Abstract
Animal cells have served as highly controllable model systems for furthering cartilage tissue engineering practices in pursuit of treating osteoarthritis. Although successful strategies for animal cells must ultimately be adapted to human cells to be clinically relevant, human chondrocytes are rarely employed in such studies. In this study, we evaluated the applicability of culture techniques established for juvenile bovine and adult canine chondrocytes to human chondrocytes obtained from fresh or expired osteochondral allografts. Human chondrocytes were expanded and encapsulated in 2% agarose scaffolds measuring Ø3–4 mm × 2.3 mm, with cell seeding densities ranging from 15 to 90 × 106 cells/mL. Subsets of constructs were subjected to transient or sustained TGF-β treatment, or provided channels to enhance nutrient transport. Human cartilaginous constructs physically resembled native human cartilage, and reached compressive Young’s moduli of up to ~250 kPa (corresponding to the low end of ranges reported for native knee cartilage), dynamic moduli of ~950 kPa (0.01 Hz), and contained 5.7 percent wet weight (%/ww) of glycosaminoglycans (≥ native levels) and 1.5 %/ww collagen. We found that the initial seeding density had pronounced effects on tissue outcomes, with high cell seeding densities significantly increasing nearly all measured properties. Transient TGF-β treatment was ineffective for adult human cells, and tissue construct properties plateaued or declined beyond 28 days of culture. Finally, nutrient channels improved construct mechanical properties, presumably due to enhanced rates of mass transport. These results demonstrate that our previously established culture system can be successfully translated to human chondrocytes.
Keywords: Cartilage, Tissue Engineering, Human, Chondrocytes, Allografts, Agarose
INTRODUCTION
Osteoarthritis is a progressive joint disease affecting over 27 million Americans and many more worldwide (Lawrence et al., 2008). Articular cartilage is recalcitrant to repair following joint injury or osteoarthritis (OA) due to its low cell population and avascular nature. Cartilage tissue engineering (CTE) is a promising strategy for treating OA by stimulating cells to produce cartilage extracellular matrix in vitro by subjecting them to favorable culture conditions (Langer and Vacanti, 1993; Vacanti and Langer, 1999). CTE techniques have improved over the years and have shown promise in animal model systems. However, cells for clinical translation of tissue engineering applications must ultimately be of human source and therefore CTE techniques that are successful in animal models must next be examined in human model systems.
When attempting to grow human cartilage in vitro, human mesenchymal stem cells (MSCs) have commonly served as cell sources (Bhumiratana et al., 2014; Bian et al., 2011; de Mara et al., 2013; Ghone and Grayson, 2012; Handorf and Li, 2011). While MSCs are readily obtained from patients from various tissues, they must be coaxed and maintained along chondrogenic lineage. An alternative is the usage of allogeneic chondrocytes harvested from tissue bank cartilage, which are ideal for CTE applications because they already possess chondrocytic phenotype and are not subject to immunological rejection by human joints. Human articular chondrocytes (hACs) are already clinically utilized in autologous chondrocyte implantation for repairing OA defects (Bentley et al., 2003; Peterson et al., 2000). Further, successes in chondrocyte-based CTE studies in animal models will likely translate best for hACs over other human cell types. Relatively few studies have utilized hACs in CTE (Adkisson et al., 2010; Dehne et al., 2009; Dell’Accio et al., 2001; Fernandes et al., 2013; Kafienah et al., 2002; Lehmann et al., 2013; Saha et al., 2013; Sittinger et al., 1994; Wang et al., 2006; Wenger et al., 2006), due perhaps to logistical challenges of obtaining donor cells in sufficient numbers and encouraging them to produce sufficient matrix. Even fewer studies have characterized functional properties of human cartilage tissue constructs such as mechanical integrity (Murphy et al., 2015; O’Connell et al., 2015; Zhao et al., 2012).
CTE has benefited from a number of advances made in animal models. Serum-free culture media permit reproducible, elevated functional properties over serum-containing media when coupled with transient TGF-β supplementation for constructs seeded with immature bovine chondrocytes (Byers et al., 2008). Agarose hydrogel scaffolds stabilize the chondrocyte phenotype (Benya and Shaffer, 1982; Buschmann et al., 1992; Mauck et al., 2000), and they promote deposition of proteoglycans that are most similar to those of native cartilage compared to other scaffold types (Mouw et al., 2005). Increasing initial cell seeding densities up to or above 60 × 106 cells/mL leads to concomitant increases in matrix synthesis, though this steepens nutrient gradients within the tissue and promotes heterogeneity (Albro et al., 2016; Bian et al., 2009a; Talukdar et al., 2011; Zhou et al., 2008). Tissue heterogeneity is mitigated by nutrient channels, which minimize nutrient diffusion distances and improve functional properties (Bian et al., 2009a; Buckley et al., 2009; Cigan et al., 2013b; Nims et al., 2016). In adult canine chondrocytes, monolayer expansion with growth factors prior to 3D culture elevates construct properties (Ng et al., 2010).
Our group has routinely reproduced native cartilage Young’s moduli (EY) of ~500 kPa and glycosaminoglycan (GAG) contents of > 4 percent of wet weight (%/ww) in juvenile bovine chondrocyte-seeded agarose constructs (Bian et al., 2009a; Cigan et al., 2013a; Cigan et al., 2014; Nims et al., 2014). Other studies have produced high-quality engineered cartilage utilizing canine or porcine cell sources (Alegre-Aguaron et al., 2014; Bian et al., 2010a; Kelly et al., 2013; Ng et al., 2010; O’Conor et al., 2014). Growth factor-aided monolayer expansion of hACs is generally necessary to obtain sufficient cell number for CTE studies (Barbero et al., 2003; Francioli et al., 2007; Jakob et al., 2001). We previously obtained EY of 100 kPa and 2.2 %/ww GAG in agarose constructs cast with passaged chondrocytes from two osteoarthritic donors at 30 × 106 cells/mL for 56 days (O’Connell et al., 2015). While these results are promising for viable long-term 3D culture of hACs, functional properties were below native levels as well as levels in bovine constructs.
The primary objective of this study was to achieve native cartilage properties in a human CTE model by translating successful techniques from our bovine and canine systems. Using chondrocytes derived from expired human osteochondral allografts, we first assessed the utility of transient versus sustained TGF-β supplementation (Figure 1). Implementing the more successful treatment, we next investigated effects of varying cell seeding density. Finally, to improve nutrient transport, we cast constructs using chondrocytes from fresh human osteochondral allografts with incorporation of nutrient channels.
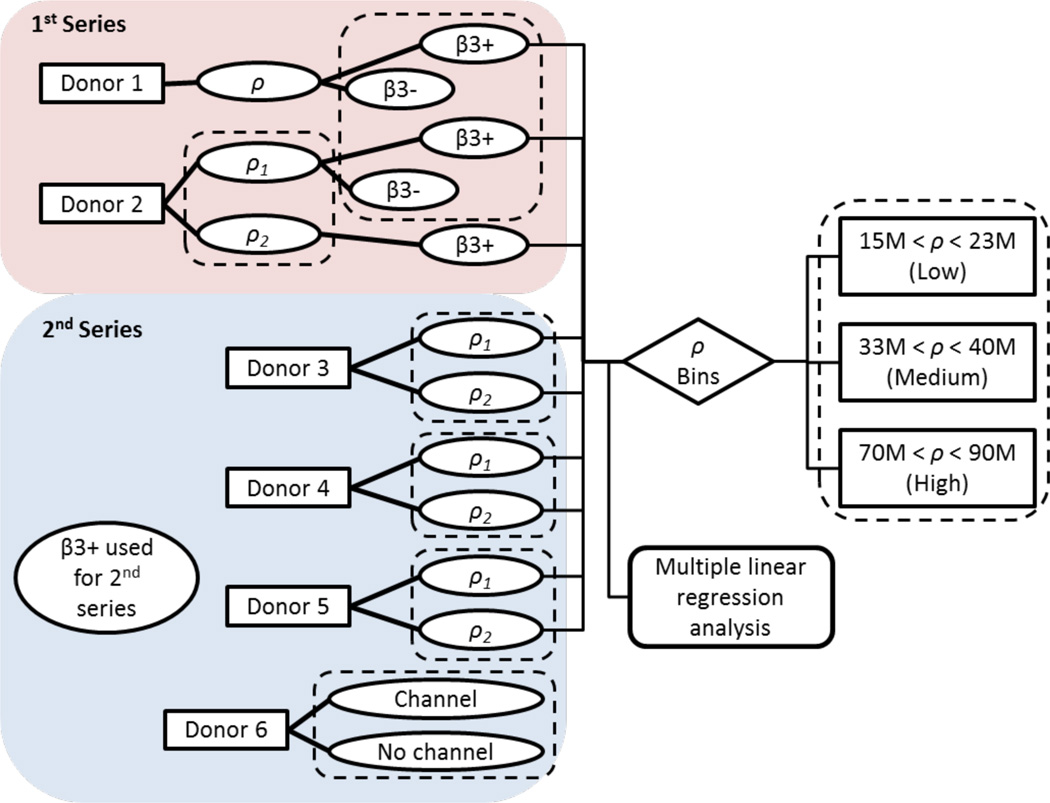
Figure 1.
Experimental design for human constructs. ρ = cell seeding density, β3+ = continuous TGF-β treatment, β3- = transient TGF-β treatment. Dotted rectangles indicate between which groups statistical comparisons were made. Culture durations were examined in all statistical analyses but for simplicity are not depicted.
METHODS
Harvest & Culture
Six osteochondral allografts (5 taluses, 1 distal femur; Figure 2) were obtained from the Musculoskeletal Transplant Foundation (mtf.org). Donors were male and female and 17–35 years old (Table 1). Tissue from each donor was cultivated separately. Grafts 1–5 were expired, having exceeded their shelf lives of approximately one month post-mortem; the 6th graft was obtained fresh (~14 days in storage). Full-thickness articular cartilage was aseptically harvested from grafts, and cartilage pieces were digested in 1000 U/mL Type IV collagenase (Sigma) for 6–8 hours at 37 °C with orbital shaking.
Figure 2.
Sample of an expired human talar allograft.
Table 1.
Donor demographics for each of the six donors, and seeding densities and total numbers of constructs (n) for each respective cast. “N/A” indicates donor demographics were not available.
| Donor | Sex | Age | Seeding Density ρ (cells/mL) |
N |
|---|---|---|---|---|
| 1 | F | 20 | 17 × 106 | 12 |
| 2 | F | 19 | ρ1: 15 × 106 | 11 |
| ρ2: 22 × 106 | 7 | |||
| 3 | M | 17 | ρ1: 23 × 106 | 2 |
| ρ2: 70 × 106 | 8 | |||
| 4 | F | 35 | ρ1: 40 × 106 | 8 |
| ρ2: 90 × 106 | 16 | |||
| 5 | M | 33 | ρ1: 22 × 106 | 16 |
| ρ2: 33 × 106 | 18 | |||
| 6 | N/A | N/A | 69 × 106 | 20 |
Released chondrocytes were plated at 20,000 cells/cm2 in expansion medium consisting of high-glucose DMEM with 10% FBS, 1% Antibiotic-antimycotic, 10 ng/mL human recombinant PDGF-ββ, 5 ng/mL human recombinant FGF-2 (Life Technologies), and 1 ng/mL human recombinant TGF-β3 (R&D Systems) (Francioli et al., 2007; Ng et al., 2010; O’Connell et al., 2015). P2 cells were trypsinized and resuspended in chemically-defined ITS medium (Cigan et al., 2013a).
The cell suspensions were mixed 1:1 with molten 4% Type VIIA agarose (Sigma) and cast between glass slides to form cellularized 2% agarose gels. A biopsy punch was employed to produce Ø3 × 2.3 mm cylindrical constructs. Constructs were cultured for 14, 28, 42, or 56 days, with 2 ≤ n ≤ 5 per time point tested, in ITS media with 0.8 Hz orbital shaking. Media were changed thrice weekly and spent media samples were frozen for subsequent analysis.
Transient TGF-β Supplementation
In the first series of casts, the efficacy of transient versus sustained TGF-β treatment in hACs was investigated (Figure 1). Samples from Donors 1 and 2 were supplemented with TGF-β for either the first 14 days (Byers et al., 2008) (β3-) or for the entire culture duration (β3+). Due to the more promising properties of the β3+ group based on these initial cultures (see Results), sustained TGF-β supplementation was used for the second series of casts (Figure 1).
Cell Seeding Density
Nine distinct casts were executed with cells from the five talar grafts. Cell densities (“ρ”) of 15, 30, and 60 × 106 cells/mL were targeted nominally; actual densities were assessed by DNA assay (Table 1). Media were supplied to constructs at approximately 0.45 mL/(106 cells · day) based upon nominal ρ (Nims et al., 2016).
Nutrient Channel Incorporation
Chondrocytes were isolated from Donor 6 cartilage and cast into Ø4 × 2.3 mm constructs at a nominal ρ of 60 × 106 cells/mL. At day 14, single nutrient channels (Ø1 mm) were made with a biopsy punch in half of the constructs (Bian et al., 2009a). Constructs were cultured and assessed similarly as those from Donors 1–5.
Mechanical, Biochemical, and Histological Characterization
See Appendix (Supplementary Data) for methods of mechanical and biochemical characterization and histology.
Statistical Analysis
Culture conditions and statistical comparisons are summarized in Figure 1. To examine effects of TGF-β supplementation, the samples modulated for TGF-β treatment were analyzed for each Donor 1 and Donor 2 by unpaired Student’s t-tests (α = 0.05) at day 42. Subsequently, the data from Donors 1–5 (omitting β3- constructs) were pooled and analyzed to identify overall trends between construct functional properties and culture parameters. Multiple linear regression analyses (STATISTICA) were performed to explore first-order relationships (detailed in Appendix, Supplementary Data).
After multiple regression analyses indicated positive, pronounced effects of ρ on construct functional properties, the data were discretized to permit analysis of variance (ANOVA) tests. Pooled data from Donors 1–5 were binned based upon whether their ρ fell below, between, or above 30 and 60 × 106 cells/mL, which have served as benchmark ρ in previous studies (Bian et al., 2009a; Cigan et al., 2013a; Cigan et al., 2014; Nims et al., 2014; Nims et al., 2016; O’Connell et al., 2015). The resulting low, medium, and high cell seeding density bins ranged from 15–23, 33–40, and 70–90 × 106 cells/mL, respectively. These bins were compared by two-way ANOVA (α = 0.05) with Bin, Day, and their interaction (Bin×Day) as independent factors; Tukey’s HSD corrected post hoc tests of the means were instituted upon determination of significance (p < 0.05). To examine whether effects of ρ were consistent when controlling for donor variability, pairwise comparisons were made between the lower and higher ρ (ρ1 and ρ2) separately for Donors 2–5 by ANOVA as described above. Donor variability was assessed by one-way ANOVA with donor as the independent factor and GAG/DNA and COL/DNA as dependent variables. Lastly, to analyze the effect of nutrient channels, constructs from Donor 6 were compared by ANOVA with CH (presence or absence of channel), Day (28 or 42), and CH×Day (their interaction) as independent factors.
RESULTS
TGF-β Supplementation
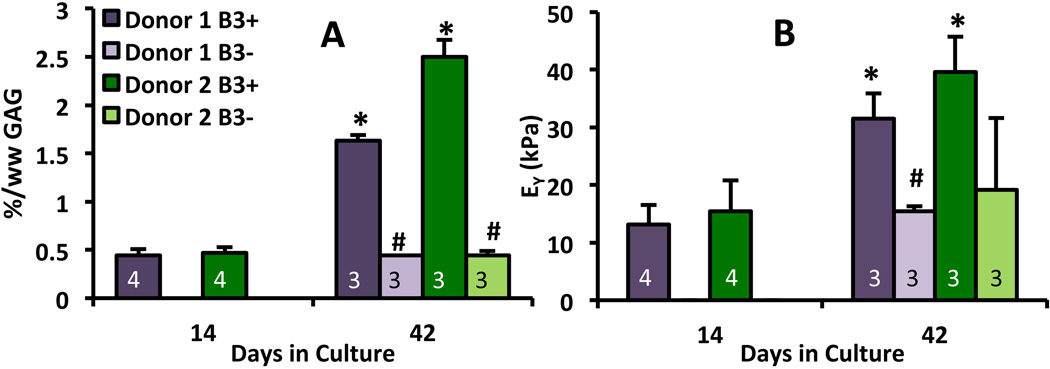
For Donors 1 and 2, β3+ constructs had significantly higher %/ww of GAG (p < 0.0005, Figure 3A) and collagen (p < 0.005) compared to β3+ constructs at day 42. Donor 1 showed significantly higher EY in the β3+ group versus β3- (p < 0.005, Figure 3B). From day 14 onward, β3- constructs did not increase appreciably in EY or GAG and collagen contents. Therefore, β3+ treatment was adopted for the second series of casts.
Figure 3.
Effects of TGF-β, both transient (treated D0-D14, “β3-”) or sustained (treated D0-D42, “β3+”) on tissue composition (A) and mechanical properties (B) in a subset of constructs from Donors 1 and 2. “*” denotes p < 0.05 vs. D14 time point (when β3- and β3+ diverge), “#” denotes p < 0.05 vs. β3+ group from same donor. Numbers of samples per group n are superimposed on bars.
Construct Functional Properties
Constructs developed a broad spectrum of functional properties. All groups tended to develop mechanical and biochemical properties concomitantly, as demonstrated by the approximately linear relationship between GAG content and EY (Figure 4A). Groups with higher ρ exhibited higher functional properties. The highest-quality constructs with cells derived from expired graft tissue were those with the highest ρ (90 × 106 cells/mL). At D28, they had the highest EY (254 ± 22 kPa, Figure 4C), G* (945 ± 65 kPa), and GAG content (4.8 ± 0.7 %/ww, Figure 4D). After 8 weeks, these constructs also had the highest COL content (1.5 ± 0.1 %/ww).
Figure 4.
Correlation between the tissue composition and mechanical properties at all time points (A), retention of GAG over time by combined constructs of Donors 1–5 (B), and EY (C) and GAG synthesis (D) of Donor 4 constructs at both low ρ (40 × 106 cells/mL) and high ρ (90 × 106 cells/mL). “*” denotes p < 0.05 vs. Day 14 time point, “†” denotes p < 0.05 vs. high ρ group, “§” denotes other p < 0.05 comparison. Numbers of samples per group n are superimposed on bars in panels B–D.
Constructs grew steadily throughout the first four weeks of culture before tending to reach a plateau or maximum for most functional properties (Figures 4B–D, 5). Indeed, ANOVA revealed that EY, G*, and collagen increased significantly overall for constructs until D28 (p < 0.05), after which no overall increases in properties were observed. GAG levels did not significantly depend on culture duration (p = 0.27), indicating that the majority of GAG deposition occurred before day 14. Furthermore, analysis of spent culture media revealed that the cumulative retention fraction of GAG by constructs decreased from day 28 to day 56 (Figure 4B). Donors exhibited similar GAG contents normalized by DNA (p = 0.11), while a significant effect of COL/DNA (p < 0.01) indicated low rates of collagen were synthesized by Donor 3 cells (Figure 6).
Figure 5.
EY (A), G* (B), GAG (C), and collagen (D) of constructs from Donors 1–5, binned by low (15–23 × 106 cells/mL), medium (33–40 × 106 cells/mL), or high (70–90 × 106 cells/mL) cell density. “*” denotes p < 0.05 for ANOVA factor, “#” denotes all bins are significantly distinct. For panels A, B, and D, individual groups not sharing a common letter (a–e) are significantly different (p < 0.05). Numbers of samples per group n are superimposed on bars.
Figure 6.
DNA-normalized GAG (A) and collagen (B) contents by donor at final time points for each culture group. Groups sharing * or # superscripts are significantly different (p < 0.05). D = Donor.
Relationships between the culture parameters, construct compositions and mechanical properties
The full results of the regression analyses are listed in Table 2. In the relationships between construct matrix contents and mechanical properties, both EY and G* were found to have strong correlations (R2 = 0.78 and 0.83, respectively) with both GAG and COL together (p < 0.0001). GAG was highly predictive of both construct mechanical properties measured (p < 0.0001), while COL was only a significant factor for G* (p <0.05). Culture duration and cell seeding density also shared positive correlations with all dependent factors (EY, G*, GAG, COL; p < 0.005), though regressions were weaker, especially that of COL (R2 = 0.19). The far stronger predictor of functional properties was ρ (p < 0.001). Culture duration was found to be less strongly correlated with functional properties, being only significantly predictive of G* (p < 0.05) and holding positive trends for EY, GAG, and COL (0.05 < p ≤ 0.08).
Table 2.
Results of multiple linear regression analyses. Given are: R2 values and p-values for overall regressions on dependent factors Y, regression coefficients A1 and A2 (± 95% confidence intervals) corresponding to dependent factors X1 and X2 (either GAG & COL or Day and ρ), and p-values for each factor X1 and X2.
| Y | R2 | p (total) | A1 (kPa) [X1 = GAG] |
p (X1) | A2 (kPa) [X2 = COL] |
p (X2) |
| EY | 0.78 | <0.0001 | 41.8±3.2 | <0.0001 | 6.1±10.4 | 0.56 |
| G* | 0.83 | <0.0001 | 156.2±10.9 | <0.0001 | 82.0±35.5 | <0.05 |
| Y | R2 | p (total) | A1 (kPa·day−1) [X1 = Day] |
p (X1) | A2 (kPa·mL·cells−1) [X2 = ρ] |
p (X2) |
| EY | 0.57 | <0.0001 | 0.52±0.30 | 0.08 | 1.81±0.17 | <0.0001 |
| G* | 0.51 | <0.0001 | 3.3±1.3 | <0.05 | 6.69±0.72 | <0.0001 |
| Y | R2 | p (total) | A1 (%/ww·day−1) [X1 = Day] |
p (X1) | A2 (%/ww·mL·cells−1) [X2 = ρ] |
p (X2) |
| GAG | 0.68 | <0.0001 | 0.014±0.007 | 0.066 | 0.04±0.003 | <0.0001 |
| COL | 0.19 | <0.005 | 0.0068±0.0036 | 0.065 | 0.0061±0.0017 | <0.001 |
Effects of Seeding Density
When the data were binned by ρ (low, medium, high) and examined by ANOVA, the most influential predictor of construct functional properties was ρ, as it had significant effects on all properties (p < 10−6, Figure 5). The bins proved to be statistically distinct with respect to both G* and GAG content (Figure 5B & C), with higher ρ associated with higher functional properties (p < 0.0005). Collagen content was lowest in constructs with low ρ (p < 0.0005), and was similar for medium and high ρ (p = 0.11, Figure 5D). EY was highest for the high ρ constructs (p < 0.0005); at day 28, these constructs had the highest EY among groups and time points (p < 0.001, Figure 5A). Among all ρ at all time points, only high ρ constructs appreciably increased in size at day 28 (1.4× day 0 wet weight) and at day 42 (1.14× day 0 wet weight). From pairwise comparisons of different ρ’s from a common donor (performed on Donors 2–5), higher ρ constructs had significantly higher functional properties (Table 3). In addition, constructs with high ρ tended to exhibit greater opacity and resemblance to native cartilage (Figure 7D, H, L).
Table 3.
Pairwise comparisons between higher (ρ2) and lower (ρ1) cell density constructs for each donor, with values averaged across all time points and associated p-values. NS = not significant, N/A = not enough samples for statistical comparison.
| EY (kPa) | G* (kPa) | GAG (%/ww) |
COL (%/ww) |
|
|---|---|---|---|---|
| Donor 2 | ρ1: 42 | ρ1: 212 | ρ1: 1.30 | ρ1: 0.38 |
| ρ2: 93 | ρ2: 433 | ρ2: 2.32 | ρ2: 0.61 | |
| p < 0.001 | p < 0.0005 | p < 0.05 | p < 0.001 | |
| Donor 3 | ρ1: 58 | ρ1: 146 | ρ1: 0.42 | ρ1: 0.18 |
| ρ2: 78 | ρ2: 453 | ρ2: 3.63 | ρ2: 0.71 | |
| NS | p < 0.05 | p < 0.005 | N/A | |
| Donor 4 | ρ1: 27 | ρ1: 168 | ρ1: 1.08 | ρ1: 0.97 |
| ρ2: 212 | ρ2: 820 | ρ2: 4.39 | ρ2: 1.00 | |
| p < 0.0005 | p < 0.0005 | p < 0.0005 | NS | |
| Donor 5 | ρ1: 21 | ρ1: 97 | ρ1: 0.55 | ρ1: 0.50 |
| ρ2: 74 | ρ2: 482 | ρ2: 2.38 | ρ2: 1.24 | |
| p < 0.0005 | p < 0.0005 | p < 0.0005 | p < 0.0005 | |
Figure 7.
Histological stains and construct gross morphology. Stains of select samples by Picrosirius Red for collagen (A–B, E–F, I–J) and Safranin O for GAG (C, G, K), and photographs of respective constructs. Samples depicted have ρ = 90 × 106 cells/mL at D28 (A–D) and D56 (E–H), and ρ = 40 × 106 cells/mL at Day 56 (I–L).
Histology & Immunohistochemistry
Safranin O stains revealed GAG distributed throughout the constructs at day 28 of culture (Figure 7C). In contrast, collagen primarily occupied the pericellular regions whereas interterritorial staining was light (Figure 7A–B). GAG staining did not improve beyond day 28 (Figure 7C, G), and collagen staining appeared to fade (Figure 7A–B, E–F). The strongest stains for both GAG and collagen were found at construct centers. Lower ρ led to lighter staining (Figure 7I–K). Immunostaining for collagen in constructs showed deposition of types I and II collagen localized at construct peripheries (Figure 8B, E). Throughout construct interiors, type II collagen showed dark staining (Figure 8D), while type I collagen was nearly absent when compared with negative control (Figure 8A, C). ELISA for type II collagen confirmed that collagens were predominantly type II (76%) when normalized to total collagen content as determined by OHP assay.
Figure 8.
Representative immunohistochemical staining for types I (A–B) and II collagen (D–E) in D27 Donor 4 constructs (90 × 106 cells/mL) along with negative control (C & F). Scale bar = 200 µm.
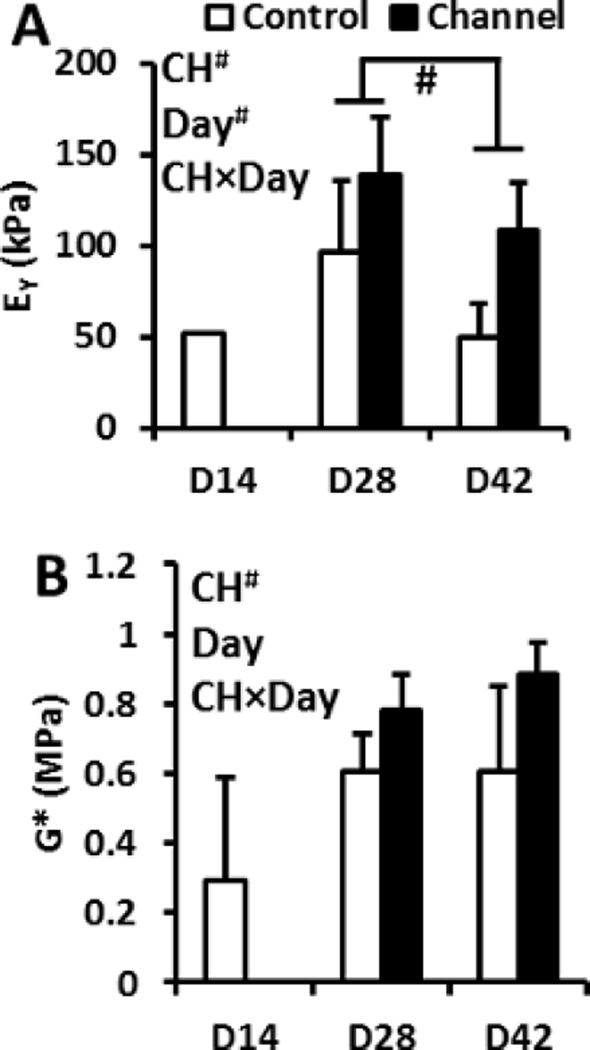
Nutrient Channel Incorporation
In constructs from Donor 6, nutrient channels were found to significantly influence mechanical properties, with both EY and G* showing marked enhancements (p < 0.005 and p < 0.01, respectively; Figure 9). Biochemical properties were not affected by channels (p > 0.05, data not shown), though constructs reached GAG and collagen levels of 5.7 ± 0.9 %/ww and 1.0 ± 0.4 %/ww, respectively. Consistent with constructs without channels from Donors 2–5, constructs from Donor 6 did not improve in mechanical properties over time beyond day 28, and EY showed a significant decline at day 42 (p < 0.05, Figure 9).
Figure 9.
EY (A) and G* (B) of constructs in presence or absence of nutrient channels. “#” denotes p < 0.05. ANOVA factors: CH = effect of channels, Day = culture duration, CH×Day = interaction. n = 4 for all groups.
DISCUSSION
In this study we present some of the most successful engineered human cartilage constructs to date. Despite sourcing most constructs’ cells from adult human osteochondral grafts that had exceeded their shelf lives, the cells were able to produce high-quality cartilage tissue constructs, the best of which rival our previous results in juvenile bovine and adult canine model systems.
Seeding density played the largest role in determining construct properties, as was revealed by multiple regression analysis and ANOVA of all samples combined (Table 2, Figure 5). Groups with lower ρ experienced less growth, particularly those with ρ similar to native human adult cartilage (~107 cells/mL)(Venn and Maroudas, 1977); this effect was most directly observed in constructs cast at different ρ from the same donor (Donors 2–5, Table 3). Impressively, constructs seeded at the highest cell density (ρ = 90 × 106 cells/mL) approached properties of human knee cartilage, with GAG levels of 5% (1–5 %/ww for native tissue) and Young’s moduli of 250 kPa (250–730 kPa for native tissue) (Fetter et al., 2006; Krishnan et al., 2003; Treppo et al., 2000). Though their collagen levels were low (1.5 %/ww) relative to native cartilage (~10 %/ww), both qualitative and quantitative analyses confirmed that the collagens produced are predominantly the type II collagens found in the native tissue (Figure 8). To the best of our knowledge, the EY and GAG levels we report are the highest for hACs in agarose to date, and are similar to those obtained for canine constructs after 28 days in culture (Bian et al., 2010a; Ng et al., 2009c). Though cells at high seeding densities may occupy appreciable fractions of the construct volume (~10–15%), any structural weaknesses potentially imposed by cells were evidently outweighed by increased levels of matrix synthesis. Synthesis rates of GAG by constructs with high ρ (80 pg GAG/cell·day) were comparable to those of juvenile bovine constructs (Nims et al., 2014). For practical considerations, Ø3 × 2.3 mm constructs were studied to permit the casting of a greater number of samples; in future studies, Ø4 mm human tissue constructs may be cultured to make more direct comparisons with bovine and canine constructs of similar size. These results strongly suggest that seeding densities in CTE studies employing hACs must be sufficiently high to reach native cartilage properties.
Although cells were sourced from six distinct donors, and some significant differences in collagen synthesis were noted in one donor (Figure 6B), we are hesitant to draw strong conclusions about donor variability. Though it may be expected that younger tissue would possess greater growth potential, the constructs exhibiting the most favorable properties had high ρ and were cast with cells originating from the oldest donor (Donor 4, Table 1), and cells from the youngest donor (Donor 3) synthesized the least collagen (Figure 6B). These observations, taken together with the higher construct properties resulting from higher ρ in pairwise comparisons for Donors 2–5 (Table 3), suggests that ρ is more impactful than donor-specific effects on construct growth. A post hoc power analysis of our results suggests that n in excess of 100 would be required to detect differences due to donor age and gender with 80% power, therefore these effects may be difficult to predict with the moderate n typically used for in vitro experiments. Therapies seeking to adapt allogeneic human constructs for clinical use would benefit from methods for prescreening and quality control of donor graft tissues.
Though hAC-laden constructs were similar in gross morphology and matrix content to bovine constructs, human constructs experienced plateauing growth. Unlike bovine constructs whose growth seemingly continues indefinitely (Nims et al., 2016; O’Connell et al., 2014), or canine constructs which have sustained growth for at least 42 days (Bian et al., 2010b), human constructs generally experienced no significant growth after four weeks in culture. This behavior is reflected by the regression analyses’ findings that only G* was positively correlated with culture duration after day 14 (Table 2). When binned by ρ, the constructs showed no significant increases in functional properties beyond day 28 (Figure 5). Histology corroborated this finding, with the intensity of GAG and collagen stains remaining similar or diminishing after day 28 (Figure 7). While the underlying causes of this growth plateau were not elucidated by this study, it is conceivable that benefits of growth factor priming during monolayer culture diminish over time. Transient TGF-β treatment, which has previously shown successes with juvenile bovine constructs, was ineffective for hACs (Figure 3), similar to our previous findings with passaged adult canine chondrocytes (Ng et al., 2008b). However, ongoing research suggests that beneficial effects of active TGF-β are limited to constructs’ surfaces and that more sophisticated delivery strategies may be necessary for homogeneous tissue growth (Albro et al., 2013; Albro et al., 2016). In these early experiments with hACs in 3D culture our knowledge of optimal growth factor treatments is not yet complete, and we are hopeful that improved delivery of TGF-β and other factors will prove useful in encouraging homogeneous and sustained growth of hAC-laden constructs.
Unlike our bovine constructs which retain up to ~90% of synthesized GAG (Nims et al., 2014), hAC-laden constructs retain less GAG (Figure 4B, D), and matrix was mostly localized at the construct center (Figure 7). Initial GAG retention of ~50% significantly decreased to ~35% at day 56 (Figure 4B). These low retention values may result from increased diffusivity of smaller GAGs typically produced by aged hACs (Buckwalter et al., 1994; Lee et al., 2013). The 2% agarose gels we commonly employ were originally titrated to optimize nutrient transport while retaining high levels of synthesized matrix by bovine cells (Ng et al., 2009a; Nims et al., 2014). Our group demonstrated that a denser agarose scaffold (3% w/v) results in significantly higher EY of hAC-laden constructs after four weeks of culture (Estell et al., 2015), suggesting that a more robust GAG network was retained by the hydrogel’s smaller pores. A more in-depth characterization of GAGs produced by these constructs could provide valuable insight for producing more robust human constructs.
The incorporation of nutrient channels elevated mechanical properties but not biochemical contents of hAC-laden constructs, reminiscent of our initial findings with channels in bovine constructs (Bian et al., 2009a). In later bovine studies, computational growth modeling and refining experimental techniques for cultivating channeled constructs revealed significant beneficial effects of channels on matrix growth (Ateshian et al., 2014; Cigan et al., 2014; Nims et al., 2016). Therefore, we believe that future studies more closely investigating the nutrient consumption rates and requirements of hAC-laden constructs could unlock the full potential of channels for this clinically relevant system.
Limitations of this culture system are similar to those we have observed in prior investigations with immature bovine and mature canine chondrocytes in agarose: the collagen content remains significantly below native levels, compromising functional mechanical properties under dynamic loading, since the dynamic compressive modulus G* correlates significantly with collagen content (Table 2). This limitation is evident in the values of G*, which peak at ~1 MPa (Figure 5), whereas native levels in human cartilage are estimated to be five to ten times greater (Krishnan et al., 2003). We previously investigated various strategies to alleviate this limitation, by subjecting constructs to dynamic compressive loading to enhance functional properties (Bian et al., 2010b; Lima et al., 2007; Mauck et al., 2000), temporarily digesting GAGs with chondroitinase ABC to delay construct swelling and mitigate steric exclusion of synthesized collagen (Bian et al., 2009b; O’Connell et al., 2014), degrading the agarose scaffold with agarase to similarly mitigate steric effects (Ng et al., 2009b), or supplementing culture media with higher levels of amino acids to enhance collagen synthesis (Ng et al., 2008a). Most of these strategies have shown statistically significant improvements, though none have raised collagen content or dynamic modulus to native levels. Therefore we will continue to investigate alternative strategies to enhance collagen content, using bovine and canine chondrocytes as testing systems prior to applying them to hACs.
This study provides an in-depth characterization of human chondrocytes in agarose for CTE, in which we expanded upon recent results (O’Connell et al., 2015) and adapted techniques that have previously proven successful in engineered bovine and canine cartilage. This technique of growing human constructs from expired graft tissue has encouraging implications for engineering allogeneic cartilage implants for treatment of osteoarthritic joints. Though we have identified new obstacles posed by these human cells, such as plateauing growth and low matrix retention by constructs, we propose promising strategies for successful growth of human tissue. High initial cell seeding density plays a key role in construct growth, and benefits of nutrient channels suggest that even greater functional properties may be achieved. The grafts used were predominantly expired, yet results were comparable to those from the single fresh graft, suggesting that expired osteochondral allografts from tissue banks may serve as reliable sources of hACs for clinical applications of CTE. Such applications may significantly reduce waste of donated tissues since expired allografts are otherwise often discarded. With the insights gleaned from these experiments, we expect that future studies will provide further successes for translating cartilage tissue engineering to human cell sources.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under Award Numbers R01AR060361, T32AR059038, R01 DE016525 and P41 EB002520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study includes research supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE 11-44155. We also thank the Musculoskeletal Transplant Foundation for providing graft tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Dr. James L. Cook serves on the Medical Board of Trustees of the Musculoskeletal Transplant Foundation. This service has facilitated our access to fresh and expired osteochondral allografts. However, the MTF was not involved in any aspect of this study. The authors have no further potential conflicts of interest associated with this study.
REFERENCES
- Adkisson HDt, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Nims RJ, Cigan AD, Yeroushalmi KJ, Alliston T, Hung CT, Ateshian GA. Accumulation of exogenous activated TGF-beta in the superficial zone of articular cartilage. Biophys J. 2013;104:1794–1804. doi: 10.1016/j.bpj.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Nims RJ, Durney KM, Cigan AD, Shim JJ, Vunjak-Novakovic G, Hung CT, Ateshian GA. Heterogeneous engineered cartilage growth results from gradients of media-supplemented active TGF-beta and is ameliorated by the alternative supplementation of latent TGF-beta. Biomaterials. 2016;77:173–185. doi: 10.1016/j.biomaterials.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre-Aguaron E, Sampat SR, Xiong JC, Colligan RM, Bulinski JC, Cook JL, Ateshian GA, Brown LM, Hung CT. Growth factor priming differentially modulates components of the extracellular matrix proteome in chondrocytes and synovium-derived stem cells. PLoS One. 2014;9:e88053. doi: 10.1371/journal.pone.0088053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateshian GA, Nims RJ, Maas S, Weiss JA. Computational modeling of chemical reactions and interstitial growth and remodeling involving charged solutes and solid-bound molecules. Biomechanics and Modeling in Mechanobiology. 2014:1–16. doi: 10.1007/s10237-014-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis & Rheumatism. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–230. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A. 2014;111:6940–6945. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Angione SL, Ng KW, Lima EG, Williams DY, Mao DQ, Ateshian GA, Hung CT. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage. 2009a;17:677–685. doi: 10.1016/j.joca.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Crivello KM, Ng KW, Xu D, Williams DY, Ateshian GA, Hung CT. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A. 2009b;15:2065–2072. doi: 10.1089/ten.tea.2008.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, Hung CT. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010a;16:1781–1790. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, Hung CT. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010b;16:1781–1790. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137–1145. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CT, Thorpe SD, Kelly DJ. Engineering of large cartilaginous tissues through the use of microchanneled hydrogels and rotational culture. Tissue Eng Part A. 2009;15:3213–3220. doi: 10.1089/ten.TEA.2008.0531. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Roughley PJ, Rosenberg LC. Age-Related changes in cartilage proteoglycans: Quantitative electron microscopic studies. Microscopy Research and Technique. 1994;28:398–408. doi: 10.1002/jemt.1070280506. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;11:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan AD, Nims RJ, Albro MB, Esau JD, Dreyer MP, Vunjak-Novakovic G, Hung CT, Ateshian GA. Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue Eng Part A. 2013a;19:1941–1948. doi: 10.1089/ten.tea.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan AD, Nims RJ, Albro MB, Hung CT, Ateshian GA. Effects of media stirring and presence of nutrient channels on functional properties of large engineered cartilage constructs. Proceedings of the ASME Summer Bioengineering Conference; Sunriver, OR, USA. 2013b. [Google Scholar]
- Cigan AD, Nims RJ, Albro MB, Vunjak-Novakovic G, Hung CT, Ateshian GA. Nutrient channels and stirring enhanced the composition and stiffness of large cartilage constructs. J Biomech. 2014;47:3847–3854. doi: 10.1016/j.jbiomech.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mara CS, Duarte AS, Sartori-Cintra AR, Luzo AC, Saad ST, Coimbra IB. Chondrogenesis from umbilical cord blood cells stimulated with BMP-2 and BMP-6. Rheumatol Int. 2013;33:121–128. doi: 10.1007/s00296-011-2328-6. [DOI] [PubMed] [Google Scholar]
- Dehne T, Karlsson C, Ringe J, Sittinger M, Lindahl A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res Ther. 2009;11:R133. doi: 10.1186/ar2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis & Rheumatism. 2001;44:1608–1619. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Estell EG, Tan AR, Bansal S, Ateshian GA, Hung CT. Modulation of hydrogel crosslinking density to promote development of functional mechanical properties in engineered cartilage. Orthopaedic Research Society 2015 Annual Meeting; Las Vegas, NV, USA. 2015. [Google Scholar]
- Fernandes AM, Herlofsen SR, Karlsen TA, Küchler AM, Fløisand Y, Brinchmann JE. Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. 2013 doi: 10.1371/journal.pone.0062994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter NL, Leddy HA, Guilak F, Nunley JA. Composition and transport properties of human ankle and knee cartilage. J Orthop Res. 2006;24:211–219. doi: 10.1002/jor.20029. [DOI] [PubMed] [Google Scholar]
- Francioli SE, Martin I, Sie CP, Hagg R, Tommasini R, Candrian C, Heberer M, Barbero A. Growth factors for clinical-scale expansion of human articular chondrocytes: relevance for automated bioreactor systems. Tissue Eng. 2007;13:1227–1234. doi: 10.1089/ten.2006.0342. [DOI] [PubMed] [Google Scholar]
- Ghone NV, Grayson WL. Recapitulation of mesenchymal condensation enhances in vitro chondrogenesis of human mesenchymal stem cells. J Cell Physiol. 2012;227:3701–3708. doi: 10.1002/jcp.24078. [DOI] [PubMed] [Google Scholar]
- Handorf AM, Li WJ. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One. 2011;6:e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kafienah We, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue engineering. 2002;8:817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- Kelly TA, Roach BL, Weidner ZD, Mackenzie-Smith CR, O’Connell GD, Lima EG, Stoker AM, Cook JL, Ateshian GA, Hung CT. Tissue-engineered articular cartilage exhibits tension-compression nonlinearity reminiscent of the native cartilage. J Biomech. 2013;46:1784–1791. doi: 10.1016/j.jbiomech.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R, Park S, Eckstein F, Ateshian GA. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. J Biomech Eng. 2003;125:569–577. doi: 10.1115/1.1610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Han L, Roughley PJ, Grodzinsky AJ, Ortiz C. Age-related nanostructural and nanomechanical changes of individual human cartilage aggrecan monomers and their glycosaminoglycan side chains. J Struct Biol. 2013;181:264–273. doi: 10.1016/j.jsb.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Martin F, Mannigel K, Kaltschmidt K, Sack U, Anderer U. Three-dimensional scaffold-free fusion culture: the way to enhanced chondrogenesis of in vitro propagated human articular chondrocytes. European journal of histochemistry: EJH. 2013:57. doi: 10.4081/ejh.2013.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Case ND, Guldberg RE, Plaas AH, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Murphy MK, Huey DJ, Hu JC, Athanasiou KA. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells. 2015;33:762–773. doi: 10.1002/stem.1890. [DOI] [PubMed] [Google Scholar]
- Ng KW, Ateshian GA, Hung CT. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Engineering Part A. 2009a;15:2315–2324. doi: 10.1089/ten.tea.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, DeFrancis JG, Kugler LE, Kelly TA, Ho MM, O’Conor CJ, Ateshian GA, Hung CT. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2008a;35:433–438. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, Kugler LE, Doty SB, Ateshian GA, Hung CT. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthritis Cartilage. 2009b:17. doi: 10.1016/j.joca.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, Lima EG, Bian L, O’Conor CJ, Jayabalan PS, Stoker AM, Kuroki K, Cook CR, Ateshian GA, Cook JL, Hung CT. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: A canine model. Tissue Eng Part A. 2009c;16:1041–1051. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, Lima EG, Bian L, O’Conor CJ, Jayabalan PS, Stoker AM, Kuroki K, Cook CR, Ateshian GA, Cook JL, Hung CT. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041–1051. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, O’Conor CJ, Lima EG, Lo SB, Ateshian GA, Cook JL, Hung CT. Primed mature canine chondrocytes can develop an engineered cartilage tissue with physiologic properties. Trans Orthop Res Soc. 2008b:599. [Google Scholar]
- Nims RJ, Cigan AD, Albro MB, Hung CT, Ateshian GA. Synthesis rates and binding kinetics of matrix products in engineered cartilage constructs using chondrocyte-seeded agarose gels. J Biomech. 2014;47:2165–2172. doi: 10.1016/j.jbiomech.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nims RJ, Durney KM, Cigan AD, Dusséaux A, Hung CT, Ateshian GA. Continuum theory of fibrous tissue damage mechanics using bond kinetics: application to cartilage tissue engineering. Interface Focus. 2016:6. doi: 10.1098/rsfs.2015.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell GD, Nims RJ, Green J, Cigan AD, Ateshian GA, Hung CT. Time and dose-dependent effects of chondroitinase ABC on growth of engineered cartilage. Eur Cell Mater. 2014;27:312–320. doi: 10.22203/ecm.v027a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell GD, Tan AR, Cui V, Bulinski JC, Cook JL, Attur M, Abramson SB, Ateshian GA, Hung CT. Human chondrocyte migration behaviour to guide the development of engineered cartilage. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clinical orthopaedics and related research. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- Saha S, Kirkham J, Wood D, Curran S, Yang XB. Informing future cartilage repair strategies: a comparative study of three different human cell types for cartilage tissue engineering. Cell and tissue research. 2013;352:495–507. doi: 10.1007/s00441-013-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittinger M, Bujia J, Minuth W, Hammer C, Burmester G. Engineering of cartilage tissue using bioresorbable polymer carriers in perfusion culture. Biomaterials. 1994;15:451–456. doi: 10.1016/0142-9612(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D–engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32:8927–8937. doi: 10.1016/j.biomaterials.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Annals of the rheumatic diseases. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Blasioli DJ, Kim HJ, Kim HS, Kaplan DL. Cartilage tissue engineering with silk scaffolds and human articular chondrocytes. Biomaterials. 2006;27:4434–4442. doi: 10.1016/j.biomaterials.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Wenger R, Hans MG, Welter JF, Solchaga LA, Sheu YR, Malemud CJ. Hydrostatic pressure increases apoptosis in cartilage-constructs produced from human osteoarthritic chondrocytes. Front Biosci. 2006;11:1690–1695. doi: 10.2741/1914. [DOI] [PubMed] [Google Scholar]
- Zhao X, Bichara DA, Ballyns FP, Yoo JJ, Ong W, Randolph MA, Bonassar LJ, Gill TJ. Properties of Cartilage Engineered from Elderly Human Chondrocytes for Articular Surface Repair. Tissue Engineering Part A. 2012;18:1490–1499. doi: 10.1089/ten.TEA.2011.0445. [DOI] [PubMed] [Google Scholar]
- Zhou S, Cui Z, Urban JPG. Nutrient gradients in engineered cartilage: metabolic kinetics measurement and mass transfer modeling. Biotechnol Bioeng. 2008;101:408–421. doi: 10.1002/bit.21887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.