Abstract
Background
Prisoners have a high prevalence of Hepatitis C virus (HCV), but case-finding may not have been cost-effective because treatment often exceeded average prison stay combined with a lack of continuity-of-care. We assess the cost-effectiveness of increased HCV case-finding and treatment in UK prisons using short-course therapies.
Methods
A dynamic HCV transmission model assesses the cost-effectiveness of doubling HCV case-finding (achieved through introducing opt-out HCV testing in UK pilot prisons) and increasing treatment in UK prisons, compared to status-quo voluntary risk-based testing (6% prison entrants/year), using currently recommended therapies (8–24 weeks) or IFN-free DAAs (8–12 weeks, 95% SVR, £3300/wk). Costs (GBP£) and health utilities (quality-adjusted life-years, QALYs) were used to calculate mean incremental cost-effectiveness ratios (ICERs). We assume 56% referral and 2.5%/25% of referred people who inject drugs (PWID)/exPWID treated within 2 months of diagnosis in prison. PWID and ex/nonPWID are in prison an average 4/8 months, respectively.
Results
Doubling prison testing rates with existing treatments produces a mean ICER of £19,850/QALY gained compared to current testing/treatment, and is 45% likely to be cost-effective under a £20,000 willingness-to-pay (WTP) threshold. Switching to 8–12 week IFN-free DAAs in prisons could increase cost-effectiveness (ICER £15,090/QALY gained). Excluding prevention benefit decreases cost-effectiveness. If >10% referred PWID are treated in prison (2.5% base-case), either treatment could be highly cost-effective (ICER<£13,000). HCV case-finding and IFN-free DAAs could be highly cost-effective if DAA cost is 10% lower or 8 weeks duration.
Conclusions
Increased HCV testing in UK prisons (such as through opt-out testing) is borderline cost-effective compared to status-quo voluntary risk-based testing under a £20,000 WTP with current treatments, but likely to be cost-effective if short-course IFN-free DAAs are used, and could be highly cost-effective if PWID treatment rates were increased.
Keywords: people who inject drugs, prison, testing, hepatitis C, economic evaluation
INTRODUCTION
Hepatitis C virus (HCV) is a blood borne virus which can result in cirrhosis, liver failure, hepatocellular carcinoma, and death. In most developed country settings the majority of transmission occurs among people who inject drugs (PWID). In the UK, >90% of ongoing transmission occurs among PWID, and >80% of chronic HCV infection is among current or ex-PWID (1). Previous treatments with pegylated interferon and ribavirin (pegIFN+RBV) were long (24–48 weeks), poorly tolerated, and cured roughly half of individuals. The advent of new short-course, better tolerated, and highly effective HCV treatments which can cure >80% of individuals makes identifying those who are chronically infected an urgent priority (2). HCV case-finding in specialist drug clinics and primary care is recommended and cost-effective, especially with increased treatment rates (3, 4).
Although there are high numbers and proportions of people who inject drugs (PWID) in prison, testing rates are relatively low (6% prison entrants were tested for HCV in England in 2013 (5)). Previous analyses suggested the cost-effectiveness of increasing HCV case-finding in UK prisons was conditional on a high level of continuity of care between prison and community, and unlikely to be cost-effective in the absence of any continuity (3). This was because of the conflict between short incarceration times for PWID (estimated average 4 months in England) and long treatment durations (24–48 weeks) required with pegIFN+RBV treatment.
Current standard of care in UK prisons is voluntary HCV testing offered to prisoners who consider themselves at risk, leading to approximately 6% of prison entrants tested per year. In 2014, opt-out testing for BBVs (HCV, HBV and HIV) was introduced in selected prisons in England (6), which doubled the numbers of HCV tests in these pilot prisons. Hence, this opt-out programme is likely to increase the numbers tested and initiated onto HCV treatment from within the prison setting. However sub-optimal treatment delivery (roughly half of HCV infected are referred to specialist care with one-quarter of those referred initiating treatment (7), of which many will have interrupted treatment due to prison release) may limit the benefit of testing interventions. Shorter duration (8–12 weeks) interferon-free direct-acting antiviral therapy (IFN-free DAAs) with high SVR rates (>90%) (2) could allow more to successfully complete treatment within their prison stay.
We assess the cost-effectiveness of increased HCV testing and treatment rates in English prisons (such as through opt-out programmes which doubled testing in UK pilot prisons), compared to status quo testing rates, combined with currently available HCV treatments or future 8–12 week IFN-free DAA therapy, including individual and prevention benefits.
METHODS
Mathematical Model
We adapt a dynamic model of incarceration and HCV transmission among PWID in England (3, 4). Full details of the model structure and calibration can be found in previous publications (3, 4) and the supplementary information. Overall, the model tracks incarceration, initiation and cessation of injecting, HCV transmission among PWID only (in both prison and the community; imprisoned PWID can only transmit to other prisoners), and HCV testing and treatment through various settings present in the UK (prison, addiction services, GP, and other). The model is dynamic, in that a PWID’s risk of acquiring HCV is proportional to the setting-specific HCV prevalence (prison/community) which can change over time. The model simulates the background rate of testing and treatment occurring in the community and prison, such that individuals can be identified in either setting.
The model includes stratification by injecting state (never PWID/PWID/former PWID); incarceration status (never/currently/formerly); contact with addiction services (in contact/not in contact); age ((15–19), (20–24), (30–54), (55–64), (65–74), (75+)); HCV infection and disease progression (never infected, spontaneously cleared, mild HCV, moderate HCV, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, liver transplant, post-transplant). HCV disease stages are further subdivided into undiagnosed or diagnosed, where those who are diagnosed can either be lost to follow-up, in referral, on antiviral treatment, achieved sustained viral response (SVR) or non-SVR). Because no data are available on continuity of care between the prison and community, we assume that those who are in referral or on treatment are lost-to followup when entering/leaving prison in line with our previous analyses (3, 4).
We include updated treatment SVR and durations with new and upcoming IFN-free DAA therapies and include progression for those in the cirrhosis SVR stage (at reduced rates compared to non-SVR) in accordance with recent data.
Model calibration
We perform a probabilistic uncertainty analysis where 1000 parameter sets were sampled from each parameter uncertainty distribution in Table 1. For each parameter set, the model was calibrated to UK epidemiological data on incarceration, injecting drug use, HCV prevalence and diagnosis. Further calibration details can be found in our previous publications (3, 4) and supplementary information. After calibration, for each of the 1000 parameter sets, the model was used to simulate the baseline and intervention scenarios.
Table 1.
Model parameters.
| Mean value | Distribution | Reference | |
|---|---|---|---|
| Transition probabilities per year (all probabilities converted to instantaneous rates) | |||
|
| |||
| Mild to moderate | 0.025 | Beta(α=38.0859, β=1485.3516) | (18) |
| Moderate to compensated cirrhosis (CC) | 0.037 | Beta(α=26.905, β=700.2582) | (18) |
| CC to decompensated cirrhosis (DC) | 0.039 | Beta(α=14.6168, β=360.1732) | (18) |
| CC/DC to HCC | 0.014 | Beta(α=1.9326, β=136.1074) | (18) |
| CC SVR to DC (Relative risk of non-SVR) | 0.07 | Lognormal (95% CI 0.03, 0.20) | (19, 20) |
| CC/DC SVR to HCC (Rel risk of non-SVR) | 0.23 | Lognormal (95%CI 0.16, 0.35) | (20) |
| DC/HCC to LT | 0.03 | Beta(α=6.5256, β=210.9945) | (18) |
| DC to death | 0.13 | Beta(α=147.03, β=983.97) | (18) |
| HCC to death | 0.43 | Beta(α=117.1033, β=155.23) | (18) |
| LT to death | 0.21 | Beta(α=16.2762, β=61.2294) | (18) |
| Post transplant to death | 0.057 | Beta(α=22.9017, β=378.8825) | (18) |
|
| |||
| Health state utilities/disutilities per year | |||
|
| |||
| Ex-PWID age 15–19 | |||
| Uninfected | 0.94 | (37) | |
| Mild | 0.77 | Beta(α=521.2375, β=155.6943) | (18, 38) |
| Moderate | 0.66 | Beta(α=168.2461, β=86.6723) | (18, 38) |
| Cirrhosis | 0.55 | Beta(α=47.1021, β=38.5381) | (18, 38) |
| Decompensated cirrhosis | 0.45 | Beta(α=123.75, β=151.25) | (18, 38) |
| Hepatocellular carcinoma | 0.45 | Beta(α=123.75, β=151.25) | (18, 38) |
| Liver transplant | 0.45 | Beta(α=123.75, β=151.25) | (18, 38) |
| Post transplant | 0.67 | Beta(α=59.2548, β=29.1852) | (38, 39) |
| Treatment IFN-containing, decrement | 0.11 | (18, 38) | |
| Treatment IFN-free, decrement | 0.06 | (8) | |
| Mild SVR | 0.82 | Beta(α=65.8678, β=14.4588) | (18, 38) |
| Moderate SVR | 0.72 | Beta(α=58.0608, β=22.5792) | (18, 33, 38) |
| Cirrhosis SVR | 0.61 | Beta(α=58.0476, β=37.1124) | (39) |
| PWID age 15–19 | |||
| Uninfected | 0.74 | Uniform (0.67,0.8) | (40) |
| HCV disease states | As in ex-PWID, but reduced by PropPWID† | Assumed | |
| Disutility with age | |||
| 20–24 | 0 | (37) | |
| 25–29 | 0.005 | (37) | |
| 30–54 | 0.049 | (37) | |
| 55–64 | 0.14 | (37) | |
| 65–74 | 0.16 | (37) | |
| 75+ | 0.21 | (37) | |
|
| |||
| Costs (£ per year, except where noted) | |||
|
| |||
| Mild diagnosed | 178 | PPI‡×Gamma(k=25.6995, θ=5.3698) | (18, 38) |
| Moderate diagnosed | 925 | PPI‡×Gamma(k=88.8502, θ=8.0698) | (18, 38) |
| Cirrhosis diagnosed | 1,468 | PPI‡×Gamma(k=24.2342, θ=46.9584) | (18, 38) |
| Decompensated cirrhosis | 11,765 | PPI‡×Gamma(k=36.0249, θ=253.1582) | (18, 38) |
| Hepatocellular carcinoma | 10,484 | PPI‡×Gamma(k=18.1081, θ=448.8045) | (18) |
| Liver transplant (per transplant) | 32,256 | PPI‡×Gamma(k=89.7536, θ=304.5004) | (18) |
| Cost of care in year of liver transplant | 12,201 | PPI‡×Gamma(k=13.7788, θ=686.4168) | (18) |
| Post transplant | 1,787 | PPI‡×Gamma(k=15.2189, θ=91.0053) | (18) |
| Mild SVR | 334 | PPI‡×Gamma(k=28.8141, θ=8.9887) | (18) |
| Moderate SVR | 925 | PPI‡×Gamma(k=88.8502, θ=8.0698) | (18) |
| Cirrhosis SVR | 1,468 | PPI‡×Gamma(k=24.2342, θ=46.9584) | (18) |
| Undiagnosed states | 0 | ||
| Drug costs, per week* | |||
| pegIFN+RBV | 228 | (23) | |
| Sofosbuvir+PR | 3143 | (23) | |
| Sofosbuvir/Ledipasvir | 3248 | NHS List price | |
| ‘Future’ IFN-free DAAs | 3300 | ||
| Treatment delivery, per week* | |||
| Ex-PWID, per week | 90 | (18) | |
| PWID (proportion ex-PWID cost) | 120% | (3, 22) | |
| Testing costs in community^ | 121 | Uniform +/− 50% | (3, 41) |
| Testing costs in prison^ | 151 | Uniform +/− 60% | (3, 41) |
| PCR RNA test (if antibody positive) | 78 | Uniform +/− 20% | (3, 41) |
|
| |||
| Testing and treatment parameters | |||
|
| |||
| Proportion PWID diagnosed (initial) | 50% | (5) | |
| Proportion PWID treated (initial) | 0% | Assumption | |
| Proportion ex-PWID diagnosed (initial) | 30% | Uniform (24%, 36%) | Assumption (14) |
| Proportion of diagnosed ex-PWID treated (initial) | 10% | Uniform (5%, 15%) | (42) |
| HCV genotype (proportion)* | |||
| Genotype 1 (G1) | 45% | (5) | |
| Genotype 2 (G2) | 5% | ||
| Genotype 3 (G3) | 45% | ||
| Genotype 4 (G4) | 5% | ||
| Sustained viral response (SVR)* | |||
| IFN/RBV G2/G3 mild/mod | 0.8 | Uniform (0.75, 0.85) | (43, 44) |
| IFN/RBV G2 cirrhosis | 0.6 | 75% reduction from mild/mod | (45) |
| Harvoni G1/G4 noncirrhosis | 0.93 | Uniform (0.9, 0.96) | (46) |
| Harvoni G1/G4 cirrhosis | 0.94 | Triangular (min 0.9, max 0.99) | (47) |
| SOF+PR G3 cirrhosis | 0.83 | Triangular (min 0.52, max 0.98) | (48) |
| Future IFN-free DAAs | 0.95 | Assumption | |
| Antiviral treatment duration (weeks)* | |||
| Harvoni G1 noncirrhosis | 8 | (9) | |
| Harvoni G1 cirrhosis, G4 | 12 | (9) | |
| pegIFN/RBV G2 | 24 | (43) | |
| pegIFN/RBV G3 non-cirrhotic | 24 | (43) | |
| SOF+PR G3 cirrhotic | 12 | (8) | |
| Proportion referred | |||
| Prison | 56% | Uniform (41%, 70%) | (51) |
| Community | 86% | Uniform (80%, 90%) | |
| Proportion referred who initiate treatment within 1 year (excl. prison) | |||
| Ex-PWID | 50% | Uniform (40%, 60%) | (34, 49) |
| PWID | 5% | Uniform (1%, 10%) | Assumption (50) |
| Treatment initiation rate after first year in referral (excl. prison) per year | |||
| Ex-PWID | 10% | Uniform (5%, 15%) | Assumption |
| PWID | 3% | Uniform (1%, 5%) | Assumption (50) |
| Proportion referred who initiate treatment in prison within 2 months | |||
| Ex-PWID | 25% | Uniform (20%, 30%) | (6, 7) |
| PWID | 2.5% | Uniform (0%, 5%) | Assumption (50) |
| Yield (proportion tests Ab+) | |||
| GP | 2.7% | § | |
| Prison | 14.7% | § | |
| Addiction services | 17.7% | § | |
| Other | 1.7% | § | |
| Distribution of PWID HCV tests | |||
| GP | 38.4% | § | |
| Prison | 11.5% | § | |
| Addiction services | 29.4% | § | |
| Other | 20.7% | § | |
PropPWID=(uninfected PWID utility value for age 15–19)/(uninfected ex-PWID utility for age 15–19).
PPI=Hospital and Community Health Services Pay and Prices Index inflation factor.
Health Protection Agency (HPA) unpublished data from the 2010 Sentinel Surveillance.
Used to calculate an average weighted treatment cost, SVR, and treatment duration.
Includes assessment, pre-test discussion, test, post-test results, and ELISA test. Includes additional assessment time in prison (20 min with nurse)HCC= hepatocellular carcinoma; LT=liver transplant; SVR=sustained viral response; pegIFN=pegylated interferon; RBV= ribavirin
Baseline
The baseline scenario assumes status quo rates of HCV voluntary risk-based testing (mean 6% of prison entrants tested annually) and treatment with current (provisionally approved by NICE) therapies (8, 9). The current therapies modeled are: Genotype 1: Sofosbuvir/Ledipasvir for Genotype 1 noncirrhotics (8 weeks) and cirrhotics (12 weeks); Genotype 2: pegIFN+RBV for 24 weeks; Genotype 3: pegIFN+RBV for 24 weeks non-cirrhotics, and SOF+pegIFN+RBV for 12 weeks cirrhotics; Genotype 4: Sofosbuvir/Ledipasvir for 12 weeks. Based on an estimated genotype distribution of 50% genotype 1/4, 5% Genotype 2, and 45% Genotype 3, the mean average SVR for this scenario is 87%.
Intervention
We examine a doubling of HCV testing in prison (to a mean 12% of prison entrants per year) due to a scale-up of opt-out testing (as achieved during Phase 1 (6)) with various treatment scenarios:
Status quo treatments: Doubling of HCV testing in prison with current treatments (as in the baseline, mean average SVR 87%) in prison and the community
8–12 week IFN-free DAAs: Doubling of HCV testing in prison with 8–12 week IFN-free DAAs with 95% SVR in prison (8 weeks G1 noncirrhotics, 12 weeks all others), and current treatments (as in the baseline) in the community
Treatments as in (1) and (2) but with treatment scale-up for PWID: The scenarios above (Status quo; 8–12 week IFN-free DAAs) but with varied levels of HCV treatment for PWID in prison (up to 25% after referral, compared with a mean of 2.5% at base-case).
Cost-effectiveness methods
We perform our analysis from the UK National Health Service (NHS) perspective as HCV testing and treatment are paid for by the NHS. Costs (in 2014 GBP,£1=$1.50) and health utilities (in quality adjusted life-years, QALYs) were attached to each model state, and discounted at 3.5% per year as per the National Institute for Health and Care Excellence guidelines. We use a 100 year time horizon to accrue both individual and population benefits. We calculate the mean incremental cost-effectiveness ratio (ICER) by dividing the difference in mean costs by the difference in mean QALYs between the intervention and its comparator. We perform an incremental analysis where the ICERs are calculated for each intervention after ranking the alternatives from least to most costly. We determine cost-effectiveness using UK willingness-to-pay thresholds of £20,000 and £30,000 per QALY gained, and denote an intervention as highly cost-effective using a WTP threshold of £13,000 in line with a recent estimate of the where the UK WTP should lie (10). Cost-effectiveness acceptability curves are presented. Multiple one-way sensitivity analyses are undertaken.
Parameterization
HCV prevalence and cascade of care
We model a scenario with a 35% baseline HCV chronic prevalence among PWID (approximately 45% antibody prevalence among PWID as found in the UK (11), assuming 26% spontaneously clear the virus (12)). Based on differential incarceration rates by age, this results in a mean fitted HCV chronic prevalence among PWID of 35% in the community and 34% in prison, and an HCV incidence of 8.8 per 100 person-years (95%I 5.9–13.4 per 100py) in the community, and 8.3 per 100 person-years (95%I 5.8–12.7 per 100py) in prison. We assume HCV prevalence among PWID is at steady-state based on the stable prevalence exhibited among PWID in contact with drugs services from 2003–2013 (13). We assume that 50% of PWID (5) and 30% of ex-PWID (14) are diagnosed initially in 2014. Setting-specific testing rates in prison and the community for PWID and ex-PWID were estimated in (3, 4); see the supplementary information for more details on community testing and cascade of care assumptions. Overall, we estimate ~6% of prison entrants tested per year, consistent with reported testing rates prior to the introduction of opt-out testing (6% in 2013 (5)). Of those tested, ~15% are HCV Ab+. A recent UK study reported a 56% referral rate from testing services to specialist care in prison (51). The time from testing to treatment initiation in prison is unknown; estimated time to referral in prison is 4 weeks (6), so we assume treatment initiation within 2 months of diagnosis in prison. Among a national survey across English prisons in 2012, 28% of patients referred to specialist care commenced treatment in the same year (7), but it is unknown what proportion were PWID. Hence, we assume 25% of ex-PWID commence treatment within 2 months of diagnosis in prison. Community PWID treatment rates in the UK are low (<25 per 1000 PWID annually), and due to the short sentences for PWID (4 months) and challenges with continuity of care after transfer or release, it is likely prison treatment rates are similarly low. Hence, we assume that 2.5% (sampled from 0–5%) of those PWID referred to specialist care from prison commence treatment within 2 months at baseline.
Incarceration parameters
The model was parameterized and calibrated to detailed UK data on incarceration patterns among PWID and the general population. We calibrate the model to 10,000 prisoners incarcerated at a given time (approximately 5,000,000 total individuals based on a 0.2% incarceration prevalence among the general population (15, 16)). See (3, 4) and the supplementary information for more details.
HCV disease progression and health utilities
Parameters for HCV disease progression and health utilities were taken from published UK economic evaluations (3, 17, 18) and recent data on progression from cirrhosis after SVR (19, 20). These assumptions result in a mean of 12% progression to cirrhosis within 20 years of infection without treatment, consistent with published estimates (21).
Costs
Costs related to HCV disease stages, HCV testing, and HCV treatment were taken from previous UK economic evaluations (3, 18, 22) and the British National Formulary (23). We assume individuals with undiagnosed HCV would not incur health care costs until progressing to decompensated cirrhosis. Costs were inflated to 2014 GBP using the Health and Community Hospital Service pay and prices index. Although we include costs for testing, we do not include additional costs for the testing scale-up as no additional funding was provided to the pilot prisons of the opt-out programme.
Sensitivity analyses
We perform matched univariate sensitivity analyses on the following parameters: discount rate (0% and 6% compared to 3.5% at base-case), IFN-free SVR (90% compared to 95% at base-case), IFN-free treatment duration (8 weeks compared to 8–12 weeks at base-case), DAA drug cost (25% reduction compared to base-case), proportion referred for treatment (100% vs 56% at base-case), time horizon (50 compared to 100 years at base-case), yield (30% reduction compared to 0% at base-case), and no prevention benefit for 2.5% and 10% referred PWID treated scenarios (2.5% referred PWID treated and prevention benefit included in base-case).
FINDINGS
Doubling of HCV testing in prison with status quo treatments
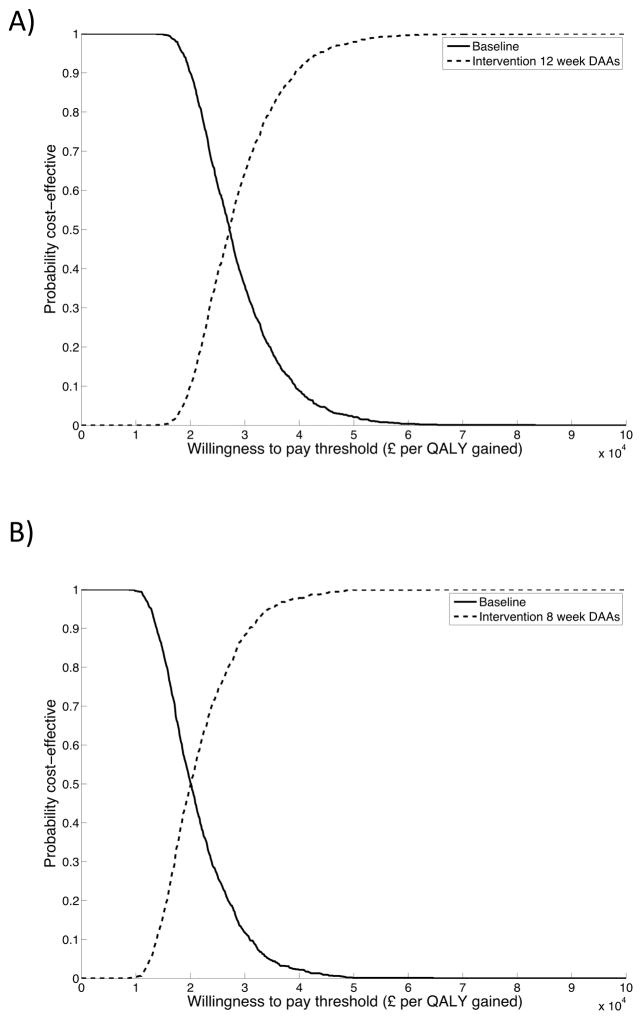
Doubling testing in prison and providing status quo HCV treatments results in approximately 2,400 HCV tests annually per 10,000 prisoners (~19,500 entrants), of which a mean of 353 are HCV Ab+, 261 are HCV RNA+, 146 are referred to treatment, and 21 are initiated onto treatment. Despite assuming a mean SVR of 87%, due to treatment interruption and assuming no continuity of care on release, the mean effective SVR is 40% (95%I 38–46). The intervention results in mean incremental costs of £8,362,599 [95%I £5,021,130–13,747,661] per 10,000 prisoners, and mean incremental QALYs gained of 421.27 [95%I 172.93–789.53] compared to status quo testing and treatment (Table 2). This strategy results in a mean ICER of £19,851 per QALY gained, and is 45% and 85% likely to be cost-effective at a £20,000 or £30,000 per QALY gained willingness to pay (WTP) threshold, respectively (Figure 1a).
Table 2.
Cost-effectiveness results.
| Mean incremental costs (£) per 10,000 prisoners* [95% Interval] | Mean incremental QALYs per 10,000 prisoners* [95% Interval] | Mean ICER (£ per QALY gained) | |
|---|---|---|---|
| Double testing and provide status quo treatment | 8,362,599† [5,021,130–13,747,661] | 421.27† [172.93–789.53] | 19,851† |
| Double testing and provide 12 week IFN-free DAA therapy in prison | 2,584,159‡ [872,364–6,078,955] | 171.25‡ [46.89–396.74] | 15,090‡ |
Compared to the status quo testing and treatment scenario.
Compared to the double testing and status quo treatment scenario.
Figure 1. Cost-effectiveness acceptability curves for doubled HCV case-finding in prison combined with (A) status quo treatments (B) 8–12 week IFN-free DAAs in prison.
Incremental comparisons shown are: (A) Doubled HCV case-finding in prison combined with status quo treatments compared to status quo testing/treatment; (B) Doubled HCV case-finding combined with 8–12 week IFN-free DAAs in prison compared to doubled case-finding with status quo treatments.
Doubling of HCV testing in prison with 8–12 week IFN-free DAAs with 95% SVR in prison
If HCV testing in prison is doubled and a switch to 8–12 week IFN-free DAA therapy is provided with 95% SVR in prison, costs increase due to provision of DAA therapy for all genotypes (mean incremental costs £2,584,159 [95%I £872,364–6,078,955] per 10,000 prisoners compared to doubled testing with status quo treatments) but QALYs are gained (171.25 [95%I 46.89–396.74]) due to a combination of increased SVR and shorter treatment duration for Genotype 2 and 3 noncirrhotics from 24 to 12 weeks (Table 2). Overall, this results in a mean ICER of £15,090 per QALY gained compared to doubled testing and status quo treatments, and is 84% and 96% likely to be cost-effective at a £20,000 or £30,000 per QALY gained willingness to pay (WTP) threshold, respectively (Figure 1b). Despite assuming a mean SVR of 95%, the mean effective SVR is 46% (95%I 43–53%). In this scenario, roughly half the benefit is due to shortening treatment; with only a shortening of treatment (but SVR rates equal to the status quo scenario) the intervention gains 79.67 QALYs [95%I 22.8–177.21]).
Doubling HCV testing with increased PWID HCV treatment rates in prison
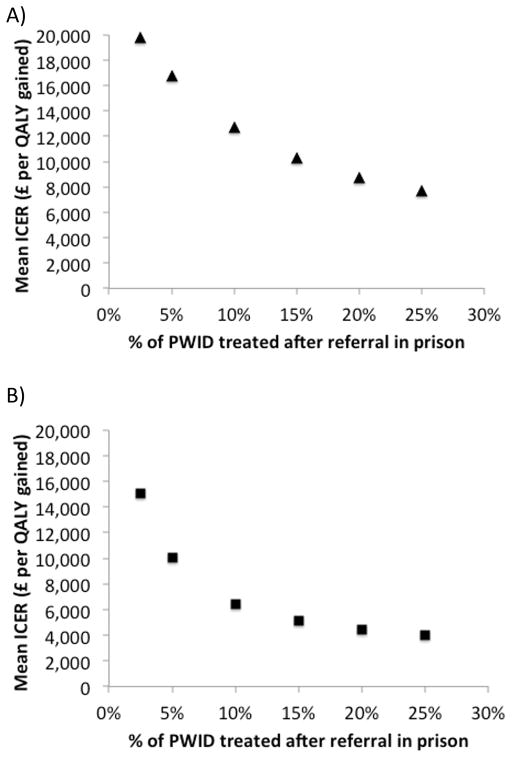
Increasing PWID treatment rates after referral in prison results in significant increases in the cost-effectiveness of doubled testing using current treatments (Figure 2a) or if 8–12 week IFN-free DAAs are introduced (Figure 2b). If 10% of PWID are treated after referral in prison, the ICERs for both treatment interventions drop below £13,000/QALY gained (£12,691/QALY with current treatments and £6,461/QALY gained with 8–12 week IFN-free DAAs). In this scenario, doubling testing with current treatments is 47% and 93% likely to be cost-effective under £13,000 and £20,000 WTP thresholds, respectively, and is 99% likely to be cost-effective under a £13,000 WTP threshold with 8–12 week IFN-free DAAs. If 25% of PWID are treated after referral, the ICERs drops to below £8,000 and £4,000 per QALY gained for the status quo treatment scenarios and 8–12 week IFN-free DAA scenarios, respectively.
Figure 2. Changes in mean ICER with increased PWID treatment rates in prison (2.5%-25% after referral).
Base-case analysis assumes a mean of 2.5% PWID treated after referral in prison. (a) Doubled testing with status quo treatments compared to status quo testing/treatment. (b) Double testing with 8–12 week IFN-free DAAs compared to doubled testing with status quo treatments.
Sensitivity analysis
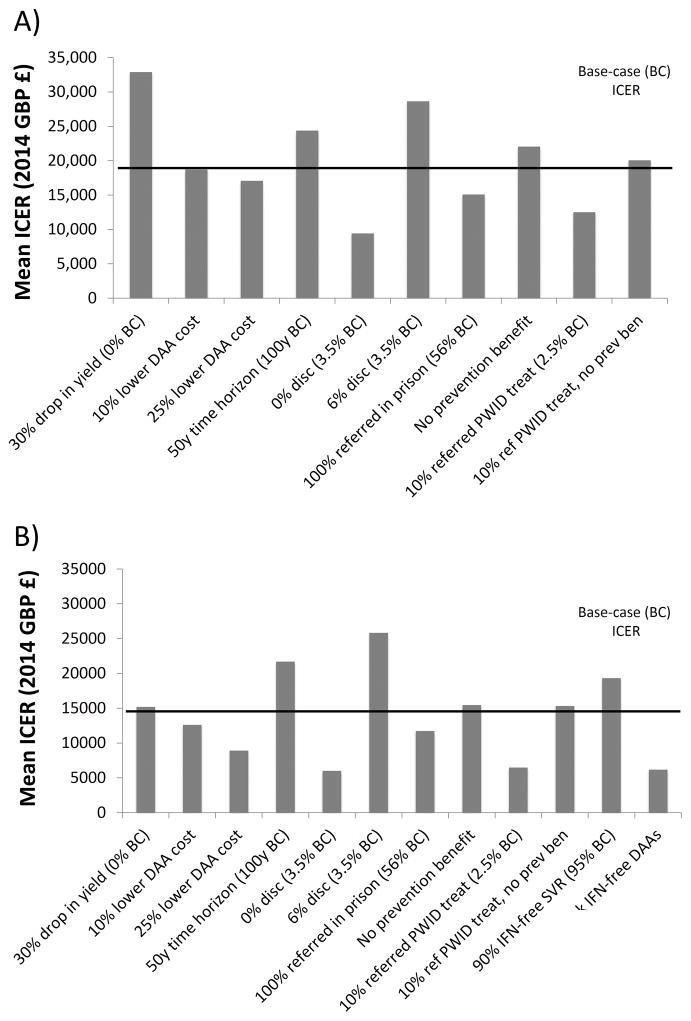
When performing a univariate sensitivity analysis on the mean ICER with status quo treatments (Figure 3a), we found that enhancements in the cascade of care increased cost-effectiveness and population prevention benefits increase as treatment rates for PWID increase. If 100% patients are referred in prison (56% at base-case), the mean ICER dropped to just over £15,000/QALY. Turning off the prevention benefit at base-case led to marginal reductions in cost-effectiveness because PWID treatment rates were very low at baseline so little prevention benefit is accrued (mean ICER £22,051/QALY gained vs £19,851/QALY gained with prevention benefits). However the prevention benefit substantially increases with increased treatment rates; treating 10% of referred PWID in prison leads to a mean ICER of £20,064/QALY gained without prevention benefits, but is highly cost-effective with prevention benefits included (mean ICER £12,495/QALY gained, Figure 3a).
Figure 3. One-way sensitivity analyses on mean ICERs.
Black horizontal line denotes the base-case ICER. (a) Doubled testing with status quo treatments compared to status quo testing/treatment. (b) Double testing with 8–12 week IFN-free DAAs compared to doubled testing with status quo treatments.
For all other analyses, the only analysis which increased the ICER above £30,000/QALY was if the doubled testing was associated with a 30% drop in yield (mean ICER £32,893; no change in base-case). Shortening the time horizon (50 years compared to 100 years at base-case), increasing the discount rate (6% compared to 3.5% at base-case) increased the mean ICER, but it remained below £30,000/QALY. (Figure 3A). Reducing the discount rate to 0% (3.5%/yr at base-case) and lowering the cost of DAAs in prison by 10 or 25% reduced the ICER (Figure 3A).
Qualitative results were similar with the sensitivity analysis on the mean ICER using 8–12 week IFN-free treatments; all scenarios led to ICERs below £30,000/QALY (Figure 3b). We additionally found that if IFN-free SVR was 90% (instead of 95%), the ICER remained below £20,000/QALY (£19,325/QALY). When switching to IFN-free DAAs, the ICER is sensitive to DAA drug cost; if the price of IFN-free DAAs is reduced by 10%, the mean ICER drops to below £13,000/QALY gained. If 8 week IFN-free therapies for all genotypes are provided in prison, the mean ICER dropped to £6,180/QALY. Although this scenario resulted in a slightly higher effective SVR due to greater treatment completion (mean effective SVR 48% overall vs 46% with 8–12 week treatments), the main improvement in cost-effectiveness was due to the reduced cost of treatment due to shorter durations.
Epidemic impact
Our model shows that baseline/existing levels of HCV treatment for PWID in prison and the community is unlikely to result in observable changes in HCV chronic prevalence or incidence among PWID in prison in the next 50 years (<9% relative reduction, supplementary information). Negligible additional impact on HCV chronic prevalence or incidence among PWID in prison (<1% relative difference from baseline) is seen with doubled HCV testing in prison due to the low baseline treatment rates for PWID in prison. Similarly, doubled prison testing would only further reduce the cumulative incidence of hepatocellular carcinoma, liver transplant, or HCV-related deaths among the entire population by an additional 1% over the next 50 years. Even with doubled HCV testing in prison and a switch to 8–12 week DAAs in prison, combined with a scale-up of HCV treatment such that 25% of PWID are treated after referral in prison, the modest reductions (12% relative reduction) in chronic prevalence or incidence occur among PWID in prison in 50 years. This is due to the low effective treatment rates for PWID given the gaps in the cascade of care from testing to SVR.
DISCUSSION
We find that increased HCV testing in prison in England – such as based on an opt out intervention which doubled HCV testing in pilot prisons - is borderline cost-effective with current treatments compared to status-quo voluntary risk based testing and is likely to be cost-effective if shorter course IFN-free DAA therapy is used in prison under a £20,000 willingness to pay (WTP) threshold. Increased HCV testing could be highly cost-effective (mean ICRER <£13,000/QALY gained) if the cascade of care is improved through increasing PWID treatment rates. For example, if >10% PWID are treated after referral in prison (four times higher than the 2.5% base-case) then doubled testing in prison is estimated to have a mean ICER of <£13,000/QALY gained. Cost-effectiveness is influenced by population prevention benefits– averting secondary infections in the community– and DAA costs. Without prevention benefits, HCV testing is above the £20,000 WTP, whereas increasing prevention benefit (through increasing PWID treatment rates) and decreasing costs could achieve cost-effectiveness below £13,000 WTP. However, even with 8-week treatments, SVR may be compromised without ensuring continuity of care or targeting treatment to people with slightly longer than average sentences.
Strengths and Limitations
A key strength of our model is the dynamic transmission component, such that cost-effectiveness includes both individual-level benefits (on prevention of disease progression) and population-level benefits (on prevention of HCV transmission among PWID). However, there are a number of limitations due to a lack of data on key parameters.
First, one of the main drivers of cost-effectiveness is the fall-off from different stages in the cascade of care, such as referral and treatment rates. Unfortunately, limited data are available on these rates across the prison system, and no data stratified by injecting risk status (24). Our referral rate in prison is based on empirical evidence from 3 prisons but may not be representative nationally, though we do include wide uncertainty. Furthermore, the model allocates treatment randomly which can compromise effective SVR. Although in some prisons patients are selected for treatment in part based on duration of stay, it is unclear how widespread this practice is, and what implications this has on PWID treatment rates (which if reduced will adversely affect cost-effectiveness but if treatment completion is increased then cost-effectiveness will improve substantially). Additionally, there is a lack of data on disease stage among prisoners which may impact treatment eligibility and treatment rates with IFN-free DAAs. Due to cost considerations, US and European guidelines recommend prioritizing HCV treatment for those with advanced liver disease (F3/F4), and recent European guidelines also recommend treating people at risk of transmission such as PWID (25) irrespective of disease stage. However, it seems more likely that UK and other countries in Europe will prioritize treatment based on disease stage (26). HCV patients within prisons (especially PWID) are generally younger with less advanced disease, so could be less likely to receive IFN-free DAA therapies. Clinicians treating such cohorts within and outside prisons may be inclined to defer therapy in the hope that they may become eligible for safer, better tolerated regimes as the guidance are revised with decline in costs over time. Additionally, cirrhosis assessment requires additional investigations which may prolong assessment time and reduce time for treatment. Indeed, despite relatively short reported times to referral from the Phase 1 ‘pathfinder prisons’ (<4 weeks), the standard against which prison performance is measured is 18 weeks, so if delays to referral and during assessment occur, this could further limit the number of PWID who can be successfully treated in prison. Therefore, shorter IFN-free DAA therapy may not necessarily translate into greater access to treatment within prison, particularly among PWID, in the absence of continuity of care arrangements with the community.
Second, we use preliminary data from the opt-out pilot program to inform our assumptions surrounding impact on testing rates, and therefore model a doubling of HCV testing rates as achieved in these pilot prisons, but the impact when fully implemented is unclear. However, other interventions to increase HCV testing in UK prisons have been unsuccessful (27).
Third, there is uncertainty regarding SVR rates with IFN-free DAA therapies among PWID and prisoners. Systematic reviews have shown comparable SVR with IFN-based therapies among PWID and non-injecting populations, and preliminary trials with IFN-free DAAs among PWID on opiate substitution therapy indicate no difference in SVR compared to non-injectors. Nevertheless, we show that IFN-free DAAs in prison are likely cost-effective even with lower SVR rates (90%) and that an important driver will be ‘effective SVR’ related to treatment completion.
Fourth, due to a lack of data we assume no continuity of treatment between prison and community such that those who are released while on treatment or in referral for treatment are assumed lost to follow-up and require re-testing and re-engagement. Providing effective continuity of care should increase the proportion successfully treated after diagnosis (whether in prison or the community) and could likely increase cost-effectiveness (3). Currently, some patients diagnosed in prison choose to defer treatment until they return to the community for such reasons such as fear of stigma within prison and the desire for a family/peer support network during treatment. Therefore, cost-effectiveness and public health impact will be affected by how well continuity of care is ensured across custodial and community settings, and efforts should be made to strengthen these transitions.
Fifth, due to a lack of data on HCV prevalence among PWID in prison, we model a 35% chronic prevalence among all PWID (11) which corresponds to a similar prevalence among incarcerated PWID based on the age distribution of incarceration rates among PWID. However, if HCV prevalence/incidence among PWID in prison is lower than the community, HCV treatment in prison may have more prevention benefit and be more cost-effective. Additionally, we assume proportional mixing by age among PWID in prison (and community) due to a lack of data to suggest otherwise. However, if PWID mix partially assortatively by age in prison, such that young PWID mix more with young PWID, this could lead to a lower HCV chronic prevalence among PWID in prison than we model, and consequently greater treatment as prevention benefits.
Sixth, we note that we assume no improvement in fibrosis score upon successful treatment with DAAs, despite evidence that fibrosis regression may occur in a portion of patients after DAA therapy although the particular patients who benefit, the degree of benefit, and timing of improvement is uncertain (28, 29). However, our analysis found HCV testing and DAA therapy highly likely to be cost-effective, and including improvement in fibrosis would increase cost-effectiveness further.
Seventh, we assume no behaviour change after HCV testing or treatment due to a lack of strong evidence in this area. Two small studies have found decreases in injecting risk behaviour with a positive HCV diagnosis (30, 31), however a recent large prospective pooled analysis among 829 PWID in Canada, the US, Netherlands, and Australia found no evidence of injecting risk behaviour change for PWID after a positive or negative HCV diagnosis (32). If HCV testing reduces risk among those with or without HCV, this would further improve any case-finding intervention. Additionally, it is possible individuals will reduce their risk following successful treatment, which would also increase the cost-effectiveness of HCV treatment.
Comparison with other studies
Two previous IFN-based studies using static models (ignoring prevention benefit in the community) suggested HCV testing in UK prisons was unlikely to be cost-effective (33, 34). Our previous analysis found that increased HCV testing in English prisons was unlikely to be cost effective (ICER ~£60,000/QALY gained) with IFN/RBV treatment due to a combination of short incarceration durations (4/8 months for PWID/non-PWID, respectively), long treatment durations (24–48 weeks), and a lack of continuity of care between prison and the community (3). In contrast our current analysis suggests that HCV case-finding in English prisons is borderline cost-effective with currently available therapies and more likely to be cost-effective with highly effective short-course IFN-free DAA therapies. Our study supports a recent dynamic modeling study in the US which found that opt-out testing in prison with DAAs is likely cost-effective, but their study assumed much higher rates of testing uptake (90% compared to 12% in our study based on pilot data) as well as behavior change after diagnosis and treatment (which we do not assume) (35).
Implications
Prisons can be an important contributor blood-borne virus risk for PWID (36), but can also play a role in public health prevention. Prisoners should receive the same standard of care as people in the community– however, interventions do need to be cost-effective. HCV case-finding among PWID in the community is cost-effective, but because continuity of care could not be guaranteed between prison and the community it was unlikely to be cost-effective in prison. We show the arrival of shorter (and more effective) HCV treatment regimens which means that more prisoners can complete treatment prior to release alters the cost-effectiveness decision. Treatment uptake in prison, as well as IFN-free DAA therapy cost, now are the main drivers of cost-effectiveness of HCV case-finding. In addition, cost-effectiveness is predicated on the “prevention benefit” of reducing HCV transmission in the community through successfully treating PWID – which requires empirical demonstration. Further, enhanced data collection of the cascade of care in prison as will be achieved through the new Health and Justice Information Service, allowing for better monitoring and evaluation of prison HCV testing and treatment programmes in the future.
Supplementary Material
Acknowledgments
Financial Support: This work was supported through a research grant from Gilead Sciences. Gilead had no influence on the design, analysis and content of the study. NM, PV, and MH acknowledge funding from National Institute for Drug Abuse R01 DA037773-01A1. NM also acknowledges funding from the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214], which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. MH and PV acknowledge funding by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at University of Bristol. SH is supported by Health Protection Scotland and the Scottish Government. Initial funding for this work was provided by NICE. The views expressed are those of the author(s) and not necessarily those of the UK NHS, the UK NIHR or the UK Department of Health
Abbreviations
- HCV
hepatitis c virus
- PWID
people who inject drugs
- pegIFN
pegylated interferon
- RBV
ribavirin
- IFN-free
interferon free
- DAA
direct acting antiviral
- HCC
hepatocellular carcinoma
- ICER
incremental cost-effectiveness ratio
- QALY
quality adjusted life-year
Footnotes
Author contributions: NKM, PV, and MH designed the study, analysed the results, drafted, edited, and approved the manuscript. NKM performed the analysis. IFB, JW, AM, WI, SS, SJH, SM, EO analysed the results, edited and approved the final manuscript.
Competing Interests: NKM has received research grants from Gilead and has received honoraria from AbbVie, Gilead, and Jannsen. IFB has received honoraria from Jannseen, AbbVie, and Gilead. SJH has received consultancy fees from Abbvie, Gilead, Janssen. SS has received financial support to attend scientific meetings from Abbvie, MSD, Roche and Gilead. MH has received unrestricted research grants from Gilead and been supported to attend and present at scientific meetings by Gilead, Janssen and Abbvie. PV has received unrestricted research grants from Gilead.
References
- 1.De Angelis D, Sweeting M, Ades AE, Hickman M, Hope V, Ramsay M. An evidence synthesis approach to estimating Hepatitis C Prevalence in England and Wales. Statistical Methods in Medical Research. 2009;18:361–379. doi: 10.1177/0962280208094691. [DOI] [PubMed] [Google Scholar]
- 2.Dore GJ, Feld J. Hepatitis C virus therapeutic development: in pursuit of perfectovir. Clinical Infectious Diseases. 2015 doi: 10.1093/cid/civ197. in press. [DOI] [PubMed] [Google Scholar]
- 3.Martin N, Hickman M, Miners A, Hutchinson S, Taylor A, Vickerman P. Cost-effectiveness of HCV case-finding for people who inject drugs via dried blood spot testing in specialist addiction services and prisons. BMJ Open. 2013;3:e003153. doi: 10.1136/bmjopen-2013-003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin N, Miners A, Vickerman P. Assessing the cost-effectiveness of interventions aimed at promoting and offering hepatitis C testing in injecting drug users: an economic modelling report. 2012 http://www.nice.org.uk/nicemedia/live/11957/59552/59552.pdf.
- 5.Health Protection Agency. Colindale. 2013. Hepatitis C in the UK 2013. [Google Scholar]
- 6.Public Health England. Blood-borne Virus Opt-Out Testing in Prisons: Preliminary Evaluation of Pathfinder Programme. Phase 1, April to September 2014. 2015 [Google Scholar]
- 7.Health Protection Agency. National survey of hepatitis C services in prisons in England. 2012. [Google Scholar]
- 8.NICE. NICE technology appraisal guidance [TA330] 2015. Sofosbuvir for treating chronic hepatitis C. [Google Scholar]
- 9.NICE. Ledipasvir-sofosbuvir for treating chronic hepatitis C Draft Guidance. 2015 https://http://www.nice.org.uk/guidance/indevelopment/gid-tag484.
- 10.Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, Devlin N, et al. CHE Research Paper 81. The University of York Centre for Health Economics; 2003. Methods for the Estimation of the NICE Cost Effectiveness Threshold. [Google Scholar]
- 11.Harris RJ, Ramsay M, Hope VD, Brant L, Hickman M, Foster GR, De Angelis D. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. European Journal of Public Health. 2011 doi: 10.1093/eurpub/ckr083. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 13.Public Health England, Health Protection Scotland, Public Health Wales, Public Health Agency Northern Ireland. Shooting Up- Infections among people who inject drugs in the United Kingdom 2013. London: 2014. [Google Scholar]
- 14.Health Protection Agency. Data tables of the Unlinked Anonymous Monitoring Survey of HIV and Hepatitis in Injecting Drug Users Surveillance Update. Jul, 2011. [Google Scholar]
- 15.Ministry of Justice. Population in Custody Tables. Aug, 2013. [Google Scholar]
- 16.Office of National Statistics. Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2013. 2013. [Google Scholar]
- 17.Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technology Assessment. 2004:8. doi: 10.3310/hta8390. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaulation. Health Technology Assessment. 2007;11:1–224. doi: 10.3310/hta11110. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer A, Veldt B, Feld J, et al. Association Between Sustained Virological Response and All-Cause Mortality Among Patients With Chronic Hepatitis C and Advanced Hepatic Fibrosis. JAMA. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 20.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of Hepatitis C Virus Infection and the Development of Hepatocellular CarcinomaA Meta-analysis of Observational Studies. Annals of Internal Medicine. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 21.Thein H-H, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: A meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 22.Martin NK, Miners A, Vickerman P, Foster G, Hutchinson S, Goldberg D, Hickman M. The cost-effectiveness of HCV antiviral treatment for injecting drug user populations. Hepatology. 2012;55:49–57. doi: 10.1002/hep.24656. [DOI] [PubMed] [Google Scholar]
- 23.British Medical Association. British National Formulary. 2014. [Google Scholar]
- 24.Humphreys C, Railton C, O’Moore É, Lombard M, Newton A. An audit of hepatitis C service provision in a representative sample of prisons in England. Journal of Public Health. 2014 doi: 10.1093/pubmed/fdu022. [DOI] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2015. 2015 doi: 10.1016/j.jhep.2022.10.006. http://www.easl.eu/research/our-contributions/clinical-practice-guidelines/detail/recommendations-on-treatment-of-hepatitis-c-2015. [DOI] [PubMed]
- 26.Innes H, Goldberg D, Dillon J, Hutchinson SJ. Strategies for the treatment of Hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut. 2014 doi: 10.1136/gutjnl-2014-308166. [DOI] [PubMed] [Google Scholar]
- 27.Craine N, Whitaker R, Perrett S, Zou L, Hickman M, Lyons M. A stepped wedge cluster randomized control trial of dried blood spot testing to improve the uptake of hepatitis C antibody testing within UK prisons. 2015:351–357. doi: 10.1093/eurpub/cku096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haseltine EL, Penney MS, George S, Kieffer TL. Successful treatment with telaprevir-based regimens for chronic hepatitis C results in significant improvements to serum markers of liver fibrosis. Journal of Viral Hepatitis. 2015;22:701–707. doi: 10.1111/jvh.12382. [DOI] [PubMed] [Google Scholar]
- 29.Poynard T, Moussalli J, Munteanu M, Thabut D, Lebray P, Rudler M, Ngo Y, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. Journal of Hepatology. 2013;59:675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Bruneau J, Zang G, Abrahamowicz M, Jutras-Aswad D, Daniel M, Roy É. Sustained Drug Use Changes After Hepatitis C Screening and Counseling Among Recently Infected Persons Who Inject Drugs: A Longitudinal Study. Clinical Infectious Diseases. 2014;58:755–761. doi: 10.1093/cid/cit938. [DOI] [PubMed] [Google Scholar]
- 31.Aspinall EJ, Weir A, Sacks-Davis R, Spelman T, Grebely J, Higgs P, Hutchinson SJ, et al. Does informing people who inject drugs of their hepatitis C status influence their injecting behaviour? Analysis of the Networks II study. International Journal of Drug Policy. 25:179–182. doi: 10.1016/j.drugpo.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Spelman T, Morris MD, Zang G, Rice T, Page K, Maher L, Lloyd A, et al. A longitudinal study of hepatitis C virus testing and infection status notification on behaviour change in people who inject drugs. Journal of Epidemiology and Community Health. 2015;69:745–752. doi: 10.1136/jech-2014-205224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutton AJ, Edmunds WJ, Sweeting MJ, Gill ON. The cost-effectiveness of screening and treatment for hepatitis C in prisons in England and Wales: a cost-utility analysis. Journal of Viral Hepatitis. 2008;15:797–808. doi: 10.1111/j.1365-2893.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- 34.Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, Stein K. The cost-effectiveness of testing for hepatitis C in former injecting drug users. Health Technology Assessment. 2006;2006:32. doi: 10.3310/hta10320. [DOI] [PubMed] [Google Scholar]
- 35.He T, Li K, Roberts MS, Spaulding AC, Ayer T, Grefenstette JJ, Chhatwal J. Prevention of Hepatitis C by Screening and Treatment in U.S. PrisonsPrevention of Hepatitis C in U.S. Prisons Annals of Internal Medicine. 2016;164:84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larney S, Kopinski H, Beckwith CG, Zaller ND, Jarlais DD, Hagan H, Rich JD, et al. The incidence and prevalence of hepatitis C in prisons and other closed settings: Results of a systematic review and meta-analysis. Hepatology. 2013 doi: 10.1002/hep.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. University of York; 2008. [Google Scholar]
- 38.Wright M, Grieve R, Roberts J, Main J, HCT Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006:10. doi: 10.3310/hta10210. [DOI] [PubMed] [Google Scholar]
- 39.Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment, or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1–210. doi: 10.3310/hta15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald S, Hutchinson SJ, Palmateer N, Allen E, Cameron S, Goldberg D, Taylor A. Decrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in Scotland. Journal of Hepatology. 2013;58:460–466. doi: 10.1016/j.jhep.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Stein K, Dalziel K, Walker A, Jenkins B, Round A, Royle P. Screening for Hepatitis C in injecting drug users: A cost utility analysis. Journal of Public Health. 2004;26:61–71. doi: 10.1093/pubmed/fdh109. [DOI] [PubMed] [Google Scholar]
- 42.Health Protection Agency Colindale. Hepatitis C in the UK 2011. 2011 Jul; [Google Scholar]
- 43.NICE. Peginterferon alfa and ribavirin for the treatment of mild chronic hepatitis C. National Institute for Health and Clinical Excellence; 2006. [Google Scholar]
- 44.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, Shafran SD, et al. Peginterferon Alfa-2a and Ribavirin for 16 or 24 Weeks in HCV Genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 45.Bruno S, Shiffman M, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. Efficacy and Safety of Peginterferon Alfa-2D (40KD) Plus Ribavirin in Hepatitis C Patients with Advanced Fibrosis and Cirrhosis. Hepatology. 2010;51:388–397. doi: 10.1002/hep.23340. [DOI] [PubMed] [Google Scholar]
- 46.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. New England Journal of Medicine. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 47.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, et al. Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. New England Journal of Medicine. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 48.Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Dvory-Sobol H, Symonds WT, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2015;61:769–775. doi: 10.1002/hep.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irving WL, Smith S, Cater R, Pugh S, Neal KR, Coupland CAC, Ryder SD, et al. Clinical pathways for patients with newly diagnosed hepatitis C - What actually happens. Journal of Viral Hepatitis. 2006;13:264–271. doi: 10.1111/j.1365-2893.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 50.Martin NK, Foster GR, Vilar J, Ryder SE, Cramp M, Gordon F, Dillon JF, et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. Journal of Viral Hepatitis. 2015;22:399–408. doi: 10.1111/jvh.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howes N, Lattimore S, Irving WL, Thomson B. Clinical care pathways for patients with hepatitis C: reducing critical barriers to effective treatment. Open Forum Infectious Diseases. 2016 doi: 10.1093/ofid/ofv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.