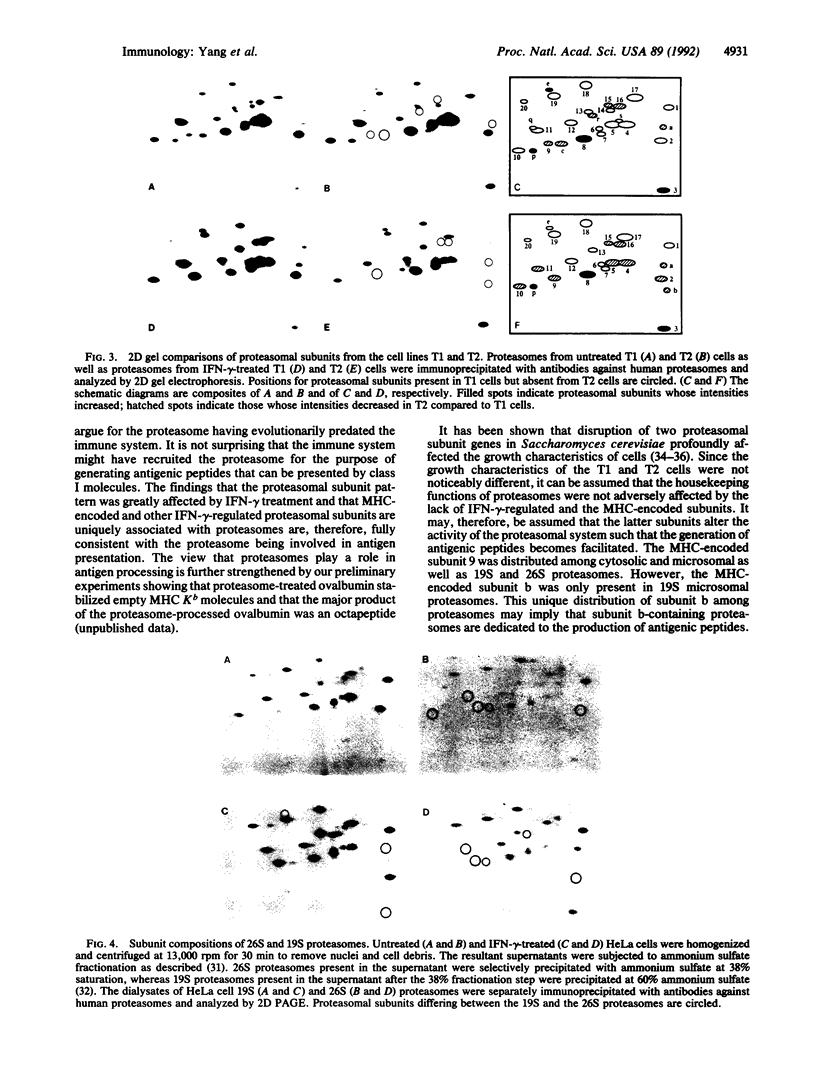
Abstract
Class I major histocompatibility complex (MHC) molecules present antigenic peptides of cytoplasmic origin to T cells. As the lengths of these peptides seem restricted to eight or nine amino acids, an unusual proteolytic system must play a role in antigen processing. Proteasomes, a major extralysosomal proteolytic system, are responsible for the degradation of cytoplasmic proteins. We demonstrate that several proteasomal subunits, including MHC-encoded subunits, are regulated by interferon gamma. These data and the finding that MHC-encoded and other interferon gamma-regulated proteasomal subunits are uniquely associated with proteasomes strongly suggest that the immune system has recruited proteasomes for antigen processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown M. G., Driscoll J., Monaco J. J. Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature. 1991 Sep 26;353(6342):355–357. doi: 10.1038/353355a0. [DOI] [PubMed] [Google Scholar]
- Deverson E. V., Gow I. R., Coadwell W. J., Monaco J. J., Butcher G. W., Howard J. C. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1990 Dec 20;348(6303):738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- Dick L. R., Moomaw C. R., DeMartino G. N., Slaughter C. A. Degradation of oxidized insulin B chain by the multiproteinase complex macropain (proteasome). Biochemistry. 1991 Mar 12;30(10):2725–2734. doi: 10.1021/bi00224a022. [DOI] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. Skeletal muscle proteasome can degrade proteins in an ATP-dependent process that does not require ubiquitin. Proc Natl Acad Sci U S A. 1989 Feb;86(3):787–791. doi: 10.1073/pnas.86.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll J., Goldberg A. L. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990 Mar 25;265(9):4789–4792. [PubMed] [Google Scholar]
- Emori Y., Tsukahara T., Kawasaki H., Ishiura S., Sugita H., Suzuki K. Molecular cloning and functional analysis of three subunits of yeast proteasome. Mol Cell Biol. 1991 Jan;11(1):344–353. doi: 10.1128/mcb.11.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Tanaka K., Orino E., Yoshimura T., Kumatori A., Tamura T., Chung C. H., Nakai T., Yamaguchi K., Shin S. Proteasomes are essential for yeast proliferation. cDNA cloning and gene disruption of two major subunits. J Biol Chem. 1990 Sep 25;265(27):16604–16613. [PubMed] [Google Scholar]
- Ganoth D., Leshinsky E., Eytan E., Hershko A. A multicomponent system that degrades proteins conjugated to ubiquitin. Resolution of factors and evidence for ATP-dependent complex formation. J Biol Chem. 1988 Sep 5;263(25):12412–12419. [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Howell K. E., Devaney E., Gruenberg J. Subcellular fractionation of tissue culture cells. Trends Biochem Sci. 1989 Feb;14(2):44–47. doi: 10.1016/0968-0004(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A., Powis S. H., Glynne R., Radley E., Beck S., Trowsdale J. Second proteasome-related gene in the human MHC class II region. Nature. 1991 Oct 17;353(6345):667–668. doi: 10.1038/353667a0. [DOI] [PubMed] [Google Scholar]
- Martinez C. K., Monaco J. J. Homology of proteasome subunits to a major histocompatibility complex-linked LMP gene. Nature. 1991 Oct 17;353(6345):664–667. doi: 10.1038/353664a0. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Interaction of human erythrocyte multicatalytic proteinase with polycations. Biochim Biophys Acta. 1990 Aug 1;1040(1):28–34. doi: 10.1016/0167-4838(90)90142-3. [DOI] [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Ortiz-Navarrete V., Seelig A., Gernold M., Frentzel S., Kloetzel P. M., Hämmerling G. J. Subunit of the '20S' proteasome (multicatalytic proteinase) encoded by the major histocompatibility complex. Nature. 1991 Oct 17;353(6345):662–664. doi: 10.1038/353662a0. [DOI] [PubMed] [Google Scholar]
- Parham P. Antigen processing. Transporters of delight. Nature. 1990 Dec 20;348(6303):674–675. doi: 10.1038/348674a0. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Natural substrates of the ubiquitin proteolytic pathway. Cell. 1991 Aug 23;66(4):615–618. doi: 10.1016/0092-8674(91)90104-7. [DOI] [PubMed] [Google Scholar]
- Rivett A. J. Purification of a liver alkaline protease which degrades oxidatively modified glutamine synthetase. Characterization as a high molecular weight cysteine proteinase. J Biol Chem. 1985 Oct 15;260(23):12600–12606. [PubMed] [Google Scholar]
- Rivett A. J. The multicatalytic proteinase of mammalian cells. Arch Biochem Biophys. 1989 Jan;268(1):1–8. doi: 10.1016/0003-9861(89)90558-4. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Howell D. N., Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Spies T., DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991 May 23;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ichihara A. Half-life of proteasomes (multiprotease complexes) in rat liver. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1309–1315. doi: 10.1016/0006-291x(89)92253-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ii K., Ichihara A., Waxman L., Goldberg A. L. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J Biol Chem. 1986 Nov 15;261(32):15197–15203. [PubMed] [Google Scholar]
- Taniguchi T. Regulation of cytokine gene expression. Annu Rev Immunol. 1988;6:439–464. doi: 10.1146/annurev.iy.06.040188.002255. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Waxman L., Fagan J. M., Goldberg A. L. Demonstration of two distinct high molecular weight proteases in rabbit reticulocytes, one of which degrades ubiquitin conjugates. J Biol Chem. 1987 Feb 25;262(6):2451–2457. [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989 Jun 2;244(4908):1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]