. Neural Control of Vergence Eye-Movements - Activity of Abducens and Oculomotor Neurons. Journal of Neurophysiology. 1984;52:743–761. doi: 10.1152/jn.1984.52.4.743. [DOI] [PubMed] [Google Scholar]
- 244.Mays LE, Porter JD, Gamlin PDR, Tello CA. Neural Control of Vergence Eye-Movements - Neurons Encoding Vergence Velocity. Journal of Neurophysiology. 1986;56:1007–1021. doi: 10.1152/jn.1986.56.4.1007. [DOI] [PubMed] [Google Scholar]
- 245.McDougal DH, Gamlin PD. The Influence of Intrinsically-Photosensitive Retinal Ganglion Cells on the Spectral Sensitivity and Response Dynamics of the Human Pupillary Light Reflex. Vision research. 2010;50:72–87. doi: 10.1016/j.visres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Melnitchenko LV, Skok VI. Natural Electrical Activity in Mammalian Parasympathetic Ganglion Neurones. Brain Res. 1970;23:277–279. doi: 10.1016/0006-8993(70)90051-x. [DOI] [PubMed] [Google Scholar]
- 247.Meriney SD, Pilar G. Cholinergic Innervation of the Smooth Muscle Cells in the Choroid Coat of the Chick Eye and Its Development. J Neurosci. 1987;7:3827–3839. doi: 10.1523/JNEUROSCI.07-12-03827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Millar C, Kaufman PL. Aqueous Humor: Secretion and Dynamics. In: Duane TD, Tasman W, Jaeger EA, editors. Duane's Foundations of Clinical Ophthalmology. Lippincott; 1998. [Google Scholar]
- 249.Miller A, Costa M, Furness JB, Chubb IW. Substance P Immunoreactive Sensory Nerves Supply the Rat Iris and Cornea. Neurosci Lett. 1981;23:243–249. doi: 10.1016/0304-3940(81)90005-7. [DOI] [PubMed] [Google Scholar]
- 250.Mione MC, Cavanagh JF, Lincoln J, Milner P, Burnstock G. Long-Term Chemical Sympathectomy Leads to an Increase of Neuropeptide Y Immunoreactivity in Cerebrovascular Nerves and Iris of the Developing Rat. Neuroscience. 1990;34:369–378. doi: 10.1016/0306-4522(90)90146-u. [DOI] [PubMed] [Google Scholar]
- 251.Mitchelson F. Muscarinic Receptor Agonists and Antagonists: Effects on Ocular Function. Handb Exp Pharmacol. 2012:263–298. doi: 10.1007/978-3-642-23274-9_12. [DOI] [PubMed] [Google Scholar]
- 252.Morgan C, DeGroat WC, Jannetta PJ. Sympathetic Innervation of the Cornea from the Superior Cervical Ganglion. An Hrp Study in the Cat. Journal of the autonomic nervous system. 1987;20:179–183. doi: 10.1016/0165-1838(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 253.Movaffaghy A, Chamot SR, Petrig BL, Riva CE. Blood Flow in the Human Optic Nerve Head During Isometric Exercise. Exp Eye Res. 1998;67:561–568. doi: 10.1006/exer.1998.0556. [DOI] [PubMed] [Google Scholar]
- 254.Mueller H. Ueber Glatte Muskeln Und Nervengeflechte Der Chorioidea Im Menschlichen Auge. Vehr physik-med Ges Wurzburg. 1859:179–192. [Google Scholar]
- 255.Muller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal Nerves: Structure, Contents and Function. Experimental Eye Research. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 256.Munch M, Kawasaki A. Intrinsically Photosensitive Retinal Ganglion Cells: Classification, Function and Clinical Implications. Curr Opin Neurol. 2013;26:45–51. doi: 10.1097/WCO.0b013e32835c5e78. [DOI] [PubMed] [Google Scholar]
- 257.Myers GA, Stark L. Topology of the near Response Triad. Ophthalmic and Physiological Optics. 1990;10:175–181. doi: 10.1111/j.1475-1313.1990.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 258.Nakai M, Ogino K. The Relevance of Cardio-Pulmonary-Vascular Reflex to Regulation of the Brain Vessels. Jpn J Physiol. 1984;34:193–197. doi: 10.2170/jjphysiol.34.193. [DOI] [PubMed] [Google Scholar]
- 259.Nakai M, Tamaki K, Ogata J, Matsui Y, Maeda M. Parasympathetic Cerebrovasodilator Center of the Facial Nerve. Circ Res. 1993;72:470–475. doi: 10.1161/01.res.72.2.470. [DOI] [PubMed] [Google Scholar]
- 260.Nakanome Y, Karita K, Izumi H, Tamai M. Two Types of Vasodilatation in Cat Choroid Elicited by Electrical Stimulation of the Short Ciliary Nerve. Exp Eye Res. 1995;60:37–42. doi: 10.1016/s0014-4835(05)80081-8. [DOI] [PubMed] [Google Scholar]
- 261.Neuhuber W, Schrodl F. Autonomic Control of the Eye and the Iris. Autonomic neuroscience : basic & clinical. 2011;165:67–79. doi: 10.1016/j.autneu.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 262.Ng YK, Wong WC, Ling EA. A Light and Electron Microscopical Localisation of the Superior Salivatory Nucleus of the Rat. Journal fur Hirnforschung. 1994;35:39–48. [PubMed] [Google Scholar]
- 263.Nicholson J, Severin CM. Afferent-Projections of the Edinger-Westphal Nucleus in the Rat. Anatomical Record. 1981;199:A183–A183. [Google Scholar]
- 264.Nicholson JE, Severin CM. The Superior and Inferior Salivatory Nuclei in the Rat. Neurosci Lett. 1981;21:149–154. doi: 10.1016/0304-3940(81)90373-6. [DOI] [PubMed] [Google Scholar]
- 265.Nickla DL, Wallman J. The Multifunctional Choroid. Progress in retinal and eye research. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nickla DL, Wildsoet C, Wallman J. Visual Influences on Diurnal Rhythms in Ocular Length and Choroidal Thickness in Chick Eyes. Experimental eye research. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 267.Nickla DL, Wildsoet CF, Troilo D. Endogenous Rhythms in Axial Length and Choroidal Thickness in Chicks: Implications for Ocular Growth Regulation. Investigative ophthalmology & visual science. 2001;42:584–588. [PubMed] [Google Scholar]
- 268.Nilsson SF. Neuropeptide Y (Npy): A Vasoconstrictor in the Eye, Brain and Other Tissues in the Rabbit. Acta Physiol Scand. 1991;141:455–467. doi: 10.1111/j.1748-1716.1991.tb09106.x. [DOI] [PubMed] [Google Scholar]
- 269.Nilsson SF. Nitric Oxide as a Mediator of Parasympathetic Vasodilation in Ocular and Extraocular Tissues in the Rabbit. Invest Ophthalmol Vis Sci. 1996;37:2110–2119. [PubMed] [Google Scholar]
- 270.Nilsson SF. The Significance of Nitric Oxide for Parasympathetic Vasodilation in the Eye and Other Orbital Tissues in the Cat. Experimental eye research. 2000;70:61–72. doi: 10.1006/exer.1999.0752. [DOI] [PubMed] [Google Scholar]
- 271.Nilsson SF, Linder J, Bill A. Characteristics of Uveal Vasodilation Produced by Facial Nerve Stimulation in Monkeys, Cats and Rabbits. Exp Eye Res. 1985;40:841–852. doi: 10.1016/0014-4835(85)90129-0. [DOI] [PubMed] [Google Scholar]
- 272.Nisida I, Okada H, Nakano O. The Activity of the Ciliospinal Centers and Their Inhibition in Pupillary Light Reflex. Jpn J Physiol. 1960;10:73–84. doi: 10.2170/jjphysiol.10.73. [DOI] [PubMed] [Google Scholar]
- 273.Nomura T, Smelser GK. The Identification of Adrenergic and Cholinergic Nerve Endings in the Trabecular Meshwork. Invest Ophthalmol. 1974;13:525–532. [PubMed] [Google Scholar]
- 274.Okada H, Nakano O, Okamoto K, Nakayama K, Nisida I. The Central Path of the Light Reflex Via the Sympathetic Nerve in the Cat. Jpn J Physiol. 1960;10:646–658. doi: 10.2170/jjphysiol.10.646. [DOI] [PubMed] [Google Scholar]
- 275.Oksala O. Effects of Calcitonin Gene-Related Peptide and Substance P on Regional Blood Flow in the Cat Eye. Experimental eye research. 1988;47:283–289. doi: 10.1016/0014-4835(88)90011-5. [DOI] [PubMed] [Google Scholar]
- 276.Oksala O, Stjernschantz J. Increase in Outflow Facility of Aqueous Humor in Cats Induced by Calcitonin Gene-Related Peptide. Exp Eye Res. 1988;47:787–790. doi: 10.1016/0014-4835(88)90045-0. [DOI] [PubMed] [Google Scholar]
- 277.Olmsted JMD. The Role of the Autonomic Nervous System in Accommodation for Far and near Vision. Journal of Nervous and Mental Disease. 1944;99:794–798. [Google Scholar]
- 278.Ostrin LA, Glasser A. Autonomic Drugs and the Accommodative System in Rhesus Monkeys. Experimental Eye Research. 2010;90:104–112. doi: 10.1016/j.exer.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ostrin LA, Glasser A. Comparisons between Pharmacologically and Edinger-Westphal-Stimulated Accommodation in Rhesus Monkeys. Investigative Ophthalmology & Visual Science. 2005;46:609–617. doi: 10.1167/iovs.04-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ostrin LA, Glasser A. Edinger-Westphal and Pharmacologically Stimulated Accommodative Refractive Changes and Lens and Ciliary Process Movements in Rhesus Monkeys. Experimental Eye Research. 2007;84:302–313. doi: 10.1016/j.exer.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Oyster CW. The Human Eye : Structure and Function. Sunderland, Mass.: Sinauer Associates; 1999. [Google Scholar]
- 282.Oyster CW. The Iris and the Pupil. In: Oyster CW, editor. The Human Eye : Structure and Function. Sunderland, Mass: Sinauer Associates; 1999. pp. 411–446. [Google Scholar]
- 283.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin Is Required for Non-Image-Forming Photic Responses in Blind Mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 284.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular Axial Length and Choroidal Thickness in Newly Hatched Chicks and One-Year-Old Chickens Fluctuate in a Diurnal Pattern That Is Influenced by Visual Experience and Intraocular Pressure Changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 285.Parelman JJ, Fay MT, Burde RM. Confirmatory Evidence for a Direct Parasympathetic Pathway to Internal Eye Structures. Transactions of the American Ophthalmological Society. 1984;82:371–380. [PMC free article] [PubMed] [Google Scholar]
- 286.Parssinen O, Leppanen E, Keski-Rahkonen P, Mauriala T, Dugue B, Lehtonen M. Influence of Tamsulosin on the Iris and Its Implications for Cataract Surgery. IOVS. 2006;47:3766–3771. doi: 10.1167/iovs.06-0153. [DOI] [PubMed] [Google Scholar]
- 287.Parver LM, Auker CR, Carpenter DO. Choroidal Blood Flow. Iii. Reflexive Control in Human Eyes. Arch Ophthalmol. 1983;101:1604–1606. doi: 10.1001/archopht.1983.01040020606021. [DOI] [PubMed] [Google Scholar]
- 288.Parver LM, Auker CR, Carpenter DO, Doyle T. Choroidal Blood Flow Ii. Reflexive Control in the Monkey. Arch Ophthalmol. 1982;100:1327–1330. doi: 10.1001/archopht.1982.01030040305021. [DOI] [PubMed] [Google Scholar]
- 289.Passatore M, Pettorossi VE. Efferent Fibers in the Cervical Sympathetic Nerve Influenced by Light. Exp Neurol. 1976;52:66–82. doi: 10.1016/0014-4886(76)90201-6. [DOI] [PubMed] [Google Scholar]
- 290.Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Brown RL. Synaptic Inputs to Retinal Ganglion Cells That Set the Circadian Clock. Eur J Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Perry VH, Cowey A. Retinal Ganglion Cells That Project to the Superior Colliculus and Pretectum in the Macaque Monkey. Neuroscience. 1984;12:1125–1137. doi: 10.1016/0306-4522(84)90007-1. [DOI] [PubMed] [Google Scholar]
- 292.Phillips NJ, Winn B, Gilmartin B. Absence of Pupil Response to Blur-Driven Accommodation. Vision Research. 1992;32:1775–1779. doi: 10.1016/0042-6989(92)90170-n. [DOI] [PubMed] [Google Scholar]
- 293.Pierson RJ, Carpenter DO. Anatomical Analysis of Pupillary Reflex Pathways in Rhesus-Monkey. Journal of Comparative Neurology. 1974;158:121–143. doi: 10.1002/cne.901580202. [DOI] [PubMed] [Google Scholar]
- 294.Pilar G, Tuttle JB. A Simple Neuronal System with a Range of Uses: The Avian Ciliary Ganglion. In: Hanin I, Goldberg AM, editors. Progress in Modern Cholinergic Biology : Model Cholinergic Synapses. New York: Raven Press; 1982. pp. 213–247. [Google Scholar]
- 295.Pintor J. Purinergic Signalling in the Eye. In: Burnstock G, Sillito AM, editors. Nervous Control of the Eye. Amsterdam: Harwood Academic; 2000. pp. 171–210. [Google Scholar]
- 296.Pitts DG. Electrical Stimulation of Oculomotor Nucleus: The Effects of Stimulus Voltage and Anesthesia. Am J Optom Arch Am Acad Optom. 1967;44:505–516. [PubMed] [Google Scholar]
- 297.Polansky JR, Zlock D, Brasier A, Bloom E. Adrenergic and Cholinergic Receptors in Isolated Non-Pigmented Ciliary Epithelial Cells. Curr Eye Res. 1985;4:517–522. doi: 10.3109/02713688509025168. [DOI] [PubMed] [Google Scholar]
- 298.Polska E, Simader C, Weigert G, Doelemeyer A, Kolodjaschna J, Scharmann O, Schmetterer L. Regulation of Choroidal Blood Flow During Combined Changes in Intraocular Pressure and Arterial Blood Pressure. Investigative ophthalmology & visual science. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- 299.Ponsford JR, Bannister R, Paul EA. Methacholine Pupillary Responses in Third Nerve Palsy and Adie's Syndrome. Brain. 1982;105(Pt 3):583–597. doi: 10.1093/brain/105.3.583. [DOI] [PubMed] [Google Scholar]
- 300.Poukens V, Glasgow BJ, Demer JL. Nonvascular Contractile Cells in Sclera and Choroid of Humans and Monkeys. Investigative ophthalmology & visual science. 1998;39:1765–1774. [PubMed] [Google Scholar]
- 301.Provencio I, Jiang GS, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An Opsin in Melanophores, Brain, and Eye. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Provencio I, Rodriguez IR, Jiang GS, Hayes WP, Moreira EF, Rollag MD. A Novel Human Opsin in the Inner Retina. Journal of Neuroscience. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of Photosensitivity by Heterologous Expression of Melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 304.Quigley HA, Silver DM, Friedman DS, He M, Plyler RJ, Eberhart CG, Jampel HD, Ramulu P. Iris Cross-Sectional Area Decreases with Pupil Dilation and Its Dynamic Behavior Is a Risk Factor in Angle Closure. J Glaucoma. 2009;18:173–179. doi: 10.1097/IJG.0b013e31818624ce. [DOI] [PubMed] [Google Scholar]
- 305.Ranson SW, Magoun HW. The Central Path of the Pupilloconstricter Reflex in Response to Light. Archives of Neurology and Psychiatry. 1933;30:1193–1204. [Google Scholar]
- 306.Reiner A, Fitzgerald MC, Li C. Neural Control of Ocular Blood Flow. In: Schmetterer L, Kiel J, editors. Ocular Blood Flow. Berlin Heidelberg: Springer; 2012. pp. 243–309. [Google Scholar]
- 307.Reitsamer HA, Kiel JW. Relationship between Ciliary Blood Flow and Aqueous Production in Rabbits. Investigative ophthalmology & visual science. 2003;44:3967–3971. doi: 10.1167/iovs.03-0088. [DOI] [PubMed] [Google Scholar]
- 308.Ripps H, Breinin GM, Baum JL. Accommodation in the Cat. Trans Am Ophthalmol Soc. 1961;59:176–193. [PMC free article] [PubMed] [Google Scholar]
- 309.Riva CE, Cranstoun SD, Petrig BL. Effect of Decreased Ocular Perfusion Pressure on Blood Flow and the Flicker-Induced Flow Response in the Cat Optic Nerve Head. Microvasc Res. 1996;52:258–269. doi: 10.1006/mvre.1996.0063. [DOI] [PubMed] [Google Scholar]
- 310.Riva CE, Falsini B, Logean E. Flicker-Evoked Responses of Human Optic Nerve Head Blood Flow: Luminance Versus Chromatic Modulation. Investigative ophthalmology & visual science. 2001;42:756–762. [PubMed] [Google Scholar]
- 311.Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal Blood Flow During Isometric Exercises. Invest Ophthalmol Vis Sci. 1997;38:2338–2343. [PubMed] [Google Scholar]
- 312.Robertson D. Primer on the Autonomic Nervous System. Boston: Academic Press; 2004. [Google Scholar]
- 313.Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M, Wood-Vasey WM, Franzen P. The Post Illumination Pupil Response Is Reduced in Seasonal Affective Disorder. Psychiatry Research. 2013;210:150–158. doi: 10.1016/j.psychres.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Roste GK, Dietrichs E. Cerebellar Cortical and Nuclear Afferents from the Edinger-Westphal Nucleus in the Cat. Anatomy and Embryology. 1988;178:59–65. doi: 10.1007/BF00305015. [DOI] [PubMed] [Google Scholar]
- 315.Ruskell GL. Access of Autonomic Nerves through the Optic Canal, and Their Orbital Distribution in Man. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:973–978. doi: 10.1002/ar.a.10108. [DOI] [PubMed] [Google Scholar]
- 316.Ruskell GL. Accommodation and the Nerve Pathway to the Ciliary Muscle: A Review. Ophthalmic Physiol Opt. 1990;10:239–242. [PubMed] [Google Scholar]
- 317.Ruskell GL. The Distribution of Autonomic Post-Ganglionic Nerve Fibres to the Lacrimal Gland in Monkeys. J Anat. 1971;109:229–242. [PMC free article] [PubMed] [Google Scholar]
- 318.Ruskell GL. Facial Parasympathetic Innervation of the Choroidal Blood Vessels in Monkeys. Exp Eye Res. 1971;12:166–172. doi: 10.1016/0014-4835(71)90085-6. [DOI] [PubMed] [Google Scholar]
- 319.Ruskell GL. Ocular Fibres of the Maxillary Nerve in Monkeys. Journal of anatomy. 1974;118:195–203. [PMC free article] [PubMed] [Google Scholar]
- 320.Ruskell GL. An Ocular Parasympathetic Nerve Pathway of Facial Nerve Origin and Its Influence on Intraocular Pressure. Exp Eye Res. 1970;10:319–330. doi: 10.1016/s0014-4835(70)80044-6. [DOI] [PubMed] [Google Scholar]
- 321.Ruskell GL. Orbital Branches of Pterygopalatine Ganglion and Their Relationship with Internal Carotid Nerve Branches in Primates. Journal of Anatomy. 1970;106 323-&. [PMC free article] [PubMed] [Google Scholar]
- 322.Ruskell GL. International Congress of Anatomists. The Orbital Distribution of the Sphenopalatine Ganglion in the Rabbit. In: Rohen JW, editor. The Structure of the Eye. Ii. Symposium; Held August 8-13, 1965 During the Eighth International Congress of Anatomists, Wiesbaden, Germany. Stuttgart: Schattauer; 1965. pp. 323–339. [Google Scholar]
- 323.Ruskell GL. Trigeminal Innervation of the Scleral Spur in Cynomolgus Monkeys. Journal of anatomy. 1994;184(Pt 3):511–518. [PMC free article] [PubMed] [Google Scholar]
- 324.Ruskell GL, Griffiths T. Peripheral Nerve Pathway to the Ciliary Muscle. Exp Eye Res. 1979;28:277–284. doi: 10.1016/0014-4835(79)90089-7. [DOI] [PubMed] [Google Scholar]
- 325.Rusu MC, Pop F, Curca GC, Podoleanu L, Voinea LM. The Pterygopalatine Ganglion in Humans: A Morphological Study. Ann Anat. 2009;191:196–202. doi: 10.1016/j.aanat.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 326.Samuels BC, Hammes NM, Johnson PL, Shekhar A, McKinnon SJ, Allingham RR. Dorsomedial/Perifornical Hypothalamic Stimulation Increases Intraocular Pressure, Intracranial Pressure, and the Translaminar Pressure Gradient. Invest Ophthalmol Vis Sci. 2012;53:7328–7335. doi: 10.1167/iovs.12-10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Saper CB, Scammell TE, Lu J. Hypothalamic Regulation of Sleep and Circadian Rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 328.Scalia F. Retinal Projections to the Olivary Pretectal Nucleus in the Tree Shrew and Comparison with the Rat. Brain, behavior and evolution. 1972;6:237–252. doi: 10.1159/000123712. [DOI] [PubMed] [Google Scholar]
- 329.Scalia F. The Termination of Retinal Axons in the Pretectal Region of Mammals. The Journal of comparative neurology. 1972;145:223–257. doi: 10.1002/cne.901450208. [DOI] [PubMed] [Google Scholar]
- 330.Scalia F, Arango V. Topographic Organization of the Projections of the Retina to the Pretectal Region in the Rat. The Journal of comparative neurology. 1979;186:271–292. doi: 10.1002/cne.901860210. [DOI] [PubMed] [Google Scholar]
- 331.Schaeffel F, Troilo D, Wallman J, Howland HC. Developing Eyes That Lack Accommodation Grow to Compensate for Imposed Defocus. Vis Neurosci. 1990;4:177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- 332.Schmerl E, Steinberg B. Central Control of Intraocular Pressure by Active Principles. Am J Ophthalmol. 1948;31:1097–1101. doi: 10.1016/0002-9394(48)92440-4. [DOI] [PubMed] [Google Scholar]
- 333.Schmidt TM, Kofuji P. Differential Cone Pathway Influence on Intrinsically Photosensitive Retinal Ganglion Cell Subtypes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:16262–16271. doi: 10.1523/JNEUROSCI.3656-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schor CM, Narayan V. Graphical Analysis of Prism Adaptation, Convergence Accommodation, and Accommodative Convergence. AM J OPTOM PHYS OPT. 1982;59:774–784. doi: 10.1097/00006324-198210000-00003. [DOI] [PubMed] [Google Scholar]
- 335.Schrodl F, Brehmer A, Neuhuber WL. Intrinsic Choroidal Neurons in the Duck Eye Express Galanin. The Journal of comparative neurology. 2000;425:24–33. [PubMed] [Google Scholar]
- 336.Schrodl F, De Laet A, Tassignon M-J, Van Bogaert P-P, Brehmer A, Neuhuber WL, Timmermans J-P. Intrinsic Choroidal Neurons in the Human Eye: Projections, Targets, and Basic Electrophysiological Data. Invest Ophthalmol Vis Sci. 2003;44:3705–3712. doi: 10.1167/iovs.03-0232. [DOI] [PubMed] [Google Scholar]
- 337.Schrodl F, Schweigert M, Brehmer A, Neuhuber WL. Intrinsic Neurons in the Duck Choroid Are Contacted by Cgrp-Immunoreactive Nerve Fibres: Evidence for a Local Pre-Central Reflex Arc in the Eye. Exp Eye Res. 2001;72:137–146. doi: 10.1006/exer.2000.0940. [DOI] [PubMed] [Google Scholar]
- 338.Schwiegerling J. Theoretical Limits to Visual Performance. Surv Ophthalmol. 2000;45:139–146. doi: 10.1016/s0039-6257(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 339.Selbach JM, Gottanka J, Wittmann M, Lutjen-Drecoll E. Efferent and Afferent Innervation of Primate Trabecular Meshwork and Scleral Spur. Investigative ophthalmology & visual science. 2000;41:2184–2191. [PubMed] [Google Scholar]
- 340.Selbach JM, Rohen JW, Steuhl KP, Lutjen-Drecoll E. Angioarchitecture and Innervation of the Primate Anterior Episclera. Current eye research. 2005;30:337–344. doi: 10.1080/02713680590934076. [DOI] [PubMed] [Google Scholar]
- 341.Selbach JM, Schonfelder U, Funk RH. Arteriovenous Anastomoses of the Episcleral Vasculature in the Rabbit and Rat Eye. J Glaucoma. 1998;7:50–57. [PubMed] [Google Scholar]
- 342.Seligsohn EE, Bill A. Effects of Ng-Nitro-L-Arginine Methyl Ester on the Cardiovascular System of the Anaesthetized Rabbit and on the Cardiovascular Response to Thyrotropin-Releasing Hormone. Br J Pharmacol. 1993;109:1219–1225. doi: 10.1111/j.1476-5381.1993.tb13752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Semmlow J, Heerema D. Synkinetic Interaction of Convergence Accomodation and Accommodative Convergence. Vision Research. 1979;19:1237–1242. doi: 10.1016/0042-6989(79)90189-5. [DOI] [PubMed] [Google Scholar]
- 344.Shi XP, Zamudio AC, Candia OA, Wolosin JM. Adreno-Cholinergic Modulation of Junctional Communications between the Pigmented and Nonpigmented Layers of the Ciliary Body Epithelium. Investigative ophthalmology & visual science. 1996;37:1037–1046. [PubMed] [Google Scholar]
- 345.Shih YF, Fitzgerald ME, Cuthbertson SL, Reiner A. Influence of Ophthalmic Nerve Fibers on Choroidal Blood Flow and Myopic Eye Growth in Chicks. Exp Eye Res. 1999;69:9–20. doi: 10.1006/exer.1999.0692. [DOI] [PubMed] [Google Scholar]
- 346.Shih YF, Fitzgerald ME, Reiner A. Effect of Choroidal and Ciliary Nerve Transection on Choroidal Blood Flow, Retinal Health, and Ocular Enlargement. Vis Neurosci. 1993;10:969–979. doi: 10.1017/s0952523800006180. [DOI] [PubMed] [Google Scholar]
- 347.Shimizu Y. Localization of Neuropeptides in the Cornea and Uvea of the Rat: An Immunohistochemical Study. Cell Mol Biol. 1982;28:103–110. [PubMed] [Google Scholar]
- 348.Sillito AM, Zbrozyna AW. The Activity Characteristics of the Preganglionic Pupilloconstrictor Neurones. J Physiol. 1970;211:767–779. doi: 10.1113/jphysiol.1970.sp009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sillito AM, Zbrożyna AW. The Localization of Pupilloconstrictor Function within the Mid-Brain of the Cat. The Journal of Physiology. 1970;211:461–477. doi: 10.1113/jphysiol.1970.sp009287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sossi N, Anderson DR. Effect of Elevated Intraocular Pressure on Blood Flow. Occurrence in Cat Optic Nerve Head Studied with Iodoantipyrine I 125. Archives of ophthalmology. 1983;101:98–101. doi: 10.1001/archopht.1983.01040010100018. [DOI] [PubMed] [Google Scholar]
- 351.Spencer SE, Sawyer WB, Wada H, Platt KB, Loewy AD. Cns Projections to the Pterygopalatine Parasympathetic Preganglionic Neurons in the Rat: A Retrograde Transneuronal Viral Cell Body Labeling Study. Brain Res. 1990;534:149–169. doi: 10.1016/0006-8993(90)90125-u. [DOI] [PubMed] [Google Scholar]
- 352.Stakenburg M. Accommodation without Pupillary Constriction. Vision Res. 1991;31:267–273. doi: 10.1016/0042-6989(91)90117-n. [DOI] [PubMed] [Google Scholar]
- 353.Steele GE, Weller RE. Subcortical Connections of Subdivisions of Inferior Temporal Cortex in Squirrel Monkeys. Visual neuroscience. 1993;10:563–583. doi: 10.1017/s0952523800004776. [DOI] [PubMed] [Google Scholar]
- 354.Steiger HJ, Buttnerennever JA. Oculomotor Nucleus Afferents in Monkey Demonstrated with Horseradish-Peroxidase. Brain Research. 1979;160:1–15. doi: 10.1016/0006-8993(79)90596-1. [DOI] [PubMed] [Google Scholar]
- 355.Steinle JJ, Krizsan-Agbas D, Smith PG. Regional Regulation of Choroidal Blood Flow by Autonomic Innervation in the Rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R202–R209. doi: 10.1152/ajpregu.2000.279.1.R202. [DOI] [PubMed] [Google Scholar]
- 356.Stelling JW, Jacob TJ. Functional Coupling in Bovine Ciliary Epithelial Cells Is Modulated by Carbachol. Am J Physiol. 1997;273:1876–1881. doi: 10.1152/ajpcell.1997.273.6.C1876. [DOI] [PubMed] [Google Scholar]
- 357.Stjernschantz J, Alm A, Bill A. Effects of Intracranial Oculomotor Nerve Stimulation on Ocular Blood Flow in Rabbits: Modification by Indomethacin. Exp Eye Res. 1976;23:461–469. doi: 10.1016/0014-4835(76)90175-5. [DOI] [PubMed] [Google Scholar]
- 358.Stjernschantz J, Bill A. Effect of Intracranial Stimulation of the Oculomotor Nerve on Ocular Blood Flow in the Monkey, Cat, and Rabbit. Invest Ophthalmol Vis Sci. 1979;18:99–9103. [PubMed] [Google Scholar]
- 359.Stjernschantz J, Bill A. Vasomotor Effects of Facial Nerve Stimulation: Noncholinergic Vasodilation in the Eye. Acta Physiol Scand. 1980;109:45–50. doi: 10.1111/j.1748-1716.1980.tb06562.x. [DOI] [PubMed] [Google Scholar]
- 360.Stjernschantz J, Geijer C, Bill A. Electrical Stimulation of the Fifth Cranial Nerve in Rabbits: Effects on Ocular Blood Flow, Extravascular Albumin Content and Intraocular Pressure. Exp Eye Res. 1979;28:229–238. doi: 10.1016/0014-4835(79)90134-9. [DOI] [PubMed] [Google Scholar]
- 361.Stjernschantz J, Sears M, Stjernschantz L. Intraocular Effects of Substance P in the Rabbit. Invest Ophthalmol Vis Sci. 1981;20:53–60. [PubMed] [Google Scholar]
- 362.Stone RA. Vasoactive Intestinal Polypeptide and the Ocular Innervation. Invest Ophthalmol Vis Sci. 1986;27:951–957. [PubMed] [Google Scholar]
- 363.Stone RA, Kuwayama Y. Substance P-Like Immunoreactive Nerves in the Human Eye. Arch Ophthalmol. 1985;103:1207–1211. doi: 10.1001/archopht.1985.01050080119031. [DOI] [PubMed] [Google Scholar]
- 364.Stone RA, Kuwayama Y, Laties AM. Regulatory Peptides in the Eye. Experientia. 1987;43:791–800. doi: 10.1007/BF01945357. [DOI] [PubMed] [Google Scholar]
- 365.Stone RA, Laties AM, Brecha NC. Substance P-Like Immunoreactive Nerves in the Anterior Segment of the Rabbit, Cat and Monkey Eye. Neuroscience. 1982;7:2459–2468. doi: 10.1016/0306-4522(82)90207-x. [DOI] [PubMed] [Google Scholar]
- 366.Stone RA, McGlinn AM. Calcitonin Gene-Related Peptide Immunoreactive Nerves in Human and Rhesus Monkey Eyes. Invest Ophthalmol Vis Sci. 1988;29:305–310. [PubMed] [Google Scholar]
- 367.Stone RA, McGlinn AM, Kuwayama Y, Grimes PA. Peptide Immunoreactivity of the Ciliary Ganglion and Its Accessory Cells in the Rat. Brain research. 1988;475:389–392. doi: 10.1016/0006-8993(88)90632-4. [DOI] [PubMed] [Google Scholar]
- 368.Stone RA, Tervo T, Tervo K, Tarkkanen A. Vasoactive Intestinal Polypeptide-Like Immunoreactive Nerves to the Human Eye. Acta Ophthalmol (Copenh) 1986;64:12–18. doi: 10.1111/j.1755-3768.1986.tb06865.x. [DOI] [PubMed] [Google Scholar]
- 369.Strohmaier CA, Reitsamer HA, Kiel JW. Episcleral Venous Pressure and Iop Responses to Central Electrical Stimulation in the Rat. Investigative ophthalmology & visual science. 2013;54:6860–6866. doi: 10.1167/iovs.13-12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sugimoto T, Itoh K, Mizuno N. Direct Projections from Edinger-Westphal Nucleus to Cerebellum and Spinal-Cord in Cat - Hrp Study. Neuroscience Letters. 1978;9:17–22. doi: 10.1016/0304-3940(78)90041-1. [DOI] [PubMed] [Google Scholar]
- 371.Sugimoto T, Itoh K, Mizuno N. Localization of Neurons Giving Rise to the Oculomotor Parasympathetic Outflow: A Hrp Study in Cat. Neurosci Lett. 1978;7:301–305. doi: 10.1016/0304-3940(78)90217-3. [DOI] [PubMed] [Google Scholar]
- 372.Sugiura S, Yamaga C. [Studies on the Adrenergic Nerve of the Cornea] Nihon Ganka Gakkai Zasshi. 1968;72:872–879. [PubMed] [Google Scholar]
- 373.Sun WS, May PJ. Organization of the Extraocular and Preganglionic Motoneurons Supplying the Orbit in the Lesser Galago. Anatomical Record. 1993;237:89–103. doi: 10.1002/ar.1092370109. [DOI] [PubMed] [Google Scholar]
- 374.Suzuki N, Hardebo JE, Owman C. Trigeminal Fibre Collaterals Storing Substance P and Calcitonin Gene-Related Peptide Associate with Ganglion Cells Containing Choline Acetyltransferase and Vasoactive Intestinal Polypeptide in the Sphenopalatine Ganglion of the Rat. An Axon Reflex Modulating Parasympathetic Ganglionic Activity? Neuroscience. 1989;30:595–604. doi: 10.1016/0306-4522(89)90154-1. [DOI] [PubMed] [Google Scholar]
- 375.Takagi M, Abe H, Toda H, Usui T. Accommodative and Pupillary Responses to Sinusoidal Target Depth Movement. OPHTHAL PHYSL OPT. 1993;13:253–257. doi: 10.1111/j.1475-1313.1993.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 376.Takemura A, Inoue Y, Kawano K, Quaia C, Miles FA. Single-Unit Activity in Cortical Area Mst Associated with Disparity-Vergence Eye Movements: Evidence for Population Coding. Journal of neurophysiology. 2001;85:2245–2266. doi: 10.1152/jn.2001.85.5.2245. [DOI] [PubMed] [Google Scholar]
- 377.Tamm ER. The Trabecular Meshwork Outflow Pathways: Structural and Functional Aspects. Experimental eye research. 2009;88:648–655. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 378.Tanaka T. Ganglion Sphenopalatinum Des Menschen. Abt Anat Inst Kais Univ (Kyoto) Ser A. 1932;3:91–115. [Google Scholar]
- 379.ten Tusscher MP, Beckers HJ, Vrensen GF, Klooster J. Peripheral Neural Circuits Regulating Iop? A Review of Its Anatomical Backbone. Doc Ophthalmol. 1994;87:291–313. doi: 10.1007/BF01203340. [DOI] [PubMed] [Google Scholar]
- 380.Ten Tusscher MP, Klooster J, Baljet B, Van der Werf F, Vrensen GF. Pre- and Post-Ganglionic Nerve Fibres of the Pterygopalatine Ganglion and Their Allocation to the Eyeball of Rats. Brain Res. 1990;517:315–323. doi: 10.1016/0006-8993(90)91043-g. [DOI] [PubMed] [Google Scholar]
- 381.Tentusscher MPM, Klooster J, Vanderwant JJL, Lamers W, Vrensen G. The Allocation of Nerve-Fibers to the Anterior Eye Segment and Peripheral Ganglia of Rats .2. The Sympathetic Innervation. Brain Research. 1989;494:105–113. doi: 10.1016/0006-8993(89)90148-0. [DOI] [PubMed] [Google Scholar]
- 382.Terenghi G, Polak JM, Allen JM, Zhang SQ, Unger WG, Bloom SR. Neuropeptide Y-Immunoreactive Nerves in the Uvea of Guinea Pig and Rat. Neuroscience letters. 1983;42:33–38. doi: 10.1016/0304-3940(83)90417-2. [DOI] [PubMed] [Google Scholar]
- 383.Terenghi G, Polak JM, Ghatei MA, Mulderry PK, Butler JM, Unger WG, Bloom SR. Distribution and Origin of Calcitonin Gene-Related Peptide (Cgrp) Immunoreactivity in the Sensory Innervation of the Mammalian Eye. J Comp Neurol. 1985;233:506–516. doi: 10.1002/cne.902330410. [DOI] [PubMed] [Google Scholar]
- 384.Terenghi G, Polak JM, Probert L, McGregor GP, Ferri GL, Blank MA, Butler JM, Unger WG, Zhang S, Cole DF, Bloom SR. Mapping, Quantitative Distribution and Origin of Substance P- and Vip-Containing Nerves in the Uvea of Guinea Pig Eye. Histochemistry. 1982;75:399–417. doi: 10.1007/BF00496742. [DOI] [PubMed] [Google Scholar]
- 385.Tervo K, Tervo T, Eranko L, Eranko O, Cuello AC. Immunoreactivity for Substance P in the Gasserian Ganglion, Ophthalmic Nerve and Anterior Segment of the Rabbit Eye. Histochem J. 1981;13:435–443. doi: 10.1007/BF01005059. [DOI] [PubMed] [Google Scholar]
- 386.Tervo K, Tervo T, Eranko L, Vannas A, Cuello AC, Eranko O. Substance P-Immunoreactive Nerves in the Human Cornea and Iris. Invest Ophthalmol Vis Sci. 1982;23:671–674. [PubMed] [Google Scholar]
- 387.Tervo T, Palkama A. Innervation of the Rabbit Cornea. A Histochemical and Electron-Microscopic Study. Acta anatomica. 1978;102:164–175. [PubMed] [Google Scholar]
- 388.Thieme H, Hildebrandt J, Choritz L, Strauss O, Wiederholt M. Muscarinic Receptors of the M2 Subtype in Human and Bovine Trabecular Meshwork. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:310–315. doi: 10.1007/s004170100288. [DOI] [PubMed] [Google Scholar]
- 389.Thomas DM, Kaufman RP, Sprague JM, Chambers WW. Experimental Studies of the Vermal Cerebellar Projections in the Brain Stem of the Cat (Fastigiobulbar Tract) J Anat. 1956;90:371–385. [PMC free article] [PubMed] [Google Scholar]
- 390.Thompson HS. The Pupil. In: Adler FH, Hart WM, editors. Adler's Physiology of the Eye: Clinical Application. 9th. St. Louis: Mosby Year Book; 1992. pp. 412–441. [Google Scholar]
- 391.Toda M, Okamura T, Ayajiki K, Toda N. Neurogenic Vasoconstriction as Affected by Cholinergic and Nitroxidergic Nerves in Dog Ciliary and Ophthalmic Arteries. Invest Ophthalmol Vis Sci. 1999;40:1753–1760. [PubMed] [Google Scholar]
- 392.Toda N, Ayajiki K, Yoshida K, Kimura H, Okamura T. Impairment by Damage of the Pterygopalatine Ganglion of Nitroxidergic Vasodilator Nerve Function in Canine Cerebral and Retinal Arteries. Circ Res. 1993;72:206–213. doi: 10.1161/01.res.72.1.206. [DOI] [PubMed] [Google Scholar]
- 393.Toda N, Toda M, Ayajiki K, Okamura T. Monkey Central Retinal Artery Is Innervated by Nitroxidergic Vasodilator Nerves. Investigative ophthalmology & visual science. 1996;37:2177–2184. [PubMed] [Google Scholar]
- 394.Toivanen M, Tervo T, Partanen M, Vannas A, Hervonen A. Histochemical Demonstration of Adrenergic Nerves in the Stroma of Human Cornea. Investigative ophthalmology & visual science. 1987;28:398–400. [PubMed] [Google Scholar]
- 395.Tornqvist G. Effect of Cervical Sympathetic Stimulation on Accommodation in Monkeys. An Example of a Beta-Adrenergic, Inhibitory Effect. Acta physiologica Scandinavica. 1966;67:363–372. doi: 10.1111/j.1748-1716.1966.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 396.Tornqvist G. The Relative Importance of the Parasympathetic and Sympathetic Nervous Systems for Accommodation in Monkeys. Invest Ophthalmol. 1967;6:612–617. [PubMed] [Google Scholar]
- 397.Toth IE, Boldogkoi Z, Medveczky I, Palkovits M. Lacrimal Preganglionic Neurons Form a Subdivision of the Superior Salivatory Nucleus of Rat: Transneuronal Labelling by Pseudorabies Virus. Journal of the Autonomic Nervous System. 1999;77:45–54. doi: 10.1016/s0165-1838(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 398.Townsend DJ, Brubaker RF. Immediate Effect of Epinephrine on Aqueous Formation in the Normal Human Eye as Measured by Fluorophotometry. Investigative ophthalmology & visual science. 1980;19:256–266. [PubMed] [Google Scholar]
- 399.Toyoshima K, Kawana E, Sakai H. Neuronal Origin of the Afferents to the Ciliary Ganglion in Cat. Brain Research. 1980;185:67–76. doi: 10.1016/0006-8993(80)90671-x. [DOI] [PubMed] [Google Scholar]
- 400.Troilo D, Wallman J. Changes in Corneal Curvature During Accommodation in Chicks. Vision Res. 1987;27:241–247. doi: 10.1016/0042-6989(87)90186-6. [DOI] [PubMed] [Google Scholar]
- 401.Tsukahara N, Kiyoara T, Ijichi Y. The Mode of Cerebellar Control of Pupillary Light Reflex. Brain research. 1973;60:244–248. doi: 10.1016/0006-8993(73)90864-0. [DOI] [PubMed] [Google Scholar]
- 402.Uddman R, Alumets J, Ehinger B, Hakanson R, Loren I, Sundler F. Vasoactive Intestinal Peptide Nerves in Ocular and Orbital Structures of the Cat. Invest Ophthalmol Vis Sci. 1980;19:878–885. [PubMed] [Google Scholar]
- 403.Unger WG. Review: Mediation of the Ocular Response to Injury. Journal of ocular pharmacology. 1990;6:337–353. doi: 10.1089/jop.1990.6.337. [DOI] [PubMed] [Google Scholar]
- 404.Usui S, Ikuno Y, Miki A, Matsushita K, Yasuno Y, Nishida K. Evaluation of the Choroidal Thickness Using High-Penetration Optical Coherence Tomography with Long Wavelength in Highly Myopic Normal-Tension Glaucoma. American journal of ophthalmology. 2012;153:10–16 e11. doi: 10.1016/j.ajo.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 405.Uusitalo H, Krootila K, Palkama A. Calcitonin Gene-Related Peptide (Cgrp) Immunoreactive Sensory Nerves in the Human and Guinea Pig Uvea and Cornea. Exp Eye Res. 1989;48:467–475. doi: 10.1016/0014-4835(89)90030-4. [DOI] [PubMed] [Google Scholar]
- 406.Uusitalo H, Lehtosalo J, Palkama A, Toivanen M. Vasoactive Intestinal Polypeptide (Vip)-Like Immunoreactivity in the Human and Guinea-Pig Choroid. Exp Eye Res. 1984;38:435–437. doi: 10.1016/0014-4835(84)90198-2. [DOI] [PubMed] [Google Scholar]
- 407.Uusitalo H, Lehtosalo JI, Palkama A. Vasoactive Intestinal Polypeptide (Vip)-Immunoreactive Nerve Fibers in the Anterior Uvea of the Guinea Pig. Ophthalmic Res. 1985;17:235–240. doi: 10.1159/000265378. [DOI] [PubMed] [Google Scholar]
- 408.Uusitalo R. Effect of Sympathetic and Parasympathetic Stimulation on the Secretion and Outflow of Aqueous Humour in the Rabbit Eye. Acta physiologica Scandinavica. 1972;86:315–326. doi: 10.1111/j.1748-1716.1972.tb05338.x. [DOI] [PubMed] [Google Scholar]
- 409.van der Werf F, Baljet B, Prins M, Otto JA. Innervation of the Lacrimal Gland in the Cynomolgous Monkey: A Retrograde Tracing Study. J Anat. 1996;188(Pt 3):591–601. [PMC free article] [PubMed] [Google Scholar]
- 410.Vilupuru AS, Glasser A. Dynamic Accommodation in Rhesus Monkeys. Vision Research. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 411.Wahlestedt C, Bynke G, Beding B, von Leithner P, Hakanson R. Neurogenic Mechanisms in Control of the Rabbit Iris Sphincter Muscle. European journal of pharmacology. 1985;117:303–309. doi: 10.1016/0014-2999(85)90003-2. [DOI] [PubMed] [Google Scholar]
- 412.Wang B, Ciuffreda KJ. Depth-of-Focus of the Human Eye: Theory and Clinical Implications. Surv Ophthalmol. 2006;51:75–85. doi: 10.1016/j.survophthal.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 413.Warwick R. The Ocular Parasympathetic Nerve Supply and Its Mesencephalic Sources. J Anat. 1954;88:71–93. [PMC free article] [PubMed] [Google Scholar]
- 414.Weber JT, Harting JK. The Efferent Projections of the Pretectal Complex: An Autoradiographic and Horseradish Peroxidase Analysis. Brain Res. 1980;194:1–28. doi: 10.1016/0006-8993(80)91315-3. [DOI] [PubMed] [Google Scholar]
- 415.Weinstein JM, Duckrow RB, Beard D, Brennan RW. Regional Optic Nerve Blood Flow and Its Autoregulation. Invest Ophthalmol Vis Sci. 1983;24:1559–1565. [PubMed] [Google Scholar]
- 416.Weinstein JM, Funsch D, Page RB, Brennan RW. Optic Nerve Blood Flow and Its Regulation. Investigative ophthalmology & visual science. 1982;23:640–645. [PubMed] [Google Scholar]
- 417.Westheimer G. Pupil Size and Visual Resolution. Vision Res. 1964;4:39–45. doi: 10.1016/0042-6989(64)90030-6. [DOI] [PubMed] [Google Scholar]
- 418.Westheimer G, Blair SM. The Parasympathetic Pathways to Internal Eye Muscles. Investigative ophthalmology. 1973;12:193–197. [PubMed] [Google Scholar]
- 419.Westphal C. Ueber Einen Fall Von Chronischer Progressiver Lähmung Der Augenmuskeln (Ophthalmoplegia Externa) Nebst Beschreibung Von Ganglienzellengruppen in Bereiche Des Oculomotoriuskerns. Arch Psychiat Nervenkr. 1887;18:846–871. [Google Scholar]
- 420.Wiederholt M, Bielka S, Schweig F, Lutjen-Drecoll E, Lepple-Wienhues A. Regulation of Outflow Rate and Resistance in the Perfused Anterior Segment of the Bovine Eye. Experimental eye research. 1995;61:223–234. doi: 10.1016/s0014-4835(05)80042-9. [DOI] [PubMed] [Google Scholar]
- 421.Wiederholt M, Schafer R, Wagner U, Lepple-Wienhues A. Contractile Response of the Isolated Trabecular Meshwork and Ciliary Muscle to Cholinergic and Adrenergic Agents. Ger J Ophthalmol. 1996;5:146–153. [PubMed] [Google Scholar]
- 422.Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of Trabecular Meshwork and Ciliary Muscle by Release of Nitric Oxide. Investigative ophthalmology & visual science. 1994;35:2515–2520. [PubMed] [Google Scholar]
- 423.Wisco JJ, Stark ME, Safir I, Rahman S. A Heat Map of Superior Cervical Ganglion Location Relative to the Common Carotid Artery Bifurcation. Anesth Analg. 2012;114:462–465. doi: 10.1213/ANE.0b013e31823b676d. [DOI] [PubMed] [Google Scholar]
- 424.Wong KY. A Retinal Ganglion Cell That Can Signal Irradiance Continuously for 10 Hours. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:11478–11485. doi: 10.1523/JNEUROSCI.1423-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Wong KY, Dunn FA, Berson DM. Photoreceptor Adaptation in Intrinsically Photosensitive Retinal Ganglion Cells. Neuron. 2005;48:1001–1010. doi: 10.1016/j.neuron.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 426.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic Influences on Rat Ganglion-Cell Photoreceptors. The Journal of physiology. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Woodhouse JM, Campbell FW. The Role of the Pupil Light Reflex in Aiding Adaptation to the Dark. Vision Res. 1975;15:649–653. doi: 10.1016/0042-6989(75)90279-5. [DOI] [PubMed] [Google Scholar]
- 428.Yamamoto R, Bredt DS, Snyder SH, Stone RA. The Localization of Nitric Oxide Synthase in the Rat Eye and Related Cranial Ganglia. Neuroscience. 1993;54:189–200. doi: 10.1016/0306-4522(93)90393-t. [DOI] [PubMed] [Google Scholar]
- 429.Ye XD, Laties AM, Stone RA. Peptidergic Innervation of the Retinal Vasculature and Optic Nerve Head. Invest Ophthalmol Vis Sci. 1990;31:1731–1737. [PubMed] [Google Scholar]
- 430.Young MJ, Lund RD. The Anatomical Substrates Subserving the Pupillary Light Reflex in Rats: Origin of the Consensual Pupillary Response. Neuroscience. 1994;62:481–496. doi: 10.1016/0306-4522(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 431.Yu Y, Koss MC. Alpha2-Adrenoceptors Do Not Mediate Reflex Mydriasis in Rabbits. Journal of Ocular Pharmacology and Therapeutics. 2004;20:479–488. doi: 10.1089/jop.2004.20.479. [DOI] [PubMed] [Google Scholar]
- 432.Zagvazdin Y, Fitzgerald ME, Reiner A. Role of Muscarinic Cholinergic Transmission in Edinger-Westphal Nucleus-Induced Choroidal Vasodilation in Pigeon. Exp Eye Res. 2000;70:315–327. doi: 10.1006/exer.1999.0791. [DOI] [PubMed] [Google Scholar]
- 433.Zamora DO, Kiel JW. Topical Proparacaine and Episcleral Venous Pressure in the Rabbit. Investigative ophthalmology & visual science. 2009;50:2949–2952. doi: 10.1167/iovs.08-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Zhang H, Clarke RJ, Gamlin PDR. Behavior of Luminance Neurons in the Pretectal Olivary Nucleus During the Pupillary near Response. Exp Brain Res. 1996;112:158–162. doi: 10.1007/BF00227189. [DOI] [PubMed] [Google Scholar]
- 435.Zhang H, Gamlin PDR. Neurons in the Posterior Interposed Nucleus of the Cerebellum Related to Vergence and Accommodation. I. Steady-State Characteristics. Journal of Neurophysiology. 1998;79:1255–1269. doi: 10.1152/jn.1998.79.3.1255. [DOI] [PubMed] [Google Scholar]
- 436.Zhang HY, Gamlin PDR. Neurons in the Posterior Interposed Nucleus of the Cerebellum Related to Vergence and Accommodation. I. Steady-State Characteristics. Journal of Neurophysiology. 1998;79:1255–1269. doi: 10.1152/jn.1998.79.3.1255. [DOI] [PubMed] [Google Scholar]
- 437.Zhang SQ, Butler JM, Cole DF. Sensory Neural Mechanisms in Contraction of the Rabbit Isolated Sphincter Pupillae: Analysis of the Responses to Capsaicin and Electrical Field Stimulation. Experimental eye research. 1984;38:153–163. doi: 10.1016/0014-4835(84)90099-x. [DOI] [PubMed] [Google Scholar]
- 438.Zhang Y, Gamlin PDR, Mays LE. Antidromic Identification of Midbrain near Response Cells Projecting to the Oculomotor Nucleus. Experimental Brain Research. 1991;84:525–528. doi: 10.1007/BF00230964. [DOI] [PubMed] [Google Scholar]
- 439.Zhang Y, Mays LE, Gamlin PDR. Characteristics of near Response Cells Projecting to the Oculomotor Nucleus. Journal of Neurophysiology. 1992;67:944–960. doi: 10.1152/jn.1992.67.4.944. [DOI] [PubMed] [Google Scholar]
- 440.Zhu BS, Gai WP, Yu YH, Gibbins IL, Blessing WW. Preganglionic Parasympathetic Salivatory Neurons in the Brainstem Contain Markers for Nitric Oxide Synthesis in the Rabbit. Neurosci Lett. 1996;204:128–132. doi: 10.1016/0304-3940(96)12338-7. [DOI] [PubMed] [Google Scholar]
- 441.Zhu BS, Gibbins IL, Blessing WW. Preganglionic Parasympathetic Neurons Projecting to the Sphenopalatine Ganglion Contain Nitric Oxide Synthase in the Rabbit. Brain Res. 1997;769:168–172. doi: 10.1016/s0006-8993(97)00844-5. [DOI] [PubMed] [Google Scholar]