Abstract
Reconstructing vegetation at hominin fossil sites provides us critical information about hominin palaeoenvironments and the potential role of climate in their evolution. Here we reconstruct vegetation from carbon isotopes of plant wax biomarkers in sediments of the Nachukui Formation in the Turkana Basin. Plant wax biomarkers were extracted from samples from a wide range of lithologies that include fluvial–lacustrine sediments and palaeosols, and therefore provide a record of vegetation from diverse depositional environments. Carbon isotope ratios from biomarkers indicate a highly dynamic vegetation structure (ca 5–100% C4 vegetation) from 2.3 to 1.7 Ma, with an overall shift towards more C4 vegetation on the landscape after about 2.1 Ma. The biomarker isotope data indicate ca 25–30% more C4 vegetation on the landscape than carbon isotope data of pedogenic carbonates from the same sequence. Our data show that the environments of early Paranthropus and Homo in this part of the Turkana Basin were primarily mixed C3–C4 to C4-dominated ecosystems. The proportion of C4-based foods in the diet of Paranthropus increases through time, broadly paralleling the increase in C4 vegetation on the landscape, whereas the diet of Homo remains unchanged. Biomarker isotope data associated with the Kokiselei archaeological site complex, which includes the site where the oldest Acheulean stone tools to date were recovered, indicate 61–97% C4 vegetation on the landscape.
This article is part of the themed issue ‘Major transitions in human evolution’.
Keywords: leaf wax, carbon isotope, pedogenic carbonate, hominin palaeoenvironment, Acheulean tools
1. Introduction
The Pleistocene Epoch in eastern Africa encompasses major events in hominin evolution that include speciation and extinction events within the genera Paranthropus and Homo in the Turkana Basin [1] as well as the advent of the Acheulean technology [2,3]. Important hominin fossils and archaeological sites that are part of this record are preserved in the Nachukui Formation, on the western side of Lake Turkana. Strata of the Nachukui Formation belong to the Omo Group, which also includes the Koobi Fora Formation on the eastern side of Lake Turkana and the Shungura Formation, located along the western side of the Omo River north of Lake Turkana. The Omo Group deposits range in age from approximately 4.3 to 0.7 Ma [4–6]. The Nachukui Formation covers from approximately 4 to 0.7 Ma and is subdivided into eight stratigraphic members in the type area, nearly all of which have a radiometrically dated volcanic tuff at their base. A rich and diverse hominin fossil record from West Turkana includes Australopithecus anamensis, Kenyanthropus platyops, Paranthropus aethiopicus, Paranthropus boisei and Homo ergaster/erectus [7–12]. Complementing the hominin record is an exceptional archaeological record of stone tools. This includes the recent discovery of the oldest stone tools dated to approximately 3.3 Ma from the Lomekwi Member, numerous Oldowan sites, and the oldest known Acheulean tools [2,3,13–16].
The environmental context of Plio-Pleistocene hominins in the Turkana Basin has received much attention because it provides an opportunity to evaluate the potential influence of climate on human evolution. The primary geochemical archive used for reconstructing vegetation in the Turkana Basin has been pedogenic carbonate in paleosols, which has been shown to reflect the proportion of C3 (trees, bushes and cool-season grasses and sedges) versus C4 (warm-season grasses and sedges) vegetation on the landscape [17,18]. The emphasis in eastern Africa, particularly in the Turkana Basin, has been on reconstructing vegetation using carbon isotope ratios of pedogenic carbonate [19–23], although some of these studies have also evaluated hydroclimate from oxygen isotopes or depth to carbonate horizons in palaeosols [19,23]. Passey et al. [24] used clumped isotope thermometry on Omo Group pedogenic carbonates to establish that the Turkana Basin has had persistently high soil temperatures of ca 33°C—similar to those observed today—over the last 4 Myr [24]. Along the western margin of Lake Turkana, palaeosol carbonate records also exist from older Late Miocene to Pliocene deposits at Lothagam (approx. 7.5–3 Ma) and Kanapoi (approx. 4.2–4 Ma) [25,26]. The pedogenic carbonate data from these older sites and from the Nachukui Formation indicate primarily C3-dominated (less than 35% C4 vegetation) to mixed C3–C4 (35–65% C4 vegetation) ecosystems until about 2 Ma, when C4-dominated (greater than 65% C4 vegetation) ecosystems become prevalent along the western margin of Lake Turkana.
Although carbon isotope data from pedogenic carbonates constitute the majority of geochemical palaeovegetation proxy data in eastern Africa, the distribution of pedogenic carbonate in Omo Group sediments is limited primarily to floodplain deposits that remained stable long enough for carbonates to form. Thus, a majority of the sediments in Omo Group lack pedogenic carbonate; this includes lacustrine deposits that were never subaerially exposed, and rapidly aggrading flood plain or littoral deposits that did not undergo significant pedogenesis. Formation of pedogenic carbonate is also favoured by a high evaporation-to-precipitation ratio, and thus humid episodes are poorly recorded by this proxy. Although the systematics of pedogenic carbonate as a recorder of vegetation are well established, there are nuances regarding the timing of carbonate precipitation that suggest seasonal biases in their formation [27,28] in addition to the depositional environment restrictions. New vegetation proxies therefore have great potential to expand our ability to characterize the vegetation of hominin palaeoenvironments. One such tool is carbon isotope analysis of plant wax biomarker molecules.
Plants produce epicuticular leaf waxes to protect the leaf tissue from abrasion by dust; attack from insects, microbes and fungi; and water loss from the leaf surface. The waxes are made up of a variety of lipids that include normal (n) alkanes and both free and esterified n-alkanoic acids and n-alcohols [29]. The basic structure of the two plant waxes used in this study, n-alkanes and n-alkanoic acids, is a linear hydrocarbon with the latter compound having a carboxylic acid group at one end of the molecule. As with bulk plant tissue, the carbon isotopic ratio of plant wax reflects a plant's photosynthetic pathway. In eastern African ecosystems, where today nearly all open-habitat grasses use the C4 pathway and trees use the C3 pathway, plant wax carbon isotopes reflect ecosystem vegetation type. Waxes from plants with the C3 pathway have δ13C values ranging from about −38 to −27‰, whereas those from plants with the C4 pathway range from −24 to −17‰ [30–32]. Plant waxes are commonly preserved in sedimentary organic matter where their resistance to diagenetic alteration and isotope exchange makes them an excellent vegetation proxy [33–35]. Several studies have used plant wax isotopes from marine [36,37] and lacustrine [38,39] sediments ranging in age from recent to ca 12 Ma to reconstruct African vegetation; however, none to date have used plant waxes in palaeosols or fluvial sediments for vegetation reconstructions in eastern Africa. Alkyl lipids in Turkana Basin sediments were first evaluated over 30 years ago [40], before the advent of compound specific isotope analysis [41]. Nonetheless, Abell & Margolis [40] identified terrestrial plant waxes from Pleistocene sediments of the Koobi Fora Formation, but also found lipids of microbial and algal origins. A more recent study added a small number (n = 4) of n-alkanoic acid carbon isotope data from Omo Group lacustrine sediments that showed an 8‰ range over a ca 40 kyr time interval [42].
Here, we present plant wax carbon isotope ratios from a wide range of Nachukui Formation sediment types to reconstruct vegetation of hominin environments, including sediments associated with the Kokiselei archaeological site complex (KASC). We analysed the carbon isotope ratios of multiple long-chain homologues of n-alkanes and n-alkanoic acids. Carbon isotope data from n-C30 alkanoic acids (hereafter C30 acids) and n-C31 alkanes (hereafter C31 alkanes) are converted to per cent C4 values using plant wax analyses of modern eastern African soils, and previously published data on C3 and C4 endmember values for plant waxes. The plant wax data from the Nachukui Formation sediments show that carbon isotopes from biomarkers are a powerful tool for reconstructing past environments, particularly in tropical ecosystems where grasses and woody vegetation can be differentiated using carbon isotopes. Our results indicate a relatively open, C4-dominated ecosystem associated with hominin sites and the oldest recovered Acheulean tools in the Nachukui Formation.
2. Methods
(a). Sampling, chronology and site localities
A majority of samples analysed in this study were collected for palaeomagnetic analyses [2] and come from three sections spanning the Kalochoro and Kaitio members of the Nachukui Formation, located along the northwestern margin of Lake Turkana (figure 1). A smaller subset of samples comes from the nearby Kaitio drainage. The tuff at the base of the Kalochoro Member has been dated to 2.33 ± 0.02 Ma [5]. The KBS Tuff (1.87 ± 0.02 Ma) marks the base of the overlying Kaitio Member, which also contains the Morutot Tuff (1.61 ± 0.02 Ma) [4]. The Olduvai Subchron (1.78 Ma) and the Reunion event (2.12 Ma) are two palaeomagnetic reversals that provide additional age control within the sequence. All samples are placed within the stratigraphic framework and age model established by Lepre et al. [2]. The subset of samples from the Kaitio drainage is assigned an age of 1.72 Ma at the base and 1.71 Ma at the top of the 2.1 m sampled section based on the stratigraphic relationship to sections in Lepre et al. [2]. Sample information, including age, lithology and facies, is given in electronic supplementary material, table S1.
Figure 1.
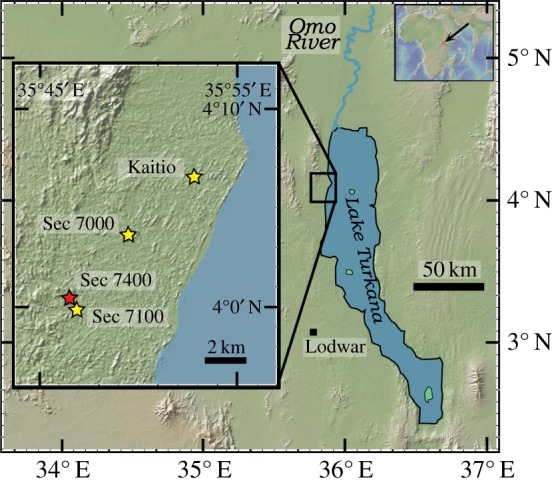
Map shows the study area and sampling sites of the Nachukui Formation along the northwestern margin of Lake Turkana. Stars indicate the locations of sampled sections in the Kalocholoro and Kaitio Members. The red star indicates the location of archaeological site KS4 where the oldest Acheulean tools were found [2,3]. Base map is from GeoMapApp [43].
Many of samples were not explicitly collected for biomarker analysis and, therefore, a rigorous physical cleaning procedure was used to ensure removal of any possible modern plant waxes. The outer surface of samples was cleaned with a Dremel® tool to remove possible modern contamination. Samples were then rinsed with dichloromethane (DCM) and crushed to a powder in mortar and pestle. Lipids were extracted from 23 to 281 g (mean: 79 g) of powdered sample with organic solvents (9 : 1 DCM : methanol) using a Dionex accelerated solvent extractor in batches of approximately 65 g of sample packed into 60 ml extraction cells. Samples were extracted with four 10 min static cycles at 100°C with a flush volume of 150% of total cell volume. An internal standard was added to the total lipid extract (TLE) which included 5α-androstane and cis-11-eicosenoic acid (∼2000 ng of each) for later quantification of lipids. In cases where water was present in the TLE, it was dried under a purified N2 stream, dissolved in DCM, approximately 1–3 g of sodium sulfate were added, and allowed to sit for at least 1 h before pipetting off the dry TLE into a new vial. Less than 20% of samples required this treatment.
The TLE was separated by solid phase extraction on silica gel columns (approx. 0.5 g solvent rinsed silica gel, 230–400 mesh). The aliphatic fraction (F1) was eluted with 4 ml of hexane, the ketone and ester fraction (F2) eluted with 4 ml of DCM and the polar fraction (F3) with 4 ml of methanol. The F3 fraction was separated through an aminopropyl column (approx. 0.5 g) where the neutral, acid (A) and polar fractions were eluted with 4 ml each of 2 : 1 DCM: iso-propanol, 4% acetic acid in diethyl ether, and methanol, respectively. Carboxylic acids in the F3A fraction were methylated (Me) with acidic methanol at 60°C for 4–12 h, yielding fatty acid methyl esters. Fatty acid methyl esters in the F3AMe fraction were isolated from molecules such as hydroxy acids containing additional functional groups using silica gel columns (as above), where the F2 fraction contained the isolated fatty acid methyl esters.
(b). Biomarker gas chromatography mass spectrometry and carbon isotopic analyses
Plant wax compounds were quantified and characterized on an Agilent gas chromatograph (Agilent 7890A GC) equipped with both a mass selective detector (5975C MSD) and flame ionization detector (FID). One microliter of sample dissolved in 100 µl hexane was injected into a multi-mode inlet injector at 60°C (0.1 min hold), which was then ramped to 320°C at 900°C min−1 and held for the duration of the analysis. Initial GC oven temperature was set at 60°C (1.5 min hold) and ramped to 150°C at 15°C min−1, then to 320°C at 4°C min−1. A helium-purged microfluidics device downstream of a DB-5 column (30 m length, 250 µm ID) quantitatively split the GC flow to the MSD and FID detectors. Compound identification was done with comparison of mass spectra and retention times to authentic standards, and quantification was done using FID peak areas. n-alkyl lipid concentrations were calculated based upon the peak area of known concentrations of internal standards added to the TLE.
Carbon isotope ratios of n-alkanes and n-alkanoic acids were analysed using a GC coupled to a Thermo Delta V isotope ratio mass spectrometer through a combustion interface at the Lamont Doherty Earth Observatory Stable Isotope Laboratory. All sample injections were interspersed injections of molecular mixtures with known isotopic values (mixes A4, A5 and F8 supplied by Arndt Schimmelmann, Univ. of Indiana) that were used for correction of carbon isotope values. The carbon isotope ratio is expressed using delta notation, where 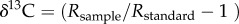 , and R = 13C/12C. n-Alkanoic acid δ13C values were corrected for the addition of the methyl group using a mass balance equation and the measured δ13C value of the methanol used for methylation.
, and R = 13C/12C. n-Alkanoic acid δ13C values were corrected for the addition of the methyl group using a mass balance equation and the measured δ13C value of the methanol used for methylation.
3. Results
(a). Biomarker concentrations and molecular distributions
Biomarker concentrations for 37 samples are presented in electronic supplementary material, tables S2 and S3. We report only concentrations from high molecular weight (HMW) compounds associated with terrestrial plants, which for n-alkanoic acids includes homologues C28–C34 and for n-alkanes C29–C35. n-alkanoic acid concentrations range from 0 to 885 ng g−1 of dry sediment, with a median value of 7 ng g−1. The range for n-alkanes is narrower at 1–76 ng g−1, also with a median value of 7 ng g−1. Grain size is the major determinant of biomarker concentration. Concentrations are highest in (lacustrine) clays followed by silts (electronic supplementary material, tables S2 and S3). Some but not all fine sands yielded sufficient concentrations for isotopic analyses. Re-worked volcanic ashes either did not have high enough concentrations or the compound distributions suggested either alteration of plant waxes or the addition of non-plant sources.
Terrestrial plant wax molecular distributions are commonly characterized by two metrics: the abundance-weighted average chain length (ACL) and carbon preference index (CPI). ACL is calculated as 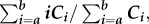 where Ci is the concentration of the molecule with chain length i (i = 28, 30, 32, 34 for n-alkanoic acids and 29, 31, 33, 35 for n-alkanes). The ranges of ACLs for n-alkanoic acids and n-alkanes were 27.6–30.3 and 28.6–31.3, respectively (electronic supplementary material, tables S2 and S3).
where Ci is the concentration of the molecule with chain length i (i = 28, 30, 32, 34 for n-alkanoic acids and 29, 31, 33, 35 for n-alkanes). The ranges of ACLs for n-alkanoic acids and n-alkanes were 27.6–30.3 and 28.6–31.3, respectively (electronic supplementary material, tables S2 and S3).
Plant waxes show a characteristic odd-over-even preference for alkanes and an even-over-odd preference for alkanoic acids resulting from their biosynthetic pathways. This preference is formalized and calculated using the CPI [44] as 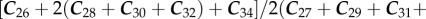
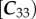 for n-alkanoic acids and
for n-alkanoic acids and 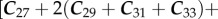
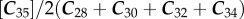 for n-alkanes. CPI in plants have a wide range (ca 0.04–99) although most yield CPIs greater than 2 [45]. Soil CPIs reported in the literature have a more limited range of about 2–10 [46], but this may be in part owing to limited sampling, because most work has focused on plants rather on soils. The ranges of CPI for n-alkanoic acids and n-alkanes in these samples were 1.5–4.9 and 1.1–4.5, respectively (electronic supplementary material, tables S2 and S3). CPI has long been thought to reflect the degree of plant wax preservation, but given the wide range observed in plants, the use of a threshold value to include or exclude a sample based on this single metric does not seem to be warranted [45].
for n-alkanes. CPI in plants have a wide range (ca 0.04–99) although most yield CPIs greater than 2 [45]. Soil CPIs reported in the literature have a more limited range of about 2–10 [46], but this may be in part owing to limited sampling, because most work has focused on plants rather on soils. The ranges of CPI for n-alkanoic acids and n-alkanes in these samples were 1.5–4.9 and 1.1–4.5, respectively (electronic supplementary material, tables S2 and S3). CPI has long been thought to reflect the degree of plant wax preservation, but given the wide range observed in plants, the use of a threshold value to include or exclude a sample based on this single metric does not seem to be warranted [45].
Evaluation of each sample chromatogram is a more robust chemical screening method than metrics such as ACL or CPI for determining the fidelity of plant waxes. Although the samples exhibited a wide range of variability in plant wax distributions, the chromatograms shown in figure 2 illustrate characteristic molecular distributions from the Nachukui Formation samples. Chromatograms of samples that exhibit molecular concentrations and distributions suitable for isotopic analysis are shown in figure 2a–c. In samples that are ideal for isotopic analysis, the time interval in the chromatogram where plant waxes elute is made up almost exclusively of a homologous series of n-alkyl lipids. In contrast, some n-alkanoic acid samples have additional (acid) compound series interspersed within the series of plant-derived n-alkanoic acids (figure 2d,e). Samples with these additional compounds were excluded from isotopic analysis. Samples that lacked sufficient compound concentrations for isotopic analysis were also excluded.
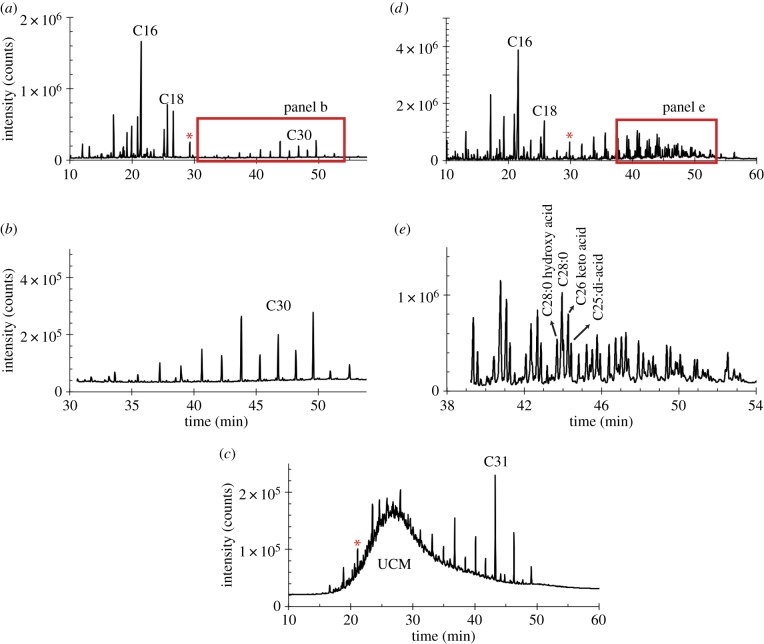
Figure 2.
Chromatograms show detector response (FID counts) versus time for Nachukui Formation samples. (a) n-alkanoic acids from sample KU107 with typical even-over-odd preference for HMW plant waxes (C26–C34) that are highlighted in (b). (c) n-alkanes from the same sample, KU107, with and odd-over-even preference in the HMW n-alkanes (C27–C35). Many n-alkane samples had an unresolved complex mixture (UCM) that eluted in the early part of the chromatogram but did not interfere with carbon isotope analysis. Panel (d) illustrates the n-alkanoic acids from KU114 where homologous series of exogenous HMW compounds not found in plant waxes were also present. The exogenous compounds are highlighted in panel (e). These include (ω – 1) keto-, (α,ω) di- acids and hydroxy acids identified from interpretation of mass spectra [47] indicative of additional (e.g. microbial) sources of alkanoic acids or degradation products of plant n-alkanoic acids. Carbon isotope ratios of plant waxes were measured in sample KU107, but not in KU114. The asterisk represents the internal standard.
(b). Carbon isotopes from plant waxes
Of the 37 samples extracted for biomarker analysis, it was possible to measure carbon isotope ratios of n-alkanoic acids in 27 samples (73%) and of n-alkanes in 18 samples (49%). In most cases where isotopic measurements were not feasible it was owing to low abundances of plant waxes. However, in several cases for n-alkanoic acid samples, isotope measurements were not made because of the presence of exogenous HMW compounds (i.e. figure 2d).
We focus on the C30 acid and C31 alkane values for our analysis and discussion, because molecular abundances compiled from modern African plants show that C31 alkane concentrations are most similar in C3 and C4 plants [32], and thus make it the most representative homologue of the actual vegetation distribution on the landscape. We assume C30 acid concentrations between C3 and C4 plants are also similar but, to the best of our knowledge, this has not been confirmed from modern plants or soils. The C30 acids range from –18.9 to –29.3‰ and the C31 alkanes from –20.3 to –30.4‰ (electronic supplementary material, figure S1). These values represent a range of C3- to C4-dominated landscapes. Carbon isotope values from other HMW even-numbered n-alkanoic acids and odd-numbered n-alkanes are presented in electronic supplementary material, tables S4 and S5, respectively. Analytical uncertainties for both compounds are ± 0.3‰.
The carbon isotope ratios from C30 acids and C31 alkanes are converted to per cent C4 vegetation on the landscape to allow for comparison to isotopic data from pedogenic carbonates. Plant wax isotope data are transformed into per cent C4 values using a linear mixing model, where per cent C4 on the landscape is calculated as 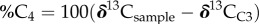
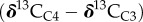 , where the subscripts ‘sample’, C3 and C4 refer to the δ13C values in the sample, C3 endmember and C4 endmember, respectively. We apply a linear mixing model to convert from δ13C values to per cent C4 vegetation rather than applying other transform functions (e.g. fraction woody cover), because the enrichment values needed to convert from lipid back to bulk plant tissue values are not well constrained for African plants, whereas the C3 and C4 endmember n-alkane values used in the linear mixing model are well defined from global and local datasets.
, where the subscripts ‘sample’, C3 and C4 refer to the δ13C values in the sample, C3 endmember and C4 endmember, respectively. We apply a linear mixing model to convert from δ13C values to per cent C4 vegetation rather than applying other transform functions (e.g. fraction woody cover), because the enrichment values needed to convert from lipid back to bulk plant tissue values are not well constrained for African plants, whereas the C3 and C4 endmember n-alkane values used in the linear mixing model are well defined from global and local datasets.
The C3 end-member values for C30 acids and C31 alkanes are −32.9±0.8 (1σ) and −31.1±1.3‰, respectively, established from modern δ13C values in soils collected from C3 riparian forests along the Omo River in the Lower Omo Valley, Ethiopia. We use –19.0‰ as a C4 endmember value for both compounds. The C4 endmember values from modern soils collected from C4-dominated ecosystems in the Lower Omo Valley are slightly more negative (–22.7 to –21.5‰ for n-alkanoic acids and n-alkanes, respectively) than the most positive values measured in the Nachukui samples (ca –19‰). Tipple et al. [48] report a mean δ13C value of –19.9 ± 2.4‰ for C31 alkanes in C4 plants, which suggests the C4 endmember value of –19‰ is reasonable as it falls well within 1 standard deviation of the mean. All δ13C endmember values have been adjusted by +1.5‰ to correct for the offset between modern (–8.0‰) and Pleistocene (approx. –6.5‰) atmospheric δ13C values [49]. As the variation in atmospheric δ13C values over the sampled interval is small [49,50], an age-dependent correction is not warranted. Per cent C4 values plotted versus age from C30 acids range from 26% to 100% and for C31 alkanes range from 5 to 90% (figure 3). Uncertainty for per cent C4 values (1σ) ranges from 6% to 11% and was calculated using the uncertainties of sample and endmember δ13C values (electronic supplementary material, tables S4 and S5).
Figure 3.
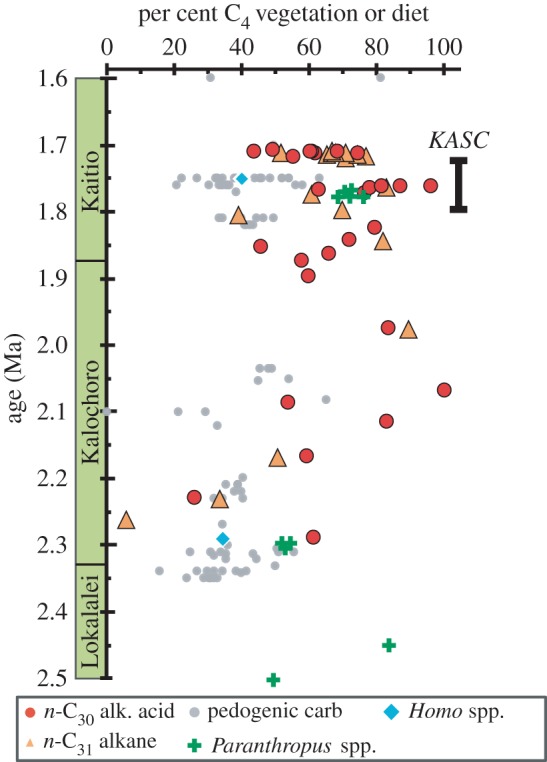
Age versus per cent C4 vegetation and per cent C4-based foods in Nachukui hominin diets. Vegetation is reconstructed from n-C30 alkanoic acid and n-C31 alkane data and from previously published pedogenic carbonate data [19,20,23]; hominin diet data are for Paranthropus and Homo [51,52]. Plant wax data indicate higher proportions of C4 vegetation on the landscape than pedogenic carbonate data. Nachukui Formation members are labelled along the age axis. KASC defines the approximate age of the Kokiselei archaeological site complex.
4. Discussion
(a). Isotopic integrity of plant waxes
Multiple lines of evidence suggest the plant wax carbon isotope values record a local, syndepositional vegetation signal. It is important to discuss this evidence as there are multiple processes that can lead to isotope-based vegetation reconstructions that are not reflective of palaeoflora. These processes include contamination of samples by modern plant biomarkers, inheritance of plant waxes from parent material and the addition or preferential degradation of biomarkers by post-depositional microbial activity.
The potential contribution of modern plant waxes to the samples was mitigated by avoiding sampling of highly fractured or porous sediments where modern roots or meteoric water could introduce modern biomarkers. In most cases, samples were taken from fresh faces excavated back from the exposed surface of the outcrop and from below the rooting zone of the modern overlying soil. Finally, abrading off the outer surface of the sample followed by an organic solvent rinse ensured removal of potential surficial contaminants. Inheritance of plant waxes from parent material is not likely in the Omo Group sediments as their primary source is rapidly weathered volcanic deposits from the Ethiopian Highlands that are devoid of any plant waxes. The contribution of plant waxes from Highlands vegetation is likely to be minimal but could occur from erosion of Highlands soils. Highlands plant waxes would be predominantly from C3 plants.
We assess a variety of characteristics of the plant wax distributions, concentrations and isotope values to evaluate the possibility that the isotope ratio has been altered by the addition or preferential degradation of lipids. The CPI and concentrations in Nachukui samples are similar to those reported in Miocene to Pleistocene age palaeosols from the Siwaliks in Pakistan [33]. Siwalik palaeosol n-alkane data record a carbon isotopic shift similar in magnitude to co-occurring pedogenic carbonates and Bengal Fan bulk organic matter that are associated with the expansion of C4 ecosystems [53].
There are no known significant microbial producers of HMW n-alkanes, although algae do produce low molecular weight compounds (C10–C22) [54]. Petrogenesis produces HMW n-alkanes, but results in a CPI of 1 and generally a mid-chain (∼C25) concentration maximum, neither of which characterizes the Nachukui samples. There are no known significant production pathways from microbial sources for HMW n-alkanoic acids in soils or lacustrine sediments, but microbes may produce small (and probably insignificant) quantities of these compounds [54]. The absence of significant post-depositional sources of HMW n-alkanoic acids or n-alkanes indicates that the compounds we have isolated and measured are in fact derived from terrestrial plant waxes. This leaves degradation of compounds as the other major pathway by which alteration of the original plant wax isotope ratio may occur.
Several studies have shown that the concentration of n-alkanes in soils decreases with time, indicating degradation of plant waxes, probably via microbial processes [55–58]. Results are conflicting however, on whether or not the isotope ratio is affected by early diagenesis, which is probably associated with microbial processes. Huang et al. [55] found no change in δ13C values of HMW n-alkanes (C27–C33) over 23 years of organic matter decomposition. In contrast, Chikaraishi & Naraoka [59] found HMW n-alkanoic acids and n-alkanes were enriched by +2.4 to +3.6‰ in modern soils compared with corresponding leaves from C3 trees at a temperate site in Japan. Although it is impossible to determine with absolute certainty if plant wax δ13C values from Nachukui Formation palaeosols have been isotopically altered or not, we can compare the δ13C values of samples from intercalated lacustrine intervals with palaeosol samples. The better-preserved lacustrine sample should not be subject to the same +2 to +4‰ enrichment from soil microbial processes as the palaeosols, and we therefore would expect them to yield more negative δ13C values if the palaeosol plant waxes have been altered. A comparison of δ13C values between the two sediment types shows little difference in median δ13C values. Median C30 acid values for palaeosol and lacustrine samples are −24.7‰ (n = 7) and −23.9‰ (n = 14), respectively. Median C31 alkane values for palaeosol and lacustrine samples are −24.0‰ (n = 8) and −22.7‰ (n = 6), respectively. In both cases, lacustrine samples are slightly more positive than palaeosols. A caveat to this comparison is that lacustrine sediments probably integrate plant waxes over a larger area than the palaeosol samples.
A second line of evidence that supports the fidelity of the plant wax isotope data is the close agreement between C30 acids and C31 alkanes where it was possible to measure both compounds on the same sample (figure 4 and electronic supplementary material, table S6). In general, the C31 alkane is enriched by ∼+1.2‰ (±1.3‰, 1σ) with respect to the C30 acid. If significant isotopic alteration occurred via microbial degradation, then such agreement between the δ13C values of acids and alkanes would probably not be preserved. While paired measurement of δ13C values on alkanes and acids is not a common practice [59], additional paired measurements from modern soils and plants in Africa would provide a framework for comparing datasets where only a single lipid class is measured, and would be useful for determining whether the original isotopic signal has been altered through the addition or degradation of lipids.
Figure 4.
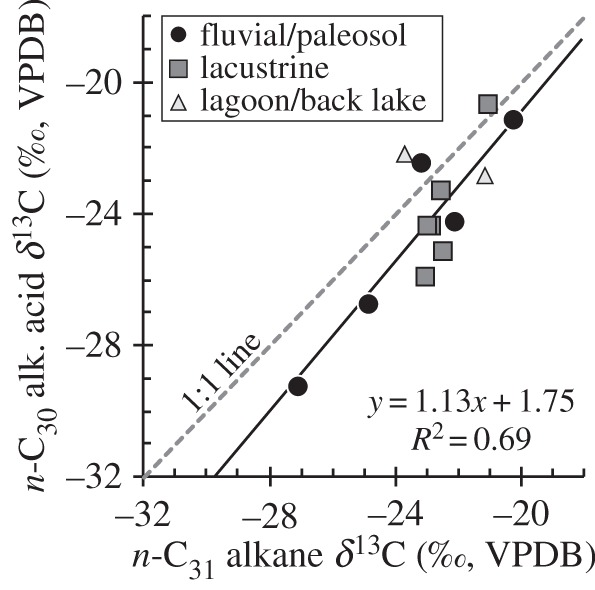
The δ13C values of n-C31 alkanes versus n-C30 alkanoic acids show close agreement in sediment samples where it was possible to measure δ13C values of both alkyl lipids. This agreement suggests preservation of the original plant wax isotopic signature in each compound. There are no clear trends based on lithology or depositional environment. The grey dashed line is a 1 : 1 line, the solid line a linear least-squares fit. Additional sample information is listed in electronic supplementary material, table S6.
(b). Palaeovegetation based on plant wax δ13C values
The plant wax δ13C values from Nachukui Formation sediments indicate a dynamic landscape that ranged between pure C3 and pure C4 landscapes from 2.3 to 1.7 Ma (figure 3). The median per cent C4 values from C30 acids and C31 alkanes are in good agreement with each other (63% and 68%, respectively). Although vegetation structure changes throughout this interval, mixed C3–C4 and C4-dominated landscapes are most common, particularly after 1.9 Ma. In fact, there are only three samples that indicate C3-dominated landscapes, all around 2.25 Ma. The data from 2.3 to 1.9 Ma are rather sparse, but can be characterized as highly variable as they span nearly the full range of vegetation. After 1.9 Ma, plant wax data show continued variability, but fall mostly between 39 and 97% C4 vegetation (figure 3).
Plant wax data suggest a secular shift after approximately 2.1 Ma towards more C4 vegetation in this part of the Turkana Basin, although the data are temporally restricted to a window of approximately 0.6 Myr. The mechanism(s) responsible for the increase in C4 vegetation is (are) not yet clear. Existing data from clumped isotope thermometry from Nachukui Formation pedogenic carbonates show relatively constant temperature from 4 to 1 Ma [24]. Atmospheric CO2 probably varied during glacial and interglacial periods, but there are no known secular decreases across this interval. Thus, it is unlikely that changes in temperature or CO2 contributed to the increase in C4 vegetation. The mean pedogenic carbonate δ18O value from 1.7 to 1.5 Ma (n = 15) is approximately 1.2‰ more positive that the mean value from 2.35 to 2.0 Ma (n = 28) [60]. Although there are essentially no δ18O data available from 2 to 1.6 Ma, one interpretation of the data is that rainfall amount may have decreased between these two periods. One climatic variable that has not been rigorously examined is seasonality of rainfall. Increases in length or intensity of the dry season favour C4 grasses over C3 vegetation and therefore make increased seasonality a potential mechanism for the observed expansion of C4 vegetation. Finally, an important factor to consider is the ecological shift associated with the transgression of Lake Lorenyang at around 2 Ma.
Detailed magnetostratigraphy combined with existing radiometric dating of tuffs in this part of the basin place the shift from fluvial to lacustrine facies at about 2.14 Ma [2,61]. A possible interpretation that considers both geochemical data and the change in lithofacies is that the fluvial sediments are associated with the ancestral Omo River or second-order drainages along the lake margin, both of which would have had riparian forests associated with them. Riparian forests would be a local source for C3 plant waxes found in the record prior to approximately 2.1 Ma. The post 2.1 Ma lacustrine sediments consist mostly of organic-rich mudstones associated with deeper-water conditions in the upper Kalochoro and Kaitio Members; however, some sandstones, probably deposited in the littoral zone or back lake/lagoon environments, are also present [2]. Plant waxes in these sediments indicate mixed C3–C4 and C4-dominated environments and may reflect a larger, basin-wide signal (figure 3). Debate continues about whether the processes that formed Lake Lorenyang were strictly climatic, tectonic or a combination of both [61,62]. What is clear is that the plant wax data show a preponderance of C4 vegetation that broadly coincides with the onset of Lake Lorenyang.
Samples from the uppermost part of the sampled section provide important information on the palaeovegetation at the KASC, which ranges in age from approximately 1.8 to 1.7 Ma. Plant wax data from a 5 m stratigraphic interval that spans much of the KASC indicate 61–97% C4 vegetation on the landscape (figure 3). The stone tool sites are associated with some of the most open environments found within the 2.3–1.7 Ma interval sampled. Tooth enamel carbon isotope data and faunal distributions from the archaeological sites KS1 and KS2 support the interpretation of C4-dominated landscapes during this period. More than 70% of all mammalian taxa sampled had δ13C values greater than −3‰, indicative of primarily C4-dominated diets [51]. The tooth enamel isotope study does not include a faunal abundance analysis or consideration of taphonomy [51], but the faunal distribution of sampled teeth is largely composed of herbivores associated with grassland to wooded grassland environments. This includes elephantids, equids, some suids, rhinocerotids and select bovids from the tribes alcelaphini, bovini and reduncini. Faunal distributions from the contemporaneous, nearby archaeological site at Naiyena Engol 1 (NY1) also support the interpretation that the former sites were more open and probably contained more C4 vegetation than the latter [63]. The presence of two browsing taxa (giraffes, deinotheres) and mixed-feeding bovids (tragelaphini and aepycerotini) at KS1 and KS2 indicates the presence of proximal wooded areas, as does recent faunal analysis [64]. Quinn et al. [20] conducted a detailed study of pedogenic carbonates associated with archaeological sites in the Turkana Basin and found that hominins may have taken advantage of the shade offered by woody environments for activities related to tool production, use or discard.
Existing carbon isotope data from hominin teeth from the Nachukui Formation provide us information on hominin diets over the interval represented by our vegetation record [51,52]. From 2.5 to 1.6 Ma, the diet of Paranthropus tracks the increase in C4 vegetation through time as determined from the plant wax data (figure 3). In contrast, the diet of Homo (n = 2) over that interval remains similar with contributions of 34–40% C4-based foods (figure 3 and electronic supplementary material, table S7). Hominin teeth found to date at or close to the KASC are attributed to Paranthropus boisei [8]. Stable isotope analyses of P. boisei teeth from site KS1 indicate diets ranging from 69 to 76% C4-based foods (figure 3 and electronic supplementary material, table S7). A single Homo sp. tooth analysed from the slightly younger NY1 site indicates a diet of 40% C4-based foods. Isotope data from teeth found in the Kalochoro and underlying Lokalalei Members, attributed to Homo sp. and Paranthropus spp., indicate diets of mixed C3–C4-based foods, with the exception of WT-17000 (P. aethiopicus; black skull), which had an estimated diet of 84% C4-based foods (figure 3) [52].
(c). Carbon isotopes and vegetation reconstruction from plant waxes and pedogenic carbonates
Carbon isotopes from plant wax biomarkers and pedogenic carbonates are both widely accepted and well-established proxies for reconstructing vegetation in past environments [17,29]. However, there are very few studies in which coexisting pedogenic carbonate and plant wax isotope data exist to enable comparison of the two vegetation proxies. The biomarker data from the Nachukui Formation provide a unique opportunity to compare plant wax data to previously published pedogenic carbonate data from the Nachukui Formation over the same time interval (electronic supplementary material, table S8, compiled from [20,60]). A useful way to directly compare biomarker and pedogenic carbonate data is to convert δ13C values to per cent C4 vegetation. Pedogenic carbonate δ13C values were first converted to organic matter δ13C values using an enrichment factor of −14‰. The per cent C4 vegetation is calculated as for the plant waxes, where C3 and C4 endmember δ13C values of organic matter are −26 and −11‰, respectively. A cumulative distribution plot of biomarker and pedogenic carbonate per cent C4 data illustrates the differences in the two vegetation proxies (figure 5).
Figure 5.
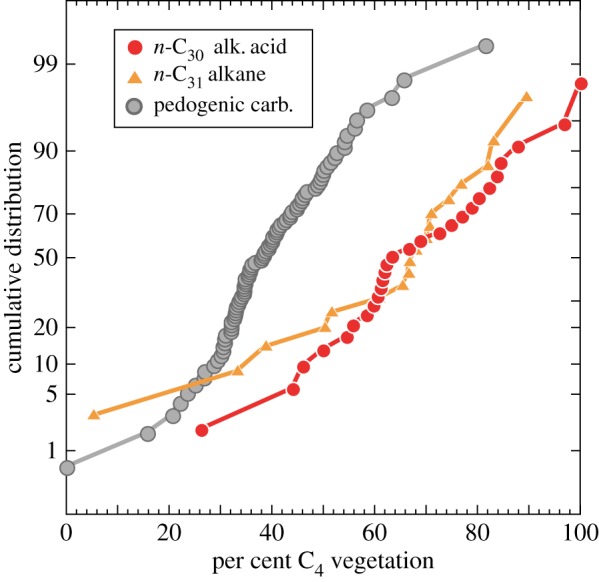
Cumulative distribution function of per cent C4 vegetation calculated from carbon isotope ratios of plant wax biomarkers and pedogenic carbonates. The 50th percentile or median values for n-C30 alkanoic acids and n-C31 alkanes are 63% and 68% C4 vegetation, respectively, whereas the median value for pedogenic carbonate is 38%.
Plant wax biomarkers yield higher median C4 vegetation values than pedogenic carbonates. The median C30 acid and C31 alkane values are 63 and 68% C4 vegetation, whereas the median pedogenic carbonate value is 38% (n = 91). Although the ranges of the biomarker and carbonate data are similar, when the extreme values (less than 3%, more than 97%) are excluded, approximately 95% of the pedogenic carbonate data fall between 20 and 66% C4 vegetation, whereas the range is higher (34–89%) for plant waxes (figure 5). The differences in distributions of per cent C4 vegetation illustrated in figure 5 persist even if more extreme values for δ13C endmember values and fractionation factors are employed to try to reconcile the two datasets. The process that led to the ca 3‰ enrichment in modern soil n-alkanes and n-alkanoic acids observed by Chikaraishi & Naraoka [59] is not a sufficient explanation for the observed offset in pedogenic carbonate and plant waxes from the Nachukui Formation. This is because about half of the n-alkanoic acid samples come from lacustrine sediments, where there is no evidence of such enrichment, and there is no systematic offset in δ13C between lacustrine and palaeosol samples.
A simple explanation for the difference is that the plant wax and pedogenic carbonate samples come from different stratigraphic horizons and as a result, represent vegetation at different times during the interval from 2.3 to 1.7 Ma. There are no paired measurements of the two proxies from the same sediment sample to make direct comparisons of carbon isotope data. It is not known whether paired measurements would be concordant or not. Without paired measurement data, we turn to evaluating proxy systematics to better understand the potential causes for the offset in the reconstructed vegetation (figure 5). Three major inter-related factors that control proxy systematics are provenance, timescale and production.
The provenance of the vegetation signal recorded in pedogenic carbonate is relatively well understood. Based on field observations, pedogenic carbonates throughout the Turkana Basin are autochthonous, and the carbonate precipitates from aqueous CO2 that has been respired from roots or released through oxidation of organic matter. Indicators of in situ carbonate production include early-stage pedogenic carbonate features (e.g. filaments) that must be autochthonous, which in places grade into stage 1–2 carbonate nodules. Plant wax provenance was previously discussed and we are confident that the primary plant wax signal is local or regional (i.e., from within the basin) and not derived from the Ethiopian Highlands. Thus, we conclude both plant waxes and pedogenic carbonate represent local vegetation, particularly for palaeosols parented on fluvial deposits. For lacustrine sediments, the plant waxes may represent a larger, basin-wide signal.
Assuming both proxies represent local vegetation, differences in the temporal integration of each proxy could explain the different vegetation signals. Pedogenic carbonates from arid to semi-arid environments are thought to form over 102–105 years [65]. For plant waxes in arid tropical ecosystems, it is not known how much time is represented in a soil sample. In addition to the total time represented in a sample, seasonal or climatic controls must also be considered, particularly for pedogenic carbonates. Several studies have documented a seasonal bias in carbonate precipitation driven by soil dewatering [27,28]. In eastern Africa, carbonate formation occurs after the rainy seasons when dewatering occurs by soil water evaporation and plant uptake of water. This is generally when peak photosynthetic activity of grasses occurs, so the bias is expected to be towards a C4 signal. The opposite is observed here, so this is an unlikely explanation for the difference.
Other temporal dynamics may be at work in these systems. As an example, we describe a process that leads to temporal offset between pedogenic carbonate and plant waxes from within the same stratigraphic horizon. In lacustrine sediments, the plant wax signal reflects regional vegetation from the catchment. If a lake level falls and the sediments are subaerially exposed and subjected to pedogenesis, then pedogenic carbonates would reflect a later time period and more local vegetation signal than the plant waxes. Other palimpsest processes are possible, and this example illustrates unexplored complexities in sedimentary processes and temporal integration that could affect the two proxy systems.
Production biases could also lead to the observed difference in reconstructed vegetation for plant waxes, we minimized this effect by calculating per cent C4 values from the C31 alkane and C30 acid, the former of which displays similar concentrations across plant functional types [32]. Production biases in the pedogenic carbonate system could be linked to seasonality or to rooting depth. If respiration rates of C3 or C4 plants differ relative to the timing of the wet season, the plant type with a higher respiration rate during the period of carbonate precipitation will be over-represented in pedogenic carbonates. In the case of a mixed C3–C4 ecosystem, the depth of carbonate formation relative to the rooting zone of grasses (shallower) and woody vegetation (deeper) could also impart a bias relative to the actual C3–C4 distribution on the landscape.
The data presented in this study provide strong evidence for a systematic offset between vegetation reconstructions based on carbon isotopes in pedogenic carbonate and plant waxes. It cannot be entirely ruled out that the difference is simply because pedogenic carbonate and plant wax samples come from different strata in this dataset. Yet the consistent relative offset towards lower per cent C4 vegetation calculated from pedogenic carbonates points to differences in how each proxy records vegetation (figure 5). Further study of the two proxies from well-constrained recent or modern ecosystems, including paired analyses of plant waxes and pedogenic carbonate from the same samples, would be the first step towards understanding the relationship between carbon isotopes and vegetation in the two different proxies.
5. Conclusion
Reconstructing the palaeoenvironment of hominins is essential for understanding the potential role of environment and environmental change on their evolution. Here we have demonstrated that carbon isotopes from plant wax biomarkers are a powerful tool for reconstructing vegetation in hominin environments using samples associated with a range of depositional environments. Plant wax biomarkers record a dynamic ecosystem with vegetation ranging from 5 to 100% C4 vegetation during the interval from 2.3 to 1.7 Ma in West Turkana. C3-dominated ecosystems are limited to strata ranging in age from 2.3 to 2.2 Ma and may be associated with riparian forests of the ancestral Omo River or smaller-order streams. After 2.1 Ma, mixed C3–C4 and C4-dominated ecosystems are prevalent. Vegetation reconstructed at the KASC indicates C4-dominated environments that ranged from 61 to 97% C4 vegetation. A majority of large herbivores and hominins associated with the KASC had C4-dominated diets, supporting the vegetation reconstruction from plant wax data. The increase in C4-based foods in the diet of Paranthropus from 2.5 to 1.7 Ma follows the increase in per cent C4 vegetation on the landscape based on the plant wax data, whereas diet of Homo does not vary.
This study demonstrates the potential for reconstructing vegetation in the Turkana Basin, and more broadly, terrestrial sediments, from plant wax carbon isotope ratios. As illustrated in this interval of the Nachukui Formation, plant waxes can be extracted from a wide range of lithologies, paving the way for continuous vegetation records across changes in depositional environments and in sediments where pedogenic carbonates are absent. The observed differences between per cent C4 vegetation determined from plant waxes and pedogenic carbonates are probably owing to differences in temporal and spatial integration of the vegetation signal into pedogenic carbonates and plant waxes in sediments. Paired measurements on both materials in recent, modern or well-constrained ecosystems could improve our ability to interpret carbon isotope data from both materials.
Supplementary Material
Supplementary Material
Acknowledgements
We dedicate this paper to Richard Leakey in honour of his 70th birthday and in acknowledgement of his contributions to the field of human evolution, particularly through his pioneering work in the Turkana Basin. In addition to his continued support of research, he is exemplary in his support of communities in the Turkana Basin. We thank Robert Foley, Marta Lahr, Lawrence Martin and Chris Stringer for the opportunity to contribute this paper. We are grateful to the members of the West Turkana Archaeological Project for support in the field and to the Government of Kenya and the National Museums of Kenya for permission to conduct research in the Turkana Basin. We thank Chris Lepre for providing many of the samples and the depositional context of them. This is Lamont-Doherty Earth Observatory contribution #8000.
Data accessibility
All organic and isotopic geochemical data for this study have been uploaded as electronic supplementary material.
Authors' contributions
K.T.U., P.J.P. and P.d.M. conceived the study and supervised the work; H.R. and S.H. oversaw WTAP fieldwork and facilitated sample collection; C.F.S. conducted fieldwork and collected samples; E.C.K., K.T.U. and P.J.P. acquired the data; K.T.U., P.J.P. E.C.K., and P.d.M. analysed and interpreted the data; K.T.U. wrote the paper and drafted the figures with input and assistance from all co-authors.
Competing interests
We have no competing interests.
Funding
Funding for fieldwork and analyses for this study came from Columbia University's Center for Climate and Life, a Climate Center Grant from the Vetlessen Foundation, and the French Ministry of Foreign Affairs. K.T.U. was supported by Lamont Postdoctoral Research Scientist Fellowship; E.C.K. was supported by the Earth Intern Programme, supported by Barnard College, The Earth Institute, Lamont-Doherty Earth Observatory and the Department of Earth and Environmental Science at Columbia University.
References
- 1.Wood B, Leakey M. 2011. The Omo-Turkana basin fossil hominins and their contribution to our understanding of human evolution in Africa. Evol. Anthropol. 20, 264–292. ( 10.1002/evan.20335) [DOI] [PubMed] [Google Scholar]
- 2.Lepre CJ, Roche H, Kent DV, Harmand S, Quinn RL, Brugal J-P, Texier P-J, Lenoble A, Feibel CS. 2011. An earlier origin for the Acheulian. Nature 477, 82–85. ( 10.1038/nature10372) [DOI] [PubMed] [Google Scholar]
- 3.Roche H, Brugal J-P, Delagnes A, Feibel C, Harmand S, Kibunjia M, Prat S, Texier P-J. 2003. Les sites archéologiques plio-pléistocènes de la formation de Nachukui, Ouest-Turkana, Kenya: bilan synthétique 1997–2001. C. R. Palevol. 2, 663–673. ( 10.1016/j.crpv.2003.06.001) [DOI] [Google Scholar]
- 4.McDougall I, Brown FH. 2006. Precise 40Ar/39Ar geochronology for the upper Koobi Fora Formation, Turkana Basin, northern Kenya. J. Geol. Soc. 163, 205–220. ( 10.1144/0016-764904-166) [DOI] [Google Scholar]
- 5.McDougall I, Brown F. 2008. Geochronology of the pre-KBS Tuff sequence, Omo Group, Turkana Basin. J. Geol. Soc. 165, 549–562. ( 10.1144/0016-76492006-170) [DOI] [Google Scholar]
- 6.McDougall I, Brown FH, Vasconcelos PM, Cohen BE, Thiede DS, Buchanan MJ. 2012. New single crystal 40Ar/39Ar ages improve time scale for deposition of the Omo Group, Omo–Turkana Basin, East Africa. J. Geol. Soc. 169, 213–226. ( 10.1144/0016-76492010-188) [DOI] [Google Scholar]
- 7.Prat S, et al. 2005. First occurrence of early Homo in the Nachukui formation (West Turkana, Kenya) at 2.3–2.4 Myr. J. Hum. Evol. 49, 230–240. ( 10.1016/j.jhevol.2005.03.009) [DOI] [PubMed] [Google Scholar]
- 8.Prat S, Brugal J-P, Roche H, Texier P-J. 2003. Nouvelles découvertes de dents d'hominidés dans le membre Kaitio de la formation de Nachukui (1,65–1,9 Ma), Ouest du lac Turkana (Kenya). C. R. Palevol. 2, 685–693. ( 10.1016/j.crpv.2003.07.001) [DOI] [Google Scholar]
- 9.Leakey MG, Feibel CS, McDougall I, Walker A. 1995. New four-million-year-old hominid species from Kanapoi and Allia Bay, Kenya. Nature 376, 565–571. ( 10.1038/376565a0) [DOI] [PubMed] [Google Scholar]
- 10.Leakey MG, Spoor F, Brown FH, Gathogo PN, Kiarie C, Leakey LN, McDougall I. 2001. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature 410, 433–440. ( 10.1038/35068500) [DOI] [PubMed] [Google Scholar]
- 11.Brown F, Harris J, Leakey R, Walker A. 1985. Early Homo erectus skeleton from west lake Turkana, Kenya. Nature 316, 788–792. ( 10.1038/316788a0) [DOI] [PubMed] [Google Scholar]
- 12.Walker A, Leakey RE, Harris JM, Brown FH. 1986. 2.5-Myr Australopithecus boisei from west of Lake Turkana, Kenya. Nature 322, 517–522. ( 10.1038/322517a0) [DOI] [Google Scholar]
- 13.Roche H, Delagnes A, Brugal J-P, Feibel C, Kibunjia M, Mourre V, Texier P-J. 1999. Early hominid stone tool production and technical skill 2.34 Myr ago in West Turkana, Kenya. Nature 399, 57–60. ( 10.1038/19959) [DOI] [PubMed] [Google Scholar]
- 14.Harmand S, et al. 2015. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521, 310–315. ( 10.1038/nature14464) [DOI] [PubMed] [Google Scholar]
- 15.Kibunjia M, Roche H, Brown FH, Leakey RE. 1992. Pliocene and Pleistocene archeological sites west of Lake Turkana, Kenya. J. Hum. Evol. 23, 431–438. ( 10.1016/0047-2484(92)90091-M) [DOI] [Google Scholar]
- 16.Roche H. 2011. The archaeology of human origins: the contribution of West Turkana, Kenya. In Casting the net wide: papers in honor of Glynn Isaac and his approach to human origins research (eds Sept J, Pilbeam DR), pp. 75–92. American School of Prehistoric Research monograph series Oakville, CT: Oxbow Books. [Google Scholar]
- 17.Cerling TE. 1984. The stable isotopic composition of modern soil carbonate and its relationship to climate. Earth Planet. Sci. Lett. 71, 229–240. ( 10.1016/0012-821X(84)90089-X) [DOI] [Google Scholar]
- 18.Cerling TE, Quade J. 1993. Stable carbon and oxygen isotopes in soil carbonates. Geophys. Monogr. 78, 217–231. ( 10.1029/gm078p0217) [DOI] [Google Scholar]
- 19.Wynn JG. 2004. Influence of Plio-Pleistocene aridification on human evolution: evidence from paleosols of the Turkana Basin, Kenya. Am. J. Phys. Anthropol. 123, 106–118. ( 10.1002/ajpa.10317) [DOI] [PubMed] [Google Scholar]
- 20.Quinn RL, et al. 2013. Pedogenic carbonate stable isotopic evidence for wooded habitat preference of early Pleistocene tool makers in the Turkana Basin. J. Hum. Evol. 123, 65–78. ( 10.1016/j.jhevol.2013.04.002) [DOI] [PubMed] [Google Scholar]
- 21.Quinn RL, Lepre CJ, Wright JD, Feibel CS. 2007. Paleogeographic variations of pedogenic carbonate δ13C values from Koobi Fora, Kenya: implications for floral compositions of Plio-Pleistocene hominin environments. J. Hum. Evol. 53, 560–573. ( 10.1016/j.jhevol.2007.01.013) [DOI] [PubMed] [Google Scholar]
- 22.Cerling TE, Bowman JR, O'Neil JR. 1988. An isotopic study of a fluvial-lacustrine sequence: the Plio-Pleistocene koobi fora sequence, East Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 63, 335–356. ( 10.1016/0031-0182(88)90104-6) [DOI] [Google Scholar]
- 23.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. 2011. Paleosol carbonates from the Omo Group: isotopic records of local and regional environmental change in East Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 307, 75–89. ( 10.1016/j.palaeo.2011.04.026) [DOI] [Google Scholar]
- 24.Passey BH, Levin NE, Cerling TE, Brown FH, Eiler JM. 2010. High-temperature environments of human evolution in East Africa based on bond ordering in paleosol carbonates. Proc. Natl Acad. Sci. USA 107, 11245 ( 10.1073/pnas.1001824107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerling T, Harris J, Leakey M. 2003. Isotope paleoecology of the Nawata and Nachukui Formations at Lothagam, Turkana Basin, Kenya. In Lothagam: the dawn of humanity in Eastern Africa (eds Leakey MG, Harris JM), pp. 605–623. New York, NY: Columbia University Press. [Google Scholar]
- 26.Wynn JG. 2000. Paleosols, stable carbon isotopes and paleoenvironmental interpretation of Kanapoi, Northern Kenya. J. Hum. Evol. 39, 411–432. ( 10.1006/jhev.2000.0431) [DOI] [PubMed] [Google Scholar]
- 27.Quade J, Eiler J, Daëron M, Achyuthan H. 2013. The clumped isotope geothermometer in soil and paleosol carbonate. Geochim. Cosmochim. Acta 105, 92–107. ( 10.1016/j.gca.2012.11.031) [DOI] [Google Scholar]
- 28.Breecker D, Sharp Z, McFadden LD. 2009. Seasonal bias in the formation and stable isotopic composition of pedogenic carbonate in modern soils from central New Mexico, USA. Geol. Soc. Am. Bull. 121, 630 ( 10.1130/B26413.1) [DOI] [Google Scholar]
- 29.Collister JW, Rieley G, Stern B, Eglinton G, Fry B. 1994. Compound-specific δ13C analyses of leaf lipids from plants with differing carbon dioxide metabolisms. Org. Geochem. 21, 619–627. ( 10.1016/0146-6380(94)90008-6) [DOI] [Google Scholar]
- 30.Rommerskirchen F, Plader A, Eglinton G, Chikaraishi Y, Rullkötter J. 2006. Chemotaxonomic significance of distribution and stable carbon isotopic composition of long-chain alkanes and alkan-1-ols in C4 grass waxes. Org. Geochem. 37, 1303–1332. ( 10.1016/j.orggeochem.2005.12.013) [DOI] [Google Scholar]
- 31.Vogts A, Moossen H, Rommerskirchen F, Rullkötter J. 2009. Distribution patterns and stable carbon isotopic composition of alkanes and alkan-1-ols from plant waxes of African rain forest and savanna C3 species. Org. Geochem. 40, 1037–1054. ( 10.1016/j.orggeochem.2009.07.011) [DOI] [Google Scholar]
- 32.Garcin Y, et al. 2014. Reconstructing C3 and C4 vegetation cover using n-alkane carbon isotope ratios in recent lake sediments from Cameroon, Western Central Africa. Geochim. Cosmochim. Acta 142, 482–500. ( 10.1016/j.gca.2014.07.004) [DOI] [Google Scholar]
- 33.Freeman KH, Colarusso LA. 2001. Molecular and isotopic records of C4 grassland expansion in the late miocene. Geochim. Cosmochim. Acta 65, 1439–1454. ( 10.1016/S0016-7037(00)00573-1) [DOI] [Google Scholar]
- 34.Polissar PJ, Freeman KH, Rowley DB, McInerney FA, Currie BS. 2009. Paleoaltimetry of the Tibetan Plateau from D/H ratios of lipid biomarkers. Earth Planet. Sci. Lett. 287, 64–76. ( 10.1016/j.epsl.2009.07.037) [DOI] [Google Scholar]
- 35.Smith FA, Wing SL, Freeman KH. 2007. Magnitude of the carbon isotope excursion at the Paleocene–Eocene thermal maximum; the role of plant community change. Earth Planet. Sci. Lett. 262, 50–65. ( 10.1016/j.epsl.2007.07.021) [DOI] [Google Scholar]
- 36.Feakins SJ, Levin NE, Liddy HM, Sieracki A, Eglinton TI, Bonnefille R. 2013. Northeast African vegetation change over 12 my. Geology 41, 295–298. ( 10.1130/G33845.1) [DOI] [Google Scholar]
- 37.Schefuß E, Schouten S, Jansen JHF, Damsté JSS. 2003. African vegetation controlled by tropical sea surface temperatures in the mid-Pleistocene period. Nature 422, 418–421. ( 10.1038/nature01500) [DOI] [PubMed] [Google Scholar]
- 38.Magill CR, Ashley GM, Freeman KH. 2013. Ecosystem variability and early human habitats in eastern Africa. Proc. Natl Acad. Sci. USA 110, 1167–1174. ( 10.1073/pnas.1206276110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castañeda IS, Werne JP, Johnson TC, Filley TR. 2009. Late Quaternary vegetation history of southeast Africa: the molecular isotopic record from Lake Malawi. Palaeogeogr. Palaeoclimatol. Palaeoecol. 275, 100–112. ( 10.1016/j.palaeo.2009.02.008) [DOI] [Google Scholar]
- 40.Abell PI, Margolis MJ. 1982. n-Paraffins in the sediments and in situ fossils of the Lake Turkana Basin, Kenya. Geochim. Cosmochim. Acta 46, 1505–1511. ( 10.1016/0016-7037(82)90310-6) [DOI] [Google Scholar]
- 41.Hayes JM, Freeman KH, Popp BN, Hoham CH. 1990. Compound-specific isotopic analyses; a novel tool for reconstruction of ancient biogeochemical processes. Org. Geochem. 16, 1115–1128. ( 10.1016/0146-6380(90)90147-R) [DOI] [PubMed] [Google Scholar]
- 42.Feakins SJ, Eglinton TI, deMenocal PB. 2007. A comparison of biomarker records of northeast African vegetation from lacustrine and marine sediments (ca. 3.40 Ma). Org. Geochem. 38, 1607–1624. ( 10.1016/j.orggeochem.2007.06.008) [DOI] [Google Scholar]
- 43.Ryan WBF, et al. 2009. Global multi-resolution topography synthesis. Geochem. Geophys. Geosyst. 10, 1–9. ( 10.1029/2008GC002332) [DOI] [Google Scholar]
- 44.Marzi R, Torkelson B, Olson R. 1993. A revised carbon preference index. Org. Geochem. 20, 1303–1306. ( 10.1016/0146-6380(93)90016-5) [DOI] [Google Scholar]
- 45.Bush RT, McInerney FA. 2013. Leaf wax n-alkane distributions in and across modern plants: implications for paleoecology and chemotaxonomy. Geochim. Cosmochim. Acta 117, 161–179. ( 10.1016/j.gca.2013.04.016) [DOI] [Google Scholar]
- 46.Bush RT, McInerney FA. 2015. Influence of temperature and C4 abundance on n-alkane chain length distributions across the central USA. Org. Geochem. 79, 65–73. ( 10.1016/j.orggeochem.2014.12.003) [DOI] [Google Scholar]
- 47.Haug P, Schnoes H, Burlingame A. 1967. Keto-carboxylic acids isolated from the Colorado Green River shale (Eocene). Chem. Commun. (Lond.), 1130–1131. ( 10.1039/C19670001130) [DOI] [PubMed] [Google Scholar]
- 48.Tipple BJ, Pagani M. 2010. A 35 Myr North American leaf-wax compound-specific carbon and hydrogen isotope record: implications for C4 grasslands and hydrologic cycle dynamics. Earth Planet. Sci. Lett. 299, 250–262. ( 10.1016/j.epsl.2010.09.006) [DOI] [Google Scholar]
- 49.Tipple B, Meyers S, Pagani M. 2010. Carbon isotope ratio of Cenozoic CO2: a comparative evaluation of available geochemical proxies. Paleoceanography 25 ( 10.1029/2009PA001851) [DOI] [Google Scholar]
- 50.Passey BH, Cerling TE, Perkins ME, Voorhies MR, Harris JM, Tucker ST. 2002. Environmental change in the Great Plains; an isotopic record from fossil horses. J. Geol. 110, 123–140. ( 10.1086/338280) [DOI] [Google Scholar]
- 51.Jehle G. 2013. An ecological snapshot of the early Pleistocene at Kokiselei, Kenya [MS]. Salt Lake City, UT: University of Utah. [Google Scholar]
- 52.Cerling TE, et al. 2013. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc. Natl Acad. Sci. USA 110, 10 501–10 506. ( 10.1073/pnas.1222568110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.France-Lanord C, Derry LA. 1994. δ13C of organic carbon in the Bengal Fan: source evolution and transport of C3 and C4 plant carbon to marine sediments. Geochim. Cosmochim. Acta 58, 4809–4814. ( 10.1016/0016-7037(94)90210-0) [DOI] [Google Scholar]
- 54.Volkman JK, Barrett SM, Blackburn SI, Mansour MP, Sikes EL, Gelin F. 1998. Microalgal biomarkers: a review of recent research developments. Org. Geochem. 29, 1163–1179. ( 10.1016/S0146-6380(98)00062-X) [DOI] [Google Scholar]
- 55.Huang Y, Eglinton G, Ineson P, Latter PM, Bol R, Harkness DD. 1997. Absence of carbon isotope fractionation of individual n-alkanes in a 23-year field decomposition experiment with Calluna vulgaris. Org. Geochem. 26, 497–501. ( 10.1016/S0146-6380(97)00027-2) [DOI] [Google Scholar]
- 56.Tu TTN, Egasse C, Zeller B, Bardoux G, Biron P, Ponge J-F, David B, Derenne S. 2011. Early degradation of plant alkanes in soils: a litterbag experiment using 13C-labelled leaves. Soil Biol. Biochem. 43, 2222–2228. ( 10.1016/j.soilbio.2011.07.009) [DOI] [Google Scholar]
- 57.Tu TTN, Derenne S, Largeau C, Bardoux G, Mariotti A. 2004. Diagenesis effects on specific carbon isotope composition of plant n-alkanes. Org. Geochem. 35, 317–329. ( 10.1016/j.orggeochem.2003.10.012) [DOI] [Google Scholar]
- 58.Huang Y, Bol R, Harkness DD, Ineson P, Eglinton G. 1996. Post-glacial variations in distributions, 13C and 14C contents of aliphatic hydrocarbons and bulk organic matter in three types of British acid upland soils. Org. Geochem. 24, 273–287. ( 10.1016/0146-6380(96)00039-3) [DOI] [Google Scholar]
- 59.Chikaraishi Y, Naraoka H. 2006. Carbon and hydrogen isotope variation of plant biomarkers in a plant–soil system. Chem. Geol. 231, 190–202. ( 10.1016/j.chemgeo.2006.01.026) [DOI] [Google Scholar]
- 60.Levin NE. 2013. Compilation of East Africa soil carbonate stable isotope data. In Integrated earth data alliance. ( 10.1594/IEDA/100231) [DOI] [Google Scholar]
- 61.Lepre CJ. 2014. Early Pleistocene lake formation and hominin origins in the Turkana–Omo rift. Quatern. Sci. Rev. 102, 181–191. ( 10.1016/j.quascirev.2014.08.012) [DOI] [Google Scholar]
- 62.Maslin MA, Brierley CM, Milner AM, Shultz S, Trauth MH, Wilson KE. 2014. East African climate pulses and early human evolution. Quatern. Sci. Rev. 101, 1–17. ( 10.1016/j.quascirev.2014.06.012) [DOI] [Google Scholar]
- 63.Brugal J-P, Roche H, Kibunjia M. 2003. Faunes et paléoenvironnements des principaux sites archéologiques plio-pléistocènes de la formation de Nachukui (Ouest-Turkana, Kenya). C. R. Palevol. 2, 675–684. ( 10.1016/j.crpv.2003.09.028) [DOI] [Google Scholar]
- 64.Brugal J-P, Roche H. 2016. Paleoecology and Paleoenvironment of Early quaternary faunal assemblages from the Nachukui Formation in Kenya: insights from the West Turkana Archaeological Project. In African Paleoecology and Human Evolution (eds Reynolds S, Bobe R). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 65.Machette MN. 1985. Calcic soils of the southwestern United States. Geol. Soc. Am. Spec. Pap. 203, 1–22. ( 10.1130/SPE203-p1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All organic and isotopic geochemical data for this study have been uploaded as electronic supplementary material.